Abstract
Exosomes are a subgroup of extracellular bilayer membrane nanovesicles that are enriched in a variety of bioactive lipids, receptors, transcription factors, surface proteins, DNA, and noncoding RNAs. They have been well recognized to play essential roles in mediating intercellular signaling by delivering bioactive molecules from host cells to regulate the physiological processes of recipient cells. In the context of heart diseases, accumulating studies have indicated that exosome-carried cellular proteins and noncoding RNA derived from different types of cardiac cells, including cardiomyocytes, fibroblasts, endothelial cells, immune cells, adipocytes, and resident stem cells, have pivotal roles in cardiac remodeling under disease conditions such as cardiac hypertrophy, diabetic cardiomyopathy, and myocardial infarction. In addition, exosomal contents derived from stem cells have been shown to be beneficial for regenerative potential of the heart. In this review, we discuss current understanding of the role of exosomes in cardiac communication, with a focus on cardiovascular pathophysiology and perspectives for their potential uses as cardiac therapies.
Keywords: cardiac remodeling, exosomes, fibrosis, inflammation, stem cells
INTRODUCTION
Extracellular vesicles (EVs) are a family of lipid bilayer bound vesicles released from cells and typically contain various bioactivated molecules including lipids, proteins, and nucleic acids. There are three major subtypes of Evs, exosomes, microvesicles, and apoptotic bodies, that differ depending on size, content, biogenesis, and function (1). In recent years there has been a dramatic rise in interest surrounding exosomes due to their ability to act as intercellular messengers as well as their disease diagnosis and therapeutic potential (2).
Initially, it was proposed that exosomes had little impact on neighboring cells and were considered by-products of cellular homeostasis by acting as a mechanism to remove cellular debris (3). However, in recent decades, it has been discovered that, following their release from cells, exosomes mediate intracellular signaling by transferring their molecular content, involved in both pathological and physiological processes, to recipient cells, resulting in functional change. Exosomes can act as a form of paracrine or endocrine signaling by acting on neighboring cells or distant cells, respectively (4).
Significant attention has focused on the role of exosomes during human diseases, including cancer (5), neurodegenerative disease (6), infectious diseases (7), and kidney disease (8). Accumulating evidence in the field of cardiovascular diseases (CVDs) suggests that exosomes play an essential role during cardiac pathophysiology. The cardiovascular system consists of numerous cell types that communicate via cell-cell interaction or paracrine interaction to maintain cardiac homeostasis and function (9). Diabetic cardiomyopathy, myocardial infarction, cardiac hypertrophy, and heart failure are just some of the pathologies that alter cardiac homeostasis and, if allowed to progress without medical intervention, will ultimately lead to death (10).
Despite significant advancements in therapeutic options, CVDs remain the leading cause of death worldwide, demonstrating the need to better understand the disease pathophysiology. Accumulating studies have shown that exosomes derived from various cardiac cells such as cardiomyocytes (11), endothelial cells (12), fibroblasts (13), immune cells (14), and resident stem cells (15) play an essential role in mediating intracellular signaling during disease pathophysiology. This suggests that they are key players in disease progression and have the potential to act as novel biomarkers or therapeutic agents (9).
This review aims to provide insight into exosome molecular structure, biogenesis, and uptake as well as strategies for their isolation. Furthermore, a comprehensive review of the current knowledge surrounding the role of exosome cell-to-cell signaling during numerous CVDs will be summarized. Finally, the feasibility of exosome-based intercellular signaling in stem-cell transplant therapy as a potential cardiac treatment will be discussed.
PHYSICAL AND PHYSIOLOGICAL PROPERTIES OF EXOSOMES
Structure and Components of Exosomes
Exosomes are nanovesicles, ranging from 30 to 100 nm in diameter, with a density of ∼1.10–1.20 g/mL (16). They are enveloped by a lipid bilayer that is enriched with a range of proteins that facilitate exosome function (17). Studies using negative staining have shown that exosomes have a cup-shaped morphology (18). However, this is due to the drying process of the technique, which results in artifact formation, and it is not a true representation of exosome structure. Instead, cryogenic transmission electron microscopy (TEM) has demonstrated that in aqueous solution exosomes have a spherical structure (19).
Due to their endocytic origin, the constituents of the exosome bilayer contain molecular characteristics similar to those of the parent cells, so they reflect the cell type from which they originate and its physiological state, either healthy or pathological (20). However, there are still some differences; for example, the endothelial marker CD144 is expressed on human umbilical vein endothelial cells but not on the exosomes released from these cells (21). Exosomes are highly diverse; it has been demonstrated that exosomes from the same cell type display a large degree of heterogeneity. Waldenström et al. (2012) showed using flow cytometry that 30% of exosomes originating from cardiomyocytes expressed carveolin-3 and 80% were positive for flotillin-1 (22).
Membrane proteins.
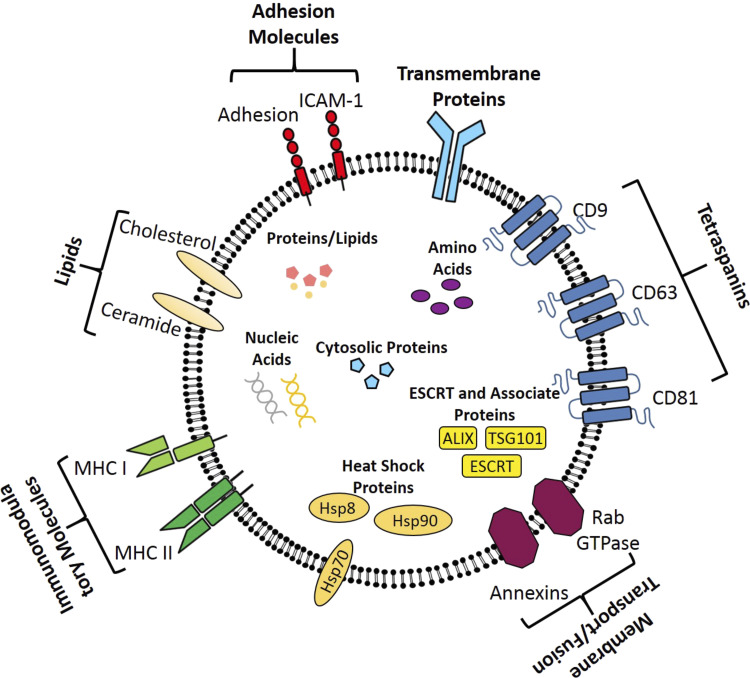
The majority of molecular studies focusing on exosome structure are limited to exosomes from a healthy state, and few have focused on exosomes in a diseased state (23). However, irrespective of the parent cell, exosomes express certain membrane proteins. The surface of exosome bilayer is enriched with tetraspanins, heat shock protein (Hsp), endosomal sorting complex required for transport (ESCRT) proteins, adhesion proteins, and other membrane proteins that facilitate exosome function (24). A summary of the exosome structure can be seen in Fig. 1.
Figure 1.
Typical structure of exosomes, including key membrane proteins and components of the exosome cargo such as nucleic acids, cytosolic proteins, amino acids, and biogenesis proteins.
Tetraspanins (notably CD9, CD63 and CD81) are abundantly expressed on the exosome membrane and are frequently used as exosome biomarkers. They are transmembrane proteins that regulate exosome migration, adhesion, and fusion to the recipient cell (25). Another key protein located on the surface of exosomes are the major histocompatibility complexes (MHCs). MHC II is restricted to exosomes derived from antigen-presenting cells including, macrophages, dendritic cells, and B cells, whereas MHC I is ubiquitously expressed on all exosomes (26). Hsp, including Hsp8 and Hsp90 have also been identified on exosomes from both healthy and diseased cells (26). Their molecular functions involve protein folding/unfolding, biosynthesis, and transport (27). Hsp70 is also present in cardiomyocyte-derived exosomes (28) and has been linked to cardiomyocyte survival following ischemia-reperfusion injury (29), whereas Hsp60 has been shown to induce apoptosis in nearby cardiomyocytes following myocardial injury (30).
Furthermore, other proteins are incorporated into the exosome membrane during biogenesis. As the majority of exosomes are produced via the endosomal sorting complexes required for transport (ESCRT) and associated protein pathway, the proteins associated with this pathway are often still present on exosomes after their release into the extracellular environment (31). Endosomal proteins such as tumor susceptibility gene 101 (TSG101) and ALG-2 interacting protein X (ALIX) are also present (32). Furthermore, proteins involved in membrane transport, fusion, and intracellular uptake including, flotillin (33), annexins (34), and Rab GTPase proteins are expressed on the surface (35). Other common proteins expressed on exosomes include GTPase proteins (EEF1A1 and EEF2) and cytoskeletal proteins (26).
In addition, adhesion molecules are abundantly expressed and regulate exosome migration, adhesion to recipient cells, and subsequent uptake (36). Key adhesion molecules include intercellular adhesion molecule 1 (ICAM-1) and integrin (37). The exosome bilayer is also enriched with an asymmetric distribution of various lipids, including ceramide, cholesterol, sphingomyelin, phosphatidylserine, phosphatidylethanolamine, and glycerophospholipids (38). They are primarily involved in exosome recognition and uptake. Of note, the expression of the various lipids can be up to four times greater on exosomes than on their parent cell (39), which aid in exosome fusion with the target cell (40).
Exosomes from different cell lineages express additional markers. For example, cardiac progenitor cell-derived exosomes also express markers CD73, CD90, and CD105 but not hematopoietic markers such as CD34 and CD45, suggesting that the presence of specific exosome surface markers could aid the identification of cardiac exosomes (41). However, currently, it is difficult to differentiate exosomes from different cell types due to a lack of established cell-specific markers (20).
Exosome content.
Exosome content differs depending on the cell of origin and the stimuli (42). Another key factor regulating exosome content is the cell disease status. The majority of cells in the cardiovascular system release exosomes; however, in response to different conditions such as inflammation or hypoxia (43), the exosome content varies to either promote improved or impaired cardiac function (44).
The typical content of exosomes includes a variety of bioactivate molecules, including DNA, RNA, mRNA, microRNA, lipids, proteins, amino acids, and metabolites. Growth factors, transcription factors, and cytokines have also been identified (1). It has been proposed that specific mechanisms are involved in regulating RNA loading into exosomes, as the maternal cell RNA content and the subsequent exosome content differ. It has been suggested that RNA-binding proteins hnRNPA2B1 (45), XP05, and MVP are key players in this process by mediating RNA sorting into exosomes (46). Cardiac exosomes also contain mitochondrial, cytosolic, and sarcomere proteins (30). An increasing number of studies have shown that inflammatory factors including TNFα and IL-6 are also present in cardiomyocyte-derived exosomes as well as Hsps, which are involved in cardiac remodeling (47), survival, and growth (48).
Biogenesis and Secretion of Exosomes
The stimulation of exosome biogenesis is initiated by numerous factors. In terms of cardiomyocytes, studies have shown that pathological conditions can stimulate exosome release, for example, hypoxia, myocardial infarction (MI) (49), inflammation (50), and glucose starvation (51).
Biogenesis.
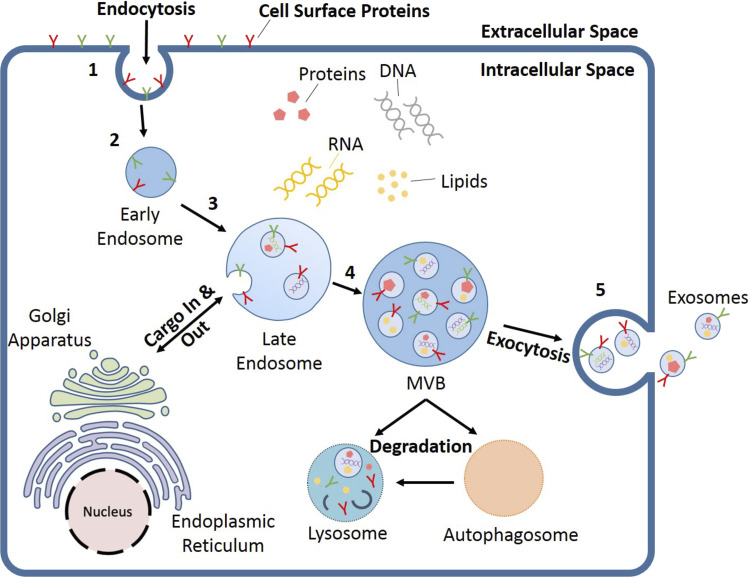
Exosome biogenesis involves the double inward invagination of the plasma membrane and the generation of multivesicular bodies (MVBs) that subsequently fuse with the plasma membrane before being released into the extracellular environment (52). The first plasma membrane invagination forms early endosomes that are enriched with specific cell membrane proteins. Early endosomes can exist independently or merged with preexisting early endosomes. They then mature into late endosomes, which involves the second inward invagination of the plasma membrane, generating MVBs containing intraluminal vesicles (ILVs). During this process, proteins, lipids, nucleic acids, and other bioactive molecules are incorporated into the ILVs. The Golgi apparatus and the endoplasmic reticulum are involved in packaging bioactive molecules into the MBVs. A single MVB often contains numerous ILVs. The majority of MVBs fuse with the cell plasma membrane, releasing the ILVs, now known as exosomes, into the extracellular environment. Alternatively, some MVBs fuse with autophagosomes or lysosomes for degradation and recycling (53). This process has been summarized in Fig. 2.
Figure 2.
Biogenesis of exosomes. Membrane invagination (1) results in the formation of an endocytic vesicle known as early endosome (2), which matures into the late endosome (3). Subsequently, the Golgi apparatus and endoplasmic reticulum incorporate various bioactive molecules into the late endosome, producing the multivesicular body (MVB) (4). The MVB can then either undergo degradation via the lysosome or autophagosome or fuse with the plasma membrane to release exosomes into the extracellular environment (5).
Secretion.
Exosome secretion into the extracellular environment involves transporting the MVBs to the plasma membrane, which is achieved by interactions between actin and the microtubule cytoskeleton (54). Live-cell imaging studies have demonstrated that knockdown of cortactin, an actin-binding protein, results in decreased exosome release (55). Several other proteins also contribute to exosome secretion, such as Rab GTPases including, Rab11, Rab27a, Rab27b, and Rab35. Both Rab11 and Rab35 facilitate the fusion of the exosome to the plasma membrane. Studies have shown that inhibition of Rab35 function results in impaired exosome secretion, resulting in their intracellular accumulation (56). Furthermore, soluble N-ethylmaleimide-sensitive fusion attachment protein receptors (SNAREs) are specifically involved in fusing MVBs with the plasma membrane (57).
It is widely accepted that the ESCRT pathway is highly involved in exosome biogenesis and secretion (58). This pathway is an evolutionarily conserved group of approximately 20 proteins that assemble into four key structures, ESCRT-0, I, II, and III, with associated proteins VPS20-associated protein-1 (VTA1), ALIX, and vacuolar protein sorting-associated protein-4 (VPS4). Physiological and pathological changes such as ischemia and inflammation trigger the ubiquitination of molecules in the cell, and this pathway packages these biomolecules into ILVs during exosome biogenesis (59). ESCRT-0, I, and II form a stable heteromultimer on the endosomal membrane by binding to the ubiquitin molecule on ubiquitylated biomolecules and the endosomal lipid phosphatidylinositol 3-phosphate (PI3P). ESCRT-I contains an elongated heterodimer that binds to ESCRT-0 and ESCRT-II at opposite ends. ESCRT-III exists in an inactive state in the cytoplasm; however, subsequent binding of ESCRT-I/II leads to ESCRT-III activation and recruitment to the late endosome. Once the associated proteins bind, the ESCRT-III complex is complete. ESCRT-III complex mediates membrane scission and vesicle budding. The ESCRT-III subunit SNF7 is involved in producing polymeric spiral-shaped constructs on the membrane of endosomes. This induces membrane curvature, producing the ILVs (60). The associated protein ALIX recruits degradation of α-4 (Doa4), a deubiquitinating enzyme, to deubiquitinate biomolecules before they enter the ILVs (61). Once the content has entered the ILVs, the VPS4-VTA1 complex disassembles the ESCRT-III complex (62).
It is now emerging that an ESCRT-independent pathway may also play a role in exosome biogenesis. This pathway usually involves protein aggregation (63), lipid rafts (64), or ceramide (65). It is also suggested that tetraspanin proteins participate in exosome biogenesis independently of the ESCRT pathway; however, this largely depends on the cell type (66). For example, studies have shown that cancer cells are capable of ESCRT-independent exosome biogenesis via the tetraspanin protein CD63 (67). However, it is currently unknown whether a similar mechanism occurs in other, noncancerous cells (66).
Esosome uptake.
The uptake of exosomes can be divided into three stages, targeting recipient cells, entering recipient cells, and release of the exosome cargo into the cell. Despite the fact that a large number of studies are investigating the use of exosomes therapeutically, the mechanisms by which they are up taken by recipient cells are not completely known.
It is unclear whether exosome uptake by recipient cells is random or specific. It is thought that the capture of exosomes depends largely on the membrane factors present on both the exosome and the target cell. Furthermore, it is not known whether the extracellular environment can influence the method of exosome internalization (68) and also whether the mode of exosome uptake by the recipient cells affects the functional outcomes or degradation of the exosome constituents (53). However, it is known that the exosomes’ uptake is not restricted to the same type of cells, as studies have shown that exosomes released from cardiomyocytes are able to be internalized by endothelial cells (69) and cardiac fibroblasts (22).
There are two key mechanisms of exosome internalization by recipient cells that have been identified, direct fusion and receptor-ligand interaction, although their exact signaling pathways are unclear (70). The direct fusion of the exosome with the plasma membrane of the recipient cells occurs via numerous fusion-associated proteins such as SNAREs and Rab GTPases (71). The lipid bilayers are brought into proximity to one another, allowing for the formation of a hemifusion stalk. Expansion of the stalk creates the hemifusion diaphragm bilayer with a fusion pore. Eventually, as the two hydrophobic cores combine, the exosome content enters the recipient cell (72).
The receptor-ligand interaction pathway usually occurs through endocytosis, when exosome surface proteins interact with molecules on the plasma membrane of the recipient cell. These molecules include integrins, proteoglycans, lectins (70), or other sphingolipid/cholesterol domains. This process can occur via either a clathrin-dependent or a clathrin-independent pathway. In the clathrin-dependent pathway, clathrin and adaptor protein 2 complex induce the invagination of the recipient cell plasma membrane, aiding exosome uptake (73). The clathrin-independent pathway mainly involves RhoA, ADP-ribosylation factor 6, or caveolin-1, which is a protein required for the formation of caveolae. These are lipid-raft-mediated plasma membrane invaginations. This process regulates exosome endocytosis using the proteins flotillin and dynamin (71).
Isolation of exosomes.
Since growing research is now focusing on the clinical utilization and therapeutic potential of exosomes, it is essential to optimize their isolation and characterization to obtain high-quality, reliable data. Exosomes have been isolated from various biological fluids, including, plasma, urine, synovial fluid, cerebrospinal fluid, nasal secretions, amniotic fluid, semen, bile, saliva, bronchoalveolar lavage, and conditioned cell culture media (66). However, biological fluids contain a range of exosomes from different origins as well as other biological components, so it is vital that effective separation occurs before further downstream analysis. Numerous approaches to isolate exosomes have been developed, including ultracentrifugation, filtration, immunoaffinity, precipitation, and chromatography, which are based on parameters such as size, mass, affinity, and density. Of note, although all these methods allow the isolation of samples enriched with exosomes, it is difficult to achieve homogenous pure isolation, leading to large amounts of variability in data (74). A summary of the various advantages and disadvantages of the various isolation techniques can be seen in Table 1.
Table 1.
Summary of the key advantages and disadvantages of the various exosome isolation techniques including ultracentrifugation, ultrafiltration, size exclusion chromatography, immunoaffinity, and polymer precipitation
| Exosome Isolation Technique | Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Differential ultracentrifugation | Density | Requires a small volume of reagents Suitable for large volumes |
Labor intensive Time consuming Large expensive equipment Not suitable for small volume samples Exosome rupture/aggregation |
(74, 75) |
| Density gradient ultracentrifugation | Density | Separates different EV subgroups Less exosome rupture/aggregation Requires a small volume of reagents Suitable for large volumes |
Labor intensive Time consuming Large expensive equipment Not suitable for small volume samples |
(76, 77) |
| Ultrafiltration | SizeMolecular weight | No large, expensive equipment Relatively fast |
Filter pore clogging Moderate purity Exosome deformation |
(78, 79, 80) |
| Size-exclusion chromatography | Size | Exosome structure and function are preserved Relatively fast Requires few reagents High Purity Little sample loss |
Lipoprotein coisolation | (78, 81) |
| Immunoaffinity | Exosome surface markers | High exosome yieldHigh purity | Antigen masking Exosome damage |
(82, 83) |
| Polymer precipitation | Precipitation | No large, expensive equipment Exosome structure and function are preserved |
Coisolation of EV, proteins, and lipoproteins Exosome aggregation |
(66, 84) |
EV, extracellular vesicles.
Characterization.
Following isolation, it is important to characterize and quantify the isolated exosomes to ensure successful separation and for downstream application. To address this, numerous techniques have been developed, including nanoparticle tracking analysis (NTA) (75), TEM (76), flow cytometry, atomic force microscopy (AFM), dynamic light scattering (DLS), and Western blot. NTA can accurately quantify the number of exosomes and calculate their relative size by analyzing the laser light-scattering characteristics of biological particles in solution undergoing Brownian motion. The exosomes can be tracked, and their hydrodynamic radius can be calculated. This approach is quick, with little sample preparation. Moreover, fluorescently labeled antibodies can bind to antigens present on the exosome surface, allowing exosome morphological characterization (85).
TEM is considered the gold standard for detecting the presence of exosomes despite the fact that it can be time consuming and requires extensive sample preparation, which may induce morphological changes to the exosomes, limiting data regarding exosome phenotype (86). However, a recent study demonstrated that cryogenic TEM was able to provide insight into exosome surface structure and morphology, indicating that it has the potential to distinguish between different subgroups of EVs as well as other biological components (76).
Conventional flow cytometry is another common technique, which involves passing a laser beam at a specific wavelength through exosome particles in a sheath fluid. As the laser hits the exosome, the light is scattered and recorded. The exosome can also be labeled with fluorescent dyes to aid morphological analysis. However, this technique is limited to detecting EVs greater then 500 nm in size; therefore, a significant number of exosomes remain undetected in a sample (21). To address this issue, a range of high-end flow cytometers with higher sensitivity have been developed that are capable of detecting exosomes ∼200 nm in size (78). These flow cytometers have shown to be comparable to TEM, overcoming the limitations associated with TEM such as sample preparation and high cost (79).
AFM can determine exosome morphology, concentration bimolecular content, and biomechanics of individual exosomes in a sample. This technique requires minimal sample preparation, and the majority of exosome remain unchanged. Exosomes are immobilized on an antibody-coated surface, and the AFM scanning tip runs over the samples, gathering data based on tip oscillation amplitude and oscillation frequency (80). Several studies have demonstrated the effectiveness of ATM by characterizing exosomes in a range of biofluids such as saliva (82) and blood (83).
DLS involves passing a monochromatic laser beam through a suspension of exosome particles. This technique is capable of measuring the size of the exosomes; however, it is unable to provide any bimolecular data. Furthermore, although this method is relatively simple, the presence of large vesicles can mask the detection of smaller exosomes. Nevertheless, the potential of this approach has been shown by characterizing the exosomes from biofluids and cultured cardiomyocytes (83, 84).
The range of exosome-specific markers such as ALIX can also be detected using Western blot analysis (81). For example, in terms of cardiomyocytes, Hsp70 is a specific cardiomyocyte-derived exosome marker. Many studies investigating cardiomyocyte-derived exosomes have also examined the expression of tetraspanins, particularly CD9 and CD81 (9). Although this approach can collectively analyze exosomes from a sample, it cannot distinguish the morphology of a single exosome. It also does not provide accurate data regarding exosome concentration (87).
Currently, the field of exosome research is restricted by the caveats associated with the isolation of exosomes and also the ability to track exosomes in vivo under an appropriate resolution. Despite these limitations, large amounts of reliable data are emerging. The role of exosomes during cardiac dysfunction and disease is also a promising field, as it could allow scientists to better understand the complex signaling underlying cardiac pathophysiology. The therapeutic potential of exosomes is also being actively explored, due to their ability to deliver specific cargo to diseased cells with minimal immune response (77). Their role as a biomarker of disease is also being explored (88).
EXOSOME-BASED INTERCELLULAR SIGNALING IN HEART DISEASES
The adult mammalian heart is built by a heterogeneous cell population where cardiomyocytes account for 70–80% of the heart volume but only 25–35% of the total cell count; and the rest is collectively named nonmyocytes, including fibroblasts, endothelial cells, pericytes (perivascular cells), and telocytes (89). Fibroblasts, the most abundant nonmyocytes (60–70%) are responsible for the turnover of an extracellular matrix and support the cardiac structure, while endothelial cells and pericytes form the innermost monolayer of the blood vessels, allowing molecular exchanges between circulating blood and surrounding tissues as well as maintaining homeostasis (90). Cardiac telocytes are a group of interstitial cajal-like cells that were first identified in 2010 by TEM. They have extremely thin and long telopodes that contribute to the epicardium and myocardium of the heart (91). Interestingly, macrophages are also an endogenous part of the myocardial tissues, where they have spindle-like shapes and are interspersed among cardiomyocytes and nonmyocytes. Further studies demonstrated that macrophages are importantly required for cardiac repair and electrical conduction in the heart (92, 93). Therefore, intercellular communication between these cell types is critically important for maintaining the structural integrity and the physiological homeostasis of the heart. It also plays a critical role in the pathogenesis of heart diseases. The role of exosome-derived miRNAs on cardiac pathology has been summarized in Table 2. The following sections will discuss the roles of exosomes in cell-to-cell communication to mediate cardiac remodeling and cardiac dysfunction.
Table 2.
Example of miRNA found in exosomes and their role in various aspects of cardiac disease, including hypertrophy, fibrosis, apoptosis, angiogenesis, and inflammation as well as their target mechanisms
| miRNA | Target | Cardiac Implication | Disease Model | References |
|---|---|---|---|---|
| ↑ miR-21* | ↓ SORBS2 and PDLIM5 expression ↑ Akt and VEGF activation |
↑ Hypertrophy ↑ Angiogenesis |
TAC Ang II minipump LAD ligation |
(96) (118) |
| ↓ miR-155 ↑ miR-155 |
↓ Socs1 expression ↑ Jaird2 and c-Ski expression ↑ STAT1 |
↓ Hypertrophy and Fibrosis ↑ Hypertrophy and fibrosis ↑ Inflammation |
Ang II minipump TAC In vitro HFD |
(98) (99) (107) (108) (132) |
| ↑ miR-200a | ↓ TSC1 and ↑ mTOR activation |
↑ Hypertrophy | In vitro | (100) |
| ↑ miR-208a | ↓ Dyrk expression and NFAT nuclear translocation | ↑ Fibrogenic gene expression | LAD ligation | (11) |
| ↑ miR-217 | ↑ PTEN signaling | ↑ Fibrosis | TAC In vitro |
(101) |
| ↑ miR-29b ↑ miR-455 |
↓ MMP-9 expression | ↓ Fibrosis | Diabetic mice | (104) |
| ↑ miR-27a ↑ miR-28-3p ↑ miR-34a |
↓ NRF2 and ↑ ROS accumulation | ↑ ROS-mediated cell damage | LAD ligation In vitro |
(110) |
| ↑ miR-144 | ↑ Antiapoptotic pathways ↓ Proapoptotic pathways e.g., Akt, GSK-3β and ERK1/2 |
↓ Apoptosis | rIPC | (113) |
| ↑ miR-24 | ↓ Bim expression | ↓ Apoptosis | Myocardial I/R injury | (114) |
| ↑ miR-30a | ↓ ATG12, beclin-1 and LC3II/LC3I ratio | ↑ Apoptosis | In vitro | (115) |
| ↑ miR-222 ↑ miR-143 |
↑ MMP-2 and MMP-9 | ↑ Endothelial cell proliferation and angiogenesis | LAD ligation | (69) |
| ↑ miR-320 | ↓ IGF-I, Hsp20, and Ets20 | ↓ Angiogenesis and tube formation | In vitro | (122) |
| ↑ miR-17 ↑ miR-19a/b ↑ miR-20a↑ miR-30v ↑ miR-126 |
↑ Proangiogenic genes | ↑ Angiogenesis | In vitro | (51) |
| ↑miR-10a↑miR-27b ↑miR-100 |
↑ VEGF and NO synthase | ↑ Angiogenesis ↓ Fibrosis |
LAD ligation In vitro |
(126) |
| ↑ miR-146a | ↑ Pro-angiogenic genes e.g., Nras, Traf6, Irak1 | ↑ Angiogenesis | In vitro Patient blood samples |
(124) |
| ↑ miR-93-5p | ↓ ATG7 and TLR4/NF-κB expression | ↓ Inflammation | LAD ligation In vitro |
(129) |
| ↓ miR-223 | ↑ IL-1β, IL-6, and CAM-1 | ↑ Inflammation | Patient blood samples In vitro |
(134) |
TAC, transverse aortic constriction; Ang II, angiotensin II; LAD, left anterior descending coronary artery; HFD, high-fat diet; rIPC, remote ischemic preconditioning; I/R, ischemia-reperfusion.
The Hypertrophic Growth-Signaling Pathway
Hypertrophic growth of the heart is an adaptive response that accompanies many kinds of CVDs, including valvular diseases, hypertension, ischemic diseases, and heart failure. This is partly due to exosome-mediated cross-talk among cardiomyocytes and other cardiac cell types in the heart, such as endothelial cells, fibroblasts, and inflammatory cells (Fig. 3).
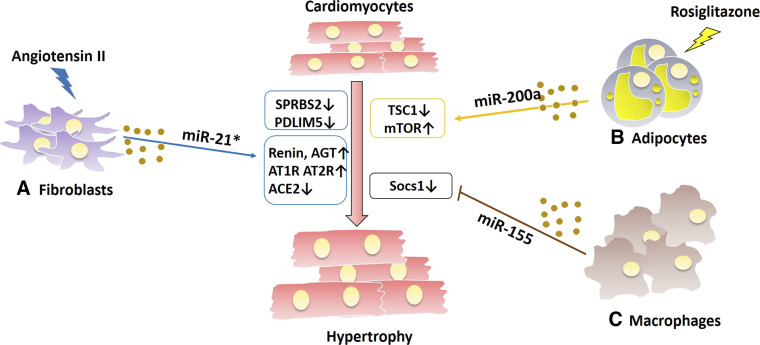
Figure 3.
Illustration of the exosome-mediated cross-talk among cardiomyocytes and other cardiac cell types in the heart, such as fibroblasts, macrophages, and adipocytes, during hypertrophic signaling.Under angiotensin II and pressure overload stresses, exosomes derived from fibroblasts (A) and macrophages (B) containing miR-21* and miR-155, respectively, can downregulate SPRBS2, PDLIM5, and Socs1 and activate several components of angiotensin signaling pathway in cardiomyocytes, eventually resulting in the development of hypertrophy. C: adipocytes could remotely enhance cardiac hypotrophy by mTOR activation-meditated exosomal miR-200a expression in response to rosiglitazone treatment.
Growth factor-treated cardiomyocytes resulted in the upregulation of exosomal transcripts involved in hypertrophic signaling pathways such as nuclear factor of activated T cells (NFAT5) and histone deacetylase-5 (HDAC5). These changes could mediate the development of hypertrophy among cardiomyocytes (42). Beyond the fibrogenic role, cardiac fibroblast proliferation is also activated in response to cardiac stress, and the enrichment in the population of cardiac fibroblasts has been proposed to mediate cross-talks between fibroblasts and cardiomyocytes to induce cardiac remodeling. In vitro experiments of coculturing between adult murine cardiomyocytes with cardiac fibroblasts or treating cardiomyocytes with conditioned fibroblast culture media caused increases in cell sizes (94, 95). In terms of the molecular mechanism, a recent study has utilized RNA sequencing to analyze the RNA content in exosomes derived from angiotensin II-treated neonatal rat cardiac fibroblasts. This led to the identification of 388 rat miRNAs and 50 miRNAs to be enriched in cardiac fibroblast-derived exosomes, of which the exclusive role of the fibroblast-derived noncanonical passenger strand (also called “star”) miRNA miR-21* has been demonstrated to be packed into exosomes and later taken up by cardiomyocytes via a receptor- or endocytosis-dependent manner. The increase in the miR-21* level downregulates the expression of structural proteins of cardiomyocytes including SORBS2 (sarcoplasmic protein sorbin and SH3 domain-containing protein 2) and PDLIM5 (PDZ and LIM domain 5), eventually resulting in the development of hypertrophy (96). Interestingly, the high level of miR-21* was also detected in pericardial fluid in TAC (transverse aortic constriction)-induced cardiac hypertrophy; thus, further studies are required to confirm whether the mechanism above is specifically mimicked in in vivo models. Another independent study has shown the role of cardiac fibroblast-derived exosomes in upregulating the expression of several components of angiotensin II-signaling pathways including renin, angiotensinogen, and angiotensin I and II receptors while suppressing the expression of angiotensin-converting enzyme 2 (ACE2), resulting in an increase in angiotensin II production in cardiomyocytes. As a result, this exacerbated angiotensin II-inducing cardiac pathological hypertrophy (97). However, further studies are needed to identify specific cargoes of fibroblast-derived exosomes that regulate the angiotensin II-signaling pathway in cardiomyocytes. These studies have provided cellular and molecular insights into the hypertrophic influence of cardiac fibroblasts on cardiomyocytes.
Needless to say, immune cells are also importantly responsible for the regulation of cardiac functions in response to cardiac stress. Interestingly, a study by Heymans et al. showed that ablated miR-155 expression in macrophages rather than in cardiomyocytes protected hearts from the development of hypertrophy in response to pressure overload by downregulating Socs1, a suppressor of cytokine signaling (98). A previous study demonstrated that miR-155 was able to enhance cardiac hypertrophy through the Jaird2 (Jumonji and AT-rich interaction domain containing 2) signaling pathway in cardiomyocytes (99).
Cardiac hypertrophy can be regulated by endocrine organs through their secreted exosomes containing hormones, paracrine factors, and inflammatory mediators. Adipose tissue is not only considered a key energy storage place but also one of the largest endocrine organs that maintain metabolic homeostasis in the body (100). The function of adipose tissues is tightly regulated by PPARγ transcription factor. Interestingly, the systemic administration of rosiglitazone, which mediates PPARγ activation led to the development of cardiac hypertrophy independently of cardiomyocyte PPARγ. Since rosiglitazone-treated adipocytes were cocultured with cardiomyocytes, molecular studies showed the activation of rosiglitazone caused the upregulation and exosome-mediated secretion of miR-200a. The uptake of adipocyte-derived exosomal miR-200a into cardiomyocytes caused suppressed TSC1 and subsequent mTOR activation, resulting in the development of hypertrophy (100).
The Fibrotic Signaling Pathway
Upon cardiac insults, fibroblasts become activated to proliferate and differentiate into myofibroblasts. This leads to an excessive synthesis and deposition of extracellular matrix (ECM)-like collagen, termed fibrosis, that eventually causes decreased myocardial compliance and increased stiffness, resulting in cardiac dysfunction. Exosomes have two faces of mechanisms, either protective or inducing fibrosis formation, depending on their original sources (Fig. 4).
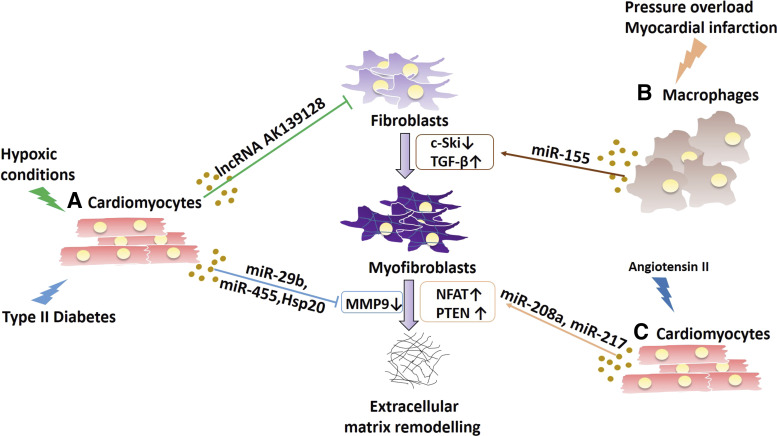
Figure 4.
The origin and the pathological stress subjected to cells can determine whether exosomes can protect against fibrosis or encourage fibrosis formation via various miRNA signaling. Cardiomyocytes (A) can induce cardioprotection by attenuating fibrogensis during hypoxic conditions and type 2 diabetes by releasing exosomes containing lncRNA AK139128 or miR-29b, miR-455, and HSP20, respectively. In contrast, exosomes derived from macrophages subjected to pressure overload or myocardial infraction (B) contain miR-155, which can exacerbate the accumulation of fibrosis by stimulating myofibroblast formation by increasing TGFβ. Similarly, exosomes released from cardiomyocytes (C) in response to angiotensin II typically contain miR-208a and miR-217 to increase extracellular matrix remodeling.
Cardiomyocyte-derived exosomes have been implicated in mediating the fibrotic response. Screening of cardiomyocyte-specific microRNAs in Sprague-Dawley rats with doxorubicin-induced and MI-induced fibrosis showed that the enrichment of miR-208a in cardiomyocyte-derived exosomes promoted fibroblast proliferation and differentiation into myofibroblasts (11). Then, molecular analysis demonstrated that miR-208a blocked Dyrk (dual specific tyrosine phosphorylated kinase) expression to induce nuclear translocation of NFAT, thus upregulating fibrogenic gene expression (11). However, under pressure overload stress, miR-217 was found to be enriched in cardiomyocyte-derived exosomes that enhanced fibroblast viability through targeting PTEN signaling cascade (101). Hps70 is a membranous marker of exosomes. During aging, downregulation of Hps70 on the exosome leads to an increase in cardiac fibrosis in old rats (102). In addition, a study by Datta et al. (2017) indicated that Hps90 is also responsible for the stimulation of collagen synthesis and the accumulation of fibrosis in response to the renal artery ligation-induced cardiac hypertrophy. Moreover, exosomes collected from IL-6-induced hypertrophic cardiomyocytes are able to reprogram fibroblasts through the activation of the STAT3 signaling pathway (47).
In contrast, a few studies also indicated the anti-fibrogenic roles of cardiomyocyte-derived exosomes. For example, Hsp20 was found abundant in cardiomyocyte-derived exosomes that were able to attenuate fibrosis through the activation of angiogenesis (103). In addition, several exosomal microRNAs including miR-29b and miR-455 have also been implicated to alleviate fibrogenesis in the model of type 2 diabetes subjected to exercise. These cardiomyocyte-derived exosomes were able to downregulate matrix metalloproteinase-9 (MMP9) expression in fibroblasts, thus preventing fibrogenesis (104). Recently, a long noncoding RNA, lncRNA AK139128, was upregulated in cardiomyocyte-derived exosomes under hypoxic conditions. A coculture of cardiomyocytes and cardiac fibroblast led to an increase in exosomal lncRNA AK139128 in cardiac fibroblasts that promoted apoptosis and inhibited proliferation of cardiac fibroblasts (105). Interestingly, simvastatin (SIM), a potent statin and potent competitive inhibitor of 5-hydroxy-3-methylglutaryl-coenzyme A reductase has been shown protect hearts against fibrosis via increasing the expression of the antifibrotic protein decorin (DCN) and inhibiting the expression of the profibrotic protein periostin (POSTN) in cardiomyocyte-derived exosomes. The uptake of these exosomes into fibroblasts led to repressed fibroblast to myofibroblast transformation, collagen production/deposition, and increased fibroblast motility (28).
Interestingly, macrophage-derived exosomal miR-155 is not only critical in the development of cardiac hypertrophy but also in the suppression of fibroblast proliferation via downregulating Socs1 protein (106). Furthermore, loss of miR-155 led to the reduction in macrophage infiltration into the myocardial tissues and the decreased expression of collagen and α-smooth muscle actin in response to angiotensin II induction (107). They also revealed that increased miR-155 expression drove the differentiation of fibroblast to myofibroblast (107). In addition, miR-155 can induce fibrogenesis through the downregulation of c-Ski protein, a negative regulator of the TGFβ signaling pathway (108).
The Antioxidant Signaling Pathway
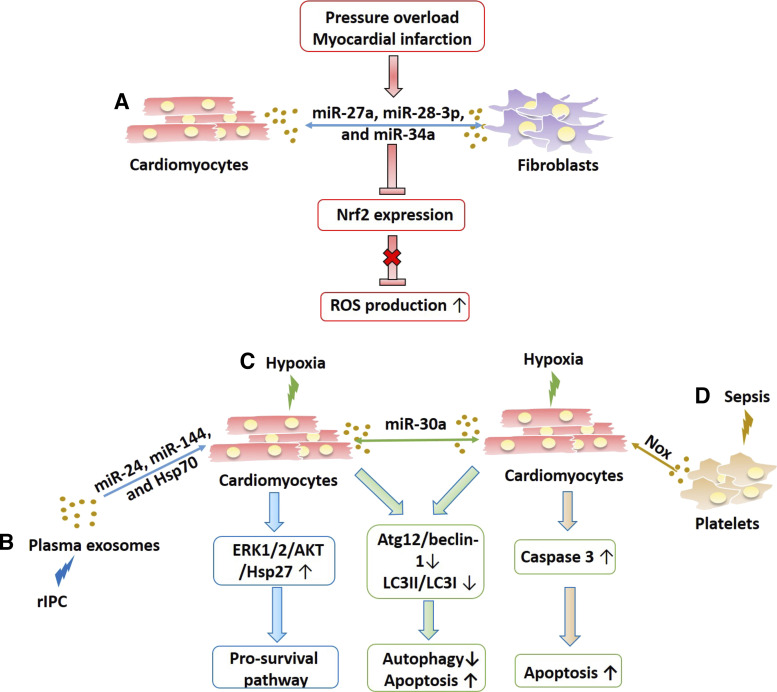
Oxidative stress is defined as an imbalance state between ROS production and the antioxidant defense. The nuclear factor erythroid 2-related factor 2 (Nrf2) pathway is an important antioxidant defense mechanism and is highly downregulated in oxidative stress-induced cardiac remodeling in chronic heart failure (CHF). In long-term MI, cardiac fibroblast-derived exosomes enriched with three microRNAs, miR-27a, miR-28-3p, and miR-34a, were absorbed by cardiomyocytes. As a result, these microRNAs were able to inhibit Nrf2 mRNA translation into protein, stopping its signaling cascade and allowing ROS to accumulate and damage the cell, which can eventually lead to CHF (109). Interestingly, TNFα treatment led to the secretion of exosomes enriched with these three microRNAs in both cardiomyocytes and fibroblasts, indicating intercellular communication of these cardiac cell types in the regulation of oxidative stress (109) (Fig. 5).
Figure 5.
The roles of exosomes in the antioxidant and apoptotic signaling pathways. A: exosome shuttling between cardiomyocytes and fibroblasts containing various miRNAs such as miR027a, miR-28-3p, and miR-34a can be attributable to reactive oxygen species (ROS) overproduction during cardiac diseases such as pressure overload and myocardial infarction by repressing Nrf2 expression, an antioxidant arm. B: in remote ischemic preconditioning (RIPC), plasma-derived exosomes can protect cardiomyocytes against apoptosis by increasing ERK1/2/Akt/Hsp27 to promote activation of prosurvival pathways. In contrast, exosomes from cardiomyocytes subjected to hypoxia (C) contain miR-30a and can cross-talk between other cardiomyocytes to decrease the autophagy response and increase apoptosis. D: platelet-derived exosomes can also induce apoptosis in cardiomyocytes by increasing caspase-3 activity to increase apoptosis.
The Apoptotic and Survival Signaling Pathway
Apoptosis-mediated loss of cardiomyocytes has long been thought to be a determining factor responsible for the progression of many cardiac ischemic diseases. Moreover, several studies have addressed the cardioprotective effects of exosomes in remote ischemic preconditioning (rIPC) studies (110, 111) (Fig. 5). Further studies have shown that plasma exosomes also confer cardioprotection via diminished cardiomyocyte apoptosis in myocardial ischemia-reperfusion injury (MIRI) (29). Moreover, rIPC increased Hsp70 levels in circulating exosomes that promoted prosurvival signaling pathways in cardiomyocytes by the activation of the Toll-like receptor 4 (TLR4)/ERK1/2/Akt/Hsp27 signaling cascade. However, the sources of exosomes were not investigated (29). Microarray studies demonstrated that IR injury decreased the miR-144 level in mouse myocardium and that rIPC led to a fourfold increase in exosomal miR-144 in the serum that exerted cardioprotective effects via the activation of several antiapoptotic and prosurvival signaling candidates including Akt, GSK-3β, and ERK1/2. Conversely, antisense-mediated repression of miR-144 abrogated its antiapoptosis and cardioprotection by rIPC (112). In another study, rIPC in rats led to increased miR-24 in plasma exosomes that resulted in decreased cardiomyocyte apoptosis, reduced infarct size, and improved systolic performance post-MI (113).
In contrast, cardiomyocyte-derived exosomes are able to induce apoptosis via suppression of autophagy via an autocrine manner in MIRI (Fig. 5). Yang and colleagues have identified the hypoxia-inducible factor (HIF)-1α-mediated enrichment of miR-30a in exosomes collected from culture medium of hypoxia-treated cardiomyocytes and the serum of acute MI patients. These exosomes were transferred between cardiomyocytes and eventually led to promoting apoptosis by reducing several autophagy-related proteins like Atg12 and beclin-1 and the LC3II/LC3I ratio (114). Likewise, Malik et al. showed that exosomes derived from hypoxia-treated cardiomyocytes were enriched with Hsp60 that activated proapoptotic pathways via TLR4 (30). These contradicting findings promote further studies to elucidate the in-depth mechanisms of exosomes derived from different sources and how they cooperate during the development of heart failure.
Sepsis cardiomyopathy is a condition where cardiac function is impaired by local severe infection, and sepsis, and exosomes are proposed to mediate this association. High levels of lipopolysaccharide (LPS) and NO production induced platelets secreting exosomes containing NAPDH oxidase, nitric oxide synthase, and protein disulfide isomerase that resulted in increased ROS production and activated caspase-3 in recipient cells such as endothelial cells and cardiomyocytes (115) (Fig. 5).
The Angiogenic Signaling Pathway
Ischemic heart diseases including MI and ischemic cardiomyopathy are associated with insufficient or absent blood flow to the myocardium due to blockage of the coronary arteries and endothelial dysfunction. Therefore, the stimulation of complete myocardial revascularization to restore blood flow into ischemic myocardial tissues has received prominent attention recently, leading to increased tissue perfusion and improved tissue recovery (116). Fundamentally, angiogenesis is a physiological process through which the existing vasculature grows and develops into new blood vessels. Mechanistically, this process is initially mediated by activation and proliferation of existing endothelial cells, in company with the increased permeability and the disruption of the basement membrane. This is followed by the immigration of endothelial cells to the extravascular space to elongate and align to re-form capillary networks. Other cell types involved in the establishment and maintenance of vascular integrity include stromal cells and endothelial progenitor cells. This process especially requires a variety of growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and angiopoietin-1 (116).
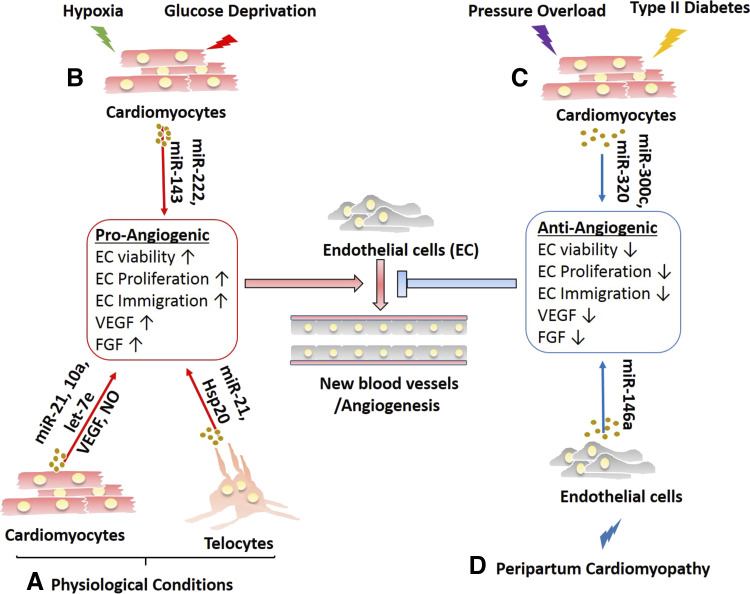
Several studies have illustrated that exosomes are beneficial for cardiac function through the promotion of angiogenesis (Fig. 6). Interestingly, intracardiac injections of exosomes derived from healthy heart explants improved cardiac functions in mice with MI compared with exosomes derived from failing heart explants. Further analysis revealed the enrichment of miR-21 in exosomes from healthy hearts that was able to promote angiogenesis through the activation of Akt and VEGF signaling pathway (117). In line with that, without stress conditions, an in vitro study showed that cardiomyocyte-derived exosomes led to increased endothelial cell proliferation, migration, and tube formation (118). Furthermore, Hsp20 was specifically enriched in these exosomes and it interacted with VEGF receptor 2 on human umbilical cord blood endothelial cells to stimulate angiogenic cascades (118). In addition, cardiomyocyte-derived exosomal Hsp20 was shown to alleviate MI in mice via activating the signaling pathway (48). A previous study demonstrated that sustained activation of Akt in endothelial cells induced the formation of blood vessels (119). Under hypoxic or ischemic conditions, cardiomyocyte-derived exosomes seem to promote endothelial cell proliferation, migration, and tubulogenesis, which paves the way toward the use of engineered exosomes as an add-on therapy to treat ischemic heart diseases. Exosomes secreted by hypoxia-treated H9C2 or primary cardiomyocytes increased proliferation and sprouting of endothelial cells, and two microRNAs, miR-222 and miR-143, were identified to become relatively abundant in ischemic exosomes that were critical for the activation of angiogenesis. Moreover, intramyocardial injection of these exosomes improved neovascularization following MI (69).
Figure 6.
Exosome-mediated signaling involved in angiogenic signaling pathways. Under physiological conditions (A), cardiomyocytes and telocytes release exosomes containing various miRNAs and bioactive molecules that promote proangiogenic signaling. Similarly, exosomes derived from cardiomyocytes subjected to hypoxic conditions or glucose deprivation (B) contain various miRNAs that increase proangiogenic signaling pathways to stimulate endothelial cells to increase angiogenesis. In contrast, exosomes derived from cardiomyocytes subjected to pressure overload or type 2 diabetes (C) typically contain miR-300c and miR-320, which promote the antiangiogenic pathway. D: similarly, in peripartum cardiomyopathy, endothelial cell-derived exosomes can induce endothelial cells to downregulate several components of the proangiogenic signaling pathway, leading to impaired angiogenesis. VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; NO, nitric oxide.
However, cardiac angiogenesis is inhibited in response to pressure overload stresses (Fig. 6). For example, miR-300c was enriched in cardiomyocyte-derived exosomes under phenylephrine and isoproterenol treatments. In vitro coculture of cardiomyocytes and endothelial cells showed reduced endothelial proliferative, migratory, and tubulogenic capacities. Inhibition of miR-300c activity alleviated the progress of cardiac hypertrophy-induced transverse aortic constriction (TAC) (120). Consistently, cardiomyocyte-derived exosomes seem to dysregulate cardiac angiogenesis under hyperglycemia. Cardiomyocyte-derived exosomes from adult Goto-Kakizaki (GK) rats, a commonly used animal model of type 2 diabetes, were enriched in miR-320 and were absorbed by endothelial cells when cocultured. miR-320 impaired endothelial cell proliferation and migration via targeting of IGF-I, Hsp20, and Ets2, resulting in inhibition of proliferation, migration, and tube formation (121). In contrast, under conditions of glucose deprivation, cardiomyocyte-derived exosomes are loaded with a broad repertoire of microRNAs, including miR-17, -19a, -19b, -20a, -30v, and -126, that are involved in the transcriptional activation of a range of proangiogenic genes (51).
Interestingly, endothelial cells can interact with each other via exosomes to regulate the establishment and maintenance of vascular integrity. Analysis of RNA content of endothelial cell-derived exosomes revealed high levels of miR-214 expression that was required for silencing the expression of ataxia telangiectasia mutated in recipient cells. This prevented the senescence of endothelial cells and facilitated cell migration and angiogenesis (122). In 16-kDa NH2-terminal prolactin fragment (16 K PRL)-induced peripartum cardiomyopathy (PPCM), endothelial cells secreted miR-146a-enriched exosomes that could be taken up by both neighboring endothelial cells and cardiomyocytes, leading to downregulation of several proangiogenic genes such as Nras, Irak1, and Traf6 and key metabolic genes like Erbb4, Notch1, and Irak1, respectively. Interestingly, exosomal miR-146a was also found relatively abundant in plasma, suggesting a potential biomarker for PPCM (123).
Besides, cardiac telocyte-derived exosomes are found to be beneficial for angiogenesis (Fig. 6). First, the transplantation of cardiac telocytes decreases MI and improves postinfarcted cardiac function in rats (124). Further analysis showed that cardiac telocyte-derived exosomes enhanced proliferation, migration, and formation of capillary-like structures of endothelial cells in vitro (125). Immunohistochemistry and qPCR revealed the upregulation of VEGF and NO synthase and several proangiogenic microRNAs (like miR-21, -10a, -let-7e, -27b, and -100) (126). Treating MI-induced rats with telocyte-derived exosomes alleviated cardiac fibrosis, improved cardiac function, and increased angiogenesis (125).
The Inflammatory Signaling Pathway
Over past few decades, increasing evidence has demonstrated that immune cells infiltrate the heart during the heart’s development and remain in the myocardial tissue where they are critically responsible for the physiological and pathological function of the heart (127). In response to MI or infectious diseases, large quantities of immune cells are employed to the heart to engulf pathogens and scavenge death cells, resulting in healing (127). Interestingly, several pieces of evidence indicate that exosomes regulate stress-induced cardioinflammation via modulating immune cell migration and cytokine releases. Liu et al. show that culture medium from cardiomyocytes exposed to ischemic conditions was able to induce bone marrow-derived dendritic cells to secrete exosomes that were targeted to splenic CD4+ T cells. This resulted in the increased release of chemokines and the inflammatory cytokines IFNγ and TNFα, thus improving cardiac performance post-MI (128). In addition, dendritic cell-derived exosomes could also induce endothelial inflammation via exosomal TNFα, thus activating the proinflammatory NF-κB signaling pathway (129). Likewise, endothelial cell-derived exosomes also have a direct impact on immune cells to modulate their morphology and proinflammatory functions. Oxidized low-density lipoprotein-treated human umbilical vein endothelial cells (HUVECs) resulted in the secretion of miR-155-enriched exosomes that significantly contributed to macrophage activation by shifting the M2 anti-inflammatory phenotype to the M1 proinflammatory phenotype (130). Interestingly, enrichment of miR-155 was also found in adipocyte-derived exosomes in obese mice that also activated M1 macrophages via upregulating the STAT1 and downregulating the Socs1 signaling pathways (131). Moreover, Liu et al. provide an insight into the cardioprotective roles of adipose-derived stromal cells (ADSCs) in acute MI. Under hypoxic conditions, ADSC-derived exosomes contained a relatively high level of miR-93-5p, which was secreted and delivered to cardiomyocytes. MiR-93-5p downregulated Atg7 and TLR4/NF-κB expression, leading to suppressing cardiac injury-mediated autophagy and inflammatory responses, respectively. Consequently, these molecular changes brought alleviating ischemia-induced cardiac injury (132). In sepsis cardiomyopathy, screening of plasma-derived exosomes in nonsurvival patients revealed a dramatically low concentration of miR-223. This might cause the upregulation of several miR-223 inflammatory targets such as IL-1β, IL-6, and CAM-1, thus impairing cardiac function (133).
Unlike apoptosis and necroptosis, pyroptosis is a mode of proinflammatory programmed cell death and is highly activated as infection occurs. This is mainly initiated by caspase-1 activation and subsequently results in converting the proforms of inflammatory cytokines such as IL-1β and IL-18 into active forms (134). In uremic heart disease, infiltrated macrophages secrete exosomes enriched in miR-155, which are absorbed by cardiomyocytes. Further analysis showed that miR-155 can directly inhibit FoxO3a activity in cardiomyocytes to promote pyroptosis and cardiac remodeling, including hypertrophy and fibrosis (135). Consistently, repression of macrophage-derived miR-155-enriched exosomes by GW4869 alleviates cardiac pyroptosis (135). In line with this, these macrophage-derived exosomes could be uptaken by fibroblasts that promoted the secretion of proinflammatory cytokines like IL-1β, IL-6, TNFα, and C-C motif ligand 2 (CCL2) expression from cardiac fibroblasts by the regulation of Socs1/Stat3 signaling cascade. Loss of miR-155 reduced susceptibility of hearts to acute MI stress by reducing inflammation and fibroblast proliferation (106). Hence, macrophage-derived exosomes could interact with different cardiac cell types to modulate the inflammatory responses in cardiac injury.
Propagation of inflammatory signaling via apoptotic exosomes.
Interestingly, several studies reported the ability of dying cells to communicate with other live cells within their microenvironment via exosomes, and that contributes pivotally to mediate proinflammatory effects. In particular, apoptotic cell-derived exosomes (ApEs) are a distinct class of exosomes that are released along with apoptotic bodies upon caspase-3 activation. Moreover, they demonstrate similar physical characteristics with exosomes and express the typical exosomal markers including CD63 and Hsp70 and the lysosomal marker lysosome-associated membrane protein-1 (LAMP1) (136). Interestingly, several studies have reported the roles of ApEs in regulating inflammation by delivering various noncoding RNAs and proteins to target cells. For instance, ApEs from mouse apoptotic endothelial cells highly expressed the active 20S proteasome core that resulted in accelerated rejection of vascular grafts (137). Further profiling of RNA contents from endothelial cells, apoptotic bodies, and ApEs revealed a distinct transcriptomic profile revealing several noncoding RNA sequences exhibiting immunostimulatory potential, such as U1 snRNA, mitochondrial tRNAs, and pathogen-like endogenous retroelements that can trigger TLRs to initiate inflammation (138). In addition, a study by Park et al. (2018) showed that these ApEs produced by HeLa cells cultured in apoptotic conditioned medium expressed unique protein markers such as sphingosine 1-phosphate receptors 1 and 3 (S1PR1/3), suggesting that ApEs are not generated through the ESCRT-dependent pathway as in conventional exosomes but from the S1P-S1PR signaling-dependent pathway (136). In addition, ApEs were able to induce the activation of p38 MAPK and NF-κB in macrophages and the subsequent release of the proinflammatory cytokine IL-1β (136). Although there are several studies investigating ApEs, our understanding of the physiological and pathological functions of ApEs is still challenging, because currently there are no biomarkers specific to ApEs. Therefore, it is technically demanding to separate ApEs from the heterogenous population of extracellular vesicles released from apoptotic cells. Also, there are different types of cell deaths apart from apoptosis, which can occur during pathological conditions such as necroptosis and necrosis, so further studies are required to examine the biological characteristics and functions of exosomes in these forms of cell deaths.
Unfortunately, there is currently no report on the function of ApEs in cardiovascular diseases. Hence, efforts to investigate the roles of ApEs on inflammation-associated heart diseases should be undertaken. However, a study of Kuwabara et al. suggested that miRNAs packaged in damaged or necrotic cardiomyocyte-derived circulating exosomes have great potential as diagnostic markers in MI. They showed that cardiac-specific miR-133a was significantly upregulated in the circulating exosomes of acute MI patients, correlating with troponin T levels found in serum (139). Further analysis showed these exosomes were released from injured cardiomyocytes within the infarct region and the peri-infarct zone (139). Although there are several other exosomal miRNAs, such as miR-21, miR-146a, miR-208, miR-499, and miR-192, found to be increased in serum of acute MI patients, their original sources were not investigated (140).
Exosomes and danger-associated molecular patterns.
The key difference between exosomes and danger-associated molecular patterns (DAMPs) is that exosomes are released from both healthy and diseased cells, whereas DAMPS are primarily released from damaged/diseased cells. Injuries during the acute phase of MI leads to activation of an immediate inflammatory response. This is initiated by the passive release of intracellular DAMPs such as several metabolic products including ATP, ions, noncoding RNA, histones, heat shock proteins, and cell debris from necrotic cell death. The DAMP-mediated inflammation occurs without pathogenic infection (i.e., sterile inflammation). DAMPs can interact with specific pattern recognition receptors (PRRs) on the surface of alive cells around the necrotic lesion, triggering the formation of an inflammasome (141). Of note, many studies described above have illustrated exosomal contents from cardiac cells that contain high levels of heat shock proteins like Hsp70 and Hsp27 (29, 142) or miRNAs such as miR-155 that can regulate apoptosis, macrophage polarization, and cytokine release. In addition, mitochondrial DNA (mtDNA) is found in circulating exosomes and acts like DAMPs to activate tissue-resident macrophages and promote leukocyte infiltration during heart diseases (143). Interestingly, mice injected with ApEs showed significantly increased levels of differentiated macrophages and various proinflammatory genes in the liver and spleen, resulting in high production of IL-1β. In contrast to the conventional notion that DAMPs can be released only by necrotic cell death and apoptosis is usually noninflammatory, the outcome of this study led to the proposal that exosomes from apoptotic cells can be considered as functional DAMPs (136). However, the mechanism underlying the inflammatory effect of ApEs remain elusive.
EXOSOME-BASED INTERCELLULAR SIGNALING IN THE STEM CELL THERAPY
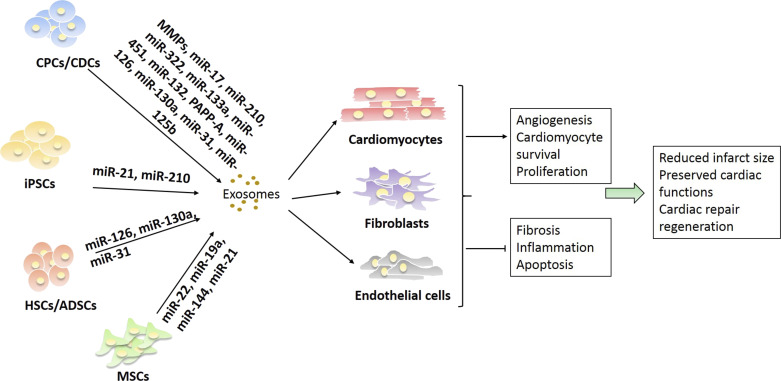
Recently, numerous studies have demonstrated that exosomes derived from various stem cells confers cardioprotection in MI models. In the following sections, we will summarize recent findings on the role of stem cell-derived exosomes as therapeutic potential in the treatment of CVDs through reduced oxidative stress and cell death, limited inflammatory response, enhanced myocardial angiogenesis, and alleviating MI size (Fig. 7). A summary of roles of the different miRNAs found in stem cell-derived exosomes can be seen in Table 3.
Figure 7.
Stem cell-derived exosomes hold potential cell-free therapies for myocardial infarction by enhancing angiogenesis, cardiomyocyte survival and proliferation, and repressing fibrosis, inflammatory responses, and apoptosis. Mesenchymal stem cells (MSCs), cardiac progenitor cells (CPCs), cardiosphere-derived cells (CDCs), induced pluripotent stem cells (iPSCs), hematopoietic stem cells (HSCs), and adipocyte-derived stem cells (ADSCs) release exosomes containing various bioactive molecules, including miRNA, which can act upon cells of the cardiovascular system including cardiomyocytes, fibroblasts, and endothelial cells. In turn, they can inhibit or promote key processes involved in cardiovascular disease to change the disease outcome.
Table 3.
Example of miRNA found in exosomes from different cardiac stem cells, their target mechanism and their role in cardiac disease
| miRNA | Cell Type | Target | Effect | Disease Model | References |
|---|---|---|---|---|---|
| ↑ miR-22 | MSCs | ↓ Mecp2 activation | Cardioprotective during ischaemia | LAD ligation In vitro |
(149) |
| ↑ miR-19a | MSCs | ↓ PTEN + Bim ↑ Akt |
Cardiomyocyte survivalPreserve mitochondrial membrane potential | LAD ligation In vitro |
(150) |
| ↑ miR-144 | MSCs | ↓ PTEN ↑ Akt activation |
↓ Apoptosis | In vitro | (151) |
| ↑ miR-21 | MSCs/CPCs/iPSCs | ↓ PTEN ↑ Akt + VEGF ↓ PDCD4 |
↑ Microvascular density ↓ Apoptosis |
LAD ligation In vitro |
(152) (166) |
| ↑ miR-210 | CPCs/CDCs/iPSCs | ↓ EFNA3 ↓ PTP1b ↑ VEGF |
↓ Apoptosis ↑ Angiogenesis |
Human myocardial sample LAD ligation In vitro |
(168) (174) |
| ↑ miR-322 | CPCs | ↑ Nox2 | ↑ Endothelial cell migration ↑ Capillary tube formation ↓ Apoptosis |
LAD ligation In vitro |
(163) |
| ↑ miR-451 | CPCs | ↓ Caspase-3/7 activation | ↓ Apoptosis | Myocardial I/R injury | (165) |
| ↑ miR-132 | CPCs | ↓ RasGap-p210 | Endothelial cell tube formation | LAD ligation Myocardial I/R injury |
(169) |
| ↑ miR-126 | CDCs/HSCs/ADSCs | ↑ VEGF + FGF ↓ Spred-1 |
↑ Angiogenesis | In vitro | (173) |
| ↑ miR-130a | CDCs/HSCs | ↓ GAX ↓ HoxA5 |
↑ Angiogenesis | In vitro | (174) |
| ↑ miR-146a | CDCs | ↓ TLR-NF-κB axis | ↓ Apoptosis | LAD ligation In vitro |
(175) |
| ↑ miR-31 | CDCs/ADSCs | ↓ Anti-angiogenic genes e.g., FIH1 | ↑ Angiogenesis | In vitro | (184) |
MSCs, mesenchymal stem cells; CPCs, cardiac progenitor cells, CDCs, cardiosphere-derived cells; iPSCs, iInduced pluripotent stem cells; HSCs, Hematopoietic stem cells; ADSCs, adipocyte-derived stem cells; LAD, left anterior descending coronary artery; I/R, ischemia-reperfusion.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are a group of multipotent stromal cells that can differentiate into different types of cells that hold great potential for cell-based therapy, including tissue repair and regeneration. Several studies have observed the beneficial effects of MSC-derived exosomes in MI and MIRI by promoting angiogenesis in company with attenuating cell death and inflammatory responses. Bian et al. has indicated the role of MSC-derived exosomes in promoting angiogenesis after MI through the stimulation of proliferation, migration, and tube formation of endothelial cells, leading to reduced infarct size and improved cardiac performance (144). Likewise, exosomes released from CXCR4-overexpressed MSCs also enhanced endothelial cells to elongate and interlink with each other to form a network of capillary-like structures, which improved angiogenesis and reduced infarcted size in a 4-wk MI model (145). Consistently, Teng et al. (2015) showed exosomes isolated from bone marrow MSCs could enhance angiogenesis and reduce inflammation by inhibiting T cell proliferation, thus preserving cardiac performance in a MI model (146). In another study, exosomes arising from Akt-overexpressed human umbilical cord blood-derived MSCs promoted endothelial cell proliferation, migration, and tube‐like structure formation in vitro, and blood vessel formation in vivo, in an acute MI model via the increased release of platelet‐derived growth factor D (PDGF-D) (147).
Several microRNAs have been implicated in the cardioprotective roles of MSCs. Human bone marrow MSCs conferred cardioprotection against ischemic injury and fibrosis in ischemic preconditioning through miR-22/methyl-CpG-binding protein-2 (Mecp2) signaling (148). On the other hand, exosomes isolated from (GATA4)-overexpressed bone marrow MSCs protected mouse hearts against MI by increasing cardiomyocyte survival and preserving mitochondrial membrane potential. This was mediated by the miR-19a and PTEN signaling pathway (149). Consistently, Wen et al. also demonstrated the beneficial effects of MSC-derived exosomes against apoptosis under hypoxic conditions through the PTEN/Akt signaling pathway. However, they explained that these effects were mediated by miR-144 (150). In addition, intramyocardial injection of human endometrium-derived MSCs also demonstrated improved microvascular density and diminished apoptosis in rats with MI through PTEN expression-targeting paracrine actions of miR-21-containing exosomes (151). Despite a variety of microRNAs proposed, it seems that MSC-derived exosomal microRNAs centrally target the PTEN/Akt signaling pathway to elicit their cardioprotective capacities. In mouse hearts with MIRI, MSC-derived exosomes increased ATP and NAPDH production, upregulated a PI3K/Akt/GSK-3β signaling cascade, and decreased JNK activities that together led to reduced oxidative stress and activated a prosurvival signaling pathway. Moreover, local and systemic inflammation were also significantly reduced after 24 h of the treatment. Consequently, the hearts had decreased infarct size and maintained cardiac functions ex vivo and in vivo in response to MIRI (152, 153). Interestingly, Liu et al. showed that bone marrow MSC-derived exosomes could reduce the adverse outcome of MI by activating AMPK/Akt/mTOR signaling-mediated autophagy (154). Besides, exosomes isolated from adipose-derived MSCs also showed increased cardiomyocyte viability in vitro in response to hypoxia/reoxygenation and improved cardiac functions in a MIRI model via activation of Wnt/β-catenin signaling. However, the key exosomal molecules involved in this regulation have not been elucidated (155).
Interestingly, long noncoding RNA is also implicated to mediate beneficial effects of MSC-derived exosomes in rats with MI. Atorvastatin was previously shown to enhance the cardioprotective effects of MSCs (156). Further analysis demonstrated atorvastatin-pretreated MSCs secreted exosomes enriched with long noncoding RNA H19 that reduced fibrosis, repressed the releases of cytokines such as IL-6 and TNFα, and enhanced angiogenesis in the peri-infarct region in vivo, thus alleviating MI. in vitro experiments showed that these exosomes increased survival and enhanced migration, the formation of network-like structure of endothelial cells (157).
Cardiac Progenitor Cells
Several studies have shown the existence of a group of endogenous stem cells in the adult heart, namely cardiac progenitor cells (CPCs), contributing to the regeneration of cardiac tissue after injury. Multiple surface markers of CPCs have been identified including c-Kit+, Sca-1+, and Isl-1+ (158). CPC-derived exosomes can also promote angiogenesis via different mechanisms. CPC-derived exosomes were able to promote endothelial cell immigration via the exosomal MMP-mediated degradation of extracellular matrix (159). The angiogenic capacity of CPC-derived exosomes is highly associated with O2 concentration. CPC-derived exosomes from hypoxic condition expressed higher levels of proangiogenic microRNAs including miR-17 and miR-210, compared with those from normoxic conditions, leading to enhanced tube formation of endothelial cells (160). Interestingly, angiogenic effects of CPC-derived exosomes were most effective during physoxia conditions (5% O2) compared with normoxic (21% O2) and hypoxic (1% O2) conditions. This could be explained by the extremely low O2 concentration, which causes a sudden increase in oxidative stress that eventually represses angiogenic signaling pathways (161). On the other hand, a study by Youn et al. provided direct evidence linking angiogenesis and reactive oxidative stress with CPC-derived exosomes. They bioengineered CPC-derived exosomes to carry the proangiogenic microRNA miR-322 and incubated those exosomes with endothelial cells. This caused greater angiogenic responses, demonstrated by increased endothelial cell migration and capillary tube formation, and in vivo delivery of these exosomes attenuated ischemic injury via enhanced angiogenesis. They further showed that this was specifically mediated by miR-322-driven activation of Nox2-derived ROS. However, the in-detailed molecular signaling behind this mechanism has not been fully explored (162). Taken together, the level of ROS in the cells may act as a key determinant on the angiogenic effect of CPC-derived exosomes.
In addition, several studies have shown that CPC-derived exosomes could inhibit apoptosis in MI models. A study by Izarra et al. revealed the role of CPC-derived exosomal miR-133a in protecting ischemic hearts from cardiac hypertrophy, fibrosis, and apoptosis via increasing cardiomyocyte proliferation and vascularization. However, those authors failed to identify downstream signaling partners responsible for these effects (163). Furthermore, analysis of the RNA content in CPC-derived exosomes revealed a relative abundance of GATA4-responsive-miR-451, and incubation of these exosomes with H9C2 cells prevented oxidative stress by inhibiting caspase-3/7 activation. Consistently, direct injection of CPC-derived exosomes into myocardial tissues alleviated cell death by 53% in mice with MIRI (164). On the other hand, H2O2-induced oxidative stress stimulated CPCs to secrete more exosomes enriched with miR-21, which downregulated programmed cell death 4 (PDCD4) expression in H9C2, thus preventing oxidative stress-induced apoptosis and injury in cardiomyocytes (165). Interestingly, in viral myocarditis, CPC-derived exosomes could also repress several proapoptotic factors like the Bim/caspase family through the utilization of the Akt/mTOR signaling pathway, thus suppressing apoptosis (166). Moreover, Barile et al. isolated CPC-derived exosomes from atrial appendage explants of patients and injected them into mice with MI. This resulted in enhanced angiogenesis, less apoptosis, and improved cardiac performance. Dissection of molecular mechanisms revealed exosomal miR-210 and miR-132 involvement. Whereas miR-132 decreased RasGAP-p120, enhancing tube formation of endothelial cells, miR-210 targeted ephrin A3 and protein tyrosine phosphatase 1B expression, inhibiting apoptosis in cardiomyocytic cells (167). Likewise, further study also showed that human CPC-derived exosomes could reduce infarct size and improve cardiac functions in murine hearts from after permanent coronary occlusion and MIRI. However, rather than microRNAs, they recruited proteomics profiling and identified the presence of pregnancy-associated plasma protein A (PAPP-A) on the exosomal membrane. This protein could enhance the production of insulin-like growth factor 1 via proteolytic cleavage of IGF-binding protein-4 (IGFBP-4), leading to the decreased activation of caspases and the increased activation of prosurvival signaling pathway including Akt and ERK1/2 phosphorylation (168).
Recently, a few studies have indicated the proliferative role of CPC-derived exosomes on cardiomyocytes and endothelial cells to protect hearts from MI. However, more studies are needed to confirm these observations using in vivo models and to unravel underlying mechanisms (169, 170). Finally, a study by Chen et al. showed that several microRNAs, like exosomal miR-1, -208, and -499 and nonexosomal miR-133, were increasingly released and circulated to bone marrow to enhance the production of circulating progenitor cells in response to acute MI, thus facilitating cardiac repair (171).
Cardiosphere-Derived Cells
Cardiosphere-derived cells (CDCs) are a kind of CPCs that form spherical aggregates as a cultured suspension. Like CPCs, these cells can also differentiate into the three major cell types in the heart: cardiomyocytes, endothelial cells, and smooth muscle cells. In vitro experiments showed CDCs under hypoxic conditions secreted exosomes enriched with several microRNAs, including miR-126, miR-130a, and miR-210, which exerted proangiogenic and antiapoptotic properties (172, 173). Myocardial injection of CDC-derived exosomes into murine hearts with MI led to enhancing regenerative capacity. Further analysis showed that the high levels of miR-146a and other microRNAs in these exosomes were synergistically responsible for this effect. Moreover, inhibition of exosome production with GW4869 blocked these beneficial effects (174). Consistently, intracardiac injection of human CDC-derived exosomes alleviated adverse remodeling and improved angiogenesis in pig models with MI (175). Interestingly, Tseliou and colleagues showed that CDC-derived exosomes induced cardiac fibroblasts to secrete stromal cell-derived factor 1 (SDF-1) and VEGF, leading to improved contractility and vessel density and reducing scar size in rats with MI (176). Moreover, recent studies analyzing CDC-derived exosomes also indicated their roles in modulating inflammatory responses. Administration of exosomes from CDCs alleviated MIRI-mediated infarct area by reducing the infiltration of macrophages in the infarcted tissue and inducing macrophage polarization, which was regulated through a miR-31/PKCδ (protein kinase Cδ) cascade. Furthermore, proteomic and miRNA profiling of exosomes from IFNγ-primed CDCs revealed the increased presence of IL-6 and miR-125b, which is upstream of the IL-6 receptor. However, further studies are required to examine the roles of these molecules on preclinical heart disease models and their therapeutic potential (79).
Induced Pluripotent Stem Cells
Since their discovery by Shinya Yamanaka in 2006, induced pluripotent stem cells (iPSCs) have offered great opportunities as a promising stem cell-based therapy for the treatment of heart diseases. Interestingly, iPSCs could also enhance cardiomyocyte survival through secreted exosomes. In vitro experiments showed that incubation of H9C2 with iPSC-derived exosomes inhibited caspase-3/7 activation in response to H2O2-induced oxidative stress. In line with that, in vivo administration of these exosomes reduced apoptosis in the ischemic myocardium. Furthermore, these exosomes also contained relatively increased levels of miR-21 and miR-210 that targeted Nanog and HIF-1α expression, respectively (177). However, the mechanism by which miR-21/Nanog and miR-210/HIF-1α cascades confer cardioprotective capacities against apoptosis is not fully understood. Interestingly, a study by Adamiak et al. showed that iPSC-derived exosomes offered a safer and more effective approach than iPSC transplantation to protect mouse hearts from a MIRI model. Consistently, suppressed apoptosis and more angiogenesis were also observed in this model (178). Besides, iPSC derivatives also exert cardioprotective effects in injured hearts through the secretion of exosomes. For example, exosomes isolated from iPSC-derived cardiomyocytes could reduce apoptosis, necrosis, fibrosis, and inflammation by inducing the clearance of injured cardiomyocytes in the peri-infarct region (179). In addition, exosomes generated by iPSC-derived cardiovascular progenitors contained 16 highly expressed microRNAs that could increase cardiomyocyte survival and endothelial cell immigration in vitro and ameliorate cardiac performance in vivo (180).
Other Stem Cells
Hematopoietic stem cells (HSCs) are a group of stem cells that can differentiate and produce other blood cells. They are identified by CD34+ antigen on the cell surface. Many studies have investigated exosomes from transplanted HSCs, A study by Sahoo et al. revealed that exosomes derived from bone marrow CD34+ stem cells exhibited proangiogenic activity in ischemic hearts by increasing the viability, proliferation, and tube-like formation of endothelial cells, leading to increased capillary density and improved cardiac function. MicroRNA profiling revealed high levels of miR-126 and miR-130a in HSC-derived exosomes, but the mechanism underlying the angiogenic regulation has remained unclear (181). Interestingly, hedgehog (Shh)-overexpressed CD34+ cells enhanced the angiogenic properties within the infarct border zone, along with reduced infarct size. This was explained by the exosome-mediated delivery of Shh to endothelial cells and subsequently activate Shh and the angiogenic signaling pathway (182). Further studies are needed to resolve the link between miR-126 and miR-130a and the Shh signaling pathway to activate angiogenesis.
Besides, Kang et al. showed that adipocyte-derived stem cells (ADSCs) could increase the migration and tube formation of endothelial cells by the release of miR-31-enriched exosomes in vitro. Further analysis showed one of the miR-31 targets was factor-inhibiting HIF-1, an antiangiogenic gene; thus, facilitating angiogenesis (183). However, ADSC-derived exosomes collected from obese patients contained low levels of cargoes including VEGF, MMP-2, and, miR-126, thus leading to impaired proangiogenic potentials of endothelial cells via inhibition of the ERK1/2 signaling cascade and upregulation of Spred1 (184).
Conclusions
Over the past decade, exosomes have become a novel field and attracted great attention as critical communicators for shuttling signaling molecules between cells in an endocrine or paracrine manner. Being considered as versatile carriers of molecular signals, exosomes from different cardiac cell types can transmit signals that are both protective and pathological in cardiac diseases. Thus, it is still not assured whether specific blockade of exosome production will be beneficial for heart diseases. Moreover, although it has been well recognized that stem cell-derived exosomes hold great potential in the treatment of cardiac diseases, the in vivo mechanistic actions of exosomes underlying their beneficial effects are not well understood. Of note, only certain cargoes, like proteins and miRNAs, are loaded into exosomes, depending on cell types and specific circumstances. This indicates the hypothesis that the sorting of cargoes is selective and tightly regulated. Future studies will be required to unravel signaling cascades involved in this step. Interestingly, with the capabilities of selectively transferring various cargoes such as proteins, noncoding RNA, and lipid between cells exosomes might become promising vectors in drug delivery by engineering host cells to overexpress a specific protein or microRNA. However, uneven distribution of exosomes in the body after systemic delivery, which remain mainly in the liver, lung, spleen, and gastrointestinal tract and a small portion in the heart, is a challenge to efficiently deliver drugs to specific cell types (185). Finally, current techniques for exosome isolation and characterization are not adequately satisfactory to achieve high yields and purity. Therefore, critical research is required to further the current understanding of signaling pathways controling specificities of cargo loading and uptake of exosomes. This understanding will provide a fundamental basis for improving the exosomes’ quality and their efficacy for therapeutic uses.
GRANTS
This study was supported by te British Heart Foundation Grants PG/19/53/34499 and FS/19/39/34447.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.Y.N. and T.A. drafted manuscript; B.Y.N. and T.A. prepared figures; B.Y.N., T.A. and X.W. edited and revised manuscript; B.Y.N., T.A. and X.W. approved final version of manuscript.
REFERENCES
- 1.Doyle L, Wang M. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8: 727, 2019. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H , et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun 7: 12277, 2016. doi: 10.1038/ncomms12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75: 193–208, 2018. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A , et al. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv 36: 328–334, 2018. doi: 10.1016/j.biotechadv.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev 34: 632–642, 2013. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Anca M, Fenoglio C, Serpente M, Arosio B, Cesari M, Scarpini EA , et al. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front Aging Neurosci 11: 232, 2019. doi: 10.3389/fnagi.2019.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues M, Fan J, Lyon C, Wan M, Hu Y. Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics 8: 2709–2721, 2018. doi: 10.7150/thno.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thongboonkerd V. Roles for exosome in various kidney diseases and disorders. Front Pharmacol 10: 1655, 2019. doi: 10.3389/fphar.2019.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Wang Z. Cardiomyocyte-derived exosomes: biological functions and potential therapeutic implications. Front Physiol 10: 1049–1049, 2019. doi: 10.3389/fphys.2019.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integr Pharm Res Pract 8: 1–11, 2019. doi: 10.2147/IPRP.S133088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Yu X, Xue F, Li Y, Liu W, Zhang S , et al. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am J Transl Res 10: 4350–4366, 2018. [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson SM, Riquelme JA, Zheng Y , et al. Endothelial cells release cardioprotective exosomes that may contribute to ischaemic preconditioning. Sci Rep 8, 15885, 2018. doi: 10.1038/s41598-018-34357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M , et al. Exosomes from cardiac stem cells amplify their own bioactivity by converting fibroblasts to therapeutic cells. J Am Coll Cardiol 66: 599–611, 2015. doi: 10.1016/j.jacc.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil M, Henderson J, Luong H, Annamalai D, Sreejit G, Krishnamurthy P , et al. The art of intercellular wireless communications: exosomes in heart disease and therapy. Front Cell Dev Biol 7: 315–316, 2019. doi: 10.3389/fcell.2019.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haider KH, Aramini B. Mircrining the injured heart with stem cell-derived exosomes: An emerging strategy of cell-free therapy. Stem Cell Res Ther 11: 23, 2020. doi: 10.1186/s13287-019-1548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lane RE, Korbie D, Anderson W, Vaidyanathan R, Trau M. Analysis of exosome purification methods using a model liposome system and tunable-resistive pulse sensing. Sci Rep 5: 7639, 2015. doi: 10.1038/srep07639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu MY, Ye ZS, Song XT, Huang RC. Differences in the cargoes and functions of exosomes derived from six cardiac cell types: a systematic review. Stem Cell Res Ther 10: 194, 2019. doi: 10.1186/s13287-019-1297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H, Mun JY. Structural analysis of exosomes using different types of electron microscopy. AM 47: 171–175, 2017. doi: 10.9729/AM.2017.47.3.171. [DOI] [Google Scholar]
- 19.Jung MK, Mun JY. Sample preparation and imaging of exosomes by transmission electron microscopy. J Vis Exp 56482, 2018. doi:10.3791/56482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol 10: 648, 2019. doi: 10.3389/fimmu.2019.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson C, Vicencio JM, Yellon DM, Davidson SM. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 228: 57–71, 2016. doi: 10.1530/JOE-15-0201. [DOI] [PubMed] [Google Scholar]
- 22.Waldenström A, Gennebäck N, Hellman U, Ronquist G. Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7: e34653, 2012. doi: 10.1371/journal.pone.0034653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J Cell Sci 118: 3631–3638, 2005. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Hong Y, Cho E, Kim GB, Kim IS. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J Extracell Vesicles 7: 1440131, 2018. doi: 10.1080/20013078.2018.1440131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andreu Z, Yáñez-Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol 5: 442, 2014. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim A, Marbán E. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 78: 67–83, 2016. doi: 10.1146/annurev-physiol-021115-104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campanella C, Bavisotto CC, Gammazza AM , et al. Exosomal heat shock proteins as new players in tumour cell-to-cell communication. J Circ Biomarkers 3, 2014. doi: 10.5772/58721. [DOI] [Google Scholar]
- 28.Kuo H-F, Hsieh C-C, Wang S-C, Chang C-Y, Hung C-H, Kuo P-L , et al. Simvastatin attenuates cardiac fibrosis via regulation of cardiomyocyte-derived exosome secretion. JCM 8: 794, 2019. doi: 10.3390/jcm8060794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S , et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65: 1525–1536, 2015. doi: 10.1016/j.jacc.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 30.Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L, Ferrara KW, Knowlton AA. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am J Physiol Heart Circ Physiol 304: 954–965, 2013. doi: 10.1152/ajpheart.00835.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morita E, Sandrin V, Chung H-Y, Morham SG, Gygi SP, Rodesch CK , et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 26: 4215–4227, 2007. [Erratum in EMBO J 31: 3228, 2012]. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willms E, Johansson HJ, Mäger I, Lee Y , et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6: 22519, 2016. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phuyal S, Hessvik NP, Skotland T, Sandvig K, Llorente A. Regulation of exosome release by glycosphingolipids and flotillins. FEBS J 281: 2214–2227, 2014. doi: 10.1111/febs.12775. [DOI] [PubMed] [Google Scholar]
- 34.Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I , et al. Exosomal annexin II promotes angiogenesis and breast cancer metastasis. Mol Cancer Res 15: 93–105, 2017. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang N, Sun B, Gupta A, Rempel H, Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J 30: 3097–3106, 2016. doi: 10.1096/fj.201600368RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimaoka M, Kawamoto E, Gaowa A, Okamoto T, Park EJ. Connexins and integrins in exosomes. Cancers (Basel) 11: 106, 2019. doi: 10.3390/cancers11010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HM, Choi E-J, Kim JH, Kim TD, Kim Y-K, Kang C , et al. A membranous form of ICAM-1 on exosomes efficiently blocks leukocyte adhesion to activated endothelial cells. Biochem Biophys Res Commun 397: 251–256, 2010. doi: 10.1016/j.bbrc.2010.05.094. [DOI] [PubMed] [Google Scholar]
- 38.Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66: 30–41, 2017. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci 14: 14240–14269, 2013. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Devhare PB, Ray RB. Extracellular vesicles: novel mediator for cell to cell communications in liver pathogenesis. Mol Aspects Med 60: 115–122, 2018. doi: 10.1016/j.mam.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos TL, Sánchez-Abarca LI, Muntión S , et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal 14: 2, 2016. doi: 10.1186/s12964-015-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gennebäck N, Gennebäck N, Hellman U, Malm L , et al. Growth factor stimulation of cardiomyocytes induces changes in the transcriptional contents of secreted exosomes. J Extracell Vesicles 2, 2013. doi: 10.3402/jev.v2i0.20167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am J Physiol Heart Circ Physiol 292: 3052–3056, 2007. doi: 10.1152/ajpheart.01355.2006. [DOI] [PubMed] [Google Scholar]
- 44.Bellin G, Gardin C, Ferroni L, Chachques J, Rogante M, Mitrečić D , et al. Exosome in cardiovascular diseases: a complex world full of hope. Cells 8: 166, 2019. doi: 10.3390/cells8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F , et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 4: 2980, 2013. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Statello L, Maugeri M, Garre E, Nawaz M, Wahlgren J, Papadimitriou A , et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One 13: e0195969, 2018. doi: 10.1371/journal.pone.0195969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Datta R, Bansal T, Rana S , et al. Myocyte-derived Hsp90 modulates collagen upregulation via biphasic activation of STAT-3 in fibroblasts during cardiac hypertrophy. Mol Cell Biol 37: e00611–e00616, 2017. doi: 10.1128/MCB.00611-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu DW, Ge PP, Liu AL, Yu XY, Liu TT. HSP20-mediated cardiomyocyte exosomes improve cardiac function in mice with myocardial infarction by activating Akt signaling pathway. Eur Rev Med Pharmacol Sci 23: 4873–4881, 2019. doi: 10.26355/eurrev_201906_18075. [DOI] [PubMed] [Google Scholar]
- 49.Loyer X, Zlatanova I, Devue C, Yin M, Howangyin K-Y, Klaihmon P , et al. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction short communication. Circ Res 123: 100–106, 2018. doi: 10.1161/CIRCRESAHA.117.311326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chistiakov D, Orekhov A, Bobryshev Y. Cardiac extracellular vesicles in normal and infarcted heart. IJMS. 17: 63, 2016. doi: 10.3390/ijms17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia NA, Ontoria-Oviedo I, González-King H, Diez-Juan A, Sepúlveda P. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One 10: e0138849–23, 2015. doi: 10.1371/journal.pone.0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38: 754–763, 2017. doi: 10.1038/aps.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science 367: eaau6977, 2020. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mittelbrunn M. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2: 282, 2011. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha S, Hoshino D, Hong NH, Kirkbride KC, Grega-Larson NE, Seiki M , et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol 214: 197–213, 2016. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu C, Morohashi Y, Yoshimura S-I, Manrique-Hoyos N, Jung S, Lauterbach MA , et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 189: 223–232, 2010. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res 114: 333–344, 2014. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 58.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P , et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci 126: 5553–5565, 2013. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 59.McNally EK, Brett CL. The intralumenal fragment pathway mediates ESCRT-independent surface transporter down-regulation. Nat Commun 9: 5358, 2018. doi: 10.1038/s41467-018-07734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang S, Henne WM, Borbat PP, Buchkovich NJ, Freed JH, Mao Y , et al. Structural basis for activation, assembly and membrane binding of ESCRT-III Snf7 filaments. Elife 4: e12548, 2015. doi: 10.7554/eLife.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henne WM, Buchkovich NJ, Emr SD. The ESCRT Pathway. Dev Cell 21: 77–91, 2011. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Mosesso N, Nagel MK, Isono E. Ubiquitin recognition in endocytic trafficking - with or without ESCRT-0. J Cell Sci 132: jcs232868, 2019. doi: 10.1242/jcs.232868. [DOI] [PubMed] [Google Scholar]
- 63.Lim YJ, Lee SJ. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol Commun 5: 64, 2017. doi: 10.1186/s40478-017-0467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Gassart A, Géminard C, Février B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood 102: 4336–4344, 2003. doi: 10.1182/blood-2003-03-0871. [DOI] [PubMed] [Google Scholar]
- 65.Verderio C, Gabrielli M, Giussani P. Role of sphingolipids in the biogenesis and biological activity of extracellular vesicles. J Lipid Res 59: 1325–1340, 2018. doi: 10.1194/jlr.R083915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tschuschke M, Kocherova I, Bryja A, Mozdziak P, Angelova Volponi A, Janowicz K , et al. Inclusion biogenesis, methods of isolation and clinical application of human cellular exosomes. JCM 9: 436, 2020. doi: 10.3390/jcm9020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P , et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 21: 708–721, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gartz M, Strande JL. Examining the paracrine effects of exosomes in cardiovascular disease and repair. J Am Heart Assoc 7: e007954, 2018. doi: 10.1161/JAHA.117.007954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ribeiro-Rodrigues TM, Laundos TL, Pereira-Carvalho R, Batista-Almeida D, Pereira R, Coelho-Santos V , et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc Res 113: 1338–1350, 2017. doi: 10.1093/cvr/cvx118. [DOI] [PubMed] [Google Scholar]
- 70.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21: 9–17, 2019. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 71.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3: 4641, 2014. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nat Struct Mol Biol 15: 675–683, 2008. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA. Exosomes: mechanisms of uptake. J Circ Biomarkers 4: 7, 2015. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burkova EE, Grigor’eva AE, Bulgakov DV, Dmitrenok PS, Vlassov VV, Ryabchikova EI , et al. Extra purified exosomes from human placenta contain an unpredictable small number of different major proteins. IJMS. 20: 2434, 2019. doi: 10.3390/ijms20102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerreiro EM, Vestad B, Steffensen LA, Aass HCD, Saeed M, Øvstebø R , et al. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS One 13: e0204276, 2018. doi: 10.1371/journal.pone.0204276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Noble JM, Roberts LM, Vidavsky N , et al. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J Struct Biol 210: 107474, 2020. doi: 10.1016/j.jsb.2020.107474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mentkowski KI, Lang JK. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci Rep 9: 10041, 2019. doi: 10.1038/s41598-019-46407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J , et al. The methods of choice for extracellular vesicles (EVs) characterization. IJMS 18: 1153, 2017. doi: 10.3390/ijms18061153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.López E, Marinaro F, de Pedro M. DLÁ, Sánchez-Margallo FM, Gómez-Serrano M, Ponath V , et al. The immunomodulatory signature of extracellular vesicles from cardiosphere-derived cells: a proteomic and miRNA profiling. Front Cell Dev Biol 8: 321–316, 2020. doi: 10.3389/fcell.2020.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma S, Leclaire M, Gimzewski JK. Ascent of atomic force microscopy as a nanoanalytical tool for exosomes and other extracellular vesicles. Nanotechnology 29: 132001, 2018. doi: 10.1088/1361-6528/aaab06. [DOI] [PubMed] [Google Scholar]
- 81.Lim J, Choi M, Lee HJae, Kim Y-H, Han J-Y, Lee ES , et al. Direct isolation and characterization of circulating exosomes from biological samples using magnetic nanowires. J Nanobiotechnology 17: 1, 2019. doi: 10.1186/s12951-018-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Korvala J, Salo T, Sormunen R , et al. Human saliva-derived exosomes: comparing methods of isolation. J Histochem Cytochem 63: 181–189, 2015. doi: 10.1369/0022155414564219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardij J, Cecchet F, Berquand A, Gheldof D, Chatelain C, Mullier F , et al. Characterisation of tissue factor-bearing extracellular vesicles with AFM: comparison of air-tapping-mode AFM and liquid peak force AFM. J Extracell Vesicles 2: 21045, 2013. doi: 10.3402/jev.v2i0.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serrano-Pertierra E, Oliveira-Rodríguez M, Rivas M, Oliva P, Villafani J, Navarro A , et al. Characterization of plasma-derived extracellular vesicles isolated by different methods: a comparison study. Bioengineering 6: 8, 2019. doi: 10.3390/bioengineering6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soo CY, Song Y, Zheng Y, Campbell EC, Riches AC, Gunn-Moore F , et al. Nanoparticle tracking analysis monitors microvesicle and exosome secretion from immune cells. Immunology 136: 192–197, 2012. doi: 10.1111/j.1365-2567.2012.03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R , et al. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost 8: 2596–2607, 2010. doi: 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- 87.Mastoridis S, Bertolino GM, Whitehouse G, Dazzi F, Sanchez-Fueyo A, Martinez-Llordella M , et al. Multiparametric analysis of circulating exosomes and other small extracellular vesicles by advanced imaging flow cytometry. Front Immunol 9: 1583, 2018. doi: 10.3389/fimmu.2018.01583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. J Control Release 228: 179–190, 2016. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 89.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R , et al. Revisiting cardiac cellular composition. Circ Res 118: 400–409, 2016. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karra R, Walter AO, Wu SM. The relationship between cardiac endothelium and fibroblasts: It’s complicated. J Clin Invest 127: 2892–2894, 2017. doi: 10.1172/JCI95492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Popescu LM, Faussone-Pellegrini MS. TELOCYTES—a case of serendipity: the winding way from interstitial cells of cajal (ICC), via interstitial cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med 14: 729–740, 2010. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res 112: 1624–1633, 2013. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A , et al. Macrophages facilitate electrical conduction in the heart. Cell 169: 510–522.e20, 2017. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol 202: 891–899, 2005. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 95.LaFramboise WA, Scalise D, Stoodley P , et al. Cardiac fibroblasts influence cardiomyocyte phenotype in vitro. Am J Physiol Cell Physiol 292: C1799–C1808, 2007. doi: 10.1152/ajpcell.00166.2006. [DOI] [PubMed] [Google Scholar]
- 96.Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A , et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124: 2136–2146, 2014. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M , et al. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 89: 268–279, 2015. doi: 10.1016/j.yjmcc.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen REW, Custers K , et al. Macrophage MicroRNA-155 promotes cardiac hypertrophy and failure. Circulation 128: 1420–1432, 2013. [Erratum in Circulation 141: e808, 2020]). doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 99.Seok HY, Chen J, Kataoka M, Huang Z-P, Ding J, Yan J , et al. Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy. Circ Res 114: 1585–1595, 2014. doi: 10.1161/CIRCRESAHA.114.303784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang X, Stroud MJ, Ouyang K, Fang L, Zhang J, Dalton ND , et al. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight 1, 2016. doi: 10.1172/jci.insight.89908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nie X, Fan J, Li H, Yin Z, Zhao Y, Dai B , et al. miR-217 promotes cardiac hypertrophy and dysfunction by targeting PTEN. Mol Ther Nucleic Acids 12: 254–266, 2018. doi: 10.1016/j.omtn.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang J, Yu XF, Li YY, Xue FT, Zhang S. Decreased HSP70 expression on serum exosomes contributes to cardiac fibrosis during senescence. Eur Rev Med Pharmacol Sci 23: 3993–4001, 2019. doi: 10.26355/eurrev_201905_17829. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Gu H, Huang W, Peng J, Li Y, Yang L , et al. Hsp20-mediated activation of exosome biogenesis in cardiomyocytes improves cardiac function and angiogenesis in diabetic mice. Diabetes 65: 3111–3128, 2016. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J Cell Mol Med 19: 2153–2161, 2015. doi: 10.1111/jcmm.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Zhang J. Exosomal lncRNA AK139128 derived from hypoxic cardiomyocytes promotes apoptosis and inhibits cell proliferation in cardiac fibroblasts. Int J Nanomedicine 15: 3363–3376, 2020. doi: 10.2147/IJN.S240660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang C, Zhang C, Liu L, A X, Chen B, Li Y , et al. Macrophage-derived miR-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther 25: 192–204, 2017. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei Y, Yan X, Yan L, Hu F, Ma W, Wang Y , et al. Inhibition of microRNA-155 ameliorates cardiac fibrosis in the process of angiotensin II-induced cardiac remodeling. Mol Med Rep 16: 7287–7296, 2017. doi: 10.3892/mmr.2017.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eissa MG, Artlett CM. The microRNA miR-155 is essential in fibrosis. ncRNA 5: 23, 2019. doi: 10.3390/ncrna5010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian C, Gao L, Zimmerman MC, Zucker IH. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol Heart Circ Physiol 314: H928–H939, 2018. doi: 10.1152/ajpheart.00602.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baranyai T, Herczeg K, Onódi Z, Voszka I, Módos K, Marton N , et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 10: e0145686, 2015. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giricz Z, Varga ZV, Baranyai T, Sipos P, Pálóczi K, Kittel Á , et al. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 68: 75–78, 2014. doi: 10.1016/j.yjmcc.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 112.Li J, Rohailla S, Gelber N, Rutka J , et al. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res Cardiol 109: 423, 2014. doi: 10.1007/s00395-014-0423-z. [DOI] [PubMed] [Google Scholar]
- 113.Minghua W, Zhijian G, Chahua H, Qiang L, Minxuan X, Luqiao W , et al. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring MIR-24 article. Cell Death Dis 9: 320, 2018. doi: 10.1038/s41419-018-0274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X , et al. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med (Berl) 94: 711–724, 2016. doi: 10.1007/s00109-016-1387-2. [DOI] [PubMed] [Google Scholar]
- 115.Gambim MH, de Oliveira do Carmo A, Marti L , et al. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care 11: R107, 2007. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 112: 1813–1824, 2005. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 117.Qiao L, Hu S, Liu S, Zhang H, Ma H, Huang K , et al. MicroRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest 129: 2237–2250, 2019. doi: 10.1172/JCI123135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang X, Wang X, Zhu H, Kranias EG, Tang Y, Peng T , et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One 7: e32765, 2012. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 4: 51–58, 2011. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ottaviani L, Sansonetti M, da Costa Martins PA. Myocardial cell-to-cell communication via microRNAs. Noncoding RNA Res 3: 144–153, 2018. [Erratum in Noncoding RNA Res 5: 220-221, 2020]. doi: 10.1016/j.ncrna.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y , et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 74: 139–150, 2014. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Balkom BWM, de Jong OG, Smits M , et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 121: 3997–4006, 2013. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 123.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen N-Q-N, Scherr M , et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 123: 2143–2154, 2013. doi: 10.1172/JCI64365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao B, Liao Z, Chen S, Yuan Z, Yilin C, Lee KKH , et al. Intramyocardial transplantation of cardiac telocytes decreases myocardial infarction and improves post-infarcted cardiac function in rats. J Cell Mol Med 18: 780–789, 2014. doi: 10.1111/jcmm.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang J, Li Y, Xue F, Liu W, Zhang S. Exosomes derived from cardiac telocytes exert positive effects on endothelial cells. Am J Transl Res 9: 5375–5387, 2017. [PMC free article] [PubMed] [Google Scholar]
- 126.Manole CG, Cismaşiu V, Gherghiceanu M, Popescu LM. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med 15: 2284–2296, 2011. doi: 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol 18: 733–744, 2018. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 128.Liu H, Gao W, Yuan J, Wu C, Yao K, Zhang L , et al. Exosomes derived from dendritic cells improve cardiac function via activation of CD4+ T lymphocytes after myocardial infarction. J Mol Cell Cardiol 91: 123–133, 2016. doi: 10.1016/j.yjmcc.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 129.Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y , et al. Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-α mediated NF-κB pathway. J Cell Mol Med 20: 2318–2327, 2016. doi: 10.1111/jcmm.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He S, Wu J, Xiao J, Li D, Sun Z, Li M. Endothelial extracellular vesicles modulate the macrophage phenotype: potential implications in atherosclerosis. Scand J Immunol 87: e12648, 2018. doi: 10.1111/sji.12648. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y, Mei H, Chang X, Chen F, Zhu Y, Han X , et al. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol 8: 505–517, 2016. doi: 10.1093/jmcb/mjw040. [DOI] [PubMed] [Google Scholar]
- 132.Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y , et al. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids 11: 103–115, 2018. doi: 10.1016/j.omtn.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ailawadi S, Wang X, Gu H, Fan GC. Pathologic function and therapeutic potential of exosomes in cardiovascular disease. Biochim Biophys Acta 1852: 1–11, 2015. doi: 10.1016/j.bbadis.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7: 99–109, 2009. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang B, Wang Z-M, Ji J-L, Gan W, Zhang A, Shi H-J , et al. Macrophage-derived exosomal Mir-155 regulating cardiomyocyte pyroptosis and hypertrophy in uremic cardiomyopathy. JACC Basic Transl Sci 5: 148–166, 2020. doi: 10.1016/j.jacbts.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park SJ, Kim JM, Kim J, Hur J, Park S, Kim K , et al. Molecular mechanisms of biogenesis of apoptotic exosome-like vesicles and their roles as damage-associated molecular patterns. Proc Natl Acad Sci U S A 115: E11721–E11730, 2018. doi: 10.1073/pnas.1811432115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B , et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med 7: 318ra200–318ra218, 2015. doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 138.Hardy MP, Audemard É, Migneault F , et al. Apoptotic endothelial cells release small extracellular vesicles loaded with immunostimulatory viral-like RNAs. Sci Rep 9: 7203, 2019. doi: 10.1038/s41598-019-43591-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M , et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 4: 446–454, 2011. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 140.Zamani P, Fereydouni N, Butler AE, Navashenaq JG, Sahebkar A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc Med 29: 313–323, 2019. doi: 10.1016/j.tcm.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 141.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA , et al. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med 20: 280–289, 2014. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakayama H, Otsu K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem J 475: 839–852, 2018. doi: 10.1042/BCJ20170714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H , et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl) 92: 387–397, 2014. doi: 10.1007/s00109-013-1110-5. [DOI] [PubMed] [Google Scholar]
- 145.Kang K, Ma R, Cai W, Huang W, Paul C, Liang J , et al. Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells Int 2015: 659890, 2015. doi: 10.1155/2015/659890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z , et al. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem 37: 2415–2424, 2015. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 147.Ma J, Zhao Y, Sun L, Sun X, Zhao X, Sun X , et al. Exosomes derived from Akt-modified human umbilical cord mesenchymal stem cells improve cardiac regeneration and promote angiogenesis via activating platelet-derived growth factor D. Stem Cells Transl Med 6: 51–59, 2017. doi: 10.5966/sctm.2016-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One 9: e88685, 2014. doi: 10.1371/journal.pone.0088685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y , et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 182: 349–360, 2015. doi: 10.1016/j.ijcard.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wen Z, Mai Z, Zhu X , et al. Mesenchymal stem cell-derived exosomes ameliorate cardiomyocyte apoptosis in hypoxic conditions through microRNA144 by targeting the PTEN/AKT pathway. Stem Cell Res Ther 11: 36, 2020. doi: 10.1186/s13287-020-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y , et al. Enhanced cardioprotection by human endometrium mesenchymal stem cells driven by exosomal microRNA-21. Stem Cells Transl Med 6: 209–222, 2017. doi: 10.5966/sctm.2015-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS , et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4: 214–222, 2010. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 153.Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE , et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10: 301–312, 2013. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 154.Liu L, Jin X, Hu C-F, Li R, Zhou Z, Shen C-X , et al. Exosomes derived from mesenchymal stem cells rescue myocardial ischaemia/reperfusion injury by inducing cardiomyocyte autophagy via AMPK and Akt pathways. Cell Physiol Biochem 43: 52–68, 2017. doi: 10.1159/000480317. [DOI] [PubMed] [Google Scholar]
- 155.Cui X, He Z, Liang Z , et al. Exosomes from adipose-derived mesenchymal stem cells protect the myocardium against ischemia/reperfusion injury through Wnt/b-catenin signaling pathway. J Cardiovasc Pharmacol 70: 225–231, 2017. doi: 10.1097/FJC.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song L, Yang Y-J, Dong Q-T, Qian H-Y, Gao R-L, Qiao S-B , et al. Atorvastatin enhances efficacy of mesenchymal stem cells treatment for swine myocardial infarction via activation of nitric oxide synthase. PLoS One 8: e65702, 2013. doi: 10.1371/journal.pone.0065702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang P, Wang L, Li Q, Tian X, Xu J, Xu J , et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res 116: 353–367, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Amini H, Rezaie J, Vosoughi A, Rahbarghazi R, Nouri MC. Progenitor cells application in cardiovascular disease. © 2017. (http//creativecommons. 9, 127–132). [DOI] [PMC free article] [PubMed]
- 159.Vrijsen KR, Sluijter JPG, Schuchardt MWL, van Balkom BWM, Noort WA, Chamuleau SAJ , et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 14: 1064–1070, 2010. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO , et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 116: 255–263, 2015. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dougherty JA, Patel N, Kumar N, Rao SG, Angelos MG, Singh H , et al. Human cardiac progenitor cells enhance exosome release and promote angiogenesis under physoxia. Front Cell Dev Biol 8: 130–112, 2020. doi: 10.3389/fcell.2020.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Youn S-W, Li Y, Kim Y-M, Sudhahar V, Abdelsaid K, Kim H , et al. Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through nox2-dependent angiogenesis. Antioxidants 8: 18, 2019. doi: 10.3390/antiox8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A , et al. MiR-133a enhances the protective capacity of cardiac progenitor cells after myocardial infarction. Stem Cell Reports 3: 1029–1042, 2014. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G , et al. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem. Biophys. Res. Commun 431: 566–571, 2013. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH , et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis 7: e2277, 2016. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li X, Yang Z, Nie W , et al. Exosomes derived from cardiac progenitor cells attenuate CVB3-induced apoptosis via abrogating the proliferation of CVB3 and modulating the mTOR signaling pathways. Cell Death Dis 10: 691, 2019. doi: 10.1038/s41419-019-1910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM , et al. Extracellular vesicles fromhuman cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function aftermyocardial infarction. Cardiovasc Res 103: 530–541, 2014. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 168.Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V , et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res 114: 992–1005, 2018. doi: 10.1093/cvr/cvy055. [DOI] [PubMed] [Google Scholar]
- 169.Maring JA, Lodder K, Mol E, Verhage V, Wiesmeijer KC, Dingenouts CKE , et al. Cardiac progenitor cell-derived extracellular vesicles reduce infarct size and associate with increased cardiovascular cell proliferation. J Cardiovasc Transl Res 12: 5–17, 2019. doi: 10.1007/s12265-018-9842-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Casieri V, Matteucci M, Pasanisi EM , et al. Ticagrelor enhances release of anti-hypoxic cardiac progenitor cell-derived exosomes through increasing cell proliferation in vitro. Sci Rep 10: 2494, 2020. doi: 10.1038/s41598-020-59225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cheng M, Yang J, Zhao X , et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun 10: 959, 2019. doi: 10.1038/s41467-019-08895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Namazi H, Mohit E, Namazi I, Rajabi S, Samadian A, Hajizadeh-Saffar E , et al. Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J Cell Biochem 119: 4150–4160, 2018. doi: 10.1002/jcb.26621. [DOI] [PubMed] [Google Scholar]
- 173.Namazi H, Namazi I, Ghiasi P, Ansari H, Rajabi S, Hajizadeh-Saffar E , et al. Exosomes secreted by normoxic and hypoxic cardiosphere-derived cells have anti-apoptotic effect. Iran J Pharm Res 17: 377–385, 2018. [PMC free article] [PubMed] [Google Scholar]
- 174.Ibrahim AGE, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2: 606–619, 2014. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R , et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38: 201–211, 2017. doi: 10.1093/eurheartj/ehw240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tseliou E, Fouad J, Reich H, Slipczuk L, de Couto G, Aminzadeh M , et al. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J Am Coll Cardiol 66: 599–611, 2015. doi: 10.1016/j.jacc.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W , et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192: 61–69, 2015. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A , et al. Induced pluripotent stem cell (iPSC)-derived extracellular vesicles are safer and more effective for cardiac repair than iPSCs. Circ Res 122: 296–309, 2018. doi: 10.1161/CIRCRESAHA.117.311769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang PC. Induced pluripotent stem cell (iPSC)-derived exosomes for precision medicine in heart failure. Circ Res 122: 661–663, 2018. doi: 10.1161/CIRCRESAHA.118.312657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.El Harane N, Kervadec A, Bellamy V, Pidial L, Neametalla HJ, Perier M-C , et al. Acellular therapeutic approach for heart failure: In vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J 39: 1835–1847, 2018. doi: 10.1093/eurheartj/ehy012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M , et al. Exosomes from human CD34+ stem cells mediate their proangiogenic paracrine activity. Circ Res 109: 724–728, 2011. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mackie AR, Klyachko E, Thorne T, Schultz KM, Millay M, Ito A , et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res 111: 312–321, 2012. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE , et al. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med 5: 440–450, 2016. doi: 10.5966/sctm.2015-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Togliatto G, Dentelli P, Gili M, Gallo S, Deregibus C, Biglieri E , et al. Obesity reduces the pro-angiogenic potential of adipose tissue stem cell-derived extracellular vesicles (EVs) by impairing miR-126 content: Impact on clinical applications. Int J Obes (Lond) 40: 102–111, 2016. doi: 10.1038/ijo.2015.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wiklander OP, Nordin JZ, O’Loughlin A , et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4: 26316, 2015. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]