Abstract
This study tested the hypothesis that early left ventricular (LV) relaxation is impaired in older obese patients with heart failure with preserved ejection fraction (HFpEF), and related to decreased peak exercise oxygen uptake (peak V̇o2). LV strain and strain rate were measured by feature tracking of magnetic resonance cine images in 79 older obese patients with HFpEF (mean age: 66 yr; mean body mass index: 38 kg/m2) and 54 healthy control participants. LV diastolic strain rates were indexed to cardiac preload as estimated by echocardiography derived diastolic filling pressures (E/e′), and correlated to peak V̇o2. LV circumferential early diastolic strain rate was impaired in HFpEF compared with controls (0.93 ± 0.05/s vs. 1.20 ± 0.07/s, P = 0.014); however, we observed no group differences in early LV radial or longitudinal diastolic strain rates. Isolating myocardial relaxation by indexing all three early LV diastolic strain rates (i.e. circumferential, radial, and longitudinal) to E/e′ amplified the group difference in early LV diastolic circumferential strain rate (0.08 ± 0.03 vs. 0.13 ± 0.05, P < 0.0001), and unmasked differences in early radial and longitudinal diastolic strain rate. Moreover, when indexing to E/e′, early LV diastolic strain rates from all three principal strains, were modestly related with peak V̇o2 (R = 0.36, −0.27, and 0.35, respectively, all P < 0.01); this response, however, was almost entirely driven by E/e′ itself, (R = −0.52, P < 0.001). Taken together, we found that although LV relaxation is impaired in older obese patients with HFpEF, and modestly correlates with their severely reduced peak exercise V̇o2, LV filling pressures appear to play a much more important role in determining exercise intolerance.
NEW & NOTEWORTHY Using a multimodal imaging approach to uncouple tissue deformation from atrial pressure, we found that left ventricular (LV) relaxation is impaired in older obese patients with HFpEF, but only modestly correlates with their severely reduced peak V̇o2. In contrast, the data show a much stronger relationship between elevated LV filling pressures and exercise intolerance, refocusing future therapeutic priorities.
Keywords: diastolic dysfunction, exercise intolerance, heart failure, heart failure with preserved ejection fraction, obesity
INTRODUCTION
Heart failure (HF) with preserved ejection fraction (HFpEF) is the fastest growing form of HF and is associated with a 65% five year mortality rate (1, 2). The primary chronic symptom in HFpEF even when clinically stable and nonedematous is decreased exercise tolerance, measured objectively as decreased peak oxygen uptake (peak V̇o2) (3–9). The physiological mechanisms underpinning the decreased peak V̇o2 in older obese patients with HFpEF has not been well characterized, however may be due in part, to impaired left ventricular (LV) diastolic function (9–12).
Diastolic dysfunction is often defined by an elevated LV end-diastolic pressure; however, this static measure—obtained from a single time-point in the cardiac cycle only at end–diastole— fails to provide insight into dynamic LV tissue relaxation during early diastole. Various imaging techniques, including Doppler ultrasound and cardiac magnetic resonance imaging (CMRI), are commonly used to assess this early period of diastole; however, they are often insensitive to detect HFpEF in outpatients with unexplained dyspnea in an early stage of the disease (13, 14); potentially leaving a large proportion of patients undetected in daily clinical practice. This may be explained by the fact that both Doppler echocardiography and CMRI measures of diastolic function are heavily influenced by loading conditions. Indeed, data from a prior CMRI study highlight the importance of controlling for preload (15). However, this proof-of-concept study used CMRI-derived volume-time profiles, which is incredibly time consuming, operator dependent, and clinically infeasible.
Myocardial feature tracking of conventional CMRI cine images alleviates many of the aforementioned limitations by reducing operator dependence and postprocessing time, and provides detailed quantitative information about myocardial relaxation (i.e., diastolic strain rate). Strain and strain rate are markers of deformation, and have been proven to be a powerful diagnostic and prognostic indicators for global and regional LV function. Although diastolic strain rate is increasingly recognized as a powerful discriminator of diastolic dysfunction, and predictive of cardiovascular events (16–18), like other imaging approaches, it remains preload dependent (19). We therefore sought to isolate diastolic relaxation from the hemodynamic driving pressure by correcting the diastolic strain rate for LV preload (estimated with Doppler ultrasound as the ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity, E/e′ ratio). Using this metric, we hypothesized that LV early diastolic strain rate indexed to LV filling pressure would be impaired in patients with HFpEF compared with controls, and be associated with peak V̇o2.
METHODS
Study Participants
All patients with HFpEF met the previously described inclusion criteria (6): ≥60 yr of age; body mass index ≥30 kg/m2; signs and symptoms of HF defined by the National Health and Nutrition Examination Survey score ≥3 (20), or the previously published criteria (21) or both; LV ejection fraction ≥50%; no segmental wall motion abnormalities; no contraindications to CMRI; and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorders that could explain the patients’ symptoms (4, 22, 23). Patients with significant pericardial and valvular disease were also excluded from the study. Healthy controls were recruited and screened, and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥140/90 mmHg), had abnormal results on the screening tests (echocardiogram, electrocardiogram, and cardiopulmonary exercise testing), or regularly undertook vigorous exercise (4, 23). All control subjects had a normal LV filling pattern (24). The study was approved by the Wake Forest University Health Sciences Institutional Review Board. All participants provided written informed consent.
Each participant was studied on two separate occasions (8 ± 9 days apart for HFpEF, 14 ± 20 days for controls: on the first visit, the participants completed an echocardiogram performed after ≥15 min of supine rest, followed by an incremental cardiopulmonary exercise test. On the second visit, participants completed a resting CMRI study. Before study visits, all participants were instructed to refrain from vigorous exercise for 24 h leading up to the laboratory visit.
Echocardiography
Echocardiography was performed with all participants resting in a semirecumbent position for ≥15 min. Doppler echocardiography was used to assess LV filling patterns, mitral septal annular velocity, and pulse-wave velocity in accordance with the American Society of Echocardiography recommendations (25), as previously described (6). All Doppler values represent the average of three cardiac cycles. The E/e′ ratio was calculated as a surrogate measure of LV filling pressures (25). Left atrial diameter was measured at the widest region of the left atrium in the 4-chamber view in end-diastole.
Cardiopulmonary Exercise Testing
Cardiopulmonary exercise testing was performed on a motorized treadmill using the modified Naughton protocol, or the Modified Bruce in control subjects with higher self-reported physical activity level, as previously described (6, 26). Participants were given detailed, standardized instructions before performing symptom-limited exhaustive test to volitional fatigue. Metabolic gas exchange was measured continuously during exercise and averaged over 15-s intervals (Medgraphics Ultima, Medical Graphics Corp., St. Paul, Minnesota) (6, 23, 26). Peak V̇o2 was calculated as the average of measures from the last 30 s during peak exercise (6, 23, 26).
Left Ventricular Morphology and Deformation by CMRI
LV mass and volumes were assessed by CMRI (1.5 T Avanto, Siemens Healthineers) from a series of multislice, multiphase gradient-echo sequences positioned perpendicular to the long-axis of the ventricles (short-axis), spanning apex to base (6). Typical CMRI imaging parameters included: flip angle 76°; repetition/echo time: 40–50/1.1–1.2 ms; slice thickness: 7–8 mm; with 25 cardiac phases. From these images, the epi- and endocardial borders of each slice were traced manually at end-diastole and end-systole, with volumes and mass derived via the method of disks. LV stroke volume and ejection fraction were calculated accordingly. All mass and volume data were expressed as absolute values and indexed to body surface area. LV concentricity was calculated by dividing LV mass by LV end-diastolic volume.
The primary end point of this investigation was LV early diastolic strain rate by myocardial feature tracking. All LV strain data was measured offline using a commercially available software package (Cvi42; v 5.3.0, Circle Cardiovascular Imaging Inc., Calgary, AB, Canada). This approach has indeed been previously validated (27–29), with excellent agreement against gold-standard CMRI tissue tagging reported by our group (30). Briefly, the endo- and epicardial borders of the previously described cine images were manually delineated at end-diastole before the feature tracking algorithm was applied. Caution was taken to avoid including slices which included the LV outflow tract and/or left atrium, apical slices without clear delineation of the LV lumen at end-systole (a minimum of 2 cm proximal to luminal obliteration), and insufficient tracking quality. Early diastolic strain rate is the slope (i.e., speed) of myocardial deformation during early diastole, reflective of early LV relaxation and diastolic function (31). Early diastolic strain rates were calculated in the longitudinal, circumferential, and radial planes. Horizontal long-axis cine images were tracked to derive longitudinal strain and strain rate, whereas short-axis cine images were used to derive circumferential and radial strain and strain rate. Strain and strain rates were obtained for each segment and the global values were defined as the mean of all segmental values. All of the data were analyzed by a single observer (T.J.S.), who was blinded to each subject’s medical history and/or group allocation. Our in-laboratory intra-rater reliability in 15 randomly selected subjects, expressed as a coefficient of variation, for each of the primary endpoints is as follows (means ± standard deviation): LV circumferential strain, 3.1% ± 2.3%; LV early diastolic circumferential strain rate, 4.9% ± 4.6%; LV radial strain, 3.6% ± 2.9%; LV early diastolic radial strain rate, 6.7% ± 7.1%; LV longitudinal strain, 4.4% ± 3.5%; and LV early diastolic longitudinal strain rate, 4.6% ± 4.4%. Moreover, our in-laboratory interrater reliability, expressed as a coefficient of variation, for each of the primary endpoints is as follows (means ± standard deviation): LV circumferential strain, 8.4% ± 9.5%; LV early diastolic circumferential strain rate, 8.8% ± 7.5%; LV longitudinal strain, 8.1% ± 6.1%; and LV early diastolic longitudinal strain rate, 10.2% ± 7.9%.
Statistical Analysis
All variables were tested for normality with histograms and quantile-quantile plots. Participant characteristics were compared between HFpEF and healthy control groups with t tests for continuous variables and chi-squared tests for categorical variables. Strain, strain rates, and strain rates indexed to E/e′ were also analyzed using analysis of covariance to adjust for group differences of age, sex, body mass index (BMI), and race. Pearson correlation coefficients were calculated on HFpEF and healthy control groups combined to analyze univariate relationships between E/e′, strain measures, and peak V̇o2. Multivariate linear regression was used to evaluate associations between strain measures and peak V̇o2 adjusted for age, sex, race, and BMI. To assess the relative contribution of E/e′ in strain rates indexed to E/e′, univariate and multivariate regression was performed with E/e′ and circumferential, radial, and longitudinal strain rates. Two-sided P < 0.05 was considered significant for all statistical tests. All statistical analyses were performed in SAS Enterprise Guide v 7.11.
RESULTS
Patient Characteristics
Patients with HFpEF were clinically stable (NYHA Class II and III) with typical characteristics of HFpEF. Relative to control participants, patients with HFpEF were more predominantly female, less frequently of white race, had increased BMI, higher resting systolic blood pressure, increased Doppler derived LV E/e′ and impaired filling patterns, and lower absolute and relative peak V̇o2 (Table 1).
Table 1.
Patient characteristics
| Variable | HFpEF | Healthy Controls | P Value |
|---|---|---|---|
| Participant characteristics | |||
| n | 79 | 54 | |
| Age, yr | 66.4 ± 5.2 | 69.1 ± 7.3 | 0.027 |
| Women, n (%) | 66 (84) | 33 (61) | 0.004 |
| White, n (%) | 42 (53) | 51 (94) | <0.001 |
| Body weight, kg | 101.3 ± 14.5 | 73.9 ± 14.8 | <0.001 |
| BSA, m2 | 2.0 ± 0.2 | 1.8 ± 0.2 | <0.001 |
| BMI, kg/m2 | 38.4 ± 5.1 | 25.9 ± 4.5 | <0.001 |
| NYHA Class II, n (%) | 52 (66) | 0 (0) | <0.001 |
| NYHA Class III, n (%) | 27 (34) | 0 (0) | <0.001 |
| Resting HR, beats/min | 73 ± 11 | 65 ± 10 | <0.001 |
| Echocardiogram measures | |||
| Ejection fraction, % | 61.0 ± 6.1 | 58.9 ± 4.8 | 0.036 |
| Relative wall thickness, mm | 0.57 ± 0.12 | 0.38 ± 0.05 | <0.001 |
| Diastolic filling pattern† | |||
| Normal, n (%) | 2 (3) | 54 (100) | <0.001 |
| Impaired relaxation, n (%) | 69 (87) | 0 (0) | <0.001 |
| Pseudonormal, n (%) | 8 (10) | 0 (0) | 0.016 |
| Restrictive, n (%) | 0 (0) | 0 (0) | 0.99 |
| E/A ratio | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.19 |
| Septal e′, cm/s | 6.1 ± 1.5 | 7.9 ± 1.6 | <0.001 |
| Lateral e′, cm/s | 7.3 ± 1.8 | 9.6 ± 2.0 | <0.001 |
| E/e′ ratio | 13.0 ± 3.6 | 9.3 ± 2.0 | <0.001 |
| LA diameter, cm | 4.0 ± 0.5 | 3.4 ± 0.5 | <0.001 |
| CMRI measures | |||
| LV EDV, mL | 116.2 ± 31.0 | 114.1 ± 25.2 | 0.69 |
| LV EDV index, mL/m2 | 56.7 ± 14.0 | 61.9 ± 11.6 | 0.027 |
| LV mass, g | 88.4 ± 22.0 | 81.7 ± 18.3 | 0.074 |
| LV mass index, g/m2 | 43.1 ± 9.6 | 44.1 ± 7.3 | 0.50 |
| LV concentricity, g/mL | 0.78 ± 0.17 | 0.74 ± 0.19 | 0.16 |
| Medical history | |||
| Current atrial fibrillation, n (%) | 2 (3) | 0 (0) | - |
| History of diabetes mellitus, n (%) | 51 (65) | 0 (0) | - |
| History of hypertension, n (%) | 75 (95) | 0 (0) | - |
| Systolic blood pressure, mmHg | 133 ± 13 | 124 ± 11 | <0.001 |
| Diastolic blood pressure, mmHg | 77 ± 8 | 75 ± 6 | 0.12 |
| Current medications | |||
| ACE-inhibitors, n (%) | 28 (36) | 0 (0) | - |
| Diuretics, n (%) | 56 (72) | 0 (0) | - |
| β-blockers, n (%) | 30 (38) | 0 (0) | - |
| Calcium antagonists, n (%) | 25 (32) | 0 (0) | - |
| Nitrates, n (%) | 6 (8) | 0 (0) | - |
| ARBs, n (%) | 26 (33) | 0 (0) | - |
| Peak V̇O2, mL·kg−1·min−1 | 14.9 ± 0.98 | 25.3 ± 0.29 | <0.001 |
| Peak V̇O2, mL/min | 1,506 ± 36 | 1,865 ± 83 | <0.001 |
Values are means ± SD, or n (%); †diastolic filling pattern determined according to the American Society of Echocardiography Criteria; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme; BMI, body mass index; BSA, body surface area; CMRI, cardiac magnetic resonance imaging; E, E-wave velocity; e′, early mitral annulus velocity (septal); HFpEF, heart failure with preserved ejection fraction; HR, heart rate; LA, left atrial; LV, left ventricle; NYHA, New York Heart Association. P values derived from t tests and χ2 tests, where appropriate.
Myocardial Feature Tracking
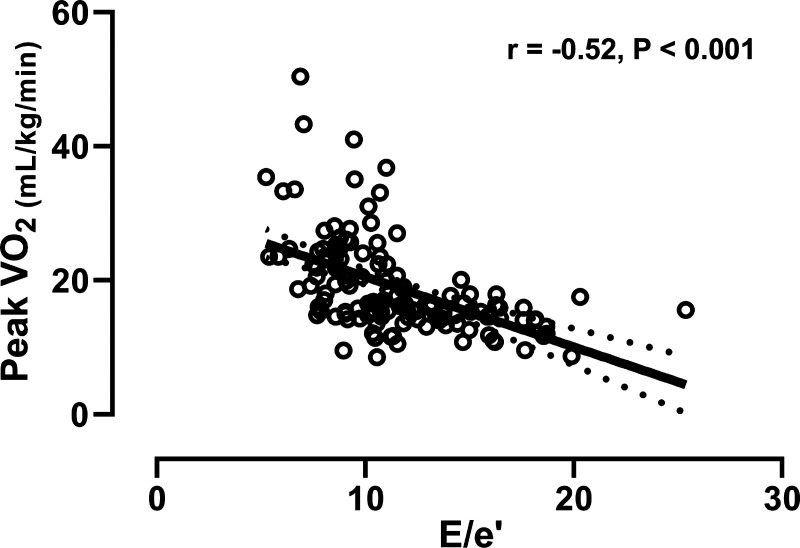
Early LV diastolic circumferential strain rate was significantly reduced in HFpEF compared to controls; however, we observed no group differences in early LV radial or longitudinal diastolic strain rates (Table 2). We also observed no significant relationships between early LV diastolic strain rate and peak V̇o2 (Fig. 1).
Table 2.
Left ventricular strain and strain rates in HFpEF and healthy controls
| Variable | Raw Means ± SD |
LS Means ± SE |
||||
|---|---|---|---|---|---|---|
| HFpEF | Control | P value | HFpEF | Control | P value | |
| Radial strain, % | 45.8 ± 1.2 | 49.3 ± 1.6 | 0.071 | 45.4 ± 1.6 | 49.9 ± 2.1 | 0.16 |
| Systolic radial SR, s-1 | 3.0 ± 0.1 | 2.6 ± 0.1 | 0.013 | 2.9 ± 0.1 | 2.7 ± 0.2 | 0.31 |
| Early diastolic radial SR, s-1 | −2.5 ± 0.1 | −2.9 ± 0.2 | 0.064 | −2.5 ± 0.2 | −2.9 ± 0.2 | 0.18 |
| Late diastolic radial SR, s-1 | −1.2 ± 0.1 | −1.0 ± 0.04 | 0.004 | −1.23 ± 0.09 | −0.92 ± 0.11 | 0.073 |
| Circumferential strain, % | −22.1 ± 0.4 | −23.4 ± 0.4 | 0.021 | −21.8 ± 0.5 | −23.8 ± 0.6 | 0.042 |
| Systolic circumferential SR, s-1 | −1.23 ± 0.03 | −1.16 ± 0.03 | 0.095 | −1.25 ± 0.04 | −1.13 ± 0.05 | 0.12 |
| Early diastolic circumferential SR, s-1 | 0.95 ± 0.04 | 1.18 ± 0.06 | 0.001 | 0.93 ± 0.05 | 1.20 ± 0.07 | 0.014 |
| Late diastolic circumferential SR, s-1 | 0.93 ± 0.05 | 0.83 ± 0.04 | 0.11 | 0.95 ± 0.05 | 0.80 ± 0.07 | 0.16 |
| Longitudinal strain, % | −20.0 ± 0.4 | −18.4 ± 0.5 | 0.025 | −19.6 ± 0.5 | −19.1 ± 0.7 | 0.64 |
| Systolic longitudinal SR, s-1 | −1.16 ± 0.03 | −1.04 ± 0.03 | 0.005 | −1.16 ± 0.03 | −1.04 ± 0.05 | 0.086 |
| Early diastolic longitudinal SR, s-1 | 0.91 ± 0.04 | 0.91 ± 0.03 | 0.92 | 0.93 ± 0.05 | 0.88 ± 0.07 | 0.61 |
| Late diastolic longitudinal SR, s-1 | 1.08 ± 0.04 | 0.79 ± 0.03 | <0.001 | 1.03 ± 0.05 | 0.86 ± 0.07 | 0.079 |
Raw data are means ± SD; LS values are means ± SE, adjusted for age, sex, BMI, and race. HFpEF, heart failure with preserved ejection fraction; SR, strain rate. P values derived from t tests and analysis of covariance to adjust for group differences in age, sex, BMI, and race. Boldface indicates significance.
Figure 1.
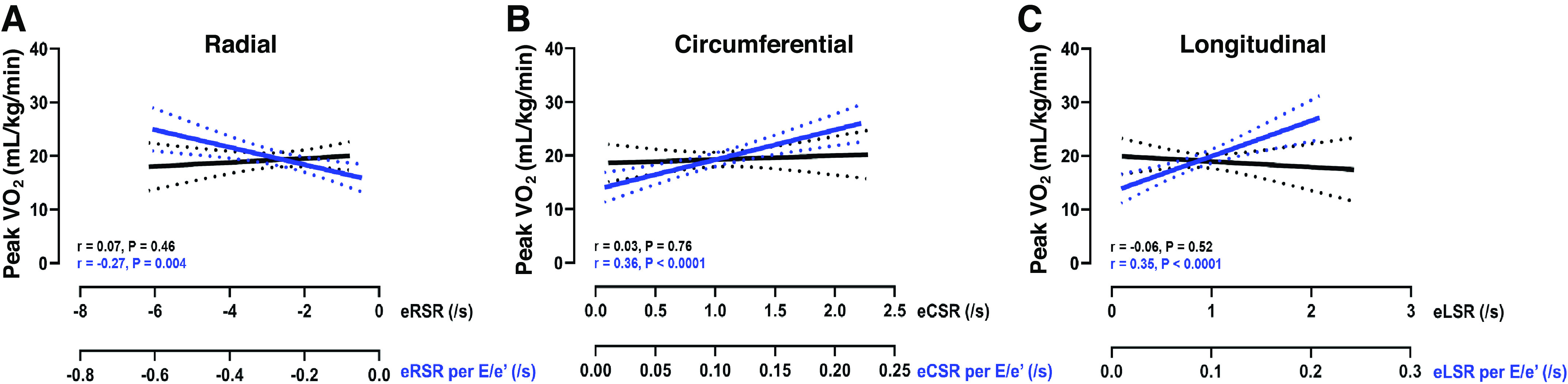
Indexing measures of early diastolic radial, circumferential, and longitudinal (A–C) strain rate to E/e′ (blue) unmasks significant relationships with peak V̇O2, not observed before indexing (black). These data highlight the importance of controlling for preload when interpreting measures of early diastolic relaxation. Data are presented as linear regression and 95% confidence intervals.
Indexing all three early LV diastolic strain rates (i.e., circumferential, radial, and longitudinal) to E/e′ exaggerated the group difference in early circumferential diastolic strain rate, and revealed significant group differences in early radial and longitudinal diastolic strain rates (Table 3). Moreover, indexing these early LV diastolic strain rates to E/e′ unmasked modest relationships between early LV relaxation and peak V̇o2, which were not otherwise observed (Fig. 1). However, this relationship appears to be driven almost entirely by E/e′, which by itself was more closely related to peak V̇o2 (Fig. 2). Indeed, in univariate regression modeling, E/e′ was significantly predictive of peak V̇o2 (β = −1.05 ± 0.15, P < 0.001), whereas early diastolic circumferential, radial, longitudinal strain rate were not. Moreover, E/e′ retained a significant relationship with peak V̇o2 in separate multivariate models that included early diastolic circumferential (β = −1.08 ± 0.17, P < 0.001), radial (β = −1.07 ± 0.17, P < 0.001), and longitudinal strain rate (β = −1.06 ± 0.15, P < 0.001), and when adjusted for age, sex, BMI, and race (Table 4).
Table 3.
Left ventricular early diastolic strain rates indexed to E/e′
| HFpEF | Healthy Controls | P Value | |
|---|---|---|---|
| n | 79 | 54 | |
| Early diastolic circumferential SR / E/e′ | 0.08 ± 0.03 | 0.13 ± 0.05 | <0.001 |
| Early diastolic radial SR / E/e′ | −0.21 ± 0.09 | −0.32 ± 0.13 | <0.001 |
| Early diastolic longitudinal SR / E/e′ | 0.08 ± 0.03 | 0.10 ± 0.04 | <0.001 |
| Adjusted for age, sex, BMI, and race | |||
| Early diastolic circumferential SR / E/e′ | 0.08 ± 0.01 | 0.13 ± 0.01 | <0.001 |
| Early diastolic radial SR / E/e′ | −0.21 ± 0.02 | −0.31 ± 0.02 | 0.003 |
| Early diastolic longitudinal SR / E/e′ | 0.08 ± 0.01 | 0.09 ± 0.01 | 0.19 |
Values are means ± SE; E/e′, early mitral inflow-to-early septal annular velocity; HFpEF, heart failure with preserved ejection fraction; SR, strain rate. P values derived from t tests.
Figure 2.
Relationship between left ventricular early mitral inflow velocity-to-early annular tissue velocity (E/e′) and peak oxygen consumption (V̇O2). Data are presented as linear regression and 95% confidence intervals.
Table 4.
Multivariate relationships between left ventricular early relaxation and peak V̇O2
| Multivariate Regressions |
||
|---|---|---|
| Adjusted for age, sex, race, and BMI | Parameter Estimates | P Value |
| E/e′ | −0.34 ± 0.11 | 0.002 |
| Early diastolic radial SR | 0.70 ± 0.35 | 0.05 |
| Early diastolic circumferential SR | −1.72 ± 1.05 | 0.10 |
| Early diastolic longitudinal SR | −1.15 ± 1.09 | 0.29 |
| Indexed data | ||
| Early diastolic radial SR / E/e′ | 0.87 ± 3.52 | 0.80 |
| Early diastolic circumferential SR / E/e′ | 3.96 ± 9.40 | 0.67 |
| Early diastolic longitudinal SR / E/e′ | 13.36 ± 10.41 | 0.20 |
Values are means ± SE. E/e′, Doppler early mitral inflow-to-early septal annular velocity; SR, strain rate. Data analyzed using multivariate linear regression.
LV systolic function by CMRI feature tracking are also detailed in Table 2. We observed a marked impairment in LV circumferential strain in patients with HFpEF compared with controls, while LV longitudinal strain was significantly higher. After adjustment for age, sex, BMI, and race, however, differences in longitudinal systolic strain were annulled.
DISCUSSION
The data herein provide novel insight into the importance of controlling for cardiac preload when interpreting measures of early diastolic relaxation, and the mechanisms contributing to impaired exercise tolerance in obese HFpEF. Isolating diastolic relaxation from the hemodynamic driving pressure, by indexing measures of early LV diastolic strain rate to E/e′, unmasked impaired early LV relaxation in our well-phenotyped cohort of obese patients with HFpEF, which was modestly related to peak V̇o2. This relationship, however, appears to be driven almost entirely by preload itself, with E/e′ being more closely related to peak V̇o2.
Impaired Diastolic Function in HFpEF
LV diastolic dysfunction is a common feature of HFpEF and is often confirmed using invasively measured end-diastolic pressures (32–34). However, this invasive measure is not always clinically feasible, and only provides insight into a single period of the cardiac cycle. Although noninvasive surrogate measures like E/e′ have been widely adopted, they are often insensitive to detect HFpEF in outpatients with unexplained dyspnea in an early stage of the disease (13, 14). Diastolic strain imaging has emerged as a powerful noninvasive tool for identifying diastolic dysfunction due to its excellent sensitivity, specificity, and ability to predict major cardiovascular events and mortality (35, 36). Indeed, adding diastolic strain rate imaging to conventional risk factors significantly improved discrimination and reclassification for HF and atrial fibrillation in a substudy of the multiethnic study of atherosclerosis (16). Together, this underscores our findings of impaired early diastolic circumferential strain rates in HFpEF compared with controls.
Deformation of the LV is strongly dependent on its geometry and on the preload and afterload to which it is subjected. A major limitation of both Doppler and diastolic strain rate imaging however, is that it they are both influenced by loading conditions. To address this limitation, we adapted a technique recently described by Heida et al. (15), and indexed our CMRI derived measures of LV relaxation to the driving force of blood entering the ventricle; thereby isolating myocardial relaxation from the hemodynamic driving pressure. This technique, 1) modestly amplified the group difference previously observed with early diastolic circumferential strain rate and 2) unmasked new group differences in early longitudinal and radial diastolic strain rates. This novel approach provides important additive insight into the interpretation, and prognostic value, of diastolic strain rates in HFpEF and other analogous conditions presenting with diastolic dysfunction.
The relationship between changes in passive and active biophysical properties of the myocardium and the resulting LV displacement and strain fields is complex. We speculate that the impairment in early LV relaxation observed herein, is likely the product of: 1) reduced titin-mediated elastic recoil and early diastolic suction (37, 38); 2) impaired ventricular interaction, such that pericardial constraint and/or pulmonary hypertension may limit diastolic mechanics (39); 3) large artery stiffness and impaired ventricular-arterial coupling, affecting calcium handling and actin-myosin cross-bridge cycling (40), and/or 4) cardiometabolic derangements, leading to ectopic myocardial fat deposition and diastolic dysfunction (9, 41). More work is needed, however, to define the specific mechanism(s) contributing to the present results.
Relationship between LV Early Relaxation and Exercise Capacity
After controlling for cardiac preload, all three early LV diastolic strain rates (radial, circumferential, and longitudinal) modestly correlated with peak V̇o2. It must be acknowledged, however, that combining early diastolic strain rates with E/e′ adds variance to the relationship with peak V̇o2, compared to E/e′ alone, redirecting the focus of future work to cardiac filling pressures and the remaining ∼73% of variance contributing to peak V̇o2 and exercise intolerance in HFpEF. Moreover, that the relationships were no longer preserved following adjustment for age, sex, BMI, and race, strongly suggests that each of these risk factors are themselves contributing to impaired early LV relaxation. That E/e′ appears to be a superior, independent, predictor of peak V̇o2, compared to early LV relaxation, is consistent with recent observations in obese patients with HFpEF describing a relationship between severely impaired peak V̇o2 and increased pulmonary capillary wedge pressure and pericardial constraint (42). We extend these observations, by defining the contribution of early LV relaxation to peak V̇o2. That early LV relaxation (indexed to E/e′) was only weakly related to peak V̇o2, and largely dependent on E/e′, adds novel mechanistic insight into the determinants of exercise intolerance in this at risk population. Specifically, we interpret these data to suggest that early LV relaxation contributes only minimally to exercise intolerance in HFpEF, whereas elevated cardiac filling pressures play a more dominant role. These data are particularly timely, given the current focus on interventions aimed at reducing cardiac filling pressures both acutely (e.g., NCT04068844) and chronically (e.g., NCT03088033).
Impaired Systolic Function in HFpEF
Patients with HFpEF often present with subclinical systolic dysfunction (10, 43). Here, we report a significant impairment in LV systolic circumferential strain [an important determinant of LV ejection fraction (44)] in HFpEF compared with controls. This is entirely consistent with previous observations, suggesting that LV circumferential strain is ∼25% lower in HFpEF than healthy age-matched control subjects (45–49), with the level of impairment related to the severity of HF diagnosis (46). In contrast to prior observations however (45–51), we found that LV longitudinal strain was greater in HFpEF than controls. Although several reasons may exist to explain these desperate results, including differences in data collection and analysis (i.e., CMRI vs. echocardiography, feature tracking vs. speckle tracking), we believe the most likely explanation relates to the fact that the group differences in longitudinal strain were abrogated once adjusted for age, sex, BMI, and race. Indeed, age, sex, and obesity are known to affect LV mechanics (52–54), and therefore likely played a key role driving the differences in systolic longitudinal deformation observed herein. That we were able to identify an altered balance between global circumferential and longitudinal shortening, despite normal LV ejection fraction, is consistent with other disease states, including Fabry disease (55).
Limitations
The study sample size was moderate, limiting the inference of these results to a wider population. Although there were some intergroup differences in participant characteristics, data were adjusted for age, sex, BMI, and race. Although the BMI of our control group was lower than that of the HFpEF cohort, we believe that a comparison with a true healthy, successful aging control group, free of other cardiovascular risk factors is important. Our analyses were also limited by the lack of invasive hemodynamic data regarding LV filling pressures. Nonetheless, the noninvasive measurement of LV filling pressure used herein is currently recommended by the consensus of the American Society of Echocardiography and European Association of Cardiovascular Imaging (25). Relatedly, our estimate of LV filling pressure could be criticized for sharing some of the same properties as our measure of early LV relaxation (namely e′ and early diastolic strain rate). Future studies that include direct pressure measurements, preferably collected simultaneously, would help to strengthen the interpretation of results. Although the CMRI and echo-Doppler were not simultaneous, they were in close proximity and there was no change in patient condition (i.e., clinically stable) between the two exams. The fact that we observed significant negative relationships between resting LV diastolic function and peak V̇o2 is a significant addition to this field. However, future work should seek to confirm and extend this work by examining the relationship between LV diastolic function and V̇o2 during maximal and sub-maximal exercise. Although the present data focused on early diastolic strain rates from CMRI, the fundamental concept proposed herein can be applied to a variety of alternative imaging modalities (e.g., speckle tracking echocardiography). In this way, the purpose of the present investigation is less about the integration of multimodel imaging, as much as it provides a unique opportunity to advance our pathophysiological understanding of exercise intolerance in HFpEF. Finally, the observational nature of our study precludes inferences of attribution.
CONCLUSIONS
Using a multimodel imaging approach, we found that uncoupling tissue deformation from cardiac preload unmasks impairments in LV relaxation in older obese patients with HFpEF that otherwise was not seen before indexing to E/e′. The data further show that impairments in early diastolic relaxation (indexed to E/e′) modestly correlate with the severely reduced peak exercise V̇o2 seen in older, obese, HFpEF. Although these data highlight the importance of controlling for cardiac preload when assessing LV relaxation, the stronger relationship observed between E/e′ and peak V̇o2 redirects future investigation and therapeutic focus toward cardiac hemodynamics.
GRANTS
This study was supported in part by National Institutes of Health Grants R01HL136601, P01HL137630, R01AG18917, R01AG045551, and R01HL107257; P30-AG21331 and U24 AG05964, and by the Kermit G. Phillips II Chair in Cardiovascular Medicine at the Wake Forest School of Medicine (to. D. W. Kitzman), and American Heart Association Grant 18PRE33960358 (to T. J. Samuel). B. Upadhya has received research funding from Novartis and Corvia Medical.
DISCLOSURES
Dr. Kitzman has been a consultant for Relypsa, Abbvie, Regeneron, GlaxoSmithKline, Merck, Corvia Medical, Bayer, and St. Luke’s Medical Center in Kansas City, Kansas, receiving grant support from Novartis, Bayer, and St. Luke’s Medical Center in Kansas City, Kansas, and owning stock in Gilead Sciences. Dr. Brubaker has been a consultant for Merck, Corvia Medical, and Boehringer Ingelheim. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.J.S., D.W.K., and M.D.N. conceived and designed research; B.U., P.B., and W.G.H. performed experiments; T.J.S., M.B.N., and M.D.N. analyzed data; T.J.S., D.W.K., M.J.H., B.U., P.B., W.G.H., and M.D.N. interpreted results of experiments; T.J.S. prepared figures; T.J.S. and M.D.N. drafted manuscript; T.J.S., D.W.K., M.J.H., B.U., P.B., M.B.N., W.G.H., and M.D.N. edited and revised manuscript; T.J.S., D.W.K., M.J.H., B.U., P.B., M.B.N., W.G.H., and M.D.N. approved final version of manuscript.
REFERENCES
- 1.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 32: 670–679, 2011. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259, 2006. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114: 2138–2147, 2006. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 4.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 58: 265–274, 2011. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 60: 120–128, 2012. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 315: 36–46, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, Haykowsky, M. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol 306: H1364–1370, 2014. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol 56: 855–863, 2010. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Mahmod M, Pal N, Rayner J, Holloway C, Raman B, Dass S, Levelt E, Ariga R, Ferreira V, Banerjee R, Schneider JE, Rodgers C, Francis JM, Karamitsos TD, Frenneaux M, Ashrafian H, Neubauer S, Rider O. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 20: 88, 2018. doi: 10.1186/s12968-018-0511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11: 507–515, 2014. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 11.Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 97: 964–969, 2011. doi: 10.1136/hrt.2010.212787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation 137: 148–161, 2018. doi: 10.1161/CIRCULATIONAHA.117.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, Seward JB, Tajik AJ. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 18: 63–68, 2005. doi: 10.1016/j.echo.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Samuel TJ, Beaudry R, Sarma S, Zaha V, Haykowsky MJ, Nelson MD. Diastolic stress testing along the heart failure continuum. Curr Heart Fail Rep 15: 332–339, 2018. doi: 10.1007/s11897-018-0409-5. [DOI] [PubMed] [Google Scholar]
- 15.Hieda M, Parker J, Rajabi T, Fujimoto N, Bhella PS, Prasad A, Hastings JL, Sarma S, Levine BD. Left ventricular volume-time relation in patients with heart failure with preserved ejection fraction. Am J Cardiol 121: 609–614, 2017. doi: 10.1016/j.amjcard.2017.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambale-Venkatesh B, Armstrong AC, Liu CY, Donekal S, Yoneyama K, Wu CO, Gomes AS, Hundley GW, Bluemke DA, Lima JA. Diastolic function assessed from tagged MRI predicts heart failure and atrial fibrillation over an 8-year follow-up period: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 15: 442–449, 2014. doi: 10.1093/ehjci/jet189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris DA, Takeuchi M, Nakatani S, Otsuji Y, Belyavskiy E, Aravind KR, Frydas A, Kropf M, Kraft R, Marquez E, Osmanoglou E, Krisper M, Kohncke C, Boldt LH, Haverkamp W, Tschope C, Edelmann F, Pieske B, Pieske-Kraigher E. Lower limit of normality and clinical relevance of left ventricular early diastolic strain rate for the detection of left ventricular diastolic dysfunction. Eur Heart J Cardiovasc Imaging 19: 905–915, 2017. doi: 10.1093/ehjci/jex185. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL, Redfield MM. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J 33: 1742–1749, 2012. doi: 10.1093/eurheartj/ehs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredholm M, Jörgensen K, Houltz E, Ricksten SE. Load-dependence of myocardial deformation variables - a clinical strain-echocardiographic study. Acta Anaesthesiol Scand 61: 1155–1165, 2017. doi: 10.1111/aas.12954. [DOI] [PubMed] [Google Scholar]
- 20.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 20: 301–306, 1992. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 21.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 333: 1190–1195, 1995. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 3: 659–667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 288: 2144–2150, 2002. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 24.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22: 107–133, 2009. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17: 1321–1360, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Scott JM, Haykowsky MJ, Eggebeen J, Morgan TM, Brubaker PH, Kitzman DW. Reliability of peak exercise testing in patients with heart failure with preserved ejection fraction. Am J Cardiol 110: 1809–1813, 2012. doi: 10.1016/j.amjcard.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustine D, Lewandowski AJ, Lazdam M, Rai A, Francis J, Myerson S, Noble A, Becher H, Neubauer S, Petersen SE, Leeson P. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 15: 8, 2013. doi: 10.1186/1532-429X-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al-Khalidi HR, Chung ES, Benson DW, Mazur W. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 3: 144–151, 2010. doi: 10.1016/j.jcmg.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Schuster A, Paul M, Bettencourt N, Morton G, Chiribiri A, Ishida M, Hussain S, Jogiya R, Kutty S, Bigalke B, Perera D, Nagel E. Cardiovascular magnetic resonance myocardial feature tracking for quantitative viability assessment in ischemic cardiomyopathy. Int J Cardiol 166: 413–420, 2013. doi: 10.1016/j.ijcard.2011.10.137. [DOI] [PubMed] [Google Scholar]
- 30.Nelson MD, Sharif B, Shaw JL, Cook-Wiens G, Wei J, Shufelt C, Mehta PK, Thomson LEJ, Berman DS, Thompson RB, Handberg EM, Pepine CJ, Li D, Bairey Merz CN. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol 40: 300–306, 2017. doi: 10.1002/clc.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Claus P, Omar AMS, Pedrizzetti G, Sengupta PP, Nagel E. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging 8: 1444–1460, 2015. doi: 10.1016/j.jcmg.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 32.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Guidelines E. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33: 1787–1847, 2012. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 33.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr,Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 68: 1476–1488, 2016. doi: 10.1161/CIR.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 34.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr,Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128: 1810–1852, 2013. 2013. doi: 10.1161/CIR.0b013e31829e8807. [DOI] [PubMed] [Google Scholar]
- 35.Dokainish H, Sengupta R, Pillai M, Bobek J, Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol 101: 1504–1509, 2008. doi: 10.1016/j.amjcard.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation 115: 1376–1383, 2007. doi: 10.1161/CIRCULATIONAHA.106.662882. [DOI] [PubMed] [Google Scholar]
- 37.Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol 294: H505–H513, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Opdahl A, Remme EW, Helle-Valle T, Edvardsen T, Smiseth OA. Myocardial relaxation, restoring forces, and early-diastolic load are independent determinants of left ventricular untwisting rate. Circulation 126: 1441–1451, 2012. doi: 10.1161/CIRCULATIONAHA.111.080861. [DOI] [PubMed] [Google Scholar]
- 39.Friedberg MK, Fernandes FP, Roche SL, Grosse-Wortmann L, Manlhiot C, Fackoury C, Slorach C, McCrindle BW, Mertens L, Kantor PF. Impaired right and left ventricular diastolic myocardial mechanics and filling in asymptomatic children and adolescents after repair of tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 13: 905–913, 2012. doi: 10.1093/ehjci/jes067. [DOI] [PubMed] [Google Scholar]
- 40.Ikonomidis I, Aboyans V, Blacher J, Brodmann M, Brutsaert DL, Chirinos JA, De CM, Delgado V, Lancellotti P, Lekakis J, Mohty D, Nihoyannopoulos P, Parissis J, Rizzoni D, Ruschitzka F, Seferovic P, Stabile E, Tousoulis D, Vinereanu D, Vlachopoulos C, Vlastos D, Xaplanteris P, Zimlichman R, Metra M. The role of ventricular-arterial coupling in cardiac disease and heart failure: assessment, clinical implications and therapeutic interventions. A consensus document of the European Society of Cardiology Working Group on Aorta & Peripheral Vascular Diseases, European Association of Cardiovascular Imaging, and Heart Failure Association. Eur J Heart Fail 21: 402–424, 2019. doi: 10.1002/ejhf.1436. [DOI] [PubMed] [Google Scholar]
- 41.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 52: 1793–1799, 2008. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 42.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136: 6–19, 2017. doi: 10.1161/CIRCULATIONAHA.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56: 845–854, 2010. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maciver DH. The relative impact of circumferential and longitudinal shortening on left ventricular ejection fraction and stroke volume. Exp Clin Cardiol 17: 5–11, 2012. [PMC free article] [PubMed] [Google Scholar]
- 45.Gregorova Z, Meluzin J, Stepanova R, Sitar J, Podrouzkova H, Spinarova L. Longitudinal, circumferential and radial systolic left ventricular function in patients with heart failure and preserved ejection fraction. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 160: 385–392, 2016. doi: 10.5507/bp.2016.007. [DOI] [PubMed] [Google Scholar]
- 46.Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, Zile MR, Voors AA, Lefkowitz MP, Packer M, McMurray JJ, Solomon SD, Investigators P. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol 63: 447–456, 2014. doi: 10.1016/j.jacc.2013.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen JW, Nazir TF, Lee L, Garvan CS, Karimi A. Speckle tracking echocardiography-determined measures of global and regional left ventricular function correlate with functional capacity in patients with and without preserved ejection fraction. Cardiovasc Ultrasound 11, 2013. doi: 10.1186/1476-7120-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C, Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography 32: 71–78, 2015. doi: 10.1111/echo.12613. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Fang F, Wai-Kwok Yip G, Sanderson JE, Feng W, Xie JM, Luo XX, Lee AP, Lam YY. Left ventricular long-axis performance during exercise is an important prognosticator in patients with heart failure and preserved ejection fraction. Int J Cardiol 178: 131–135, 2015. doi: 10.1016/j.ijcard.2014.10.130. [DOI] [PubMed] [Google Scholar]
- 50.Angadi SS, Jarrett CL, Sherif M, Gaesser GA, Mookadam F. The effect of exercise training on biventricular myocardial strain in heart failure with preserved ejection fraction. ESC Heart Fail 4: 356–359, 2017. doi: 10.1002/ehf2.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kosmala W, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of abnormal left ventricular functional reserve with outcome in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging 11: 1737–1746, 2017. [DOI] [PubMed] [Google Scholar]
- 52.Andre F, Steen H, Matheis P, Westkott M, Breuninger K, Sander Y, Kammerer R, Galuschky C, Giannitsis E, Korosoglou G, Katus HA, Buss SJ. Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 17: 25–25, 2015. doi: 10.1186/s12968-015-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hollingsworth KG, Blamire AM, Keavney BD, MacGowan GA. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol 302: H885–H892, 2012. doi: 10.1152/ajpheart.00985.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 110: 3081–3087, 2004. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 55.Cheng-Baron J, Chow K, Pagano JJ, Punithakumar K, Paterson DI, Oudit GY, Thompson RB. Quantification of circumferential, longitudinal, and radial global fractional shortening using steady-state free precession cines: a comparison with tissue-tracking strain and application in Fabry disease. Magn Reson Med 73: 586–596, 2015. doi: 10.1002/mrm.25166. [DOI] [PubMed] [Google Scholar]