Abstract
Introduction:
Older patients with myeloid neoplasms (MN) receiving outpatient chemotherapy are at risk of experiencing treatment-related toxicities such as functional decline. A mobile health (mHealth) exercise intervention may ameliorate these toxicities. This qualitative study aimed to inform the design of a mHealth exercise intervention for this population.
Methods:
This was a qualitative study of thirteen patients aged ≥60 years receiving hypomethylating agents for MN. EXCAP©® is a home-based walking and progressive resistance exercise program. We combined EXCAP©® with a mobile app; the combination (GO-EXCAP Mobile App) has not been previously tested. A brief verbal description about the intervention was provided to the participants but they did not perform it. Participants were interviewed and inductive thematic analysis was used to analyze the data.
Results:
Mean age was 71.6 (SD 8.5). Three themes were identified: 1) Perceptions of the intervention feasibility, 2) Ways to leverage the app to deliver the exercise intervention, and 3) Personalized exercise goals. Walking and resistance exercises were perceived to be feasible. Patients were comfortable initiating the intervention in cycle 2 of chemotherapy, with exercise increments occurring from week 2-4 of the cycle. Ways to leverage the app to deliver EXCAP©® include 1) Video feature for exercise demonstration and interactions, and 2) Exercise data and symptom surveys to be communicated to the exercise physiologist and primary oncology team. Preservation of existing function and activity was an important goal to participants.
Conclusions:
Our findings provide insights about the preferences of older adults with MN for a mHealth exercise intervention.
Keywords: Mobile health, exercise intervention, geriatric hematology, myeloid neoplasms
Introduction
Myeloid neoplasms are a group of diseases that include acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and MDS/myeloproliferative neoplasm (MPN) overlap syndromes. Over 60% of myeloid neoplasms are diagnosed in adults aged ≥60 years.1 Functional decline and symptoms (e.g., fatigue) are common toxicities experienced by older patients with myeloid neoplasms.2-4 These toxicities can lead to reduced quality of life, treatment interruptions, and shorter survival.5,6 In a prospective study of 49 older patients with AML receiving intensive chemotherapy, functional impairments were noted in up to 62% of patients at baseline prior to treatment and increased to 82% within eight weeks of discharge from induction hospitalization.2 In a separate cohort of patients with AML, older age was associated with slower recovery of functional status.4
Walking and resistance training exercises prevent functional decline and improve symptoms such as fatigue in patients with cancer,7-10 and their effects may be dose-dependent (i.e., duration, frequency, and intensity). For example, improvement in psychological health may be seen with a daily step count of >3000 or 2-3 days per week of resistance training exercise,8 whereas improvement or maintenance of strength are more likely to be seen with a daily step count of >10,000 or 3-4 days per week of resistance training exercise.11,12 Improvement of cardiovascular health is also more likely to be seen with a daily step count of >10,000.11,12 Therefore, ensuring adherence to exercise is important to facilitate the associated health benefits.
Exercise adherence can be a challenge in older patients with myeloid neoplasms, however, due to comorbidities, symptoms, lack of exercise self-efficacy (belief in the ability to organize and execute the courses of action required for producing a given outcome), and limited access to exercise oncology professionals and facilities.13-16 Mobile health (mHealth) has the potential to improve exercise adherence by enhancing exercise self-efficacy and by helping to address barriers to exercise in real-time such as a lack of motivation.17-19 Prior studies have mainly focused on younger patients or older patients receiving inpatient intensive chemotherapy.10,20 Older patients receiving outpatient chemotherapy, a population that is generally more vulnerable than those receiving intensive chemotherapy, have received scant attention. This population may benefit more from a mHealth exercise intervention in the outpatient setting.
The purpose of this qualitative study was to inform the design of a mHealth exercise intervention for older patients receiving hypomethylating agent-based regimen, the most common outpatient chemotherapy for myeloid neoplasms, for a subsequent single-arm pilot study. Specifically, we were interested in understanding how to leverage a mobile app to deliver an evidence-based exercise intervention and enhance exercise adherence in preparation for a clinical trial.
Methods
Study Design, Setting, and Participants
We conducted a qualitative study in older patients with myeloid neoplasms recruited from an academic cancer center [University of Rochester Medical Center/Wilmot Cancer Institute (URMC/WCI), Rochester, NY, USA)]. Eligible patients were: 1) aged ≥60 years, 2) diagnosed with a myeloid neoplasm, 3) received at least one cycle of hypomethylating agent-based treatment, 4) had a physician-verified Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) between 0 and 2, 5) had no medical contraindications to exercise per the treating oncologist, 6) able to walk four meters as part of the Short Physical Performance Battery (SPPB; balance and strength tests were also performed to provide a total score but they were not used to determine eligibility),21 7) English-speaking, and 8) capable of providing informed consent. Consecutive sampling was used. Patient who were seen at URMC/WCI September 2019 to January 2020 were screened, and those who met the aforementioned eligibility criteria were approached. Subject eligibility was confirmed by the treating physician and principal investigator (KPL). The study was approved by the University of Rochester Research Subjects Review Board.
Study Procedures
Study personnel screened provider clinic and infusion schedules. Once eligibility was confirmed, study personnel approached potential participants face-to-face. After obtaining informed consent, study personnel (CS; trained by KPL) conducted a 20- to 60-minute semi-structured interview with each patient in a private space. Patients also completed demographic forms and the SPPB with study personnel. The SPPB consists of gait speed, chair stand, and balance tests, with a score ranging from 0 to 12. A score of nine or below is considered impaired.21 Recruitment continued until theoretical saturation was achieved (i.e., no new themes emerged from the interviews).Interviews were audio-recorded, uploaded for transcription, and subsequently deleted from the audio-recorder. Transcripts were not provided to participants for comments, corrections, or feedback. All patients received a $30 gift card for their participation.
Intervention Development and Description
During the interview, patients were provided with a verbal description of the proposed intervention (described below). They were given explanations of the types of exercise that encompass EXercise for CAncer Patients (EXCAP©®) and how this would be combined with use of the mobile app. Patients were shown the mobile app home screen but were not asked to enter any data into the mobile app or perform the exercises.
EXCAP©®
EXCAP©® is an individually tailored, low to moderate intensity, home-based exercise program consisting of progressive walking and resistance band exercises. The intervention is delivered by American College of Sports Medicine-certified exercise physiologists. EXCAP©® has been utilized in prior studies and shown to be feasible.8,22 The original EXCAP©® intervention has no mHealth components other than a pedometer to track daily steps and the pedometer is not linked to an app. Participants record their exercises in paper-and-pencil forms.
PointClickCare Mobile Application (App)
The PointClickCare Mobile App allows activities (e.g., symptom surveys, daily step count) to be entered through a remote Web portal and then displayed on a tablet. Information on the Web portal is transferred to the mobile app and vice versa.23 We have previously shown that the mobile app is feasible for use in older patients with myeloid neoplasms.23
Geriatric Oncology-EXercise for CAncer Patients (GO-EXCAP) Mobile App
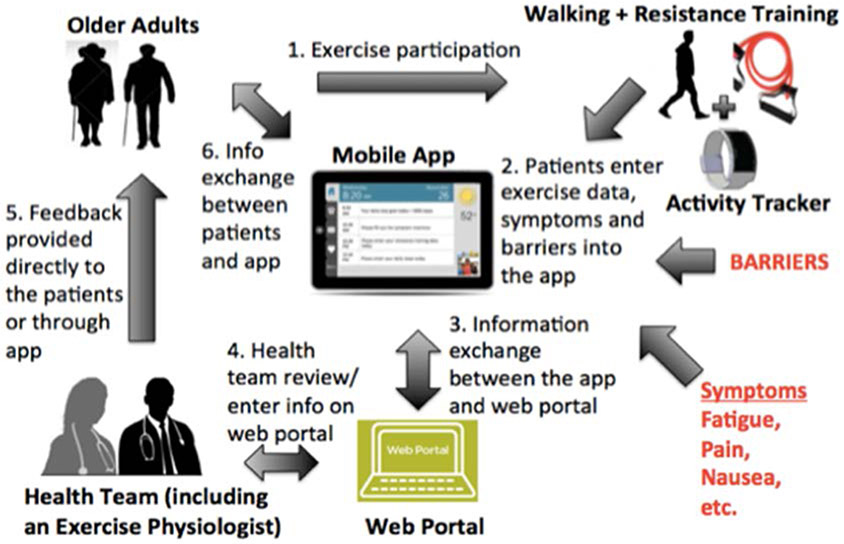
We combined EXCAP©® and PointClickCare into a novel mHealth exercise intervention, Geriatric Oncology-EXCAP (GO-EXCAP Mobile App) (Figure 1). The proposed intervention had not been tested in combination; it consists of EXCAP©®, an activity tracker, and a tablet with the PointClickCare mobile app. The activity tracker and resistance bands would be utilized for the individualized walking and resistance band exercise prescriptions as per the original EXCAP©® protocol.8,11,12 The PointClickCare mobile app would serve as a platform for patients to track exercise data, rate of perceived exertion (RPE; intensity of exercise from 1 to 10; 1 being the least intense and 10 being the most intense),24 and symptoms. It would also serve as a communication tool for exercise physiologists to adjust and increase exercise prescriptions.
Figure 1:
Geriatric-Oncology Exercise for Cancer Patients (GO-EXCAP) mobile exercise intervention
Qualitative interview with patients on proposed GO-EXCAP Mobile App
Our interviews focused on three areas. First, as EXCAP©® has not been tested in older patients with myeloid neoplasm, we specifically inquired about their perceptions of the feasibility of the walking and resistance band exercises during treatment (see Appendix Table 1 for interview script). Topics inquired included frequency (e.g., number of times per day, number of days), duration, and intensity (RPE) of exercise as well as the starting point in relation to treatment (e.g., baseline daily steps or distance walked prior to and during treatments). Second, we obtained feedback on how best to leverage the mobile app to deliver the EXCAP©® intervention, promote exercise adherence, and overcome barriers to exercise. Examples of questions included preferred communication with an exercise physiologist, frequency of communication, and type of activity tracker. Lastly, we sought general feedback on exercises and/or mobile apps beyond our proposed intervention (e.g., other exercises that would be of interest to them other than walking and resistance band exercises).
Analyses
A priori, we estimated that our sample size would be around 10 based on recommendations from prior studies 25,26 The number was subsequently increased to 13 to obtain additional feedback on ways to leverage the mobile app to deliver the exercise intervention based upon feedback from prior participants.
We used descriptive statistics to summarize demographic and clinical characteristics. Interviews were qualitatively analyzed using inductive thematic analysis, and major themes were identified from each of the three interviewed areas (perceptions of the feasibility of EXCAP©®, ways to leverage the mobile app to deliver EXCAP©®, and general feedback). Codes were derived from the data. Transcripts were analyzed by two independent coders (CS and GD) with MAXQDA 2018 (VERBI Software GmbH, Berlin, Germany), confirmed with a third investigator (KPL), and data were further consolidated and summarized. Any disagreements were discussed and resolved via group consensus.
Results
Patient Demographics
Of the 22 eligible participants approached, seventeen consented and five declined to participate (one patient had already spent too much time in the hospital, one patient perceived that it was too much effort, one patient was not mentally up for it, one patient was hard of hearing, and one patient was not interested without providing a reason). Of those who consented, four patients subsequently withdrew prior to the interview (one patient had too much going on, one patient perceived the study being too involved, one patient felt like it wasn’t right for him, and one patient moved), resulting in a total of thirteen participants. The average age of enrolled participants was 71.6 (SD 8.5) years. Among the thirteen participants, 61.5% (8/13) were male, 92.3% (11/13) were white, 53.8% were married (7/13), and 53.8% (7/13) lived with a partner (Table 1). Over half (8/13; 61.5%) of participants had a diagnosis of AML, 23.1% (3/13) MDS, and 15.4% (2/13) MDS/MPN overlap. The average number of comorbidities was 3.6 (SD 1.7) (Table 1). The mean SPPB score was 9.5 (SD 1.6).
Table 1:
Patient demographic and clinical information
| Variables | N=13 | |
|---|---|---|
| Age in years, mean (SD) | 71.6 (8.5) | |
| Gender, N (%) | Male | 8 (61.5) |
| Female | 5 (38.5) | |
| Race, N (%) | White | 12 (92.3) |
| Black or African American | 1 (7.7) | |
| Ethnicity, N (%) | Not Hispanic or Latino | 13 (100) |
| Marital status, N (%) | Married | 7 (53.8) |
| Long term, committed significant other | 1 (7.7) | |
| Divorced or widowed | 3 (23.1) | |
| Single | 2 (15.4) | |
| Education, N (%) | High school or below | 2 (15.4) |
| At least some college | 7 (53.9) | |
| Postgraduate level | 4 (30.8) | |
| Living arrangement, N (%) | Partner (spouse/significant other) | 7 (53.8) |
| Child/Children | 1 (7.7) | |
| Other | 1 (7.7) | |
| None | 4 (30.8) | |
| Diagnosis, N (%) | Acute myeloid leukemia | 8 (61.3) |
| Myelodysplastic syndrome | 3 (23.1) | |
| Myelodysplastic syndrome/Myeloproliferative neoplasm overlap | 2 (15.4) | |
| Number of comorbidities, mean (SD) | 3.6 (1.7) | |
| Comorbidities, N (%) | Diabetes mellitus | 7 (53.9) |
| Hypertension | 7 (53.9) | |
| Gastroesophageal refluex disease | 4 (30.8) | |
| Atrial fibrillation | 4 (30.8) | |
| Hyperlipidemia | 4 (30.8) | |
| Arthritis | 4 (30.8) |
Perceptions of the feasibility of EXCAP©®
Walking and resistance band exercises were perceived as feasible forms of exercise during treatment. Prior to treatment initiation, patients estimated that they walked on average between 400 and 15,000 steps daily (if patients reported distance walked instead, these were converted to approximate number of steps; 1 mile ≅ 2000 steps), and the number of steps generally decreased during treatment due to fatigue, lack of motivation (due to low blood counts and shortness of breath), and lack of time given frequent traveling for treatments. One participant (Patient 08, 69 year-old, AML) said: “It is hard to motivate yourself when your blood cell counts are down so low you just – it’s hard to get dressed, you know?” While the range of average daily steps varied widely, over half walked between 2000-6000 steps prior to treatment initiation. Patients felt comfortable with an initial starting point of 500-2,000 steps per day. Many were comfortable increasing the number of steps walked if they were able to, generally in an increment of 200-500 steps every few days. More than half (7/11; 63.6%) of the participants stated that moderate RPE during walking exercises would be feasible; 9.1% (1/11) preferred RPE to be weak and 9.1% (1/11/) strong/very strong. Two patients stated that their RPE would depend on their energy levels and two patients did not provide a specific RPE.
Patients preferred performing the resistance band exercises for 5-30 minutes, with varying frequency per week. Seven participants perceived that they were able to perform at least three times per week. For RPE, 40.0% (4/10) stated that moderate RPE would be feasible, 30.0% (3/11) preferred a strong/very strong RPE, and 10.0% (1/10) preferred a weak RPE. The remaining (2/10; 20.0%) stated that their RPE would depend on their energy levels. They preferred increments to occur in several minutes weekly.
All participants were comfortable starting the exercise intervention in cycle 2 of chemotherapy. The majority expressed challenges in exercising on days of chemotherapy infusion (the first 5 to 7 days of a cycle), therefore preferring exercise increments to occur between week 2 and 4 of a chemotherapy cycle.
Ways to leverage the mobile app to deliver EXCAP©®
Participants liked the idea of watching a video on the mobile app that demonstrates the exercises. Alternatively, having someone (e.g., an exercise physiologist) demonstrating and talking through these exercises on the mobile app via a video conference was also recommended. The mobile app can also facilitate interactions periodically between participants and an exercise physiologist, especially when they encounter challenges and would like to talk through the problems. One patient mentioned that the exercise physiologist should not make them feel guilty if they cannot do the exercises.
Patient 10, 82 year-old, AML: “I don’t have a problem meeting with them, as long as they don’t push me into doing things I don’t want to do…They shouldn’t make me feel guilty if I can’t do something.”
Suggested frequency and format for these interactions varied. One example that was proposed by the participants was that they could meet with an exercise physiologist in-person in the beginning then interactions can be facilitated through the mobile app during the intervention program. Another option included in-person interaction a few weeks after starting the intervention program. One patient mentioned flexibility as a key factor for patient engagement.
Many liked the idea of exercise alone due to personal preference, long travel, busy schedule, and other competing priorities such as caregiving. However, having someone or a community/group with whom to exercise helps both to keep patients accountable and to overcome the lack of motivation to exercise and loneliness. A mobile app could help facilitate this through a variety of ways, such as organizing live exercise sessions via video conferencing periodically, displaying data from other participants on the app, and arranging group sessions with other participants to share successes and challenges as well as to provide support to each other. Participants were enthusiastic about doing the exercises if its importance was communicated and that the data would be reviewed by their oncologist. One participant also mentioned the incorporation of a survey on sleep and diet as they may be associated with exercise adherence.
Interviewer: “Is there anything you think we should be considering while we’re creating a program like this?
Patient 12, 64 year-old, MDS: “I personally would say maybe sleep patterns because that does affect me… I would say that might be something you might want to ask people, do you get a full night’s sleep? That would affect some mornings. Some days I’m not in the mood to walk even around the block. That would be one thing I can think of. Does diet really affect?”
Another participant would also like the exercise physiologist to be aware of fatigue during the first week of the chemotherapy cycle. Forgetfulness or “chemo brain” was mentioned as a barrier to exercise and therefore having a mobile app providing reminders to exercise may be helpful.
Personalized exercise goals
Preservation of existing function and activity was an important goal to participants (Patient 10, 82 year-old, AML: “…your goal should be what is it you can help preserve during those periods of time so you don’t lose function.”) Related to this, setting specific target goals such as being able to walk to the mailbox, clean the house, and walk from one place to another may promote adherence. As one participant (Patient 8, 69 year-old, AML) said, “the benefit is personal.” Two participants mentioned the fear of falling. They proposed that ways to help them regain confidence with walking may be helpful (e.g., the need to motivate them to keep trying and be aware of their surroundings, start with simple exercises such as exercise ball).
Patient 1, 61 year-old, AML: “You keep trying. And you have to be confident and you have to be aware. I think mental awareness is one of the biggest things… So you have to make, and you have to be aware of your…your step.”
Patient 10, 82 year-old, AML: “That was fine until I became unsteady on my feet, and then it was unsafe for me to do that. Was I willing to do that? Yes, I was willing to do that, but was I willing to break my hip in the process? No…. There are some simple things that I might be wiling to do that don’t take much time; the balls that are soft but heavy, I don’t know how much they weigh, but it’s relatively easy to throw them from one hand to the other.”
Discussion
Older patients with myeloid neoplasms are underrepresented in supportive care studies, especially in interventions delivered via technologies.17 In order to optimize a behavioral intervention for this vulnerable population, we sought participant input early in the development and adaptation process. We were able to incorporate some of the feedback in an ongoing single-arm pilot study to further optimize the GO-EXCAP Mobile App intervention as well as to evaluate its feasibility and usability (ClinicalTrials.gov identified: NCT04035499). Feedback gathered from this qualitative study may also be applicable to the development of other mHealth exercise intervention.
Three published pilot studies evaluated exercise in 50 older patients with myeloid neoplasms and suggested that exercise may improve physical function, fatigue, mood disturbances, and quality of life.10,20,27 Importantly, exercise was safe in this population. However, two of these studies focused on patients receiving inpatient induction chemotherapy, a fitter population, and the exercise intervention was delivered primarily in the inpatient setting. Our study, therefore, provides important insights on developing an exercise intervention for a vulnerable population of myeloid neoplasms treated in the outpatient setting. Specifically, the low- to moderate- intensity exercises were perceived to be feasible by this population. Our proposed GO-EXCAP intervention has the potential to improve exercise adherence and address barriers to exercise during outpatient chemotherapy. While barriers identified in our population (e.g., fatigue and environmental factors) were similar to our studies of patients with cancer,28,29 interventions to address these barriers may be different and need to be tailored to their needs, ultimately improving outcomes that are important to them such as maintaining functional independence.
Studies have shown that walking and resistance training exercises improve outcomes.7-10 Given the benefits of these exercises may be dose-dependent, our study provides important insights into leveraging a mobile app to incrementally increase the number of steps walked and resistance training exercises during treatment. While participants reported that their number of steps walked generally decreased during treatment, their self-reported pre-treatment daily steps (or distance walked) suggest that it may be feasible to achieve the minimum number of steps to achieve benefits (e.g., >3000 steps) with the support of a mobile app. Doses needed to achieve benefits from resistance training exercises were also perceived to be feasible. Using participant feedback, we have incorporated some of the suggestions in our ongoing single-arm pilot study. These include: 1) Initiating the intervention in cycle two of chemotherapy to maximize accrual and to reduce participant burden in the context of starting a new treatment regimen or clinical trial. In addition, cycle one of chemotherapy is the time when participants frequently have low blood counts and feel fatigued and short of breath so exercising may be challenging; 2) Providing an option to the patient where exercises are taught on a video conference call by an exercise physiologist at the beginning of the intervention; 3) Providing motivational messages through the app by the exercise physiologist; and 4) Incorporating surveys that inquire about symptoms such as fatigue and sleep that are sent to the primary oncology team on a weekly basis. These can also be reviewed by an exercise physiologist so he/she is aware of symptoms and can modify exercise prescription if needed.
We continue to obtain feedback from participants on how to organize sessions for group exercises or discussions about successes and challenges as part of our single-arm pilot study. Ongoing efforts include: 1) Developing videos for the exercises; 2) Building self-management algorithms for fatigue and other symptoms to facilitate exercises; 3) Involving oncologists periodically during the program so that they can reinforce the importance of exercises; and 4) Incorporating specific target goals that are personal to the patient. These efforts will be used to inform future trials. Specific examples include: 1) Exercise videos will be incorporated into the mHealth app so patients can review them anytime, 2) Patients will be provided with automated self-management strategies for common symptoms associated with myeloid neoplasms and their treatments; and 3) Patient-specific goals (e.g., being able to walk to the mailbox) will be inquired and assessed prior to and after the intervention to assess for improvement.
A major strength of this study was the inclusion of stakeholders that should facilitate the development of a patient-centered mHealth exercise intervention. Inclusion of stakeholder feedback when developing and pilot testing behavioral interventions are commonly used approaches by researchers.30-33 Given limited data on mHealth exercise intervention in our population, we believe that gathering stakeholder feedback even prior to pilot testing is important. There were also limitations. For example, most of our participants were White and well-educated. Additionally, given our small sample size, we could not determine subgroup preferences or response (e.g., sex-specific). We also recruited patients from an academic cancer center and therefore our sample may not reflect those treated in community oncology practices. We plan to continue collecting feedback from patients seen at WCI and affiliated oncology practices in our ongoing single-arm pilot study in order to obtain feedback from a broader geographical area (e.g., rural vs. urban).
In conclusion, this study serves to inform the development and adaptation of our mHealth exercise intervention for older patients with myeloid neoplasms. Our findings provide important insights about the preferences of older adults living with MN for a mHealth exercise intervention.
Supplementary Material
Acknowledgements:
We wish to acknowledge Dr. Susan Rosenthal, MD for her editorial assistance.
Financial Support: The work was supported by the National Cancer Institute at the National Institute of Health (UG1 CA189961; K99CA237744 to KPL, K07CA221931 to IRK), the National Institute of Aging at the National Institute of Health (K24 AG056589 to SGM), the National Institute of Nursing Research at NIH (K24NR018621 to RS) and the Wilmot Research Fellowship Award (to KPL). We would like to acknowledge those who provided feedback as part of the Transdisciplinary Research in Energetics and Cancer Research Education Program (TREC Training Workshop; R25CA203650). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Conflict of Interest: Dr. Loh serves as a consultant to Pfizer and Seattle Genetics. Dr. Mohile received research funding from Carevive for other projects. All other authors have no relevant conflicts of interest to report.
References
- 1.SEER Cancer Statistics Factsheets: Acute Myeloid Leukemia. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/amyl.html. [Google Scholar]
- 2.Klepin HD, Tooze JA, Pardee TS, et al. Effect of Intensive Chemotherapy on Physical, Cognitive, and Emotional Health of Older Adults with Acute Myeloid Leukemia. Journal of the American Geriatrics Society. 2016;64(10):1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alibhai SM, Leach M, Kowgier ME, Tomlinson GA, Brandwein JM, Minden MD. Fatigue in older adults with acute myeloid leukemia: predictors and associations with quality of life and functional status. Leukemia. 2007;21(4):845–848. [DOI] [PubMed] [Google Scholar]
- 4.Alibhai SM, Breunis H, Timilshina N, et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol. 2015;6(4):262–271. [DOI] [PubMed] [Google Scholar]
- 5.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tinsley SM, Sutton SK, Thapa R, Lancet J, McMillan SC. Treatment Choices: A Quality of Life Comparison in Acute Myeloid Leukemia and High-risk Myelodysplastic Syndrome. Clin Lymphoma Myeloma Leuk. 2017;17s:S75–s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sajid S, Dale W, Mustian K, et al. Novel physical activity interventions for older patients with prostate cancer on hormone therapy: A pilot randomized study. J Geriatr Oncol. 2016;7(2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loh KP, Kleckner IR, Lin PJ, et al. Effects of a Home-based Exercise Program on Anxiety and Mood Disturbances in Older Adults with Cancer Receiving Chemotherapy. J Am Geriatr Soc. 2019;67(5):1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31(26):3233–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klepin HD, Danhauer SC, Tooze JA, et al. Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. J Geriatr Oncol. 2011;2(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin P-J, Loh KP, Inglis JE, et al. Effects of exercise on cancer-related fatigue and muscular strength in patients with breast cancer. Journal of Clinical Oncology. 2019;37(15_suppl):11507–11507. [Google Scholar]
- 12.Mustian KM, Peppone L, Darling TV, Palesh O, Heckler CE, Morrow GR. A 4-week home-based aerobic and resistance exercise program during radiation therapy: a pilot randomized clinical trial. J Support Oncol. 2009;7(5):158–167. [PMC free article] [PubMed] [Google Scholar]
- 13.Courneya KS, Segal RJ, Reid RD, et al. Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J Clin Epidemiol. 2004;57(6):571–579. [DOI] [PubMed] [Google Scholar]
- 14.Moschny A, Platen P, Klaassen-Mielke R, Trampisch U, Hinrichs T. Barriers to physical activity in older adults in Germany: a cross-sectional study. Int J Behav Nutr Phys Act. 2011;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egerton T, Chastin SF, Stensvold D, Helbostad JL. Fatigue May Contribute to Reduced Physical Activity Among Older People: An Observational Study. J Gerontol A Biol Sci Med Sci. 2016;71(5):670–676. [DOI] [PubMed] [Google Scholar]
- 16.Sjors C, Bonn SE, Trolle Lagerros Y, Sjolander A, Balter K. Perceived reasons, incentives, and barriers to physical activity in Swedish elderly men. Interact J Med Res. 2014;3(4):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaffer K, Panneerselvam N, Loh KP, et al. Systematic Review of Randomized Controlled Trials of Exercise Interventions Using Digital Activity Trackers in Patients With Cancer. J Natl Compr Canc Netw. 2019;17(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela T, Okubo Y, Woodbury A, Lord SR, Delbaere K. Adherence to Technology-Based Exercise Programs in Older Adults: A Systematic Review. J Geriatr Phys Ther. 2018;41(1):49–61. [DOI] [PubMed] [Google Scholar]
- 19.Bandura A: Self-efficacy: The exercise of control. 1997, New York, NY: W.H. Freeman. [Google Scholar]
- 20.Alibhai SM, O'Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012;36(10):1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-Extremity Function in Persons over the Age of 70 Years as a Predictor of Subsequent Disability. New England Journal of Medicine. 1995;332(9):556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleckner IR, Kamen C, Gewandter JS, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018;26(4):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loh KP, Ramsdale E, Culakova E, et al. Novel mHealth App to Deliver Geriatric Assessment-Driven Interventions for Older Adults With Cancer: Pilot Feasibility and Usability Study. JMIR Cancer. 2018;4(2):e10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American College of Sports Medicine (2010) ACSM’s guidelines for exercise testing and prescription. Lippincott, Williams, & Wilkins, Baltimore. [DOI] [PubMed] [Google Scholar]
- 25.Virzi RA. Refining the Test Phase of Usability Evaluation: How Many Subjects Is Enough? Human Factors. 1992;34(4):457–468. [Google Scholar]
- 26.Nielsen J, Landauer TK. A mathematical model of the finding of usability problems. CHI ’93 Conference on Human Factors in Computing Systems, Amsterdam, The Netherlands. 1993. [Google Scholar]
- 27.Schuler MK, Hentschel L, Gobel J, et al. Effects of a home-based exercise program on physical capacity and fatigue in patients with low to intermediate risk myelodysplastic syndrome-a pilot study. Leuk Res. 2016;47:128–135. [DOI] [PubMed] [Google Scholar]
- 28.Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH. Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology. 2013;22(1):186–194. [DOI] [PubMed] [Google Scholar]
- 29.Fisher A, Wardle J, Beeken RJ, Croker H, Williams K, Grimmett C. Perceived barriers and benefits to physical activity in colorectal cancer patients. Support Care Cancer. 2016;24(2):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parra DC, Wetherell JL, Van Zandt A, Brownson RC, Abhishek J, Lenze EJ. A qualitative study of older adults' perspectives on initiating exercise and mindfulness practice. BMC Geriatr. 2019;19(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alkawaldeh MY, Jacelon CS, Choi J. Older adults' experiences with a tablet-based self-management intervention for diabetes mellitus type II: A qualitative study. Geriatr Nurs. 2020;41(3):305–312. [DOI] [PubMed] [Google Scholar]
- 32.Shelef DQ, Rand C, Streisand R, et al. Using stakeholder engagement to develop a patient-centered pediatric asthma intervention. J Allergy Clin Immunol. 2016;138(6):1512–1517. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs JM, Walsh EA, Rapoport CS, et al. Development and Refinement of a Telehealth Intervention for Symptom Management, Distress, and Adherence to Adjuvant Endocrine Therapy after Breast Cancer. J Clin Psychol Med Settings. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.