Abstract
AIM
To quantify the microstructural differences in the cervical-thoracic spinal cord of adults with cerebral palsy (CP).
METHOD
Magnetic resonance imaging of the proximal spinal cord (C6–T3) was conducted on a cohort of adults with CP (n=13; mean age=31y 11mo, standard deviation [SD] 8y 7mo; range=20y 8mo–47y 6mo; eight females, five males) and population norm adult controls (n=16; mean age=31y 4mo, SD 9y 9mo; range=19y 4mo–49y 5mo; seven females, nine males). The cross-sectional area (CSA) of the spinal cord, gray and white matter, magnetization transfer ratio (MTR), and fractional anisotropy of the cuneatus and corticospinal tracts were calculated.
RESULTS
The total spinal cord CSA and proportion of the spinal cord gray matter CSA were significantly decreased in the adults with CP. The corticospinal tracts’ MTR was lower in the adults with CP. Individuals that had reduced gray matter also tended to have reduced MTR in their corticospinal tracts (r=0.42, p=0.029) and worse hand dexterity clinical scores (r=0.53, p=0.004).
INTERPRETATION
These results show that there are changes in the spinal cord microstructure of adults with CP. Ultimately, these microstructural changes play a role in the extent of the hand sensorimotor deficits seen in adults with CP.
Cerebral palsy (CP) consists of a general class of movement disorders that result from an insult to the developing brain.1 A substantial portion of these individuals have a life expectancy of at least 58 years,2 indicating that many individuals in this population survive well into late adulthood. Lack of participation in activities, weight gain, fatigue, and accelerated sarcopenia each contribute to progressive deterioration of the musculoskeletal system that occurs throughout the lifespan in CP.3,4 Such deterioration is presumed to impact the ability to perform upper extremity tasks of daily living (i.e. buttoning shirt, brushing teeth), as completing such tasks often becomes progressively more difficult with age.5 Despite these recognized functional declines, there is an incredible lack of specialized treatments for adults with CP.6 This lack of treatments at least partially exists because we have limited insight on the neurophysiological changes seen in the aging population with CP.
Previous transcranial magnetic stimulation studies have established that the perinatal brain insults that occur in persons with CP can induce activity-dependent neuroplastic changes. When a unilateral brain insult occurs, the ipsilateral hemisphere may assume control of the affected side of the body.7 Essentially, the paretic hand may become either partially, or fully, controlled by corticospinal tract projections from the ipsilateral motor cortex. The likelihood of this occurring is often dependent on the timing of the insult and size of the lesion in the developing brain. Smaller lesions are less likely to result in corticospinal tract reorganization, and intermediate lesions result in partial reorganization where the paretic limb is controlled by both the contralateral and ipsilateral hemisphere. Alternatively, large lesions are the most likely to lead to complete reorganization, where the paretic limb is completely controlled by ipsilateral connections. While the motor cortex is susceptible to reorganization, the ascending somatosensory tracts do not appear to reorganize.8,9 This creates a dissociation between the motor and somatosensory cortices, such that the somatosensory information is being processed in a different hemisphere than the hemisphere controlling movement for the paretic limb. This dissociation likely helps to explain why the amount of reorganization present within the motor cortex has consistently been associated with increased sensorimotor impairments.7
Numerous animal studies have expanded on these findings by demonstrating that changes in brain activity can also result in alterations within the structure of the spinal cord. For example, the outcomes from several animal models have shown that inactivation of one hemisphere can result in the corticospinal neurons terminating more dorsally in the spinal cord gray matter, potentially leading to competition for the dorsal horn real-estate where the sensory neurons typically reside.10,11 Thus, the structural integrity of the spinal interneuronal pool is partly dependent upon cortical activity and the integrity of the corticospinal tracts. Despite these novel insights, the translation of the outcomes from these animal models to what is seen in humans with CP is largely lacking.
No studies to date have examined the microstructural changes within the spinal cord of individuals with CP. The lack of this information also creates difficulties in interpreting functional brain imaging results, as little is known about the integrity of the fibers connecting the brain to the body in this population. The objective of the current investigation was to begin to address this knowledge gap by using high-resolution magnetic resonance imaging (MRI) to quantify the potential spinal cord microstructural differences between adults with CP and a cohort of population norm controls. Specifically, we aimed to assess the gray and white matter cross-sectional area (CSA), the fractional anisotropy, and the magnetization transfer ratio (MTR) within the upper spinal cord. Analysis of the white and gray matter CSA can provide information regarding the total number of cell bodies and myelination within the spinal cord. Fractional anisotropy describes the movement of water molecules, in which a value of 0 represents isotropic diffusion and a value of 1 indicates complete anisotropic movement. Fractional anisotropy tends to be higher in regions of greater uniformity (i.e. fiber bundles) and lower in regions in which there is no specific orientation of cell shape or diffusion.12 Therefore, it is a viable outcome measure that can be used to assess the integrity and overall uniformity of the descending and ascending sensorimotor tracts.13
MTR is a measurement that can be used as an accessory to magnetic resonance angiography or to improve gadolinium enhancement from T1-weighted scans, and it can also be used in conjunction with T2-weighted images in order to assess the potential gray matter damage and loss of myelination persistent in the spinal cord. Briefly, there is a bound pool and a free pool of hydrogen nuclei that are involved in the generation of MRI signals. The bound pool has extremely short relaxation times that are not normally detectable by standard MRI. However, applying a magnetization transfer pulse that selectively saturates protons in the bound pool results in energy transfer and saturation of the free pool. Subsequently, when a radiofrequency pulse is applied, the measured signal from the free pool will be lower because of the partial saturation.14 Thus, depending on the macromolecular composition of the tissue, the signal will be more or less affected by a magnetization transfer pulse, allowing for inferences to be made regarding the underlying microstructure. For example, extensive gray matter damage and significant demyelination has been associated with a lower MTR.15,16 Previously, MTR in the spinal cord has also been associated with the degree of sensorimotor impairments in patients with spinal cord injury17 and clinical outcomes after surgical interventions in patients with degenerative cervical myelopathy.18 Assessing how these various microstructural outcome measures may be altered in adults with CP can provide insight into the neurophysiological origins of the upper extremity impairments seen in this population.
METHOD
A cohort of adults with CP that had a spastic bilateral presentation (n=13; mean age=31y 11mo, standard deviation [SD] 8y 7mo; range=20y 8mo–47y 6mo; eight females, five males, Manual Ability Classification System levels I–III) and population norm adult controls (n=16; mean age=31y 4mo, SD 9y 9mo; range=19y 4mo–49y 5mo; seven females, nine males) completed this study. Further details on the individuals with CP are presented in Table 1. The participants with CP had not undergone upper extremity surgeries, had not had botulinum neurotoxin A injections in the past year, and were not on antispasmodic medication. The Institutional Review Board (i.e. a research ethics review panel) reviewed and approved this investigation. Written informed consent was acquired from all of the adult participants.
Table 1:
Demographics of the adults with cerebral palsy
| Participant age (y:mo) |
Sex | MACS level |
Type | Assistive mobility |
|---|---|---|---|---|
| 47:6 | F | I | Spastic bilateral | None |
| 37:6 | F | I | Spastic bilateral | None |
| 20:8 | F | II | Spastic bilateral | Forearm crutches |
| 35:11 | F | II | Spastic bilateral | Forearm crutches |
| 35:5 | M | III | Spastic bilateral | Powered chair |
| 27:10 | F | II | Spastic bilateral | Walker |
| 20:11 | M | I | Spastic bilateral | None |
| 31:10 | M | I | Spastic bilateral | None |
| 35:10 | M | I | Spastic bilateral | Wheelchair |
| 21:2 | M | I | Spastic bilateral | None |
| 43:4 | F | II | Spastic bilateral | None |
| 33:2 | F | I | Spastic bilateral | None |
| 24:2 | F | II | Spastic bilateral | Wheelchair |
MACS, Manual Ability Classification System.
MRI acquisition and data processing
Cervical-thoracic spinal cord MRI scans were acquired with a Siemens Prisma 3T scanner (Siemens, Malvern, PA, USA). High-resolution T1-weighted axial magnetization-prepared rapid acquisition with gradient echo images were obtained with a 64-channel head/neck coil (voxel size=1.0mm × 1.0mm × 1.0mm; repetition time/echo time 2000/3.72ms; flip angle=9°; field of view=320mm × 320mm; slice thickness: 1mm slice; slices=192, scan time=56s). T2-weighted images were collected across C1 to T6 (voxel size=0.8mm × 0.8mm × 0.8mm, slices=64, repetition time/echo time=1500/120ms, flip angle=120°, and field of view=256mm × 256mm, scan time=240s). Gradient recalled echo T2* images were collected across C6 to T3 (voxel size=0.5mm × 0.5mm × 5.0mm, slices=15, repetition time/echo time=600/14ms, flip angle=30°, and field of view=224mm × 224mm, scan time=283s). Diffusion weighted images were collected across C6 to T3 (voxel size=0.4mm × 0.4mm × 5.0mm; slices=15, repetition time/echo time=610/60ms, b-values=0 s/mm2, 100 s/mm2, 30 non-collinear gradient directions, scan time=122s). Magnetization transfer scans were also collected across C6 to T3 (voxel size=0.9mm × 0.9mm × 5.0mm; slices=22, repetition time/echo time=35/3.13ms, flip angle=9°, and field of view=230mm × 230mm, scan time=130s) using a vendor-equipped saturation pulse.
The partially automated Spinal Cord Toolbox (version 3.2.2, Polytechnique, Montreal, Canada) was subsequently used for spinal cord and gray and white matter segmentation, vertebral labeling, cross-sectional measurement, and template registration of the spinal tracts.19 After vertebral labeling, the spinal cord was initially straightened, as described by De Leener et al.,20 which was then followed by inferior-superior affine alignment based on vertebral levels. The spinal cord centerline was then aligned between the template and the participant using the center of mass of the spinal cord segmentation, which was then followed by a non-linear within-plane BSplineSyN registration.21 The spinal cord internal structure was accounted for by assuming a linear deformation based on the outer shape of the spinal cord.
The total CSA across C6 to T3 was extracted from the T2 images and the T2* was used for gray and white matter extraction. In order to determine the relative proportion of gray and white matter, these values were normalized to the total spinal cord CSA. The PAM50 template was registered to the diffusion weighted images and magnetization transfer images after motion correction, and the diffusion tensors for the respective spinal cord tracts were calculated. The fractional anisotropy values from the left and right corticospinal and cuneatus tracts were subsequently calculated from the diffusion weighted images. The MTR of the corticospinal tract and cuneatus tracts were additionally calculated from the magnetization transfer scans.
Lastly, each participant completed the Box and Block Test of hand dexterity.22 For this test, the participant was instructed to move as many blocks as possible from one compartment to another in a 60 second time period. We assessed whether the number of blocks moved was correlated with the respective spinal cord outcome measures.
Statistical methods
The respective outcome measures were tested for normality using the Shapiro–Wilk test, and the data that were not normally distributed were log transformed before further analysis. In order to test for equality of variance between samples, Levene’s test was used. When the samples did not have equal variances, Welch’s t-tests were used to compare outcome measures between groups. When the samples did have equal variances, independent samples t-tests were used to compare outcome measures between groups. We also ran Pearson Product-Moment Correlation analyses to assess whether the amount of gray matter, white matter, fractional anisotropy, or MTR measures were associated with each other. All results are presented as mean (SD). JASP software was used for statistical analysis (Version 0.12.2.0; Psychological Methods, Amsterdam, the Netherlands).
RESULTS
An independent samples t-test revealed that the adults with CP and population norm controls did not differ by age (p=0.860). Outcome measures were successfully extracted for all 13 adults with CP and 16 adult control participants that completed the study. Figure 1 shows exemplary images from the processing pipeline where the spinal cord was segmented (Fig. 1a) and the gray and white matter in the respective segments was parcellated (Fig. 1b). The results indicated that the total CSA of the C6 to T3 portion of the spinal cord was smaller in the adults with CP compared with the controls (CP=66.22mm2, SD 8.60mm2, 95% confidence interval [CI] 61.55–70.89; controls=76.75mm2, SD 8.77mm2, 95% CI 72.45–81.05, p=0.002, Fig. 1c). In addition, the gray matter CSA (CP=9.42mm2, SD 1.41mm2, 95% CI 8.62–10.22; controls=12.22mm2, SD 1.29mm2, 95% CI 11.59–12.85, p<0.001, Fig. 1d) and white matter CSA (CP=61.93mm2, SD 8.81mm2, 95% CI 57.14–66.72; controls=69.27mm2, SD 8.59mm2, 95% CI 65.06–73.48, p=0.032, Fig. 1e) were also significantly lower in individuals with CP. When normalized to the total spinal cord CSA, the gray matter remained smaller in the adults with CP relative to the controls (CP=14.2%, SD 1.9%, 95% CI 13.13–15.28]; controls=16.2%, SD 2.7%, 95% CI 14.88–17.52, p=0.041), but the white matter no longer differed between the respective groups (CP=93.7%, SD 8.4%, 95% CI 89.13–98.27; controls=90.2%, SD 4.6%, 95% CI 87.95–92.45, p=0.208).
Figure 1:
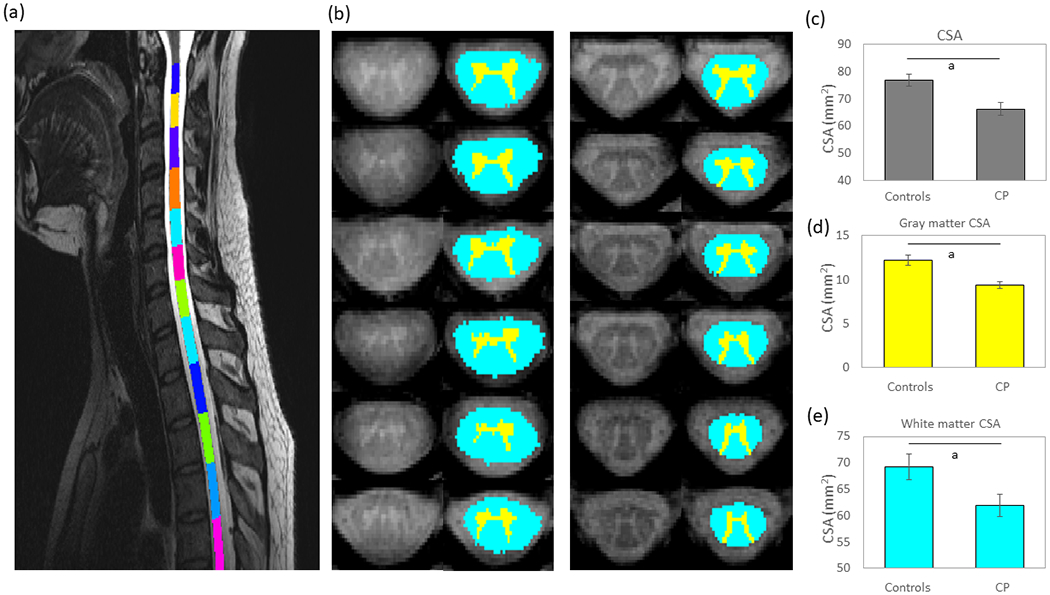
(a) The sagittal view of the cervical-thoracic spinal cord segmentation for a single representative adult with cerebral palsy (CP). (b) Parcellations of the cross-sectional area (CSA) from C6 to T3 for a representative adult participant with CP (left) and population norm adult control (right). The aqua represents white matter while the yellow represents gray matter. (c) Average spinal cord CSA for the adults with CP and adult controls. The CSA was significantly less for the adults with CP. (d) Average gray matter CSA for the adults with CP and adult controls. As shown, the CSA was significantly less for the adults with CP. (e) Average white matter CSA for the adults with CP and controls. The white matter CSA was also significantly less for the adults with CP. Bar graphs represent the mean and the standard error of the mean. ap≤0.05.
Figure 2 displays a fractional anisotropy map (Fig. 2a) and MTR scan (Fig. 2b), from representative participants. The MTR was significantly lower in the corticospinal tracts in the adults with CP (CP=42.61%, SD 5.69%, 95% CI 39.52–45.70; controls=46.64%, SD 3.18%, 95% CI 45.082–48.20, p=0.023; Fig. 3a), but not in the cuneatus tracts (CP=46.71%, SD 6.73%, 95% CI 43.05–50.37; controls=47.09%, SD 3.34%, 95% CI 45.45–48.73, p=0.712). The fractional anisotropy values were not significantly different between groups in either the corticospinal (CP=0.63, SD 0.09, 95% CI 0.58–0.68; controls=0.66, SD 0.06, 95% CI 0.63–0.69, p=0.207) or cuneatus tracts (CP=0.65, SD 0.07, 95% CI 0.61–0.69; controls=0.66, SD 0.08, 95% CI 0.62–0.70, p=0.800). We found a moderate positive correlation between the MTR in the corticospinal tracts and gray matter CSA across all participants (r=0.42, p=0.027, Fig. 3b). This correlation implies that participants who had less gray matter also tended to have lower MTR values. We also found a positive correlation between the Box and Block Test and the gray matter CSA (r=0.53, p=0.004; Fig. 3c), as well as the total CSA (r=0.40, p=0.031), indicating that the individuals that moved more blocks tended to have more gray matter and total CSA within their upper spinal cord. No other correlations between outcome measures were significant (p>0.05).
Figure 2:
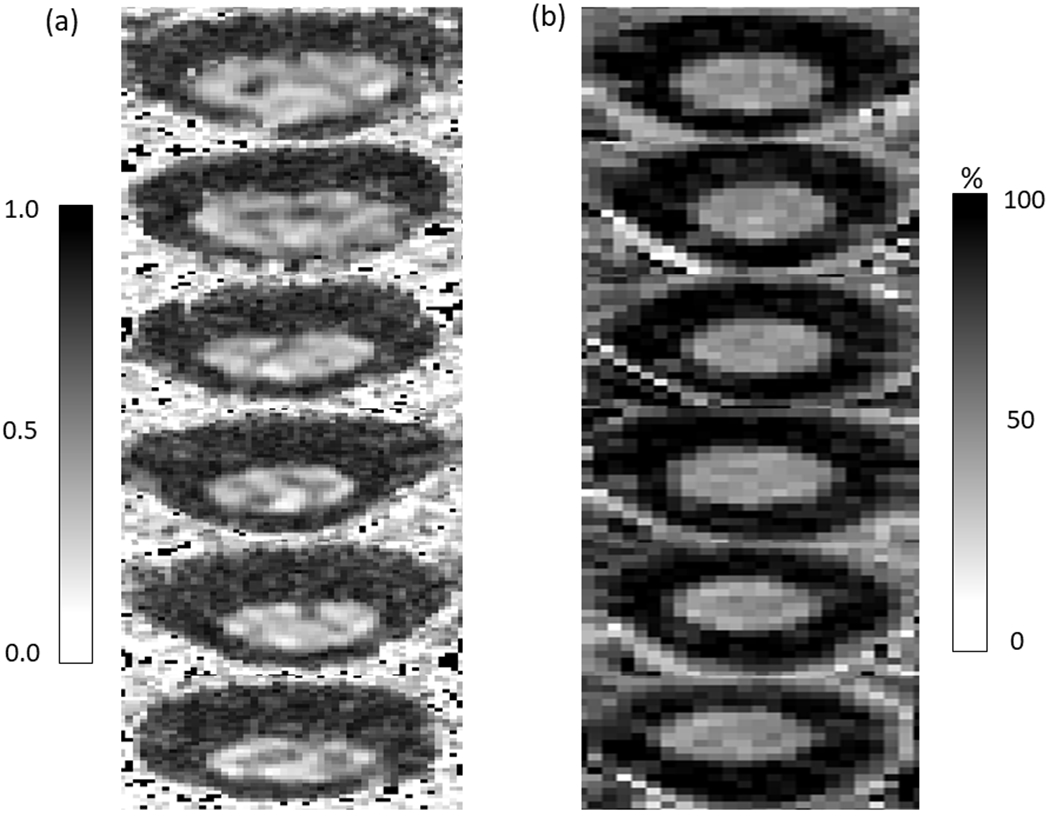
(a) Example fractional anisotropy map from a representative participant. (b) Example magnetization transfer ratio images from a representative participant. The two images are from different participants.
Figure 3:
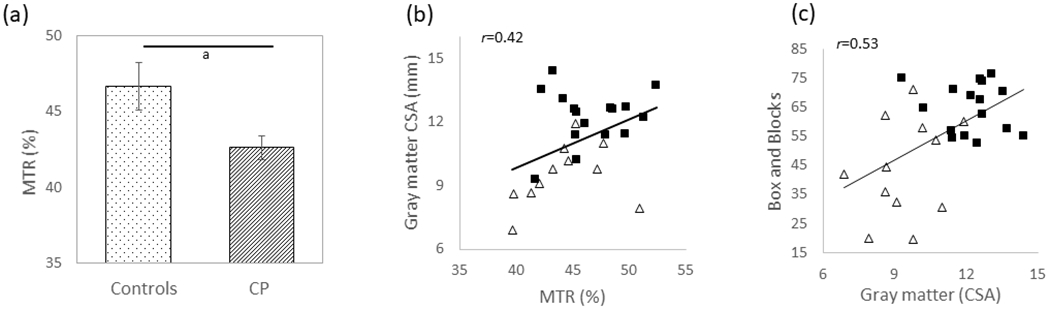
(a) The magnetization transfer ratio (MTR) in the corticospinal tracts for the adults with cerebral palsy (CP) and population norm adult controls. The adults with CP had significantly lower MTR in the corticospinal tracts, suggesting greater microstructural disruptions. (b) Scatter plot depicting the positive relationship between the corticospinal tract MTR and gray matter cross-sectional area (CSA). Adults with CP are depicted with white triangles, and the adult controls are depicted with black squares. Less gray matter was associated with a reduction in the corticospinal tract MTR, suggesting that participants with less grey matter CSA tended to have greater damage to the corticospinal tract microstructure. ap≤0.05. (c) Scatter plot depicting the relationship between the gray matter CSA and Box and Block Test scores. More gray matter was associated with improved hand dexterity.
DISCUSSION
We used high-resolution MRI to image the cervical-thoracic spinal cord (C6–T3) in order to quantify the microstructural differences between adults with CP and a cohort of controls. Our results revealed that the spinal cord CSA was smaller in those with CP, including specific CSA reductions in both white and gray matter. The spinal cord CSA and gray matter CSA were each associated with improved clinical scores of hand dexterity. Furthermore, the relative proportion of gray matter within the spinal cord was also notably less in the adults with CP. Our imaging approach also indicated that the MTR of the corticospinal tracts was lower in adults with CP, which suggests that there were greater microstructural disruptions. Further discussion of the implications of these results are detailed in the following sections.
The spinal cord CSA was notably smaller in the adults with CP, and our normalization analysis suggested that this reduction was primarily due to a reduction in the gray matter, which indicates that those with CP likely have fewer neural cell bodies within the spinal cord. This gray matter reduction likely reflects a reduction in GABAergic interneuronal network activity within the spinal cord. As GABAergic interneuronal cells are reciprocally connected with glutamatergic cells,23,24 this would lead to heightened glutamatergic activity and ultimately increased spinal cord excitability, which has often been reported in individuals with CP based on assessments of the Hoffmann reflex.25
Alternatively, the decrease in gray matter may partially be a result of activity-dependent competition and reorganization within the spinal cord. Using an animal model, Friel et al. demonstrated that inactivation of one hemisphere of the brain early in development can result in structural changes within the spinal cord.10 Specifically, the non-affected hemisphere may assume control over the paretic limb, and the descending motor tract terminations shift towards occupying the real-estate of the dorsal horn of the spinal cord.10,11 Thus, an insult to the developing brain can result in the motor tracts terminating in areas that are normally occupied by the sensory neurons. In turn, this may result in activity-dependent competition for space in the dorsal horn of the spinal cord that leads to an overall pruning of corticospinal and sensory neurons. In either case, we suggest that the reduction in gray matter may also be a result of the activity-dependent plastic changes that occur as a downstream effect from the insult to the developing brain. This may ultimately contribute to sensorimotor impairments, which is supported by the observation that the individuals that had lower Box and Block Test scores tended to have decreased gray matter.
Although the total white matter CSA differed between groups, the proportion of the white matter that comprised the spinal cord CSA was not different. However, the MTR of the corticospinal tracts was notably less in the adults with CP. This implies that there are likely microstructural disruptions that could affect the transmission of the descending motor signals between the brain and the spinal cord interneurons. In addition, participants that had a reduction in the spinal cord gray matter also tended to have diminished MTR values for the corticospinal tracts. These results further support the notion that disruptions in the fidelity of the corticospinal tract signals may influence their spinal cord terminations and ultimately the organization of the spinal cord interneurons.10 In other patient populations, a decreased MTR has been associated with inflammation and the extent of gray matter lesions.26 Hence, it is plausible that the lower MTR values seen in our adults with CP might reflect similar underlying pathophysiology, as it is well recognized that CP can be associated with chronic inflammatory responses in the central nervous system, which are associated with neural cell apoptosis.27
Despite our novel findings, this study had several limitations. Our cohort consisted only of adults with spastic bilateral CP, making it difficult to draw conclusions about how these findings may extend to children or other types of CP. Even with the novel information diffusion tensor imaging can provide regarding the integrity and organization of the spinal cord, several limitations on its use within the spinal cord exist. First, the signal to noise ratio decreases when imaging further down the cord, likely due to the morphology of the body and geometry of the surface coil.28 This may lead to an overestimation of the fractional anisotropy values in areas in which the signal to noise ratio is lower, resulting in lower fractional anisotropy values further down the spine. Additionally, fractional anisotropy values differ greatly between gray and white matter, indicating that populations with reduced gray matter may have altered fractional anisotropy values as a result of volume differences. In our study, there were differences in gray and white matter distribution within the upper spinal cord, which could potentially alter the fractional anisotropy values. MTR comes with several limitations as well. MTR scans are generally susceptible to motion error and they can have high variability depending on the properties of the magnetization transfer pulse (i.e. the shape, bandwidth, frequency offset).
In conclusion, our results are the first to identify microstructural changes in the spinal cord of adults with CP. The CSA of the spinal cord and proportion of the gray matter were markedly reduced, and this was related to reduced hand dexterity. Additionally, the MTR implied that there were notable disruptions in the fidelity of the corticospinal tracts, and these were directly linked to the gray matter structural changes. Based on previous animal literature and H-reflex/transcranial magnetic stimulation studies in humans,10,11,25 we suspect that the initial insult to the developing brain induces long-lasting downstream effects in the spinal cord microstructure. Ultimately, the disrupted spinal cord microstructure likely plays a prominent role in the sensorimotor deficits and spasticity seen in adults with CP.
What this paper adds.
Adults with cerebral palsy (CP) have a reduced spinal cord cross-sectional area (CSA).
Spinal cord gray matter is reduced in adults with CP.
Spinal cord CSA is associated with hand dexterity.
Magnetization transfer ratio of corticospinal tracts was lower in adults with CP.
Acknowledgements
This work was partially supported by the National Institutes of Health (1R01-HD086245, 1R01-HD101833, R21-HD096390). The authors have stated they had no interests that might be perceived as posing a conflict or bias.
ABBREVIATIONS
- CSA
Cross-sectional area
- MTR
Magnetization transfer ratio
REFERENCES
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl 2007; 109: 8–14. [PubMed] [Google Scholar]
- 2.Blair E, Langdon K, McIntyre S, Lawrence D, Watson L. Survival and mortality in cerebral palsy: observations to the sixth decade from a data linkage study of a total population register and National Death Index. BMC Neurol 2019; 19: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundh S, Nasic S, Riad J. Fatigue, quality of life and walking ability in adults with cerebral palsy. Gait Posture 2018; 61: 1–6. [DOI] [PubMed] [Google Scholar]
- 4.Morgan P, McGinley J. Gait function and decline in adults with cerebral palsy: a systematic review. Disabil Rehabil 2014; 36: 1–9. [DOI] [PubMed] [Google Scholar]
- 5.Strauss D, Ojdana K, Shavelle R, Rosenbloom L. Decline in function and life expectancy of older persons with cerebral palsy. NeuroRehabilitation 2004; 19: 69–78. [PubMed] [Google Scholar]
- 6.Liptak GS. Health and well being of adults with cerebral palsy. Curr Opin Neurol 2008; 21: 136–42. [DOI] [PubMed] [Google Scholar]
- 7.Staudt M Reorganization after pre- and perinatal brain lesions. J Anat 2010; 217: 469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staudt M, Braun C, Gerloff C, Erb M, Grodd W, Krageloh-Mann I. Developing somatosensory projections bypass periventricular brain lesions. Neurology 2006; 67: 522–5. [DOI] [PubMed] [Google Scholar]
- 9.Wilke M, Staudt M, Juenger H, Grodd W, Braun C, Krageloh-Mann I. Somatosensory system in two types of motor reorganization in congenital hemiparesis: topography and function. Hum Brain Mapp 2009; 30: 776–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friel KM, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci 2007; 27: 11083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friel KM, Chakrabarty S, Martin JH. Pathophysiological mechanisms of impaired limb use and repair strategies for motor systems after unilateral injury of the developing brain. Dev Med Child Neurol 2013; 55(Suppl. 4): 27–31. [DOI] [PubMed] [Google Scholar]
- 12.Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 2007; 28: 226–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 1995; 8: 333–44. [DOI] [PubMed] [Google Scholar]
- 14.Boer R Magnetization Transfer Contrast. Part 1: MR Physics. MedicaMundi 1995; 40: 64–73. [Google Scholar]
- 15.Dousset V, Grossman RI, Ramer KN, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992; 182: 483–91. [DOI] [PubMed] [Google Scholar]
- 16.Dousset V Magnetization transfer imaging in vivo study of normal brain tissues and characterization of multiple sclerosis and experimental allergic encephalomyelitis lesions. J Neuroradiol 1993; 20: 297. [PubMed] [Google Scholar]
- 17.Cohen-Adad J, El Mendili MM, Lehéricy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 2011; 55: 1024–33. [DOI] [PubMed] [Google Scholar]
- 18.Paliwal M, Weber KA, Hopkins BS, et al. Magnetization transfer ratio and morphometrics of the spinal cord associates with surgical recovery in patients with degenerative cervical myelopathy. World Neurosurg 2020; 144: e939–e947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Leener B, Levy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017; 145: 24–43. [DOI] [PubMed] [Google Scholar]
- 20.De Leener B, Mangeat G, Dupont S, et al. Topologically preserving straightening of spinal cord MRI. J Magn Reson Imaging 2017; 46: 1209–19. [DOI] [PubMed] [Google Scholar]
- 21.Tustison NJ, Avants BB. Explicit B-spline regularization in diffeomorphic image registration. Front Neuroinform 2013; 7: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther 1985; 39: 386–91. [DOI] [PubMed] [Google Scholar]
- 23.Muthukumaraswamy SD, Myers JF, Wilson SJ, et al. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage 2013; 66: 36–41. [DOI] [PubMed] [Google Scholar]
- 24.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8: 45–56. [DOI] [PubMed] [Google Scholar]
- 25.Hodapp M, Klisch C, Mall V, Vry J, Berger W, Faist M. Modulation of soleus H-reflexes during gait in children with cerebral palsy. J Neurophysiol 2007; 98: 3263–8. [DOI] [PubMed] [Google Scholar]
- 26.Schmierer K, Parkes HG, So PW, et al. High field (9.4 Tesla) magnetic resonance imaging of cortical grey matter lesions in multiple sclerosis. Brain 2010; 133: 858–67. [DOI] [PubMed] [Google Scholar]
- 27.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 2012; 71: 444–57. [DOI] [PubMed] [Google Scholar]
- 28.Vedantam A, Jirjis MB, Schmit BD, Wang MC, Ulmer JL, Kurpad SN. Characterization and limitations of diffusion tensor imaging metrics in the cervical spinal cord in neurologically intact subjects. J Magn Reson Imaging 2013; 38: 861–7. [DOI] [PubMed] [Google Scholar]


