Abstract
Proton Bragg peak irradiation has a higher ionizing density than conventional photon irradiation or the entrance of the proton beam profile. Whether targeting the DNA damage response could enhance vulnerability to the distinct pattern of damage induced by proton Bragg peak irradiation is currently unknown. Here we performed genetic or pharmacologic manipulation of key DNA damage response elements and evaluated DNA damage signaling, DNA repair, and tumor control in cell lines and xenografts treated with the same physical dose across a radiotherapy linear energy transfer spectrum. Radiotherapy consisted of 6 MV photons and the entrance beam or Bragg peak of a 76.8 MeV spot scanning proton beam. More complex DNA double strand breaks induced by Bragg peak proton irradiation preferentially underwent resection and engaged homologous recombination (HR) machinery. Unexpectedly, the ATM inhibitor AZD0156 but not an inhibitor of ATR rendered cells hypersensitive to more densely ionizing proton Bragg peak irradiation. ATM inhibition blocked resection and shunted more double strand breaks to processing by toxic ligation through nonhomologous end-joining, whereas loss of DNA ligation via XRCC4 or Lig4 knockdown rescued resection and abolished the enhanced Bragg peak cell killing. Proton Bragg peak monotherapy selectively sensitized cell lines and tumor xenografts with inherent HR defects, and the repair defect induced by ATM inhibitor co-administration showed enhanced efficacy in HR proficient models. In summary, inherent defects in HR or administration of an ATM inhibitor in HR proficient tumors selectively enhance the relative biological effectiveness of proton Bragg peak irradiation.
Keywords: ATM, proton, radiation, homologous recombination, end joining
Introduction
Radiotherapy is an integral component of the multidisciplinary management of malignancies, with over 50% of all cancer patients receiving radiotherapy at some point during their disease course[1]. In recent years, there has been a rapid expansion in the use of protons for the delivery of therapeutic radiation. Compared to conventional photon-based treatment, protons have physical characteristics which result in a distinct dose deposition profile, defined by the Bragg peak, which is located at the end of the proton path where the ions come to a stop. Whereas photons slowly deposit energy as they pass through tissue, including beyond the target, the large majority of the energy from each proton is deposited at the Bragg peak within the target which can result in better sparing of normal tissue. Because of these distinct physical properties and resulting organ-sparing capacity, proton therapy is being investigated across multiple malignancies as a promising strategy to reduce the acute and late adverse effects associated with radiotherapy and has emerged as a standard of care for the treatment of most pediatric tumors[2].
Photons and protons are considered sparse ionizing density forms of radiation. Indeed, relative to heavy ions such as carbon, photons and protons have a comparably low Linear Energy Transfer (LET), which impacts the complexity of DNA damage and the repair capacity of treated cells, with protons having a slightly higher LET than photons [3, 4]. Consistently, a generic Relative Biologic Effectiveness (RBE, the dose of x-rays divided by the dose of protons required for equal biological effect) value of 1.1 has been used for proton therapy treatment planning in the clinic, which means in order to achieve an equivalent biological effect, the physical dose delivered with protons must be 10% lower[5]. Although the LET of protons is similar to photons for much of the proton beam profile, the LET rises within the proton Bragg peak, which results in clustering of lesions in closer proximity on the DNA strands[6]. In turn, studies suggest that the more difficult to repair damage induced by elevated LET proton dose deposition at the Bragg peak results in a RBE greater than 1.1 [7, 8]. As a consequence, clinicians routinely avoid proton beam angles with critical organs immediately distal to the target along the beam path in order to limit areas of elevated LET Bragg peak dose deposition and subsequent risk of normal tissue toxicity in these structures [9–14]. Whether inherent defects in DNA repair render tumors more susceptible to the distinct DNA damage created by elevated LET Bragg peak irradiation, or whether the cellular DNA damage response can be targeted pharmacologically to induce vulnerabilities to this damage remains to be elucidated.
The DNA double strand break (DSB) is the most lethal form of DNA damage to which cells must respond following radiotherapy. Radiation-induced, cytotoxic, DNA double-strand breaks (DSBs) are predominantly repaired by two pathways. Homologous recombination (HR) is employed preferentially during the G2- and S-phase when a sister chromatid is available as a repair template to enable error-free repair. In contrast, non-homologous end joining (NHEJ) involves removal of damaged bases followed by re-ligation, which can lead to reduced genome integrity. NHEJ can occur throughout the cell cycle but predominates in G1 since NHEJ does not require a template sister chromatid[15]. A key step in DNA DSB repair that promotes HR repair over NHEJ is DNA end resection, which is facilitated by BRCA1 and creates 3’ single-stranded DNA (ssDNA) overhangs[16]. Following end resection, ssDNA is immediately coated by replication protein A (RPA) and subsequently replaced by RAD51. The formation of RAD51 nucleoprotein filaments, which requires the activity of BRCA1 and BRCA2, drives the key HR steps of homology search and strand invasion of the corresponding sister chromatid that enables high-fidelity repair[17]. Pathogenic mutations in BRCA1, BRCA2, or other components of the HR pathway result in HR-deficiency, regardless of which component of the HR pathway is inactivated[18]. While most widely appreciated in breast and ovarian cancer, HR deficiency by genetic or epigenetic means has also been reported in a number of other malignancies, such as photon-incurable osteosarcomas and chordomas [19–21].
Ataxia-Telangiectasia Mutated (ATM), ATM and Rad3-related (ATR) and the catalytic subunit of DNA-dependent protein kinase (DNA-PKcs) are considered master regulators of DNA damage response (DDR) signaling networks that orchestrate the cellular response to DNA damage. Although each kinase has multiple targets and there is considerable cross-talk between pathways, ATM largely promotes DSB repair by activating DNA end resection[22], whereas ATR and DNAPK primary responsibilities lie in activating the cellular response to replication stress and mediating NHEJ, respectively[17]. Small molecule inhibitors of ATM, ATR, and DNAPK are being investigated to induce pharmacologic repair deficiency in tumors and augment the effects of cancer therapy[23]. Fundamental to genomic instability and tumorigenesis, inherent tumor defects in DNA repair can also confer increased sensitivity to specific therapies, the most prominent example being the unique sensitivity of BRCA1 and BRCA2 deficient tumors to poly (ADP-ribose) polymerase (PARP) inhibition, which is already an approved treatment strategy in multiple malignancies[24].
In this study, we extend this paradigm to particle therapy. We demonstrate that repair deficiency induced by co-treatment with an ATM inhibitor, but not inhibitors of ATR and DNAPK, selectively enhances the efficacy of elevated LET proton therapy delivered at the Bragg peak by inhibiting resection and promoting ‘toxic’ NHEJ-mediated DSB repair[22]. A similar phenotype of enhanced RBE is also observed with Bragg peak irradiation alone specifically in tumors with inherent HR deficiency. Our results suggest that pharmacologic inhibition of ATM or inherent HR deficiency create a unique vulnerability to the distinct damage induced by Bragg peak proton irradiation and create a framework for the development of ‘precision’ particle radiotherapy in the clinic.
Materials and Methods
Cell lines
BT549 and U2OS cell lines were purchased from ATCC (Manassas, VA). MDA-MB-436 (BRCA1−/−) and MDA-MB-436 BRCA1+/+ complemented cell lines were gifted from Neil Johnson and V79 and VC8 were gifted by Fergus Couch. Cells were grown in DMEM RPMI1640 or McCoy’s 5A with 10% FBS and were transfected with Polyetherimide (PEI) or TransIT-X2 (Mirus). All cell types were routinely cultured and kept in humidified and normoxic conditions at 37°C and 5% CO₂. The cells were tested for mycoplasma contamination routinely every 2–3 months and were maintained below passage number 20.
shRNA knockdown
XRCC4 shRNA (NM_022406), LIG4shRNA (NM_022406) and RAD51shRNA (NM_002875) were purchased from Sigma-Aldrich. PLKO.1 control shRNA (#10879) was purchased from Addgene. The following is the sequence information of shRNAs: XRCC4, TGTGTGAGTGCTAAGGAAGCT; PLKO.1 shRNA GACTATCATATGCTTACCGT. LIG4 shRNA, CCGGGCCTATCTCATGACCATATTGCTCGAGCAATATGGTCATGAGATAGGCTTTTTG; RAD51 shRNA, CCGGCGGTCAGAGATCATACAGATTCTCGAGAATCTGTATGATCTCTGACCGTTTTT. Lentiviral shRNAs were made according to the protocol from Sigma-Aldrich.
In vitro setup and proton irradiation
Cells were irradiated with a 76.8 MeV spot scanning proton beam (Pro Beat V, Hitachi, Japan), a mini pyramid filter and 25 mm range shifter in the nozzle. Four cell culture 6-well plates fit into a phantom, with two plates in the entrance and two plates at the Bragg peak positions of the proton Bragg curve. These two positions along the proton beam profile were selected in order to compare the biological effects of low LET (dose-averaged LET [LETd] = 2.2 keV/μm]) vs. high LET (LETd = 7.0 keV/μm) protons at the entrance and Bragg peak, respectively, while also maintaining a high level of dosimetric accuracy. Though points along the distal fall off region of the Bragg peak have higher associated LETd values, the dosimetric uncertainty is much larger. The phantom was placed on a modified treatment couch top attached to the robotic arm for accurate positioning. A posterior field encompassing all four 6-well plates was delivered with variable MU depending on the physical dose delivered.
Clonogenic cell survival assay
Cells were plated and kept in an incubator for 4–6 hours prior to irradiation with photons or protons to ensure cells were adherent to the plate bottom. Cells were plated at various densities ranging from 100 – 10000 cells depending on physical dose and cell type. Plates were then exposed to photons or protons, various DNA repair inhibitors, or a combination repair inhibitors administered one hour prior to irradiation and then returned to the incubator for 14 days. Control plates also went through mock treatment and were incubated for the same period of time. Plates were fixed, stained with Giemsa, and colonies (> 50 cells) were counted manually. Survival data was fit with the linear quadratic (LQ) model, using Χ2; nonlinear regression to solve for α and β values in MINUIT (CERN, Geneva, Switzerland) [25]. Proton RBE was calculated directly from the raw data by taking the ratio of surviving fractions at 2 Gy:
For our experiments, Scontrol referred to surviving fraction following 2 Gy using 6MV photons. Error was calculated from the survival data by taking one standard deviation from three independent clonogenic cell survival experiments.
Chemicals and antibodies
AZD0156, AZD6738 were provided by astra Zeneca and VX-984, an agent proprietary of EMD Serono, was provided by the Division of Cancer Treatment and Diagnosis, National Cancer Institute. Anti-γ-H2AX (05–636, RRID AB_309864) antibodies for immunofluorescence (IF) were purchased from Millipore. Anti-RAD51 (ab133534, RRID AB_2722613) antibodies for IF were purchased from Abcam. Anti-RPA32 kDa subunit antibodies (9H8) (SC-56770 RRID AB_785534) for IF were purchased from Santa Cruz. Anti-BrdU antibodies (347580 RRID AB_400326) for IF were purchased from BD Biosciences. Anti-β-Actin (A1978 RRID AB_476692) antibodies for Western blot were purchased from Sigma. Anti-phospho-RPA32 S4/S8 (A300–245A RRID AB_210574) for Western blot was purchased from Bethyl Laboratories. Anti-phospho-ATR (Ser428) antibody 2853 (RRID AB_2290281), anti-phospho-Chk2 (Thr68) (C13C1) rabbit mAb 2197 RRID AB_2080501, anti-phospho-Chk1 (Ser345) (133D3) rabbit mAb 2348 RRID AB_331212, anti-phospho-histone H2A.X (Ser139) (20E3) rabbit mAb 9718 RRID AB_10694395, anti-Chk1 (2G1D5) mouse mAb RRID AB_2080356and anti-Chk2 (D9C6) XP rabbit mAb #6334 RRID AB_ 11178526 for Western blot were purchased from Cell Signaling Technology. XRCC4 antibody(15817–1-AP) and Lig4 antibody(12695–1-AP) were purchased from Proteintech.
Immunofluorescent staining
Immunofluorescent staining was performed using standard procedures. Briefly, cells were seeded in 6 well plates containing coverslips. Following irradiation and adequate incubation time to allow for repair, cells were fixed by 3% paraformaldehyde for 30 minutes, washed 3 times in 1×PBS, and then extracted with 0.5% Triton X-100 PBS solution for 5 to 10 minutes. After blocking with PBS containing 1% bovine serum albumin, cells were incubated with indicated primary antibodies overnight. Then cells were washed three times with 1×PBS and incubated with Alexa Fluor 488 or Alexa Fluor 594 conjugated second primary antibodies (Thermo Fisher Scientific) for 30 minutes to one hour at room temperature. Next, cells were stained with 100ng/ml 4, 6-diamidino-2-phenylindole (DAPI) for 3–5 minutes to visualize nuclear DNA. The cover slips were mounted onto glass slides using anti-fade solution, and the slides were visualized using a Leica ECLIPSE E800 fluorescence microscope with a 40×objective lens (NA 1.30).
In vivo xenograft lines and study design
All animal procedures were performed according to NIH guidelines and approved by the Mayo Clinic Institutional Animal Care and Use Committee and Biosafety Committee. The triple negative breast cancer patient-derived xenograft model, MC-BR-BTY-0030, was established in the context of the Breast Cancer Genome Guided Therapy Study, BEAUTY, a prospective Institutional Review Board–approved preoperative chemotherapy clinical trial, which has previously been reported[26, 27]. MC-BR-BTY-0030 was established from the chemoresistant surgical specimen of a patient with TNBC after standard of care anthracycline and taxane-based preoperative chemotherapy. Histology was confirmed by hematoxylin and eosin and immunohistochemical stains.
MC-BR-BTY-0030 PDX tumors grown to 1 cm in diameter were resected and manually disaggregated into a single cell suspension using a 1-cc syringe. Two million tumor cells suspended in 100 μL of matrigel and PBS were then injected subcutaneously into the nape of the neck of 4–5 week old female athymic nude mice (Hsd:Athymic Nude-Foxn1nu from Envigo). Once tumors reached 50 mm3, the mice were randomized into the following groups: (1) Sham RT + vehicle, (2) 5 Gy photons + vehicle, (3) 5 Gy proton entrance + vehicle, (4) 5 Gy proton Bragg peak + vehicle, (5) sham RT + AZD0156, (6) 5 Gy photons + AZD0156, (7) 5 Gy proton entrance + AZD0156, (8) 5 Gy proton Bragg peak + AZD0156. AZD-0156 (10mg/kg) or tocopheryl polyethylene glycol succinate (TPGS) vehicle was administered one hour prior to and 24 following irradiation by intraperitoneal injection.
Similar to above, two million MDA-MB-436 (BRCA1−/−) or MDA-MB-436 (BRCA1+/+) were injected into athymic nude mice and once the tumors reached 50 mm3 the mice were randomized to (1) Sham RT, (2) photons, (3) proton entrance, (4) proton Bragg peak. Photon and proton irradiation was a single 5 Gy physical dose fraction.
Irradiation of animals
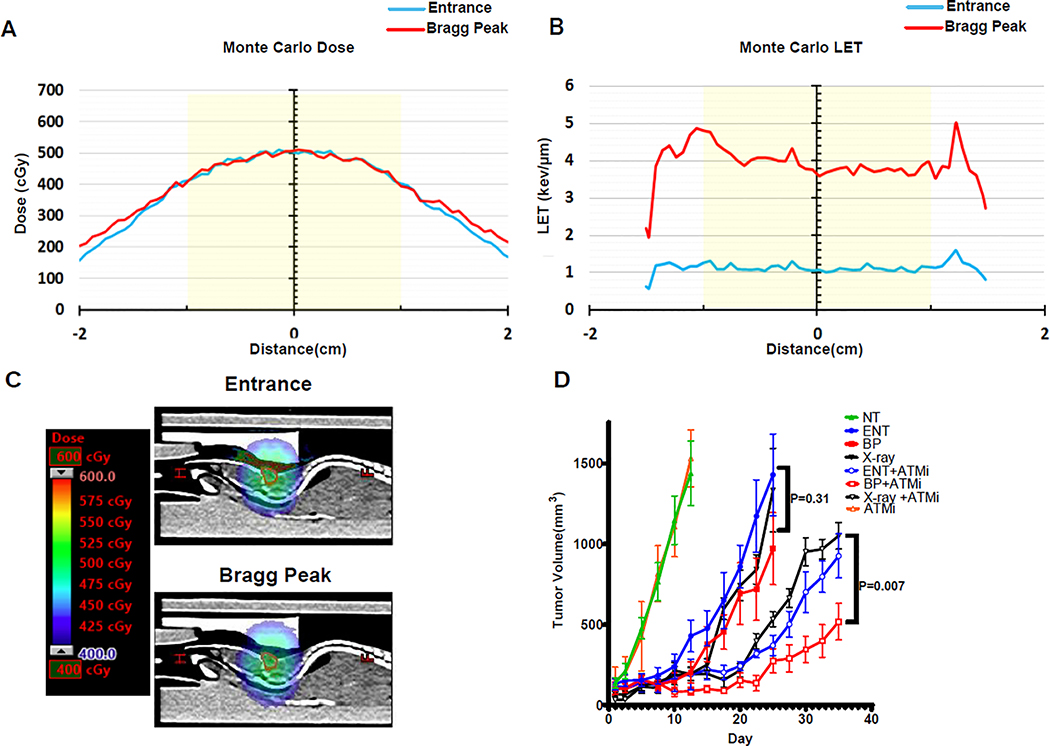
Three separate treatment plans were generated in Eclipse (Varian Medical Systems, Palo Alto, CA, USA) to deliver a 5 Gy physical dose to the tumor: 1) a photon plan generated with parallel opposed IMRT 6 MV fields; 2) a proton plan generated with the entrance portion of a 76.8 MeV Bragg curve using two parallel opposed fields; 3) an LET-enhanced proton plan generated to treat with the middle of a 1.5 cm wide spread out Bragg peak (SOBP) using two parallel opposed fields. The photon plan was delivered with a Truebeam linear accelerator (Varian medical systems, Palo Alto, CA, USA) using MLC’s to shape the delivered dose to the tumor. All proton plans were delivered using a spot scanning proton system (Pro Beat V, Hitachi, Japan); a 45 mm range shifter was used for the Bragg peak irradiation. The LETd was calculated using an in-house GPU-based Monte Carlo[14], where values across the target were 1.1 keV/μm and 4.0 keV/μm for the entrance and Bragg peak plans, respectively. Monte Carlo calculations also served as a verification of the dose reported in the treatment planning system. Representative Monte Carlo dose and LETd distributions across the tumor are shown in figure 5A–C.
Figure 5. ATM inhibition promotes the efficacy of Proton Bragg peak irradiation in vivo.
A-D MC-BR-BTY-0030 triple negative breast cancer patient-derived xenograft cells were subcutaneously injected into the midline of athymic nude mice. After tumors reached 50mm3 animals were randomized to sham (no treatment [NT]), or 5 Gy (500 cGy) delivered with 6 MV photons (X-ray), 76.8 MeV protons at the entrance (ENT) portion of the Bragg curve, or 76.8 MeV protons at the middle of a 1.5 cm wide spread-out Bragg peak (BP or LET-optimized protons) in a single fraction, with or without pre-treatment one hour prior with the ATM inhibitor, AZD0156 (10mg/kg). AZD0156 was also administered a second time at 24 hours. A, Monte Carlo dose calculation for the entrance and Bragg peak proton plans showing the same physical dose administered. B, Monte Carlo LETd calculation for the entrance and Bragg peak proton plans. An approximate 4-fold increase in LETd with the Bragg peak plan was achieved. Yellow shade in A and B denotes a 2 cm diameter easily encompassing the tumor. C, Sagittal CT image from the entrance and Bragg peak proton plans demonstrating the same physical dose administered, as in A. The tumor volume is delineated in red. D, Tumor volume was assessed over time with day 1 denoting the first dose of AZD0156 and day of irradiation. Shown are the representative data (mean ± SEM) from biologically independent samples (n=7). P values are displayed for the comparison of photons and Bragg peak protons obtained using the two-sided unpaired t-test.
For irradiation, animals were placed in a mouse specific immobilization box with attached anesthesia lines. Inhalation anesthesia was a mixture of 2.5% isoflurane and 1 L/min oxygen using a Diamed vaporizer and 3D printed custom nose cone with an active charcoal scavenger. Mice were irradiated in the head first prone position and treated six at one time. A representative sample of mice with embedded tumors was scanned in their setup position with a clinical CT scanner (Siemens) to ensure robustness of treatment delivery. Mice were observed daily and tumors were measured by digital calipers thrice weekly. Tumor volume (V) was calculated as: V = (4/3) × π × length ×width × height. Animals were monitored until the tumors became ulcerated, erythematous, impaired movement, or exceeded 1,500 mm3, at which time the mice were euthanized.
Results
DNA repair of proton Bragg peak-induced damage is delayed
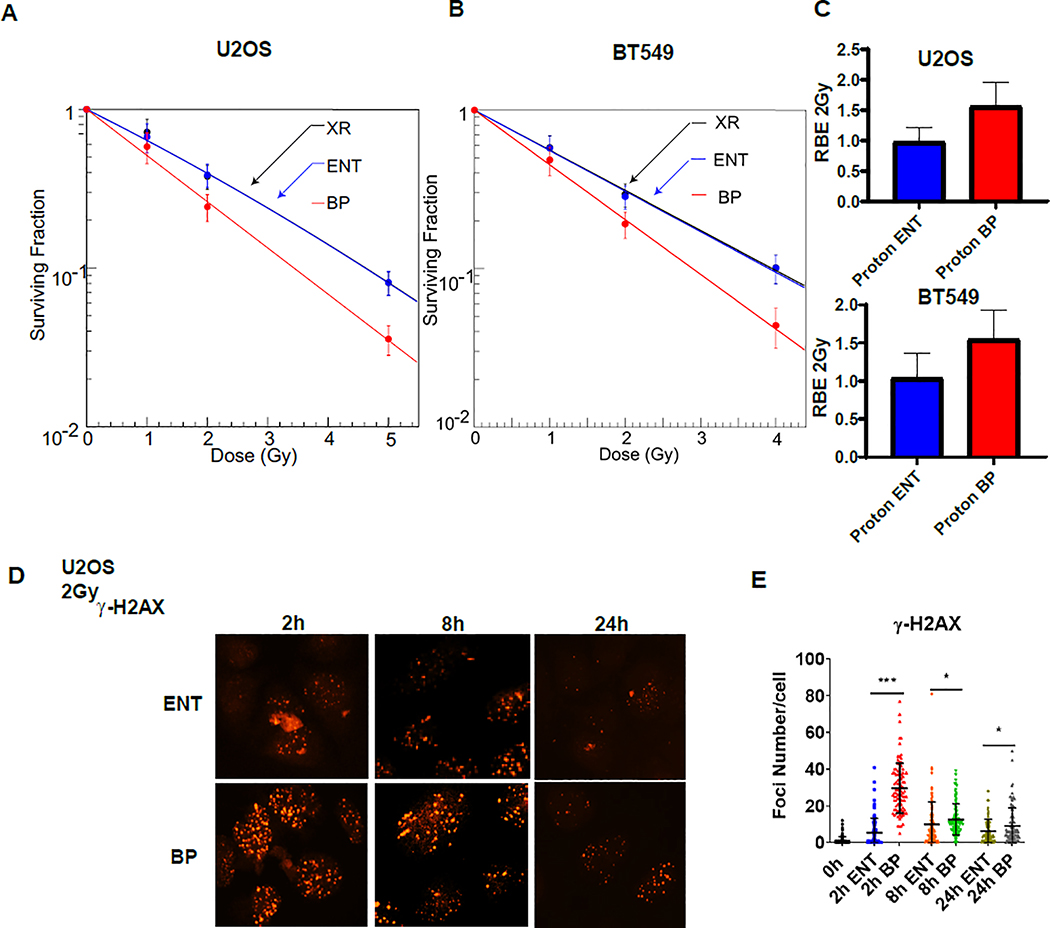
In order to better define the relationship between LET and RBE of proton therapy at distinct points along the proton beam profile, we previously built and validated a specialized, high precision acrylic jig with a cell plate holder to enable highly reproducible placement of the cell surface within the beam profile [28]. Using this robust model, we sought to investigate differences in DNA damage response and repair between photons and protons. We began by irradiating U2OS and BT549 cells at the same dose levels by using 6 MV photons (LETd = 0.31 keV/μm)[29] or 76.8 MeV protons delivered at either the entrance (LETd = 2.2 keV/μm) or the Bragg peak (LETd = 7.0 keV/μm) of the proton beam profile and assessed the effects using clonogenic survival assays. As displayed in figure 1A–B, survival curves overlapped for cells irradiated with photons or protons at the entrance of the beam profile, whereas there was a significant reduction in clonogenic cell survival for cells irradiated at the proton Bragg peak. The proton Bragg peak RBE2Gy (the ratio between the surviving fraction after a delivered physical dose of 2 Gy from 6 MV photons and Bragg peak protons) was 1.5 and 1.6 for BT549 and U2OS cells, respectively. The corresponding proton entrance RBE2Gy (the ratio between the surviving fraction after a delivered physical dose of 2 Gy from 6MV photons and entrance protons) values were 1.0 and 1.0 (Figure 1C). These results were consistent with increased proton Bragg peak RBE observed in prior studies arising as a result of the higher LET at the Bragg peak[30].
Figure 1. A-B Proton Bragg peak DNA damage is more difficult to repair.
A-B, U2OS and BT549 cells irradiated with 6MV photons or 76.8 MeV protons delivered at the entrance (ENT) or the Bragg peak (BP) of the proton beam profile and clonogenic survival was assessed. Data are presented as the mean ± SEM from three independent experiments. C, The proton entrance and proton Bragg peak RBE2Gy for the experiments in A and B was quantified. D-E, U2OS cells were treated with 2 Gy using 76.8 MeV protons delivered at ENT or the BP and the formation and resolution of γ-H2AX (D-E) were assessed using immunofluorescence. Representative images (D) and quantification (E) are displayed, (mean ± SEM) from three independent experiments. P values were calculated using ANOVA; *p <0.05, ***p<0.001.
U2OS are osteosarcoma cells commonly employed in DNA repair studies, as repair proteins recruited into ionizing radiation-induced foci can be readily visualized with immunofluorescence in this cell line. DNA DSB repair activity can be monitored indirectly by measuring the formation and resolution of phospho-SER139-H2AX (γ-H2AX)[31]. Consistent with the idea that elevated LET results in more complex and difficult to repair DNA damage, higher induction and persistence of damage-inducible γ-H2AX were observed with proton Bragg peak irradiation compared to proton entrance (Figure 1D–E, Supplementary Figure 1A–B). Thus, the RBE of proton Bragg peak irradiation is modestly elevated and the repair of DNA damage induced by this part of the proton beam is delayed.
Proton Bragg peak irradiation upregulates DNA DSB end-resection and HR
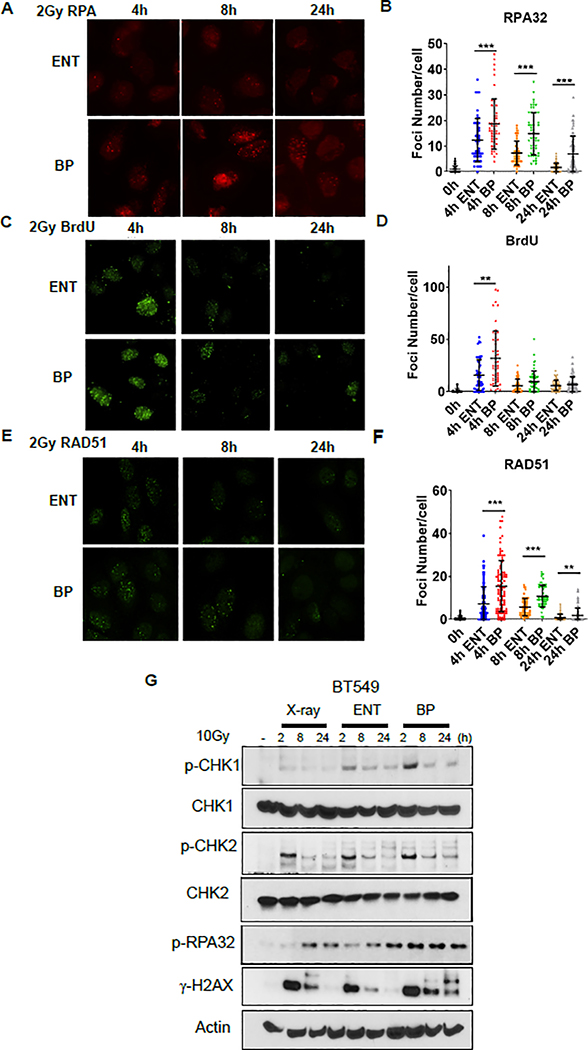
The enhanced cell killing, upregulated induction and delayed resolution of γ-H2AX foci following proton irradiation at the Bragg peak raised the possibility that Bragg peak irradiation may also differentially activate the DNA damage response. The processing of DSB ends to create ssDNA overhangs is an early determinant of DNA DSB repair pathway choice and required for repair to proceed by HR. Resection has been reported to be upregulated in response to the more complex DNA damage induced by heavy ion irradiation[32], and we hypothesized that the density of DNA lesions from the modest elevation in LET of proton Bragg peak irradiation might also preferentially engage DNA resection machinery compared to the entrance of the proton beam profile [32]. In order to quantify resection indirectly, we assessed RPA foci at various time points following the same dose of entrance and Bragg peak proton irradiation, as ssDNA is immediately coated by RPA following DSB resection. As displayed in figure 2A–B and supplementary figure 1C–D, there were a greater number of RPA foci per cell following proton Bragg peak irradiation compared to entrance. These differences persisted at 24 hours post-irradiation. Enhanced resection was also confirmed by assessment of BrdU foci, which can only be detected by an anti-BrdU antibody at resection sites following BrdU incorporation during single stranded DNA synthesis (Figure 2 C–D)[33].
Figure 2. Proton Bragg peak irradiation upregulates resection and ATR pathway signaling.
A-F U2OS cells were irradiated with 2Gy using 76.8 MeV protons delivered at the entrance (ENT) or the Bragg peak (BP) of the proton beam profile and RPA (A-B), BrdU (C-D) and RAD51 (E-F) foci were assessed using immunofluorescence at the indicated time points. Representative images are shown in A, C, and E, and data quantification is displayed in B, D, and F (mean ± SEM, n = 3). P values were calculated using ANOVA; **p<0.01, ***p<0.001. G, BT549 cells were irradiated with 10Gy using 6 MV photons or 76.8 MeV protons delivered at ENT or the BP. Lysates were collected after indicated time and Western blot analysis was performed using the indicated antibodies. Actin was used as a loading control.
Following resection and the coating of ssDNA, RPA must be replaced by RAD51, the key DNA recombinase that drives homology search and strand invasion of the corresponding sister chromatid, for repair to proceed by HR. We measured RAD51 foci formation following irradiation with entrance or Bragg peak protons. RAD51 recruitment was increased at 4, 8, and 24 hours following proton Bragg peak irradiation, relative to proton entrance (Figure 2 E–F, supplementary Figure 1E–F). Altogether, these results suggested that DNA DSB resection and RAD51 recruitment is increased in response to the modest elevation in LET from Bragg peak proton irradiation.
Proton Bragg peak irradiation activates ATR pathway signaling
The coating of ssDNA by RPA leads to the recruitment of ATR to stalled replication forks or resected DSBs, where ATR is activated[34]. Activated ATR phosphorylates CHK1. In addition, ATR phosphorylates RPA, which promotes RAD51 recruitment [35–37]. Given the increased recruitment of RPA and RAD51 to damage sites with Bragg peak irradiation in the above studies, we postulated that ATR pathway signaling may also be preferentially upregulated in response to proton Bragg peak irradiation. We evaluated the expression of phosphorylated CHK1 and phosphorylated RPA32, an RPA subunit, in BT549 cells as readouts of ATR pathway activity. In accordance with the observed increased RPA recruitment (Figure 2 A–B, supplementary figure 1C–D), there was early upregulated phosphorylation of CHK1 and RPA32, consistent with greater ATR activity downstream of resected DNA DSBs following proton Bragg peak irradiation relative to the same physical dose administered with photons or the entrance of the proton beam profile (Figure 2 G, supplementary figure 1G). In contrast, there were no differences across the radiation types with regards to phosphorylation of CHK2, downstream from ATM (Figure 2 G, supplementary figure 1G)[38]. We concluded from this set of experiments that ATR pathway signaling could be upregulated following proton Bragg peak irradiation.
Inhibition of ATM selectively enhances the effectiveness of proton Bragg peak irradiation
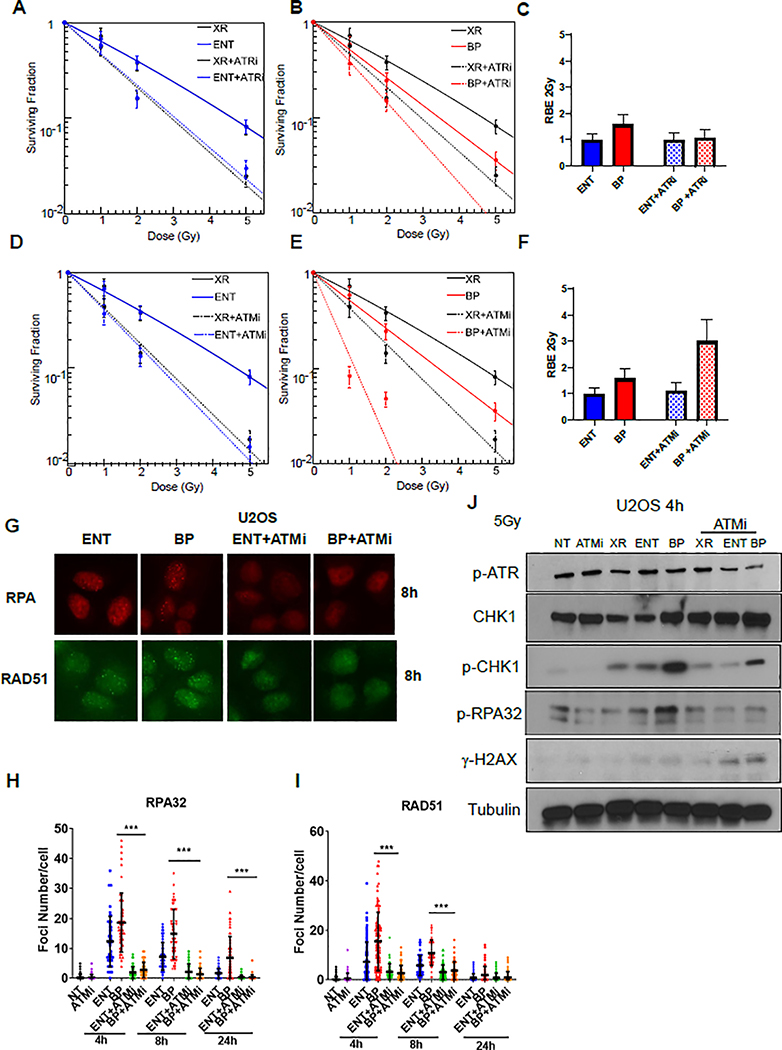
Based on the above findings demonstrating upregulated HR and ATR pathway activity following proton Bragg peak irradiation, we hypothesized that pharmacologic ATR inhibition may enhance the proton Bragg peak RBE. However, co-treatment with the ATR inhibitor, AZD6738, uniformly sensitized cells to photon, proton entrance, and proton Bragg peak irradiation, without changing the RBE (Figure 3A–C). Co-treatment with the DNA-PK inhibitor, VX-984, minimally increased the proton Bragg peak RBE2Gy (Supplementary Figure 2A–C). In contrast, co-treatment with the ATM inhibitor, AZD0156, resulted in a striking doubling of the proton Bragg peak RBE2Gy from 1.5 (± 0.4) to 3.0 (± 0.8) whereas the proton entrance beam RBE did not change (Figure 3D–F). Relative enhancement of proton Bragg peak cell killing was also observed with ATM inhibition in a non-cancerous cell line, V79, although the effect was much less pronounced (proton Bragg peak RBE2Gy 1.45 [± 0.3] (Supplementary Figure 5E).
Figure 3. A. ATM inhibition enhances the relative efficacy of proton Bragg peak irradiation.
A-D, U2OS cells were pre-treated with the ATR inhibitor AZD6738 (200nM, A-C) or the ATM inhibitor AZD0156 (2nM, D-F) for one hour prior to irradiation with 6MV photons (XR) or 76.8 MeV protons delivered at the entrance (ENT) or the Bragg peak (BP) of the proton beam profile and clonogenic survival was assessed, (mean ± SEM) from three independent experiments. C, F The proton entrance and proton Bragg peak RBE2Gy for the experiments in A-B (C) and C-D (F) was quantified. G-I, U2OS cells were pre-treated with AZD0156 for one hour prior to irradiation with 2Gy using 76.8 MeV protons delivered at ENT or the BP of the proton beam profile and RPA (G-H) and RAD51 (G, I) foci were assessed using immunofluorescence and quantified at the indicated time points. Representative images are shown in G and data quantification is displayed for RPA in H and RAD51 in I (mean ± SEM, n = 3). P values were calculated using ANOVA; ***p<0.001. J, U2OS cells were pretreat with DMSO or AZD0156 and irradiated with 5Gy using 6 MV photons or 76.8 MeV protons delivered at ENT or the BP. Lysates were collected after 4 hours and Western blot analysis was performed using the indicated antibodies. Tubulin was used as a loading control.
ATM promotes HR-mediated DNA DSB repair by activating DSB end resection and other HR factors [39]. Consistent with inhibition of this pathway, ATM inhibition resulted in a reduction in RPA and RAD51 foci (Fig. 3G–I) and p-RPA and p-CHK1 levels after proton irradiation (Figure 3J). Osteosarcomas and other subsets of tumor histologies are inherently resistant to conventional radiation with photon and proton therapy. These findings raised the possibility that ATM inhibitors might be combined with Bragg peak irradiation to improve tumor control.
NHEJ ligase activity is required for ATM inhibitor-induced enhancement of Bragg peak proton efficacy
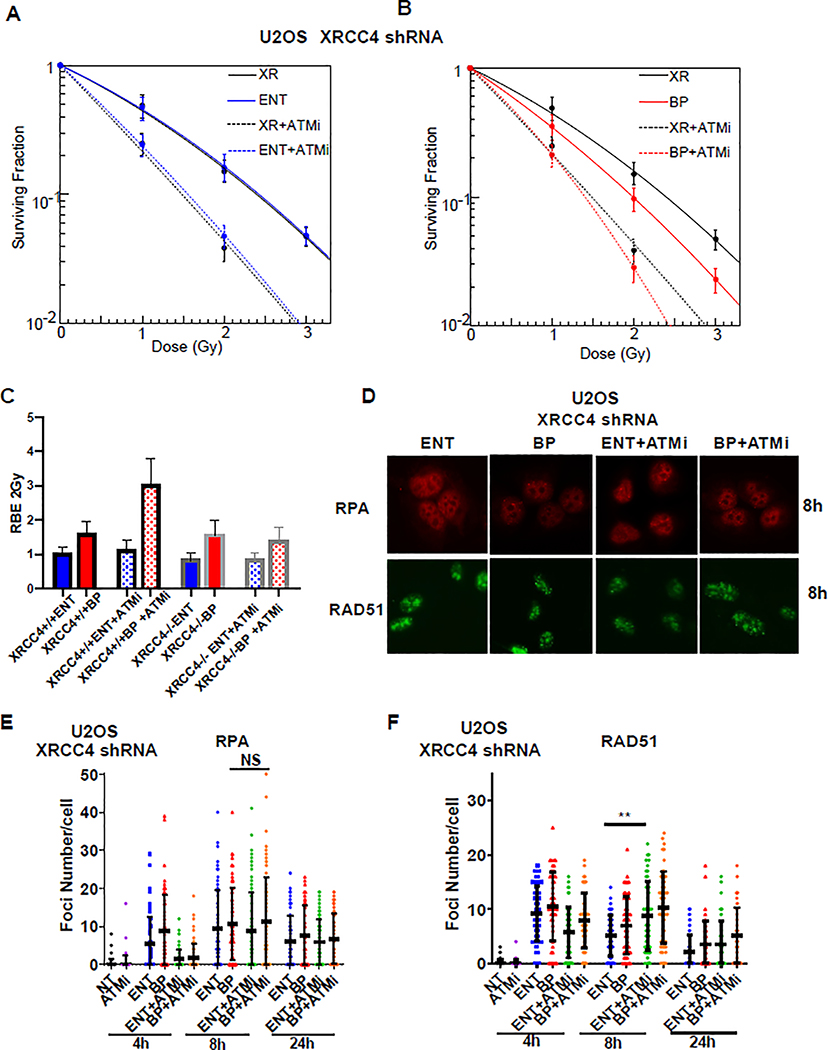
Recently, ATM was found to promote survival following DSBs induced by DNA topoisomerase 1 (TOP1) and PARP inhibition by suppressing toxic NHEJ-mediated DSB repair and cell death[22]. Given that ATM inhibition blocked RPA and RAD51 foci formation and downregulated p-RPA and p-CHK1 levels (Figure 3G–J), we hypothesized that the RBE of LET-optimized proton therapy in ATM inhibitor -treated tumors was dependent on reduced DSB resection and shunting of more complex DNA lesions into the error-prone, toxic NHEJ repair pathway. In order to evaluate the impact of NHEJ on proton Bragg peak RBE we used shRNAs to disrupt the core NHEJ factors XRCC4 and Lig4, which promote DNA ligation, and assessed cell survival following photon, proton entrance and proton Bragg peak irradiation alone or in combination with ATM inhibition. As expected due to the important role of NHEJ in handling many radiation-induced DSBs, XRCC4 and Lig4 knockdown sensitized cells to photons, proton entrance, and proton Bragg peak irradiation (Supplementary Figure 3A–H). However, XRCC4 and Lig4 knockdown had minimal impact on the RBE of proton entrance or Bragg peak irradiation relative to wild-type cells (Supplementary Figure 3D,H). Of note, cell survival following ATM inhibitor co-administration was not impacted in Entrance irradiated and only modestly impacted in Bragg peak irradiated XRCC4 knockdown (Figure 4A–B) and Lig4 knockdown cells (Supplementary Figure 4A–B). This finding was in stark contrast to wild-type cells, which were dramatically sensitized to ATM inhibition when irradiated at the proton Bragg peak (Figure 3E). These series of experiments revealed a striking reduction in the proton Bragg peak RBE in ATM inhibitor treated XRCC4 and Lig4 knockdown cells compared to wild-type cells (Figure 4C, Supplementary Figure 4C). Together, these findings were consistent with the possibility that ATM inhibition may enhance the proton Bragg peak RBE by inhibiting resection and upregulating toxic NHEJ.
Figure 4. Loss of NHEJ blocks ATM inhibitor-induced RBE enhancement of Bragg peak proton.
A-B, U2OS cells were infected with a lentivirus encoding XRCC4 shRNA. Cells were then pre-treated with the ATM inhibitor AZD0156 (2nM) for one hour prior to irradiation with 6MV photons or 76.8 MeV protons delivered at the entrance (ENT) or the Bragg peak (BP) of the proton beam profile and clonogenic survival was assessed. Data are presented as the mean ± SEM from three independent experiments. C The proton entrance and proton Bragg peak RBE2Gy for the experiments in A-B was quantified. Also displayed for comparison are the proton entrance and proton Bragg peak RBE2Gy for wild-type U2OS cells from Figure 3D. D-F, XRCC4 knockdown U2OS cells were pre-treated with AZD0156 for one hour prior to irradiation with 2Gy using 76.8 MeV protons delivered at entrance or the Bragg peak of the proton beam profile and RPA (D-E) and RAD51 (D, F) foci were assessed using immunofluorescence and quantified at the indicated time points. Representative images are shown in D and data quantification is displayed for RPA in E and RAD51 in F (mean ± SEM, n = 3). P values were calculated using ANOVA; ***p<0.001.
NHEJ loss promotes resection and HR repair of elevated LET proton Bragg peak lesions
The experiments described above suggested that loss of NHEJ ligase activity could block the RBE enhancement of proton Bragg peak irradiation observed when co-treated with an ATM inhibitor. Given that NHEJ and HR compete for repair of DSBs, we hypothesized that loss of XRCC4 may increase resection and error-free HR repair of the more complex DSB lesions induced by the ATM inhibitor plus proton Bragg peak combination. In order to address this hypothesis, we again analyzed RPA and RAD51 foci formation as readouts of resection and HR, respectively, following proton entrance and proton Bragg peak irradiation of XRCC4 knockdown cells. Compared to wild-type cells treated with proton therapy alone, which displayed early and robust recruitment of RPA and RAD51, particularly when cells were treated with proton Bragg peak irradiation (Figure 3G–I), the recruitment of RPA and RAD51 following proton irradiation of XRCC4 knockdown cells pre-treated with ATM inhibitor was delayed (Figure 4D–F), suggestive of delayed DSB end resection and HR. Despite the delay in resection and HR repair, loss of XRCC4 still inhibited the RBE of enhancement of ATM inhibitor co-treatment with proton Bragg peak irradiation (Figure 4B–C). Collectively, these findings suggested that resection, as a mediator of DSB repair pathway choice, and toxic DSB repair by NHEJ are important determinants of the heightened sensitivity of elevated LET proton Bragg peak irradiation.
ATM inhibition enhances the efficacy of ‘LET-optimized’ proton Bragg peak irradiation in vivo
We have demonstrated the feasibility of using Monte Carlo simulation to create LET-optimized proton therapy plans that capitalize on a convergence of Bragg peaks to enhance the LET in the target volume [40]. Using this system, we developed a robust entrance beam proton plan (LETd 1 keV/μm) and Bragg peak (LET-optimized) plan (LETd 4 keV/μm) for irradiation of heterotopic xenografts (Figure 5A–C). Both plans were designed to administer a single fraction of 5 Gy (500 cGy, Figure 5A, C), with either low (entrance) or elevated (Bragg peak) LET (Figure 5B). We also designed a similar 6MV photon plan for administration of the same dose. We postulated that LET-optimized proton combined modality therapy would be most relevant for overcoming therapeutically resistant disease. Therefore, we selected a patient-derived xenograft model for investigation, MC-BR-BTY-0030, which was generated from the chemoresistant surgical specimen of a patient with residual triple negative breast cancer after preoperative anthracycline and taxane-based chemotherapy [26, 27, 41]. Irradiation of this HR proficient model with Bragg peak protons or entrance protons resulted in comparable delay in tumor regrowth as photon irradiation (p=0.31 for the comparison of photons vs Bragg peak protons); in contrast, while concomitant AZD0156 enhanced the efficacy of all three radiation arms, the most profound improvement in tumor control was achieved when the ATM inhibitor was combined with Bragg peak proton irradiation (p=0.007 for the comparison of photons vs Bragg peak protons, Figure 5D). These data are consistent with the enhanced RBE of proton Bragg peak irradiation in vitro when co-treated with an ATM inhibitor (Figure 3D–F) and demonstrate that ATM inhibitors can be combined with Bragg peak (i.e. LET optimized) proton therapy to achieve enhanced tumor control in vivo.
Proton Bragg peak irradiation is more effective in BRCA1 deficient cells and tumors
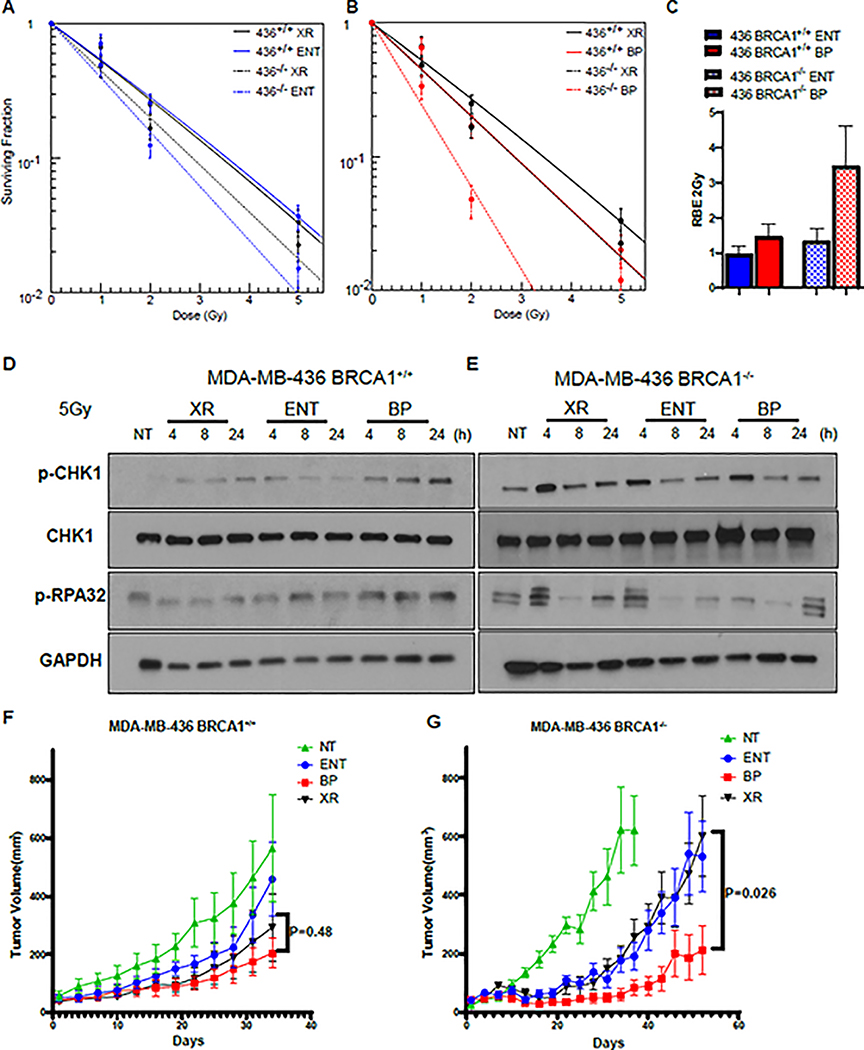
BRCA1 is a central HR factor that antagonizes 53BP1’s function of protecting DNA ends in order to promote end-resection during the S and G2 of the cell cycle[16, 42]. BRCA1 also has an additional prominent role at a later stage of HR in promoting RAD51 recruitment, in collaboration with BRCA2[16]. Studies have suggested that deficiencies in DSB repair by HR may also render cells more sensitive to protons, but the impact of BRCA1 and other HR genes on the relationship between LET and RBE is not well established [43–45]. In addition, whether the elevated LET of proton Bragg peak irradiation can be harnessed to more effectively target HR deficient tumors in vivo has not been addressed. In order to evaluate the impact of BRCA1 on the relationship between LET and RBE we irradiated BRCA1 mutant MDA-MB-436 (BRCA1−/−) breast cancer cells and the wild-type BRCA1 complemented MDA-MB-436 (BRCA1 +/+) isogenic pair with photons or protons at entrance or the Bragg peak and evaluated cell survival with clonogenic assays. While cell kill was modestly increased in BRCA1−/− cells following photon or proton entrance beam irradiation, there was a marked increase in cell kill with Bragg peak irradiation (RBE2Gy = 3.5) in the BRCA1−/−, but not in the BRCA1+/+ sub-line (RBE2Gy = 1.5, Figure 6A–C). BRCA1+/+ cells treated with Bragg peak irradiation, but not BRCA1−/− cells, exhibited upregulated phosphorylated CHK1 and RPA32 following Bragg peak irradiation, consistent with defective end resection in Bragg peak irradiated BRCA1−/− cells (Figure 6D–E)[16]. We further observed enhanced proton Bragg peak RBE when we irradiated the BRCA2 mutant Chinese hamster cell line, VC8, relative to its wild-type isogenic pair, V79, and following knockdown of RAD51 in U2OS cells (Supplementary Figure 5A–D). ATM inhibitor co-treatment of HR deficient VC8 did not enhance the Bragg peak RBE (Supplementary Figure 5F). These results suggested that ATM inhibition and HR deficiency may be epistatic with respect to proton Bragg peak RBE enhancement.
Figure 6. The RBE of proton Bragg peak irradiation is enhanced in BRCA1 deficient tumor cells.
A-B, MDA-MB-436 BRCA1+/+ and BRCA−/− sub-lines were irradiated with 6MV photons or 76.8 MeV protons delivered at the entrance (ENT) or the Bragg peak (BP) of the proton beam profile and clonogenic survival was assessed, (mean ± SEM) from three independent experiments are plotted. C The proton entrance and Bragg peak RBE2Gy for the experiments in A-B was quantified. D, E MDA-MB-436 BRCA1+/+ and BRCA1−/− cells were irradiated with 5 Gy using 6 MV photons(X-ray, XR) or 76.8 MeV protons delivered at ENT or the BP. Lysates were collected at the indicated time points and Western blot analysis was performed using the indicated antibodies. Tubulin was used as a loading control. F,G MDA-MB-436 BRCA1+/+ cells (D) or MDA-MB-436 BRCA1−/− cells were subcutaneously injected into the midline of NOD-SCID mice. After tumors reached 50mm3 animals were randomized to sham (no treatment [NT]), or 5 Gy delivered with 6 MV photons (XR), 76.8 MeV protons at entrance (ENT), or 76.8 MeV protons at the Bragg peak (BP) (LET-optimized protons) in a single fraction. Tumor volume was assessed over time. Shown are the representative data (mean ± SEM) from biologically independent samples (n=9). P values are displayed for the comparison of x-rays and Bragg peak protons obtained using the two-sided unpaired t-test.
In order to determine whether tumor inherent HR deficiency could also enhance the RBE of proton Bragg peak irradiation in vivo, we next injected MDA-MB-436 (BRCA1−/−) and wild-type BRCA1 complemented MDA-MB-436 (BRCA1 +/+) cells subcutaneously into nude mice and irradiated established tumors with 5 Gy using the same system of photon, proton entrance, and proton Bragg peak (LET-optimized) plans, as described above. As displayed in Figure 6F, regrowth delay of MDA-MB-436 (BRCA1+/+) tumors was comparable when tumors were irradiated with photons, proton entrance or proton Bragg peak plans (p=0.48). In contrast, the tumor regrowth delay was significantly greater following Bragg peak irradiation, but not proton entrance irradiation, in the BRCA1−/− tumors (Figure 6G, p=0.026). Together, these findings suggested that LET-optimized proton therapy is feasible, and when used to treat HR-deficient tumors, may be a tumor specific radiosensitizing strategy that can potentially improve tumor control.
Discussion
In the present study, we discovered that the characteristic DNA damage and distinct downstream signaling induced by elevated LET proton Bragg peak irradiation can be exploited, in combination with a selective inhibitor of ATM, to enhance the efficacy of therapy. Our data suggests that under physiologic conditions a greater number of DNA DSBs induced by Bragg peak irradiation preferentially undergo resection and repair by HR. In contrast, ATM inhibition shunts these lesions into the error-prone and toxic NHEJ repair pathway, thereby enhancing the proton Bragg peak RBE. Consistently, Bragg peak irradiation provides marked proton RBE enhancement in HR deficient, but not HR proficient cancer models. We have previously shown that LET-optimized (Bragg peak) proton plans can be generated to increase the proton LET within tumor targets while avoiding normal tissues[40]. Our results reported here support a potential paradigm for precision medicine with LET-optimized proton therapy. Our findings suggest the therapeutic ratio may be enhanced by selecting HR deficient tumors for LET-optimized proton therapy because non-cancerous cells have intact HR and would be expected to be less vulnerable to the damage induced by LET-optimized protons. Further, LET-optimized proton therapy could be combined with an ATM inhibitor to enhance the RBE and overcome therapeutic resistance in HR proficient malignancies.
Our studies have also highlighted a potential mechanism of resistance to DNA damage induced by Bragg peak proton irradiation. Indeed, the enhanced proton Bragg peak RBE with co-administration of an ATM inhibitor was abolished when the DNA ligation step of NHEJ was inactivated by loss of XRCC4, enabling repair to eventually proceed by HR. Interestingly, the enhanced efficacy of proton Bragg peak irradiation was still lost in the absence of XRCC4 despite a substantial delay in resection and HR activation, as assessed by RPA and RAD51 foci induction. In addition, ATR facilitates repair by HR[37], and ATR pathway signaling was upregulated following Bragg peak proton irradiation. However, ATR inhibition unexpectedly did not enhance the proton Bragg peak RBE. Prior work has demonstrated that NHEJ rates are unchanged in the absence of ATR kinase activity[46]. Collectively, our findings suggest that the DNA ligation step of NHEJ, rather than an inability to recruit RAD51, may be the key determinant of the observed enhancement in the Bragg peak irradiation RBE. These results are consistent with the work of Balmus et al., who demonstrated resistance to inhibitors of TOP1 and PARP when NHEJ was prevented in ATM-deficient cells[22]. Whether the efficacy of proton Bragg peak irradiation can also be enhanced or abrogated by manipulating other key regulators of DNA DSB repair pathway choice is an important area of further investigation[47–49].
Prior in vitro work has suggested that HR deficiency from loss of XRCC3, BRCA2, RAD51, SLX, and MUS81, FANCA, BRCA1, and FANCD2 may render cells more susceptible to proton therapy [43–45, 48]. By irradiating cells at both the entrance and the Bragg peak of the proton beam profile, we were able to examine the relationship between LET and RBE in HR deficient and HR proficient models. We observed marked RBE enhancement in HR deficient models in the LET range of proton Bragg peak irradiation, but little if any enhancement at the entrance of the proton beam profile. These findings suggest that planning strategies which maximize the number of Bragg peaks deposited within the tumor, termed LET-optimized proton therapy, will be most efficacious in treating HR deficient tumors[40, 50]. Of note, for consistency we used 76.8 MeV beams for the in vitro studies reported here. However, we have previously reported comparably enhanced RBE at the Bragg peak using a range of proton energies that may be used clinically, including a 160 MeV beam[25]. Collectively, our in vitro and in vivo studies demonstrating enhanced efficacy of proton Bragg peak irradiation in the setting of HR deficiency provide strong justification for the prospective evaluation of LET-optimized proton therapy in HR deficient tumors in the clinic.
Many HR deficient tumors result from bi-allelic alterations in HR genes, whereas surrounding normal tissues typically have a ‘single hit’ which does not typically result in clinically significant deficiency in HR function[51, 52]. This discrepancy in DNA repair capacity between tumor and normal tissues forms the basis of the exquisite sensitivity of PARP inhibition in multiple HR-deficient tumor types, as well the excellent tolerability of these agents[24, 53]. Consistently, we observed markedly less enhancement in the RBE of Bragg peak irradiation in HR proficient, compared with HR deficient models. Along similar lines, co-administration of an ATM inhibitor with LET-optimized protons appeared to enhance the RBE to the greatest extent in the cancerous lines assessed, which have a higher level of genomic instability than non-cancerous cells. Nevertheless, given the profound effects of ATM inhibitors as radiosensitizers and the potential for increased toxicity, investigation of this combination strategy will likely be most appropriate in therapeutically resistant tumors that have poor outcomes with traditional radiotherapy. Importantly, proton therapy enables highly conformal radiotherapy plans with limited dose deposition into normal tissues outside of the target volume. Carefully designed phase 1b studies with adequate follow-up to assess for tolerability will be needed to identify the most appropriate dosing regimens for the ATM inhibitor + LET optimized proton therapy combination.
Emerging data suggests that loss of Fanconi anemia genes results in hypersensitivity to ATM inhibition[54]. Whether tumors with Fanconi anemia pathway or other genetic alterations could provide additional tumor specificity with the ATM inhibition plus Bragg peak irradiation combination warrants additional investigation. Further work is necessary to identify tumors with true functional and targetable repair deficiencies at the time clinical decision making in order optimize the selection of patients for clinical trials investigating LET-optimized protons, PARP inhibition, and other potential repair targeted therapies[51, 55–57].
Carbon ion therapy is being investigated in centers in Asia and Europe as the potential higher LETd achievable with carbon ions (>100 keV/μm) compared with photons (0.2 keV/μm) or protons (1–10 keV/μm) is hypothesized to provide superior anti-tumor effects for treating otherwise highly radio-resistant tumors[58]. However, patient access to carbon ion therapy is exceptionally limited. Notably, we achieved ‘carbon-like’ RBE enhancement (i.e. RBE 2–3) with proton Bragg peak irradiation in HR-deficient tumors and HR-proficient tumors co-treated with an ATM inhibitor. Given the high costs associated with carbon ion therapy, an important area for future research will be to determine whether the combination of LET-optimized proton therapy with an ATM inhibitor can achieve comparable or improved disease control outcomes in radiation resistant malignancies. Prior studies have also suggested that the more complex DNA damage induced by higher LET particles such as carbon may promote resection, ATR pathway activation, and HR[32, 59, 60]. The close proximity of DNA DSBs induced by high LET particles may lead to reduced Ku and DNAPKcs binding and inefficient DNAPK kinase activation at these shorter fragments [61, 62]. Whether ATM inhibition can further enhance the RBE of carbon ion therapy, or whether the RBE enhancement observed in our studies is unique to the intermediate LET range achievable with proton Bragg peak irradiation requires additional investigation[63–65].
In summary, tumors with genetic or epigenetic defects in HR or co-administration of an ATM inhibitor in repair proficient tumors present a new opportunity for precision medicine in the field of particle therapy. We are designing clinical trials to target the DNA damage response and exploit the unique relationship between LET and RBE of proton Bragg peak irradiation.
Supplementary Material
Statement of Significance.
Co-administration of an ATM inhibitor re-wires DNA repair machinery to render cancer cells uniquely hypersensitive to DNA damage induced by the proton Bragg peak, which is characterized by higher density ionization.
Acknowledgments
The authors would like to acknowledge Astra Zeneca for provision of pharmaceutical agents and to Stephen Durant for scientific input and dosing recommendations. The authors would also like to acknowledge Ann C. Tuma, Gasper J. Kitange, Katrina K. Bakken, Danielle Burgenske, Brett L. Carlson, Mark A. Schroeder, and Shiv K. Gupta for their assistance with experimental techniques, and radiation therapists Courtney Vinsand, Jill Frericks, Adam Boyer, Brooke Tebo, Alex Kehren, Tracy Gadient, Mackenzie Breedon, Jaimee Sullivan, Nicole Johnson, Rashad Momoh and Kristen Dezell for their assistance with proton irradiation. This work was supported in part by R01 CA203561 (ZL), and the American Society for Radiation Oncology, the National Cancer Institute of the National Institutes of Health under Award Number P50CA116201, the Mayo Clinic Research Pipeline K2R Award and K12 HD065987 (RWM).
Footnotes
The authors report no conflicts of interest to disclose
References
- 1.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–253. [DOI] [PubMed] [Google Scholar]
- 2.Weber DC, Habrand JL, Hoppe BS, Hill Kayser C, Laack NN, Langendijk JA et al. Proton therapy for pediatric malignancies: Fact, figures and costs. A joint consensus statement from the pediatric subcommittee of PTCOG, PROS and EPTN. Radiother Oncol 2018; 128: 44–55. [DOI] [PubMed] [Google Scholar]
- 3.Prise KM, Ahnstrom G, Belli M, Carlsson J, Frankenberg D, Kiefer J et al. A review of dsb induction data for varying quality radiations. Int J Radiat Biol 1998; 74: 173–184. [DOI] [PubMed] [Google Scholar]
- 4.Pastwa E, Neumann RD, Mezhevaya K, Winters TA. Repair of radiation-induced DNA double-strand breaks is dependent upon radiation quality and the structural complexity of double-strand breaks. Radiat Res 2003; 159: 251–261. [DOI] [PubMed] [Google Scholar]
- 5.Paganetti H, Blakely E, Carabe-Fernandez A, Carlson DJ, Das IJ, Dong L et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys 2019; 46: e53–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickoloff JA, Sharma N, Taylor L. Clustered DNA Double-Strand Breaks: Biological Effects and Relevance to Cancer Radiotherapy. Genes (Basel) 2020; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paganetti H Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014; 59: R419–472. [DOI] [PubMed] [Google Scholar]
- 8.Cuaron JJ, Chang C, Lovelock M, Higginson DS, Mah D, Cahlon O et al. Exponential Increase in Relative Biological Effectiveness Along Distal Edge of a Proton Bragg Peak as Measured by Deoxyribonucleic Acid Double-Strand Breaks. Int J Radiat Oncol Biol Phys 2016; 95: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones B, Wilson P, Nagano A, Fenwick J, McKenna G. Dilemmas concerning dose distribution and the influence of relative biological effect in proton beam therapy of medulloblastoma. Br J Radiol 2012; 85: e912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeler CR, Mirkovic D, Titt U, Blanchard P, Gunther JR, Mahajan A et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol 2016; 121: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giantsoudi D, Adams J, MacDonald SM, Paganetti H. Proton Treatment Techniques for Posterior Fossa Tumors: Consequences for Linear Energy Transfer and Dose-Volume Parameters for the Brainstem and Organs at Risk. Int J Radiat Oncol Biol Phys 2017; 97: 401–410. [DOI] [PubMed] [Google Scholar]
- 12.Underwood TSA, Grassberger C, Bass R, MacDonald SM, Meyersohn NM, Yeap BY et al. Asymptomatic Late-phase Radiographic Changes Among Chest-Wall Patients Are Associated With a Proton RBE Exceeding 1.1. Int J Radiat Oncol Biol Phys 2018; 101: 809–819. [DOI] [PubMed] [Google Scholar]
- 13.Mutter RW, Jethwa KR, Wan Chan Tseung HS, Wick SM, Kahila MM, Viehman JK et al. Incorporation of Biologic Response Variance Modeling Into the Clinic: Limiting Risk of Brachial Plexopathy and Other Late Effects of Breast Cancer Proton Beam Therapy. Pract Radiat Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan Chan Tseung H, Ma J, Beltran C. A fast GPU-based Monte Carlo simulation of proton transport with detailed modeling of nonelastic interactions. Med Phys (Research Support, Non-U.S. Gov’t) 2015; 42: 2967–2978. [DOI] [PubMed] [Google Scholar]
- 15.Pannunzio NR, Watanabe G, Lieber MR. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J Biol Chem 2018; 293: 10512–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen CC, Feng W, Lim PX, Kass EM, Jasin M. Homology-Directed Repair and the Role of BRCA1, BRCA2, and Related Proteins in Genome Integrity and Cancer. Annu Rev Cancer Biol 2018; 2: 313–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell 2010; 40: 179–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer 2016; 16: 110–120. [DOI] [PubMed] [Google Scholar]
- 19.Kovac M, Blattmann C, Ribi S, Smida J, Mueller NS, Engert F et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nature Communications 2015; 6: 8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng X, Pleasance E, Zhao EY, Ng T, Grewal JK, Mohammad N et al. Therapeutic Implication of Genomic Landscape of Adult Metastatic Sarcoma. JCO precision oncology 2019: 1–25. [DOI] [PubMed] [Google Scholar]
- 21.Heeke AL, Pishvaian MJ, Lynce F, Xiu J, Brody JR, Chen WJ et al. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO precision oncology 2018; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmus G, Pilger D, Coates J, Demir M, Sczaniecka-Clift M, Barros AC et al. ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks. Nat Commun 2019; 10: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrassa L, Damia G. DNA damage response inhibitors: Mechanisms and potential applications in cancer therapy. Cancer Treat Rev 2017; 60: 139–151. [DOI] [PubMed] [Google Scholar]
- 24.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med 2017; 377: 523–533. [DOI] [PubMed] [Google Scholar]
- 25.Howard M, Beltran C, Sarkaria J, Herman MG. Characterization of relative biological effectiveness for conventional radiation therapy: a comparison of clinical 6 MV X-rays and 137Cs. J Radiat Res 2017; 58: 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetz MP, Kalari KR, Suman VJ, Moyer AM, Yu J, Visscher DW et al. Tumor Sequencing and Patient-Derived Xenografts in the Neoadjuvant Treatment of Breast Cancer. J Natl Cancer Inst 2017; 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Qin B, Moyer AM, Sinnwell JP, Thompson KJ, Copland JA 3rd et al. Establishing and characterizing patient-derived xenografts using pre-chemotherapy percutaneous biopsy and post-chemotherapy surgical samples from a prospective neoadjuvant breast cancer study. Breast Cancer Res 2017; 19: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard ME, Beltran C, Anderson S, Tseung WC, Sarkaria JN, Herman MG. Investigating Dependencies of Relative Biological Effectiveness for Proton Therapy in Cancer Cells. Int J Part Ther 2018; 4: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Report 16. Journal of the International Commission on Radiation Units and Measurements 2016; os9(1). [Google Scholar]
- 30.Paganetti H Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014; 59: R419–472. [DOI] [PubMed] [Google Scholar]
- 31.Surovtseva YV, Jairam V, Salem AF, Sundaram RK, Bindra RS, Herzon SB. Characterization of Cardiac Glycoside Natural Products as Potent Inhibitors of DNA Double-Strand Break Repair by a Whole-Cell Double Immunofluorescence Assay. J Am Chem Soc 2016; 138: 3844–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Averbeck NB, Ringel O, Herrlitz M, Jakob B, Durante M, Taucher-Scholz G. DNA end resection is needed for the repair of complex lesions in G1-phase human cells. Cell Cycle 2014; 13: 2509–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee B, Tomimatsu N, Burma S. Immunofluorescence-based methods to monitor DNA end resection. Methods Mol Biol 2015; 1292: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saldivar JC, Cortez D, Cimprich KA. The essential kinase ATR: ensuring faithful duplication of a challenging genome. Nat Rev Mol Cell Biol 2017; 18: 622–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy AK, Fitzgerald M, Ro T, Kim JH, Rabinowitsch AI, Chowdhury D et al. Phosphorylated RPA recruits PALB2 to stalled DNA replication forks to facilitate fork recovery. J Cell Biol 2014; 206: 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vassin VM, Anantha RW, Sokolova E, Kanner S, Borowiec JA. Human RPA phosphorylation by ATR stimulates DNA synthesis and prevents ssDNA accumulation during DNA-replication stress. J Cell Sci 2009; 122: 4070–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buisson R, Niraj J, Rodrigue A, Ho CK, Kreuzer J, Foo TK et al. Coupling of Homologous Recombination and the Checkpoint by ATR. Mol Cell 2017; 65: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 2013; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balmus G, Pilger D, Coates J, Demir M, Sczaniecka-Clift M, Barros AC et al. ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks. Nature communications 2019; 10: 87–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan Chan Tseung HS, Ma J, Kreofsky CR, Ma DJ, Beltran C. Clinically Applicable Monte Carlo-based Biological Dose Optimization for the Treatment of Head and Neck Cancers With Spot-Scanning Proton Therapy. Int J Radiat Oncol Biol Phys 2016; 95: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 41.Tu X, Kahila MM, Zhou Q, Yu J, Kalari KR, Wang L et al. ATR Inhibition Is a Promising Radiosensitizing Strategy for Triple-Negative Breast Cancer. Mol Cancer Ther 2018; 17: 2462–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol 2014; 15: 7–18. [DOI] [PubMed] [Google Scholar]
- 43.Liu Q, Underwood TSA, Kung J, Wang M, Lu HM, Paganetti H et al. Disruption of SLX4-MUS81 Function Increases the Relative Biological Effectiveness of Proton Radiation. Int J Radiat Oncol Biol Phys 2016; 95: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, Ghosh P, Magpayo N, Testa M, Tang S, Gheorghiu L et al. Lung cancer cell line screen links fanconi anemia/BRCA pathway defects to increased relative biological effectiveness of proton radiation. Int J Radiat Oncol Biol Phys 2015; 91: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 45.Grosse N, Fontana AO, Hug EB, Lomax A, Coray A, Augsburger M et al. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys 2014; 88: 175–181. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, Wang H, Powell SN, Iliakis G, Wang Y. ATR affecting cell radiosensitivity is dependent on homologous recombination repair but independent of nonhomologous end joining. Cancer Res 2004; 64: 7139–7143. [DOI] [PubMed] [Google Scholar]
- 47.Bunting SF, Callen E, Wong N, Chen HT, Polato F, Gunn A et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 2010; 141: 243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fontana AO, Augsburger MA, Grosse N, Guckenberger M, Lomax AJ, Sartori AA et al. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother Oncol 2015; 116: 374–380. [DOI] [PubMed] [Google Scholar]
- 49.Noordermeer SM, Adam S, Setiaputra D, Barazas M, Pettitt SJ, Ling AK et al. The shieldin complex mediates 53BP1-dependent DNA repair. Nature 2018; 560: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao W, Khabazian A, Yepes PP, Lim G, Poenisch F, Grosshans DR et al. Linear energy transfer incorporated intensity modulated proton therapy optimization. Phys Med Biol 2017; 63: 015013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mutter RW, Riaz N, Ng CK, Delsite R, Piscuoglio S, Edelweiss M et al. Bi-allelic alterations in DNA repair genes underpin homologous recombination DNA repair defects in breast cancer. J Pathol 2017; 242: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riaz N, Blecua P, Lim RS, Shen R, Higginson DS, Weinhold N et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat Commun 2017; 8: 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med 2019; 381: 2391–2402. [DOI] [PubMed] [Google Scholar]
- 54.Cai MY, Dunn CE, Chen W, Kochupurakkal BS, Nguyen H, Moreau LA et al. Cooperation of the ATM and Fanconi Anemia/BRCA Pathways in Double-Strand Break End Resection. Cell Rep 2020; 30: 2402–2415 e2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Longo DL. Personalized Medicine for Primary Treatment of Serous Ovarian Cancer. N Engl J Med 2019; 381: 2471–2474. [DOI] [PubMed] [Google Scholar]
- 56.Davies H, Glodzik D, Morganella S, Yates LR, Staaf J, Zou X et al. HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med 2017; 23: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sztupinszki Z, Diossy M, Krzystanek M, Borcsok J, Pomerantz MM, Tisza V et al. Detection of Molecular Signatures of Homologous Recombination Deficiency in Prostate Cancer with or without BRCA1/2 Mutations. Clin Cancer Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vogin G, Wambersie A, Koto M, Ohno T, Uhl M, Fossati P et al. A step towards international prospective trials in carbon ion radiotherapy: investigation of factors influencing dose distribution in the facilities in operation based on a case of skull base chordoma. Radiat Oncol 2019; 14: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okayasu R, Okada M, Okabe A, Noguchi M, Takakura K, Takahashi S. Repair of DNA damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-joining pathway. Radiat Res 2006; 165: 59–67. [DOI] [PubMed] [Google Scholar]
- 60.Xue L, Furusawa Y, Okayasu R, Miura M, Cui X, Liu C et al. The complexity of DNA double strand break is a crucial factor for activating ATR signaling pathway for G2/M checkpoint regulation regardless of ATM function. DNA Repair (Amst) 2015; 25: 72–83. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Zhang X, Wang P, Yu X, Essers J, Chen D et al. Characteristics of DNA-binding proteins determine the biological sensitivity to high-linear energy transfer radiation. Nucleic Acids Res 2010; 38: 3245–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pang D, Winters TA, Jung M, Purkayastha S, Cavalli LR, Chasovkikh S et al. Radiation-generated short DNA fragments may perturb non-homologous end-joining and induce genomic instability. J Radiat Res 2011; 52: 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gerelchuluun A, Manabe E, Ishikawa T, Sun L, Itoh K, Sakae T et al. The major DNA repair pathway after both proton and carbon-ion radiation is NHEJ, but the HR pathway is more relevant in carbon ions. Radiat Res 2015; 183: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitajima S, Nakamura H, Adachi M, Ijichi K, Yasui Y, Saito N et al. AT cells show dissimilar hypersensitivity to heavy-ion and X-rays irradiation. J Radiat Res 2010; 51: 251–255. [DOI] [PubMed] [Google Scholar]
- 65.Xue L, Yu D, Furusawa Y, Cao J, Okayasu R, Fan S. ATM-dependent hyper-radiosensitivity in mammalian cells irradiated by heavy ions. Int J Radiat Oncol Biol Phys 2009; 75: 235–243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.