Abstract
Stromal fibrosis activates pro-survival and pro-epithelial-to-mesenchymal transition (EMT) pathways in pancreatic ductal adenocarcinoma (PDAC). In patient tumors treated with neoadjuvant stereotactic body radiation therapy (SBRT), we found upregulation of fibrosis, extracellular matrix (ECM), and EMT gene signatures, which can drive therapeutic resistance and tumor invasion. Molecular, functional, and translational analysis identified two cell surface proteins, A disintegrin and metalloprotease 10 (ADAM10) and ephrinB2, as drivers of fibrosis and tumor progression after RT. RT resulted in increased ADAM10 expression in tumor cells, leading to cleavage of ephrinB2, which was also detected in plasma. Pharmacologic or genetic targeting of ADAM10 decreased RT-induced fibrosis and tissue tension, tumor cell migration, and invasion, sensitizing orthotopic tumors to radiation killing and prolonging mouse survival. Inhibition of ADAM10 and genetic ablation of ephrinB2 in fibroblasts reduced the metastatic potential of tumor cells after RT. Stimulation of tumor cells with EphrinB2 FC-protein reversed the reduction in tumor cell invasion with ADAM10 ablation. These findings represent a model of PDAC adaptation that explains resistance and metastasis after radiation therapy and identifies a targetable pathway to enhance RT efficacy.
Introduction:
Pancreatic adenocarcinoma (PDAC) is a deadly malignancy with poor outcomes despite advances in surgery, radiation, and chemotherapy (1). It is characterized by acellular tumors with a desmoplastic stroma containing a complex milieu of extracellular matrix (ECM), cancer-associated fibroblasts, pro- and anti-inflammatory immune cells, and tumor vasculature(2). This stromal microenvironment has been shown to have a major impact on patient survival, with multiple groups discovering stromal gene signatures that portend a poor prognosis in PDAC patients (3–5). The stromal component of PDACs leads to increased tissue tension, which has been shown to activate cellular pathways in tumor cells leading to more aggressive tumor behavior and resistance to chemo- and radiotherapy (6,7). Additionally, extracellular collagens deposited during fibrosis have been shown to activate pathways driving epithelial to mesenchymal transition (EMT) and metastasis (8).
Despite trials with older adjuvant radiation therapy methods demonstrating a survival disadvantage using adjuvant radiation therapy (RT) for PDAC (9), neoadjuvant stereotactic body radiation therapy (SBRT) is frequently used to facilitate surgery in patients with borderline resectable pancreatic cancer, where surgery remains the only curative option(10). Though its optimal role, technique, and efficacy are still being investigated, SBRT has been used in the locally advanced pancreatic setting with favorable local control and overall survival outcomes, particularly when higher of doses of radiation can be delivered to the tumor using advanced techniques(11). The ALLIANCE A021501 trial just recently reported inferior outcomes with neoadjuvant SBRT in addition to neoadjuvant chemotherapy(12,13), raising the concern that even with modern delivery, the aggressive biology of PDAC may overcome the cytotoxic effect of SBRT, with radiation even stimulating more aggressive behavior. This stands in contrast though with smaller series delivering much higher doses of RT using new image guided adaptive techniques, which have achieved preliminary outcomes far surpassing historical controls(14) and suggest its ideal implementation is still being optimized. Fibrosis is a known sequela of RT (15), and in PDAC where fibrosis contributes to aggressive behavior and therapeutic resistance, this early cytotoxic benefit may be partially offset by an increase in resistance and EMT in residual tumor clonogens. Fibrosis has an impact on immune cell infiltration, which alters the efficacy of RT mediated tumor killing. Late tissue fibrosis after RT can also increase the technical difficulty of surgery, and is known to contribute to normal tissue toxicity in other cancer types(16). Targeting pathways of fibrosis common to PDAC tumors and the effects of RT presents the possibility of enhancing the efficacy of SBRT and improving oncologic outcomes while reducing treatment toxicity.
The Ephrin receptor proteins (EPHs) are the largest family of receptor tyrosine kinases (RTK) that, along with their membrane bound ligands, the ephrins, mediate a large variety of developmental processes and have been implicated in carcinogenesis, particularly angiogenesis, but also fibrosis, EMT, and immune infiltration(17). We have shown that ephrinB2 expression confers poor prognosis in pancreatic cancer (18), and that inhibition of the interaction between ephrinB2 and its cognate receptor, EphB4, reduces RT-induced fibrosis and enhance tumor killing by RT in PDAC xenograft tumors (18). Recent studies have shown that cleavage of the ephrin-B2 ectodomain by the A Disintegrin and Metalloproteinase domain-containing protein 10 (ADAM10) results in the generation of a pro-fibrotic, soluble ephrin-B2 ectodomain that drives fibroblast activation in lung, skin, and cardiac fibrosis (19,20). Previous studies have shown that ADAM10 is involved in EMT and metastasis in colorectal (21) and breast cancer (22). In addition, ADAM10 has been shown to be increased in vascular endothelium in response to RT (23). These together suggest a role for ADAM10 in driving fibrogenesis and EMT following RT, prompting us to investigate this pathway in the context of SBRT-induced tumor fibrosis in PDAC. Here, we investigate the role of the ADAM10-ephrinB2 pathway in driving pancreatic tumor fibrosis following SBRT and the therapeutic potential of targeting ADAM10 to inhibit fibrosis and tumor progression.
We demonstrate upregulation of gene expression signatures associated with fibrosis and EMT in 29 human patient PDAC tumor samples treated with SBRT. Patients with high expression of ADAM10 and ephrinB2 following SBRT have a worse prognosis than those with lower levels. Mechanistically, we demonstrate that ADAM10 is upregulated in tumor cells following SBRT in PDAC cells in vitro and in vivo, and that pharmacologic inhibition as well as genetic knockout of ADAM10 abrogates post-radiation fibrosis and delays tumor progression in orthotopic and metastatic mouse models of PDAC. We found that ADAM10 knockout prevents proteomic changes consistent with matrisome activation after RT, and ADAM10 deficient cells have a reduction in fibroblast dependent migration and invasion which reciprocally is stimulated by the addition of ephrinB2 FC protein. We also detect soluble ephrinB2 in plasma samples collected from our in vivo models, as well as in pancreatic cancer patients treated with SBRT on clinical trial NCT02873598, in a dose-dependent manner. These data suggest a utility for anti-fibrotic therapy targeting ADAM10 to block RT-induced fibrosis and improve outcomes in PDAC, as well as ephrinB2 a serum marker for RT-induced fibrosis that could direct treatment selection and monitor therapeutic efficacy.
Materials and methods:
RNA Seq
For RNA Seq library preparation, a TempO-Seq FFPE Assay 96 sample kit was used (BioSpyder,Carlsbad, CA). FFPE slides of FNA biopsy and post-neoadjuvant patient samples were obtained from the University of Colorado biorepository. All the patients had borderline resectable pancreatic cancer, treated with neoadjuvant chemotherapy followed by restaging and treatment with 30–33.6Gy SBRT to pancreatic tumors, followed by pancreaticoduodenectomy and adjuvant chemotherapy. Tumor samples were reviewed by a pathologist, and areas of tumor cellularity identified and marked. Areas of tumor cellularity were scraped and processed per above BioSpyder standard protocol. Samples were pooled and run in 2 sequencing lanes using NextSeq high throughput sequencing instrument (Illumina, San Diego, CA). Reads were aligned and counts generated using Biospyder TempoSeqr platform. Genes with <1 mean raw counts or <1 mean counts per million (CPM) were removed from the dataset. Differential expression was calculated using the limma R package (24). The resulting fold-change was used with the fgsea R package (25) to perform gene set enrichment analysis for Hallmark and C2 Curated gene sets (26). Heatmap displaying Z-score transformed gene expression was generated using the ComplexHeatmap R package (27) (v1.20.0) and hierarchical clustering using Euclidean distance with complete linkage. CPM normalized gene expression of post-SBRT samples was used to group patients into high- and low-expression groups using a median split for ADAM10 and EFNB2. Survival was calculated using the survival R package, and significance was calculated with a log-rank test.
TCGA analysis
Gene expression data was obtained from The Cancer Genome Atlas for 179 pancreatic adenocarcinoma patients. Expression of ephrinB2 and ADAM10 were plotted and separated into groups having above or below median expression. Overall survival and disease-free survival for these groups were calculated using Kaplan-Meier method, using log rank tests for comparisons of groups.
Cell Culture
Mouse KPC pancreatic cancer cell lines FC1242 and PK5L1940 were passaged in RPMI1640 supplemented with 10% FBS. Mouse NIH-3T3 fibroblasts were passaged in RPMI1640 supplemented with 10% FBS. All cells were passaged every 2–3 days when sub-confluent, at a density of 1:3–1:8. Cells were not allowed to grow beyond passage 15. PK5L1940 cells were kindly provided by laboratory of Michael Gough (Earle A. Chiles Research Institute, Providence Cancer Institute). Mycoplasma testing performed by University of Colorado core facility every 3 months.
ADAM10 inhibitor
ADAM10 inhibitor GI254023X was obtained from Sigma Aldrich(St Louis, MO). Inhibitor was added in in vitro culture experiments at a concentration of 20nM. In in vivo mouse experiments, inhibitor was delivered by intraperitoneal injection at a concentration of 20mg/kg, every 72 hours.
CRISPR Knockout
The following oligos were used for ADAM10 gRNA: Adam10-For--caccGGTTTCATCAAGACTCGTGG Adam10-Rev—aaacCCACGAGTCTTGATGAAACC. The oligos were annealed and cloned into BbsI-digested PX458 (Addgene Plasmid #48138). The ligation were transformed into NEB® Stable Competent E. coli (NEB Cat# C3040H) and plated on ampicillin-agar plates. Plasmid from colonies were sequenced using the U6-for primer: gagggcctatttcccatgattcc. PX458-gRNA plasmids were transfected into FC1242, PK5L1940 and NIH-3T3 cells and flow sorted for GFP expression. GFP positive clones were grown in culture and knockout was confirmed by western blotting.
Human tissue and trial specimens
Informed written consent was obtained for all tumor sample collection, studies were performed in accordance with U.S. Common Rule, and approved by institutional review board. Patient archival tumor samples were identified and obtained from biorepository, collected per COMIRB13–0315. Patients were selected from all borderline resectable pancreatic cancer patients seen through University of Colorado pancreas multidisciplinary clinic between 1/2013–12/2018, who were then treated with neoadjuvant chemotherapy and SBRT. SBRT doses were 30–33Gy in 5 fractions. All patients received either FOLFIRINOX or gemcitabine based chemotherapy per supplemental table 1. Following neoadjuvant therapy patients received surgery followed by further adjuvant chemotherapy. Surgical samples were scored for inflammation and fibrosis by pathologist.
Patient plasma samples were collected as part of a Phase I radiation dose escalation clinical trial (NCT02873598) before, during (6hr post) and post (6 weeks). Prior to SBRT these patients all received neoadjuvant chemotherapy. SBRT doses ranged from 9Gy x 3 fractions to 11Gy x3 fractions. Primary outcome measure of this study is MTD of SBRT, with local control, progression free survival, overall survival, small intestine changes and vascular and cellular changes as secondary endpoints. This study is still active. Samples were collected and analyzed per COMIRB19–0328.
Cell Migration Assays.
Cells were passaged to sub-confluency and then scratched using Incucyte cell scratch apparatus. Media was changed and cells were then cultured in Incucyte live cell imaging incubator and images were collected every 2 hours. Scratch confluency was calculated using Incucyte software (Essen Bioscience, Ann Arbor, MI).
Generation of KO Cell lines
Cell lines were generated by CRISPR-CAS9 KO using GFP expressing plasmids obtained from University of Colorado Functional Genomics facility. Cells were transfected with plasmid using standard Lipofectamine 2000 transfection, cells were then flow sorted and grown in individual colonies. Cell lysates were then screened by western blotting.
Protein lysate preparation and immunoblotting
PK5L1940 cells and FC1242 KPC Cells were lysed using RIPA lysis buffer (Millipore, Billerica, MA, USA), containing protease inhibitor cocktail (Thermo Fisher Scientific Inc., IL, USA) and phosphatase inhibitor (Sigma, MO, USA) on ice for 30 min. Homogenates were centrifuged at 4°C at 13,000 rpm for 20 min, and lysates collected. For western blotting, lysates (50 μg) were loaded onto 10% SDS-PAGE gels. Membranes were probed overnight at 4°C in 1% BSA in PBS-T. Anti-ADAM10 (Abcam 1997), Anti-ephrinB2 (Abcam 150411), Anti-EphB4 (generous gift from the Vasgene Therapeutics, Inc) and anti-Vimentin (Cell Signaling, Catalog #5741S) antibodies were used. Horseradish peroxidase (HRP)–conjugated secondary antibodies were obtained from Sigma (St. Louis, MO, USA).
Subcellular Fractionation
To determine the subcellular localization of ADAM10, cells were treated with 10 Gy and cultured for 7 days post-radiation. After 7 days, cells were scraped in cold PBS and subjected to subcellular fractionation (Thermo Scientific Cat #78840) according to the manufacturer’s protocol.
Immunohistochemistry
Tumors were harvested from flank xenograft experiments in which PK5L1940 cells were injected into the flanks of BL6 mice. Tumors were excised at the time of sacrifice and fixed in formalin for 24 hours. Formalin-fixed tumors were sliced and embedded tumor and mounted onto glass slides in paraffin. For immunohistochemical analysis, these mounted tissues were deparaffinized and hydrated followed by antigen retrieval as described earlier (28). Full IHC procedure described in supplementary methods.
Multiplexed IHC analysis was performed on Vectra platform (Caliper Life Sciences, Hopkinton, MA), described in detail in (29). Full multiplexed IHC analysis described in supplementary methods.
Picrosirius and Trichrome Staining
Tumors were harvested from flank xenograft experiments in which PK5L1940 cells were injected into the flanks of BL6 mice. These formalin-fixed tumors were sliced, embedded, and mounted onto glass slides in paraffin. Picrosirius staining and Trichrome staining were performed using standard conditions by University of Colorado Cancer Center Pathology Core. Picrosirius birefringence was imaged using Nikon microscope polarized filter. Birefringence signal was quantitated using ImageJ software.
EphrinB2 ELISA
ELISA was performed on human plasma samples obtained from patients with locally advanced pancreatic cancer on protocol COMIRB 16–1139. Mouse and Human ELISA kits were obtained from LSBio (LSBio, Seattle, WA). Standard kit protocol was used for ELISA assay. Described in further detail in supplementary methods.
Mass Spectrometry analysis
Briefly, snap frozen tumor samples of approximately 5mg were pulverized in liquid nitrogen. High salt extraction was used to make the cell fraction. Guanidine extraction was used to make the sECM fraction. Hydroxylamine extraction was used to make the iECM fraction. Fractions were digested with trypsin. 600ng of protein was analyzed nano-UHPLC-MS/MS (Easy-nLC1200, Orbitrap Fusion™ Lumos™Tribrid™, Thermo Fisher Scientific). Files were loaded into Proteome Discoverer 2.2 and were searched against Swissprot mouse and human database. In Excel, protein abundances from the three fractions were summed together per protein. Data was visualized in excel and MetaboAnalyst 4.0(30). Mass spectrometry analysis described in greater detail in Supplementary Methods.
Orthotopic and metastatic in vivo models and radiotherapy
Female C57BL6 (6 weeks old) were purchased from Jackson Laboratories (Indianapolis, In, USA). All the mice were cared for in accordance with the ethical guidelines and conditions set and overseen by the University of Colorado, Anschutz Medical Campus Animal Care and Use Committee. ADAM10-flox Pdgfra-Cre mice were obtained from David Lagares (Massachusetts General Hospital, Boston MA). The protocols used for animal studies were reviewed and approved by the Institutional Animal Care and Use committee at the University of Colorado, Anschutz Medical Campus.
For orthotopic experiments, The protocol is described in detail in (31). Described in further detail in supplementary methods.
Metastatic implantation is described in detail in (32). Described in further detail in supplementary methods.
Image-guided radiotherapy was performed using the X-Rad SmART small animal irradiator (Precision X-Ray, North Bradford CT) at 225kVp, 20mA with 0.3 mm Cu filter. For animal experiments, the mice were positioned in the prone orientation and a CT scan was acquired. Radiation was delivered at a dose rate of 5.6Gy/min. A single 16Gy dose of x-ray radiation was delivered to mouse pancreata using 10mm square beam with field edges at mouse midline and below left ribs. Described in further detail in supplementary methods.
ADAM10 Knockout Mice
We crossed mice with ADAM10 flanked by loxP sites (adam10loxP/loxP mice) (33) to mice that express a tamoxifen-inducible Cre recombinase driven by the mouse promoter of the platelet derived growth factor receptor, alpha polypeptide (PDGFRα-Cre-ERT transgenic mice)(34). Our results indicate that tamoxifen treatment of offspring that were homozygous for the ‘floxed’ adam10 allele and hemizygous for the PDGFαR-Cre-ERT transgene (adam10loxP/loxP;PDGFRα-Cre-ERT ), led to the deletion of the adam10 gene in pancreatic fibroblasts and the generation of adam10 conditional knockout mice, herein referred to as adam10-CKO mice. Described in further detail in supplementary methods.
Atomic Force Microscopy
Tumor tissue was fresh frozen, embedded in OCT. Atomic force microscopy (AFM) was performed on 20 μm LV sections. The nanomechanical and topographical properties of frozen, non-fixed tumor sections placed on glass slides were characterized using a NanoWizard® 4a (JPK Instruments, Carpenteria, CA, USA). Briefly, the samples were immersed in PBS for 15 min to remove OCT and allowed to thaw. The sections were then covered with protease inhibitor at 1X (HALT, Thermo Fisher Scientific) and tissue stiffness was determined using a qp-BioAC-1 (NanoandMore) cantilever with a force constant in the range of 0.15 to 0.55 N/m. Calibration of the cantilever was made using the thermal oscillation method before each experiment. Tissue mapping was performed by quantitative imaging (QITM) mode. Tumor sections were scanned in a 20 X 20 mm area using a set point of 3nN, a Z-length of 3.5 mm, and a pixel time of 30 ms with a resolution of 256 × 256 pixels. At least four scans were performed on each tumor section. The Hertz model was used to determine the mechanical properties of the tissues using the JPK software(35).
Establishment of PDAC tumor organoids.
All PDAC tumor specimens were obtained from University of Colorado Hospital patients with informed consent after approval by the ethical committee (COMIRB 08–0439). PDAC tumor organoids were established either from fresh surgical PDAC specimens or from patient-derived xenograft (PDX) models using established protocols with some modifications (Sato et al., Gastroenterology 2011 and Seino et al., Cell Stem Cell 2018). Once PDAC tumor organoids were established, short tandem repeat and mycoplasma analysis was performed, and stocks cryopreserved. Described in further detail in supplementary methods.
Organoid Viability Assay
Organoid viability was quantified using the CellTiter-Glo 3D Viability Assay (Cat #G9681) according to the manufacturer’s protocol. Briefly, cells were lysed and ATP levels, a marker of metabolic activity, were quantified to determine the number of viable cells.
Organoid Growth Assay
Organoids were plated at approximately 200 cells per well and cultured in an Incuctye Live-Cell Analysis incubator for 7 days with images collected every 4 hours. Organoid growth was quantified using Incucyte imaging software.
Statistical significance
In vitro experiments were conducted for a minimum of n=2 times in duplicates or triplicates. Quantitative analyses were performed using a Student’s t-test, Mann-Whitney test, One-Way ANOVA, or the Mantel-Cox test for survival using GraphPad Prism. p-values of <0.05 was considered statistically significant.
Results:
Neoadjuvant chemotherapy and SBRT upregulates EMT and fibrosis gene signatures in PDAC.
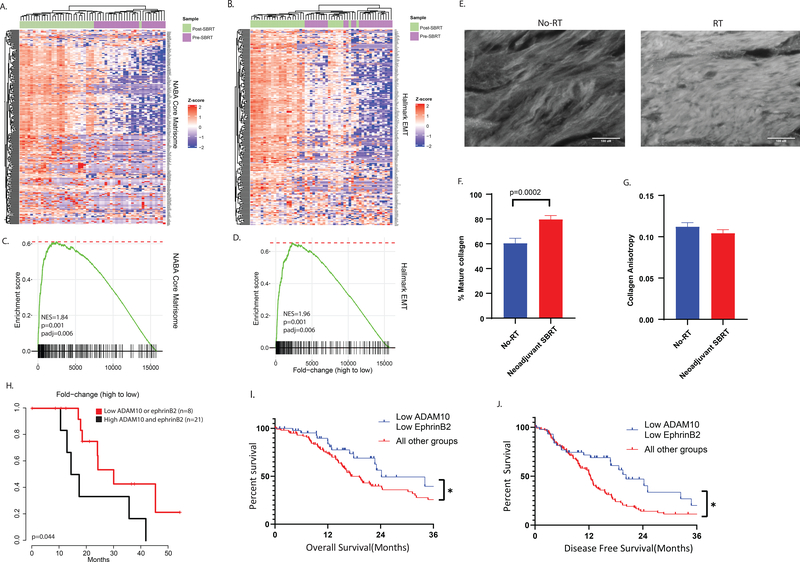
We reasoned that there would be global gene expression changes consistent with fibrosis after SBRT in patients with PDAC and performed RNA seq analysis of tumor cellular regions of 29 BRPC tumors treated with neoadjuvant combination chemotherapy and SBRT in the University of Colorado pancreas multidisciplinary clinic, comparing global gene expression to 26 pre-neoadjuvant therapy biopsy samples (patient, tumor and chemotherapy characteristics in Supplemental Table 1, representative images of surgical slides before in Sup. Fig. 1a,c,e, and after region collection in Sup. Fig. 1b,d,f). We performed GSEA of Naba matrisome genes, a set of ECM-associated genes compiled by domain-based organization and associated with activated ECM and fibrosis (36) and found global upregulation of matrisomal genes (Figure 1a,b) as well as collagens (Sup. Fig. 1g, 1h) after treatment with SBRT. This change was accompanied by upregulation of hallmark EMT genes (Fig. 1c, 1d), suggestive of a more motile and invasive phenotype. To examine induction of fibrosis by a different method, we examined mature collagen formation by picrosirius red staining in surgical samples from 5 patients without neoadjuvant RT and 5 with neoadjuvant RT (Supplemental Table 2) and found increased stromal collagen deposition in samples treated with RT (Figure 1e, 1f). Both sets had similar collagen anisotropy, consistent with the desmoplastic stroma of these tumors (Figure 1g).
Figure 1: Neoadjuvant SBRT induces gene expression changes consistent with activated matrisome, EMT in BRPC, and ADAM10 and ephrinB2 together are predictive of survival.
RNA sequencing gene expression in human biopsy and tumor samples treated with neoadjuvant SBRT before surgical resection. Heatmaps were generated with A. Z-score transformed gene expression and B. GSEA was performed using the Hallmark EMT gene set. C. Z-score transformed gene expression and D. GSEA was performed using the Core Matrisome gene set. Purple represents pre-SBRT samples, and green represents post-SBRT samples. The genes are ordered by the fold-change of post-SBRT samples compared to pre-SBRT. E. Representative immunofluorescence images of picrosirius stained tumor slides from patients with PDAC, either treated with surgical resection without RT(left), or with neoadjuvant SBRT followed by surgical resection(right). F. Quantification of area per slide positive for collagen staining in no-RT(blue) and neoadjuvant SBRT(red) samples. 5 patients per group, >10 fields of view collected per patient. P-value calculated with Students T-Test. G. Quantification of collagen anisotropy in no-RT(blue) and neoadjuvant SBRT(red) samples. 5 patients per group, >10 fields of view collected per patient, 4 regions of interest per FOV. H. Kaplan-Meyer analysis of overall patient survival stratified by expression of ADAM10 and EFNB2, black curve represents patients with post-neoadjuvant tumors with high expression of both ADAM10 and EFNB2, red curve represents patients with low expression of either. NES, normalized enrichment score. I. Kaplan-Meyer analysis of OS of 147 TCGA patients with PDAC stratified by above or below median expression of ADAM10 and EFNB2 shows that patients with below median expression of ADAM10 and EFNB2 have a positive prognosis compared to above median expression of either gene product. J. Kaplan-Meyer analysis of DFS of 147 TCGA patients with PDAC stratified by above or below median expression of ADAM10 and EFNB2 shows that patients with below median expression of ADAM10 and EFNB2 have a positive prognosis compared to above median expression of either gene product. P-values calculated by Mantel-Cox test.
ADAM10 and EFNB2 correlate with response to neoadjuvant SBRT and prognosis in PDAC.
We have previously shown that inhibition of ephrinB2 ligand binding with EPHB4 decreases RT induced fibrosis and sensitized PDAC to RT. It was recently reported that ADAM10 induction led to ephrinB2 cleavage in a bleomycin model of pulmonary fibrosis, driving the fibrotic phenotype, leading us to hypothesize that ADAM10 may be induced following RT and facilitate ephrinB2 function in PDAC tumors. We evaluated pancreatic cancer patients’ survival following neoadjuvant SBRT stratified by ADAM10 and ephrinB2 expression in our 29 post-surgical human PDAC patient samples. High expression of ADAM10 and ephrinB2 post-SBRT resulted in significantly worse prognosis (Fig. 1h). We also observed enriched matrisome protein expression in these patients (Sup. Fig. 1i). Examination of ADAM10 and ephrinB2 expression in RT-naïve PDAC patients in the cancer genome atlas (TCGA) revealed that patients with high ADAM10 or high ephrinB2 expression had worse prognosis compared to patients with low expression of both ADAM10 and EFNB2, with worse median OS (median: 24.2 months, vs. median 17.25 months, HR 1.403, 95% CI of HR 0.8235–2.392, p=0.04)(Fig. 1i) and DFS (Median: 20.3 months vs. median 12.3 months, HR 1.645, 95% CI of HR 1.045–2.591, p=0.02) (Fig. 1j). Interestingly, individual expression of ADAM10 or ephrinB2 alone were not independently prognostic(Sup fig. 1j, 1k). Additionally, correlations were observed between ADAM10 (Sup. Fig. 2a) (R2=0.21, p=1.73×10−11) and EPHB4 expression (Sup. Fig. 2b) (R2=0.15, p=3.13×10−8) with EFNB2, suggesting commonality to aggressive tumors. To ensure that expression of these proteins was independently predictive of survival and not only associated with other tumor cellularity, we examined tumor purity between tumors in the TCGA with low and high ADAM10 and ephrinB2 expression. We found no correlation between ADAM10 expression and tumor purity (Sup. Fig. 2c), while EFNB2 expression was associated with higher tumor purity (Sup. Fig. 2d, 2e). However, no correlation was observed between tumor purity and patient prognosis (Sup. Fig. 2f). We also tested whether ADAM10 and EFNB2 expression were associated with known tumor subtypes. We found that high EFNB2 was associated with a difference in tumor subtype, as characterized in Bailey et al, containing more squamous and progenitor types, versus low ephrinB2 expression, which associated with more immunogenic and ADEX types(3). ADAM10 was not associated with differences in the Bailey et al subtypes(3) (Sup. Fig. 2g), and neither ADAM10 nor EFNB2 were associated with enrichment for the basal and classical subtypes characterized in Moffitt et al(4) (Sup. Fig. 2j, 2k). High EFNB2 expression was associated with the progenitor Bailey et al subtype (Sup. Fig. 2h). Interestingly, neither Bailey nor Moffitt subtypes were predictive of survival in the TCGA cohort (Sup. Fig. 2i, 2l). These together suggest that ADAM10 and ephrinB2 coexpression are independently predictive of survival and not a consequence of other known tumor characteristics such as cellularity or published subtype.
ADAM10, EPHB4, and ephrinB2 proteins are expressed on tumor cells and stroma.
Using multispectral imaging analysis, we found ADAM10, EphB4, and ephrinB2 to be expressed in human pancreatic tumor samples (Fig. 2a). ADAM10 displayed patchy expression on some tumor islands, as determined by morphology (Fig. 2a.) and cytokeratin staining (Sup. Fig. 3a), with little stromal expression, as determined by Vimentin and α-SMA (Fig. 2a, Sup Fig. 3b, 3c). EphB4 displayed consistent expression on tumor islands whereas, ephrinB2 displayed punctate patchy tumor cell as well as stromal expression (Fig. 2a, Sup. Fig. 3a, 3b, 3c). Using the Human Protein Atlas, we found ADAM10 and EphB4 to be expressed on tumor cells, while ephrinB2 occurred on stroma and some tumor cells by morphology (Sup. Fig. 3d). When we examined syngeneic mouse flank tumors (using the KPC cell line PK5L1940) by IHC, we observed ADAM10 and EphB4 to have a cytoplasmic/membranous expression pattern on morphologic tumor islands, while ephrinB2 displayed more spindle shaped expression between clusters of tumor cells, consistent with predominantly stromal expression (Sup. Figure 3e). Consistent with these findings, using multispectral imaging of orthotopically implanted KPC tumors, we found EphB4 to be expressed on cell clusters not expressing vimentin (Sup fig 3f) Additionally, by sequential immunofluorescence analysis, ADAM10 staining was observed in regions of EpCAM staining in these tumors(Sup. Fig. 3g.). Examination of ADAM10 (Sup. Fig. 4a), EFNB2 (Sup. Fig. 4b), and EPHB4 (Sup. Fig. 4c) gene expression in tumors in the UCSC Cell Browser Treehouse Cancer Compendium dataset(37) revealed that all had higher than average expression in PDAC tumors compared to other tumor types. Also, in the UCSC Cell Browser Adult Pancreas dataset, ADAM10 and EPHB4 had higher ductal expression in normal pancreas, consistent with the cell type of origin of PDAC (Sup. Fig. 4d, 4e), while EFNB2 had both ductal and mesenchymal expression (Sup. Fig. 4f). We also examined expression of all 3 proteins in published scRNA seq datasets of human PDAC tumors (Sup. Fig. 5a, 5b, 5c)(38) and mouse PDAC tumors (Sup. Fig. 5d, 5e, 5f)(39) and found all 3 to be expressed in subsets of ductal, endothelial, and fibroblast cells.
Figure 2: ADAM10 upregulation leads to ephrinB2 cleavage, fibrosis, tumor survival after RT in PDAC tumors.
A. VECTRA multiplexed immunohistochemistry analysis of human PDAC tissue samples. Top panel shows false color reconstruction of individual immunostains. ADAM10 is shown in green, ephrinB2 in cyan, EPHB4 in yellow, a-SMA in white, CD-31 in orange, Vimentin in magenta, bottom panel shows true color immunofluorescence image before unmixing. B. Western blotting for ADAM10 in KPC cells 7 days following treatment with increasing doses of ionizing radiation as shown. B-actin shown as loading control. C. quantitation of b. D. Schema for orthotopic pancreatic implantation and in vivo irradiation of KPC derived tumors in immunologically intact syngeneic mice, Created with BioRender.com. E. western blotting of ADAM10 and ephrinB2 from WT and ADAM10 knockout orthotopic tumors 2 weeks following treatment with increasing doses of RT. F. quantitation of ADAM10 from e. G. Representative immunofluorescence images of picrosirius stained tumor slides from WT and ADAM10 KO orthotopic tumors treated with 0 or 16Gy RT. H. Quantification of area per slide positive for collagen staining in WT 0Gy (blue), WT16Gy(red), ADAM10KO 0Gy(green) and ADAM10 KO 16Gy(purple) samples. 5 patients per group, >10 fields of view collected per patient. P-value calculated with Students T-Test. I. Quantification of collagen anisotropy in no-RT(blue) and neoadjuvant SBRT(red) samples. 5 patients per group, >10 fields of view collected per patient, 4 regions of interest per FOV. J. Volume and K. Mass of orthotopic tumors collected at sacrifice 2 weeks following treatment with RT as per schema in d. WT 0Gy(blue), WT 16gy, ADAM10 KO 0Gy (green), ADAM10 KO 16gy(purple). L. Volume and M. mass of PK5L1940 tumors implanted and treated as per schema in d. treated with either vehicle or ADAM10 inhibitor GI254023x every 72 hours prior to sacrifice, starting at time of RT. p values calculated by student’s T-test. ≥5 mice per group. N. Schema for survival analysis of mice implanted with WT or ADAM10 KO tumors followed by in vivo irradiation, Created with BioRender.com. O. Kaplan-Meyer survival analysis of mice with orthotopic pancreatic implantation of WT or ADAM10 KO PK5L1940 cells followed by in vivo irradiation with 0 or 16Gy RT. Groups are WT 0Gy(black), WT 16Gy (red), ADAM10 KO 0Gy (blue), ADAM10 KO 16Gy(red). ≥10 mice per group. P-values calculated by Mantel-Cox test. *indicates P<0.05, **<0.02.
ADAM10 is induced in PDAC cells after RT treatment leading to cleavage of EphrinB2
To determine if ADAM10 was induced by RT, we examined ADAM10 expression in PK5L1940 PDAC cells in vitro 1 week following irradiation and found that expression of precursor and active isoforms of ADAM10 increased with increasing RT doses (Fig. 2b, 2c). We then generated a CRISPR-CAS9 knockout of ADAM10 in PK5L1940 KPC cells to test the impact of ADAM10 on fibrosis and survival following RT (Sup. Fig. 5g). We performed orthotopic tumor implantation to properly recapitulate the local tumor microenvironment with the host stromal contribution, followed by in vivo irradiation with an image guided small animal irradiator (Fig. 2d). Wildtype (WT) and ADAM10 KO graft tumors were implanted into BL/6 mice and protein analysis revealed ADAM10 upregulation in WT tumors with increasing doses of RT. A cleaved band of ephrinB2 was also observed in WT tumors at the 16Gy dose of RT, but was absent in the ADAM10 KO grafts (Fig. 2e, f). Interestingly this cleavage did not occur at a dose of 8Gy, suggestive of a threshold effect. There was still some detectable ADAM10 in these tumors consistent with some stromal contribution by the host mouse.
Inhibition of ADAM10 decreases RT-induced fibrosis and enhances tumor growth delay by RT.
To examine whether ADAM10 was driving fibrosis in response to radiation, we implanted PK5L1940 PDAC WT or ADAM10 KO cells orthotopically into syngeneic immunocompetent mouse pancreata, followed by in vivo treatment with RT (Fig 2d.). Analysis of mature collagen formation by Massons Trichrome and Picrosirius red staining (Fig. 2g) demonstrated RT induced mature collagen formation (Fig. 2h) and increased collagen organization (by anisotropy) in WT tumors, but not in ADAM10 knockout tumors (Fig. 2i). These results were validated in flank PK5L1940 tumors treated with RT and the ADAM10 specific inhibitor GI254023x. Treatment with the ADAM10 inhibitor blocked RT-induced fibrosis (Supplemental Fig. 5h, 5i, 5j), and when combined with RT, resulted in a reduction in tumor growth compared to untreated tumors (Sup. Fig. 5k). This reduction in tumor growth was also observed with the combination of RT and ADAM10 inhibition using another KPC cell line, FC1242 (Sup. Fig. 5l).
ADAM10 knockout on cancer cells improves survival in orthotopically implanted pancreatic tumors.
Examining the effect of ADAM10 KO on orthotopic pancreatic tumor size 2 weeks following in vivo irradiation, we found that tumor mass and volume was reduced in ADAM10 KO tumors, while the combination of ADAM10 knockout and RT further reduced tumor mass beyond ADADM10 KO or RT alone (Fig 2j, k). We also observed a significant reduction in the growth rate of flank tumors with the combination of ADAM10 KO and RT (Sup. Fig. 5m). Similarly, we found that treatment with the combination of ADAM10 inhibitor GI254023x and RT resulted in a reduction in orthotopic tumor volume and mass beyond inhibitor alone, while treatment with RT alone resulted in a significant reduction in tumor mass only (Fig 2 l, m). Examining median survival, we found that ADAM10 knockout (35 days) or treatment with RT alone (33 days) did not significantly improve survival above untreated WT tumors (28 days) (Fig. 2n, o). However, the combination of ADAM10 KO and RT resulted in a significant improvement in survival (48 days) suggesting a synergistic effect of the two treatments and demonstrating the importance of intact ADAM10 expression for resistance to RT(Fig. 2n, o).
RT induces proteomic changes consistent with fibrosis with increasing dose, which is dependent on ADAM10.
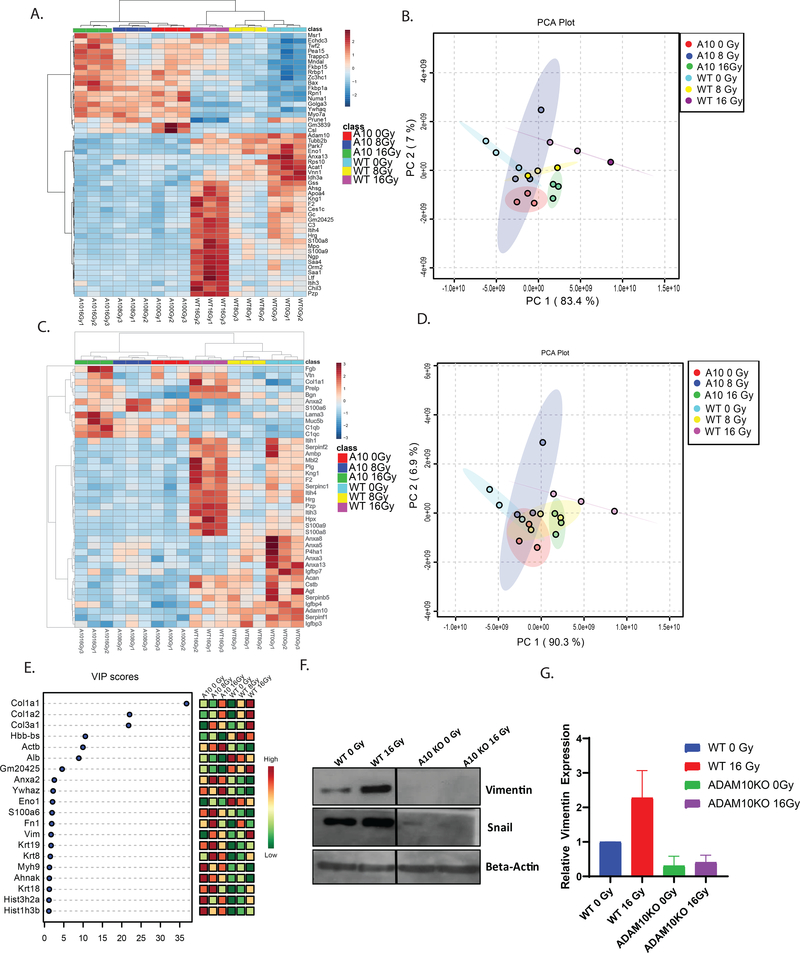
To determine whether activation of the extracellular matrix was occurring with RT in an ADAM10-dependent manner, we performed mass spectrometry on WT and ADAM10 KO tumors treated with RT. Hierarchical clustering resulted in the discovery of global proteomic changes occurring in WT but not ADAM10 KO tumors (Fig. 3a, 3b), indicating that ADAM10 is required for changes in the proteomic expression of tumor stroma following high dose RT. Using the core matrisome gene set (40), we discovered that numerous matrisome proteins had increased expression with higher dose of RT (Fig. 3c, 3d). These proteins did not change in the ADAM10 KO tumors, suggesting that RT-induced ECM activation and fibrosis is dependent on ADAM10 activity. Notably, on partial least square analysis, the protein with the greatest significance was Collagen I (Fig. 3e), which is known to regulate invasion, metastasis, and apoptotic pathways in PDAC (8). Re-interrogating our human post-SBRT surgical samples, we found an enrichment of matrisome proteins upregulated in our human samples treated with RT versus the pre-SBRT biopsy samples (Sup. Fig. 5n, 5o), indicating conservation of a common set of upregulated ECM genes following RT. We also examined the effect of ADAM10 inhibition in the presence of RT on proteomic expression in tumors by treating WT grafts with GI254023x and found there were ADAM10 activity dependent changes in matrisome proteins after RT (Sup. Fig. 6a-d). Reactome pathway analysis of our WT-ADAM10 KO comparison found the 5 most overrepresented pathways in WT tumors treated with SBRT included ECM organization and collagen formation (Sup. Fig. 6e), consistent with ADAM10 driving RT-induced ECM activation and fibrosis. The most overrepresented pathways in ADAM10 KO tumors included Notch signaling, the intrinsic apoptosis pathway, and interferon signaling. Additionally, we found a large number of negatively prognostic proteins downregulated by ADAM10 inhibition, as well as genes involved in promoting cell motility, invasion, and metastasis (Sup. Table 3).
Figure 3: RT induces activated matrisome and stromal fibrosis proteins in ADAM10 dependent manner.
A. Clustering analysis of matrisome proteins detected by mass spectrometry in mouse flank KPC tumors treated with 0, 8, 16Gy RT (cyan:WT 0Gy, yellow:WT 8Gy, magenta:16Gy, red:ADAM10KO 0Gy, blue:ADAM10KO 8Gy, red:ADAM10KO 16Gy). B. Principle component analysis(PCA) of most highly differentially regulated genes from analysis in panel a. C. Clustering analysis of 50 most highly regulated proteins detected by compartment resolved mass spectrometry in mouse flank KPC tumors treated with 0, 8, 16Gy RT (cyan:WT 0Gy, yellow:WT 8Gy, magenta:16Gy, red:ADAM10KO 0Gy, blue:ADAM10KO 8Gy, red:ADAM10KO 16Gy) D. PCA of matrisome proteins from analysis in panel c. E. Variable importance in projection (VIP) scores from partial least square (PLS) analysis of mass spectrometry of mouse flank tumors treated as in panel a. F. Western blotting for EMT markers in WT or ADAM10 KO tumors 2 weeks following treatment with 0 or 16Gy RT. G. Quantitation of vimentin in f n=3.
To confirm our proteomic analysis, we examined markers of EMT by western blotting following RT, and found induction of vimentin and snail to occur in WT tumors treated with 16Gy RT. When we examined ADAM10 KO tumors(Fig. 3f, 3g)we see a reduction in expression of these markers as compared to control RT treated tumors, confirming this induction of EMT is mediated by ADAM10. We also see a reduction in these markers after treatment with GI254023X (Sup. Fig. 6f).
RT increases physical tension of PDAC tumors in ADAM10 dependent manner
We examined whether these ADAM10 dependent matrisomal changes had an effect on the tissue tension within PDAC tumors, as that has been shown to drive global gene expression changes and subsequent aggressive tumor behavior (7). Using Atomic Force Microscopy (AFM), we found the nanomechanical stiffness of WT PDAC tumors increased after RT (Fig. 4A, 4B, 4C). This increase with RT did not occur in the ADAM10 KO tumors (Fig. 4B, 4D), and compared to WT tumors, stiffness was lower in ADAM10 KO tumors with or without RT (Fig. 4B, 4D).
Figure 4. ADAM10 KO reduces PDAC tissue stiffness after RT, as well as migratory and invasive capacity of KPC cells.
A. Representative images of stiffness maps from atomic force microscopy of WT and ADAM10 knockout tumors treated with 0 or 16Gy RT B. Comparison of frequency of Young’s Modulus measurements between 0Gy and 16Gy treated WT KPC tumors. C. Comparison of frequency of Young’s Modulus measurements between WT and ADAM10 KO tumors treated with 16Gy RT. D. Comparison of mean Young’s Modulus between groups. E. MTT assay of WT and ADAM10 PK5L1940 cells. F. Wound healing in WT and ADAM10 KO PK5L1940 KPC cells. Y axis represents tumor cell migration 12 hours following wound formation. N>5 for all samples G. Wound healing in WT PK5L1940 cells, +/− coculture with 3T3 cells for 1 week. H. Wound healing in ADAM10 KO cells +/− coculture with 3T3 cells. I. Wound healing in WT cells treated with EPHB4 inhibitor TNYL-RAW (B4i), +/− coculture with 3T3 cells. J. Wound healing in WT cells 1 week after treatment with 0 or 10 Gy RT. (k.) Wound healing in ADAM10KO knockout cells 1 week after treatment with 0 or 10Gy RT. L. Wound healing in WT cells 1 week after treatment with 10Gy RT, with or without coculture with fibroblasts. M. Wound healing in cocultured WT cells 1 week after treatment with 0 or 10Gy RT. N. Wound healing in cocultured ADAM10 KO cells 1 week after treatment with 0 or 10Gy RT. O. Xcelligence assay for transwell invasion through Matrigel 1 in WT PK5L1940 cells 1 week following treatment with 0 or 10Gy RT. P. Xcelligence assay for transwell invasion through Matrigel 1 in ADAM10 KO cells 1 week following treatment with 0 or 10Gy RT or 10Gy RT with addition of EPHB4 activator ephrinB2 FC protein. Q. MTT assay of WT PK5L1940 cells 1 week following treatment with 0 or 10Gy. R. MTT assay of ADAM10 KO cells 1 week following treatment with 0 or 10Gy RT, or 10Gy RT and EPHB4 activator ephrinB2 FC Protein. N ≥3. P-values calculated using students T-test, all error bars show SEM of measurements. * indicates p<0.05, ** p<0.02 **** indicates p<0.0001
ADAM10 knockout on cancer cells reduces migratory ability of KPC cells, and RT increases migration in an ADAM10 dependent manner.
We next examined the effect of ADAM10 on the migration of the KPC cell line PK5L1940. Firstly, we found no difference in cell proliferation by MTT assay between WT and ADAM10 KO cells to explain differences in migratory capacity (Fig 4e). In wound-healing assays of KPC cells, cell migration was greater in WT cells compared to ADAM10 KO cells, consistent with ADAM10 activity enhancing migration (Fig. 4f). To determine if this migration could be further enhanced by fibroblast-expressed ephrinB2, we incubated KPC cells in co-culture with NIH-3T3 fibroblasts for 1 week prior to scratch assay. Fibroblasts enhanced the motility of WT KPC tumor cells (Fig. 4g), an effect that was not observed in ADAM10 KO cells (Fig. 4h). To determine if migration was dependent on the stimulatory effect of ephrinB2-EphB4 interaction, we treated PK5L1940 cells with a pegylated peptide inhibitor (TNYL-RAW, labeled B4i) that blocks the ephrinB2-EphB4 interaction(41). In the presence of TNYL-RAW, there was no increase in motility with the addition of fibroblasts (Fig. 4i). These suggest that ADAM10 enhances motility, and the addition of fibroblasts enhances tumor cell motility in ADAM10 and ephrinB2-EphB4 dependent manners. When we treated WT cells with RT, we found an increase in migration (Fig. 4j), which did not occur in ADAM10KO cells(Fig. 4k). We also observed an increase in migratory capacity of WT cells treated with RT with the addition of fibroblasts (Fig. 4l). Furthermore, when we treated fibroblast cocultured WT cells with RT, we detected a significant increase in their migratory capacity (Fig. 4m), whereas with cocultured ADAM10KO cells, this difference was no longer observed (Fig 4n).
RT decreases invasion of ADAM10 KO cells, which is rescued by addition of EphrinB2 FC protein.
To test whether RT affected the invasive capacity of our KPC tumor cells in a manner dependent on ADAM10 cleavage of ephrinB2, we cocultured WT or ADAM10 KO cells with fibroblasts for 1 week following in vitro irradiation and examined invasion by Xcelligence invasion assay 24 hours following removal of fibroblasts and initiation of serum starvation. We found a trend towards an increase in invasion in WT cells with the addition of RT (Fig. 4o). However, in our ADAM10 KO cells, a significant reduction in invasion after treatment with RT occurred, which was reversed with the addition of EPHB4 stimulating ephrinB2 mimetic ephrinB2 FC protein(Fig. 4p). We did not observe a significant difference in cell viability in recovered cells following RT(Fig. 4q). This suggests that preservation of invasion capability following RT is dependent on ADAM10 and ephrinB2. We did not observe a reduction in ADAM10 KO cell viability with RT, or an increase in viability with the addition of ephrinB2 FC(Fig. 4r), suggesting this invasion effect is not a function of cell survival.
ADAM10 and EPHB4 inhibition enhances growth inhibitory effect of RT in human tumor organoids.
We utilized human tumor organoid line Panc193, cocultured with human CAFs (ephrinB2 expression shown in Sup. Fig. 6g) to determine whether ADAM10 inhibition could enhance the growth delay seen with RT in a human tumor model. A significant decrease in tumor growth constant (k) was observed with RT in both the vehicle treated and GI254023x treated tumor organoids (Fig. 5a), with a trend towards more reduction of tumor growth in the presence of inhibitor treated. Bioluminescent organoid viability was not reduced by RT alone, but the combination of ADAM10 inhibition with RT was sufficient to significantly reduce viability (Fig. 5b). Using the EphB4-ephrinB2 inhibitor, TNYL-RAW (labeled B4i), a similar reduction in growth in organoid viability was observed (Fig. 5c). These data indicate that both ADAM10 inhibition and EphB4 inhibition enhanced the tumor cell killing effect of RT in this system.
Figure 5. ADAM10 KO and RT reduce growth of metastatic and orthotopically implanted KPC tumors.
A. Exponential growth rate (k) of human organoid line Panc193, in co-culture with human CAFs, measured by Incucyte live cell analyzer after treatment with 0 or 10Gy RT, in the presence or absence of ADAM10 inhibitor GI254023x. B. Cell viability of human Panc193 organoids, in coculture with human CAFs, 1 week following treatment with 0 or 10Gy RT, in presence or absence of ADAM10 inhibitor GI254023x. C. Cell viability of human Panc193 organoids, in coculture with human CAFs, 1 week following treatment with 0 or 10Gy RT, in presence or absence of EPHB4 inhibitor TNYL-Raw(B4i)). D. Schema of hemispleen metastatic liver tumor model utilized in following slides. E. Representative images of mouse livers collected at sacrifice, 2 weeks following implantation with fibroblast cocultured WT or ADAM10 KO PK5L1940 cells which had been treated with 0 or 10Gy RT 1 week prior, Created with BioRender.com. F. Number of metastatic liver lesions counted at necropsy, 14 days after hemispleen implantation of fibroblast cocultured WT or ADAM10 KO PK5L1940 cells after treatment with 10Gy RT. G. Proportion of liver surface area comprised of tumor at day 14 post implant of WT or ADAM10 knockout PK5L1940 cells after treatment with 10Gy RT. H. Survival in mice treated with hemispleen implantation of metastatic liver tumors, using fibroblast cocultured WT and ADAM10 KO PK5L1940 cells, N=5. P values shown derived from Mantel-Cox log rank analysis. I. Liver mass of mice 14 days after hemispleen implantation with WT PK5L1940 cells co-cultured with WT or EphrinB2 KO NIH 3T3 cells. J. Proportion of liver surface area comprised of tumor 14 days after hemispleen implantation with WT PK5L1940 cells co-cultured with WT or EphrinB2 KO NIH 3T3 cells. K. Tumor volume, 27 days following implantation of WT or ADAM10KO tumors into flanks of PDGFRa-ADAM10 fl mice or littermate controls. L. Tumor volume of WT tumors 27 days following implantation into flanks of PDGFRa-ADAM10 fl mice or littermate controls, all treated with 16Gy RT 7 days following implantation. N>3, error bars represent SEM. P values calculated using student’s T-test. * indicates p<0.05, ** p<0.02, *** indicates p<0.001, **** indicates p<0.0001
ADAM10 knockout on cancer cells abrogates metastasis formation in liver metastasis model.
To test the effect of ADAM10 KO on metastasis of KPC cells in vivo, we utilized a hemispleen model of liver metastases (Fig. 5d)(32). WT and ADAM10 KO cells were irradiated in vitro (10Gy or 0Gy) and co-cultured with NIH-3T3 fibroblasts prior to mouse injection to mimic the stimulatory effect seen in our motility assays (Fig. 5d). Two weeks post-implantation, the number of metastatic lesions and area of metastases were reduced in the livers of mice implanted with ADAM10 knockout cells treated with RT (Fig. 5e, 5f, 5g). Neither ADAM10 KO nor treatment of WT cells with RT improved median survival compared to untreated WT cells (Fig. 5h). Combination RT with ADAM10 KO, however, led to a significant improvement in overall survival (Fig. 5 h.). These data suggest that inhibiting ADAM10 induction following RT can improve survival by reducing the metastatic potential of tumor cells, though the modest delay in mortality in this experiment suggests that these tumor cells retain their proliferation potential once metastases are seeded.
EphrinB2 knockout on cocultured fibroblasts reduces metastatic potential of PDAC cells.
We next generated a CRISPR-CAS9 knockout of ephrinB2 on NIH-3T3 fibroblasts to determine whether the contribution of ephrinB2 from fibroblasts was important for the metastatic potential of KPC cells. We cocultured PK5L1940 KPC tumor cells with ephrinB2 KO fibroblasts (Sup. Fig. 6h) and performed hemispleen implantations to analyze the rate of liver metastasis formation. We found that 14 days following implantation, both liver weight and surface area of PDAC metastases were reduced significantly by ephrinB2 knockout on the cocultured fibroblasts (Fig. 5i, 5j), confirming the role of stromal contributed ephrinB2 in metastasis formation.
Fibroblast-specific ADAM10 -deficient mice are not protected from tumorigenesis in vivo, and have no difference in sensitivity to RT.
In order to further demonstrate that tumor cell and not host stromal ADAM10 was responsible for the resistant EMT phenotype we observed, we then utilized a mouse knockout model of ADAM10 for tumor implantation experiments. As mice that are globally ADAM10-deficient die at day 9.5 of embryogenesis with multiple defects of the developing central nervous system, somites, and cardiovascular system,(42) we utilized mice in which we could conditionally delete ADAM10 in pancreatic fibroblasts. Mice with ADAM10 flanked by loxP sites (adam10loxP/loxP mice) (33) were crossed with mice that express a tamoxifen-inducible Cre recombinase driven by the mouse promoter of the platelet derived growth factor receptor, alpha polypeptide (PDGFRα-Cre-ERT transgenic mice)(34). PDGFRα-Cre mice have been used to label and/or target genes in glial progenitor cells in the CNS(34) as well as dermal (43), lung(44), muscle(45) and liver(46) fibroblasts during the development of fibrosis. PDGFRα has been also used to investigate CAF functions in vivo during the development of cancer due to its ability to target a distinct subset of tumor resident CAFs (47),(48) compared to Acta2-Cre transgenic mouse (which targets both myofibroblasts and smooth muscle cells) or FSP-Cre transgenic mouse (which targets both fibroblasts and an subset of myeloid cells). Our results indicate that tamoxifen treatment of offspring that were homozygous for the ‘floxed’ adam10 allele and hemizygous for the PDGFαR-Cre-ERT transgene (adam10loxP/loxP;PDGFRα-Cre-ERT ), led to the deletion of the adam10 gene in pancreatic fibroblasts and the generation of adam10 conditional knockout mice, herein referred to as ADAM10-CKO mice. Littermates treated with corn oil vehicle alone were used as controls and are herein referred to as adam10-C mice.
To determine the contribution of ADAM10 on stromal fibroblasts to tumor growth, we implanted adam10-C and adam10-CKO Mice with WT or ADAM10 KO flank tumors and compared their growth rate to tumors implanted in WT mice. While there was a reduction in tumor growth 27 days following implantation of ADAM10 KO tumor cells compared to WT tumor cells in both WT and fibroblast-specific ADAM10-deficient mice, there was no tumor growth delay in the ADAM10-CKO mice (Fig. 5k, Sup. Fig. 6i). Similarly, we found no difference in size of WT tumors implanted in WT or ADAM10-CKO mice 20 days after treatment with 16Gy RT (Fig. 5l). These together suggest ADAM10 expression on tumor cells, rather than fibroblasts, is required for the observed pro-tumorgenic, pro-survival effect.
Soluble ephrinB2 is increased in pancreatic cancer patient and mouse plasma following high dose tumor-directed RT.
To determine whether serum soluble ephrinB2 could be detected following SBRT, we tested plasma locally advanced pancreatic cancer patient on clinical trial NCT02873598 with samples collected before, during, and after treatment with 3 fractions of increasing doses of SBRT for ephrinB2 expression by ELISA (Fig. 6a). We could detect ephrinB2 in pancreatic cancer patients’ plasma and found that this detection increased with time after SBRT and with increasing doses of SBRT (Fig. 6A). We also tested serum collected from tumor bearing mice 2 weeks following RT and found increasing quantities of soluble plasma ephrinB2 with increasing dose (Fig. 6b). We compared serum ephrinB2 in mice with WT or ADAM10 KO PDAC tumor grafts and found that ADAM10 KO significantly reduced the levels of serum ephrinB2 (Fig. 6c). Our findings that RT induced ADAM10 cleaves ephrinB2 with increasing doses suggest that ephrinB2 cleavage may be a useful serum marker for RT-induced fibrosis and its disappearance a marker of efficacy of ADAM10-directed therapy.
Figure 6. EphrinB2 is released into patient plasma and mouse plasma after high dose RT to PDAC tumors.
A. ELISA for ephrinB2 in patient plasma samples collected from patients with LAPC, prior to, during, and after treatment with SBRT. X axis shows dose per fraction of SBRT, patients were treated with 3 fractions. Y axis represents ephrinB2 plasma concentration determined by standard curve. *p<0.05 B. ELISA for ephrinB2 in mouse plasma collected 2 weeks following increasing single fraction doses of RT to flank grafts, Y axis represents absorbance at 450nm minus negative control. N=6 replicates. C. ELISA for ephrinB2 in mice implanted with WT KPC flank grafts, vs. ADAM10 KO KPC grafts, Y axis represents absorbance at 450nm minus negative control N=6 replicates. P values calculated using Student’s T-Test.
Discussion:
Cytotoxic therapies, including RT, are core tools in the oncology armamentarium. RT is a powerful locoregional modality that can both directly ablate tumor cells as well as stimulate local and systemic immune anti-tumor responses(49), which are both crucial for local and distant control of pancreatic cancer. However, any cytotoxic therapy can apply selective pressure to a tumor population, and may be made more effective by targeting the mechanisms of adaptation tumors use to become resistant to these therapies. This is especially relevant in the context of a cancer whose tumorigenesis parallels the late effects of radiation, and while the role of dose escalation is still being investigated, a recent major trial of of SBRT failed in improving outcomes and may in fact worsen them(13). It should be noted that preclinical models of RT have shown activation of adaptive resistance mechanisms in other tumor histologies. Fractionated RT has been shown to lead to EMT in breast cancer through Notch-mediated activation of JAK/STAT3 signaling (50), which is also a key regulator of the desmoplastic stroma in pancreatic cancer (7). As EMT is fundamental to normal wound healing (51), it follows that cytotoxic therapies would lead to EMT given the ensuing local inflammatory response (52). This is more significant in pancreatic cancer where it has been shown that chronic inflammation is central to the disease’s natural history (53), with ECM activation further enhancing its aggressiveness (3,7). Here, we found ECM and EMT pathways were upregulated following RT in patient and mouse pancreatic cancer tumors, but were able to abrogate these changes by targeting ADAM10. ADAM10 inhibition was able to block ephrinB2 cleavage, mature collagen deposition, and tumor cell motility, enhancing tumor killing by radiation and decreasing metastasis formation. These findings suggest that targeting the cellular and microenvironmental response to radiation may enhance the efficacy of standard treatments, and may raise the possibility of cure with cutting edge radiation delivery.
There has been interest in the targeting of metalloproteinases (MMPs), as these proteins have been found to play roles in multiple pathways implicated in tumor aggression (54). Trials of MMP inhibitors, to which ADAM10 enzymatic activity is related, did not lead to large scale adoption in part due to their non-specificity and ubiquitous nature, resulting in a broad range of effects on many tissues. This led to dose-limiting toxicities, notably musculoskeletal pain, which, while reversible, made it an unattractive option in a long-term maintenance setting(55). Importantly, as in the case with ADAM10, inhibition was not inherently cytotoxic and without combination with a cytotoxic agent would not result in tumor killing. While solid tumors are prone to dysregulation of major cellular pathways to increase their invasive potential such as the TGF-B, Wnt, Notch, and Hedgehog pathways (56), targeting of these pathways does not typically result in durable cytotoxicity as in the case of oncogene-addicted hematologic malignancies (57). The lack of cytotoxicity does not discount the fact that therapies targeting mediators of fibrosis and EMT may have utility in enhancing the effect of traditional cytotoxic therapies by blocking the phenotypic changes that allow for tumor adaptation and survival.
We previously discovered that ephrinB2-EphB4 inhibition reduces stromal fibrosis in pancreatic tumors and leads to improved tumor killing by radiation therapy. Other Eph-ephrins have been shown to be regulated by ADAM10 cleavage in development, and the action of ephrinB2 has been shown to be both positively (19) and negatively (58) regulated by ADAM10 cleavage. ADAM10 activity has been found to be dependent on EPH function in other developmental contexts (59). Fibroblast activation by ephrinB2 driving myofibroblast differentiation and resulting pulmonary fibrosis was found to be dependent on the activity of ADAM10 in response to bleomycin (19). One novel finding of that publication was the detection of a shorter soluble cleaved isoform of ephrinB2 that could function in a paracrine manner to induce this myofibroblast differentiation. ADAM10 has also been seen to be induced in endothelial cells in response to RT, leading to an increase in vascular permeability (23), itself an important step in initiating tissue fibrosis (60). Our detection of soluble ephrinB2 in this context has significant implications, given the prior finding that ephrinB2 could stimulate fibrosis, and the importance of fibrosis in driving the invasive nature of pancreatic cancer (61). While preliminary, the clinical and mechanistic significance of systemic ephrinB2 warrants further study, and could represent a therapeutic target to enhance the efficacy of RT and decrease tumor resistance and normal tissue toxicity.
Fibrosis of normal tissues is a normal consequence of radiation therapy (15) which has traditionally been thought to be related to damage to tissues residing in the treated field (62). But as outcomes improve and advanced technologies allowing higher doses per treatment such as SBRT come into greater use, it will be important to understand the impact of cytotoxic stimulation of fibrosis has on the tumor microenvironment and systemic response. Both of these potential sequelae highlight the potential benefit of combining agents targeting tumor fibrosis, through pathways such as ADAM10-ephrinB2, in not only improving local outcomes, but preventing adaptive resistance and decreasing normal tissue toxicity.
Supplementary Material
Significance:
Targeting a previously unidentified adaptive resistance mechanism to radiation therapy(RT) in PDAC tumors in combination with RT could increase survival of the 40% of PDAC patients with locally advanced disease.
Acknowledgement
This work was supported by Cancer Center Support Grant (P30CA046934), R01-DE028282 (Karam), R01-DE028529 (Karam), Paul Sandoval Funds (Mueller), RSNA Resident Research Grant (Mueller), Cancer League of Colorado Grant (Mueller) and by the Wings of Hope Foundation (Karam, Goodman). D.L. gratefully acknowledges funding support from the NIH (grant R01 HL147059-01).
Footnotes
Conflict of Interest Statements
Dr. Karam receives clinical trial research funding unrelated to this work from AstraZeneca.
References:
- 1.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52 [DOI] [PubMed] [Google Scholar]
- 4.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolle R, Blum Y, Marisa L, Loncle C, Gayet O, Moutardier V, et al. Pancreatic Adenocarcinoma Therapeutic Targets Revealed by Tumor-Stroma Cross-Talk Analyses in Patient-Derived Xenografts. Cell Rep 2017;21:2458–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice AJ, Cortes E, Lachowski D, Cheung BCH, Karim SA, Morton JP, et al. Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 2017;6:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 2016;22:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weniger M, Honselmann KC, Liss AS. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers (Basel) 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans DB, Hess KR, Pisters PW. ESPAC-1 trial of adjuvant therapy for resectable adenocarcinoma of the pancreas. Ann Surg 2002;236:694; author reply −6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman KA. Stereotactic Body Radiation Therapy for Pancreatic Cancer. Cancer J 2016;22:290–5 [DOI] [PubMed] [Google Scholar]
- 11.Reyngold M, Parikh P, Crane CH. Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol 2019;14:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran NH, Sahai V, Griffith KA, Nathan H, Kaza R, Cuneo KC, et al. Phase 2 Trial of Neoadjuvant FOLFIRINOX and Intensity Modulated Radiation Therapy Concurrent With Fixed-Dose Rate-Gemcitabine in Patients With Borderline Resectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2020;106:124–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz MHG. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. In: Matthew H. G. Katz QSJPMJMHMCBMWSARdWMLHSSBW, x, Reilly, The University of Texas Md Anderson Cancer Center HTX, Mayo Clinic RMN, Miami Cancer Institute MFL, et al. , editors2021; Gastrointestinal Cancers Symposium. American Society of Clinical Oncology. [Google Scholar]
- 14.Rudra S, Jiang N, Rosenberg SA, Olsen JR, Roach MC, Wan L, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med 2019;8:2123–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM. Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol 2015;141:1985–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng J, Wulff-Burchfield EM, Murphy BA. Late Soft Tissue Complications of Head and Neck Cancer Therapy: Lymphedema and Fibrosis. J Natl Cancer Inst Monogr 2019;2019 [DOI] [PubMed] [Google Scholar]
- 17.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell 2008;133:38–52 [DOI] [PubMed] [Google Scholar]
- 18.Oweida A, Bhatia S, Hirsch K, Calame D, Griego A, Keysar S, et al. Ephrin-B2 overexpression predicts for poor prognosis and response to therapy in solid tumors. Mol Carcinog 2017;56:1189–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagares D, Ghassemi-Kakroodi P, Tremblay C, Santos A, Probst CK, Franklin A, et al. ADAM10-mediated ephrin-B2 shedding promotes myofibroblast activation and organ fibrosis. Nat Med 2017;23:1405–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Vorst EP, Jeurissen M, Wolfs IM, Keijbeck A, Theodorou K, Wijnands E, et al. Myeloid A disintegrin and metalloproteinase domain 10 deficiency modulates atherosclerotic plaque composition by shifting the balance from inflammation toward fibrosis. Am J Pathol 2015;185:1145–55 [DOI] [PubMed] [Google Scholar]
- 21.Gavert N, Sheffer M, Raveh S, Spaderna S, Shtutman M, Brabletz T, et al. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res 2007;67:7703–12 [DOI] [PubMed] [Google Scholar]
- 22.Wozniak J, Ludwig A. Novel role of APP cleavage by ADAM10 for breast cancer metastasis. EBioMedicine 2018;38:5–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabacik S, Raj K. Ionising radiation increases permeability of endothelium through ADAM10-mediated cleavage of VE-cadherin. Oncotarget 2017;8:82049–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergushichev AA. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv 2016:060012 [Google Scholar]
- 26.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics 2011;27:1739–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016;32:2847–9 [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Sharma J, Bukkapatnam S, Oweida A, Lennon S, Phan A, et al. Inhibition of EphB4-Ephrin-B2 Signaling Enhances Response to Cetuximab-Radiation Therapy in Head and Neck Cancers. Clin Cancer Res 2018;24:4539–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang W, Hennrick K, Drew S. A colorful future of quantitative pathology: validation of Vectra technology using chromogenic multiplexed immunohistochemistry and prostate tissue microarrays. Hum Pathol 2013;44:29–38 [DOI] [PubMed] [Google Scholar]
- 30.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018;46:W486–W94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu W, Su GH. Development of orthotopic pancreatic tumor mouse models. Methods Mol Biol 2013;980:215–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soares KC, Foley K, Olino K, Leubner A, Mayo SC, Jain A, et al. A preclinical murine model of hepatic metastases. J Vis Exp 2014:51677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian L, Wu X, Chi C, Han M, Xu T, Zhuang Y. ADAM10 is essential for proteolytic activation of Notch during thymocyte development. Int Immunol 2008;20:1181–7 [DOI] [PubMed] [Google Scholar]
- 34.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010;68:668–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pena B, Bosi S, Aguado BA, Borin D, Farnsworth NL, Dobrinskikh E, et al. Injectable Carbon Nanotube-Functionalized Reverse Thermal Gel Promotes Cardiomyocytes Survival and Maturation. ACS Appl Mater Interfaces 2017;9:31645–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 2012;11:M111 014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivian J, Rao AA, Nothaft FA, Ketchum C, Armstrong J, Novak A, et al. Toil enables reproducible, open source, big biomedical data analyses. Nat Biotechnol 2017;35:314–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, et al. Author Correction: Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res 2019;29:777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov 2019;9:1102–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 2012;4:a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noberini R, Mitra S, Salvucci O, Valencia F, Duggineni S, Prigozhina N, et al. PEGylation potentiates the effectiveness of an antagonistic peptide that targets the EphB4 receptor with nanomolar affinity. PLoS One 2011;6:e28611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet 2002;11:2615–24 [DOI] [PubMed] [Google Scholar]
- 43.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013;504:277–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miwa H, Era T. Generation and characterization of PDGFRalpha-GFPCreERT2 knock-In mouse line. Genesis 2015;53:329–36 [DOI] [PubMed] [Google Scholar]
- 45.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 2015;21:786–94 [DOI] [PubMed] [Google Scholar]
- 46.Hayes BJ, Riehle KJ, Shimizu-Albergine M, Bauer RL, Hudkins KL, Johansson F, et al. Activation of platelet-derived growth factor receptor alpha contributes to liver fibrosis. PLoS One 2014;9:e92925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer 2020;146:895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raz Y, Cohen N, Shani O, Bell RE, Novitskiy SV, Abramovitz L, et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med 2018;215:3075–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol 2018;10:1758834017742575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim RK, Kaushik N, Suh Y, Yoo KC, Cui YH, Kim MJ, et al. Radiation driven epithelial-mesenchymal transition is mediated by Notch signaling in breast cancer. Oncotarget 2016;7:53430–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, et al. Epithelial-mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 2016;365:495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm Genome 2018;29:843–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kitajima S, Thummalapalli R, Barbie DA. Inflammation as a driver and vulnerability of KRAS mediated oncogenesis. Semin Cell Dev Biol 2016;58:127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knapinska AM, Estrada CA, Fields GB. The Roles of Matrix Metalloproteinases in Pancreatic Cancer. Prog Mol Biol Transl Sci 2017;148:339–54 [DOI] [PubMed] [Google Scholar]
- 55.Goffin JR, Anderson IC, Supko JG, Eder JP Jr., Shapiro GI, Lynch TJ, et al. Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin Cancer Res 2005;11:3417–24 [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 2014;7:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haque S, Morris JC. Transforming growth factor-beta: A therapeutic target for cancer. Hum Vaccin Immunother 2017;13:1741–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji YJ, Hwang YS, Mood K, Cho HJ, Lee HS, Winterbottom E, et al. EphrinB2 affects apical constriction in Xenopus embryos and is regulated by ADAM10 and flotillin-1. Nat Commun 2014;5:3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Janes PW, Wimmer-Kleikamp SH, Frangakis AS, Treble K, Griesshaber B, Sabet O, et al. Cytoplasmic relaxation of active Eph controls ephrin shedding by ADAM10. PLoS Biol 2009;7:e1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakai N, Bain G, Furuichi K, Iwata Y, Nakamura M, Hara A, et al. The involvement of autotaxin in renal interstitial fibrosis through regulation of fibroblast functions and induction of vascular leakage. Sci Rep 2019;9:7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas D, Radhakrishnan P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer 2019;18:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reisz JA, Bansal N, Qian J, Zhao W, Furdui CM. Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 2014;21:260–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.