Abstract
Objectives
The effect of the use of immunomodulatory drugs on the risk of developing hospital-acquired bloodstream infection (BSI) in patients with COVID-19 has not been specifically assessed. We aim to identify risk factors for, and outcomes of, BSI among hospitalized patients with severe COVID-19 pneumonia.
Methods
We performed a severity matched case–control study (1:1 ratio) nested in a large multicentre prospective cohort of hospitalized adults with COVID-19. Cases with BSI were identified from the cohort database. Controls were matched for age, sex and acute respiratory distress syndrome. A Cox proportional hazard ratio model was performed.
Results
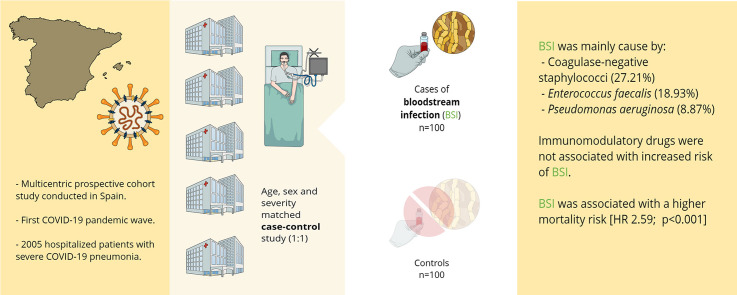
Of 2005 patients, 100 (4.98%) presented 142 episodes of BSI, mainly caused by coagulase-negative staphylococci, Enterococcus faecalis and Pseudomonas aeruginosa. Polymicrobial infection accounted for 23 episodes. The median time from admission to the first episode of BSI was 15 days (IQR 9–20), and the most frequent source was catheter-related infection. The characteristics of patients with and without BSI were similar, including the use of tocilizumab, corticosteroids, and combinations. In the multivariate analysis, the use of these immunomodulatory drugs was not associated with an increased risk of BSI. A Cox proportional hazard ratio (HR) model showed that after adjusting for the time factor, BSI was associated with a higher in-hospital mortality risk (HR 2.59; 1.65–4.07; p < 0.001).
Discussion
Hospital-acquired BSI in patients with severe COVID-19 pneumonia was uncommon and the use of immunomodulatory drugs was not associated with its development. When adjusting for the time factor, BSI was associated with a higher mortality risk.
Keywords: Bacteraemia, COVID-19, Hospital-acquired infection, SARS-CoV-2 pneumonia, Immunomodulatory therapy
Graphical abstract
Introduction
To date, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 116 million cases and more than 2.5 million deaths worldwide [1]. After a decade of austerity in the public health system, and with as many as 3 164 983 cases and 71 727 confirmed deaths up to 9 March 2021, Spain has been particularly badly hit by the COVID-19 pandemic [2,3].
In this setting, various therapeutic strategies have been implemented. These approaches include immunomodulatory drugs such as corticosteroids and monoclonal antibodies, which may potentially increase the risk of infectious complications. Indeed, corticosteroid treatment has previously been associated with viral clearance delay, and anti-interleukin-6 drugs with a suppressed innate immune response [4,5]. Significantly, during the COVID-19 pandemic it has been difficult to maintain infection control standards due to the overburdening of the healthcare systems. In particular, the shortage of staff and critical care resources [6], the proliferation of newly created critical care units with less experienced personnel, the increase in the healthcare worker–patient ratio [7], and the difficulty of conducting adequate antimicrobial stewardship programs may have facilitated the development of hospital-acquired infections, including bloodstream infections (BSIs).
To date, coinfection and superinfection in patients with COVID-19 have been analysed globally as secondary outcomes in a small number of COVID-19 series [[8], [9], [10], [11], [12], [13]]. However, these studies have mainly analysed community-acquired and hospital-acquired infections together, which hinder an accurate assessment of the impact of hospital-acquired infections.
BSI is a complication that has been reported in approximately 5.2% of patients admitted to the intensive care unit (ICU) [14] and is associated with high in-hospital mortality and increased use of antibiotic therapy. To our knowledge, only one study has addressed the burden of BSI in COVID-19 patients, in the United States [15]. However, that study did not correct the immortal time bias or perform severity stratification matching, which may have overestimated the real impact of risk factors such as the use of immunomodulatory drugs. Therefore, we aim to address risk factors and outcomes of COVID-19 patients with hospital-acquired BSI, focusing on the use of immunomodulatory drugs, in a multicentre cohort of consecutive hospitalized patients with COVID-19.
Materials and methods
Study design
We performed a severity matched case–control study (1:1 ratio) nested in a multicentre prospective cohort of hospitalized adults with COVID-19 (COVID-Metrosud). In order to avoid mortality bias, we only included the hospitals of the COVID-Metrosud cohort with ICU units, which are Bellvitge University Hospital, a 700-bed university centre that serves nearly 1 000 000 inhabitants in Catalonia, and the Moisés Broggi Hospital Complex, a 350-bed public hospital that serves 425 000 inhabitants in the same area. All patients were adults (>18 years old) admitted with PCR-proven SARS-CoV-2 infection and severe COVID-19 pneumonia for at least 48 hr between 28 February and 25 April 2020.
For the purpose of the study, patients who developed BSI (cases) were compared with those admitted for the same reason but who did not present this complication (controls). Controls were matched based on age, sex and severity according to PaO2/FiO2 (PaFi) and randomly selected using the R statistical package to reduce selection bias. All controls had negative blood cultures. Patients were followed up to 90 days from admission. When the study was designed, there were no published studies about BSI incidence and possible risk factors in COVID-19 patients. For this reason, we considered the study by Prowle et al. [14] regarding BSI in ICU patients the power of the sample size. Accordingly, we estimated that the incidence of BSI in patients with COVID-19 would be about 5%. The sample size was calculated for a confidence or certainty level of 95% and an expected OR of 2.00, with a statistical power of 80%. Therefore a sample of 100 patients with BSI and 100 controls (1:1 design) was regarded as the best choice.
The study was approved by the Ethics Committee of the coordinating centre in accordance with Spanish legislation, following the ethical standards of the Helsinki Declaration (PR140/20). Due to the observational and anonymous nature of the study, the need for informed consent was waived by the local Ethics Committee.
Data collection and definitions
Data were collected from electronic health records into a secure web-based software platform (REDCAP).
Hospital-acquired BSI was defined as the growth of a non-skin flora commensal in one or more than one blood cultures >48 hr after admission. In the case of the presence of a skin flora commensal such as coagulase-negative staphylococci, growth was required in at least two blood cultures. The study of bacterial pathogens in blood was performed as described (please see supplementary material). Polymicrobial episodes were defined as having >1 clinically significant blood culture isolate occurring within 2 days of each other. Clinical significance of each BSI was categorized as either true bloodstream infection, contamination or unknown significance by one of our researchers. These assessments were made based on the number of positive cultures, presence of plausible source and concordant clinical manifestations. Further definitions and the criteria for the use of immunomodulatory therapy can be found elsewhere (please see supplementary material).
Statistical analysis
Continuous and categorical variables were presented as medians (interquartile range) and absolute numbers (percentage) respectively. Kolmogorov–Smirnoff test was used to evaluate normality, and the Mann–Whitney U-test, chi-squared test and Fisher's test were used to compare differences between patients presenting BSI and controls.
Multivariate analysis was performed with all variables that achieved statistical significance (p < 0.05; CI 95%) in the univariate analysis and the variables considered clinically relevant for this study. To deal with death as a competing risk, a cause-specific Cox regression model was estimated to identify risk factors for hospital-acquired BSI. Participants were censored at death or hospital discharge. The Cox proportional hazards model was used to perform univariate and multivariate survival analyses, which are reported as HR and 95% confidence interval. To avoid immortal time bias, during the time between emergency department assessment and hospital-acquired bloodstream infection patients with BSI were also classified as controls. The proportionality of risks in the Cox models was verified using the Schoenfeld residuals. Statistical analyses were performed with SPSS version 20 (IBM 20.0) and R software 3.6.3 (cran.r-project.org).
Results
Of 2005 patients included, 100 (4.98%) presented 142 episodes of BSI. As shown in Table 1 , there were no differences in demographic and clinical characteristics nor laboratory findings except for D-dimer, which was higher in the BSI group than in controls (975 μg/L vs. 483ug/L; p < 0.001).
Table 1.
Baseline characteristics of all patients compared by groups
| Patients with BSI n = 100 | Controls n = 100 | p | |
|---|---|---|---|
| Agea (median; IQR) | 64.50 (57–71.75) | 64 (57–72) | 1.000 |
| Gender: malea | 66; 66% | 66; 66% | 1.000 |
| Baseline conditions | |||
| CCI (mean; IQR) | 3 (2–4) | 3 (2–4) | 1.000 |
| Obesity | 39; 39% | 36; 36% | 0.770 |
| Diabetes | 27; 27% | 24; 24% | 0.776 |
| Hypertension | 53; 53% | 47; 47% | 0.480 |
| Dyslipaemia | 43; 43% | 42; 42% | 1.000 |
| Ischemic cardiac disease | 8; 8% | 5; 5% | 0.568 |
| Atrial fibrillation | 1; 1% | 6; 6% | 0.118 |
| COPD | 4; 4% | 6; 6% | 0.748 |
| OSA | 14; 14% | 10; 10% | 0.515 |
| Asthma | 4; 4% | 4; 4% | 1.000 |
| Chronic kidney disease | 11; 11% | 9; 9% | 0.748 |
| Haematological malignancy | 0; - | 3; 3% | 0.246 |
| Solid malignancy | 9; 9% | 10; 10% | 1.000 |
| Ictus | 5; 5% | 3; 3% | 0.721 |
| Vascular disease | 1; 1% | 3; 3% | 0.621 |
| Dementia | 1; 1% | 2; 2% | 1.000 |
| Chronic hepatitis | 0; - | 3; 3% | 0.246 |
| Connective tissue disease | 3; 3% | 4; 4% | 1.000 |
| HIV | 1; 1% | 0; - | 0.364 |
| Solid organ transplant | 2; 2% | 0; - | 0.497 |
| Immunosuppressive treatment | 3; 3% | 4; 4% | 1.000 |
| Laboratory findings | |||
| PaFia | 244 (87–286.50) | 242 (137.25–295) | 0.861 |
| Leucocytes (×109/L (median, IQR) | 7.82 (5.38–10.70) | 7.50 (5.27–10.76) | 0.884 |
| Neutrophils (/mm3)) (median, IQR) | 6560 (4000–9445) | 5835 (3540–9142) | 0.466 |
| Lymphocytes (/mm3) (median, IQR) | 860 (642.5–1000) | 830 (560–1157) | 0.768 |
| Platelets (×109/L) (median, IQR) | 205 (159.5–250.7) | 182 (154–253) | 0.122 |
| Albumin (g/L) (median, IQR) | 35 (28.5–37.7) | 34 (31.75–37) | 0.785 |
| AST (U/L) (median, IQR) | 54.5 (33.7–71) | 50.8 (31–94.7) | 0.364 |
| C-reactive protein (mg/L) (median, IQR) | 149 (83.3–235.2) | 128 (63.6–218.5) | 0.157 |
| D-dimer (μg/L)(median, IQR) | 975 (647–1562) | 483 (312–955) | <0.001 |
| Ferritin (μg/L) (median, IQR) | 806 (308–1167) | 990 (267 -1428) | 0.490 |
| Radiological findings | |||
| Bilateral involvement | 88; 88% | 83; 83% | 0.422 |
| Severity scores | |||
| PSI score | 3 (2–4) | 3 (2–4) | 0.461 |
| CURB-65 score | 1 (1–2) | 1 (1 -2) | 0.237 |
| MuLBSTA score | 9 (7–11) | 9 (5–9) | 0.324 |
BSI, bloodstream infection; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea; HIV, human immunodeficiency virus; AST, alanine aspartate aminotransferase; IQR, interquartile range, SD, standard deviation; ICU, intensive critical unit; PSI score, Pneumonia Severity Index or PORT score; CURB-65 score, Confusion, Urea, Respiratory rate, Blood pressure <90, Age ≥65; pneumonia severity score; MuLBSTA score, Mortality Risk in Patients with Viral Pneumonia.
Matched variables.
Table 2 shows that the median length of hospital admission until first BSI was 15 (9–20) days. Most episodes (87.3%) occurred in the ICU, and 30.98% developed in newly created critical care units. The most frequent focus of BSI was catheter infection (47.1%), followed by unknown (23.9%) and respiratory origin (21.8%). The most frequently isolated microorganisms were coagulase-negative staphylococci (27.2%), followed by Enterococcus faecalis (18.9%) and Pseudomonas aeruginosa (8.8%). Up to 16.1% of BSI were polymicrobial, with Gram-positive combined with Gram-negative bacteraemia being the most frequent isolation (52.17%). Finally, 10.65% of cases were due to antibiotic-resistant microorganisms. The prevalence of the different microorganisms depending on the presumed focus can be consulted found in the supplementary material.
Table 2.
Clinical and microbiological characteristics of 142 health-acquired bloodstream infection episodes
| Patients with BSI (n = 100) Number of episodes = 142 | |
|---|---|
| BSI per patient (median; IQR) | 1; (1–2) |
| Length of hospital admission until BSI episode in days (median; IQR) | 15 (9–20) |
| BSI acquisition | |
| ICU | 124; 87.32% |
| General ward | 18; 12.67% |
| BSI sources | |
| Catheter infection | 67; 47.18% |
| Unknown origin | 34; 23.94% |
| Respiratory tract | 31; 21.83% |
| Urinary tract | 9; 6.33% |
| Skin and soft tissue infection | 1; 0.70% |
| Total of microorganisms isolated (n = 169) | |
| Gram-positive microorganisms | 100; 59.17% |
| Coagulase-negative staphylococci | 46; 27.21% |
| Enterococcus faecalis | 32; 18.93% |
| Enterococcus faecium | 10; 5.91% |
| Staphylococcus aureus | 9; 5.32% |
| Streptococcus pneumoniae | 2; 1.18% |
| Streptococcus mitis | 1; 0.59% |
| Gram-negative microorganisms | 64; 37.86% |
| Pseudomonas aeruginosa | 15; 8.87% |
| Klebsiella pneumoniae | 10; 5.91% |
| Serratia marcescens | 9; 5.32% |
| Escherichia coli | 8; 4.73% |
| Enterobacter cloacae | 8; 4.73% |
| Enterobacter aerogenes | 7; 4.14% |
| Other Klebsiella spp. | 3; 1.77% |
| Achromobacter spp. | 1; 0.59% |
| Proteus mirabilis | 1; 0.59% |
| Stenotrophomonas maltophilia | 1; 0.59% |
| Fungi | 5; 2.95% |
| Candida albicans | 2; 1.18% |
| Candida glabrata | 1; 0.59% |
| Candida parapsilopsis | 1; 0.59% |
| Candida tropicalis | 1; 0.59% |
| Anaerobes | |
| Bacteroides fragilis | 2; 1.18% |
| Polymicrobial | 23; 16.19% |
| Gram-positive combinationa | 9; 39.13% |
| Gram-positive and negative combinationa | 12; 52.17% |
| Gram-negative combinationa | 2; 8.69% |
| Antibiotic-resistant microorganisms | 18; 10.65% |
| ESBL-producing Enterobacteralesb | 10; 55.55% |
| Vancomycin-susceptible ampicillin-resistant Enterococcus spp.b | 3; 16.66% |
| ESBL-producing and carbapenem-resistant Pseudomonas spp.b | 2; 11.11% |
| Methicillin-resistant S. aureusb | 2; 11.11% |
| Vancomycin-resistant Staphylococcus epidermidisb | 1; 5.55% |
BSI, bloodstream infection; IQR, Interquartile range; SD, standard deviation; ICU, intensive care unit; ESBL, extended-spectrum β-lactamase.
(n; % of total of polymicrobial episodes).
(n; % of total antibiotic-resistant microorganisms).
As shown in Table 3 , we found no association between BSI the use of tocilizumab alone or combined with corticosteroids. Of note, local guidelines allowed for only one dose of Tocilizumab. Nevertheless, patients with BSI received corticosteroids for longer than the control group (14.5 days vs. 8.7 days, p 0.01). Antibiotic treatment was also significantly longer (17.7 days vs. 7 days; p < 0.001) in this group of patients. Patients with BSI received broad-spectrum antibacterial therapy more often (76% vs. 40%; p < 0.001), but, there were no differences regarding DOT/1000 patient-days between the groups (p 0.564).
Table 3.
Antiviral, anti-inflammatory and antimicrobial treatment and outcomes
| Patients with BSI n = 100 | Controls n = 100 | p | |
|---|---|---|---|
| Antiviral treatment | |||
| Hydroxychloroquine monotherapy | 59; 59% | 45; 45% | 0.066 |
| Lopinavir monotherapy | 1; 1% | 0 | 1.000 |
| Lopinavir and hydroxychloroquine | 29; 29% | 39; 39% | 0.179 |
| Remdesivir | 6; 6% | 5; 5% | 0.764 |
| Anti-inflammatory treatment | |||
| Tocilizumab | 45; 45% | 45; 45% | 1.000 |
| Corticosteroids | 65; 65% | 58; 58% | 0.383 |
| >1 mg/kg | 42; 42% | 30; 30% | 0.106 |
| LOT in days (median; SD) | 14.50 ± 20.72 | 8.78 ± 11.23 | 0.010 |
| Tocilizumab and corticosteroids | 33; 33% | 31; 31% | 0.880 |
| Antibiotic therapy | |||
| Broad-spectrum antibiotic therapy | 76 (76%) | 40 (40 %) | <0.001 |
| LOT antibiotic therapy (median; IQR) | 17 (7–32) | 7 (4 –13) | <0.001 |
| DOT antibiotic therapy (median; IQR) | 71 (46–101) | 41 (31–88) | 0.564 |
| Outcomes | |||
| ICU admission | 91; 91% | 91; 91% | 1.000 |
| Length of ICU stay (median; IQR) | 24 (13.5–41) | 10 (5–16) | <0.001 |
| Septic shocka | 79; 57.7% | 58; 42.3% | 0.002 |
| Mechanical ventilationa | 88; 56.1% | 69; 43.9% | 0.002 |
| Days of mechanical ventilation (median; IQR) | 23 (13–38) | 9 (4–14) | <0.001 |
| Total length of hospital stay (median; IQR) | 33 (18–53) | 18 (10–29) | <0.001 |
| In-hospital mortality | 49; 49% | 46; 46% | 0.777 |
| 30-day case-fatality rate | 31; 31% | 16; 16% | 0.013 |
BSI, bloodstream infection; SD, standard deviation; LOT, length of treatment; DOT, days of treatment; IQR, interquartile range; ICU, intensive care unit.
(n; % of ICU admitted patients).
In terms of outcomes, patients with BSI presented longer hospital stay (33.5 days vs. 18 days; p < 0.001) and longer ICU stay (24 days vs. 10 days; <0.001) than patients in the control group. Furthermore, patients with BSI and COVID-19 presented significantly more septic shock during hospitalization (57.7% vs. 42.3%) and more received mechanical ventilation (56.1% vs. 43.9%; p < 0.001. We did not find an association between BSI and global in-hospital case fatality rate (49% vs. 46%; p 0.777). Nevertheless, the 30-day case-fatality rate was significantly higher in the BSI group (31% vs. 16%; p 0.013).
The cause-specific multivariate analysis did not reveal the use of immunomodulatory drugs as significant risk factors for the development of BSI (Table 4 ). Instead, the d-dimer >700 μg/L appears as the only independent predictor of BSI (HR 2.68; p < 0.001). Among episodes of BSI, advanced age (69 years vs. 60 years; HR 1.09) and lymphopenia (830/mm3 vs. 1000/mm3; HR 1.00) were independent risk factors for in-hospital mortality (please see supplementary material).
Table 4.
Risk factors for bloodstream infection development in patients with COVID-19 by multivariate analysis
| Patients with BSI n = 100 | Controls n = 100 | p | OR; CI 95% | p | |
|---|---|---|---|---|---|
| Agea (median; IQR) | 64.50 (57–71.75) | 64 (57–72) | 1.000 | 1.05 (0.96–1.16) | 0.256 |
| Gender: Male | 66; 66% | 66; 66% | 1.000 | 1.12 (0.71–1.75) | 0.631 |
| D-dimer (>700 μg/L) | 42; 42% | 9; 9% | <0.001 | 2.68 (1.61–4.44) | <0.001 |
| Tocilizumab | 45; 45% | 45; 45% | 1.000 | 1.23 (0.73–2.07) | 0.447 |
| Corticosteroids (>10 days) | 39; 39% | 36; 36% | 0.770 | 1.22 (0.68–2.20) | 0.509 |
| Tocilizumab and corticosteroids (>10 days) | 21; 21% | 22; 22% | 0.880 | 0.74 (0.44–1.78) | 0.500 |
| Previous Broad-spectrum antibiotic therapy | 61 (61%) | 33 (33%) | <0.001 | 1.23 (0.80–1.88) | 0.347 |
| ICU admission | 91; 91% | 91; 91% | 1.000 | 1.15 (0.43–3.08) | 0.779 |
OR, odds ratio; CI, confidence interval; IQR, interquartile range; SD, standard deviation; LOT, length of treatment; ICU, Intensive Care Unit; IQR, interquartile range.
5 year increase in age.
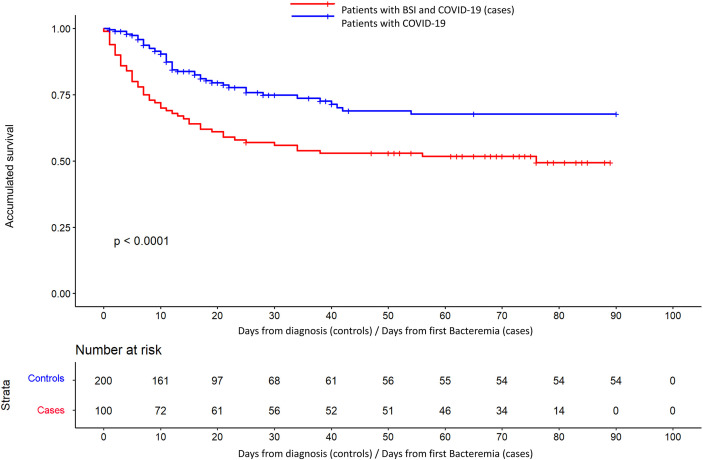
When mortality was adjusted for time of exposure from the event (Fig. 1 and Table 5 ), it was significantly higher in the group of patients with BSI (HR 2.59; p < 0.001). Other independent factors for in-hospital mortality were advanced age (HR 1.05; p 0.003) and ICU admission (HR 4.01; p 0.005). The univariate analysis of the risk factors for in-hospital mortality can be found in the supplementary material.
Fig. 1.
Survival curves for the Cox proportional regression model in BSI (cases) and controls groups.
Table 5.
Risk factors for in-hospital mortality by time-adjusted Cox proportional hazards ratio model
| In-hospital mortality (n = 95; 47.50%) | Survivors (n = 105; 52.5%) | p | HR; CI 95% | p | |
|---|---|---|---|---|---|
| Group: Cases | 49; 51.6% | 51; 48.6% | 0.777 | 2.59 (1.65–4.07) | <0.001 |
| Agea (median; IQR) | 69 (61.50–72) | 60 (53–67) | 0.016 | 1.05 (1.02–1.09) | 0.003 |
| Gender: Women | 43; 45.3% | 54; 51.4% | 0.085 | 0.96 (0.58–1.61) | 0.894 |
| Charlson Comorbidity Index (median; IQR) | 3 (2–4) | 2 (1–3) | 0.054 | 1.06 (0.91–1.24) | 0.431 |
| PSI group (median; IQR) | 3 (2–4) | 2 (2 - 3) | 0.002 | 1.27 (0.77–2.09) | 0.342 |
| CURB-65 (median; IQR) | 2 (1–2) | 1 (1–2) | <0.001 | 1.14 (0.54–2.40) | 0.778 |
| Tocilizumab | 45; 47.4% | 44; 41.9% | 0.478 | 0.72 (0.29–1.83) | 0.538 |
| Corticosteroids | 53; 55.8% | 70; 66.7% | 0.146 | 0.45 (0.24–0.84) | 0.010 |
| Tocilizumab and corticosteroids | 32; 33.7% | 32; 30.5% | 0.649 | 2.29 (0.76–6.96) | 0.168 |
| ICU admission | 90; 94.7% | 92; 87.6% | <0.089 | 3.96 (1.47–10.68) | 0.010 |
HR, hazard ratio; IQR, interquartile range; PSI, Pneumonia Severity Index; LOT, length of treatment; ICU, Intensive Care Unit.
5 year increase in age.
Discussion
In our large multicentre cohort of hospitalized severity-matched patients with pneumonia due to SARS-CoV-2, we found that hospital-acquired BSI was uncommon, mainly limited to patients admitted to the ICU, and not associated with immunomodulatory therapy.
Most studies conducted so far have analysed community-acquired and hospital-acquired co-infection together [[10], [11], [12], [13]]. However, these two scenarios should be analysed separately since they represent different clinical presentations requiring different approaches. On the one hand, empirical antibiotic treatment is usually given in cases of suspected coinfection; however, this does not seem to be justified in view of the low rates of bacterial coinfection observed on hospital admission [16], which are lower than those reported during the H1N1 influenza pandemic [17]. On the other, nosocomial infection may be more closely related to host characteristics and to the management during hospitalization. In this regard, the prevalence of BSI in the setting of our cohort of patients with severe SARS-CoV-2 pneumonia was similar to that reported in other critically ill patients [14,18]. However, one recent multicentre case-cohort study, based on a large ICU cohort in France, found that the ICU-BSI risk was higher for COVID-19 than non-COVID-19 critically ill patients after seven days of ICU stay [19].
One interesting finding in our study was the longer median time to the first BSI episode in our cohort compared with reports of H1N1 influenza infection and other COVID-19 studies mentioned above. In addition, in our study Staphylococcus aureus was less frequently recorded than in influenza pneumonia [20]. Interestingly, we identified a higher rate of polymicrobial BSI including Enterococcus spp. from an unknown source than other series involving general population [21]. Nevertheless, our rates are similar to those for enterococcal bacteraemia in COVID-19 patients admitted to the ICU [22] reported in an observational Italian study, which reached 55.8%. These findings may be partially explained by the fact that SARS-CoV-2 also affects the gastrointestinal tract and could facilitate bacterial translocation due to mucosal damage. Moreover, broad-spectrum antimicrobial treatment depletes the normal gastrointestinal flora and favours enterococcal proliferation, particularly in frail patients [23]. As we have reported elsewhere, overall antimicrobial consumption increased dramatically during the study period [24]. In this regard, although the indication of antimicrobials in COVID-19 patients continues to be controversial, we believe that antimicrobial stewardship must be prioritized [25]. However, the ratio of BSI due to antibiotic-resistant microorganisms in our study (~11%) was lower than previously observed in our centres (~18%). Of note, between 2015 and 2018 one of the two participating centres presented an outbreak of NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae [26], which could have been a determinant factor in this differences. Studies are needed on the real impact of the COVID-19 pandemic on antimicrobial resistance.
Even though no differences were found regarding baseline conditions and clinical presentations, D-dimer levels on admission were higher in patients with BSI. This finding has been associated with the development of culture-proven BSI [27]. It can be speculated that d-dimer could act as a surrogate marker for endothelial activation and posterior BSI due to skin bacteria in COVID-19 patients. Significantly, immunomodulatory drugs did not seem to be a risk factor for BSI development. On the one hand, and in line with the previous trials with Tocilizumab and other drugs such as baricitinib, immunomodulatory treatment was not associated with an increased risk of BSI [28,29]. On the other, and even though corticosteroids have been identified as risk factors for BSI in COVID-19 and non-COVID-19 patients, this association was not borne out in our analysis [15]. A plausible explanation is that we applied a severity-matched stratification, and also the duration of corticosteroid treatment was longer in patients presenting BSI.
We also found that when adjusting for time to avoid immortal time bias, BSI was significantly associated with higher mortality. Interestingly, as broadly reported, treatment with corticosteroids was identified as a protective factor for in-hospital mortality in patients with severe COVID-19. These results are similar to those reported in the French study mentioned above [19]. Furthermore, in agreement with previous studies [12], advanced age and lymphopenia were identified as predictors of mortality among episodes of BSI.
We hypothesize that the difficulty of achieving optimal compliance with infection control measures may have played a role in the prevalence of BSI in COVID-19 patients. In this regard, the incorporation of untrained critical care personnel, an overburdened healthcare system, and the difficulty in complying with standard hand hygiene recommendations may have affected the incidence of BSI in COVID-19 patients. Furthermore, the relocation of staff to front-line work and the extra burden in managing COVID-19 control measures, the daily activities carried out by infection control departments were seriously affected [30].
This study has several limitations that should be acknowledged. Firstly, the prospective cohort in which this study was conducted was not designed for study of this kind. Secondly, the study was conducted in a limited geographical area; although the prospective database of the COVID-Metrosud cohort includes five hospitals, only two were selected for this analysis. Thirdly, relevant data regarding infection control parameters were not obtained. Lastly, controls were not matched for the time at risk for developing BSI. Nevertheless, this is the first large prospective, severity-matched, case–control multicentre study evaluating the role of immunomodulatory therapies in the development of BSI as a complication of severe COVID-19. Also, since each patient included in the study was individually evaluated by an infectious diseases specialist, the data are reliable and focused on BSI. Furthermore, we applied a cause-specific Cox regression model to deal with death as a potential competing risk and a Cox proportional hazards model to avoid immortal time bias regarding the outcomes analysis.
In summary, this study is the first severity-matched case-control study addressing BSI as a complication of patients admitted with COVID-19. We found that BSI occurred later than in other critical care conditions and that polymicrobial flora and Enterococcus spp. aetiology were frequently isolated. When matched for severity, immunomodulatory drugs were not identified as an independent risk for the development of BSI. Nevertheless, BSI in severe COVID-19-patients was associated with higher mortality risk. Hygiene measures and infection control surveillance are key steps to reduce BSI in patients with COVID-19.
Transparency declaration
All authors have completed the ICMJE uniform disclosure form. G.A.A. reports a predoctoral research grant from the 201808-10 project, funded by La Marató de TV3. A.R. is receiving a predoctoral research grant from the Instituto de Salud Carlos III, Spanish Ministry of Science, Innovation and Universities, (PFIS grant FI18/00183). A.R.M. is currently leading a study on Parkinson's disease granted by AbbVie (outside the submitted work). J.C., G.A.A., A.R. report participating in F. Hoffmann–La Roche and Gilead Sciences financed clinical trials. This study was supported by La Marató de TV3 (grant no. 201808-10). We thank CERCA Programme/Generalitat de Catalunya for institutional support. This study was also supported by the Plan Nacional de I+D+I 2013–2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (grant. REIPI RD16/0016/0008). The study was cofinanced by European Development Regional Fund “A way to achieve Europe”, Operative program Intelligent Growth 2014–2020. This study was also supported by a research grant from the Instituto de Salud Carlos III, Spanish Ministry of Science, Innovation and Universities, through the 2018 call for predoctoral contracts for training in health research (FI18/00183).
Author contributions
G.A.A., A.R. and C.G. contributed to the concept and design of the study. The inclusion, data collection and interpretation was performed by G.A.A., A.R., I.O., A.S., A.C., A.R.M., E.I., V.D.B., IG, MR, AB, LG, AB, CAP. AP reviewed the antimicrobial use analysis. A.P., S.B. and C.T. supervised and performed statistical analysis. C.G., A.R. and J.C. contributed greatly to the writing of this paper. All authors have read and approved the final version of this manuscript.
Access to data
Data collected for the study—including de-identified individual participant data and a data dictionary defining each field in the set—will be made available to researchers who provide a methodologically sound proposal to the corresponding author with a signed data access agreement at any point.
Acknowledgements
The authors would like to thank all the health and non-health professionals of five hospitals conforming COVID-Metrosud cohort their work during the last year. We would also want to remember all the patients admitted and all those family members who, still unable to visit their own, encouraged us to continue this and other research projects.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.06.041.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.WHO Coronavirus (COVID-19) Dashboard WHO coronavirus disease (COVID-19) dashboard. https://covid19.who.int/
- 2.García-Basteiro A., Alvarez-Dardet C., Arenas A., Bengoa R., Borrell C., Del Val M., et al. The need for an independent evaluation of the COVID-19 response in Spain. Lancet. 2020;396:529–530. doi: 10.1016/S0140-6736(20)31713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health of Spain Update No. 328. Coronavirus disease (COVID-19) https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_328_COVID-19.pdf
- 4.Pawar A., Desai R.J., Solomon D.H., Santiago Ortiz A.J., Gale S., Bao M., et al. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis. 2019;78:456–464. doi: 10.1136/annrheumdis-2018-214367. [DOI] [PubMed] [Google Scholar]
- 5.Yang J.W., Fan L.C., Miao X.Y., Mao B., Li M.H., Lu H.W., et al. Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect. 2015;21:956–963. doi: 10.1016/j.cmi.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Cimiotti J.P., Aiken L.H., Sloane D.M., Wu E.S. Nurse staffing, burnout, and health care-associated infection. Am J Infect Contr. 2012;40:486–490. doi: 10.1016/j.ajic.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Needleman J., Buerhaus P., Mattke S., Stewart M., Zelevinsky K. Nurse-Staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346:1715–1722. doi: 10.1056/NEJMsa012247. [DOI] [PubMed] [Google Scholar]
- 8.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Vidal C., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., et al. Incidence of co-infections and superinfections in hospitalised patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ripa M., Galli L., Poli A., Oltoni C., Spagnuolo V., Mastrangelo A., et al. Secondary infections in patients hospitalized with COVID-19: incidence and predictive factors. Clin Microbiol Infect. 2021;27:451–457. doi: 10.1016/j.cmi.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nori P., Cowman K., Chen V., Bartash R., Szymczak W., Madaline T., et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Contr Hosp Epidemiol. 2021;42:84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prowle J.R., Echeverri J.E., Ligabo E.V., Sherry N., Taori G.C., Crozier T.M., et al. Acquired bloodstream infection in the intensive care unit: incidence and attributable mortality. Crit Care. 2011;15:R100. doi: 10.1186/cc10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt P.J., Shiau S., Brunetti L., Xie Y., Solanki K., Khalid S., et al. Risk factors and outcomes of hospitalized patients with severe coronavirus disease 2019 (COVID-19) and secondary bloodstream infections: a multicenter case-control study. Clin Infect Dis. 2021;72:e995–e1003. doi: 10.1093/cid/ciaa1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Loeches I., Schultz M.J., Vincent J.L., Alvarez-Lerma F., Bos L.B., Solé-Violán J., et al. Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med. 2017;43:48–58. doi: 10.1007/s00134-016-4578-y. [DOI] [PubMed] [Google Scholar]
- 18.Giacobbe D.R., Battaglini D., Ball L., Brunetti B., Codda G., Crea F., et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50 doi: 10.1111/eci.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buetti N., Ruckly S., de Montmollin E., Reignier J., Terzi N., Cohen Y., et al. COVID-19 increased the risk of ICU-acquired bloodstream infections: a case-cohort study from the multicentric OUTCOMEREA network. Intensive Care Med. 2021;47:180–187. doi: 10.1007/s00134-021-06346-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muscedere J., Ofner M., Kumar A., Long J., Lamontagne F., Cook R., et al. The occurrence and impact of bacterial organisms complicating critical care illness associated with 2009 influenza A(H1N1) infection. Chest. 2013;144:39–47. doi: 10.1378/chest.12-1861. [DOI] [PubMed] [Google Scholar]
- 21.Pavlaki M., Poulakou G., Drimousis P., Adamis G., Apostolidou E., Gatselis N.K., et al. Polymicrobial bloodstream infections: epidemiology and impact on mortality. J Glob Antimicrob Resist. 2013;1:207–212. doi: 10.1016/j.jgar.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Bonazzetti C., Morena V., Giacomelli A., Oreni L., Casalini G., Galimberti L.R., et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: an observational study. Crit Care Med. 2021;49:e31–e40. doi: 10.1097/CCM.0000000000004748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gudiol C., Ayats J., Camoez M., Domínguez M.A., García-Vidal C., Bodro M., et al. Increase in bloodstream infection due to vancomycin-susceptible Enterococcus faecium in cancer patients: risk factors, molecular epidemiology and outcomes. PLoS One. 2013;8:74734. doi: 10.1371/journal.pone.0074734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abelenda-Alonso G., Padullés A., Rombauts A., Gudiol C., Pujol M., Álvarez-Pouso C., et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Contr Hosp Epidemiol. 2020;41:1371–1372. doi: 10.1017/ice.2020.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huttner B., Catho G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw E., Rombauts A., Tubau F., Padullés A., Càmara J., Lozano T., et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2018;73:1104–1106. doi: 10.1093/jac/dkx496. [DOI] [PubMed] [Google Scholar]
- 27.Schwameis M., Steiner M.M., Schoergenhofer C., Lagler H., Buchtele N., Jilma-Stohlawetz P., et al. D-dimer and histamine in early stage bacteremia: a prospective controlled cohort study. Eur J Intern Med. 2015;26:782–786. doi: 10.1016/j.ejim.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Rosas I.O., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMullen K.M., Smith B.A., Rebmann T. Impact of SARS-CoV-2 on hospital acquired infection rates in the United States: predictions and early results. Am J Infect Contr. 2020;48:1409. doi: 10.1016/j.ajic.2020.06.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.