Abstract
We report a case of minimal change disease (MCD) with severe acute kidney injury (AKI) following the first injection of the ChAdOx1 nCoV-19 (AZD1222) vaccine from Oxford-AstraZeneca against coronavirus disease 2019 (COVID-19). A 71-year-old man with a history of dyslipidemia and a baseline serum creatinine of 0.7 mg/dL presented with nephrotic syndrome, AKI, and severe hypertension 13 days after receiving the Oxford-AstraZeneca vaccine. Refractory hyperkalemia and hypervolemia with oligoanuria prompted initiation of hemodialysis. His serum albumin was 2.6 g/dL and his urinary protein-creatinine ratio was 2,321 mg/mmol. Given a high suspicion for rapidly progressive glomerulonephritis, empirical glucocorticoid treatment was initiated (3 methylprednisolone pulses followed by high-dose prednisone). A kidney biopsy showed MCD and acute tubular injury. Kidney function and proteinuria subsequently improved, and hemodialysis was discontinued 38 days after the start of therapy. This case describes de novo MCD after the Oxford-AstraZeneca vaccine. It adds to the few published case reports of MCD after the Pfizer-BioNTech COVID-19 vaccine. Further reports and studies will be needed to elucidate whether MCD is truly associated with COVID-19 vaccination.
Index Words: acute kidney injury (AKI), acute tubular injury (ATI), case report, adverse event, coronavirus disease 2019 (COVID-19), COVID-19 vaccine, minimal change disease (MCD), renal biopsy, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), vaccine safety
Introduction
The coronavirus 2019 (COVID-19) pandemic has been associated with an increased mortality worldwide. New vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were thus developed to limit contagion and mortality. Major side effects of these vaccines are uncommon. However, de novo minimal change disease (MCD) following the Pfizer-BioNTech COVID-19 vaccine has been reported.1, 2, 3
We report the case of a 71-year-old man with nephrotic syndrome and acute kidney injury (AKI) following the administration of the ChAdOx1 nCoV-19 (AZD1222) vaccine, which was developed by the University of Oxford, AstraZeneca, and the Serum Institute of India, and is based on a replication-incompetent chimpanzee adenovirus vector that expresses the SARS-CoV-2 spike protein. His kidney biopsy demonstrated MCD and acute tubular injury.
Case Report
A 71-year-old man presented to the emergency room with anasarca, AKI, and severe hypertension. He was only known to have dyslipidemia, which was treated with rosuvastatin, 20 mg daily. His usual blood pressure was 130/80 mm Hg. He had no prior history of SARS-CoV-2 infection or chronic kidney disease. His baseline serum creatinine was 0.7 mg/dL. He was not taking any other medications including nonsteroidal anti-inflammatory drugs. Thirteen days before presentation, he received the first injection of the Oxford-AstraZeneca COVID-19 vaccine. One day after vaccination, and 12 days before presentation, he developed new-onset hypertension (>190/>100 mm Hg) and progressive facial and upper extremity edema. He was seen by his family physician who prescribed amlodipine; he then presented to the emergency department when his edema worsened.
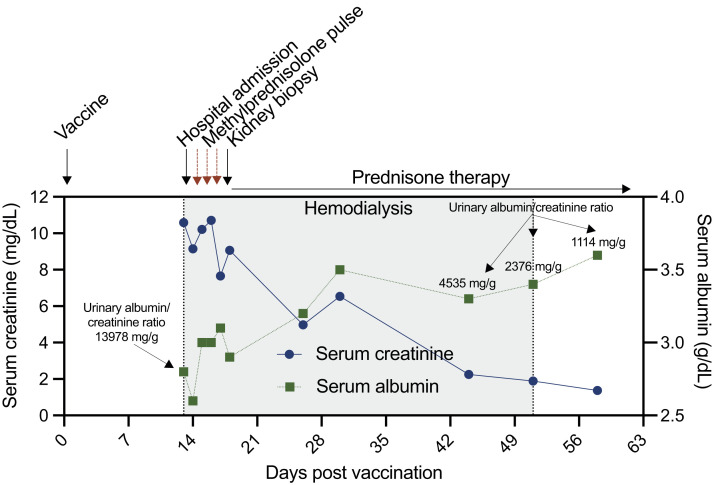
On admission, his blood pressure was 204/102 mm Hg. Physical examination revealed bilateral pitting edema in all extremities. His serum albumin and creatinine were 2.8 g/dL and 10.6 mg/dL, respectively. His serum urea nitrogen was 103.8 mg/dL. Serum sodium (138 mmol/L), calcium (9 mg/dL), and bicarbonate (25 mmol/L) were within reference ranges, but serum magnesium (2.9 mg/dL), potassium (5.5 mmol/L), and phosphorus (9.6 mg/dL) were elevated. Hemoglobin (13.4 g/L) and bilirubin (0.18 mg/dL) were within reference ranges, and haptoglobin was not decreased (3.05 g/L). The initial urinary protein-creatinine ratio (UPCR) was 2321 mg/mmol. On urine microscopy, 6 to 10 red blood cells per high-power field and granular casts were seen. Polymerase chain reaction testing for SARS-CoV-2 was negative. The clinical course and important laboratory studies are summarized in Figure 1 .
Figure 1.
Timeline of clinical events from vaccination to the last hemodialysis session.
To exclude infection and neoplasm, an abdominal ultrasound, thoracic computed tomography, and blood cultures were performed and were unremarkable. Negative results were found upon testing for antinuclear and anti–double-stranded DNA antibodies, antineutrophil cytoplasmic autoantibodies, as well as for antibodies to glomerular basement membrane and M-type phospholipase A2 receptor (PLA2R). Levels of complement factors C3 and C4 were normal. Tests for HIV and hepatitis B and C virus were negative. Serum free light chains levels and protein electrophoresis with immunofixation were normal.
One day after admission, the patient was oligoanuric and his hyperkalemia and hypervolemia became refractory to intravenous diuretics, prompting hemodialysis to be initiated. Given the presence of AKI, proteinuria, hematuria, edema, and hypertension, a rapidly progressive glomerular disease could not be excluded. Empirical steroid therapy was thus initiated 1 day after admission, starting with 3 daily pulses of 1 g of methylprednisolone followed by prednisone, 60 mg per day.
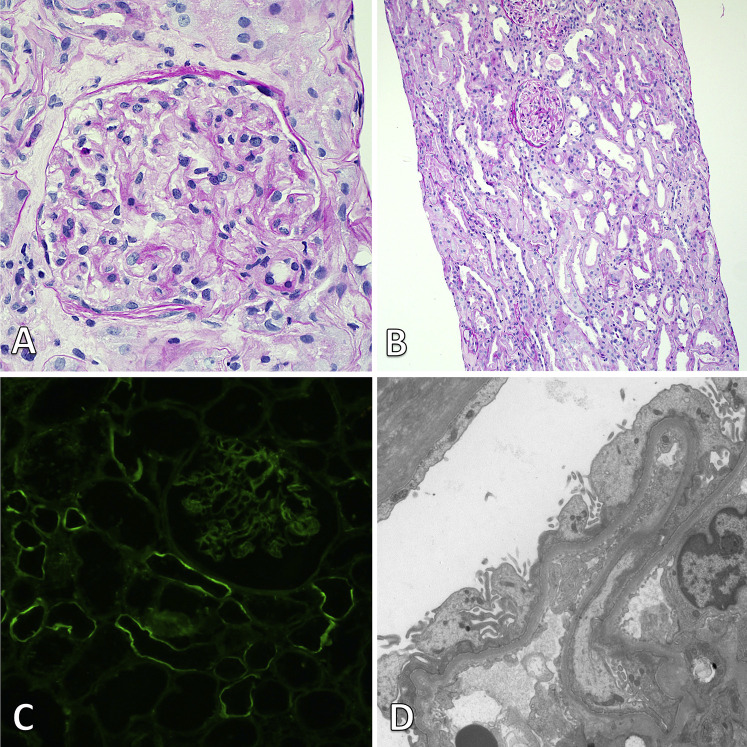
A kidney biopsy was performed 4 days after admission and 17 days after vaccination. There were 24 glomeruli sampled for light microscopy, including 2 globally sclerotic. Viable glomeruli showed a normal morphology. In the cortex, diffuse acute tubular epithelial injury was observed, with epithelial flattening, loss of brush border, and cell sloughing. Interstitial fibrosis was mild, accompanied by mild mononuclear interstitial infiltrate. Arteriolar hyalinosis was moderate, and arterial sclerosis was mild.
Immunofluorescence for immunoglobulin A (IgA), IgG, IgM, κ and λ light chain, C3, C1q, fibrin, and albumin was performed on 10 glomeruli and did not show any specific glomerular staining. Segmental linear staining along the tubular basement membranes was noted with IgG and κ light chain (1+ on a scale of 0 to 3+). The electron microscopy report showed diffuse podocyte foot-process effacement (>80%) with focal microvillous transformation. No glomerular or tubular immune-complex–type deposits or organized deposits were seen (Fig 2 ). There were no tubuloreticular inclusions.
Figure 2.
Kidney biopsy findings. (A) Light microscopy shows a normal glomerulus (periodic acid–Schiff; original magnification, ×400). (B) At a lower power view, diffuse acute tubular injury is present with tubular dilatation and epithelial simplification (periodic acid–Schiff; original magnification, ×100). (C) Immunofluorescence staining for IgG shows segmental linear staining along some tubular basement membranes without any specific glomerular staining (×200). (D) Electron microscopy reveals diffuse foot-process effacement with focal microvillous transformation of the podocytes and no electron-dense deposits (original magnification, ×8,000).
Together, these findings were compatible with a diagnosis of MCD with acute tubular injury. Hemodialysis therapy was stopped after 38 days of therapy. At dialysis cessation, serum creatinine went down to 1.4 mg/dL. At the last follow-up evaluation, 30 days after the end of hemodialysis, the UPCR had decreased to 28 mg/mmol, and serum creatinine was 1.2 mg/dL.
Discussion
We described the case of a 71-year-old man who presented with nephrotic syndrome, new-onset hypertension, and AKI 13 days after receiving his first dose of the Oxford-AstraZeneca COVID-19 vaccine. We diagnosed MCD with acute tubular injury. The patient was not known to have any disease that could cause secondary MCD. Thus, we suspect that his presentation might be related to the Oxford-AstraZeneca COVID-19 vaccine. Although our case describes de novo MCD related to the Oxford-AstraZeneca COVID-19 vaccine, cases of both de novo and relapsing MCD have been described with the Pfizer-BioNTech COVID-19 vaccine.4 Two cases of relapsing MCD have been reported with the Oxford-AstraZeneca COVID-19 vaccine.5
Lebedev et al1 described a 50-year-old previously healthy man presenting with nephrotic syndrome and AKI 10 days after receiving the Pfizer-BioNTech COVID-19 vaccine. They diagnosed MCD with acute tubular injury on kidney biopsy, and the patient responded well to steroids 5 days after initiation of therapy. He did not need dialysis.
D’Agati et al2 described a 77-year-old man with a 15-year history of type 2 diabetes without retinopathy and coronary artery disease who presented with nephrotic syndrome and AKI 7 days after receiving the Pfizer-BioNTech COVID-19 vaccine. The kidney biopsy showed MCD with concurrent acute tubular injury and mild diabetic changes. The patient did not respond to steroids after 3 weeks of treatment, with a reported serum creatinine of 3.74 mg/dL. He did not receive dialysis treatment during the described clinical course.
Maas et al3 described a patient in his eighties with history of venous thromboembolism and taking no medications who presented 7 days after his first dose of Pfizer-BioNTech COVID-19 vaccine with nephrotic syndrome and AKI. The kidney biopsy also showed MCD and acute tubular injury. The patient did not need dialysis and responded to steroids therapy in 10 days.
Finally, Kervella et al5 and Komaba et al6 presented 2 different cases of MCD relapse after a Pfizer-BioNTech COVID-19 vaccine dose. Remission was achieved with a course of corticosteroids in the first case, and treatment with corticosteroids and cyclosporin in the second. Morlidge et al4 reported 2 cases of MCD relapsing within 2 days after receiving the Oxford-AstraZeneca COVID-19 vaccine. Both cases responded to prednisolone therapy.
MCD has also been previously described following vaccination against other pathogens. There are several case reports of MCD in the literature after vaccination for influenza,7 , 8 tetanus-diphtheria-poliomyelitis,9 and hepatitis B virus.10 , 11
Classically, MCD presents with nephrotic syndrome and a preserved kidney function. AKI has been described in the setting of MCD,12 but this is not a usual presentation. Moreover, hypertension and hematuria are not classically associated with MCD. Our patient required dialysis, which is rare in the context of newly diagnosed MCD. Interestingly, AKI has been reported so far in all published MCD cases suspected to be in relation to the Pfizer-BioNTech COVID-19 vaccine.
It is obviously difficult to prove definite causality in the case we present here as well as in other previously published cases. Even if secondary causes of MCD were excluded and the presentation was atypical for primary MCD, de novo MCD after vaccination might only be coincidental.
Several hypotheses can be formulated on how a vaccine could trigger MCD. Considering that existing reports involve both messenger RNA–based (Pfizer-BioNTech) and adenovirus vector-based (Oxford-AstraZeneca) vaccines points to a potential mechanism that probably does not implicate a direct effect of the vaccine itself. Instead, a T-cell process ignited by the vaccine might lead to podocyte injury. Furthermore, there are at least 3 reported cases of MCD that occurred after SARS-CoV-2 infection,13, 14, 15 suggesting a molecular mimicry mechanism between SARS-CoV-2 spike protein and self-antigens on the podocytes. Another hypothesis is aberrant activation of the immune system in predisposed individuals. Further reports and studies will be needed to elucidate whether MCD is truly associated with COVID-19 vaccination.
In conclusion, we describe a 71-year-old man diagnosed with MCD and AKI after the first dose of the Oxford-AstraZeneca COVID-19 vaccine. He needed short-term hemodialysis and responded well to high-dose steroids. This case adds to the few published cases that associate the Pfizer-BioNTech COVID-19 vaccine with MCD. Our report suggests a potential relationship between MCD and the Oxford-AstraZeneca COVID-19 vaccine. Because vaccination seems to be the most promising way out of the current COVID-19 global pandemic, millions of doses of different COVID-19 vaccines will be administered around the world in a near future. Thus, adult and pediatric nephrologists should be aware of this rare but generally reversible potential complication of COVID-19 vaccination.
Article Information
Authors’ Full Names and Academic Degrees
Simon Leclerc, MD, Virginie Royal, MD, Caroline Lamarche, MD, MSc, and Louis-Philippe Laurin, MD, MSc.
Support
There were no expenses related to this study. Dr Laurin is a Fonds de recherche du Québec-Santé Junior 2 Scholar. The funder had absolutely no role in the preparation of this case report.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
The authors wish to thank Dr Joannie Lefebvre and Dr Michel Vallée for their input regarding the patient clinical presentation and outcome.
Patient Protections
This case report was approved by CIUSSS-EMTL Hôpital Maisonneuve-Rosemont Research Ethics Committee. The authors declare that they have obtained consent from the patient reported in this article for publication of the information about him that appears within this Case Report.
Peer Review
Received May 26, 2021. Evaluated by 2 external peer reviewers, with direct editorial input from the Pathology Editor, an Associate Editor, and a Deputy Editor. Accepted in revised form June 25, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Lebedev L., Sapojnikov M., Wechsler A., et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(1):142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agati VD, Kudose S, Bomback AS, Adamidis A, Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. Published online May 15, 2021. https://doi.org/10.1016/j.kint.2021.04.035 [DOI] [PMC free article] [PubMed]
- 3.Maas R.J., Gianotten S., van der Meijden W.A.G. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(2):312. doi: 10.1053/j.ajkd.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morlidge C, El-Kateb S, Jeevaratnam P, Thompson B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. Published online, June 10, 2021. https://doi.org/10.1016/j.kint.2021.06.005 [DOI] [PMC free article] [PubMed]
- 5.Kervella D, Jacquemont L, Chapelet-Debout A, Deltombe C, Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. Published online May 5, 2021. https://doi.org/10.1016/j.kint.2021.04.033 [DOI] [PMC free article] [PubMed]
- 6.Komaba H., Wada T., Fukagawa M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(3):469–470. doi: 10.1053/j.ajkd.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutiérrez S., Dotto B., Petiti J.P., et al. Minimal change disease following influenza vaccination and acute renal failure: just a coincidence? Nefrologia. 2012;32(3):414–415. doi: 10.3265/Nefrologia.pre2012.Feb.11370. [DOI] [PubMed] [Google Scholar]
- 8.Kielstein J.T., Termühlen L., Sohn J., Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol. 2000;54(3):246–248. [PubMed] [Google Scholar]
- 9.Clajus C., Spiegel J., Bröcker V., Chatzikyrkou C., Kielstein J.T. Minimal change nephrotic syndrome in an 82 year old patient following a tetanus-diphteria-poliomyelitis-vaccination. BMC Nephrol. 2009;10:21. doi: 10.1186/1471-2369-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Işlek I., Cengiz K., Cakir M., Küçüködük S. Nephrotic syndrome following hepatitis B vaccination. Pediatr Nephrol. 2000;14(1):89–90. [PubMed] [Google Scholar]
- 11.Ozdemir S., Bakkaloğlu A., Oran O. Nephrotic syndrome associated with recombinant hepatitis B vaccination: a causal relationship or just a mere association? Nephrol Dial Transplant. 1998;13(7):1888–1889. doi: 10.1093/oxfordjournals.ndt.a027900. [DOI] [PubMed] [Google Scholar]
- 12.Jennette J.C., Falk R.J. Adult minimal change glomerulopathy with acute renal failure. Am J Kidney Dis. 1990;16(5):432–437. doi: 10.1016/s0272-6386(12)80055-2. [DOI] [PubMed] [Google Scholar]
- 13.Kudose S., Batal I., Santoriello D., et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada M., Rastogi P., Ince D., et al. Minimal change disease with nephrotic syndrome associated with coronavirus disease 2019 after apolipoprotein L1 risk variant kidney transplant: a case report. Transplant Proc. 2020;52(9):2693–2697. doi: 10.1016/j.transproceed.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta R.K., Bhargava R., Shaukat A.A., Albert E., Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21(1):326. doi: 10.1186/s12882-020-01970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]