Abstract
Background
We aimed to assess the safety and immunogenicity of an inactivated vaccine against COVID-19 in Chinese adults aged ≥18 years.
Methods
This is an ongoing randomized, double-blind, placebo-controlled, phase 1/2 clinical trial among healthy adults aged ≥18 years in Henan Province, China. Participants (n = 336 in 18–59 age group and n = 336 in ≥60 age group) were enrolled between April 12 and May 17 2020, and were equally randomized to receive vaccine or placebo (aluminum hydroxide adjuvant) in a three-dose schedule of 2·5, 5, or 10 µg on days 0, 28, and 56. Another 448 adults aged 18–59 years were equally allocated to four groups (a one-dose schedule of 10 µg, and two-dose schedules of 5 µg on days 0 and 14/21/28) and received vaccine or placebo (ratio 3:1 within each group). The primary outcomes were 7-day post-injection adverse reactions and neutralizing antibody titres on days 28 and 90 after the whole-course vaccination. Trial registration: www.chictr.org.cn #ChiCTR2000031809.
Findings
The 7-day adverse reactions occurred in 4·8% to 32·1% of the participants in various groups, and most adverse reactions were mild, transient, and self-limiting. Twenty participants reported 68 serious adverse events which were judged to be unrelated to the vaccine. The 90-day post-injection geometric mean titres of neutralizing antibody ranged between 87 (95% CI: 61–125) and 129 (99–169) for three-dose schedule among younger and older adults; 20 (14–27), 53 (38–75), and 44 (32–61) in 5 µg days 0 and 14/21/28 groups, respectively, and 7 (6–9) in one-dose 10 µg group. There were no detectable antibody responses in all placebo groups.
Interpretation
The inactivated vaccine against COVID-19 was well tolerated and immunogenic in both younger and older adults. The two-dose schedule of 5 µg on days 0 and 21/28 and three-dose schedules on days 0, 28, and 56 could be further evaluated for long-term safety and efficacy in the phase 3 trials.
Research in context.
Evidence before this study
By May 10, 2021, results of the clinical trials in different phases of 24 SARS-CoV-2 vaccines (seven protein subunit, six inactivated, six recombinant, three mRNA, one DNA, and one virus-like particle vaccine) have been reported. For the inactivated vaccine developed by the Sinopharm (Wuhan, China), we have previously reported the interim results of the phase 1 trial of three-dose groups and phase 2 trial of days 0 and 14/21 groups among adults aged 18–59 years. It was reported that 6·0% to 25·0% of the participants had adverse reactions, and neutralizing antibody titres ranged between 121 and 316 on day 14 after the whole-course vaccination.
Added value of this study
This study reported safety profile at 292–327 days after the first injection of an inactivated SARS-CoV-2 vaccine and antibody responses at 90 days after the whole-course vaccination, which showed that the vaccine was safe, tolerable, and immunogenic in healthy adults. Only 15% of participants experienced adverse reactions, which were mostly mild-to-moderate and self-limiting, and no severe adverse reaction was reported. Two-dose schedules of 5 µg on days 0 and 21/28 and three-dose schedules of 2·5 µg, 5 µg, and 10 µg on days 0, 28, and 56 induced neutralizing antibodies in over 87% and 71% of recipients on days 28 and 90 after the whole-course vaccination, respectively.
Implications of all the available evidence
The inactivated SARS-CoV-2 vaccine had a good safety profile and demonstrated immunogenicity among adults. Two-dose schedules of 5 µg on days 0 and 21/28 and three-dose schedules of 2·5 µg, 5 µg, and 10 µg on days 0, 28, and 56 are potential options for phase 3 clinical trials and future applications in the real world. Extended follow-ups and phase 3 clinical trials are still warranted to evaluate the long-term safety, immunogenicity, and efficacy of the inactivated vaccine..
Alt-text: Unlabelled box
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to affect everyone's life and is responsible for more than 3·7 million deaths worldwide as of June 9, 2021 [1]. Unprecedented efforts have been made to rapidly develop vaccines against the COVID-19 by pharmaceutical companies, academic researchers, and government agencies [2]. According to the draft landscape of COVID-19 candidate vaccines by the World Health Organizations (WHO), there were over 185 candidate vaccines in preclinical evaluations and 102 undergoing clinical evaluations in different phases till June 8 [2]. Among them, the results of phase 1/2 trials of 24 candidate vaccines have been published or made available on preprint servers, including seven protein subunit [3], [4], [5], [6], [7], [8], [9], six inactivated [10], [11], [12], [13], [14], [15], six recombinant, [16], [17], [18], [19], [20], [21] three mRNA, [22], [23], [24] one DNA vaccine, [25] and one virus-like particle vaccine [26]. All 24 vaccines demonstrated acceptable safety profiles and immunogenicity. Among them, seven also published the interim results of the phase 3 trial [27], [28], [29], [30], [31], [32], [33].
Since age has been identified as an independent risk factor for critical illness or death of COVID-19 and there is a possibility of lower immune response [34,35], the safety and immunogenicity of the vaccines should be particularly assessed in older people. Previous studies indicated that the safety profile of vaccines against the COVID-19 was similar or even better among older adults compared with younger adults [4,12,14,[18], [19], [20],24,29,[36], [37], [38]]. As for antibody responses, several studies reported lower antibody responses among older adults [4,19,24,38], while others reported similar antibody responses between older and younger adults [12,14,18,36,37]. Therefore, it is necessary to evaluate the safety and immunogenicity of each vaccine against COVID-19 in both young and old adults.
We have previously reported interim results of an ongoing phase 1/2 trial of an inactivated vaccine against COVID-19 among healthy adults aged 18–59 years old for the 28-day post-vaccination adverse events and 14-day post-vaccination humoral immunogenicity [13]. The report included participants who received three doses of 2·5 μg, 5 μg, or 10 μg in the phase 1 trial and two doses of 5 μg on days 0 and 14 or days 0 and 21 in the phase 2 trial [13]. In the current analysis, we included all adult groups that were designed in the original protocol (Supplementary Appendix Section 1) of the phase 1/2 trial including studies among those aged 60 years or older, and updated the antibody response till 90 days after the whole-course vaccination as well as serious adverse events till March 5, 2021 with a maximum follow-up of 327 days since the first injection.
2. Methods
2.1. Study design and participants
The phase 1/2, randomized, double-blind, placebo-controlled trial was designed by the Wuhan Institute of Biological Products Co. Ltd. and Henan Province center for Disease Control and Prevention (CDC), and the study protocol was approved by the institutional review board of Henan CDC. All participants provided written informed consent before enrollment. Investigators at the Wuzhi county CDC, Henan Province, performed the ongoing trial and collected data since April 12, 2020. An independent data and safety monitoring board (DSMB) monitored the safety data and evaluated risks among the participants during the trial. The trial was registered with www.chictr.org.cn (ChiCTR2000031809) and included participants aged ≥6 years; however, interventions among children and adolescents were only initiated after evaluating the 28-day post-vaccination safety and immunogenicity results among adults. Therefore, the trial among children and adolescents is still ongoing and data are not yet available. The current analysis included only adults aged 18 years and above.
Healthy, non-pregnant adults aged 18 years or older who had no known history of SARS-CoV-1 (via on-site inquiry) or SARS-CoV-2 infection (via serological and nucleic acid test); had not been to Hubei province, regions outside China, or other regions with confirmed COVID-19 cases; and had not contacted with confirmed or suspected cases were assessed for eligibility. Key exclusion criteria included respiratory diseases or symptoms within 14 days before vaccination; abnormalities in laboratory examinations; history of severe allergic reaction or allergy to ingredients in the vaccine; taking certain treatments or medications or other vaccines before the study as specified in Supplementary Appendix Section 1; and history of severe comorbidities that may affect the adherence. Details of the inclusion and exclusion criteria are described in the previous publication [13] and Supplementary Appendix Section 1. All the authors had full access to all the data in the study.
2.2. Randomization and masking
Sequential computer-generated randomization numbers were assigned to participants, and stratified block randomization by age and doses was adopted (block size 8). Within each randomization block, the ratio of vaccine to placebo (aluminum hydroxide adjuvant) was 3:1.
2.3. Procedures
In the phase 1 trial, participants were stratified by 18–59 (younger adults) and ≥60 years old (older adults) and randomly allocated to receive the inactivated SARS-CoV-2 vaccine at 2·5 μg, 5 μg, or 10 μg, or placebo on days 0, 28, and 56 via intramuscular injections (24 participants in each group). To accelerate the development of vaccine and provide relevant data for the phase 3 trial, the phase 2 trial was designed at the same time with the phase 1 trial, and same group assignment approach (i.e., vaccine at 2·5 μg, 5 μg, and 10 μg, and placebo on days 0, 28, and 56 among younger and older adults, respectively) was used with a larger sample size for each group (60 participants in each group). With the purpose of quickly increasing vaccine coverage in the population, four more groups in those aged 18–59 years old were added in the phase 2 trial to explore whether less doses or shorter between-dose intervals could also be immunogenic, i.e., two-dose schedule of 5 μg on days 0 and 14, on days 0 and 21, or on days 0 and 28, or a one-dose schedule of 10 μg on day 0 (vaccine:placebo = 3:1 in each group).
Participants were sequentially enrolled and received injections in an age- and dose-escalating manner. The first group of participants aged 18–59 years in the phase 1 trial received the inactivated vaccine at 2·5 μg first, and adverse events were monitored for 7 days after the first injection. If no severe adverse events were observed, the second group of participants aged 18–59 years in the phase 1 trial received the inactivated vaccine at 5 μg; meanwhile, a group of participants aged ≥60 years in the phase 1 trial and participants aged 18–59 years in the phase 2 trial received the inactivated vaccine at 2·5 μg. Similar sequences were used for 5 μg and 10 μg groups (Supplementary Appendix, Fig. 1). Participants, investigators, and laboratory personnel were blinded to the intervention allocation.
Details of the vaccine development and production were reported previously [13,39]. Briefly, a SARS-CoV-2 strain (WIV04 strain, GenBank accession number MN996528) was isolated from a patient in the Jinyintan Hospital, Wuhan. The virus strains were cultivated in qualified Vero cell lines for proliferation, followed by purification and β-propanolide inactivation (1:4000 vol/vol) at 2–8 °C for 48 h. A second inactivation was subsequently performed after clarification of cell debris and ultrafiltration, then the vaccine was adsorbed to 0·5 mg aluminum hydroxide and packed into pre-filled syringes in 0·5 mL of sterile phosphate-buffered saline without preservative. The placebo only contained 0·5 mg aluminum hydroxide in 0·5 mL sterile phosphate-buffered saline. All the vaccines and placebos were approved by the National Institutes for Food and Drug Control of China, and provided in coded, identical-appearing, single-dose vials.
2.4. Outcomes
Participants were requested to record any injection-site-specific adverse events (e.g., pain, redness, and swelling), systemic adverse events (e.g., fever, headache, and fatigue), and unsolicited adverse events on diary cards (within 7 days) or contact cards (8 days and after) of each injection. After each dose, participants were observed for 30 min to record any adverse events. Within 7 days after the first dose, researchers visited the participants on a daily basis and guided the participants to correctly report the adverse events on the diary card. After the second and third dose, researchers made a phone call within 24 h, a face-to-face visit within 1–3 days, a phone call within 4–7 days, a weekly phone call between days 8 and 28, and a monthly phone call after 28 days following the whole-course vaccination to collect information on the adverse events. The participants could also actively report adverse events to the researchers at any time. The grading of adverse events and relationships between vaccinations and adverse events were decided by investigators according to the standard guidelines issued by the National Medical Products Administration of China, and details are shown in Supplementary Appendix Section 1. The 7-day adverse reactions were the primary safety outcome, while all adverse events within 28 days and serious adverse events after 28 days, regardless of relations with the vaccine, were considered as the secondary safety outcome. A total of 13 trained investigators were involved in adjudications of the relations of the serious adverse events with vaccine and determined the severity according to medical records. To assess any acute toxic effects after vaccination, laboratory safety tests (including blood routine tests, total bilirubin, liver enzymes, urea nitrogen, creatinine, and urine sugar, protein, and occult blood) were also performed before and on day 4 after each injection in the phase 1 trial and were considered as secondary safety outcomes.
The primary humoral immunogenicity outcomes were neutralizing antibody titres and specific IgG binding antibody titres measured on days 28 and 90 after the whole-course vaccination. Antibody titres at the same time point in the phase 1 and 2 trials were combined and analysed together. In the phase 1 trial, blood samples were also collected before each dose, on day 4 after each injection, and on day 14 after the first and second injections, and neutralizing antibody titres and specific IgG binding antibody titres measured at these time points were secondary immunogenicity outcomes. The schedule of blood collections is described in Supplementary Appendix Fig. 2. Plaque reduction neutralization test (PRNT) was used to measure the neutralization capacity induced by the vaccine against live SARS-CoV-2 (BetaCoV/Wuhan/AMMS01/2020 activated), and the PRNT50 values were used to determine the maximal dilution factors when SARS-CoV-2 plaque formation could be reduced by 50%. An in-house developed ELISA kit was used to measure specific IgG-binding antibody titres with the inactivated whole SARS-CoV-2 as coating antigen. The specificity, recovery, and inter-assay and intra-assay coefficients of variation of the ELISA assay were 100%, 96·0–105·3%, and 6·4–10·2%, respectively. Detailed immunogenicity assays have been reported previously [13]. The low detection limit was 5 for the neutralizing antibody titre and 10 for the specific IgG-binding antibody titre, and those below the detection limit were respectively assigned to 5 or 10 for further analysis. Seroconversion rate on days 28 and 90 after the whole-course vaccination was defined as at least a four-fold increase of antibody titres over baseline (i.e., 20 for neutralizing and 40 for IgG-binding antibody titres) and was considered as a secondary immunogenicity outcome.
2.5. Statistical analysis
The phase 1 and 2 trials were designed concurrently, thus the sample size was not calculated according to the statistical power calculation. Instead, the sample size was determined according to recommendations from the National Medical Products Administration of China, i.e., a minimum of 20–30 participants in a phase 1 vaccine trial and a roughly 3-fold sample size in a phase 2 vaccine trial. The sample size in the current study was in the similar range compared to other clinical trials of COVID-19 vaccines [[12], [13], [14],19,40,41].
The safety analysis was conducted in all participants receiving at least one injection. The immunogenicity analysis was conducted in participants receiving at least one injection and having results of antibody titre measurements at the designated time points. Rates of adverse reactions, unsolicited adverse events, abnormal laboratory indicators, and seroconversion between vaccine and placebo groups and among different dose groups were compared using χ2 test or Fisher's exact test (for sparse outcomes). Log10-transformed antibody titres between vaccine and placebo groups were compared using Student's t-test or Mann-Whitney U test (for non-normally distributed data). We also made post-hoc comparisons of antibody titres across 2·5 μg, 5 μg, and 10 μg days 0, 28, and 56 groups as well as across two-dose and one-dose group through ANOVA and Student-Newman-Keuls Tests. Results were presented by different age groups (18–59 and ≥60 years old) as specified as in Supplementary Appendix Section 1, and post-hoc stratified analyses of the immune responses were conducted by sex and body mass index (BMI; <24 kg/m2 versus ≥24 kg/m2 according to the Chinese criterion for determining overweight/obesity) [42]. Analyses were performed using SPSS software, version 25·0 (IBM SPSS Inc., Chicago, IL), and hypothesis testing was two-sided with an alpha value of 0·05. The results of secondary endpoints and post-hoc analyses should be interpreted cautiously given the potential type 1 error due to multiple comparisons.
2.6. Role of the funding source
The China National Biotec Group Company Limited and the Wuhan Institute of Biological Products Co. Ltd. were the study sponsors and designed the trial, provided the study product, and oversaw all trial operations. The sponsors used contract clinical research organizations to coordinate interactions with regulatory authorities and oversee clinical site operations. Data were collected by the clinical site research staff, managed by a blinded contract research organization data management team, monitored by a contract research organization, and overseen by the sponsor and an independent data and safety monitoring board. The analyses were performed after the data were checked and locked before unblinding by an independent statistician who was not involved in the trial. Manuscript preparation was performed by the study authors and the decision to submit the manuscript for publication was made by the study authors. All the authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Participants
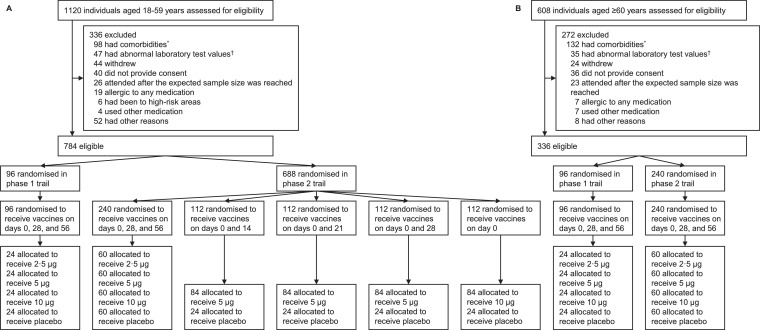
Between April 12 and May 17, 2020, a total of 1120 volunteers aged 18–59 years old (younger adults) and 608 volunteers aged ≥60 years old (older adults) were screened, and 784 and 336, respectively, were finally enrolled in the phase 1 and 2 trials (Fig. 1). All participants completed the first injection while five did not complete the second or third injection. All participants provided blood samples before the first injection, while a total of 8 and 9 participants did not provide blood samples on days 28 and 90, respectively, after the whole-course vaccination. Data in the three-dose schedule groups in both phase 1 and 2 trials were combined, and the baseline characteristics of the participants stratified by age groups (18–59 and ≥60 years old), dose groups (2·5 μg, 5 μg, 10 μg, and placebo) and schedule groups (three doses on days 0, 28, and 56, two doses on days 0 and 14/21/28, and one dose on day 0) are shown in Table 1. The mean (standard deviation) age was 43·1 (9·6) years in participants aged 18–59 years and 66·7 (4·3) in those aged ≥60 years (79·2% aged 60–69 years); and there were 462 (58·9%) and 136 (40·5%) women in the two age groups, respectively.
Fig. 1.
Screening, randomization, and inclusion in safety and immunogenicity analyses stratified by age groups
*The comorbidities in the exclusion criteria included cardiovascular disease, cancer, respiratory disease, autoimmune disease, tuberculosis, severe liver disease, congenital malformation, mental illness, nervous system diseases, uncontrolled hypertension and diabetes, severe malnutrition, fever within 14 days, and other diseases that could affect participation and compliance in the trial as judged by the investigators. †Laboratory tests included routine blood tests, liver enzymes, total bilirubin, creatinine, urea nitrogen, urine protein, urine sugar, and urinary occult blood. Details of the list and definition of abnormal values are provided in Supplementary Appendix Section 1. ‡One participant aged 18–59 years who received placebo on days 0, 28, and 56 in the phase 2 trial did not provide blood sample on days 28/90 after the third dose for antibody measures. One participant aged 18–59 years who received vaccines at 5 μg on days 0 and 21 in the phase 2 trial received two injections but did not provide blood sample on day 90 after the second dose. One participant aged 18–59 years who received vaccines at 5 μg on days 0 and 28 in the phase 2 trial did not provide blood sample on days 28/90 after the second dose. One participant aged 18–59 years who was allocated to receive placebo on days 0 and 28 in the phase 2 trial did not receive the second dose because of positive pregnancy test and thus did not provide blood sample on days 28/90 after the second dose. One participant aged ≥60 years who was allocated to receive vaccines at 5 μg on days 0, 28, and 56 in the phase 1 trial withdrew from the study before the second dose. One participant aged ≥60 years who was allocated to receive vaccines at 10 μg on days 0, 28, and 56 in the phase 1 trial withdrew from the study before the second dose. One participant aged ≥60 years who received vaccines at 5 μg on days 0, 28, and 56 in the phase 1 trial did not provide blood sample on days 28/90 after the third dose for antibody measures. Two participants aged ≥60 years who were allocated to receive vaccines at 10 μg on days 0, 28, and 56 in phase 2 trial withdrew from the study before the third dose.
Table 1.
Baseline characteristics of the study participants in the phase 1 and 2 trials*.
| Adults aged 18–59 years (phase 1 & 2 combined) |
Adults aged 60 years or older (phase 1 & 2 combined) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days 0, 28, and 56 |
Days 0, 28, and 56 |
||||||||
| 2·5 μg (n = 84) | 5 μg (n = 84) | 10 μg (n = 84) | Placebo (n = 84) | 2·5 μg (n = 84) | 5 μg (n = 84) | 10 μg (n = 84) | Placebo (n = 84) | ||
| Age, years | |||||||||
| Mean (SD) | 43·4 (9·5) | 43·0 (10·2) | 44·3 (8·9) | 43·9 (9·2) | 66·6 (4·0) | 67·8 (5·2) | 66·3 (3·6) | 66·4 (4·2) | |
| 18–29 | 8 (9·5) | 9 (10·7) | 8 (9·5) | 10 (11·9) | ·· | ·· | ·· | ·· | |
| 30–39 | 19 (22·6) | 23 (27·4) | 14 (16·7) | 16 (19·1) | ·· | ·· | ·· | ·· | |
| 40–49 | 36 (42·9) | 27 (32·1) | 35 (41·7) | 33 (39·3) | ·· | ·· | ·· | ·· | |
| 50–59 | 21 (25·0) | 25 (29·8) | 27 (32·1) | 25 (29·8) | ·· | ·· | ·· | ·· | |
| 60–69 | ·· | ·· | ·· | ·· | 70 (83·3) | 61 (72·6) | 68 (81·0) | 67 (79·8) | |
| ≥70 | ·· | ·· | ·· | ·· | 14 (16·7) | 23 (27·4) | 16 (19·1) | 17 (20·2) | |
| Sex | |||||||||
| Women | 51 (60·7) | 49 (58·3) | 48 (57·1) | 55 (65·5) | 35 (41·7) | 35 (41·7) | 39 (46·4)† | 27 (32·1) | |
| Men | 33 (39·3) | 35 (41·7) | 36 (42·9) | 29 (34·5) | 49 (58·3) | 49 (58·3) | 45 (53·6) | 57 (67·9) | |
| Height, m | 163·3 (8·7) | 164·8 (7·0) | 165·1 (8·2) | 164·2 (7·7) | 161·7 (8·3) | 161·5 (8·5) | 161·6 (8·5)† | 163·8 (8·7) | |
| Weight, kg | 69·4 (11·9) | 70·9 (11·4) | 68·7 (11·2) | 68·0 (11·8) | 68·9 (10·2) | 68·3 (11·0) | 67·3 (10·2) | 69·3 (10·3) | |
| Body mass index, kg/m2 | 26·0 (4·2) | 26·1 (3·6) | 25·2 (3·4) | 25·2 (3·5) | 26·3 (3·2) | 26·1 (2·9) | 25·8 (3·6) | 25·8 (2·9) | |
| Systolic blood pressure, mmHg | 123·1 (11·9) | 124·6 (9·9) | 125·9 (11·3) | 123·9 (11·3) | 132·4 (6·8) | 129·7 (9·5)† | 131·5 (7·4) | 132·2 (6·6) | |
| Diastolic blood pressure, mmHg | 81·4 (6·0) | 80·3 (5·8) | 82·9 (5·3) | 80·8 (6·4) | 83·3 (4·0) | 81·0 (5·6) | 82·6 (4·4) | 83·4 (3·9) | |
| Adults aged 18–59 years (phase 2 only) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days 0 and 14 |
Days 0 and 21 |
Days 0 and 28 |
Day 0 |
||||||
| 5 μg (n = 84) | Placebo (n = 28) | 5 μg (n = 84) | Placebo (n = 28) | 5 μg (n = 84) | Placebo (n = 28) | 10 μg (n = 84) | Placebo (n = 28) | ||
| Age, years | |||||||||
| Mean (SD) | 43·7 (8·8) | 45·9 (8·7) | 41·4 (9·3)† | 46·2 (8·9) | 42·6 (9·8) | 44·1 (10·7) | 41·0 (10·9) | 42·1 (9·8) | |
| 18–29 | 5 (6·0) | 2 (7·1) | 13 (15·5) | 1 (3·6) | 11 (13·1) | 4 (14·3) | 15 (17·9) | 5 (17·9) | |
| 30–39 | 23 (27·4) | 6 (21·4) | 18 (21·4) | 3 (10·7) | 23 (27·4) | 4 (14·3) | 21 (25·0) | 5 (17·9) | |
| 40–49 | 38 (45·2) | 9 (32·1) | 39 (46·4) | 14 (50·0) | 22 (26·2) | 10 (35·7) | 28 (33·3) | 12 (42·9) | |
| 50–59 | 18 (21·4) | 11 (39·3) | 14 (16·7) | 10 (35·7) | 28 (33·3) | 10 (35·7) | 20 (23·8) | 6 (21·4) | |
| Sex | |||||||||
| Women | 52 (61·9) | 19 (67·9) | 52 (61·9) | 19 (67·9) | 47 (56·0) | 16 (57·1) | 43 (51·2) | 11 (39·3) | |
| Men | 32 (38·1) | 9 (32·1) | 32 (38·1) | 9 (32·1) | 37 (44·1) | 12 (42·9) | 41 (48·8) | 17 (60·7) | |
| Height, m | 163·9 (8·3) | 161·8 (7·2) | 162·7 (8·4) | 160·3 (10·2) | 164·9 (8·5) | 162·9 (8·0) | 164·9 (8·5) | 167·1 (8·1) | |
| Weight, kg | 66·5 (11·2) | 67·8 (11·6) | 67·9 (13·1) | 65·8 (12·5) | 69·8 (13·9) | 66·4 (11·5) | 68·6 (13·4) | 73·7 (16·4) | |
| Body mass index, kg/m2 | 24·7 (3·4) | 25·8 (3·9) | 25·6 (4·0) | 25·5 (3·4) | 25·6 (4·2) | 24·9 (3·1) | 25·2 (4·1) | 26·2 (4·8) | |
| Systolic blood pressure, mmHg | 123·5 (11·2) | 125·5 (9·8) | 124·6 (9·7) | 123·0 (9·6) | 122·5 (12·3) | 120·1 (10·8) | 123·7 (12·4) | 123·9 (11·6) | |
| Diastolic blood pressure, mmHg | 79·8 (6·7) | 80·1 (5·6) | 80·5 (5·7) | 80·0 (5·9) | 79·5 (6·5) | 78·1 (6·3) | 81·3 (6·4) | 82·2 (5·4) | |
Data are mean (SD) or n (%). †Differences between vaccine and placebo group were statistically insignificant (p>0·05), which were tested using t-test for continuous variables and χ2 test for categorical variables.
3.2. Safety
Injection-site and systemic reaction symptoms (according to solicited reports) are shown in Table 2. Within 7 days after each injection, the number of participants reporting adverse reactions was 12 (14·3%), 18 (21·4%), 21 (25·0%), and 14 (16·7%) in the 2·5 μg, 5 μg, 10 μg, and placebo groups among younger adults with three doses, respectively; and the corresponding number was 4 (4·8%), 13 (15·5%), 6 (7·1%), and 14 (16·7%) in the age group of ≥60 years. The proportion of adverse reactions was similar between vaccine and placebo groups (P >0·099), except that the proportion of total adverse reactions was larger among those receiving placebo compared with 2·5 μg on days 0, 28, and 56 among older adults (P = 0·042). Besides, the proportion of adverse reactions was higher in 5 μg group compared with 2·5 μg or 10 μg group among older adults receiving three doses (P = 0·012), and no statistically significant differences were observed for other comparisons among different dose groups (P >0·056). In the phase 2 trial among younger adults, the number of participants reporting adverse reactions was 5 (6·0%), 4 (14·3%), 16 (19·1%), 5 (17·9%), 17 (20·2%), 9 (32·1%), 9 (10·7%), and 3 (10·7%) in 5 μg days 0 and 14, placebo days 0 and 14, 5 μg days 0 and 21, placebo days 0 and 21, 5 μg days 0 and 28, placebo days 0 and 28, 10 μg day 0, and placebo day 0 groups, respectively. No statistically significant differences of incident adverse reactions were observed between vaccine and placebo groups (P > 0·099); however, incidence rates of total and injection-site adverse reactions were lower among 5 μg days 0 and 14 group compared with 5 μg days 0 and 21/28 groups (P < 0·013).
Table 2.
7-day adverse reactions among study participants who received at least one dose in the phase 1 and 2 trials*.
| Adults aged 18–59 years |
Adults aged 60 years or older |
|||||||
|---|---|---|---|---|---|---|---|---|
| Days 0, 28, and 56 |
Days 0, 28, and 56 |
|||||||
| 2·5 μg (n = 84) | 5 μg (n = 84) | 10 μg (n = 84) | Placebo (n = 84) | 2·5 μg (n = 84) | 5 μg (n = 84) | 10 μg (n = 84) | Placebo (n = 84) | |
| Total adverse reactions | 12 (14·3; 7·6–23·6) | 18 (21·4; 13·2–31·7) | 21 (25·0; 16·2–35·6) | 14 (16·7; 9·4–26·4) | 4 (4·8; 1·3–11·8)† | 13 (15·5; 8·5–25·0)‡ | 6 (7·1; 2·7–14·9) | 14 (16·7; 9·4–26·4) |
| Grade 3 total adverse reactions | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Injection-site reactions | 11 (13·1; 6·7–22·2) | 15 (17·9; 10·4–27·7) | 21 (25·0; 16·2–35·6) | 10 (11·9; 5·9–20·8) | 2 (2·4; 0·3–8·3) | 11 (13·1; 6·7–22·2) | 3 (3·6; 0·7–10·1) | 10 (11·9; 5·9–20·8) |
| Induration | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 |
| Itching | 1 (1·2; 0·0–6·5) | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 |
| Pain | 11 (13·1; 6·7–22·2) | 15 (17·9; 10·4–27·7) | 21 (25·0; 16·2–35·6) | 10 (11·9; 5·9–20·8) | 2 (2·4; 0·3–8·3) | 11 (13·1; 6·7–22·2) | 3 (3·6; 0·7–10·1) | 9 (10·7; 5·0–19·4) |
| Redness | 0 | 0 | 2 (2·4; 0·3–8·3) | 1 (1·2; 0·0–6·5) | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 |
| Swelling | 1 (1·2; 0·0–6·5) | 0 | 2 (2·4; 0·3–8·3) | 1 (1·2; 0·0–6·5) | 0 | 1 (1·2; 0·0–6·5) | 0 | 1 (1·2; 0·0–6·5) |
| Systemic reactions | 3 (3·6; 0·7–10·1) | 4 (4·8; 1·3–11·8) | 1 (1·2; 0·0–6·5) | 4 (4·8; 1·3–11·8) | 2 (2·4; 0·3–8·3) | 4 (4·8; 1·3–11·8) | 4 (4·8; 1·3–11·8) | 6 (7·1; 2·7–14·9) |
| Grade 3 systemic reactions | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) | 0 | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 |
| Coughing | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 2 (2·4; 0·3–8·3) | 2 (2·4; 0·3–8·3) | 0 | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) | 0 | 1 (1·2; 0·0–6·5) |
| Fever | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) | 2 (2·4; 0·3–8·3) | 1 (1·2; 0·0–6·5) | 2 (2·4; 0·3–8·3) | 3 (3·6; 0·7–10·1) | 3 (3·6; 0·7–10·1) |
| Grade 3 fever | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Headache | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nausea | 1 (1·2; 0·0–6·5) | 1 (1·2; 0·0–6·5) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pruritus (non-inoculated site) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) |
| Vomiting | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 | 0 | 0 |
| Other reactions | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 |
| Severe adverse reactions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Days 0 and 14 |
Days 0 and 21 |
Days 0 and 28 |
Day 0 |
|||||
|---|---|---|---|---|---|---|---|---|
| 5 μg (n = 84) | Placebo (n = 28) | 5 μg (n = 84) | Placebo (n = 28) | 5 μg (n = 84) | Placebo (n = 28) | 10 μg (n = 84) | Placebo (n = 28) | |
| Total adverse reactions | 5 (6·0; 2·0–13·4) | 4 (14·3; 4·0–32·7) | 16 (19·1; 11·3–29·1) | 5 (17·9; 6·1–36·9) | 17 (20·2; 12·3–30·4) | 9 (32·1; 15·9–52·4) | 9 (10·7; 5·0–19·4) | 3 (10·7; 2·3–28·2) |
| Injection-site reactions | 2 (2·4; 0·3–8·3) | 3 (10·7; 2·3–28·2) | 13 (15·5; 8·5–25·0) | 4 (14·3; 4·0–32·7) | 11 (13·1; 6·7–22·2) | 5 (17·9; 6·1–32·0) | 6 (7·1; 2·7–14·9) | 3 (10·7; 2·3–28·2) |
| Induration | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Itching | 0 | 0 | 1 (1·2; 0·0–6·5) | 1 (3·6; 0·1–18·4) | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Pain | 2 (2·4; 0·3–8·3) | 3 (10·7; 2·3–28·2) | 12 (14·3; 7·6–23·6) | 4 (14·3; 4·0–32·7) | 11 (13·1; 6·7–22·2) | 4 (14·3; 4·0–32·7) | 5 (6·0; 2·0–13·4) | 3 (10·7; 2·3–28·2) |
| Redness | 0 | 0 | 0 | 1 (3·6; 0·1–18·4) | 0 | 0 | 0 | 0 |
| Swelling | 0 | 0 | 1 (1·2; 0·0–6·5) | 1 (3·6; 0·1–18·4) | 0 | 1 (3·6; 0·1–18·4) | 0 | 0 |
| Systemic reactions | 4 (4·8; 1·3–11·8) | 2 (7·1; 0·9–23·5) | 4 (4·8; 1·3–11·8) | 2 (7·1; 0·9–23·5) | 6 (7·1; 2·7–14·9) | 3 (10·7; 2·3–28·2) | 4 (4·8; 1·3–11·8) | 0 |
| Anorexia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Constipation | 0 | 0 | 0 | 0 | 0 | 1 (3·6; 0·1–18·4) | 0 | 0 |
| Coughing | 1 (1·2; 0·0–6·5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 1 (3·6; 0·1–18·4) | 0 | 0 |
| Fatigue | 1 (1·2; 0·0–6·5) | 0 | 0 | 0 | 2 (2·4; 0·3–8·3) | 1 (3·6; 0·1–18·4) | 1 (1·2; 0·0–6·5) | 0 |
| Fever | 4 (4·8; 1·3–11·8) | 1 (3·6; 0·1–18·4) | 2 (2·4; 0·3–8·3) | 1 (3·6; 0·1–18·4) | 4 (4·8; 1·3–11·8) | 0 | 0 | 0 |
| Headache | 1 (1·2; 0·0–6·5) | 1 (3·6; 0·1–18·4) | 0 | 1 (3·6; 0·1–18·4) | 0 | 1 (3·6; 0·1–18·4) | 2 (2·4; 0·3–8·3) | 0 |
| Nausea | 0 | 0 | 1 (1·2; 0·0–6·5) | 1 (3·6; 0·1–18·4) | 0 | 2 (7·1; 0·9–23·5) | 0 | 0 |
| Pruritus (non-inoculated site) | 0 | 0 | 0 | 1 (3·6; 0·1–18·4) | 0 | 0 | 1 (1·2; 0·0–6·5) | 0 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 1 (3·6; 0·1–18·4) | 0 | 0 |
| Other reactions | 0 | 0 | 0 | 0 | 1 (1·2; 0·0–6·5) | 2 (7·1; 0·9–23·5) | 0 | 0 |
| Severe adverse reactions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Data are n (%; 95% confidence interval), and 95% confidence interval was calculated by the Clopper-Pearson (exact) method. Data from the phase 1 and 2 trials were pooled. †Difference between vaccine and placebo groups was statistically significant (P = 0·042), all other P values>0·085. ‡Difference among different dose groups was statistically significant (P = 0·012), and all other P values>0·056.
The most common adverse reaction was injection-site pain (126 out of 1120 participants), followed by fever (26 out of 1120 participants). Most adverse reactions were mild (grade 1/2), transient and self-limiting. Unsolicited adverse events with 28 days (regardless of relations with the vaccination) are shown in the Supplementary Appendix Tables 1 and 2. Up to March 5, 2021, a total of 68 severe adverse events from 20 participants occurred during the follow-up (median 306 days after the first injection, range 292–327 days; Supplementary Appendix, Table 3), but none were judged to be related to the vaccination.
In the phase 1 trial, there were 4 (16·7%; 4·7%−37·4%; among older adults receiving vaccines at 2·5 μg) to 10 (41·7%; 22·1%−63·4%; among older adults receiving vaccines at 10 μg) participants in each group having abnormalities in blood biochemical tests or urinalysis tests before and on day 4 after each injection (Supplementary Appendix, Table 4), and all abnormalities were transient which needed no special treatment. A similar pattern was also found in younger adults and was reported in our previous publication [13].
3.3. Immunogenicity
None of the participants had any measurable neutralizing antibody response at baseline, and none in the placebo group had any measurable neutralizing antibody response during the follow-up. Differences of neutralizing antibody tires and specific binding antibody titres on days 28 and 90 after the whole-course vaccination between vaccine and placebo groups were statistically significant (P ≤ 0·0004). Among younger adults with three injections, the geometric mean titre (GMT) of neutralizing antibody at day 28 after the third injection was 149 (95% CI, 110–202), 238 (188–302), and 216 (181–257) in the 2·5 μg, 5 μg, and 10 μg group, respectively (Table 3). The GMT for older adults was 161 (118–219), 161 (118–221), and 187 (138–253) in the 2·5 μg, 5 μg, and 10 μg group, respectively. In the phase 2 trial among younger adults, the GMT was 39 (29–53) in the 5 μg days 0 and 14 group, 134 (104–174) in the 5 μg days 0 and 21 group, 91 (71–115) in the 5 μg days 0 and 28 group, and 24 (17–34) in 10 μg day 0 group. Among participants receiving three doses, seroconversion rates of neutralizing antibody on day 28 after the whole-course vaccination ranged between 90·5% (82·1%−95·8%) and 100% (95·7%−100·0%) among younger adults and ranged between 87·8% (78·7%−94·0%) and 90·1% (83·6%−96·6%) among older adults. In the phase 2 trial among younger adults, the seroconversion rate was 72·6% (61·8%−81·8%) in 5 μg days 0 and 14 group, 90·5% (82·1%−95·8%) in 5 μg days 0 and 21 group, 91·6% (83·4%−96·5%) in 5 μg days 0 and 28 group, and 51·2% (40·0%−62·3%) in 10 μg day 0 group.
Table 3.
Antibody responses of vaccine groups 28 and 90 days after the whole-course vaccination in the phase 1 and 2 trials*.
| 18–59 years |
60 years or older |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days 0, 28, and 56 |
Days 0 and 14 | Days 0 and 21 | Days 0 and 28 | Day 0 | Days 0, 28, and 56 |
|||||||
| 2·5 μg | 5 μg | 10 μg | 5 μg | 5 μg | 5 μg | 10 μg | 2·5 μg | 5 μg | 10 μg | |||
| Neutralizing antibodies to live SARS-CoV-2 28 days after the whole-course vaccination | ||||||||||||
| Participants | 84 | 84 | 84 | 84 | 84 | 83 | 84 | 84 | 82 | 81 | ||
| Geometric mean titre | 149 (110–202) | 238 (188–302) | 216 (181–257) | 39 (29–53) | 134 (104–174) | 91 (71–115) | 24 (17–34) | 161 (118–219) | 161 (118–221) | 187 (138–253) | ||
| Seroconversion rate | 76 (90·5; 82·1–95·8) | 82 (97·6; 91·7–99·7) | 84 (100·0; 95·7–100·0) | 61 (72·6; 61·8–81·8) | 76 (90·5; 82·1–95·8) | 76 (91·6; 83·4–96·5) | 43 (51·2; 40·0–62·3) | 74 (88·1; 79·2–94·1) | 72 (87·8; 78·7–94·0) | 73 (90·1; 83·6–96·6) | ||
| Specific binding antibody responses to whole SARS-CoV-2 antigen 28 days after the whole-course vaccination | ||||||||||||
| Geometric mean titre | 238 (205–275) | 297 (256–344) | 271 (237–311) | 42 (35–50) | 136 (115–160) | 151 (125–182) | 29 (25–35) | 186 (156–220) | 242 (202–290) | 346 (297–403) | ||
| seroconversion rate | 84 (100·0; 95·7–100·0) | 84 (100·0; 95·7–100·0) | 84 (100·0; 95·7–100·0) | 54 (64·3; 53·1–74·5) | 82 (97·6; 91·7–99·7) | 79 (95·2; 88·1–98·7) | 37 (44·1; 33·2–55·3) | 83 (98·8; 93·5–100·0) | 82 (100·0; 95·6–100·0) | 81 (100·0; 95·6–100·0) | ||
| Neutralizing antibodies to live SARS-CoV-2 90 days after the whole-course vaccination | ||||||||||||
| Participants | 84 | 84 | 84 | 84 | 83 | 83 | 84 | 84 | 82 | 81 | ||
| Geometric mean titre | 87 (61–125) | 128 (95–173) | 129 (99–169) | 20 (14–27) | 53 (38–75) | 44 (32–61) | 7 (6–9) | 99 (70–139) | 99 (69–144) | 120 (84–172) | ||
| Seroconversion rate | 67 (79·8; 69·6–87·8) | 74 (88·1; 79·2–94·1) | 77 (91·7; 83·6–96·6) | 42 (50·0; 38·9–61·1) | 59 (71·1; 60·1–80·5) | 59 (71·1; 60·1–80·5) | 12 (14·3; 7·6–23·6) | 68 (81·0; 70·9–88·7) | 64 (78·1; 67·5–86·4) | 67 (82·7; 72·7–90·2) | ||
| Specific binding antibody responses to whole SARS-CoV-2 antigen 90 days after the whole-course vaccination| | ||||||||||||
| Geometric mean titre | 164 (145–185) | 224 (197–255) | 234 (203–270) | 34 (29–40) | 99 (83–117) | 96 (82–113) | 23 (20–26) | 130 (107–159) | 196 (157–245) | 265 (226–311) | ||
| Seroconversion rate | 84 (100·0; 95·7–100·0) | 84 (100·0; 95·7–100·0) | 84 (100·0; 95·7–100·0) | 51 (60·7; 49·5–71·2) | 79 (95·2; 88·1–98·7) | 78 (94·0; 86·5–98·0) | 27 (32·1; 22·4–43·2) | 77 (91·7; 83·6–96·6) | 78 (95·1; 88·0–98·7) | 81 (100·0; 95·6–100·0) | ||
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. * Geometric mean titres are shown with 95% confidence intervals. Seroconversion rates are n (%; 95% confidence interval), and 95% confidence interval was calculated by the Clopper-Pearson (exact) method. Data from the same immunization procedure groups in the phase 1 and 2 trials were pooled. The antibodies were not measurable at baseline for all groups and days 28 and 90 after the whole-course vaccination for the alum-only group, and thus data were not shown. All T tests showed that the differences of neutralizing antibody tires and specific binding antibody titres between vaccine and placebo groups were statistically significant (P ≤ 0·0004). Seroconversion rate was defined as at least a four-fold increase of antibody titres over baseline (i.e., 20 for neutralizing and 40 for IgG-binding antibody titres). Results of ANOVA, Student-Newman-Keuls Test, and Fisher's exact test are reported in the apendix (p 8).
The GMT of neutralizing antibody titres on day 90 after the whole-course vaccination were 87 (95% CI, 61–125). 128 (95–173), and 129 (99–169) in the 2·5 μg, 5 μg, and 10 μg groups among younger adults, respectively, and the corresponding figures were 99 (70–139), 99 (69–144), and 120 (84–172) among older adults; in the phase 2 trial among younger adults, the GMT was 20 (14–27) in the 5 μg days 0 and 14 group, 53 (38–75) in the 5 μg days 0 and 21 group, 44 (32–61) in the 5 μg days 0 and 28 group, and 7 (6–9) in the 10 μg day 0 group. The GMT of neutralizing antibody titres declined by 35·8% (10 μg days 0, 28, and 56 group among older adults) to 70·8% (10 μg day 0 group) from day 28 to day 90 after the whole-course vaccination; however, we did not observe statistically different seroconversion rate of neutralizing antibody on day 90 after the whole-course vaccination among those receiving three doses at different dosages, and the seroconversion rates were 79·8% (69·6%−87·8%) to 91·7% (83·6%−96·6%) among younger adults and 78·1% (67·5%−86·4%) to 82·7% (72·7%−90·2%) among older adults. Among four exploratory 2-dose or 1-dose groups, seroconversion rates were 71·1% (60·1%−80·5%) in 5 μg days 0 and 21/28 groups, which were higher than 50·0% (38·9%−61·1%) in 5 μg days 0 and 14 group and 14·3% (7·6%−23·6%) in 10 μg day 0 group (Supplementary Appendix, Table 5). A similar pattern was found for the specific IgG-binding antibody levels.
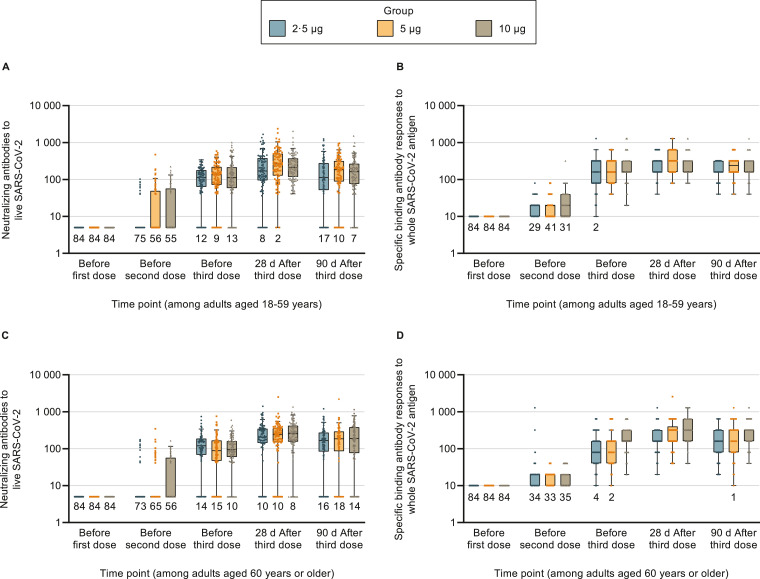
The dynamic changes of the antibody responses in the 0/28/56 groups in the phase 1 and 2 trials were further examined (Fig. 2). The antibody responses with more time points in the phase 1 trial among younger adults were shown before [13], and Supplementary Appendix Table 6 shows the results for older adults. Most participants started to generate antibody responses after the second injection, which remained at high levels 28 days after the third injection, but antibody titres declined 90 days after the whole-course vaccination. Results of subgroup analyses by sex and BMI groups are reported in Supplementary Appendix Figs. 3 and 4, and all subgroups could generate neutralizing antibody and specific IgG-binding antibody on days 28 and 90 after the whole-course vaccination.
Fig. 2.
Antibody responses of participants receiving 3 doses of vaccines in the phase 1 and 2 trials at different time points
Data from the phase 1 and 2 trials were combined. Placebo groups are not shown since no measurable antibody responses could be detected in these groups at any time. There were 84 participants in each group. Among participants aged ≥60 years in the medium-dose group, 1, 1, and 2 participants did not provide blood samples before the second dose, before the third dose, and on days 28 and 90 after the whole-course vaccination. Among participants aged ≥60 years in the high-dose group, 1, 1, and 3 participants did not provide blood samples before the second dose, before the third dose, and on days 28 and 90 after the whole-course vaccination. The dots represent individual participant values. The boxes and horizontal bars denote interquartile range (IQR) and median value. The whisker's upper endpoint equaled to 75th percentile + 1•5 × IQR, and the whisker's lower endpoint equaled to 25th percentile - 1•5 × IQR. The numbers below the boxes indicate the number of participants with values below the detection limit. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
4. Discussion
In this phase 1/2 clinical trial among healthy adults, the SARS-CoV-2 inactivated vaccine was safe and well-tolerated in all age and dose groups, and 15·1% of participants experienced adverse reactions. The most common injection-site adverse reaction was injection-site pain, and the most common systemic adverse reaction was fever, which were mostly mild-to-moderate and self-limiting. No serious adverse reactions related to the vaccine occurred. The inactivated vaccine could effectively elicit humoral responses, and the GMT of neutralizing antibody titres on days 28 and 90 after the third injection ranged from 149 to 238 and 87 to 129, respectively among participants receiving three doses of vaccines. In general, the antibody response was weaker in 5 μg days 0 and 14 group and 10 μg day 0 group. Given that large-scale production of the inactivated vaccine would be achievable and the vaccine could be stored at 2–8 °C, the inactivated vaccine could be an optimal option for many developing countries and regions where cold storage and distribution capacity was relatively poor.
The incidence rates of adverse reactions to the inactivated vaccine were generally comparable across different vaccine groups with no clear dose-response associations and similar when compared to the placebo group, indicating that the adverse reactions may not be entirely due to the vaccine but may be because of intramuscular injections or the aluminum hydroxide adjuvant that was contained in both the vaccines and placebos. The incidence rates of adverse reactions were similar to what we have reported before in selected groups with a smaller sample size [13], as well as recent reports of other whole-virus inactivated vaccine candidates [[10], [11], [12],14,40]. In the current analysis, we found similar incidence rates of adverse reactions among older adults (11·0%) compared with younger adults (19·3%) who received three injections on days 0, 28 and 56. This pattern is consistent with reports of another inactivated vaccine candidate [12,40]. Nevertheless, the current studies could not provide firm evidence of the superiority and inferiority in terms of safety profile among different vaccines or between older and younger adults, because of small sample sizes in phase 1 and 2 trials, short follow-up duration, and varied populations, settings, and methodologies across trials. Phase 3 trials with long-term follow-up as well as larger sample size are still needed to provide the full safety profile of the vaccines.
The vaccine-enhanced diseases, i.e., antibody-dependent enhancement (ADE) and vaccine-associated enhanced respiratory disease (VAERD) [43,44], have been frequently raised as concerns for the COVID-19 vaccine candidates, particularly for inactivated vaccines. However, current phase 1/2 trials are not designed to assess these outcomes given that none of the participants was infected with SARS-CoV-2 after vaccination. Although published phase 3 trials of other vaccines did not report ADE and VAERD [27], [28], [29], [30], [31], [32], [33], the safety of the inactivated vaccine still need to be closely monitored in the subsequent follow-up visits as well as in the phase 3 trial and real-world observations.
We have previously reported the interim results of antibody responses for some groups from the phase 1 (2·5 μg, 5 μg, and 10 μg with three injections) and phase 2 (5 μg on days 0 and 14/21) trials up to 14 days after the whole-course vaccination. The current analysis had an expanded sample size (e.g., 84 in each group rather than 24 in different dose groups with three injections), longer follow-up (90 rather than 14 days post injection), additional age groups (i.e., those aged ≥60 years old), and more schedule groups (i.e., 5 μg days 0 and 28 group and 10 μg day 0 group). Neutralizing antibody responses were generally similar among younger and older adults in our study, and the GMTs of neutralizing antibody on day 90 after three doses were 87–129 and 99–120 among younger and older adults, respectively, which was also observed in other vaccines [12,14,18,36,37]. Again, the efficacy in different age groups needs to be tested in the phase 3 clinical trials. The GMT of neutralizing antibody level was lower for those receiving vaccines at 5 μg on days 0 and 14/21 on days 28 and 90 compared to day 14 after the whole-course vaccination. Although seroconversion rates of neutralizing antibody on day 90 after the whole-course vaccination were over 70% among participants receiving 2·5 μg, 5 μg, or 10 μg on days 0, 28, and 56 or those receiving 5 μg on days 0 and 21/28, the results still raised a concern whether the antibody levels would decline over time, and longer follow-up is still needed to confirm the durability of antibody against SARS-CoV-2 and the efficacy of the vaccine. Moreover, current epidemiological evidence regarding the temporal change of antibody level against SARS-CoV-2 after infection remained debatable, and most were from studies with relatively small sample sizes. Some studies reported stable neutralizing or specific binding antibody over 6 months, while others found the antibody titre significantly declined and even turned to be undetectable in some participants [45], [46], [47], [48], [49], [50]. Besides, two studies reported antibody titres on days 90 or 180 after the whole-course vaccination. The phase 1 trial of an inactivated vaccine, the BBV152 vaccine, did not observe declining antibody titres between days 14 and 90 after the whole-course vaccination, and seroconversion rates of neutralizing antibody on day 90 after the whole-course vaccination ranged between 73·1 and 81·1% among those receiving vaccines [51]. Another study in 33 adults found specific binding antibody titres induced by mRNA-1273 vaccine remained high in 6 months after vaccination, and nearly all participants had measurable neutralizing antibody titres [52]. Accordingly, future studies are still warranted to test the durability of humoral and cellular immune induced by different vaccines against SARS-CoV-2.
The antibody titres on day 90 after the whole-course vaccination were low among those receiving 10 μg on day 0, and the seroconversion rates were only 32·1% for specific IgG binding antibody and 14·3% for neutralizing antibody, indicating the poor performance of this approach. Our results also indicated that a relatively longer interval (e.g., 21 or 28 days) between injections would be preferred, because the 5 μg days 0 and 14 group had lower antibody titres and seroconversion rates (60·7% for specific IgG binding antibody and 50·0% for neutralizing antibody). Nevertheless, the efficacy of the three-dose and two-dose schedules is unclear, and the optimal or minimum antibody titres that could protect people from COVID-19 are yet to be established. Besides, the sample size of current phase 2 trial is still limited, and the comparison among groups of different immune procedures might be underpowered, and thus the results should be interpreted cautiously. Subgroup analyses by intervals between injections should be performed in a study with a larger sample size to further verify the current results.
Another concern is that whether current vaccines could protect against infection of emerging SARS-CoV-2 variants, such as the B.1.1.7 lineage (first detected in the UK), B.1.351 lineage (South Africa), and P.1 lineage (Brazil). Phase 3 clinical trials showed that two-dose regimen of ChAdOx1 nCoV-19 vaccine manifested no protection against mild-to-moderate COVID-19 caused by B.1.351 lineage and reduced efficacy against symptomatic infection caused by B.1.1.7 lineage [53,54]. As for inactivated vaccines, previous studies reported that serum collected from participants receiving BBIBP-CorV vaccines had a higher neutralizing antibody titre against B.1.1.7 lineage but similar or lower neutralizing antibody titre against B.1.351 lineage, compared with neutralizing antibody titre against wild-type virus [55,56]; while serum from those receiving CoronaVac vaccines have lower neutralizing antibody titre against both B.1.1.7 and B.1.351 lineage [56]. neutralizing capacity against emerging SARS-CoV-2 variants of this investigated inactivated vaccine is unavailable now but is planned to be tested to facilitate the understanding of its effects on different variants.
This study has several limitations. First, the long-term safety and immunogenic responses are still unclear and extended follow-up visits are underway. Second, the results from subgroup and secondary analyses should be interpreted cautiously due to the limited sample size. Third, cellular immune responses and antigen-specific antibody binding responses were not measured in the current study, and data of neutralizing antibody titres against emerging variants of SARS-CoV-2 are unavailable. Fourth, data from older adults were mainly from those aged 60–69, and evidence from the oldest old is still warranted. Besides, the trial among children and adolescents is still ongoing and data are not yet available. Fifth, since we did not collect and measure antibody titres in convalescent sera as a comparator in this study, it is difficult to compare the humoral immune responses induced by the inactivated vaccine versus natural infection. Given varied PRNT and ELISA assays across epidemiological studies and clinical trials, it was also inappropriate to directly compare antibody titres in our study with other studies. However, a previous study of the phase 1/2 trials of BBV152, another inactivated vaccine, showed that the neutralizing antibody titres generated by vaccine were similar to the titres in human convalescent serum [11].
In conclusion, the preliminary results of the phase 1/2 trial indicated that the inactivated vaccine against SARS-CoV-2 was safe and immunogenic among adults, including those aged 60 years or older. The results also suggested that the two-dose schedule of 5 µg on days 0 and 21/28 could be further tested for efficacy in phase 3 trial given the better immunogenicity profile compared to a single high dose (10 µg on days 0) and the two-dose schedule with a shorter between-dose interval (5 µg on days 0 and 14). A third boost dose could generate even higher antibody titres as expected, but this may reduce the number of people who can get vaccinated and cause inadequate vaccine supplies. Extended follow-up is ongoing, and phase 3 trials have been launched in multiple countries using the two-dose schedule of 5 µg with 21–28 days of interval, which would provide additional evidence on the long-term safety and efficacy of the inactivated vaccine.
Contributors
Xiaoming Yang and Shengli Xia are the principal investigators. Xiaoming Yang, Shengli Xia, Shuo Shen, Wanshen Guo, Kai Duan, Yuntao Zhang, Zhiming Yuan, Xinguo Li, Wei Chen, Cong Wu, Lianghao Zhang, and Yunkai Yang designed the trial and study protocol. An Pan, Yan-Bo Zhang, and Zejun Wang contributed to the literature search. All authors had access to data and Xiaoming Yang and Shengli Xia verified the data. Yong-Li Yang, Zejun Wang, Yan-Bo Zhang, and An Pan contributed to the analyses. Yan-Bo Zhang, An Pan, and Shihe Huang wrote the first draft of the manuscript. All authors contributed to the data interpretation and revision of the manuscript. Xiaoming Yang and Shengli Xia monitored the trial. Wei Chen and Zejun Wang were responsible for the laboratory analysis.
Funding
The study was funded by the National Program on Key Research Project of China (2020YFC0842100) and Major Science and Technology Project of the National New Drug Development of China (2018ZX09734-004).
Data sharing statement
The phase 1/2 trials are still ongoing, and the trial data will be made available 6 months after the trial is complete upon reasonable request of the corresponding author and approval by the institutional review board.
Declaration of Competing Interest
Xiaoming Yang report grants from National Program on Key Research Project of China during the conduct of the study; and has patent 202,010,559,132.3 pending.
Kai Duan report grants from National Program on Key Research Project of China during the conduct of the study. Additionally, Kai Duan has patent 202,010,559,132.3 pending and is an employee of Wuhan Institute of Biological Products Co Ltd.
Yong-Li Yang reports personal fees from Wuhan Institute of Biological Products Co Ltd, during the conduct of the study.
Cong Wu, Jia Lu, Jing Guo, Lianghao Zhang, Shihe Huang, Wei Chen, Wei Zhou, Wenhui Wang, Xin Wan, and Xuanxuan Nian report being employees of Wuhan Institute of Biological Products Co Ltd.
Qian Wang, Xue-Wei Wang, Xu-Qin Yang, Yunkai Yang report being employees of the China National Biotec Group Company Limited.
Lili Huang reports grants from Major Science and Technology Project of the National New Drug Development of China, during the conduct of the study.
Zhiming Yuan reports grants from National Program on Key Research Project of China, during the conduct of the study.
Shengli Xia and Wanshen Guo reports grants from National Program on Key Research Project of China, grants from Major Science and Technology Project of the National New Drug Development of China, during the conduct of the study.
Shuo Shen reports grants from National Program on Key Research Project of China during the conduct of the study, and is an employee of Wuhan Institute of Biological Products Co Ltd.
Xinguo Li reports being an employee of Wuhan Institute of Biological Products Co Ltd, and has a patent 202,010,559,132.3 pending.
Yuntao Zhang reports grants from National Program on Key Research Project of China, during the conduct of the study and is an employee of China National Biotec Group Company Limited.
Zejun Wang reports grants from National Program on Key Research Project of China and is an employee of Wuhan Institute of Biological Products Co Ltd.
An Pan, Cheng Peng, Dongyang Zhao, Huajun Zhang, Wangyang You, Wei Zhang, Xiaoxiao Gao, Yan-Bo Zhang, Yanxia Wang, Zhengli Shi, and Zhiqiang Xie have nothing to disclose.
Jianhui Du and Xing-Hang Li report being students of Wuhan Institute of Biological Products Co Ltd.
Acknowledgments
The vaccine was developed and the study was sponsored by the China National Biotec Group Company Limited and the Wuhan Institute of Biological Products Co. Ltd. We are grateful for all investigators at the Henan Center for Disease Control and Prevention and Wuzhi county Center for Disease Control and Prevention who contributed to the site work of the trials. We appreciate the contributions of the members in the data and safety monitoring board, including Dr. Xiaoju Zhang and Dr. Qiuxing Zhang from the Henan Province People's Hospital, and Dr. Guoli Yan from the Henan University of Chinese Medicine. They received remuneration for their work. We thank all the participants in the trial for their altruism and dedication to the trial.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101010.
Contributor Information
Shuo Shen, Email: shenshuo1@sinopharm.com.
Shengli Xia, Email: 1792865518@qq.com.
An Pan, Email: panan@hust.edu.cn.
Xiaoming Yang, Email: yangxiaoming@sinopharm.com.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO coronavirus disease (COVID-19) dashboard. 2021. https://covid19.who.int/ (accessed June 10, 2021).
- 2.World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed June 10, 2021).
- 3.Keech C., Albert G., Cho I. Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richmond P., Hatchuel L., Dong M. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang-Monteagudo A., Ochoa-Azze R., Climent-Ruiz Y., et al. A single dose of SARS-CoV-2 FINLAY-FR-1A dimeric-RBD recombinant vaccine enhances neutralization response in COVID-19 convalescents, with excellent safety profile. A preliminary report of an open-label phase 1 clinical trial. medRxiv 2021; published online Mar 3. http://medrxiv.org/content/early/2021/03/03/2021.02.22.21252091.abstract (preprint). [DOI] [PMC free article] [PubMed]
- 6.Chappell K.J., Mordant F.L., Li Z. Safety and immunogenicity of an MF59-adjuvanted spike glycoprotein-clamp vaccine for SARS-CoV-2: a randomized, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00200-0. published online Apr 19. In press. https://pubmed.ncbi.nlm.nih.gov/33887208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goepfert P.A., Fu B., Chabanon A.L. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00147-X. published online Apr 19. In press. https://pubmed.ncbi.nlm.nih.gov/33887209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh S.M., Liu W.D., Huang Y.S., et al. First-in-human trial of a recombinant stabilized prefusion SARS-CoV-2 spike protein vaccine with adjuvant of aluminum hydroxide and CpG 1018. medRxiv 2021; published online Apr 6. http://medrxiv.org/content/early/2021/04/06/2021.03.31.21254668.abstract (preprint).
- 9.Yang S., Li Y., Dai L. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00127-4. published online Mar 24. In press. https://pubmed.ncbi.nlm.nih.gov/33773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Che Y., Liu X., Pu Y. Randomized, double-blinded and placebo-controlled phase II trial of an inactivated SARS-CoV-2 vaccine in healthy adults. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1703. published online Nov 9. In press. https://www.ncbi.nlm.nih.gov/pubmed/33165503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ella R., Vadrevu K.M., Jogdand H. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;21:637–646. doi: 10.1016/S1473-3099(20)30942-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z., Hu Y., Xu M. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803–812. doi: 10.1016/S1473-3099(20)30987-7. https://www.ncbi.nlm.nih.gov/pubmed/33548194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia S., Duan K., Zhang Y. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia S., Zhang Y., Wang Y. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan H.X., Liu J.K., Huang B.Y. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults: randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chin Med J. 2021;134(11):1289–1298. doi: 10.1097/CM9.0000000000001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett J.R., Belij-Rammerstorfer S., Dold C. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 17.Logunov D.Y., Dolzhikova I.V., Zubkova O.V. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadoff J., Le Gars M., Shukarev G. Interim results of a phase 1-2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021;384(19):1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu F.C., Guan X.H., Li Y.H. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanini S., Capone S., Antinori A., et al. GRAd-COV2, a gorilla adenovirus based candidate vaccine against COVID-19, is safe and immunogenic in young and older adults. medRxiv 2021; published online Apr 13. http://medrxiv.org/content/early/2021/04/13/2021.04.10.21255202.abstract (preprint). [DOI] [PubMed]
- 21.Sieling P., King T., Wong R., et al. Single prime hAd5 spike (S) + nucleocapsid (N) dual antigen vaccination of healthy volunteers induces a ten-fold increase in mean S- and N- T-Cell Responses Equivalent to T-Cell responses from patients previously infected with SARS-CoV-2. medRxiv 2021; published online Apr 7. http://medrxiv.org/content/early/2021/04/07/2021.04.05.21254940.abstract (preprint).
- 22.Jackson L.A., Anderson E.J., Rouphael N.G. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kremsner P., Mann P., Bosch J., et al. Phase 1 assessment of the safety and immunogenicity of an mRNA- lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. medRxiv 2020; published online Nov 9. http://medrxiv.org/content/early/2020/11/09/2020.11.09.20228551.abstract (preprint).
- 24.Walsh E.E., Frenck R.W., Falsey A.R. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tebas P., Yang S., Boyer J.D. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClin Med. 2021;31 doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward B.J., Gobeil P., Séguin A., et al. Phase 1 trial of a candidate recombinant virus-like particle vaccine for Covid-19 disease produced in plants. medRxiv 2020; published online Nov 6. http://medrxiv.org/content/early/2020/11/06/2020.11.04.20226282.abstract (preprint).
- 27.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bueno S.M., Abarca K., González P.A., et al. Interim report: safety and immunogenicity of an inactivated vaccine against SARS-CoV-2 in healthy Chilean adults in a phase 3 clinical trial. medRxiv 2021; published online Apr 1. http://medrxiv.org/content/early/2021/04/01/2021.03.31.21254494.abstract (preprint).
- 29.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadoff J., Gray G., Vandebosch A. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voysey M., Clemens S.A.C., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Kaabi N., Zhang Y., Xia S. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021 doi: 10.1001/jama.2021.8565. published online May 26. In press. https://pubmed.ncbi.nlm.nih.gov/34037666/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramasamy M.N., Minassian A.M., Ewer K.J. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson E.J., Rouphael N.G., Widge A.T. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Formica N., Mallory R., Albert G., et al. Evaluation of a SARS-CoV-2 vaccine NVX-CoV2373 in younger and older adults. medRxiv 2021; published online Mar 1. http://medrxiv.org/content/early/2021/03/01/2021.02.26.21252482.abstract (preprint). [DOI] [PMC free article] [PubMed]
- 39.Wang Z.J., Zhang H.J., Lu J. Low toxicity and high immunogenicity of an inactivated vaccine candidate against COVID-19 in different animal models. Emerg Microbes Infect. 2020;9:2606–2618. doi: 10.1080/22221751.2020.1852059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Zeng G., Pan H. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu F.C., Li Y.H., Guan X.H. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou B.F. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83–96. [PubMed] [Google Scholar]
- 43.Eroshenko N., Gill T., Keaveney M.K., Church G.M., Trevejo J.M., Rajaniemi H. Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat Biotechnol. 2020;38:789–791. doi: 10.1038/s41587-020-0577-1. [DOI] [PubMed] [Google Scholar]
- 44.Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368:945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 45.Dan J.M., Mateus J., Kato Y. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaebler C., Wang Z., Lorenzi J.C.C. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartley G.E., Edwards E.S.J., Aui P.M. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Science immunology. 2020;5 doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang C., Huang L., Wang Y. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan Y., Liu F., Xu X. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front Med. 2020;14:746–751. doi: 10.1007/s11684-020-0822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J., Liang B., Chen C. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12:1813. doi: 10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ella R., Reddy S., Jogdand H. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00070-0. published online Mar 8. In press. https://pubmed.ncbi.nlm.nih.gov/33705727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doria-Rose N., Suthar M.S., Makowski M. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021 doi: 10.1056/NEJMc2103916. https://pubmed.ncbi.nlm.nih.gov/33822494 published online Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emary K.R.W., Golubchik T., Aley P.K. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madhi S.A., Baillie V., Cutland C.L. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang B., Dai L., Wang H., et al. Neutralization of SARS-CoV-2 VOC 501Y.V2 by human antisera elicited by both inactivated BBIBP-CorV and recombinant dimeric RBD ZF2001 vaccines. bioRxiv 2021 https://www.biorxiv.org/node/1767047.abstract (preprint).
- 56.Wang G.L., Wang Z.Y., Duan L.J. Susceptibility of circulating SARS-CoV-2 variants to neutralization. N Engl J Med. 2021;384(24):2354–2356. doi: 10.1056/NEJMc2103022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.