Abstract
Objectives
We conducted a systematic review and meta-analysis with meta-regression of creatine kinase-MB (CK-MB), a biomarker of myocardial injury, in COVID-19 patients.
Methods
We searched PubMed, Web of Science and Scopus, for studies published between January 2020 and January 2021 that reported CK-MB, COVID-19 severity and mortality (PROSPERO registration number: CRD42021239657).
Results
Fifty-five studies in 11,791 COVID-19 patients were included in the meta-analysis. The pooled results showed that CK-MB concentrations were significantly higher in patients with high disease severity or non-survivor status than patients with low severity or survivor status (standardized mean difference, SMD, 0.81, 95% CI 0.61 to 1.01, p<0.001). The rate of patients with CK-MB values above the normal range was also significantly higher in the former than the latter (60/350 vs 98/1,780; RR = 2.84, 95%CI 1.89 to 4.27, p<0.001; I2 = 19.9, p = 0.254). Extreme between-study heterogeneity was observed (I2 = 93.4%, p<0.001). Sensitivity analysis, performed by sequentially removing each study and re-assessing the pooled estimates, showed that the magnitude and direction of the effect size was not modified (effect size range, 0.77 to 0.84). Begg's (p = 0.50) and Egger's (p = 0.86) t-tests did not show publication bias. In meta-regression analysis, the SMD was significantly and positively associated with the white blood count, aspartate aminotransferase, myoglobin, troponin, brain natriuretic peptide, lactate dehydrogenase, and D-dimer.
Conclusions
Higher CK-MB concentrations were significantly associated with severe disease and mortality in COVID-19 patients. This biomarker of myocardial injury might be useful for risk stratification in this group.
Keywords: Creatine kinase-MB, Creatine kinase-MB values Above the normal range, COVID-19, Disease severity, Mortality
1. Introduction
Several clinical and demographic factors have been shown to be significantly associated with measures of coronavirus disease 2019 (COVID-19) severity, based on clinical and imaging findings and/or the need for aggressive single- and multi-organ support, and mortality [1,2]. The evidence of excessive inflammatory activity in severe COVID-19, captured during the early phases of the pandemic, prompted the investigation of the clinical role of specific biomarkers of inflammatory and immunomodulating pathways, particularly C-reactive protein (CRP), white blood cell count (WBC), neutrophils, lymphocytes, platelets, procalcitonin, ferritin, and serum amyloid A [[3], [4], [5], [6]]. Additional research has shown that patients with COVID-19 can also experience structural and functional abnormalities of specific organs and systems, e.g., cardiovascular, hematological, gastrointestinal, and neurological, in addition to the well-known respiratory compromise, characterized by the development of interstitial pneumonia and acute respiratory distress syndrome (ARDS) [[7], [8], [9]]. In particular, COVID-19 patients might present with subclinical or overt evidence of myocardial necrosis, which might manifest as acute coronary syndrome, myocarditis, arrhythmias, or heart failure [10]. While the exact mechanisms involved in the pathogenesis of cardiac complications in COVID-19 remain to be established, the presence of myocardial necrosis has been shown to be independently associated with more severe disease, transfer to the intensive care unit (ICU) and mortality [[11], [12], [13]]. Biomarkers of myocardial damage, particularly creatine kinase-MB (CK-MB) and troponin, have been increasingly investigated in COVID-19 patients in terms of their predictive capacity and potential to assist with clinical decisions [14]. A number of systematic reviews and meta-analyses on troponin and COVID-19 have been published [[15], [16], [17], [18]]. Similarly, meta-analyses have sought to critically appraise the available evidence regarding the clinical role of CK-MB [[19], [20], [21], [22]], with the largest meta-analysis identifying a total of 25 studies in 5,626 COVID-19 patients [21]. While CK-MB is less used in the diagnosis and the monitoring of myocardial necrosis in contemporary clinical practice since the advent of high-sensitivity troponin, additional retrospective and prospective studies have since been published on the associations between serum CK-MB concentrations, COVID-19 severity, and adverse outcomes. Furthermore, the assessment of CK-MB in COVID-19 patients might provide specific clinical information, independent of myocardial necrosis and cardiac complications, for early risk stratification in this group. We sought to address these issues by conducting an updated systematic review and meta-analysis of studies reporting serum CK-MB concentrations in COVID-19 patients with different disease severity, based on clinical guidelines or need for hospitalization, mechanical ventilation, or transfer to the ICU, and survival status during follow up. Additionally, a meta-regression analysis was performed to investigate possible associations between the effect size of the differences in CK-MB concentrations and a number of plausible clinical, demographic and biochemical factors.
2. Materials and methods
2.1. Search strategy, eligibility criteria, and study selection
We conducted a systematic literature search using the terms “CK-MB” or “creatine kinase MB” and “coronavirus disease 19” or “COVID-19”, in the electronic databases PubMed, Web of Science and Scopus, for peer-reviewed studies published from January 2020 to January 2021 that reported serum CK-MB concentrations in COVID-19 patients (PROSPERO registration number: CRD42021239657). The reference lists of the retrieved articles were also searched to identify additional studies. Eligibility criteria included: (a) studies reporting continuous data on CK-MB concentrations in COVID-19 patients, (b) articles investigating COVID-19 patients with different degrees of disease severity and/or survival status, (c) adult patients, (d) English language, (e) ≥10 COVID-19 patients, and (f) full-text available. Two investigators independently screened the abstracts. If relevant, the full articles were independently reviewed. The Newcastle-Ottawa scale was used to assess the quality of each study [23]. A score ≥6 indicated good quality when converting the scale to the Agency for Healthcare Research and Quality standards [24].
2.2. Statistical analysis
Standardized mean differences (SMD) and 95% confidence intervals (CIs) were calculated to build forest plots of continuous data and to evaluate differences in CK-MB concentrations between COVID-19 patients with low vs. high disease severity or survivor vs. non-survivor status. A p-value <0.05 was considered statistically significant. If studies reported CK-MB concentrations as median and interquartile range (IQR) or median and range, the corresponding means and standard deviations were estimated as previously described [25,26]. The Q-statistic was used to assess the heterogeneity of SMD values across studies with a significance level set at p<0.10. Inconsistency across studies was evaluated through the I2 statistic, with I2 values <25% indicating no heterogeneity, between 25 and 50% moderate heterogeneity, between 50 and 75% large heterogeneity, and >75% extreme heterogeneity [27,28]. A random-effect model was used to calculate the pooled SMD and corresponding 95% CIs in the presence of significant heterogeneity. In sensitivity analyses, the influence of each study on the overall effect size was assessed using the leave-one-out method [29]. The presence of publication bias was assessed using the Begg’s adjusted rank correlation test and the Egger’s regression asymmetry test at the p<0.05 level of significance [30,31], and further evaluated using the Duval and Tweedie “trim and fill” procedure. The latter, a funnel-plot-based method of testing and adjusting for publication bias, is a nonparametric (rank-based) data augmentation technique that increases the observed data, so that the funnel plot is more symmetric, and recalculates the pooled SMD based on the complete data [32]. To explore possible contributors to the between-study variance, we investigated the effects of several biologically and/or clinically plausible factors on the SMD by univariate meta-regression analysis. These factors included age, gender, clinical endpoint, month of recruitment commencement, diabetes, hypertension and cardiovascular disease, biomarkers of inflammation (CRP, WBC), liver damage (aspartate aminotransferase - AST, alanine aminotransferase - ALT, albumin), cardiac damage (myoglobin, troponin, brain natriuretic peptide - BNP), renal damage (serum creatinine), tissue damage and sepsis (lactate dehydrogenase - LDH, procalcitonin), and pro-thrombotic tendency (D-dimer). Statistical analyses were performed using Stata 14 (STATA Corp., College Station, TX, USA). The study was fully compliant with the PRISMA statement regarding the reporting of systematic reviews and meta-analyses [33].
3. Results
3.1. Literature search and study selection
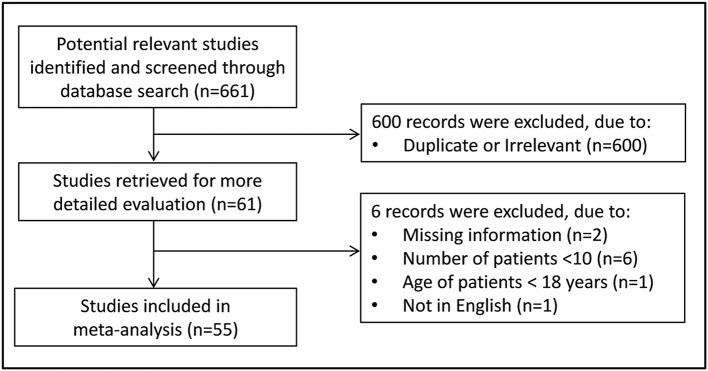
Fig. 1 describes the flow chart of the screening process. We initially identified 661 studies. A total of 600 studies were excluded after the first screening because they were either duplicates or irrelevant. After a full-text revision of the remaining 61 articles, 6 were further excluded because they did not meet the inclusion criteria. Thus, 55 studies in 11,791 COVID-19 patients, 9,596 (48% males, mean age 54 years) with low severity or survivor status and 2,195 (62% males, mean age 66 years) with high severity or non-survivor status during follow up, were included in the meta-analysis (Table 1 ) [14,[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87].
Fig. 1.
Flow chart of study selection.
Table 1.
Characteristics of the selected studies.
| Mild disease or survivor |
Severe disease or non-survivor |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author, |
Study |
NOS |
n | Age |
Gender |
CK-MB |
n | Age |
Gender |
CK-MB |
|
| Country [reference] | design | Endpoint | (stars) | (Years) | (M/F) | U/L |
(Years) | (M/F) | U/L |
||
| (Mean ±SD) | (Mean ±SD) | ||||||||||
| Aladağ N et al., | R | Survival status | 7 | 35 | 68 | 22/13 | 29 ± 23 | 15 | 68 | 6/9 | 33 ± 25 |
| Turkey [34] | |||||||||||
| Cao L et al., | R | Survival status | 7 | 78 | 68 | 33/45 | 0.8 ± 0.4a | 22 | 81 | 12/10 | 4.9 ± 5.4a |
| China [35] | |||||||||||
| Chen FF et al., | R | Survival status | 7 | 577 | 63 | 297/280 | 1.1 ± 0.6a | 104 | 73 | 65/39 | 3.7 ± 3.1a |
| China [36] | |||||||||||
| Chen J et al., | R | Mechanical ventilation | 7 | 68 | 67 | 38/30 | 36 ± 77 | 30 | 68 | 18/12 | 10 ± 13 |
| China [37] | |||||||||||
| Deng M et al., | R | Disease severity | 7 | 53 | 35 | 24/29 | 10 ± 5 | 12 | 33 | 12/0 | 10 ± 13 |
| China [38] | |||||||||||
| Deng P et al., | R | Survival status | 7 | 212 | 63 | 97/115 | 1.1 ± 0.6a | 52 | 75 | 33/19 | 2.0 ± 0.5a |
| China [39] | |||||||||||
| Dong X et al., | R | Survival status | 7 | 65 | 54 | 30/35 | 24 ± 15 | 54 | 70 | 38/16 | 39 ± 29 |
| China [40] | |||||||||||
| Du RH et al., | R | ICU admission | 5 | 58 | 68 | 38/20 | 3.7 ± 4.1a | 51 | 79 | 36/15 | 5.4 ± 6.7a |
| China [41] | |||||||||||
| Feng X et al., | P | Composite endpointb | 7 | 94 | 63 | 58/36 | 18 ± 6 | 20 | 69 | 13/7 | 21 ± 14 |
| China [42] | |||||||||||
| Feng Y et al., | R | Disease severity | 5 | 352 | 51 | 190/162 | 13 ± 4 | 124 | 60 | 81/43 | 16 ± 7 |
| China [43] | |||||||||||
| Gao S et al., | R | Survival status | 5 | 175 | 70 | 79/96 | 9 ± 4 | 35 | 74 | 22/13 | 13 ± 8 |
| China [44] | |||||||||||
| Gong J et al., | R | Disease severity | 5 | 161 | 45 | 72/89 | 12 ± 5 | 28 | 64 | 16/12 | 16 ± 17 |
| China [45] | |||||||||||
| Guo H et al., | R | Survival status | 7 | 28 | 59 | NR | 6 ± 21a | 46 | 72 | NR | 11 ± 49a |
| China [46] | |||||||||||
| Guo J et al., | R | Survival status | 6 | 43 | 60 | 22/21 | 11 ± 4 | 31 | 68 | 21/10 | 18 ± 7 |
| China [47] | |||||||||||
| Han H et al., | R | Disease severity | 5 | 198 | 60 | 71/127 | 1.0 ± 0.6a | 75 | 59 | 26/49 | 1.3 ± 1.1a |
| China [48] | |||||||||||
| He B et al., | NR | Disease severity | 5 | 32 | 42 | 15/17 | 0.6 ± 0.2a | 21 | 57 | 13/8 | 1.6 ± 1.2a |
| China [49] | |||||||||||
| Hu J et al., | R | Disease severity | 7 | 130 | 63 | 58/72 | 13 ± 17 | 52 | 64 | 42/10 | 42 ± 93 |
| China [50] | |||||||||||
| Hu X et al., | R | Disease severity | 7 | 175 | 41 | 80/95 | 9.2 ± 4.5 | 38 | 53 | 22/16 | 8.9 ± 4.4 |
| China [51] | |||||||||||
| Jang JG et al., | R | Disease severity | 7 | 87 | 68 | 34/53 | 1.9 ± 1.8a | 23 | 54 | 14/9 | 5.3 ± 4.1a |
| Korea [52] | |||||||||||
| Ji L et al., | P | Composite endpointc | 7 | 243 | 52 | 121/122 | 0.4 ± 0.2 | 37 | 71 | 20/17 | 14 ± 20 |
| China [53] | |||||||||||
| Li N et al., | R | Disease severity | 7 | 103 | 61 | 48/55 | 0.8 ± 0.7a | 35 | 67 | 23/12 | 2.7 ± 2.9a |
| China [54] | |||||||||||
| Li Y et al., | R | Survival status | 7 | 64 | 54 | 30/34 | 11.7 ± 3.7 | 37 | 72 | 23/14 | 22.3 ± 16.3 |
| China [55] | |||||||||||
| Liu SL et al., | R | Disease severity | 5 | 194 | 43 | 91/103 | 9.3 ± 3.4 | 31 | 64 | 17/14 | 13.7 ± 7.4 |
| China [56] | |||||||||||
| McRae MP et al., | R | Survival status | 5 | 117 | 63 | 52/65 | 5.5 ± 7.5a | 43 | 73 | 30/13 | 8.6 ± 11.8a |
| USA [57] | |||||||||||
| Mertoglu C et al., | R | ICU admission | 7 | 532 | 48 | 306/226 | 16.4 ± 5.5 | 23 | 59 | 13/10 | 49 ± 52 |
| Turkey [58] | |||||||||||
| Qin W et al., | R | Survival status | 7 | 239 | 63 | 113/126 | 0.9 ± 0.4a | 23 | 69 | 10/13 | 2.0 ± 1.3a |
| China [59] | |||||||||||
| Tao Z et al., | R | Disease severity | 7 | 202 | 54 | 72/130 | 4.1 ± 5.5a | 20 | 65 | 8/12 | 2.5 ± 2.2a |
| China [60] | |||||||||||
| Tuo H et al., | R | Survival status | 5 | 96 | 51 | 38/58 | 0.8 ± 0.6a | 52 | 69 | 29/23 | 2.8 ± 2.3a |
| China [61] | |||||||||||
| Wang B et al., | R | Survival status | 7 | 54 | 62 | 40/14 | 24 ± 23 | 50 | 72 | 40/10 | 29 ± 22 |
| China [62] | |||||||||||
| Wang C et al., | R | Disease severity | 6 | 35 | 38 | 17/18 | 16 ± 13 | 10 | 43 | 6/4 | 20 ± 7 |
| China [63] | |||||||||||
| Wang D et al., | R | Disease severity | 5 | 72 | 44 | 29/43 | 13 ± 5 | 71 | 65 | 44/27 | 14 ± 8 |
| China [64] | |||||||||||
| Wang F et al., | R | Disease progression | 7 | 253 | 41 | 109/144 | 0.6 ± 0.6a | 70 | 60 | 45/25 | 0.7 ± 0.7a |
| China [65] | |||||||||||
| Wang G et al., | NR | Disease severity | 7 | 193 | 42 | 95/98 | 60 ± 55 | 16 | 54 | 10/6 | 8.4 ± 4.6 |
| China [66] | |||||||||||
| Wang JH et al., | R | Survival status | 7 | 1074 | 61 | 502/572 | 0.8 ± 0.4a | 61 | 74 | 43/18 | 2.5 ± 2.7a |
| China [67] | |||||||||||
| Wang K et al., | P | Survival status | 7 | 277 | 46 | 129/148 | 14 ± 4 | 19 | 66 | 11/8 | 21 ± 8 |
| China [68] | |||||||||||
| Wang Z et al., | R | Survival status | 7 | 100 | 58 | 44/56 | 1.0 ± 0.5 | 56 | 72 | 32/24 | 6.4 ± 8.6 |
| China [69] | |||||||||||
| Wu C et al., | R | Presence of ARDS | 6 | 117 | 48 | 68/49 | 15.3 ± 5.2 | 84 | 59 | 60/24 | 16.8 ± 5.6 |
| China [70] | |||||||||||
| Xie J et al., | NR | Disease progression | 6 | 75 | 51 | 45/30 | 19.3 ± 5.9 | 29 | 66 | 18/11 | 22 ± 7.4 |
| China [71] | |||||||||||
| Xie Y et al., | R | Disease severity | 5 | 38 | 61 | 14/24 | 0.6 ± 0.4 | 24 | 72 | 13/11 | 0.8 ± 0.5 |
| China [72] | |||||||||||
| Xu B et al., | R | Survival status | 5 | 117 | 56 | 59/58 | 14 ± 6 | 28 | 73 | 17/11 | 21 ± 10 |
| China [73] | |||||||||||
| Yang A et al., | R | Disease severity | 5 | 99 | 49 | 49/50 | 11 ± 10 | 15 | 60 | 7/8 | 12.1 ± 7.2 |
| China [74] | |||||||||||
| Yang C et al., | R | Survival status | 7 | 145 | 57 | 77/68 | 13 ± 6 | 58 | 67 | 38/20 | 19 ± 7 |
| Chin [75] | |||||||||||
| Yang J et al., | R | Survival status | 5 | 332 | 55 | 170/162 | 1.2 ± 1.0a | 25 | 75 | 15/10 | 7 ± 6.7a |
| China [14] | |||||||||||
| Yao Q et al., | R | Survival status | 6 | 96 | 51 | 36/60 | 16 ± 5 | 12 | 65 | 7/5 | 23 ± 10 |
| China [76] | |||||||||||
| Yu Z et al., | R | Survival status | 7 | 123 | 80 | 46/77 | 7.3 ± 3.7 | 18 | 84 | 11/7 | 8.8 ± 6.1 |
| China [77] | |||||||||||
| Zeng Z et al., | R | ICU admission | 7 | 406 | 43 | 206/200 | 10.6 ± 5.5 | 55 | 60 | 33/22 | 12 ± 6.4 |
| China [78] | |||||||||||
| Zhang C et al., | R | Disease severity | 7 | 56 | 44 | 24/32 | 0.5 ± 0.7 | 24 | 65 | 9/15 | 1.1 ± 1.2 |
| China [79] | |||||||||||
| Zhang G et al., | R | Disease severity | 5 | 166 | 51 | 73/93 | 12.3 ± 3.7 | 55 | 62 | 35/20 | 22.3 ± 15.6 |
| China [80] | |||||||||||
| Zhang JJ et al., | R | Survival status | 7 | 240 | 53 | 119/121 | 1.2 ± 1.0 | 49 | 69 | 25/14 | 2.3 ± 1.9 |
| China [81] | |||||||||||
| Zhang Q et al., | R | Disease severity | 7 | 47 | 61 | 18/29 | 9.3 ± 3.0 | 27 | 72 | 18/9 | 16.3 ± 8.9 |
| China [82] | |||||||||||
| Zhang XB et al., | R | Survival status | 7 | 410 | 53 | 219/191 | 7.1 ± 7.6 | 22 | 66 | 11/11 | 19.4 ± 19.6 |
| China [83] | |||||||||||
| Zhao Y et al., | R | Disease severity | 7 | 336 | 43 | 145/191 | 0.8 ± 0.4 | 81 | 56 | 53/28 | 1.1 ± 0.4 |
| China [84] | |||||||||||
| Zhou C et al., | R | Disease severity | 7 | 95 | 35 | 38/57 | 6.9 ± 3.7 | 28 | 40 | 17/11 | 8.0 ± 2.0 |
| China [85] | |||||||||||
| Zhou J et al., | NR | ICU admission | 5 | 156 | 40 | 75/81 | 11.1 ± 6.2 | 45 | 57 | 27/18 | 9.3 ± 4.7 |
| China [86] | |||||||||||
| Zhu Y et al., | R | Survival status | 5 | 73 | 73 | 39/34 | 1.8 ± 1.3 | 29 | 62 | 19/20 | 7.2 ± 8.7 |
| China [87] | |||||||||||
Abbreviations: ICU, intensive care unit; NOS, Newcastle-Ottawa quality assessment scale for case–control studies; NR, Not Reported; P, prospective; R, retrospective; ARDS, acute respiratory distress syndrome.
-ng/mL or μg/L.
-survival status on discharge, disease severity, and mechanical ventilation.
-survival status on discharge, presence of ARDS.
Two studies were conducted in Turkey [34,58], 1 in the USA [57], 1 in Korea [52], and the remaining 51 in China [14,[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51],[53], [54], [55], [56],[59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87]]. Three studies were prospective [42,53,68], 47 retrospective [[34], [35], [36], [37], [38], [39], [40], [41],[43], [44], [45], [46], [47], [48],[50], [51], [52],[54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65],67,[69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85],87], whereas the remaining 5 did not report information regarding the study design [14,49,66,71,86]. Clinical endpoints included disease severity based on current clinical guidelines in 21 studies [38,43,45,[48], [49], [50], [51], [52],54,56,60,63,64,66,72,74,79,80,82,84,85], admission to ICU in 4 [41,58,78,86], clinical progress in 2 [65,71], presence of ARDS in 1 [70], need for mechanical ventilation in 1 [37], and combined clinical outcomes in 2 [42,53], and survival status in 24 [14,[34], [35], [36],39,40,44,46,47,55,57,59,61,62,[67], [68], [69],73,[75], [76], [77],81,83,87]. Only 1 study reported the highest CK-MB serum concentrations during hospitalization [61], whereas the remaining 54 reported a single CK-MB concentration within the first 24–48 h from admission.
3.2. Meta-analysis
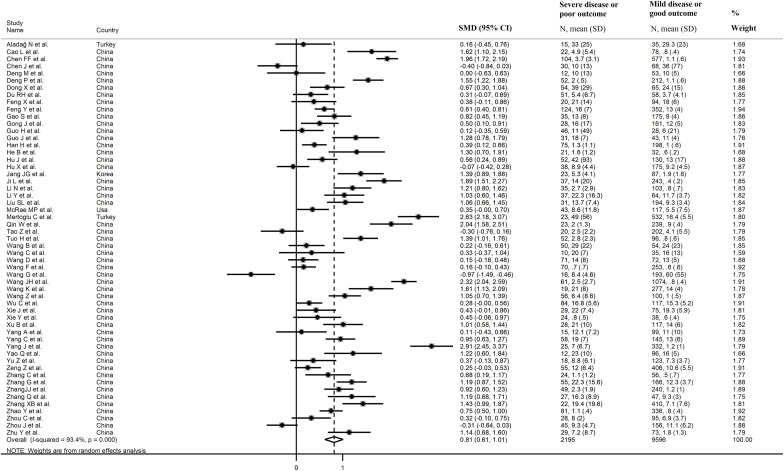
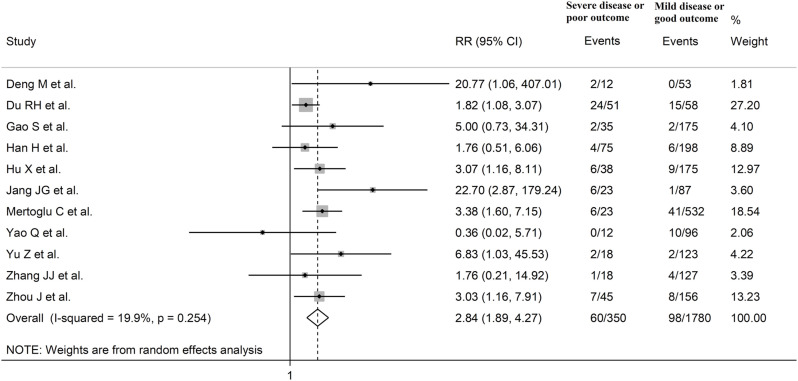
The overall SMD in CK-MB concentrations between COVID-19 patients with low vs. high disease severity or survivor vs. non-survivor status during follow up is shown in Fig. 2 . In 5 studies, patients with high severity or non-survivor status had lower CK-MB concentrations when compared to those with low severity or survivor status (mean difference range, -0.07 to -0.97) [37,51,60,66,86], although only 1 study reported a statistically significant difference [66]. In 1 study, no difference was observed (mean difference 0.00) [38], whereas in the remaining 49 studies the CK-MB concentrations were lower in patients with low severity or survivor status (mean difference range, 0.11 to 2.91), with a non-significant difference in 17 [34,38,41,42,46,57,[62], [63], [64], [65],[70], [71], [72],74,77,78,85]. The pooled results confirmed that the CK-MB concentrations were statistically significantly higher in patients with high disease severity or non-survivor status during follow up (SMD 0.81, 95% CI 0.61 to 1.01, p<0.001) (Fig. 2). An extreme heterogeneity between studies was observed (I2 = 93.4%, p<0.001). A sub-group of 11 studies reported the proportion of patients with CK-MB concentrations above the normal range in COVID-19 patients with high severity or non-survivor status vs. those with low severity or survivor status during follow-up [38,41,44,48,51,52,58,76,77,81,86]. The rate of participants with CK-MB values above the normal range was statistically significantly higher in the former than the latter (60/350 vs 98/1,780; RR = 2.84, 95%CI 1.89 to 4.27, p<0.001; I2 = 19.9, p = 0.254) (Fig. 3 ).
Fig. 2.
Forest plot of studies reporting CK-MB concentrations in patients with COVID-19.
Fig. 3.
Forest plot of studies reporting the proportion of patients with CK-MB concentrations above the normal range in COVID-19 patients with high severity or non-survivor status vs. those with low severity or survivor status during follow-up.
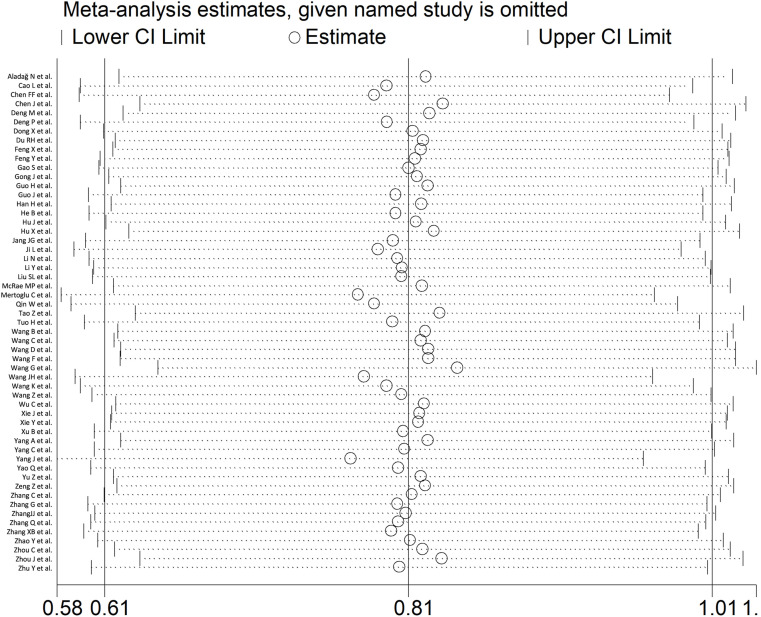
Sensitivity analysis, performed by sequentially removing each study and re-assessing the pooled estimates, showed that the magnitude and the direction of the effect size were not influenced (effect size range, between 0.77 and 0.84) (Fig. 4 ). CK-MB concentrations remained statistically significantly higher (SMD 0.72, 95% CI 0.54 to 0.90, p<0.001; I2 = 90.2%, p<0.001) in patients with high severity or non-survivor status after excluding 5 relatively large studies that accounted for nearly 28% of the overall sample size [36,58,67,78,83].
Fig. 4.
Sensitivity analysis of the association between CK-MB and COVID-19 disease. The influence of individual studies on the overall standardized mean difference (SMD) is shown. The middle vertical axis indicates the overall SMD and the two vertical axes indicate the 95% confidence intervals (CIs). The hollow circles represent the pooled SMD when the remaining study is omitted from the meta-analysis. The two ends of each broken line represent the 95% CIs.
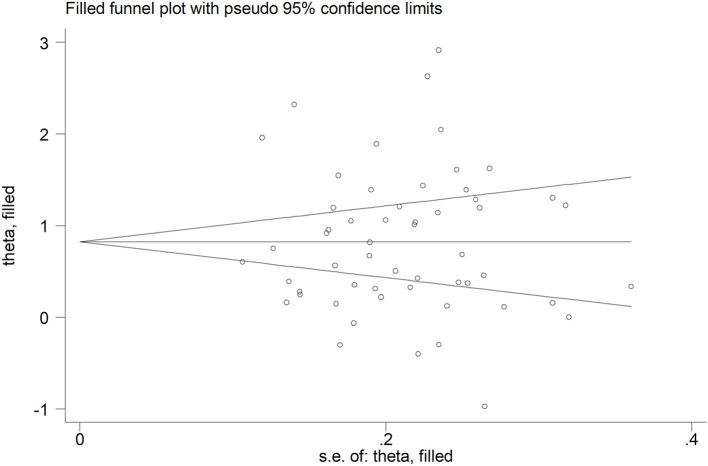
No publication bias was observed with the Begg's (p = 0.50) and Egger's (p = 0.86) t-tests. Accordingly, the trim-and-fill analysis showed that no study was missing or should be added (Fig. 5 ).
Fig. 5.
Funnel plot of studies investigating disease severity of survival status after trimming and filling. Dummy studies and genuine studies are represented by enclosed circles and free circles, respectively.
3.3. Meta-regression analysis
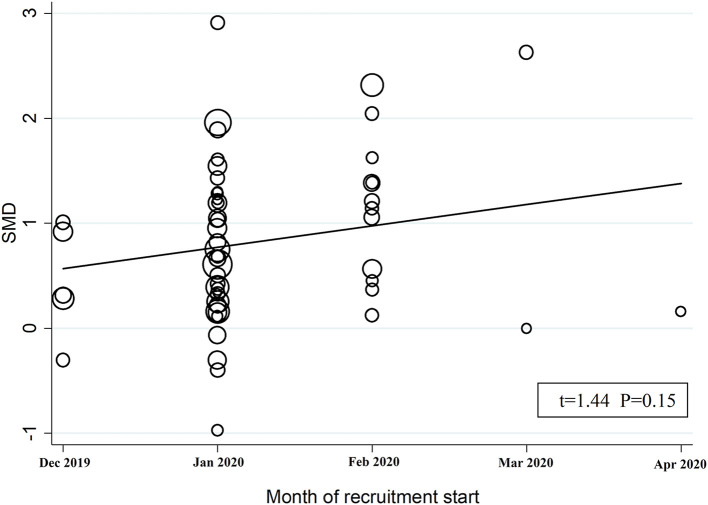
In univariate meta-regression analysis, the SMD was statistically significantly and positively associated with the WBC (t = 3.51, p = 0.001), AST (t = 2.81, p = 0.007), myoglobin (t = 2.91, p = 0.01), troponin (t = 3.83, p = 0.001), BNP (t = 2.67, p = 0.02), LDH (t = 2.40, p = 0.02), and D-dimer (t = 2.47, p = 0.02) (Table 2 ). By contrast, no statistically significant associations were observed between the SMD and age (t = 0.43, p = 0.67), gender (t = −0.54, p = 0.59), month of recruitment commencement (t = 1.44, p = 0.15; Fig. 6 ), CRP (t = 1.17, p = 0.25), ALT (t = −0.73, p = 0.47), creatinine (t = 1.17, p = 0.25), procalcitonin (t = 1.75, p = 0.10), albumin (t = −1.53, p = 0.13), diabetes (t = 0.56, p = 0.58), hypertension (t = −0.80, p = 0.43) and cardiovascular disease (t = 0.59, p = 0.56) (Table 2).
Table 2.
Univariate meta-regression analysis between effect size and possible contributors to heterogeneity.
| Number of studies | t | p-value | Explained heterogeneity | |
|---|---|---|---|---|
| Age | 54 | 0.43 | 0.67 | 0% |
| Gender | 54 | −0.54 | 0.59 | 0% |
| Time of recruitment commencement | 54 | 1.44 | 0.15 | 3% |
| White blood cell count | 44 | 3.51 | 0.001 | 22% |
| C-reactive protein | 46 | 1.17 | 0.25 | 1% |
| Procalcitonin | 23 | 1.75 | 0.10 | 9% |
| Aspartate aminotransferase | 47 | 2.81 | 0.007 | 15% |
| Alanine aminotransferase | 48 | −0.73 | 0.47 | 0% |
| Albumin | 37 | −1.53 | 0.13 | 4% |
| D-dimer | 40 | 2.47 | 0.02 | 13% |
| Troponin | 26 | 3.83 | 0.001 | 39% |
| Myoglobin | 17 | 2.91 | 0.01 | 36% |
| Brain natriuretic peptide | 19 | 2.67 | 0.02 | 29% |
| Creatinine | 43 | 1.17 | 0.25 | 5% |
| Lactate dehydrogenase | 34 | 2.40 | 0.02 | 15% |
| Diabetes | 37 | 0.56 | 0.58 | 0% |
| Cardiovascular disease | 34 | −0.80 | 0.43 | 0% |
| Hypertension | 39 | 0.59 | 0.56 | 0% |
Fig. 6.
Bubble plot reporting univariate meta-regression analysis between the month of recruitment commencement and SMD. Each study is represented by a circle (bubble) with the size area proportional to the study precision (the inverse of its within-study variance).
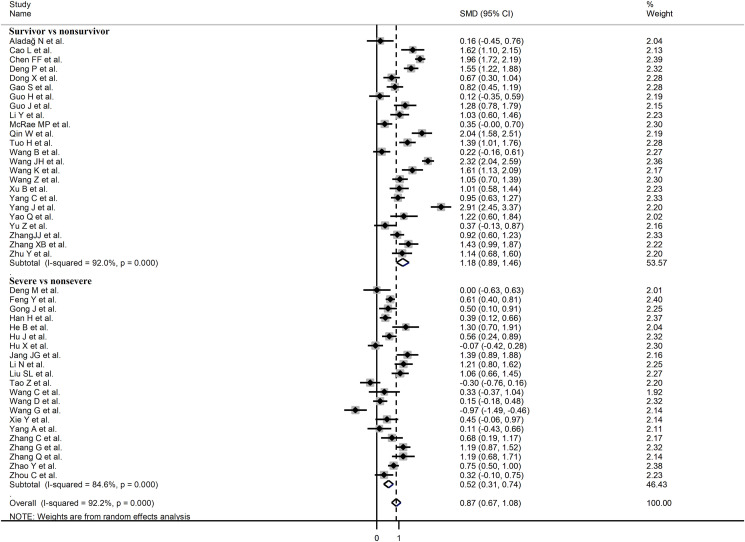
In sub-group analysis, the pooled SMD in studies assessing disease severity (SMD 0.52, 95% CI 0.32 to 0.54, p<0.001; I2 = 84.6, p<0.001) was statistically significantly lower than that observed in studies assessing survival status (SMD 1.18, 95% CI 0.89 to 1.46, p<0.001; I2 = 92.0, p<0.001; t = 3.38, p = 0.002) (Fig. 7 ). However, the between-study variance remained extreme regardless of the type of endpoint studied.
Fig. 7.
Forest plot of studies examining CK-MB concentrations in patients with COVID-19 according to disease severity or survival status. The diamond represents the point estimate and confidence intervals after combining and averaging all individual studies. The vertical line through the vertical points of the diamond represents the point estimate of the averaged studies.
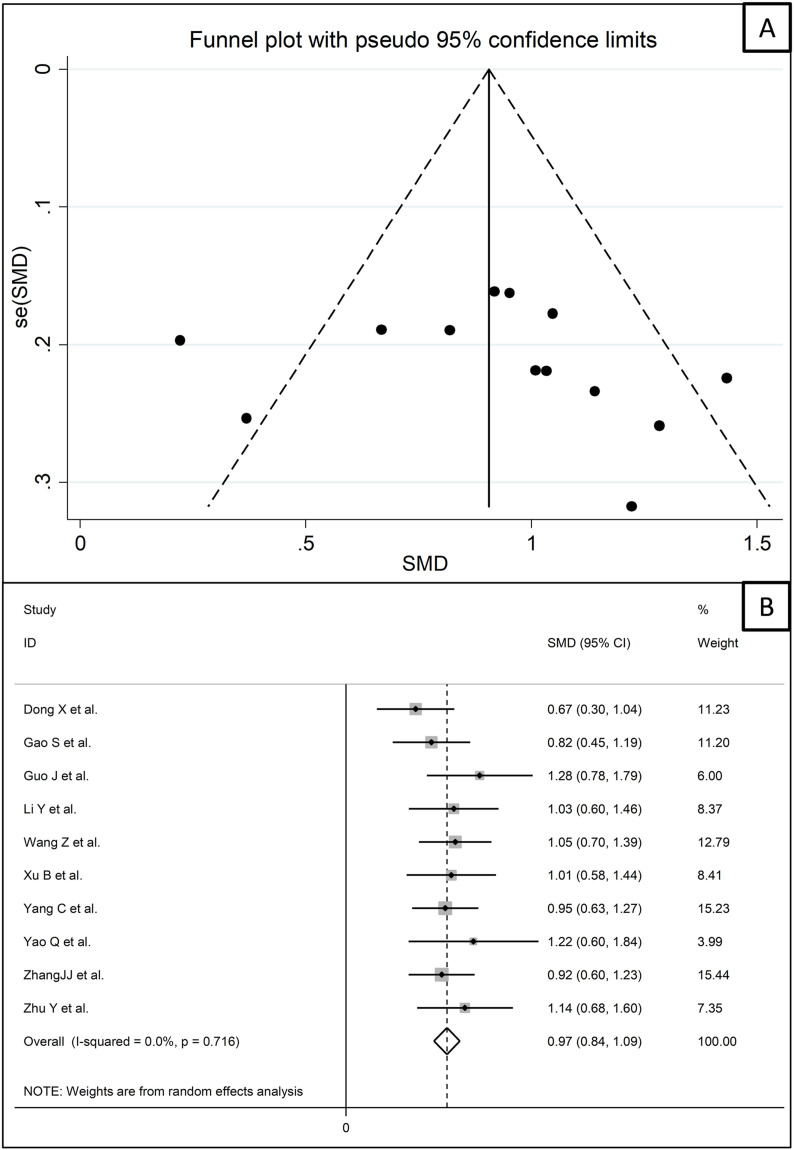
We further sought to identify additional, more homogeneous, study sub-groups according to endpoint reported, study design (retrospective or prospective), country, and assay type (measurement of CK-MB on the basis of activity or mass). In particular, a sub-group of 13 retrospective studies conducted in China [40,44,47,55,62,69,73,[75], [76], [77],81,83,87] assessed survival and measured CK-MB using an assay based on activity evaluation. Funnel plot analysis showed that 3 of these studies were located outside of the plot (Fig. 8 A) [62,77,83]. After removing these studies, the effect size of the remaining 10 studies confirmed that the CK-MB concentrations were statistically significantly higher in patients with high disease severity or non-survivor status during follow up (SMD 0.97, 95% CI 0.84 to 1.09, p<0.001) (Fig. 8B). However, a substantially lower heterogeneity between studies was observed (I2 = 0.0%, p = 0.72).
Fig. 8.
Funnel plot of a sub-group of 13 studies that were homogeneous for endpoint, country, study design and assay (A). Forest plot of CK-MB concentrations after removing the three studies that were outside the funnel plot (B).
4. Discussion
In this updated systematic review and meta-analysis, the serum concentrations of CK-MB, measured within 24–48 h from admission in virtually all studies, were statistically significantly higher in patients with COVID-19 who had a more severe clinical picture, based on available guidelines, disease progress, need for mechanical ventilation, or transfer to ICU, or did not survive, during follow up. The magnitude of the observed SMD value suggests the presence of a clinically relevant between-group difference in CK-MB concentrations [88]. Whilst the study heterogeneity was extreme, the sequential omission of individual studies did not exert tangible effects on the SMD value. Furthermore, there was no evidence of publication bias according to the Begg’s and Egger’s t-tests and the trim and fill analysis. Previous systematic reviews and meta-analyses have reported associations between serum CK-MB concentrations and COVID-19 severity and mortality [[19], [20], [21], [22]]. Our meta-analysis has captured more than double the number of studies, 55 vs. 25, and participants, 11,791 vs. 5,626, than the largest previously published meta-analysis [21]. Furthermore, we investigated possible associations between the observed SMD and a number of biologically and clinically plausible factors. Using meta-regression analysis, there were statistically significant and positive associations between the SMD and the WBC and the concentrations of AST, myoglobin, troponin, BNP, LDH, and D-dimer.
CK-MB, one of the three CK isoenzymes, is present in high concentrations in the myocardium although it can also be detected in the brain and the skeletal muscle [89]. The release of CK-MB in the circulation, and its consequent increase in serum concentrations, has been used for many years to diagnose myocardial necrosis and its clinical manifestations, e.g., myocardial infarction, until more sensitive and specific biomarkers, i.e., high-sensitivity troponin, were introduced in clinical practice [89,90]. The results of our meta-analysis might primarily reflect the presence of significant myocardial damage in COVID-19 patients with more compromised clinical presentations and high-risk of mortality. However, the associations observed in meta-regression indicate that the clinical information provided by CK-MB might complement, rather than duplicate, that of other cardiac biomarkers. While, not surprisingly, the CK-MB SMD was statistically significantly associated with troponin, myoglobin, and BNP, the additional associations observed suggest that the elevations of CK-MB in high-risk COVID-19 patients can also reflect a state of excess inflammation (WBC), single- and multi-organ damage (AST and LDH), and pro-thrombotic tendency (D-dimer). Notably, these biomarkers have, in turn, shown significant associations with COVID-19 severity and adverse outcomes [16,91].
4.1. Limitations of the study
The extreme heterogeneity observed between the studies, together with the exclusion of articles written in non-English language, e.g., Chinese, is a limitation of our meta-analysis. However, the trend and magnitude of the reported differences in CK-MB were maintained, in presence of a significantly reduced heterogeneity, in a sub-group of 10 studies that were homogeneous for endpoint, study design, country, and assay used. In addition, it is important to emphasize that there was no evidence of publication bias and that the overall effect size was not affected in sensitivity analyses. A number of unreported factors might have contributed to the heterogeneity, particularly the coexistence of rhabdomyolysis, reported in COVID-19 patients and a common cause of increased serum CK-MB concentrations [[92], [93], [94]], and the fact that virtually all selected studies reported a single measurement of CK-MB rather than serial assessments.
5. Conclusions
In conclusion, our updated systematic review and meta-analysis with meta-regression has shown that higher serum CK-MB concentrations are significantly associated with worse clinical status and reduced survival in patients with COVID-19. Further research is warranted to establish the clinical utility of this biomarker, in conjunction with other patient characteristics, for early risk stratification and acute management in this group.
Financial disclosure
The authors have no funding to disclose.
The Author contribution
Study design: Angelo Zinellu.
Data collection: Angelo Zinellu.
Statistical analysis: Angelo Zinellu, Salvatore Sotgia.
Data interpretation: Angelo Zinellu, Salvatore Sotgia, Alessandro Fois, Arduino Mangoni.
Manuscript preparation: Angelo Zinellu, Salvatore Sotgia, Alessandro Fois, Arduino Mangoni.
Literature search: Angelo Zinellu, Arduino Mangoni.
Funds collection: n/a.
Declaration of competing interest
The authors declare no conflict of interests.
References
- 1.Huang C., Soleimani J., Herasevich S., Pinevich Y., Pennington K.M., Dong Y., Pickering B.W., Barwise A.K. Clinical characteristics, treatment, and outcomes of critically ill patients with COVID-19: a scoping review. Mayo Clin Proc. 2021;96:183–202. doi: 10.1016/j.mayocp.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pijls B.G., Jolani S., Atherley A., Derckx R.T., Dijkstra J.I.R., Franssen G.H.L., Hendriks S., Richters A., Venemans-Jellema A., Zalpuri S., Zeegers M.P. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383:2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paliogiannis P., Zinellu A., Scano V., Mulas G., De Riu G., Pascale R.M., Arru L.B., Carru C., Pirina P., Mangoni A.A., Fois A.G. Laboratory test alterations in patients with COVID-19 and non COVID-19 interstitial pneumonia: a preliminary report. J Infect Dev Ctries. 2020;14:685–690. doi: 10.3855/jidc.12879. [DOI] [PubMed] [Google Scholar]
- 5.Gao Y.D., Ding M., Dong X., Zhang J.J., Kursat Azkur A., Azkur D., Gan H., Sun Y.L., Fu W., Li W., Liang H.L., Cao Y.Y., Yan Q., Cao C., Gao H.Y., Bruggen M.C., van de Veen W., Sokolowska M., Akdis M., Akdis C.A. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 6.Zinellu A., Paliogiannis P., Carru C., Mangoni A.A. Serum amyloid A concentrations, COVID-19 severity and mortality: an updated systematic review and meta-analysis. Int J Infect Dis. 2021 doi: 10.1016/j.ijid.2021.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur V., Ratho R.K., Kumar P., Bhatia S.K., Bora I., Mohi G.K., Saxena S.K., Devi M., Yadav D., Mehariya S. Multi-organ involvement in COVID-19: beyond pulmonary manifestations. J Clin Med. 2021;10 doi: 10.3390/jcm10030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perisetti A., Gajendran M., Mann R., Elhanafi S., Goyal H. COVID-19 extrapulmonary illness - special gastrointestinal and hepatic considerations. Dis Mon. 2020;66 doi: 10.1016/j.disamonth.2020.101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu W., Sun N.N., Gao H.N., Chen Z.Y., Yang Y., Ju B., Tang L.L. Risk factors analysis of COVID-19 patients with ARDS and prediction based on machine learning. Sci Rep. 2021;11:2933. doi: 10.1038/s41598-021-82492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Guan B., Su T., Liu W., Chen M., Bin Waleed K., Guan X., Gary T., Zhu Z. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106:1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shchendrygina A., Nagel E., Puntmann V.O., Valbuena-Lopez S. COVID-19 myocarditis and prospective heart failure burden. Expert Rev Cardiovasc Ther. 2021;19:5–14. doi: 10.1080/14779072.2021.1844005. [DOI] [PubMed] [Google Scholar]
- 13.Linschoten M., Peters S., van Smeden M., Jewbali L.S., Schaap J., Siebelink H.M., Smits P.C., Tieleman R.G., van der Harst P., van Gilst W.H., Asselbergs F.W., consortium C-Cc. Cardiac complications in patients hospitalised with COVID-19. Eur Heart J Acute Cardiovasc Care. 2020;9:817–823. doi: 10.1177/2048872620974605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J., Liao X., Yin W., Wang B., Yue J., Bai L., Liu D., Zhu T., Huang Z., Kang Y. Elevated cardiac biomarkers may be effective prognostic predictors for patients with COVID-19: a multicenter, observational study. Am J Emerg Med. 2021;39:34–41. doi: 10.1016/j.ajem.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippi G., Lavie C.J., Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H., Wooster L., Rotter J.I., Guo X., Malhotra R. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toraih E.A., Elshazli R.M., Hussein M.H., Elgaml A., Amin M., El-Mowafy M., El-Mesery M., Ellythy A., Duchesne J., Killackey M.T., Ferdinand K.C., Kandil E., Fawzy M.S. Association of cardiac biomarkers and comorbidities with increased mortality, severity, and cardiac injury in COVID-19 patients: a meta-regression and decision tree analysis. J Med Virol. 2020;92:2473–2488. doi: 10.1002/jmv.26166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X., Pan X., Li Y., An N., Xing Y., Yang F., Tian L., Sun J., Gao Y., Shang H., Xing Y. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: a meta-analysis and systematic review. Crit Care. 2020;24:468. doi: 10.1186/s13054-020-03183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal A., Kumar A., Patel D., Puri R., Kalra A., Kapadia S.R., Reed G.W. Meta-analysis comparing outcomes in patients with and without cardiac injury and coronavirus disease 2019 (COVID 19) Am J Cardiol. 2021;141:140–146. doi: 10.1016/j.amjcard.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker C., Deb S., Ling H., Wang Z. Assessing the elevation of cardiac biomarkers and the severity of COVID-19 infection: a meta-analysis. J Pharm Pharmaceut Sci. 2020;23:396–405. doi: 10.18433/jpps31501. [DOI] [PubMed] [Google Scholar]
- 22.Parohan M., Yaghoubi S., Seraji A. Cardiac injury is associated with severe outcome and death in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J Acute Cardiovasc Care. 2020;9:665–677. doi: 10.1177/2048872620937165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G.A., Shea B., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. 2020. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses the ottawa hospital research Institute2013.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp 20/11/2020. [Google Scholar]
- 24.Shamsrizi P., Gladstone B.P., Carrara E., Luise D., Cona A., Bovo C., Tacconelli E. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria-producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowden J., Tierney J.F., Copas A.J., Burdett S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. 2011;11:41. doi: 10.1186/1471-2288-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin. 1999;47:15–17. https://econpapers.repec.org/article/tsjstbull/y_3a1999_3av_3a8_3ai_3a47_3asbe26.htm [Google Scholar]
- 30.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 31.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 32.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aladağ N., Atabey R.D. The role of concomitant cardiovascular diseases and cardiac biomarkers for predicting mortality in critical COVID-19 patients. Acta Cardiol. 2021 Apr;76(2):132–139. doi: 10.1080/00015385.2020.1810914. [DOI] [PubMed] [Google Scholar]
- 35.Cao L., Zhang S., Luo X., Wang E., Bai Y., Li Z., Li F., Ma J., Liu H. Myocardium injury biomarkers predict prognosis of critically ill coronavirus disease 2019 (COVID-19) patients. Ann Palliat Med. 2020;9:4156–4165. doi: 10.21037/apm-20-2112. [DOI] [PubMed] [Google Scholar]
- 36.Chen F.F., Zhong M., Liu Y., Zhang Y., Zhang K., Su D.Z., Meng X., Zhang Y. The characteristics and outcomes of 681 severe cases with COVID-19 in China. J Crit Care. 2020;60:32–37. doi: 10.1016/j.jcrc.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Zhu Y.F., Du Z.Q., Li W.F., Zhang M.J., Zhao S.D., Ying J.W., Li Z., Miao H.J. Predictors of mechanical ventilation for COVID-19: combined data from three designated hospitals. Eur Rev Med Pharmacol Sci. 2020;24:13065–13071. doi: 10.26355/eurrev_202012_24214. [DOI] [PubMed] [Google Scholar]
- 38.Deng M., Qi Y., Deng L., Wang H., Xu Y., Li Z., Meng Z., Tang J., Dai Z. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity. 2020;28:1815–1825. doi: 10.1002/oby.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng P., Ke Z., Ying B., Qiao B., Yuan L. The diagnostic and prognostic role of myocardial injury biomarkers in hospitalized patients with COVID-19. Clin Chim Acta. 2020;510:186–190. doi: 10.1016/j.cca.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong X., Sun L., Li Y. Prognostic value of lactate dehydrogenase for in-hospital mortality in severe and critically ill patients with COVID-19. Int J Med Sci. 2020;17:2225–2231. doi: 10.7150/ijms.47604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du R.H., Liu L.M., Yin W., Wang W., Guan L.L., Yuan M.L., Li Y.L., Hu Y., Li X.Y., Sun B., Peng P., Shi H.Z. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in wuhan, China. Ann Am Thorac Soc. 2020;17:839–846. doi: 10.1513/AnnalsATS.202003-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng X., Li P., Ma L., Liang H., Lei J., Li W., Wang K., Song Y., Li S., Yang W., Yang C. Clinical characteristics and short-term outcomes of severe patients with COVID-19 in wuhan, China. Front Med. 2020;7:491. doi: 10.3389/fmed.2020.00491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., Xiong W., Yang D., Chen R., Lu F., Lu Y., Liu X., Chen Y., Li X., Li Y., Summah H.D., Lin H., Yan J., Zhou M., Lu H., Qu J. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao S., Jiang F., Jin W., Shi Y., Yang L., Xia Y., Jia L., Wang B., Lin H., Cai Y., Xia Z., Peng J. Risk factors influencing the prognosis of elderly patients infected with COVID-19: a clinical retrospective study in Wuhan, China. Aging (Albany NY) 2020;12:12504–12516. doi: 10.18632/aging.103631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong J., Ou J., Qiu X., Jie Y., Chen Y., Yuan L., Cao J., Tan M., Xu W., Zheng F., Shi Y., Hu B. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in wuhan and guangdong, China. Clin Infect Dis. 2020;71:833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo H., Shen Y., Wu N., Sun X. Myocardial injury in severe and critical coronavirus disease 2019 patients. J Card Surg. 2021;36:82–88. doi: 10.1111/jocs.15164. [DOI] [PubMed] [Google Scholar]
- 47.Guo J., Zhou B., Zhu M., Yuan Y., Wang Q., Zhou H., Wang X., Lv T., Li S., Liu P., Yang Y., He P., Zhang P. CURB-65 may serve as a useful prognostic marker in COVID-19 patients within Wuhan, China: a retrospective cohort study. Epidemiol Infect. 2020;148:e241. doi: 10.1017/S0950268820002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han H., Xie L., Liu R., Yang J., Liu F., Wu K., Chen L., Hou W., Feng Y., Zhu C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He B., Wang J., Wang Y., Zhao J., Huang J., Tian Y., Yang C., Zhang H., Zhang M., Gu L., Zhou X., Zhou J. The metabolic changes and immune profiles in patients with COVID-19. Front Immunol. 2020;11:2075. doi: 10.3389/fimmu.2020.02075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J., Zhou J., Dong F., Tan J., Wang S., Li Z., Zhang X., Zhang H., Ming J., Huang T. Combination of serum lactate dehydrogenase and sex is predictive of severe disease in patients with COVID-19. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000022774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X., Hu C., Yang Y., Chen J., Zhong P., Wen Y., Chen X. Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China. Ther Adv Respir Dis. 2020 Jan-Dec;14 doi: 10.1177/1753466620963035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang J.G., Hur J., Choi E.Y., Hong K.S., Lee W., Ahn J.H. Prognostic factors for severe coronavirus disease 2019 in daegu, Korea. J Kor Med Sci. 2020;35 doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji L., Cao C., Gao Y., Zhang W., Xie Y., Duan Y., Kong S., You M., Ma R., Jiang L., Liu J., Sun Z., Zhang Z., Wang J., Yang Y., Lv Q., Zhang L., Li Y., Zhang J., Xie M. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit Care. 2020;24:700. doi: 10.1186/s13054-020-03416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li N., Kong H., Zheng X.Z., Li X.Y., Ma J., Zhang H., Wang D.X., Li H.C., Liu X.M. Early predictive factors of progression from severe type to critical ill type in patients with Coronavirus Disease 2019: a retrospective cohort study. PloS One. 2020;15 doi: 10.1371/journal.pone.0243195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Y., Yang S., Peng D., Zhu H.M., Li B.Y., Yang X., Sun X.L., Zhang M. Predictive value of serum cystatin C for risk of mortality in severe and critically ill patients with COVID-19. World J Clin Cases. 2020;8:4726–4734. doi: 10.12998/wjcc.v8.i20.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu S.L., Wang S.Y., Sun Y.F., Jia Q.Y., Yang C.L., Cai P.J., Li J.Y., Wang L., Chen Y. Expressions of SAA, CRP, and FERR in different severities of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:11386–11394. doi: 10.26355/eurrev_202011_23631. [DOI] [PubMed] [Google Scholar]
- 57.McRae M.P., Simmons G.W., Christodoulides N.J., Lu Z., Kang S.K., Fenyo D., Alcorn T., Dapkins I.P., Sharif I., Vurmaz D., Modak S.S., Srinivasan K., Warhadpande S., Shrivastav R., McDevitt J.T. Clinical decision support tool and rapid point-of-care platform for determining disease severity in patients with COVID-19. Lab Chip. 2020;20:2075–2085. doi: 10.1039/d0lc00373e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mertoglu C., Huyut M.T., Arslan Y., Ceylan Y., Coban T.A. How do routine laboratory tests change in coronavirus disease 2019? Scand J Clin Lab Invest. 2021;81:24–33. doi: 10.1080/00365513.2020.1855470. [DOI] [PubMed] [Google Scholar]
- 59.Qin W.D., Bai W., Liu K., Liu Y., Meng X., Zhang K., Zhang M. Clinical course and risk factors of disease deterioration in critically ill patients with COVID-19. Hum Gene Ther. 2021 doi: 10.1089/hum.2020.255. [DOI] [PubMed] [Google Scholar]
- 60.Tao Z., Xu J., Chen W., Yang Z., Xu X., Liu L., Chen R., Xie J., Liu M., Wu J., Wang H., Liu J. Anemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol. 2020 doi: 10.1002/jmv.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tuo H., Li W., Tang L., He B., Yao B., Mao P., Tang Q. Cardiac biomarker abnormalities are closely related to prognosis in patients with COVID-19. Int Heart J. 2021;62:148–152. doi: 10.1536/ihj.20-180. [DOI] [PubMed] [Google Scholar]
- 62.Wang B., Zhong F., Zhang H., An W., Liao M., Cao Y. Risk factor Analysis and nomogram construction for non-survivors among critical patients with COVID-19. Jpn J Infect Dis. 2020;73:452–458. doi: 10.7883/yoken.JJID.2020.227. [DOI] [PubMed] [Google Scholar]
- 63.Wang C., Deng R., Gou L., Fu Z., Zhang X., Shao F., Wang G., Fu W., Xiao J., Ding X., Li T., Xiao X., Li C. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. 2020;8:593. doi: 10.21037/atm-20-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang D., Li R., Wang J., Jiang Q., Gao C., Yang J., Ge L., Hu Q. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. BMC Infect Dis. 2020;20:519. doi: 10.1186/s12879-020-05242-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang F., Qu M., Zhou X., Zhao K., Lai C., Tang Q., Xian W., Chen R., Li X., Li Z., He Q., Liu L. The timeline and risk factors of clinical progression of COVID-19 in Shenzhen, China. J Transl Med. 2020;18:270. doi: 10.1186/s12967-020-02423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G., Wu C., Zhang Q., Wu F., Yu B., Lv J., Li Y., Li T., Zhang S., Wu C., Wu G., Zhong Y. C-reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis. 2020;7:153. doi: 10.1093/ofid/ofaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J.H., Chen R.D., Yang H.K., Zeng L.C., Chen H., Hou Y.Y., Hu W., Yu J.S., Li H. Inflammation-associated factors for predicting in-hospital mortality in patients with COVID-19. J Med Virol. 2021 doi: 10.1002/jmv.26771. [DOI] [PubMed] [Google Scholar]
- 68.Wang K., Zuo P., Liu Y., Zhang M., Zhao X., Xie S., Zhang H., Chen X., Liu C. Clinical and laboratory predictors of in-hospital mortality in patients with coronavirus disease-2019: a cohort study in wuhan, China. Clin Infect Dis. 2020;71:2079–2088. doi: 10.1093/cid/ciaa538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z., Wang Z. Identification of risk factors for in-hospital death of COVID - 19 pneumonia -- lessions from the early outbreak. BMC Infect Dis. 2021;21:113. doi: 10.1186/s12879-021-05814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, China. JAMA Intern Med. 2020 Jul 1;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. Erratum in: JAMA Intern Med. 2020 Jul 1;180(7):1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie J., Shi D., Bao M., Hu X., Wu W., Sheng J., Xu K., Wang Q., Wu J., Wang K., Fang D., Li Y., Li L. A predictive nomogram for predicting improved clinical outcome probability in patients with COVID-19 in Zhejiang province, China. Engineering (Beijing) 2020 Jun 6 doi: 10.1016/j.eng.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie Y., You Q., Wu C., Cao S., Qu G., Yan X., Han X., Wang C., Zhang H. Impact of cardiovascular disease on clinical characteristics and outcomes of coronavirus disease 2019 (COVID-19) Circ J. 2020;84:1277–1283. doi: 10.1253/circj.CJ-20-0348. [DOI] [PubMed] [Google Scholar]
- 73.Xu B., Fan C.Y., Wang A.L., Zou Y.L., Yu Y.H., He C., Xia W.G., Zhang J.X., Miao Q. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81:e51–e60. doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang A., Qiu Q., Kong X., Sun Y., Chen T., Zuo Y., Yuan D., Dai W., Zhou J., Peng A. Clinical and epidemiological characteristics of COVID-19 patients in chongqing China. Front Public Health. 2020;8:244. doi: 10.3389/fpubh.2020.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang C., Liu F., Liu W., Cao G., Liu J., Huang S., Zhu M., Tu C., Wang J., Xiong B. Myocardial injury and risk factors for mortality in patients with COVID-19 pneumonia. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao Q., Wang P., Wang X., Qie G., Meng M., Tong X., Bai X., Ding M., Liu W., Liu K., Chu Y. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130:390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 77.Yu Z., Ke Y., Xie J., Yu H., Zhu W., He L., Zheng Q., Li C., Lu J., Li S., Wen S., Wei S., Liu N., Wei L., Bai R. Clinical characteristics on admission predict in-hospital fatal outcome in patients aged >/=75 years with novel coronavirus disease (COVID-19): a retrospective cohort study. BMC Geriatr. 2020;20:514. doi: 10.1186/s12877-020-01921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeng Z., Ma Y., Zeng H., Huang P., Liu W., Jiang M., Xiang X., Deng D., Liao X., Chen P., Chen Y. Simple nomogram based on initial laboratory data for predicting the probability of ICU transfer of COVID-19 patients: multicenter retrospective study. J Med Virol. 2020 doi: 10.1002/jmv.26244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang C., Qin L., Li K., Wang Q., Zhao Y., Xu B., Liang L., Dai Y., Feng Y., Sun J., Li X., Hu Z., Xiang H., Dong T., Jin R., Zhang Y. A novel scoring system for prediction of disease severity in COVID-19. Front Cell Infect Microbiol. 2020;10:318. doi: 10.3389/fcimb.2020.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J., Peng Z., Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J.J., Cao Y.Y., Tan G., Dong X., Wang B.C., Lin J., Yan Y.Q., Liu G.H., Akdis M., Akdis C.A., Gao Y.D. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. 2020 doi: 10.1111/all.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Q., Wei Y., Chen M., Wan Q., Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabet Complicat. 2020;34 doi: 10.1016/j.jdiacomp.2020.107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang X.B., Hu L., Ming Q., Wei X.J., Zhang Z.Y., Chen L.D., Wang M.H., Yao W.Z., Huang Q.F., Ye Z.Q., Cai Y.Q., Zeng H.Q. Risk factors for mortality of coronavirus disease-2019 (COVID-19) patients in two centers of Hubei province, China: a retrospective analysis. PloS One. 2021;16 doi: 10.1371/journal.pone.0246030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao Y., Wang F., Dong G., Sheng Q., Feng S. A disease progression prediction model and nervous system symptoms in coronavirus disease 2019 patients. Am J Transl Res. 2020;12:8192–8207. [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou C., Huang Z., Tan W., Li X., Yin W., Xiao Y., Tao Z., Geng S., Hu Y. Predictive factors of severe coronavirus disease 2019 in previously healthy young adults: a single-center, retrospective study. Respir Res. 2020;21:157. doi: 10.1186/s12931-020-01412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou J., Sun J., Cao Z., Wang W., Huang K., Zheng F., Xie Y., Jiang D., Zhou Z. Epidemiological and clinical features of 201 COVID-19 patients in Changsha city, Hunan, China. Medicine (Baltim) 2020;99 doi: 10.1097/MD.0000000000021824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y., Du Z., Zhu Y., Li W., Miao H., Li Z. Evaluation of organ function in patients with severe COVID-19 infections. Med Clin. 2020;155:191–196. doi: 10.1016/j.medcli.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen J. second ed. Erlbaum; Hillsdale, NJ, USA: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 89.Saenger A.K., Jaffe A.S. The use of biomarkers for the evaluation and treatment of patients with acute coronary syndromes. Med Clin North Am. 2007;91:657–681. doi: 10.1016/j.mcna.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Wu A.H., Wang X.M., Gornet T.G., Ordonez-Llanos J. Creatine kinase MB isoforms in patients with skeletal muscle injury: ramifications for early detection of acute myocardial infarction. Clin Chem. 1992;38:2396–2400. [PubMed] [Google Scholar]
- 91.Paliogiannis P., Mangoni A.A., Dettori P., Nasrallah G.K., Pintus G., Zinellu A. D-dimer concentrations and COVID-19 severity: a systematic review and meta-analysis. Front Public Health. 2020;8:432. doi: 10.3389/fpubh.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khosla S.G., Nylen E.S., Khosla R. Rhabdomyolysis in patients hospitalized with COVID-19 infection: five case series. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620984603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geng Y., Ma Q., Du Y.S., Peng N., Yang T., Zhang S.Y., Wu F.F., Lin H.L., Su L. Rhabdomyolysis is associated with in-hospital mortality in patients with COVID-19. Shock. 2021 doi: 10.1097/SHK.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Torres P.A., Helmstetter J.A., Kaye A.M., Kaye A.D. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15:58–69. [PMC free article] [PubMed] [Google Scholar]