Abstract
Objective
A major coronavirus disease 2019 (COVID-19) outbreak occurred in Northeastern France in spring 2020. This single-center retrospective observational cohort study aimed to compare patients with severe COVID-19 and those with non-severe COVID-19 (survivors vs. non-survivors, ICU patients vs. non-ICU patients) and to describe extrapulmonary complications.
Patients and methods
We included all patients with a confirmed diagnosis of COVID-19 admitted to Colmar Hospital in March 2020.
Results
We examined 600 patients (median age 71.09 years; median body mass index: 26.9 kg/m2); 57.7% were males, 86.3% had at least one comorbidity, 153 (25.5%) required ICU hospitalization, and 115 (19.1%) died. Baseline independent factors associated with death were older age (> 75 vs. ≤ 75 years), male sex, oxygen supply, chronic neurological, renal, and pulmonary diseases, diabetes, cancer, low platelet and hemoglobin counts, and high levels of C-reactive protein (CRP) and serum creatinine. Factors associated with ICU hospitalization were age < 75 years, oxygen supply, chronic pulmonary disease, absence of dementia, and high levels of CRP, hemoglobin, and serum creatinine. Among the 600 patients, 80 (13.3%) had an acute renal injury, 33 (5.5%) had a cardiovascular event, 27 (4.5%) had an acute liver injury, 24 (4%) had venous thromboembolism, eight (1.3%) had a neurological event, five (0.8%) had rhabdomyolysis, and one had acute pancreatitis. Most extrapulmonary complications occurred in ICU patients.
Conclusion
This study highlighted the main risk factors for ICU hospitalization and death caused by severe COVID-19 and the frequency of numerous extrapulmonary complications in France.
Keywords: COVID-19, SARS-CoV-2, Outcome, Extrapulmonary COVID-19, Mortality
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in China in December 2019, causing coronavirus disease 2019 (COVID-19), which is a polymorph disease that mainly affects the respiratory tract [1]. However, COVID-19 is associated with extrapulmonary complications [2], including neurological disorders (encephalopathy, Guillain-Barré syndrome, stroke, encephalitis) [3]; thrombotic disorders (mainly deep vein thrombosis and pulmonary embolism), acute coronary syndrome, myocarditis and arrhythmia [4], [5], [6]; acute kidney injury (AKI) and acute liver injury (ALI) [7], and rhabdomyolysis [8]. These events, especially pulmonary events with acute respiratory distress syndrome (ARDS), are detrimental as they lead to hospitalization in the intensive care unit (ICU) or even to death. Classic risk factors for severe outcomes such as older age or male sex are now well-established but risk factors for extrapulmonary complications need better evaluation.
France identified its first three COVID-19 cases in January 2020 [9], and a major outbreak occurred in March 2020, beginning in Northeastern France. Colmar public hospital (Hôpitaux Civils de Colmar [HCC]), a 1,000-bed facility, was one of the most affected by this first wave. We aimed to conduct a single-center retrospective cohort study of all patients with a confirmed diagnosis of COVID-19 in the HCC in March 2020 to assess the risk of death, ICU hospitalization, and extrapulmonary events in COVID-19 patients. We focused on risk factors at baseline associated with these severe outcomes.
2. Patients and methods
2.1. Study design
We retrospectively analyzed data of all consecutive patients with COVID-19 in the HCC between March 1 and March 31, 2020. COVID-19 was confirmed by a positive result by real-time reverse transcriptase polymerase chain reaction (PCR) for SARS-CoV-2. This study aimed to describe the characteristics of patients with severe COVID-19 (ICU hospitalization or death) and to assess those with and without extrapulmonary complications. The study was approved by the Ethics Committee of Medicine Odontology and Pharmacy Faculties and Hospitals (University Hospital of Strasbourg; No. CE-2020-32).
2.2. Laboratory test, demographics, and medical history
Nasopharyngeal swabs, sputum, tracheal aspiration, and bronchoalveolar lavage specimen were collected in the emergency department or in other departments, including the ICU. SARS-CoV-2 positivity was assessed by specific in-house real-time PCR in which the primer and probe sequences targeted two regions of the RNA-dependent RNA-polymerase gene and were specific to SARS-CoV-2. Approximately 10 copies/reaction were produced to analyze assay sensitivity (Institut Pasteur, Paris, France).
2.3. Data collection/endpoints
We retrospectively collected all data from computer-based patient records (Crystal Link®): sex, age, body mass index (BMI), medical history, symptoms at admission, and routine blood examinations (complete blood count, serum creatinine, transaminases, gamma-glutamyl transferase, alkaline phosphatase, total bilirubin, creatine phosphokinase [CPK], lactate dehydrogenase, electrolytes, calcium, phosphorus, C-reactive protein [CRP], and procalcitonin). The type of hospitalization (ICU or non-ICU, with dates and need for invasive mechanical ventilation) and the patient's end of follow-up status (death, transfer to a rehabilitation unit, discharge status, with the date of each of these events) at the cutoff date (July 31, 2020) were identified together with extrapulmonary complications during hospitalization. These extrapulmonary complications included thromboembolic events (phlebitis and pulmonary embolism), AKI (according to KDIGO definition [10]), ALI (ASAT ≥ 3 times the upper limit of the normal range [UNR]), rhabdomyolysis (CPK ≥ 3 UNR without recent fall or trauma history), neurological events (stroke, Guillain-Barré syndrome, meningitis, and encephalitis), and cardiac events (arrhythmia, myocarditis, pericarditis, acute ischemia, and Takotsubo syndrome). All extrapulmonary events were validated by a senior physician.
2.4. Statistical analysis
Overall baseline characteristics are presented and classified according to the patient's survival status (survivors vs. non-survivors) at the cutoff date (July 31, 2020) and according to the type of hospitalization (ICU vs. non-ICU). Continuous variables were summarized by median and first and third quartiles (Q1, Q3) and compared by Wilcoxon rank sum test. Categorical data are presented by the number of missing values and absolute and relative counts, and compared by Fisher's exact test. Furthermore, the death rate curve with 95% confidence interval (CI) and the number of patients at risk was determined using the nonparametric Kaplan–Meier method. Baseline independent factors likely to explain severe COVID-19 (death and ICU hospitalization) or non-pulmonary events (AKI, ALI, cardiac and thrombotic events) were selected using backward logistic regression, with a minimum significance level of 0.20. Selected variables were considered independent when Spearman's rank correlation coefficient was < 0.30. We also presented the odds ratios (OR) with two-sided 95% CI of the final models, excluding correlated variables. Statistical data were analyzed using the SAS 9.4 software (SAS Institute Cary, NC, USA).
3. Results
3.1. Demographic and clinical characteristics at baseline
Patients’ main characteristics and laboratory results at baseline are presented in Table 1, Table 2 . Patients were mostly males (57.7%), with a median age of 71 years [Q1 60.9, Q3 81.3 yrs.] and a median BMI of 26.9 kg/m2 [Q1 23.8, Q3 31.0 kg/m2]. Most patients (86.3%) had at least one comorbidity. The median duration between symptom onset and hospitalization was 7 days.
Table 1.
Patients’ baseline characteristics and laboratory findings by survival status (survivors vs. non-survivors).
| Survivors (n = 485) |
Non-survivors (n = 115) |
All patients (n = 600) |
P-value | ||
|---|---|---|---|---|---|
| Sex | Male | 272 (56.1%) | 74 (64.3%) | 346 (57.7%) | NS b |
| Female | 213 (43.9%) | 41 (35.7%) | 254 (42.3%) | ||
| Age (years) | Median [Q1, Q3] | 69.00 [58.8, 78.5] | 79.93 [70.5, 87.6] | 71.09 [60.9, 81.3] | < 0.001 a |
| BMI (kg/m2) | n (%) | 298 (61.44%) | 62 (53.91%) | 360 (60.00%) | NS a |
| Median [Q1, Q3] | 26.84 [24.0, 30.9] | 27.46 [22.9, 31.3] | 26.90 [23.8, 31.0] | ||
| Risk factors | At least one risk factor | 409 (84.3%) | 109 (94.8%) | 518 (86.3%) | 0.002 b |
| Chronic renal disease | 28 (5.8%) | 20 (17.4%) | 48 (8.0%) | < 0.001 b | |
| Chronic liver disease | 5 (1.0%) | 1 (0.9%) | 6 (1.0%) | NS b | |
| Chronic cardiovascular disease | 308 (63.5%) | 91 (79.1%) | 399 (66.5%) | 0.001 b | |
| Chronic pulmonary disease | 91 (18.8%) | 34 (29.6%) | 125 (20.8%) | 0.015 b | |
| Diabetes | 114 (23.5%) | 42 (36.5%) | 156 (26.0%) | 0.006 b | |
| Cancer | 77 (15.9%) | 32 (27.8%) | 109 (18.2%) | 0.004 b | |
| Immunodeficiency | 8 (1.6%) | 1 (0.9%) | 9 (1.5%) | NS b | |
| Pregnancy | 2 (0.4%) | - | 2 (0.3%) | NS b | |
| Symptom duration (days) | n (%) | 447 (92.16%) | 101 (87.83%) | 548 (91.33%) | < 0.001 a |
| Median [Q1, Q3] | 7.0 [10, 3] | 3.0 [7, 1] | 7.0 [9, 3] | ||
| Body temperature (°C) | n (%) | 269 (94.39%) | 64 (96.97%) | 333 (94.87%) | NSa |
| Median [Q1, Q3] | 38.00 [37.1;38.6] | 38.00 [37.1;38.6] | 38.00 [37.1;38.6] | ||
| Ventilation | n (%) | 456 (94.0%) | 115 (96.0%) | 567 (94.5%) | < 0.001 b |
| Spontaneous ambient air | 302 (66.2%) | 46 (41.4%) | 348 (61.4%) | ||
| O2 | 154 (33.8%) | 65 (58.6%) | 219 (38.6%) | ||
| O2 flow (L/min) | n (%) | 154 (100.00%) | 65 (100.00%) | 219 (100.00%) | 0.002 a |
| Median [Q1, Q3] | 4.0 [2, 9] | 8.0 [3, 15] | 5.0 [3, 9] | ||
| Hemoglobin (g/L) | n (%) | 476 (100.00%) | 110 (97.35%) | 586 (99.49%) | 0.014 a |
| Median [Q1, Q3] | 13.50 [12.2, 14.6] | 12.80 [11.4, 14.4] | 13.40 [12.0, 14.5] | ||
| Platelets (G/L) | n (%) | 473 (99.37%) | 110 (97.35%) | 583 (98.98%) | 0.001 a |
| Median [Q1, Q3] | 198.0 [159, 257] | 177.0 [140, 231] | 195.0 [155, 253] | ||
| Leucocytes (G/L) | n (%) | 476 (100.00%) | 110 (97.35%) | 586 (99.49%) | < 0. 001 a |
| Median [Q1, Q3] | 6.180 [4.63, 8.38] | 7.615 [5.53, 9.63] | 6.405 [4.76, 8.60] | ||
| Neutrophils (G/L) | n (%) | 472 (99.16%) | 107 (94.69%) | 579 (98.30%) | < 0. 001 a |
| Median [Q1, Q3] | 4.640 [3.25, 6.78] | 6.370 [4.23, 8.30] | 4.970 [3.32, 7.16] | ||
| Lymphocytes (G/L) | n (%) | 470 (98.74%) | 105 (92.92%) | 575 (97.62%) | < 0. 001 a |
| Median [Q1, Q3] | 0.790 [0.55, 1.09] | 0.600 [0.43, 0.85] | 0.750 [0.51, 1.06] | ||
| Creatinine (μmol/L) | n (%) | 465 (97.69%) | 113 (100.00%) | 578 (98.13%) | < 0. 001 a |
| Median [Q1, Q3] | 80.0 [65, 101] | 117.0 [83, 174] | 84.0 [66, 115] | ||
| ASAT (IU/L) | n (%) | 318 (66.81%) | 72 (63.72%) | 390 (66.21%) | NS a |
| Median [Q1, Q3] | 41.0 [28, 60] | 45.0 [30, 61] | 42.0 [28, 60] | ||
| ALAT (IU/L) | n (%) | 370 (77.73%) | 85 (75.22%) | 455 (77.25%) | 0.008 a |
| Median [Q1, Q3] | 31.0 [19, 50] | 25.0 [17, 39] | 30.0 [19, 48] | ||
| Bilirubin (μmol/L) | n (%) | 367 (77.10%) | 87 (76.99%) | 454 (77.08%) | NS a |
| Median [Q1, Q3] | 8.20 [5.9, 11.7] | 9.00 [5.9, 13.5] | 8.30 [5.9, 11.7] | ||
| CPK (IU/L) | n (%) | 49 (10.29%) | 13 (11.50%) | 62 (10.53%) | NS a |
| Median [Q1, Q3] | 129.0 [73, 330] | 373.0 [118, 635] | 147.0 [79, 423] | ||
| LDH (IU/L) | n (%) | 227 (47.69%) | 55 (48.67%) | 282 (47.88%) | NS a |
| Median [Q1, Q3] | 324.0 [236, 449] | 339.0 [243, 444] | 325.5 [239, 449] | ||
| Calcium (mmol/L) | n (%) | 68 (14.29%) | 11 (9.73%) | 79 (13.41%) | NS a |
| Median [Q1, Q3] | 2.105 [2.00, 2.18] | 2.090 [1.96, 2.16] | 2.100 [2.00, 2.18] | ||
| Phosphate (mmol/L) | n (%) | 43 (9.03%) | 4 (3.54%) | 47 (7.98%) | NS a |
| Median [Q1, Q3] | 0.9 [1, 1] | 1.1 [1, 1] | 0.9 [1, 1] | ||
| CRP (mg/L) | n (%) | 469 (98.53%) | 113 (100.00%) | 582 (98.81%) | < 0.001 a |
| Median [Q1, Q3] | 73.0 [39, 123] | 96.0 [53, 189] | 75.0 [42, 135] | ||
| PCT (μg/L) | n (%) | 167 (35.08%) | 43 (38.05%) | 210 (35.65%) | 0.039 a |
| Median [Q1, Q3] | 0.200 [0.10, 0.39] | 0.300 [0.20, 0.63] | 0.230 [0.11, 0.41] |
Results are presented as N (%), median [Q1, Q3], % are calculated for non-missing values only. BMI: body mass index; ASAT: aspartate aminotransferase; ALAT: alanine aminotransferase; CPK: creatinine phosphokinase; LDH: lactate dehydrogenase; CRP: C-reactive protein; PCT: procalcitonin.
Wilcoxon rank sum test.
Fisher's exact test.
Table 2.
Patients’ baseline characteristics and laboratory findings according to hospitalization type (ICU vs. non-ICU).
| Non-ICU (n = 447) |
ICU (n = 153) |
All patients (n = 600) |
P-value | ||
|---|---|---|---|---|---|
| Sex | Male | 237 (53.0%) | 109 (71.2%) | 346 (57.7%) | <0.001 b |
| Female | 210 (47.0%) | 44 (28.8%) | 254 (42.3%) | ||
| Age (years) | Median [Q1, Q3] | 73.13 [60.2;83.9] | 68.70 [62.2;73.2] | 71.09 [60.9;81.3] | <0.001 a |
| BMI (kg/m2) | n (%) | 278 (62.19%) | 82 (53.59%) | 360 (60.00%) | 0.011 a |
| Median [Q1, Q3] | 26.66 [23.46;30.80] | 28.03 [24.82;33.52] | 26.90 [23.77;31.02] | ||
| Risk factors | At least one risk factor | 393 (87.9%) | 125 (81.7%) | 518 (86.3%) | NS b |
| Chronic renal disease | 38 (8.5%) | 10 (6.5%) | 48 (8.0%) | NS b | |
| Chronic liver disease | 4 (0.9%) | 2 (1.3%) | 6 (1.0%) | NS b | |
| Chronic cardiovascular disease | 298 (66.7%) | 101 (66.0%) | 399 (66.5%) | NS b | |
| Chronic pulmonary disease | 88 (19.7%) | 37 (24.2%) | 125 (20.8%) | NS b | |
| Diabetes | 114 (25.5%) | 42 (27.5%) | 156 (26.0%) | NS b | |
| Cancer | 83 (18.6%) | 26 (17.0%) | 109 (18.2%) | NS b | |
| Immunodeficiency | 8 (1.8%) | 1 (0.7%) | 9 (1.5%) | NS b | |
| Pregnancy | 2 (0.4%) | - | 2 (0.3%) | NS b | |
| Symptom duration (days) | n (%) | 399 (89.26%) | 149 (97.39%) | 548 (91.33%) | < 0.001 a |
| Median [Q1, Q3] | 6.0 [9; 2] | 7.0 [10; 5] | 7.0 [9; 3] | ||
| Body temperature (°C) | n (%) | 241 (94.88%) | 92 (94.85%) | 333 (94.87%) | 0.018 a |
| Median [Q1, Q3] | 38.00 [37.0;38.5] | 38.00 [37.5;39.0] | 38.00 [37.1;38.6] | ||
| Ventilation | n (%) | 421 (94.18%) | 146 (95.46%) | 567 (94.50%) | <0.001 b |
| Spontaneous ambient air | 280 (66.5%) | 68 (46.6%) | 348 (61.4%) | ||
| O2 | 141 (33.5%) | 78 (53.4%) | 219 (38.6%) | ||
| O2 flow (L/min) | 141 | 78 | 219 | <0.001 a | |
| n (%) | (100.00%) | (100.00%) | (100.00%) | ||
| Median [Q1, Q3] | 3.0 [2;6] | 9.0 [6;15] | 5.0 [3;9] | ||
| Hemoglobin (g/L) | n (%) | 435 (99.54%) | 151 (99.34%) | 586 (99.49%) | <0.001 a |
| Median [Q1, Q3] | 13.20 [11.8;14.4] | 13.80 [12.5;14.8] | 13.40 [12.0;14.5] | ||
| Platelets (G/L) | n (%) | 432 (98.86%) | 151 (99.34%) | 583 (98.98%) | NS a |
| Median [Q1, Q3] | 194.0 [155;250] | 196.0 [155;258] | 195.0 [155;253] | ||
| Leucocytes (G/L) | n (%) | 435 (99.54%) | 151 (99.34%) | 586 (99.49%) | 0.004 a |
| Median [Q1, Q3] | 6.010 [4.55;8.25] | 7.460 [5.67;9.86] | 6.405 [4.76;8.60] | ||
| Neutrophils (G/L) | n (%) | 431 (98.63%) | 148 (97.37%) | 579 (98.30%) | <0.001 a |
| Median [Q1, Q3] | 4.530 [3.20;6.61] | 6.075 [4.29;8.41] | 4.970 [3.32;7.16] | ||
| Lymphocytes (G/L) | n (%) | 431 (98.63%) | 144 (94.74%) | 575 (97.62%) | NS a |
| Median [Q1, Q3] | 0.750 [0.51;1.07] | 0.720 [0.50;1.02] | 0.750 [0.51;1.06] | ||
| Creatinine (μmol/L) | n (%) | 428 (98.17%) | 150 (98.04%) | 578 (98.13%) | <0.001 a |
| Median [Q1, Q3] | 81.0 [64;107] | 95.0 [73;152] | 84.0 [66;115] | ||
| ASAT (IU/L) | n (%) | 289 (66.28%) | 101 (66.01%) | 390 (66.21%) | <0.001 a |
| Median [Q1, Q3] | 38.0 [27;55] | 53.0 [41;79] | 42.0 [28;60] | ||
| ALAT (IU/L) | n (%) | 334 (76.61%) | 121 (79.08%) | 455 (77.25%) | <0.001 a |
| Median [Q1, Q3] | 27.0 [17;43] | 38.0 [25;54] | 30.0 [19;48] | ||
| Bilirubin (μmol/L) | n (%) | 331 (75.92%) | 123 (80.39%) | 454 (77.08%) | 0.023 a |
| Median [Q1, Q3] | 8.10 [5.8;11.5] | 8.90 [6.5;13.3] | 8.30 [5.9;11.7] | ||
| CPK (IU/L) | n (%) | 43 (9.86%) | 19 (12.42%) | 62 (10.53%) | NS a |
| Median [Q1, Q3] | 138.0 [74;635] | 155.0 [84;327] | 147.0 [79;423] | ||
| LDH (IU/L) | n (%) | 198 (45.41%) | 84 (54.90%) | 282 (47.88%) | <0.001 a |
| Median [Q1, Q3] | 286.0 [222;381] | 434.5 [325;571] | 325.5 [239;449] | ||
| Calcium (mmol/L) | n (%) | 54 (12.39%) | 25 (16.34%) | 79 (13.41%) | 0.010 a |
| Median [Q1, Q3] | 2.13 [2.06;2.20] | 2.06 [1.92;2.13] | 2.10 [2.00;2.18] | ||
| Phosphate (mmol/L) | n (%) | 24 (5.50%) | 23 (15.03%) | 47 (7.98%) | NS a |
| Median [Q1, Q3] | 1.0 [1;1] | 0.9 [1;1] | 0.9 [1;1] | ||
| CRP (mg/L) | n (%) | 431 (98.85%) | 151 (98.69%) | 582 (98.81%) | <0.001 a |
| Median [Q1, Q3] | 66.0 [31;107] | 115.0 [66;191] | 75.0 [42;135] | ||
| PCT (μg/L) | n (%) | 126 (28.90%) | 84 (54.90%) | 210 (35.65%) | <0.001 a |
| Median [Q1, Q3] | 0.160 [0.10;0.31] | 0.290 [0.19;0.70] | 0.230 [0.11;0.41] |
Results are presented as N (%), median [Q1, Q3], % are calculated for non-missing values only. BMI: body mass index; ASAT: aspartate aminotransferase; ALAT: alanine aminotransferase; CPK: creatinine phosphokinase; LDH: lactate dehydrogenase; CRP: C-reactive protein; PCT: procalcitonin.
Wilcoxon rank sum test.
Fisher's exact test.
3.2. Deaths
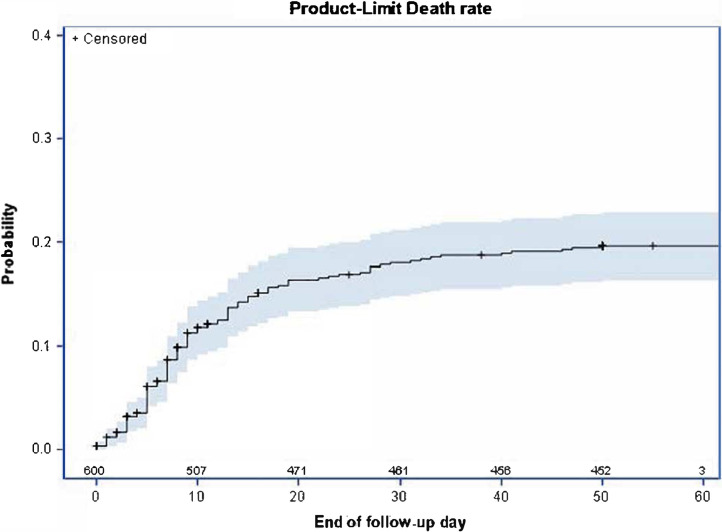
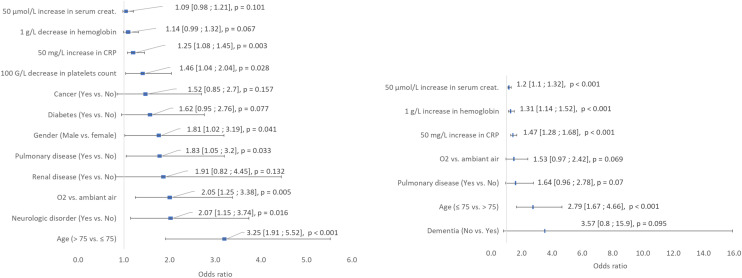
A total of 115 patients (19.1%) died, of whom 44 (38.3%) were hospitalized in the ICU. The overall case fatality was 9.1%, 15.6%, and 17.3% on days 7, 14 and 21, respectively (Fig. 1 ). Baseline characteristics and univariate analysis of survivors and non-survivors are presented in Table 1. Fig. 2 illustrates the results of the multivariate logistic regression to identify baseline variables possibly associated with patients’ death. Patients aged > 75 years (OR 3.25 [95% CI 1.91, 5.52]) and male patients (OR 1.81 [95% CI 1.02, 3.19]) as well as those with concomitant neurological disorders (OR 2.07 [95% CI 1.15, 3.74]), renal disease (OR 1.91 [95% CI 0.82, 4.45]), pulmonary disease (OR 1.83 [95% CI 1.05, 3.20]), diabetes (OR 1.62 [95% CI 0.95, 2.76]), or cancer (OR 1.52 [95% CI 0.85, 2.70]), and those who required oxygen by face mask (OR 2.05 [95% CI 1.25, 3.38]) had a higher risk of death in our cohort. The risk of death was also high in those with higher CRP (OR 1.25 [95% CI 1.08, 1.45], 50 mg/L increase) or serum creatinine (OR 1.09 [95% CI 0.98, 1.21], 50 μmol/L increase) levels and in those with low hemoglobin (OR 1.14 [95% CI 0.99, 1.32], 1 g/L decrease) or platelet (OR 1.46 [95% CI 1.04, 2.04], 100 G/L decrease) count.
Fig. 1.
Death rate curve–Product limit estimate (Kaplan–Meier) with two-sided confidence interval and number of patients at risk. Day 0 is the day of hospitalization. Patients alive at the end of follow-up were censored at the date of last information.
Fig. 2.
Results of the multivariate logistic regression analysis of death (left) and ICU (right) presented as odds ratios with 95% confidence interval. All baseline variables significant at the 0.20 level were included in the model. Creat.: Creatinine; CRP: C-Reactive Protein.
3.3. ICU hospitalization
A total of 153 patients (25.5%) required ICU hospitalization, of whom 139 (92.1%) required invasive mechanical ventilation. Table 2 summarizes the baseline characteristics and univariate analysis of patients according to hospitalization type (ICU or non-ICU). Fig. 2 depicts the multivariate logistic regression results to identify baseline variables that likely explained the need for ICU hospitalization. Patients aged ≤ 75 years (OR 2.79 [95% CI 1.67, 4.66]), those with concomitant pulmonary disease (OR 1.64 [95% CI 0.96, 2.78]), those without dementia (OR 3.57 [95% CI 0.8, 15.9]), and those requiring oxygen by face mask (OR 1.53 [95% CI 0.97, 2.42]) were at higher risk of being hospitalized in the ICU. Higher CRP (OR 1.47 [95% CI 1.28, 1.68], 50 mg/L increase), hemoglobin (OR 1.31 [95% CI 1.14, 1.52], 1 g/L increase), and serum creatinine (OR 1.20 [95% CI 1.10, 1.32], 50 μmol/L increase) levels also increased the risk of ICU hospitalization.
3.4. Extrapulmonary complications
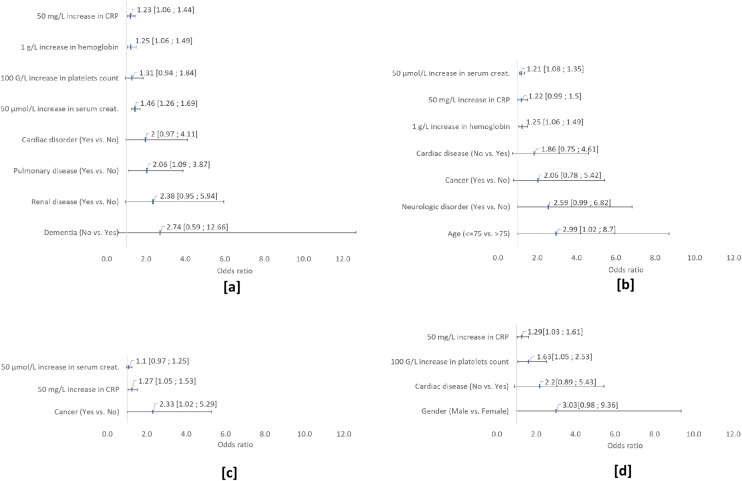
Among the 600 patients, 80 (13.3%) experienced AKI (stage 1, n = 42; stage 2, n = 15; stage 3, n = 23), 33 (5.5%) experienced a cardiovascular event (atrial fibrillation, n = 17; flutter, n = 4; ventricular tachycardia, n = 3; ischemic cardiac event, n = 8; Takotsubo syndrome, n = 3; lower limb ischemia, n = 1; pericarditis, n = 2), 27 (4.5%) exhibited ALI, 24 (4%) had venous thromboembolism, eight (1.3%) had a neurological event (ischemic stroke, n = 7; Guillain–Barré syndrome, n = 1), five (0.8%) had rhabdomyolysis, and one had acute pancreatitis, all during hospitalization (Table 3 ). Most extrapulmonary events occurred in ICU patients. When comparing extrapulmonary complications among alive (n = 485) and dead patients (n = 115) we found a higher rate of pulmonary embolism (6 vs 10; P = 0.02), renal failure (24 vs 56; P = 0.011), and rhabdomyolysis (3 vs 2; P = 0.04) among non-survivors. The median [Q1, Q3] time between symptom onset and extrapulmonary event was 11 days (range: 5-16 days), 11 days (range: 6-15 days), 4 days (range: 1-43 days) and 18 days (range: 10-35 days) for AKI, ALI, cardiac events, and thromboembolic events, respectively. Fig. 3 presents the results of the multivariate logistic regression to identify baseline variables that likely explained the four most frequent extrapulmonary events. Patients with concomitant renal disease (OR 2.38 [95% CI 0.95, 5.94]), pulmonary disease (OR 2.06 [95% CI 1.05, 3.20]) or cardiac disorder (OR 2 [95% CI 0.97, 4.11]) had a higher risk of AKI. AKI risk was also high in those with higher CRP (OR 1.23 [95% CI 1.06, 1.44], 50 mg/L increase), hemoglobin (OR 1.25 [95% CI 1.06, 1.49], 1 g/L increase) or platelet (OR 1.31 [95% CI 0.94, 1.84], 100 G/L increase) count. ALI patients aged > 75 years (OR 2.99 [95% CI 1.02, 8.7]) as well as those with concomitant cancer (OR 2.06 [95% CI 0.78, 5.42]) and cardiac disease (OR 1.86 [95% CI 0.75, 4.61]), those with higher CRP (OR 1.22 [95% CI 0.99, 1.5], 50 mg/L increase) or serum creatinine (OR 1.21 [95% CI 1.08, 1.35], 50 μmol/L increase) levels and those with high hemoglobin (OR 1.25 [95% CI 1.06, 1.49], 1 g/L increase) had a higher risk of ALI. Cardiac patients with concomitant cancer (OR 2.33 [95% CI 1.02, 5.29] or higher CRP (OR 1.27 [95% CI 1.05, 1.53], 50 mg/L increase) or serum creatinine (OR 1.1 [95% CI 0.97, 1.25], 50 μmol/L increase) levels had a higher risk of cardiac event. Patients were at higher risk of thromboembolic events if they were males (OR 3.03 [95% CI 0.98, 9.36]), had a concomitant cardiac disease (OR 2.2 [95% CI 0.89, 5.43]), had a higher CRP level (OR 1.29 [95% CI 1.03, 1.61], 50 mg/L increase) or higher platelet count (OR 1.63 [95% CI 1.05, 2.53], 100 G/L increase).
Table 3.
Extrapulmonary complications according to hospitalization type (ICU vs. non-ICU).
| Non-ICU (n = 447) |
ICU (n = 153) |
All patients (n = 600) |
P-value | |
|---|---|---|---|---|
| At least one event | 66 (14.8%) | 70 (45.8%) | 136 (22.7%) | < 0.001a |
| Thromboembolic event | 8 (1.8%) | 16 (10.5%) | 24 (4.0%) | < 0.001a |
| Deep vein thrombosis | 2 (25.0%) | 11 (68.8%) | 13 (54.2%) | NSa |
| Pulmonary embolism | 7 (87.5%) | 9 (56.3%) | 16 (66.7%) | NSa |
| Renal failure | 29 (6.5%) | 51 (33.3%) | 80 (13.3%) | < 0.001a |
| Rhabdomyolysis | 1 (0.2%) | 4 (2.6%) | 5 (0.8%) | 0.016a |
| Hepatic disorder | 12 (2.7%) | 15 (9.8%) | 27 (4.5%) | < 0.001a |
| Cardiovascular event | 12 (2.7%) | 21 (13.7%) | 33 (5.5%) | < 0.001a |
| Myocardial infarction | 6 (50.0%) | 2 (9.5%) | 8 (24.2%) | 0.015a |
| Arrhythmia | 4 (33.3%) | 18 (85.7%) | 22 (66.7%) | 0.005a |
| Atrial fibrillation | 3 (25.0%) | 14 (66.7%) | 17 (51.5%) | 0.032a |
| Flutter | 1 (8.3%) | 3 (14.3%) | 4 (12.1%) | NSa |
| Ventricular Tachycardia | - | 3 (14.3%) | 3 (9.1%) | NSa |
| Lower limb ischemia | - | 1 (4.8%) | 1 (3.0%) | NSa |
| Takotsubo syndrome | 2 (16.7%) | 1 (4.8%) | 3 (9.1%) | NSa |
| Pericarditis | 1 (8.3%) | 1 (4.8%) | 2 (6.1%) | NSa |
| Neurological disorder | 5 (1.1%) | 3 (2.0%) | 8 (1.3%) | NSa |
| Number of extrapulmonary events | ||||
| 0 | 381 (85.2%) | 83 (54.2%) | 464 (77.3%) | < 0.001a |
| 1 | 55 (12.3%) | 39 (25.5%) | 94 (15.7%) | |
| 2 | 10 (2.2%) | 21 (13.7%) | 31 (5.2%) | |
| 3 | 1 (0.2%) | 7 (4.6%) | 8 (1.3%) | |
| 4 | - | 3 (2.0%) | 3 (0.5%) |
Results are presented as n (%), % are calculated for non-missing values only.
Fisher's exact test.
Fig. 3.
Results of the multivariate logistic regression analysis of extrapulmonary event ([a] AKI, [b] ALI, [c] cardiac events, [d] thromboembolic events) presented as odds ratios with 95% confidence interval. All baseline variables significant at the 0.20 level were included in the models.
4. Discussion
In this single-center large retrospective cohort study of patients hospitalized with a confirmed diagnosis of COVID-19 in France, we described risk factors for ICU hospitalization and death of patients with severe COVID-19. With a median age of 71 years and a higher male proportion (57.7%), our study population is similar to that described in previous large cohort studies focusing on patients with COVID-19 [11], [12], [13]. Major serious complication of COVID-19 is severe ARDS, which leads to ICU hospitalization and/or death. In this study, 153 patients (25.5%) were hospitalized in the ICU and 115 (19.1%) died. Among those who died, 44 were previously hospitalized in the ICU (28.7% of ICU patients). The death rate of patients with COVID-19 in this study is within the range of previously published studies: 13.2%–32.5% in China [14], [15], [16], 22% in Germany [17], 28% (32% in the ICU) in the United Kingdom [11], and 16.3%–39% in the United States [12], [13]. However, we found that time to death was an important parameter. In our study, the death rate on day 7 was approximately 50% of what it was on day 21. The latter result is similar to the death rate on day 20 reported by Cecconi et al. (15.1%) [18]. Parameters contributing to serious COVID-19 are multiple and difficult to analyze. Considering the retrospective nature of our study and the absence of published guidelines for COVID-19 treatment in March 2020, we did not assess the impact of treatments that were administered (antiviral therapies, antibiotics, corticosteroids) for serious COVID-19 cases. However, only a few patients from our cohort received potential treatments for COVID-19. Such treatments included the administration of lopinavir/ritonavir in 11 patients (1.8%), hydroxychloroquine in 57 patients (9.5%), remdesivir in two patients (0.33%), and corticosteroids in 38 (6.3%) patients. Pressure on hospital beds in various departments, especially the ICU, regarding the COVID-19 epidemic is another factor that might have contributed to the severe outcome.
Unlike previous studies [1], [16], [18], we analyzed the risk factors for ICU hospitalization separately from the risk factors of patients who died from severe COVID-19. In addition, older or highly comorbid patients such as patients with dementia were not transferred to the ICU. An analysis including other criteria such as those explained by the WHO working study groups [19], notably the use of high-flow nasal oxygenation, could have been of interest to appreciate the severity of patients. However in March 2020, we only had a few ventilators available in Colmar for this type of oxygenation and all were dedicated to mechanical ventilation. Therefore, no high-flow ventilation was performed during the first wave of the epidemic.
Previous studies using univariate analyses reported age, male sex, and all comorbidities as important risk factors for death [11], [15], [20]. In the present study, the multivariate analysis also revealed that male sex, age > 75 years (frequently used to define geriatric patients), and several previous high-risk concomitant conditions (including neurological, renal, and pulmonary diseases, diabetes, and cancer) were risk factors for death. These results are similar to those reported by Docherty et al. who identified neurological disorders, chronic renal, respiratory diseases and malignancy as prognostic factors for death [11].
According to the univariate analyses of previous studies, higher leukocytes, neutrophils, CRP, PCT, ASAT, ALAT, and LDH levels or lower creatinine, lymphocyte, and platelet levels indicate severe COVID-19 [21], [22], [23], [24]. D-dimers, BNP, and troponin are also important prognostic biomarkers [13], [16], [25], but they were not included in our study because they were not routinely assessed at the start of the epidemic. Using multivariate analyses, we could quantify the impact of increased baseline CRP or creatinine levels on death or ICU hospitalization. A 50 μmol/L increase in serum creatinine levels increased the risks for death and ICU hospitalization, with OR values of 1.09 and 1.20, respectively. Likewise, a 50 mg/L increase in CRP levels increased the risks for death and ICU hospitalization, with OR values of 1.25 and 1.47, respectively. Therefore, elevated CRP or serum creatinine levels are risk factors for severe COVID-19 but of lower magnitude than age or concomitant diseases.
Considering that SARS-CoV-2 can affect several organs [2], we analyzed the extrapulmonary complications of COVID-19. Specific viral damages in the cells of human tissues can cause further ACE2 viral receptor expression, immune-mediated inflammation (cytokine storm), hypoxia, or drug toxicity, resulting in organ failure [2]. Extrapulmonary events occurred more frequently in ICU patients [12], [26]. We focused on biological complications or well-defined events, excluding complications such as confusion or heart failure, which are less discriminating, especially in the context of retrospective data collection. The median time between COVID-19 symptom onset and extrapulmonary events was quite similar for AKI, ALI, and thromboembolic events but was shorter for cardiovascular events. Acute infection with fever can cause arrhythmia, which may explain this discrepancy. For all events, in three out of four complications, elevated CRP and creatinine levels appeared to be independent risk factors, highlighting the role of inflammation and cytokine storm in these events.
AKI was the most frequent extrapulmonary event, affecting 13% of our patients. The occurrence rate of AKI ranges from 0.5% to 30% [2], [24], and this condition may even affect > 30% of patients in the United States [12], [27] or even 78% in ICU patients [28] compared with 33% of ICU patients in our study. Cardiovascular events affected 4.5% of our COVID-19 patients, and 67% of these events were arrhythmia cases. As reported by Zeng et al. [29], arrhythmia occurred more frequently in ICU patients. Arrhythmia needs to be identified because of its major implications on other complications, such as stroke and cardiac failure. Ischemic and Takotsubo syndromes are well-known COVID-19 complications. Troponin and BNP use, routine ECG, and echocardiography could probably improve the management of these cardiac events [2].
Moreover, seven patients presented with neurological events, of which six experienced ischemic stroke and one had Guillain–Barré syndrome. Guillain–Barré syndrome, which is an inflammatory polyradiculoneuropathy, is regularly associated with viral infection, as described in numerous case reports [30], [31]. Stroke has also been reported in patients with COVID-19 [3].
Rhabdomyolysis, which is an already known COVID-19 extrapulmonary complication [8], rarely occurred in our study population. It was only reported in five patients after exclusion of nine patients before admission because of a fall or trauma. In addition, one patient experienced acute pancreatitis, which is also a rare extrapulmonary complication of COVID-19.
This study has limitations as well as potential biases. It is a retrospective study with inhomogeneous data collected at baseline at a time when guidelines for treatment and extrapulmonary complication screening were lacking. Nonetheless, our data are robust, exhaustively recorded and easy to find in patients’ records. Other parameters, such as heart failure, co-infection or confusion (indicating potential encephalopathy) could be of interest. However, these parameters were difficult to confirm or to identify in the patients’ records because medical data collection was not yet standardized in March 2020. Despite having a large cohort of 600 patients, researchers should still be cautious in interpreting the results, particularly for events occurring in < 30 patients. Of note, the multivariate results that are presented in this paper are purely descriptive and should not be considered predictive.
5. Conclusion
Based on one of the largest single-center cohorts in France, this study describes the main risk factors for ICU hospitalization and death caused by COVID-19 and the frequency of numerous extrapulmonary complications. The baseline independent factors associated with death were older age, male sex, oxygen supply, chronic neurological disease, renal disease, pulmonary disease, diabetes, cancer, low platelet count, low hemoglobin level, high CRP level, and high serum creatinine level. Meanwhile, factors such as age < 75 years, oxygen supply, chronic pulmonary disease and high levels of CRP, hemoglobin, and serum creatinine were associated with ICU hospitalization. Extrapulmonary complications mostly occurred in ICU patients, with AKI and cardiovascular events being the most common. This study highlighted the main risk factors for ICU hospitalization and death caused by severe COVID-19 and the frequency of a wide range of extrapulmonary complications in France, suggesting that these complications should be prevented and monitored for better patient follow-up and outcomes.
Authors’ contributions
MM conceived and designed the study, analyzed the data, and drafted the article. SG, TB, MMZ, DK, CI, ME, APS, JO, JD, EH, JDK and the authors of the Center Alsace Covid-19 Study Group collected and analyzed the data. CK analyzed the data and performed statistical analyses. All authors reviewed and approved the article.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
The authors sincerely thank all healthcare and non-healthcare professionals who provided care to patients infected with SARS-CoV-2 at the Civil Hospitals of Colmar during the COVID-19 pandemic.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Wang M., Zhou Y., Chang J., Xian Y., Mao L., et al. 2020. Acute Cerebrovascular Disease Following COVID-19: A Single Center, Retrospective, Observational Study. [htpps://papers.ssrn.com/sol3/papers.cfm?abstract_id=3550025. preprint] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rismanbaf A., Zarei S. Liver and Kidney Injuries in COVID-19 and Their Effects on Drug Therapy; a Letter to Editor. Arch Acad Emerg Med. 2020;8(1):e17. [PMC free article] [PubMed] [Google Scholar]
- 8.Jin M., Tong Q. Rhabdomyolysis as Potential Late Complication Associated with COVID-19. Emerg Infect Dis. 2020;26(7):1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernard Stoecklin S., Rolland P., Silue Y., Mailles A., Campese C., Simondon A., et al. First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020. Euro Surveill. 2020;25(6.) doi: 10.2807/1560-7917.ES.2020.25.6.2000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 11.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch J.S., Ng J.H., Ross D.W., Sharma P., Shah H.H., Barnett R.L., et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J., Zhang S., Wu Z., Shang Y., Dong X., Li G., et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. 2020;10(1):99. doi: 10.1186/s13613-020-00706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecconi M., Piovani D., Brunetta E., Aghemo A., Greco M., Ciccarelli M., et al. Early Predictors of Clinical Deterioration in a Cohort of 239 Patients Hospitalized for Covid-19 Infection in Lombardy, Italy. J Clin Med. 2020;9(5) doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Characterisation WHOWGotC, Management of C-i. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H., et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol. 2020;92(10):1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alnor A., Sandberg M.B., Gils C., Vinholt P.J. Laboratory Tests and Outcome for Patients with Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. J Appl Lab Med. 2020;5(5):1038–1049. doi: 10.1093/jalm/jfaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner F.S., McCulloch D.J., Atluri V., Blain M., McGuffin S.A., Nalla A.K., et al. Clinical Features and Outcomes of 105 Hospitalized Patients With COVID-19 in Seattle, Washington. Clin Infect Dis. 2020;71(16):2167–2173. doi: 10.1093/cid/ciaa632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng J.H., Wu W.B., Qu J.X., Wang Y., Dong C.F., Luo Y.F., et al. Cardiac manifestations of COVID-19 in Shenzhen, China. Infection. 2020;48(6):861–870. doi: 10.1007/s15010-020-01473-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Canavero I., Ravaglia S., Valentino F., Micieli G. Mini-review: Guillain-Barre syndrome and myelitis associated with SARS-CoV-2 infection. Neurosci Lett. 2021;759:136040. doi: 10.1016/j.neulet.2021.136040. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koike H., Chiba A., Katsuno M. Emerging Infection, Vaccination, and Guillain-Barresyndrome: A review. Neurol Ther. 2021:1–15. doi: 10.1007/s40120-021-00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]