Highlights
-
•
Immunity from natural SARS-CoV-2 infection is protective in healthcare workers
-
•
Secondary infection is associated with low or absent serum neutralizing titer
-
•
Anti-Spike IgG were not significantly lower in subjects with secondary infections
-
•
Secondary infection is usually asymptomatic or mildly symptomatic
-
•
Vaccination of SARS-CoV-2-seronegative subjects might be prioritized
Keywords: SARS-CoV-2 infection, secondary infection, neutralizing antibody, immune protection
Abstract
Objective
The protection from SARS-CoV-2 infection induced by SARS-CoV-2 anti-S1 and anti-S2 IgG antibody positivity resulting from natural infection was evaluated.
Methods
The frequency of SARS-CoV-2 infection (as determined by virus RNA detection) was evaluated in a group of 1,460 seropositive and a control group of 8,150 seronegative healthcare workers in three Centres of Northern Italy in the period June-November 2020. Neutralizing serum titers were analyzed in seropositive subjects with or without secondary SARS-CoV-2 infection.
Results
During the 6-month survey, 1.78% seropositive subjects developed secondary SARS-CoV-2 infection while 6.63% seronegative controls developed primary infection (odds ratio: 0.26; 95% confidence interval: 0.17-0.38). Secondary infection was associated with low or absent serum neutralizing titer (p<0.01) and was mildly symptomatic in 45.8% cases vs 71.4% symptomatic primary infections (odds ratio: 0.34; 95% confidence interval: 0.16-0.78).
Conclusions
Immunity from natural infection appears protective from secondary infection; therefore, vaccination of seronegative subjects might be prioritized.
Introduction
Natural SARS-CoV-2 infection elicits humoral (Percivalle et al., 2020; Ni et al., 2020; Muecksch et al., 2021) and cellular responses (Grifoni et al., 2020). However, little is known about protection against secondary infection. Recently, a longitudinal study conducted in the United Kingdom (Lumley et al., 2021) showed that SARS-CoV-2 seropositivity was associated with a lower SARS-CoV-2 RNA detection rate.
Furthermore, some authors reported cases of SARS-CoV-2 secondary infection (To et al., 2020; Van Elslande et al., 2020(Muecksch et al., 2021); Tillett et al., 2021), suggesting that previous exposure might not guarantee complete protection.
On the other side, the recently introduced vaccines showed high efficacy in preventing COVID-19 disease (Baden et al., 2021; Voysey et al., 2021), and the hypothesis of vaccination for previously SARS-CoV-2 infected subjects has been debated.
We analyzed data from a large retrospective observational case-control study, providing results on immune protection from secondary SARS-CoV-2 infection in seropositive subjects.
Methods
A cohort of 9,610 healthcare workers (2,567 male and 7,043 female; median age 47 years, range 21-70 years) from three hospitals in Northern Italy (Fondazione IRCCS Policlinico San Matteo, Pavia; Alessandro Manzoni Hospital, Lecco; Guglielmo da Saliceto Hospital, Piacenza), involved in Covid-19 diagnosis and clinical care, were stratified according to SARS-CoV-2 seropositivity in the period April 29th -May 29th. Data on the role of the workers are reported in Table 1.
Table 1.
Role of the study participants
| Staff group | Fondazione IRCCS Policlinico San Matteo Pavia | Alessandro Manzoni Hospital Lecco | Guglielmo da Saliceto Hospital Piacenza | All participants |
|---|---|---|---|---|
| nursing or health care assistant | 1647 | 1374 | 1661 | 4682 |
| doctor | 924 | 514 | 571 | 2009 |
| pharmacist, biologist, chemist, physicist | 82 | 16 | 259 | 357 |
| researcher | 31 | 0 | 0 | 31 |
| healthcare technicians | 608 | 336 | 199 | 1143 |
| administrative | 306 | 287 | 325 | 918 |
| other | 164 | 0 | 306 | 470 |
| Total | 3762 | 2527 | 3321 | 9610 |
Data were matched with SARS-CoV-2 RNA positivity in nasopharyngeal swabs from June 01st -November 30th, 2020.
Detection of SARS-CoV-2 RNA in seronegative subjects was defined as a primary infection, whereas, according to the ECDC case definition (ECDC, 2021), detection of SARS-CoV-2 RNA ≥60 days following previous positive serology (anti-spike IgG antibodies) was defined as a secondary infection.
Serological analysis was performed using chemiluminescent assay (Liason SARS-CoV-2 S1/S2 IgG, Diasorin, Saluggia, Italy) to measure SARS-CoV-2 anti-S1 and anti-S2 IgG antibodies. Neutralizing antibody serum titer was determined as previously reported (Percivalle et al., 2020).
The health condition of all workers was constantly monitored in all three hospitals. The PCR assays to detect SARS-CoV-2 RNA were performed in the laboratories of each center. Nasopharyngeal sampling was scheduled every 14 days for healthcare workers in fragile wards in Pavia and Lecco hospitals and every 7 days in Piacenza hospital. In addition, in Piacenza hospital, monitoring was scheduled every 14 days in Covid-19 wards. In all centers, nasopharyngeal sampling was collected from all the symptomatic individuals and contacts. Data on Symptoms were collected during an interview by a physician and inserted into a specific database.
Results
At the end of the first epidemic wave, in the period April 29th -May 29th, 2020, 9,610 venous blood samples of healthcare workers were collected to evaluate their SARS-CoV-2 anti-S1 and anti-S2 IgG antibody status. 1,460 health care workers (15.2%) were SARS-CoV-2 seropositive (cases) while 8,150 were not (controls). During the second epidemic wave, SARS-CoV-2 infection was detected in 26/1,460 SARS-CoV-2-seropositive subjects (1.78%), and in 540/8,150 SARS-CoV-2-seronegative controls (6.63% primary infections), with an odds ratio of 0.26 (95% confidence interval [CI] 0.17-0.38). The populations from each center considered independently showed a similar trend (Table 2). The median age was similar in subjects with primary (47 years, range 23-64) or secondary infections (49 years, range 26-59), as well as the rate of subjects with direct contact with patients (75.66% vs. 71.43%, respectively; p=0.68).
Table 2.
Occurrence of SARS-CoV-2 infection in seropositive and seronegative healthcare workers
| Center | SARS-CoV-2 serostatus | No. subjects (%) | No. subjects with SARS-CoV-2 RNA positive swab (% of seropositive or seronegative subjects) | Odds ratio (95% confidence interval) |
|---|---|---|---|---|
| Pavia | Positive | 321 (8.5) | 7 (2.18) | 0.33 (0.15-0.69) |
| Negative | 3441 (91.5) | 219 (6.36) | ||
| Piacenza | Positive | 965 (29.1) | 14 (1.45) | 0.24 (0.14-0.42) |
| Negative | 2356 (70.9) | 136 (5.77) | ||
| Lecco | Positive | 174 (6.9) | 5 (2.87) | 0.35 (0.15-0.81) |
| Negative | 2353 (93.1) | 185 (7.86) | ||
| Total | Positive | 1460 (15.2) | 26 (1.78) | 0.26 (0.17-0.38) |
| Negative | 8150 (74.8) | 540 (6.63) |
Data on symptoms were available in 24/26 (92.3%) secondary infections and in 391/540 (72.4%) primary infections. Mild symptoms were reported in 11/24 (45.8%) secondary vs. 279/391 (71.4%) primary infections (odds ratio 0.34, 95% CI 0.16-0.78, p=0.012), while the other subjects were asymptomatic.
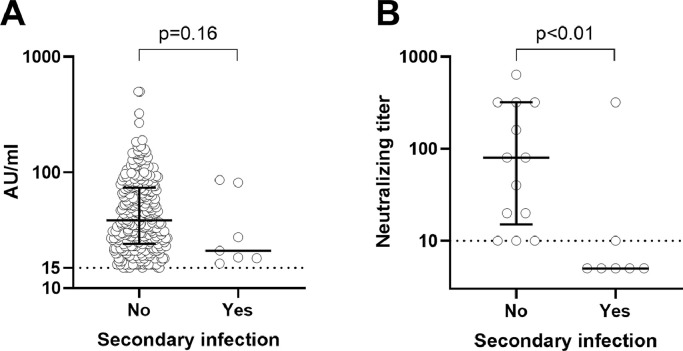
Data on anti-Spike IgG antibody quantitative levels were available for 313 seropositive subjects without and 7 seropositive subjects with a SARS-CoV-2 secondary infection (Fig 1A), all from the Pavia hospital; for the other two hospitals, only qualitative results were available. Although the difference between the two groups was not significant (p=0.16; Mann-Whitney U-test), 5/7 subjects with SARS-CoV-2 secondary infection had anti-Spike IgG antibody levels within the lower quartile of subjects with no subsequent positive swab. Neutralizing serum titer was determined for the 7 subjects with and 13 seropositive subjects without secondary infection (Fig 1B). Neutralizing serum titer was significantly lower in the subjects with secondary infection (p<0.01; Mann-Whitney U-test) and, in particular, was undetectable in 5 of them.
Figure 1.
Anti-SARS-CoV-2 S1/S2 IgG antibody serum levels (A) and neutralizing serum titers (B) in SARS-CoV-2-seropositive subjects with no SARS-CoV-2 secondary infection (n=313 for anti- SARS-CoV-2 S1/S2 IgG antibody and n=13 for neutralizing antibody) or with secondary infection (n=7). Median levels (and interquartile range for subjects with no secondary infection) are shown. Dotted horizontal lines represent cutoff levels for positive results (≥15 arbitrary units (AU)/ml for anti-SARS-CoV-2 S1/S2 IgG antibody and ≥1:10 neutralizing serum titer). Each symbol represents an individual.
Discussion
The results of this case-control study confirmed that immunity resulting from natural infection is associated with significant protection from secondary infection. It could be hypothesized that ineffective SARS-CoV-2 immunity was elicited in the subjects developing a secondary infection. Indeed, SARS CoV-2 neutralizing antibodies were undetectable in 5/7 (71.4%) subjects analyzed with secondary infection. While we cannot rule out false-positive serostatus results in these individuals, it could also be speculated that the presence of high SARS-CoV-2 neutralizing titers might be crucial for protection.
During the survey period, the odds for developing SARS-CoV-2 infection were 4 times lower in seropositive subjects. Therefore, we can assume that immunity elicited by natural infection is around 75% protective, similar to what is observed with an adenovirus-vectored vaccine (70%), and slightly lower than what reported for mRNA vaccines (95%) (Baden et al., 2021; Voysey et al., 2021). However, it is difficult to directly compare the protective effect of natural with vaccine-induced immunity, as there is a difference in the immune response that they elicit. In our study, about half of the individuals with SARS-CoV-2 secondary infection were asymptomatic virus carriers, diagnosed occasionally due to screening of contacts of infected subjects. Therefore, it appears that immunity occurring after natural infection is highly protective from symptomatic SARS-CoV-2 secondary infection. Because of vaccination strategies, prioritizing the immunization of seronegative individuals while deferring it in previously infected individuals would reduce the need for vaccine doses and speed up the process to reach immune protection in the population.
Significant limitations of this study were its retrospective nature, the availability of quantitative anti-SARS-CoV-2 IgG antibody levels from only one center, and the testing of neutralization titers in a limited subset. On the other hand, this study supports and further extends the results of the large SIREN study conducted in England on healthcare workers (Hall et al., 2021) and other recent studies conducted on healthcare workers or the general population (reviewed by O Murchu et al., 2021). However, these studies did not evaluate the levels of neutralizing antibodies in subjects with secondary infections. Only one study evaluated the role of neutralizing antibodies for SARS-CoV-2 reinfection, in ferrets (Kim Y et al., 2021). Kim et al. reported that SARS-CoV-2 reinfected ferrets showed active virus replication in the upper respiratory and gastrointestinal tracts. The high neutralizing antibody titer group showed attenuated viral replication and rapid viral clearance. Furthermore, direct-contact transmission was observed only from reinfected ferrets with low neutralizing antibody titers (<20) and not from other groups. More data are needed to evaluate the protective role of neutralizing antibodies in humans.
In conclusion, we confirmed that immunological memory elicited by SARS-CoV-2 infection is protective from secondary infections up to 6 months. Further analysis in the general population is warranted.
Acknowledgments
Conflict of Interest
The authors have no conflict of interest to declare.
Funding Source
This work was supported by Fondazione Cariplo [grant CoVIM, no. 2020-1374] and Ministero della Salute, Ricerca Finalizzata [grant BIAS no. 2020-12371760] and Ricerca Corrente, [grant no. 80206] and from European Commission – Horizon 2020 [EU project 101003650 – ATAC]
Ethical Approval
The SARS CoV-2 antibody status of health care workers was performed in Lombardy Region (Pavia and Lecco Hospitals) in agreement with a specific Regional screening protocol (Circolare Regione Lombardia “Utilizzo test sierologici”, protocollo G1.2020.0017959, 22 April 2020). In Emilia-Romagna Region (Piacenza Hospital) screening was performed in agreement with a specific Regional protocol (Emilia-Romagna Region, Giunta Regionale, “COVID-19: disciplina dei test sierologici”, Delibera N° 350, 16 April 2020). Specific written informed consent was collected.
References
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . ECDC; Stockholm: 2021. Reinfection with SARS-CoV-2: implementation of a surveillance case definition within the EU/EEA. April 08th, 2021. [Google Scholar]
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.015. 1489-501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative healthcare workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YII, Kim SM, Park SJ, Kim EH, Yu KM, Chang JH. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg Microbes Infect. 2021;10(1):152–160. doi: 10.1080/22221751.2021.1872352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C. Longitudinal Serological Analysis and Neutralizing Antibody Levels in Coronavirus Disease 2019 Convalescent Patients. J Infect Dis. 2021;223(3):389–398. doi: 10.1093/infdis/jiaa659. February 13th. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Ye F, Cheng ML, Feng Y, Deng YQ, Zhao H. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity. 2020;52 doi: 10.1016/j.immuni.2020.04.023. 971-7.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O Murchu E, Byrne P, Carty PG, De Gascun C, Keogan M, O'Neill M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev Med Virol. 2021:e2260. doi: 10.1002/rmv.2260. May 27Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percivalle E, Cambiè G, Cassaniti I, Nepita EV, Maserati R, Ferrari A. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as of April 06th, 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2021;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KK, Hung IF, Ip JD, Chu AW, Chan WM, Tam AR. COVID-19 reinfection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole-genome sequencing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1275. ciaa1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elslande J, Vermeersch P, Vandervoort K, Wawina-Bokalanga T, Vanmechelen B, Wollants E. Symptomatic SARS-CoV-2 reinfection by a phylogenetically distinct strain. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1330. ciaa1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]