Abstract
Background
COVID-19, which is a disease caused by the SARS-CoV-2 virus, has spread around the world since late 2019. Studies have found associations between the rising levels of TNF-α and severe COVID-19 cases. Hence, TNF-α blocking can possibly be a favorable intervention in modifying COVID-19. To this end, in order to manage pneumonia caused by COVID-19, adalimumab may potentially be considered as a potential therapeutic agent. The present study aimed to investigate the potential therapeutic role of adalimumab in treating COVID-19 cases in combination therapy with remdesivir and dexamethasone.
Methods
Among the 68 patients who were included in the current randomized controlled trial, 34 were assigned to the adalimumab group and the remaining 34 were assigned to the control group. Adalimumab at a dose of 40 mg, subcutaneous for once, was used for the intervention group. Both the intervention and control groups received remdesivir, dexamethasone, and supportive care. The data gathered to make comparisons of the groups included demographic information, the rate of mortality, mechanical ventilation requirement, length of stay in hospital and Intensive Care Unit (ICU), and imaging findings.
Results
There was no significant difference between the two groups in the terms of mortality rate (P-value = 1) and mechanical ventilation requirement (P-value = 1). The length of hospital and ICU stay as well as radiologic changes were not affected either (P-value = 1, 0.27, and 0.53, respectively).
Conclusions
Our findings did not support the use of adalimumab in combination with remdesivir and dexamethasone in the treatment of severe COVID-19 cases.
Keywords: Adalimumab, TNF-α, COVID-19, Coronavirus, Pulmonary infection
1. Introduction
The spread of COVID-19, a disease caused by the SARS-CoV-2 virus, around the world began in late 2019. The disease has a high rate of mortality. Virus immunology and its effects on the immune system have been studied in several studies [1]. Despite lots of trials for evaluating the effectiveness of different potential medications, therapeutic options for treating COVID-19 with promising effects are still lacking. There is still no general agreement on what protocols to apply for treatment of the disease [2]. In addition, since the disease is tremendously contagious, its clinical features are diverse. symptoms in the patients are highly severe, and successful handling of the conditions is a challenging effort [3], [4], [5]. Clinical trials are nowadays conducted to carry out experiments with medications such as antivirals, corticosteroids, immunomodulatory agents, and anti-helminthics. Despite this, there is still no optimal strategy for treatment and definite cure, specifically in dealing with severe cases [2], [6], [7], [8]
Evidence indicates the contribution of the overactivity of the immune system, including cytokine release syndrome, to more severe symptoms and multiorgan failure of patients diagnosed with COVID-19 [9], [10]. Moreover, studies have found associations between the rise of TNF-α and severe COVID-19 cases [11], [12]. Accordingly, TNF-α blocking can possibly be a favorable intervention in modifying COVID-19 [12].
By binding to TNF-α, Adalimumab as a recombinant fully human IgG1 monoclonal antibody inhibits the interaction of this inflammatory cytokine with TNF receptors on the p55 and p75 cell surface [13]. Adalimumab has been proven to suppress C-reactive protein, IL-6, and matrix metalloproteinases (MMP-1 and MMP-3) by tissue remodeling and matrix destruction [14]. Moreover, reducing the activities of MMPs may be effective in controlling damage to tissues by COVID-19 [15]. According to the existing body of knowledge and given evidence, adalimumab can have valuable therapeutic potential in managing COVID-19 pneumonia. Hence, the present study aimed to investigate the potential therapeutic roles of adalimumab in treating COVID-19 cases in combination therapy with remdesivir and dexamethasone.
2. Materials and Methods
2.1. Setting
This study was a randomized prospective clinical trial conducted at the Dr. Masih Daneshvari Hospital, affiliated and selected referral center for COVID-19 patients, Tehran, Iran.
2.2. Patients
Patients with severe illness at ages from 18 to 70 years old with COVID-19 were considered as the cases for study. The criteria for identifying the cases were Reverse Transcription-Polymerase Chain Reaction (RT-PCR) reports and Computed Tomography (CT) scan confirming bilateral pulmonary infiltration. Severe or critical cases of COVID-19 were determined based on the SpO2 ≤ 93% at room air, Heart rate ≥ 125 min, respiratory rate ≥ 30/min, The evidence of shock, the need for mechanical ventilation or vasopressors, clinically significant acute hepatic, renal or neurological dysfunction due to COVID-19 and patients with acute respiratory distress syndrome [16].
The following were regarded for the exclusion criteria: denying to sign the consent form; acute or chronic kidney disease proven by a rise in serum creatinine by more than 0.3 mg/dl during 48 h or glomerular filtration rate lower than 30 ml/min; the history of liver failure (Child-Pugh stage C and D or more than 5 times rise above the upper limit of normal in liver function tests or 3 times in patients with symptoms of liver failure); the history of malignancy; the history of heart failure; patients with latent or active tuberculosis or any active infection; patients receiving medications affecting IL-6 or TNF-α levels; patients with active peptic ulcer disease; patients with a history of an allergic reaction to adalimumab or developing allergic reaction while receiving the medication; mildly ill patients; and pregnancy or breastfeeding.
2.3. Informed consent and approval
Signing the consent form by one’s own or a legal representative was the necessary condition for including a patient in the present study. This research was approved with the ethics code number of IR.SBMU.NRITLD.REC.1399.154 by the ethics committee of Shahid Beheshti University of Medical Sciences. Moreover, the trial was registered in the Iranian Registry of Clinical Trials with the registration number of IRCT20151227025726N23.
2.4. Interventions
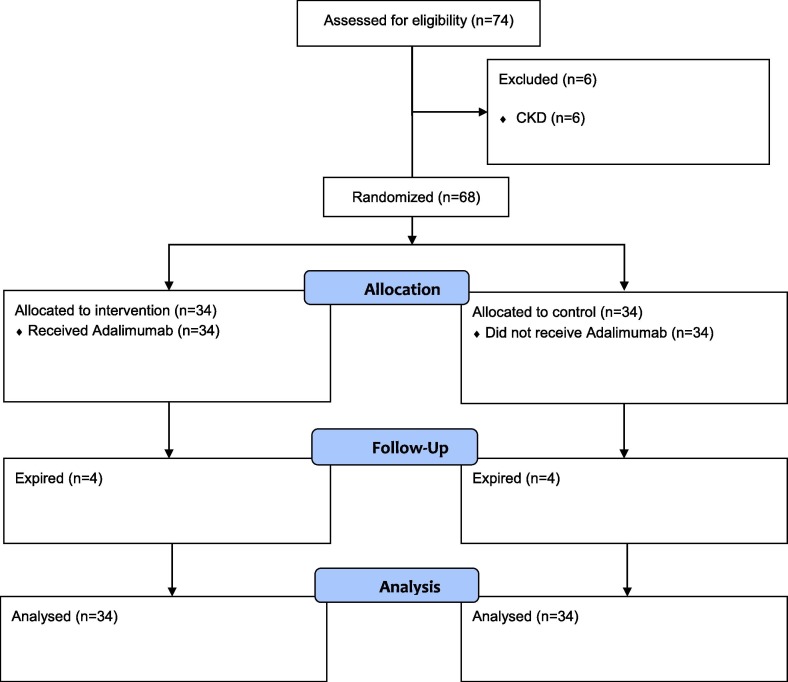
In total, the study included 68 patients that were randomly categorized into adalimumab and control groups (Fig. 1 ). We considered that the proportion of patients needing mechanical ventilation in the control group to be 0.5 and in the adalimumab group to be 0.15. Considering α error probability of 0.05 and power of 0.8, 27 patients were calculated in each arm. We enrolled 34 patients in each group considering the drop-out rate. The patients were randomized via permuted block randomization. Those in the adalimumab group received adalimumab (CinnoRA®, CinnaGen, Iran), 40 mg, single-dose, subcutaneously in prefilled syringe form, while those in the control group did not receive adalimumab. Both groups received oxygen and fluid support, remdesivir 200 mg stat followed by 100 mg intravenously daily for five to ten days, and dexamethasone 6 mg intravenously daily for ten days or up to the point of discharge.
Fig. 1.
CONSORT flowchart.
2.5. Outcomes
Requiring invasive mechanical ventilation, the necessity of admission to the Intensive Care Unit (ICU), and the rate of mortality were considered as the primary outcomes of the study. These outcomes were assessed until death or discharge from the hospital. For the secondary outcomes, length of stay in the ICU or hospital and improvement observed in the chest CT scan were incorporated. Data collection was done using medical records of patients regarding age, gender, underlying diseases, results of laboratory tests, and CT imaging findings.
2.6. Statistical analysis
SPSS v.25.0 software was used for analyzing the results. The difference in binary variables was evaluated through Chi-square tests and for assessing the normality of the data distribution, the Shapiro-Wilk test was conducted. Finally, the student-t or Mann-Whitney U test was performed to compare quantitative variables for both groups.
3. Results
Table 1 represents demographic data and past medical history of the patients. No meaningful difference was observed between the two groups of patients in terms of age, gender, and past medical history.
Table 1.
Age, gender, and past medical history of the population under study.
| Adalimumab group (n = 34) | Control group (n = 34) | P-value | |
|---|---|---|---|
| Age | 53.15 ± 12.9 | 56.12 ± 11.51 | 0.32 |
| Gender (Male) | 22 (64.7%) | 18 (52.94%) | 0.46 |
| Smoking History | 4 (11.7%) | 1 (2.9%) | 0.32 |
| Diabetes | 7 (20.58%) | 12 (35.29%) | 0.17 |
| Hypertension | 8 (23.52%) | 12 (35.29%) | 0.28 |
| Ischemic Heart Disease | 0 (0%) | 3 (8.82%) | 0.23 |
| Chronic Obstructive Pulmonary Disease | 1 (1.92%) | 0 (0%) | 1 |
| Malignancy | 0 (0%) | 1 (2.94%) | 0.5 |
| Chronic Kidney Disease | 0 (0%) | 1 (2.94%) | 0.5 |
| Rheumatoid Arthritis | 1 (2.94%) | 1 (2.94%) | 0.75 |
| Data are presented as percent or mean ± SD | |||
Table 2 gives the mean time in days from when the patients were admitted to the hospital to when adalimumab was administered.
Table 2.
The time from admission to administration of adalimumab for the intervention group.
| Frequency | Minimum | Maximum | Mean ± SD | |
|---|---|---|---|---|
| Time from admission to administration (in days) | 34 | 2 | 11 | 4.35 ± 1.73 |
| SD: Standard Deviation | ||||
In order to compare the two groups with regard to the necessity of invasive mechanical ventilation, admission to the ICU, the improvement level observed in CT scan, and the rate of mortality, the Chi-square test was carried out. Mann-Whitney U or student t-test was also conducted in order to comparatively analyze the population under study with regard to the length of stay in the hospital and ICU. Table 3 provides the results achieved by both tests.
Table 3.
Differences between the two groups regarding the necessity of invasive mechanical ventilation, admission to the ICU, improvement in the chest CT scan, the rate of mortality, and stay length in ICU and hospital.
| Adalimumab Group (n = 34) | Control Group (n = 34) | P-value (95% CI) | |
|---|---|---|---|
| Mechanical ventilation requirement | 4 (11.7%) | 3 (8.8%) | 1 |
| The need for non-invasive ventilation | 4 (11.7%) | 2 (5.8%) | 0.67 |
| Nasal or face mask or nasal oxygen therapy | 28 (82.3%) | 31 (91.1%) | 0.42 |
| The need for admission to the ICU | 5 (14.7%) | 5 (14.7%) | 1 |
| More than 50% improvement in chest CT scan | 8 (23.5%) | 5 (14.7%) | 0.74 |
| Length of hospital stay (day) | 12.18 ± 4.64 | 10.85 ± 5.29 | 0.27 (-1.08–3.73) |
| Length of ICU stay | 13 (8–18.5) | 9 (6.5–19.5) | 0.53 (-10.47–12.47) |
| Mortality (expired) | 4 (11.7%) | 4 (11.7%) | 1 |
Data are presented in numbers for qualitative data and median plus 25%-75% percentile for quantitative data. ICU: Intensive Care Unit; CT: Computed Tomography
Data regarding the improvement of symptoms in both groups are shown in Table 4 . Symptoms were evaluated at baseline, Day 3, and Day 7 after adalimumab treatment. The Table indicates that there was no difference between the two groups in terms of symptoms recovery. The study also found no significant differences between the two groups regarding CRP and TNF-α levels.Table 5 . (SEE)
Table 4.
The trend of symptoms improvement and lab tests between the two groups.
| Adalimumab group | Total | Control group | Total | P-Value | |
|---|---|---|---|---|---|
| Fever baseline | 2 (6.06%) | 33 | 2 (5.88%) | 34 | 1 |
| Fever day 3 | 0 (0%) | 33 | 0 (0%) | 32 | – |
| Fever day 7 | 0 (0%) | 17 | 1 (6.25%) | 16 | 0.48 |
| Cough baseline | 21(61.76%) | 34 | 17(50%) | 34 | 0.32 |
| Cough day 3 | 13(39.39%) | 33 | 13(38.2%) | 30 | 0.75 |
| Cough day 7 | 2(5.88%) | 17 | 3 (43.33%) | 13 | 0.62 |
| Dyspnea baseline | 27 (79.41%) | 34 | 24 (70.5%) | 34 | 0.4 |
| Dyspnea day 3 | 14(42.42%) | 33 | 16 (53.33%) | 30 | 0.38 |
| Dyspnea day 7 | 2 (12.5%) | 16 | 1(7.69%) | 13 | 0.76 |
| Myalgia baseline | 3(9.09%) | 33 | 7 (20.58%) | 34 | 0.3 |
| Myalgia day 3 | 2(6.06%) | 33 | 6(20%) | 30 | 0.13 |
| Myalgia day 7 | 0 (0%) | 16 | 0(0%) | 13 | – |
| Chest pain baseline | 8 (24.24%) | 33 | 13 (38.23%) | 34 | 0.21 |
| Chest pain day 3 | 6 (18.18%) | 33 | 8 (26.66%) | 30 | 0.41 |
| Chest pain day 7 | 0 (0%) | 16 | 0(0%) | 13 | – |
| Headache baseline | 6 (18.18%) | 33 | 7 (20.58%) | 34 | 1 |
| Headache day 3 | 1(3.03%) | 33 | 2 (6.66%) | 30 | 0.6 |
| Headache day 7 | 0 (0%) | 16 | 0(0%) | 13 | – |
| Diarrhea baseline | 7 (21.21%) | 33 | 4 (11.76%) | 34 | 0.29 |
| Diarrhea day 3 | 3 (9.09%) | 33 | 0 (0%) | 30 | 0.24 |
| Diarrhea day 7 | 0 (0%) | 16 | 0(0%) | 13 | – |
| Sore throat baseline | 3 (9.09%) | 33 | 3 (8.82%) | 34 | 1 |
| Sore throat day 3 | 2 (6.06%) | 33 | 3 (10%) | 30 | 0.66 |
| Sore throat day 7 | 0 (0%) | 16 | 0(0%) | 13 | – |
Data are presented as Mean ± SD; CRP: C-Reactive Protein; IL-6: Interleukin-6
Table 5.
Difference in lab tests for the two groups.
| Adalimumab group | Total | Control group | Total | P-Value | |
|---|---|---|---|---|---|
| WBC before adalimumab | 7.7(4.21–10.58) | 25 | 6.48(4.62–9.96) | 25 | 0.82 |
| WCB 3 days after adalimumab | 9.29 ± 3.79 | 14 | 10.8 ± 5.1 | 14 | 0.38 |
| D-Dimer before adalimumab | 806(405–1490) | 25 | 460(308–720) | 21 | 0.059 |
| D-Dimer 3 days after adalimumab | 888(435.5–1619) | 18 | 405(264.25–1086.75) | 16 | 0.03 |
| Ferritin before adalimumab | 900.5(463.75–1373.75) | 28 | 523 (295–1030) | 31 | 0.18 |
| Ferritin after 3 days after adalimumab | 1273.88 ± 561.29 | 18 | 689.81 ± 567.91 | 16 | 0.005 |
| CRP before adalimumab | 70(54–81) | 31 | 56(38–73) | 34 | 0.053 |
| CRP 3 days after adalimumab | 12(4.75–22.25) | 30 | 22 (11–42) | 23 | 0.025 |
| IL-6 before adalimumab | 22.25 ± 12.01 | 29 | 16.1 ± 8.96 | 33 | 0.02 |
| IL-6 3 days after adalimumab | 17.8 ± 10.15 | 21 | 19.1 ± 11.51 | 17 | 0.71 |
| TNF-α before adalimumab | 6.5 (5.5–10.1) | 27 | 7.45(6.02–10.45) | 26 | 0.68 |
| TNF-α 3 days after adalimumab | 6.9 (5.15–11.4) | 20 | 6.8 (5.9–10.2) | 21 | 0.91 |
Data are presented as Mean ± SD; CRP: C-Reactive Protein; IL-6: Interleukin-6
4. Discussion
Our study did not find any therapeutic benefits of administering Adalimumab for the severe cases of COVID-19 in combination with dexamethasone and remdesivir in terms of the need for mechanical ventilation, admission to the ICU and hospital. The improvement level in the chest CT and the trend of improvement in symptoms were not affected either.
Some studies have evaluated the possible therapeutic advantages of adalimumab for COVID-19. The disease is shown to be less severe in patients who have rheumatologic disorders and are already on TNF-α inhibitors [17]. According to Tursi et al., adalimumab might have advantages for not only improving the symptoms of Crohn’s disease but also treating COVID-19 in such patients [18]. In a study conducted by Conti et al., a patient with psoriasis under adalimumab therapy was observed. no symptoms that could be related to COVID-19 were detected for him. This is while the patient under study was in close contact with the infected patients [19]. Several research studies have proven positive effects for anti-TNF-α agents with regard to monocytes proliferation, which expresses TNF-α in patients with severe COVID-19 [20], [21]. In the report offered by Valent et al., a psoriatic man who underwent adalimumab therapy for almost a year every two weeks recovered from COVID-19 rapidly [19].
However, using adalimumab in combination with remdesivir and dexamethasone was not proven effective in our results. The levels of TNF-α were not affected either. Some experts believe in associations between severe cases of COVID-19 and increased levels of TNF-α levels [22]; hence, anti-TNF-α therapy may lead to better outcomes. However, we could not find any significant difference in TNF-α levels 3 days after adalimumab therapy. There is limited evidence regarding the onset of lowering TNF-α levels with adalimumab in COVID-19 patients. It is noteworthy that TNF-α was not measured for all patients before and after adalimumab therapy and it is possible that in the case of larger samples with valid data, significant results could be achieved. As a limitation of our study, we were not able to measure TNF-α in all the patients. It seemed that despite developing severe COVID-19, TNF-α levels did not increase that much and due to this fact, TNF-α inhibitor adalimumab was not effective in enhancing the disease outcomes. It is suggested to measure TNF-α levels prior to decide whether the patient will benefit from adalimumab therapy or not.
Increased TNF-α levels higher than 35 pg/ml are associated with disease severity and help determine survival and morbidity [23]. However, it seems that TNF-α levels do not rise in all severe COVID-19 cases and it is important to consider TNF-α levels before deciding to administer adalimumab. There may be a role for this medication in severe COVID-19 cases with increased TNF-α levels. CRP levels were significantly lower in the adalimumab group after 3 days, which may indicate anti-inflammatory effects of adalimumab in this regard. Considering the inflammatory effects of COVID-19 in the lungs, TNF-α levels might be higher in the lungs than the systemic levels and this could explain the rational for reduced CRP levels after 3 days in the adalimumab group. The number of white blood cells and TNF-α levels were decreased after 3 days. However, this reduction was not statistically significant. There was a possibility of statistical significance if the sample size was larger. It seems that the study was underpowered to show statistical differences regarding these parameters.
Interestingly, the level of ferritin was meaningfully higher in the adalimumab group after treatment. However, this result is of poor value to interpret as the number of samples with documented ferritin levels involved in the analysis was low.
To mention the main limitation of the presented research study, we could not collect lab results for all the 68 patients included in the study. This issue happened because of the emergence of administration in some situations which caused some data missing and made the interpretation of lab results difficult.
In our study, it was hypothesized that administration of adalimumab could reduce mechanical ventilation by 35% with 80% power and 0.05 α. As a result, this study failed to meet the proposed hypothesis. However, adalimumab may still be effective in reducing mechanical ventilation by less than 35%. Hence, conducting the study with a larger sample size to detect smaller changes may illustrate the significant difference between the two groups.
Another point to consider is that we evaluated the adalimumab effects combined with remdesivir and dexamethasone. The administration of remdesivir and dexamethasone generally seems to work very well for the patients without any further treatments [24]. Due to the ethical issues, the potential therapeutic effects of adalimumab as monotherapy were not determined in our study. Besides considering the results of a case report that achieved a rapid response following a single dose of adalimumab in a post-coronary artery bypass graft surgery patient [12], we decided to administer one single 40 mg dose only. However, there is a possibility of different results with different dosing regimens and an increased number of administrations. There is a study which has concluded that long-term adalimumab therapy in patients with inflammatory bowel diseases resulted in the development of less severe COVID-19. This study may necessitate the need for an increased number of administrations.
5. Conclusion
We did not find any therapeutic benefits for adalimumab in combination with remdesivir and dexamethasone in severe COVID-19 cases. It seems that increased levels of TNF-α may lead to better predictions of the efficacy of anti-TNF-α therapy. Our patients did not have increased levels of TNF-α despite being the severe cases of COVID-19.
CRediT authorship contribution statement
Atefeh Fakharian: Conceptualization, Methodology, Writing - review & editing. Saghar Barati: Methodology, Writing - original draft, Formal analysis. Maryam Mirenayat: Methodology, Writing - review & editing. Mitra Rezaei: Writing - review & editing. Sara Haseli: Writing - review & editing. Pooria Torkaman: Methodology, Writing - review & editing. Sahar Yousefian: Data curation, Software, Writing - review & editing. Alireza Dastan: Writing - review & editing. Hamidreza jamaati: Methodology, Writing - review & editing. Farzaneh Dastan: Conceptualization, Methodology, Writing - review & editing, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was approved and supported by Shahid Beheshti University of Medical Sciences.
References
- 1.Vabret N., et al. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen A.A., et al. Immunoglobulins in the treatment of COVID-19 infection: Proceed with caution! Clinical Immunology. 2020;216 doi: 10.1016/j.clim.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verity R., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan. China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guan W.-J., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.-C., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. International Journal of Antimicrobial Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu R., et al. An Update on Current Therapeutic Drugs Treating COVID-19. Current pharmacology reports. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozkan G., et al. How does colistin-induced nephropathy develop and can it be treated? Antimicrobial agents and chemotherapy. 2013;57(8):3463–3469. doi: 10.1128/AAC.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dastan, F., et al., Promising effects of tocilizumab in COVID-19: A non-controlled, prospective clinical trial. International immunopharmacology, 2020. 88: p. 106869-106869. [DOI] [PMC free article] [PubMed]
- 11.Chen G., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. The Journal of clinical investigation. 2020;130(5) doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fakharian A., et al. Successful Management of COVID-19 With Adalimumab in a Post-Coronary Artery Bypass Graft Surgery Patient. J Cardiothorac Vasc Anesth. 2021 doi: 10.1053/j.jvca.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmann M., et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. The Lancet. 2020;395(10234):1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collange O., et al. Coronavirus Disease 2019: Associated Multiple Organ Damage. Open Forum Infect Dis. 2020;7(7):p. ofaa249. doi: 10.1093/ofid/ofaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solun B., Shoenfeld Y. Inhibition of metalloproteinases in therapy for severe lung injury due to COVID-19. Med Drug Discov. 2020;7 doi: 10.1016/j.medidd.2020.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden L.R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito C.A., et al. COVID-19 in patients with rheumatological diseases treated with anti-TNF. Annals of the rheumatic diseases. 2020 doi: 10.1136/annrheumdis-2020-218171. [DOI] [PubMed] [Google Scholar]
- 18.Tursi A., et al. COVID-19 infection in Crohn’s disease under treatment with adalimumab. Gut. 2020;69(7):1364–1365. doi: 10.1136/gutjnl-2020-321240. [DOI] [PubMed] [Google Scholar]
- 19.Conti A., et al. Evolution of COVID-19 infection in 4 psoriatic patients treated with biological drugs. Journal of the European Academy of Dermatology and Venereology. 2020 doi: 10.1111/jdv.16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tufan A., Avanoglu Guler A., Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI-1):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tursi, A., L.M. Vetrone, and A. Papa, Anti-TNF-α Agents in Inflammatory Bowel Disease and Course of COVID-19. Inflammatory Bowel Diseases, 2020. 26(7): p. e73-e73. [DOI] [PMC free article] [PubMed]
- 22.Chen, X.Y., B.X. Yan, and X.Y. Man, TNFα inhibitor may be effective for severe COVID-19: learning from toxic epidermal necrolysis. Ther Adv Respir Dis, 2020. 14: p. 1753466620926800. [DOI] [PMC free article] [PubMed]
- 23.Del Valle D.M., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai C., et al. Updated guidance on the management of COVID-19: from an American Thoracic Society/European Respiratory Society coordinated International Task Force (29 July 2020) Eur Respir Rev. 2020;29(157) doi: 10.1183/16000617.0287-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]