Abstract
In Italy, the COVID-19 vaccination campaign started in December 2020 with the vaccination of healthcare workers (HCW). To analyse the real-life impact that vaccination is having on this population group, we measured the association between week of diagnosis and HCW status using log-binomial regression. By the week 22–28 March, we observed a 74% reduction (PPR 0.26; 95% CI 0.22–0.29) in the proportion of cases reported as HCW and 81% reduction in the proportion of symptomatic cases reported as HCW, compared with the week with the lowest proportion of cases among HCWs prior to the vaccination campaign (31 August-7 September). The reduction, both in relative and absolute terms, of COVID-19 cases in HCWs that started around 30 days after the start of the vaccination campaign suggest that COVID-19 vaccines are being effective in preventing infection in this group.
Keywords: COVID-19, Vaccines, Healthcare workers, Pandemic
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic continues to affect European countries despite the wide range of interventions implemented since early 2020. One of the most affected countries has been Italy with 3.4 million people diagnosed with SARS-CoV-2 infection and over 106,000 associated deaths recorded up to 28 March 2021 [1]. By March 2021, four vaccines have been approved by the European Medicines Agency (EMA) and licensed for use in Italy: BNT162b2 (BioNTech-Pfizer) [2], mRNA-1273 (Moderna) [3], Oxford–AstraZeneca [4] and Ad26.COV2.S (Johnson & Johnson) [5]. In the European Union (EU), procurement of vaccines for COVID-19 follows a “centralised approach” and is being managed by the European Commission [6], [7], [8]. By 28 March 2021, Italy had received 11,197,915 vaccine doses (68.5% BioNTech-Pfizer, 24.2% Oxford–AstraZeneca and 7.3% Moderna). A two-dose vaccination strategy is being used, in accordance with the summary of product characteristics of the specific vaccines, which recommends an interval of 21 days for the BioNTech-Pfizer vaccine, 28 days for Moderna and 10–12 weeks for Oxford-AstraZeneca. The Italian vaccination campaign started on 27 December 2020 and planned to vaccinate approximately 1.8 million HCWs and 570,287 residents of long-term care facilities (LTCF) in the first phase, followed by persons over 80 years of age [9]. Although efficacy of COVID-19 vaccines against symptomatic disease has been proven in clinical trials, there is a need to evaluate the effectiveness of vaccination campaigns in real-life settings. We describe the short-term impact of the Italian vaccination campaign on COVID-19 diagnosis in health care workers (HCW).
2. Methods
To describe the impact of vaccination on SARS-CoV-2 infections among HCWs, we analysed data on vaccine doses retrieved from the national government’s open data directory [10], and weekly data on SARS-CoV-2 infections among HCW since 31 August 2020 from the Italian national case-based COVID-19 surveillance system [11]. This system collects information on all RT-PCR confirmed cases of SARS-CoV-2 infection and, since 15 January 2021, also on cases confirmed through rapid antigen tests (RAT). The analysis was restricted to cases aged 20–65 years old.
We evaluated the association between week of diagnosis and HCW status using log-binomial regression and adjusting for sex, age group (20–34, 35–49, and 50–65 years), region of diagnosis, and regional hospitalisation rate in the previous week. The strength of the associations is described using prevalence proportion ratios (PPR) and their 95% confidence intervals (CI). Because HCWs are tested for SARS-CoV-2 infection more often than non-HCWs, we also carried the analysis restricting it to symptomatic cases.
HCW status (yes/no) is routinely collected from cases reported to the surveillance system for COVID-19. However, this information was missing in 32.7% out of the 2,149,709 cases included. We treated these cases as non-HCW, but we conducted a sensitivity analysis restricted to cases diagnosed in five regions (accounting for 50.5% of the total cases) where the proportion of missing information on HCW status was less than 2% (Supplementary Material 1). Data on COVID-19 hospital bed occupancy were retrieved from the Italian Ministry of Health (9).
3. Results
From 27 December 2020 to 28 March 2021, 2.98 million vaccine doses were administered to HCWs, 93.3% of which BioNTech-Pfizer vaccines, 4.4% Oxford-AstraZeneca and 2.2% Moderna. The cumulative weekly number of doses administered to HCWs increased during the first weeks of the vaccination campaign, reaching an average of 60,585 doses per day in the week from 5 to 11 January 2021 (Table 1 ).
Table 1.
Trends in the number of reported COVID-19 cases -total and among healthcare workers (HCW), COVID-19 hospitalisation rates, and vaccine doses administered to HCW, by week. Italy, 1 September 2020 – 28 March 2021.
| Week of diagnosis | Number of reported COVID-19 cases |
Number of reported symptomatic COVID-19 cases |
COVID-19 Hospitalisation rate (× 100,000 residents)* | Cumulative number of doses of COVID-19 vaccine administered to HCW | ||||
|---|---|---|---|---|---|---|---|---|
| Total | HCW | HCW (%) | Total | HCW | HCW (%) | |||
| 31 Aug − 6 Sep 2020 | 7055 | 245 | 3.5 | 2841 | 110 | 3.9 | 14.1 | 0 |
| 7–13 Sep 2020 | 7198 | 269 | 3.7 | 3097 | 142 | 4.6 | 17.4 | 0 |
| 14–20 Sep 2020 | 7606 | 310 | 4.1 | 3626 | 155 | 4.3 | 18.2 | 0 |
| 21–27 Sep 2020 | 8786 | 390 | 4.4 | 3898 | 154 | 4.0 | 22.2 | 0 |
| 28 Sep − 4 Oct 2020 | 12,151 | 623 | 5.1 | 5473 | 310 | 5.7 | 27.6 | 0 |
| 5–11 Oct 2020 | 25,144 | 1282 | 5.1 | 11,673 | 609 | 5.2 | 34.9 | 0 |
| 12–18 Oct 2020 | 49,860 | 2830 | 5.7 | 21,362 | 1386 | 6.5 | 55.2 | 0 |
| 19–25 Oct 2020 | 95,615 | 5249 | 5.5 | 38,234 | 2413 | 6.3 | 104.6 | 0 |
| 26 Oct − 1 Nov 2020 | 138,803 | 7382 | 5.3 | 53,796 | 2950 | 5.5 | 214.4 | 0 |
| 2–8 Nov 2020 | 165,633 | 8374 | 5.1 | 65,057 | 3129 | 4.8 | 407.6 | 0 |
| 9–15 Nov 2020 | 162,783 | 8115 | 5.0 | 68,202 | 3292 | 4.8 | 645.2 | 0 |
| 16–22 Nov 2020 | 142,136 | 7081 | 5.0 | 62,296 | 3221 | 5.2 | 835.1 | 0 |
| 23–29 Nov 2020 | 104,917 | 6116 | 5.8 | 45,756 | 2887 | 6.3 | 900.1 | 0 |
| 30 Nov − 6 Dec 2020 | 82,836 | 5941 | 7.2 | 39,234 | 3022 | 7.7 | 870.4 | 0 |
| 7–13 Dec 2020 | 69,315 | 5275 | 7.6 | 35,730 | 2944 | 8.2 | 786.4 | 0 |
| 14–20 Dec 2020 | 68,881 | 4946 | 7.2 | 36,416 | 2718 | 7.5 | 705.9 | 0 |
| 21–27 Dec 2020 | 60,286 | 4247 | 7.0 | 32,056 | 2448 | 7.6 | 626.1 | 6298 |
| 28 Dec 2020–3 Jan 2021 | 76,165 | 4460 | 5.9 | 41,030 | 2593 | 6.3 | 550.2 | 108,909 |
| 4–10 Jan 2021 | 78,892 | 4325 | 5.5 | 43,008 | 2529 | 5.9 | 501.2 | 544,734 |
| 11–17 Jan 2021 | 58,887 | 3408 | 5.8 | 30,597 | 1954 | 6.4 | 478.2 | 889,688 |
| 18–24 Jan 2021 | 52,894 | 2908 | 5.5 | 27,672 | 1655 | 6.0 | 444.5 | 1,049,841 |
| 25–31 Jan 2021 | 51,800 | 2088 | 4.0 | 27,719 | 1086 | 3.9 | 417.5 | 1,488,085 |
| 1–7 Feb 2021 | 52,052 | 1562 | 3.0 | 27,859 | 802 | 2.9 | 386.0 | 1,876,472 |
| 8–14 Feb 2021 | 52,525 | 1093 | 2.1 | 28,693 | 574 | 2.0 | 359.8 | 2,098,363 |
| 15–21 Feb 2021 | 57,241 | 848 | 1.5 | 32,171 | 460 | 1.4 | 342.9 | 2,261,837 |
| 22–28 Feb 2021 | 78,564 | 1088 | 1.4 | 43,714 | 530 | 1.2 | 327.0 | 2,385,642 |
| 1–7 Mar 2021 | 92,258 | 1076 | 1.2 | 52,001 | 505 | 1.0 | 339.8 | 2,580,392 |
| 8–14 Mar 20 | 101,360 | 1179 | 1.2 | 58,173 | 554 | 1.0 | 385.0 | 2,761,142 |
| 15–21 Mar 2021 | 96,907 | 1138 | 1.2 | 55,772 | 559 | 1.0 | 463.8 | 2,873,534 |
| 22 Mar − 28 Mar 2021 | 91,159 | 1089 | 1.2 | 47,169 | 517 | 1.1 | 583.5 | 2,977,506 |
Hospitalisation rate is presented with one week lag and refers to the previous week as indicated in the first column. This is to account for the delay between infection and diagnosis in HCW.
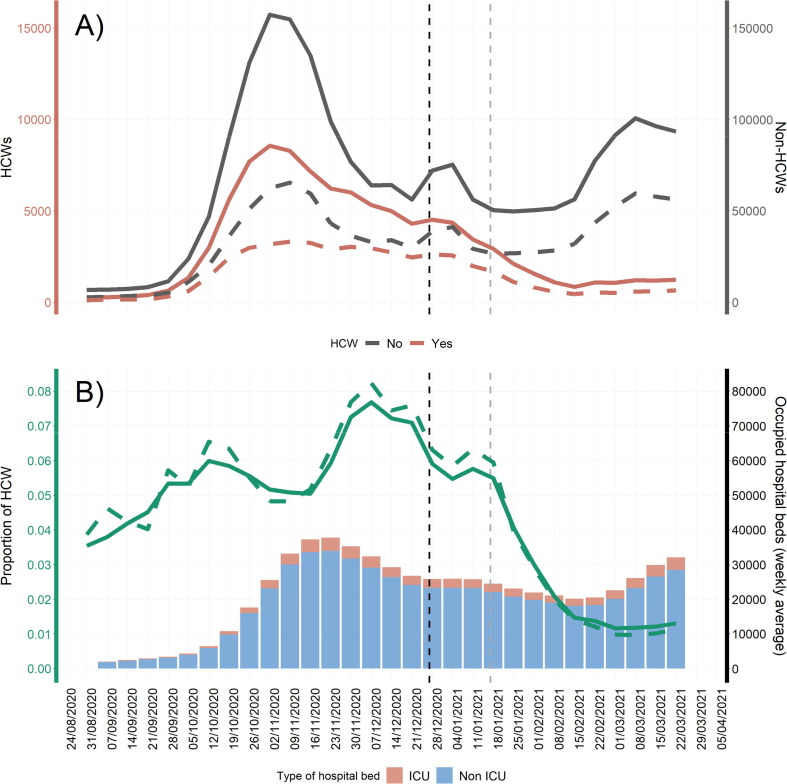
The total number of cases, as well as the number of cases reported as HCWs, increased from the first weeks of September until the first week of November, and decreased after that with a similar temporal trend until January 2021. However, the two curves started to diverge at the end of January, approximately 30 days after the start of the vaccination campaign, with the number of cases reported as HCWs decreasing and non-HCWs remaining stable until mid-February. At this time point, a new wave of COVID-19 affected Italy, with cases of SARS-CoV-2 infection increasing in non-HCWs, but remaining stable in HCWs. The same pattern was observed when only symptomatic cases were taken into account (Fig. 1 A). Equally, similar trends were also observed for ICU admission and mortality in HCWs compared with non-HCWs (Supplementary Material 2).
Fig. 1.
Trends in the weekly number of COVID-19 cases (solid line) and symptomatic COVID-19 cases (dashed line) reported as healthcare workers (HCW) and not HCWs (A). Trend in the proportion of total cases (solid line) and symptomatic cases (dashed line) reported as HCWs with the average daily occupation of hospital beds per week, in Italy from 1 September to 28th of March (B). Vertical black dotted line: Start of the COVID-19 vaccine campaign (27th of december 2020). Vertical grey dotted line: Start of administration of second doses of COVID-19 vaccines (17th of January 2021).
Similarly, the proportion of cases reported as HCWs followed a similar trend to the hospitalization rate from COVID-19, with a time lag of approximately one week. The proportion of cases reported as HCWs peaked at weeks 7 to 13 December 2020 (7.6%). However, after the start of the vaccination campaign, the proportion of cases and symptomatic cases reported as HCWs decreased faster than hospitalization rates (Fig. 1B).
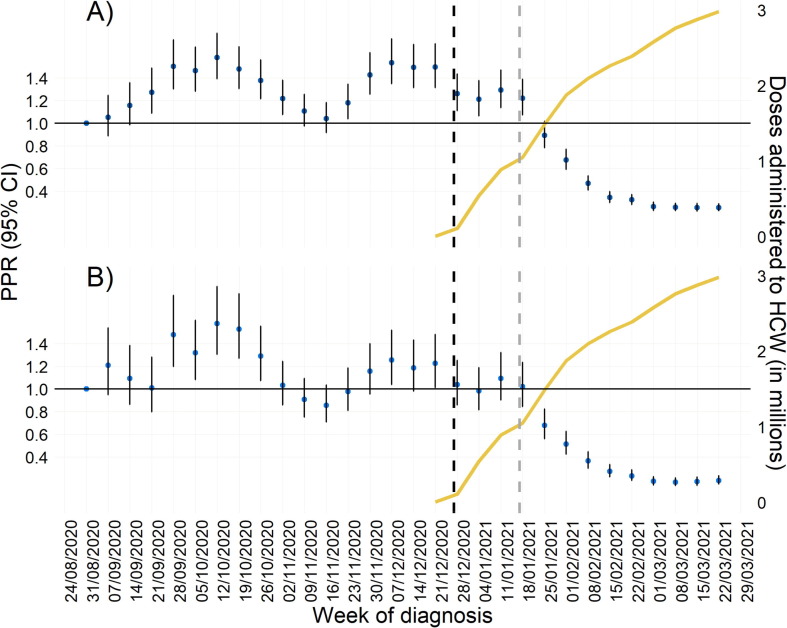
These changes in trends were confirmed with results from the multivariable analysis. After adjusting, the proportion of HCWs among cases fluctuated, but no consistent trend was observed until the week of 18 to 24 January 2021. From this date onwards, the proportion of cases reported as HCW decreased consistently and significantly in the following nine weeks (Fig. 2 ), with an estimated reduction of 74% (PPR = 0.26, 95% CI: 0.22–0.29) when considering all cases and of 81% when considering only symptomatic cases (PPR = 0.19, 95% CI 0.16–0.24) in the week from the 22 to 28 of March compared to the week from the 31 August to 7 of September (i.e. the week showing the lowest proportion of HCWs among diagnosed cases during the period prior the vaccination campaign).
Fig. 2.
Adjusted prevalence proportion ratio (PPR) of HCWs who tested positive for SARS-CoV-2 infection among all cases (A) and among symptomatic cases (B) by week, with 95% Confidence Intervals (CI); and cumulative number of COVID-19 vaccine doses administered to HCW in Italy (yellow line). 1 September 2020 to 28th of March 2021. The reference was set to the week of the 03–09 November. PPR was adjusted for sex, age group (i.e., 20–34, 35–49, and 50–65 years), region of diagnosis, and regional hospitalisation rate in the previous week. Vertical black dotted line: Start of administration of the first doses of the COVID-19 vaccine (27th of december 2020). Vertical grey dotted line: Start of administration of the second doses of the COVID-19 vaccine (17th of January 2021). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Our findings suggest a reduction in the number of SARS-CoV-2 infection among HCWs in Italy, both in absolute terms and in relative terms as a proportion of total and symptomatic cases, following the start of the COVID-19 vaccination campaign in this population group.
Our results coincide with those reported in one medical centre of the US, where they observed a drastic reduction in the percentage of positive tests among vaccinated HCWs [12]. In Israel, where 45.3% of the population had received at least one dose of the vaccine as of 06 February 2021, a 49% reduction in the number of cases and a 36% reduction in hospitalizations from COVID-19 has been reported one month and a half after the start of the vaccination campaign [13]. Similarly, in the UK, rates of infection and hospitalizations are falling faster among groups who were offered first the vaccine [14]. Both countries have found high estimates of vaccine effectiveness against symptomatic infection seven days after the second dose: 86% (95 %CI 76–97) in the UK [15]; and 94% (95% CI 87–98) in Israel [16].
Although vaccine effectiveness has been reported as early as within the first 20 days after the first dose [16], we only saw a reduction in the proportion of cases reported as HCWs in the week of 25–31 January 2021, around 30 days after the start of the vaccination campaign. This is longer than the 21-day period from the start of the campaign that passed in Israel before the effect of vaccination started to be observed. One likely explanation is the different speed at which vaccination campaigns were rolled-out in both countries, with Israel being able to achieve higher vaccine coverage in a shorter period of time.
Our analysis has several limitations. Firstly, there could be differences among regions on which professional categories fall under the definition of HCW, both in terms of reporting to the epidemiological surveillance and to the national monitoring of the vaccination campaign. This heterogeneity meant that we did not have a reliable indicator to compare the incidence of infection of this group with the rest of the population over time. Secondly, data openly available on vaccinations does not allow to disaggregate both by category (e.g. healthcare workers) and by type of dose (first or second), therefore, from the date when second doses started to be administered (17 January 2021) we cannot know how many healthcare workers had each type of dose. Finally, these results show the impact of the BioNTech-Pfizer alone and we cannot assess the impact of the other two vaccines approved for use in the EU, given that they accounted for less than 3% of the doses administered to HCWs.
5. Conclusions
This early analysis shows that the number and the proportion of cases reported as healthcare workers started to drop significantly on the week starting on the 25th January 2021, 30 days after the start of the COVID-19 vaccination campaign. Since then, a consistent reduction in such proportion has been observed, suggesting that vaccination is being effective in reducing infections in this group. These results could be used by public health authorities to promote adhesion to the vaccination campaign in areas with relative low coverage among healthcare workers.
Ethical statement
This study was conducted using three sources of data. Data on covid-19 vaccinations and on bed occupancy from COVID-19 are publicly available. Data from the Italian national integrated COVID-19 surveillance routinely collected and analysed within the mandate of the Italian National Institute of Health. The scientific dissemination of COVID-19 surveillance data was authorised by the Italian Presidency of the Council of Ministers (Ordinance n. 640, 27/02/2020) (10).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
AMU, MDM, XA, PP and MF designed the paper and planned the analysis. XA, MS, DP and AB obtained the data and carried out the analysis, coordinated by MF.
AMU, MDM, FDA, AF, FR, MCR and MFV wrote the manuscript, which was then revised by PP and MF. All authors read and approved the last version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank the COVID-19 working group from the ISS and the Regional Authorities:
Stefania Bellino, Stefano Boros, Ornella Punzo, Alessandra Ciervo, Corrado Di Benedetto, Stefania Giannitelli, Paola Stefanelli, Marco Tallon, Roberta Urciuoli (Istituto Superiore di Sanità);
Regional representatives: Antonia Petrucci (Abruzzo), Michele La Bianca (Basilicata), Anna Domenica Mignuoli (Calabria), Pietro Buono (Campania), Erika Massimiliani (Emilia-Romagna), Fabio Barbone (Friuli Venezia Giulia), Francesco Vairo (Lazio), Camilla Sticchi (Liguria), Danilo Cereda (Lombardia), Lucia di Furia (Marche), Francesco Sforza (Molise), Pierpaolo Bertoli (P.A. Bolzano), Pier Paolo Benetollo (P.A. Trento), Chiara Pasqualini (Piemonte), Lucia Bisceglia (Puglia), Maria Antonietta Palmas (Sardegna), Salvatore Scondotto (Sicilia), Lucia Pecori (Toscana), Enrica Ricci (Umbria), Mauro Ruffier (Valle d’Aosta), Filippo Da Re (Veneto).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.07.003.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.EpiCentro. COVID-19 integrated surveillance data in Italy, https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-dashboard (accessed 7 April 2021).
- 2.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoll M.D., Wonodi C. Oxford–AstraZeneca COVID-19 vaccine efficacy. The Lancet. 2021;397:72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J, Le Gars M, Shukarev G, et al. Interim Results of a Phase 1–2a Trial of Ad26.COV2.S Covid-19 Vaccine. N Engl J Med. Epub ahead of print 13 January 2021. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed]
- 6.PINHO AC. EMA recommends first COVID-19 vaccine for authorisation in the EU. European Medicines Agency, https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (2020, accessed 7 April 2021).
- 7.CZARSKA-THORLEY D. COVID-19 Vaccine Moderna. European Medicines Agency, https://www.ema.europa.eu/en/medicines/human/EPAR/covid-19-vaccine-moderna (2021, accessed 7 April 2021).
- 8.PINHO AC. EMA recommends COVID-19 Vaccine AstraZeneca for authorisation in the EU. European Medicines Agency, https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-astrazeneca-authorisation-eu (2021, accessed 7 April 2021).
- 9.Ministero della Salute. Piano strategico nazionale dei vaccini per la prevenzione delle infezioni da SARS-CoV-2, https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=78657&parte=1%20&serie=null (accessed 7 April 2021).
- 10.Open Data su consegna e somministrazione dei vaccini anti COVID-19 in Italia. Developers Italia, https://github.com/italia/covid19-opendata-vaccini (2021, accessed 7 April 2021).
- 11.Ocdpc n. 640 del 27 febbraio 2020. Ulteriori interventi urgenti di protezione civile in relazione all’emergenza relativa al rischio sanitario connesso all’insorgenza di patologie derivanti da agenti virali trasmissibili. - Normativa. Dipartimento della Protezione Civile, http://www.protezionecivile.gov.it/amministrazione-trasparente/provvedimenti/dettaglio/-/asset_publisher/default/content/ocdpc-n-640-del-27-febbraio-2020-ulteriori-interventi-urgenti-di-protezione-civile-in-relazione-all-emergenza-relativa-al-rischio-sanitario-connesso-a (accessed 7 April 2021).
- 12.Daniel W, Nivet M, Warner J, et al. Early Evidence of the Effect of SARS-CoV-2 Vaccine at One Medical Center. N Engl J Med. Epub ahead of print 7 April 2021. doi: 10.1056/NEJMc2102153. [DOI] [PMC free article] [PubMed]
- 13.Rossman H, Shilo S, Meir T, et al. Patterns of COVID-19 pandemic dynamics following deployment of a broad national immunization program. medRxiv 2021; 2021.02.08.21251325.
- 14.PHE. PHE monitoring of the early impact and effectiveness of COVID-19 vaccination in England. PHE, https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/963532/COVID-19_vaccine_effectiveness_surveillance_report_February_2021_FINAL.pdf (2021, accessed 7 April 2021).
- 15.Hall VJ, Foulkes S, Saei A, et al. Effectiveness of BNT162b2 mRNA Vaccine Against Infection and COVID-19 Vaccine Coverage in Healthcare Workers in England, Multicentre Prospective Cohort Study (the SIREN Study). SSRN Scholarly Paper ID 3790399, Rochester, NY: Social Science Research Network, https://papers.ssrn.com/abstract=3790399 (22 February 2021, accessed 7 April 2021).
- 16.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. Epub ahead of print 7 April 2021. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.