Abstract
Our aging population is growing and developing treatments for age-related diseases such as Alzheimer’s and Parkinson’s disease has taken on an increasing urgency and is accompanied by high public awareness. The already high and rising incidence of acute kidney injury (AKI) in the elderly, however, has received relatively little attention despite the potentially fatal outcomes associated with an episode of AKI in this age group. When discussing AKI and aging, one should consider two aspects: first, elderly patients have an increased susceptibility to an AKI episode, and second, they have decreased kidney repair after AKI given the high incidence of progression to chronic kidney disease (CKD). It is unclear if the same factors that drive the increased susceptibility to AKI could be playing a role in the decreased repair capacity or if they are totally different and unrelated. This review will examine current knowledge on the risk factors for the increased susceptibility to AKI in the elderly and will also explore potential aspects that might contribute to a decreased kidney repair response in this age group.
Keywords: Acute kidney injury, aging, senescence, renal aging, chronic kidney disease, elderly
Introduction
The world population is estimated to be 7.3 billion with 9% being age 65 and older, and this population segment is projected to increase to 17% by 2050 [1] with this trend occurring across all continents. In North America it is estimated that 21% of the total population will be 65 and older. Aging is thought to be a factor in the decline in kidney function seen in older patients. The macro- and micro-structural changes that occur in renal aging have been well described [2, 3]; however, the exact molecular mechanisms that drive these changes are not completely understood, but over the last decade there has been progress in understanding this process at the molecular level [4]. Clinical studies have shown that elderly patients are at higher risk of acute kidney injury (AKI) and they have poorer outcomes after an episode of AKI [5–8]. It is unknown what the factors are that lead to a decreased repair capacity in the aging kidney after injury, but animal studies have started to shed light on possible pathways that could be playing a role [9–11]. This review will discuss current knowledge on the effects of aging in the susceptibility and response to AKI.
Prevalence of AKI
The incidence of AKI is variable, in part due to different criteria defining AKI and the lack of a “gold standard” for its identification. AKI has been estimated to be 21% in low to middle income countries and between 3.0% and 18.3% in high-income countries [12]. The 2018 CDC report looking at hospitalization trends for AKI in the US from 2000 to 2014 indicates that the rate of AKI hospitalizations increased, going from 3.5 to 11.7 per 1000 persons, which is a 230% increase [13].
In the ICU setting it is estimated that one-third to two-thirds of patients experience AKI with 10–15% of patients requiring kidney replacement therapy (KRT) and within this group the mortality is 50% [12]. Another multinational study reported that over half of ICU patients encounter an AKI episode [14]. Compared to studies 25 years ago, current studies indicate that the ICU patient population is older [15, 16] and has more comorbid conditions, which not only increase their risk of developing AKI, but also lead to poorer outcomes. These troubling statistics are not unexpected given the rising elderly population worldwide. Feest et al. showed that there is a 3- to 8-fold, age-dependent increase in the frequency of community-acquired AKI in patients older than 60 years [17]. Data from the United States Renal Data System (USRDS) 2018 report indicate that the in-hospital mortality rate was 8.2% among Medicare patients aged older than 66 who had a first AKI hospitalization; in stark contrast, the mortality rate was 1.8% in non-AKI hospitalizations. When patients who were discharged to hospice were included in the mortality rate, the numbers show an striking increase of 13.2% vs. 3.8% for AKI vs. non-AKI hospitalizations in this age group [18]. These statistics stress the significant risk that elderly patients can encounter during AKI. Furthermore, elderly patients with AKI requiring dialysis have been reported to have mortality rates ranging from 31 to 80% [19]. Of those who survive an AKI episode, it is estimated that 31.3% did not recover kidney function compared to 26% of younger patients. These patients eventually progress to develop chronic kidney disease (CKD) and are at risk of kidney failure [19, 20]. Similar statistics were reported in the USRDS 2018 report, where Medicare patients aged 66 and older who were hospitalized for AKI had a 36% cumulative probability of a recurrent AKI hospitalization within one year and 30.8% were diagnosed as having CKD in the year following an AKI hospitalization [18]. The study by Ishani et al., found that Medicare beneficiaries aged 67 and older with no history of CKD and an episode of AKI had a 54% risk of developing kidney failure (hazard ratio 13.00) and this risk rose to 216% (hazard ratio 41.19) for patients with underlying CKD [5].
In the case of children with progeria who experience premature aging, intriguingly, they have been reported to have normal kidney function based on clinical parameters such as serum creatinine [21]; however, the study by Delahunt et al., suggests that there are some histological changes suggestive of renal aging [22]. Their study described the autopsy findings in the kidneys of two patients with progeria. One patient was an 11-year-old male and the other patient a 20-year-old female. The kidney from the young patient did not have any features suggestive of renal aging; however, the older female kidney had focal glomerulosclerosis and tubular atrophy. They also performed a review of published autopsy cases and noted that progeria patients who survived into the teenage years and beyond commonly had abnormal renal histological features [22]. Recently, a group generated the first large animal model for progeria, a Yucatan minipig carrying the heterozygous LMNA mutation. This model recapitulates all the main clinical manifestations of progeria. In their characterization of the model, they described that the vast majority of the minipigs showed dilation of the cortical tubules and different degrees of glomerulosclerosis [23]. Therefore, there is suggestion that progeria patients over time may develop features of renal aging; although perhaps not detectable by clinical parameters such as creatinine measurement. With these observations in mind, it is possible that they might also be at risk for AKI similarly to our elderly population.
Risk factors for increased susceptibility to AKI in the elderly
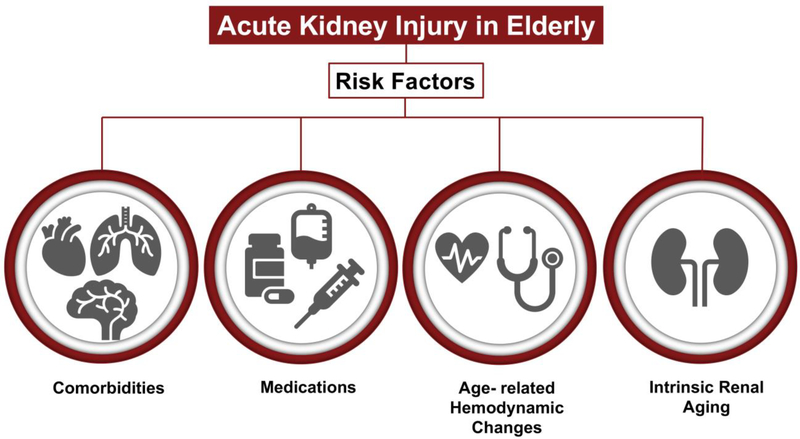
As described above, the prevalence of AKI in elderly patients is high compared to younger adults and next I will describe some of the risk factors that can predispose elderly patients to AKI (Figure 1 and Table 1).
Figure 1. Risk factors for increased susceptibility to AKI in the elderly.
Epidemiological studies have highlighted the increased incidence of AKI in elderly patients and their poor outcomes. Comorbidities, medications, age-related hemodynamic changes and intrinsic aging of the kidney are some of the possible risk factors for the increased susceptibility of elderly patients to AKI.
Table 1.
Risk factors for increased susceptibility to AKI in the elderly
| Risk Factor | Summary | References |
|---|---|---|
| Comorbidities | CKD prevalence is highest in the elderly, ranging between 38–44% | [24, 25] |
| Age-related hemodynamic changes | There is progressive increase in blood pressure with aging | [26–33] |
| Medications | Nephrotoxins account for a large percentage of AKI cases in the elderly | [34–40] |
| Intrinsic kidney changes with aging | Glomerulosclerosis: one of the main histological changes driving CKD progression | [43–47] |
| Tubular atrophy: decreased sodium reabsorption and urine concentrating capacity increases the risk of AKI | [48–55] | |
| Reduced GFR: secondary to reduced plasma flow and histological changes | [56–61] |
Comorbidities
As lifespan increases so does the incidence of comorbid conditions such as diabetes mellitus, hypertension, CKD, atherosclerosis, systolic and/or diastolic dysfunction, prostatic disease, and malignancy. Diabetes mellitus and hypertension can lead to the development of CKD, which in itself can be a risk factor for AKI. Data from the CDC suggest that CKD prevalence is highest in the elderly, with a 38% prevalence rate in those older than 65 years [24]. In a study by Stevens et al. looking at the CKD prevalence in elderly patients in both NHANES and the Kidney Early Evaluation Program (KEEP), they found the CKD prevalence to be 44% in both cohorts with about 69–77% patients having stage 3 and approximately 5% having stage 4 or 5 [25].
Age-related hemodynamic changes
Previous studies have pointed out the importance of renal hemodynamics during essential hypertension [26]. They established that hypertensive patients exhibited increased renal vascular resistance and decreased renal blood flow preferentially in the outer cortex. Therefore, hemodynamic changes can lead to kidney damage in themselves due to the decreased renal blood flow and can also lead to magnification of an ischemic event or an ineffective repair response.
The Framingham and NHANES studies demonstrated that there is a progressive increase in blood pressure with aging and systolic blood pressure rises until the eighth or ninth decades of life [27–29]. Diastolic blood pressure on the other hand remains constant or declines after the fifth or sixth decade, leading to an increase in pulse pressure [28]. The increasing pulse pressure and decreasing diastolic blood pressure are considered surrogate measurements for large artery stiffness that becomes a dominant hemodynamic factor in both normotensive and hypertensive subjects [28]. Large artery stiffness can lead to renal microvascular damage given that the kidney has the highest flow rate and lowest vascular resistance of any large organ; therefore, making it susceptible to trauma [30]. A study proposed that increased pulsatile pressure from aortic stiffening is transmitted to the peripheral glomeruli and causes excessive tensile stress leading to glomerular hypertension and sclerosis [31]. These kidney structural changes can set the stage for the development of CKD.
Isolated systolic hypertension can lead to left ventricular hypertrophy and diastolic dysfunction. Management of the latter sometimes requires use of diuretics, which can predispose to AKI due to volume depletion. Furthermore, neuroendocrine changes due to aging may lead to orthostatic hypotension, large blood pressure variability and reduced heart rate variability [32], which can cause renal hypoperfusion and risk for AKI. It has also been proposed that a disbalance between vasoconstrictor and vasodilator factors leads to increased kidney susceptibility to toxic substances [33].
Medications
As described previously, with advanced age there is increased development of chronic conditions such as hypertension, diabetes mellitus, atherosclerosis, and heart failure; these in turn will also lead to an increase in medication use. Polypharmacy is a recognized problem in the elderly population, and this poses an increased risk for adverse events [34]. It is estimated that 20% of the AKI episodes are due to nephrotoxic drugs and specifically in the elderly population is estimated to be as high as 66% [35].
Cardiovascular drugs are one of the categories that can potentially affect kidney function either by causing volume depletion (diuretics), or by interaction with other drugs (diuretics + ACE inhibitors + NSAIDs). Furthermore, with increasing age there is reduced β-adrenoceptor responsiveness and reduced baroreflex function, which can increase the sensitivity to hypotension from diuretics and vasodilators [36], and thus predispose to AKI.
Non-steroidal anti-inflammatory drugs (NSAIDs) are often prescribed in the elderly population during management of osteoarthritis. One study reported that the prevalence of NSAID use in patients over 65 years of age is as high as 96% [37]. NSAID nephrotoxicity can be further exacerbated if there is concomitant use of ACE inhibitors and/or diuretics, which can be often the case as they are standard medications in the management of hypertension and heart failure, which are common comorbidities in elderly patients.
Contrast-induced nephropathy (CIN) is considered the third most common cause of AKI in hospitalized patients [38]. One group performed a prospective observational study over the course of one year and looked at the incidence of CIN in critically ill older patients [39]. They enrolled 26 critically ill patients with stable kidney function who needed computed tomography with intravenous contrast and categorized them in two groups: < 65 years old (n = 13) and > 65 years old (n = 13) with no statistically significant differences between the groups. They found that in the older group, 38.5% fulfilled the criteria for CIN (25% or greater increase from baseline serum creatinine or an absolute increase by 0.5 mg/dL until the 5th day after the infusion of contrast agent) while there were no CIN cases in the younger group. Therefore, elderly patients are at increased risk of nephrotoxicity from contrast, and standard protective measures such hydration and avoidance of nephrotoxic drugs prior to and post contrast exposure may help decrease this risk. A recently published study looked at the incidence of AKI among older adults undergoing coronary angiography for acute myocardial infarction (AMI) that were part of the SILVER-AMI cohort study [40]. SILVER-AMI was a multi-center study in the US that evaluated risk factors for hospital readmission, morbidity and mortality among patients age 75 or older hospitalized for AMI. They evaluated 2212 patients (mean age 81.3 years) who underwent coronary angiography and found that 421 developed AKI, which is nearly 1 in 5 participants. They also corroborated prior studies and found that AKI was associated with increased mortality and hospital readmission at 6 months. It is worth pointing out this study found that risk factors for AKI, namely heart failure, BMI > 30, low hemoglobin and non-white race, were consistent with previous studies of younger patients; however, none of the aging-related conditions seem to be predictive of risk of AKI, leading the authors to suggest that geriatric conditions mediate their influence through other risk factors.
Intrinsic kidney changes with aging
The kidneys decrease in size during aging with 10% decrease in kidney mass per decade after the age of 50 [41]. The volume loss is thought to be due primarily to cortical tissue loss secondary to glomerulosclerosis; however, the medulla remains relatively unchanged [42]. Other histological changes include tubular atrophy with dilation, interstitial fibrosis, and infiltration of mononuclear cells [2, 3].
Glomerulosclerosis:
Glomerulosclerosis is considered one of the main histological changes driving progression of CKD along with interstitial fibrosis; however, it has also been described as part of the normal aging process [43]. Podocyte injury and loss has been recognized as a key driver of glomerulosclerosis [44] and a previous study suggested that the glomerulosclerosis of aging is secondary to early podocyte damage, though the mechanism is unknown [45]. Atherosclerosis has also been implicated as a cause for the development of glomerulosclerosis. Kasiske looked at the renal histology of 57 autopsy patients with mild systemic atherosclerosis and compared them to sex- and aged-matched patients with moderated to severe atherosclerosis [46]. The study showed that there was a correlation between atherosclerosis and glomerulosclerosis: individuals with severe systemic atherosclerosis had a greater amount of intrarenal vascular disease. This finding along with age was closely associated with glomerulosclerosis. A more recent study using three-dimensional digital imaging looked at healthy aged kidneys and observed that small arterial changes were critical to the development of glomerulosclerosis and interstitial damage [47]. Therefore, it is possible that macro- and microvascular changes in arteries and arterioles lead to luminal occlusion and ischemia with the subsequent development of glomerulosclerosis. Furthermore, changes in blood vessel tone, which causes an attenuated vasodilator response, but enhanced vasoconstriction, might potentially cause kidney damage in the smaller vascular beds and lead to development of glomerulosclerosis [41]. Another factor that has been proposed to contribute to the development of glomerulosclerosis is large-artery stiffness, as described above. The pulsatile pressure and blood flow are transmitted to the glomeruli causing damage with development of albuminuria and reduced glomerular filtration rate [30, 31].
Tubular atrophy:
The decreased blood flow to the kidney cortex due to hemodynamic or vascular changes can potentially result in hypoperfusion in the peritubular capillary beds, leading to tubular cell death and consequently tubular atrophy. Furthermore, glomerulosclerosis can cause atrophy of the attached tubule since the glomerular capillary network is connected to the peritubular capillaries and damage upstream can affect the capillaries downstream [48]. A reduction in the density of peritubular capillaries, also known as peritubular capillary rarefaction, has been implicated as an inciting event in the development of tubular atrophy, due to decreased blood flow and concomitant development of hypoxia and tubular cell death [49, 50]. The aging tubular epithelium has decreased sodium reabsorption, potassium excretion, and urine concentrating capacity [51–54]. These changes can increase the susceptibility of elderly patients to AKI. For instance, elderly persons are not able to dilute or concentrate their urine and thus more prone to volume depletion and therefore are at risk of AKI. Furthermore, they have lower levels of serum renin and aldosterone, and thus their response to a hypovolemic state might be blunted leading to an even higher risk of kidney injury [55].
Reduced glomerular filtration rate:
Previous studies have shown that compared to young healthy subjects, healthy elderly patients have a reduced GFR even in the absence of underlying kidney disease [56]. These studies have also shown that elderly patients have a lower effective renal plasma flow (ERFP) and a higher filtration fraction [56], the latter possibly explained by the renal cortical loss and preservation of juxtamedullary nephrons with higher filtration fraction [57]. Furthermore, Fliser et al. noted that the decrease in ERFP and increase in filtration fraction is more pronounced in elderly patients with heart failure and hypertension [57]. It is unclear if these hemodynamic changes could predispose elderly patients to kidney injury or progression to CKD. Another study by Hollenberg et al. used the Xenon washout technique to characterize the effect of aging on the renal vasculature in potential transplant donors. They included 207 normal human subjects with ages ranging from 17 to 76 years. They observed a reduction in mean blood flow per unit mass with advancing age [58]. They suggested three possible factors that contribute to the reduced GFR. First, reduced RPF decreases cortical perfusion rate and thus limits filtration. Second, based on morphological data, age-related atrophy involves the kidney cortex more than the medulla and in turn glomeruli are especially affected. Third, vascular lesions primarily involve small arteries rather than arterioles, which results in a reduction of filtration pressure in large numbers of glomeruli. One could also speculate that the structural changes seen in renal aging, such as glomerulosclerosis and tubular atrophy, eventually translate to a decrease in GFR due to overall nephron loss. This functional loss can potentially leave the elderly with a reduced renal reserve in the event of an insult and therefore increased susceptibility to AKI and progression to CKD. It is important to highlight that studies looking at renal functional reserve (RFR), defined as the capacity of the kidney to increase GFR under certain physiological or pathological conditions, have shown that during aging there is a progressive decline in the RFR, without any major alteration in baseline GFR [59–61]. However, once the renal reserve is gone, a kidney insult will be much more noticeable as a rise in Cr, as there is no compensatory mechanism. This loss of RFR might account for the higher incidence of AKI noted in elderly patients.
Another interesting finding of the Hollenberg study is that elderly patients had a blunted vasodilator response to acetylcholine, but an intact vasoconstrictor response to angiotensin, and this has led to the suggestion that elderly patients may be more susceptible to AKI in a low perfusion state because of an attenuated vasodilatory response and an augmented vasoconstrictor response [55].
One aspect to consider when evaluating GFR in elderly patients is that they have reduced creatinine production due to reduced muscle mass and the serum creatinine may appear normal, but in fact there is reduced GFR [62]. If this is overlooked it may place elderly patients at risk for nephrotoxicity if medications are not dosed appropriately. This fact also makes it difficult to estimate GFR based on well-known formulas, as some formulas may overestimate GFR in the elderly [63]. A recent study tried to determine if other filtration makers such as Cystatin C and β2M could accurately estimate GFR in older adults with and without type 1 diabetes; however, they found that their performance was lower when compared to creatinine [64].
Factors that might contribute to decreased repair in the aging kidney
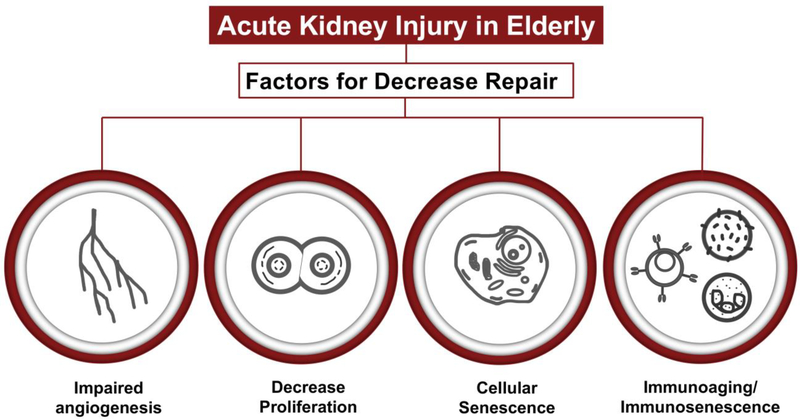
Currently there are ongoing research efforts trying to identify drivers that impair the regenerative response in the aging kidney after injury. Although progress has been made, it is still an underexplored area and most of what is known so far comes from preclinical studies and extrapolation from aging studies in other organs. Below I will describe some of the processes that could be contributing to decreased repair in the aging kidney (Figure 2 and Table 2).
Figure 2. Factors that might contribute to decreased repair in the aging kidney.
It is well-established from clinical studies that elderly patients are at higher risk of progression to CKD after an episode of AKI. This risk is likely due to decreased repair capacity after injury, although it is unclear what the drivers for this failed repair are. Possible mechanisms driving this failed repair include impaired angiogenesis, decreased proliferation, cellular senescence, and impaired immune response.
Table 2.
Factors that might contribute to decreased repair in the aging kidney
| Factors for decreased repair | Summary | References |
|---|---|---|
| Impaired angiogenesis | Preclinical and clinical studies have shown that there is an imbalance in pro-angiogenic factors in the aged kidney | [65–67] |
| Decreased proliferative capacity | In rodent studies, aged kidneys that sustain an injury have less tubular epithelium proliferation compared to young injured kidneys | [68–73] |
| Cellular senescence | Cells are permanently arrested and have a secretory phenotype that releases pro-inflammatory factors | [74–83] |
| Impaired immune response | Two states noted: chronic level of inflammation known as “inflammaging” and decreased immune function also known as “immunosenescence” | [84–85] |
Impaired angiogenesis
Kang et al. had previously described that the aging kidney in rats is characterized by both glomerular and peritubular capillary loss [65]. They observed a significant reduction in glomerular and peritubular capillary endothelial proliferation thus indicating that the aging kidney has an impaired angiogenic response. They studied if there was an imbalance in angiogenic factors and found that the aged kidney has decreased expression of vascular endothelial growth factor (VEGF, a driver of angiogenesis) in the outer medulla and increased renal Thrombospondin-1 expression (TSP-1, an inhibitor of blood-vessel formation); therefore explaining the observed impaired angiogenesis [65]. One could extrapolate these results and hypothesize that impaired angiogenesis at baseline could be playing a role in the decreased reparative capacity of the aging kidney as peritubular capillary loss takes place in AKI [66], and thus is a potential driver for failed repair. Interestingly a recently published study from the TRIBE-AKI Consortium looked at the association of angiogenesis markers and outcomes after AKI [67]. In a prospective cohort of 1444 adult patients who underwent cardiac surgery, the study looked at the plasma concentration of two pro-angiogenic markers, VEGF and PGF (placental growth factor), and one anti-angiogenic marker, VEGFR1 (soluble VEGF receptor 1), that were measured within 6 hours before and after surgery. They observed that higher levels of VEGF and PGF postoperatively were associated with lower AKI and mortality risk. On the other hand, high VEGFR1 levels were associated with higher risk for AKI and mortality. Therefore, these observations suggest that angiogenesis is an important event in the kidney repair response and thus decreased VEGF levels in the aging kidney could be potentially driving the higher incidence of AKI and increased mortality seen in the elderly.
Decreased proliferative capacity
The kidney is a slow cycling organ, with < 1% cells proliferating at a given time under basal conditions [68]. After an injury, the tubular epithelium enters a proliferative phase to repair the damaged tubules [69]. A recent study using a novel mouse model that allows genetic labeling of injured tubular epithelial cells demonstrated that surviving tubular epithelial cells have proliferative capacity and contribute to kidney repair [70]. Furthermore, translational profiling of these injured tubular epithelial cells showed upregulation of genes related to cell cycle, thus suggesting these cells are primed to proliferate. Schmitt et al. observed that aged mice after ischemic renal injury had less tubular proliferation than young mice [71]. This lack of proliferative response might reflect cell senescence, a state where epithelia are metabolically active but not able to undergo further cell division. P16INK4a, a tumor suppressor and cell cycle regulator, has been found to accumulate with aging in different rodent and human organs including the kidney, and is considered a biomarker of cell senescence [72]. P16INK4a is a Cyclin Dependent Kinase (CDK) inhibitor that inhibits the kinase activity of CDK4 and CDK6, both of which play a fundamental role in regulation of the mammalian cell cycle. Inhibition of CDK4 and CDK6 inhibits the phosphorylation of the Retinoblastoma protein and causes G1 arrest. P16INK4a expression has been noted in the tubules of aged mouse kidneys and therefore this might be responsible for the decreased proliferation in this renal compartment [72]. Braun et al. deleted p16INK4a and showed an escape from p16INK4a-dependent senescence, with less interstitial fibrosis and tubular atrophy after unilateral ischemia-reperfusion injury as compared to wild type mice [73]. There was also increased tubular cell proliferation and reduced senescent tubular cells in the mice where p16INK4a was deleted [73]. These preclinical studies suggest that reduced tubular epithelial proliferation after injury, probably driven by cellular senescence, might be playing a role in the decreased repair capacity of the aging kidney. However, more studies are needed to further explore this possibility and methods to evaluate proliferation in human kidney samples after injury. Perhaps with the advent of single cell profiling, new insights into the proliferative capacity of the aging kidney can be elucidated.
Cellular senescence
Cellular senescence describes a state where cells are permanently arrested, but still metabolically active. It can take place in many different cell types, tissues, and organs. Although it has been recognized and described, there are still no available tools to reliably identify these cells, especially in vivo. Senescence-associated β-galactosidase staining (SA-β-gal) is frequently used as a marker of senescence. Recently, it has been proposed that the use of a combination of molecular markers representing different senescence characteristics might increase the validity of identifying senescent cells [74]. Candidate senescent markers include: γH2A, p53BP1 (activation of DNA-damage response), p16, p21, p53 (cell-cycle arrest), Ki67, BrdU (lack of proliferation markers) and Senescence-associated heterochromatin foci (SAHF). Cellular senescence has been described in various contexts raging from embryonic development to disease and regeneration [75]. Possible triggers include DNA damage, oncogenic stimuli, mechanical stress, reactive oxygen species, and telomere shortening.
Several studies have shown that accumulation of senescent cells leads to age-related organ dysfunction due to cell cycle block and due to a complex pro-inflammatory response termed the senescence-associated secretory phenotype (SASP) [76]. The SASP includes the secretion of pro-inflammatory cytokines (IL-6), chemokines (MCP-1), granulocyte-macrophage colony-stimulating factors, proteases, and growth factors (TGF-β). Studies over the past few years have implicated the SASP as an inducer of senescence in normal neighboring cells via a paracrine effect [75, 77]. TGF-β family ligands, VEGF, CCL2, and CCL20 are some of the SASP components identified as mediators of paracrine senescence [75, 77], which can exert a paracrine effect in healthy neighboring cells, leading to an inflammatory and pro-fibrotic local environment. Senescent cells have been identified in the aging kidneys of rodent models more specifically in tubular epithelial cells [78, 79]. In human kidneys, senescent cells have been identified in donor kidneys and the levels of pre-transplant senescent cells correlate with the development of interstitial fibrosis and tubular atrophy [80, 81]. Furthermore, kidney allografts from older donors are more vulnerable to ischemic injury and delayed graft function post-transplant [82, 83]. Therefore, it is plausible that the presence of senescent tubular epithelial cells leads to impaired tubular reparative response due to inability to enter the cell cycle after injury and the pro-inflammatory and pro-fibrotic environment surrounding the remaining healthy tubular cells. A recent study by Jin et al. found that in 3 different models of AKI, mice showed the presence of senescent cells mostly in the proximal tubule and these cells were found surprisingly early on and slowly increase over time [11]. They also observed that senescent tubular cells are the source of profibrotic signals, such as the Hedgehog ligands, thus adding further evidence to the role of these cells in the development of organ fibrosis.
Impaired immune response
With aging there is a low, chronic level of inflammation not associated with infection and this has been termed “inflammaging” [84]. It is unknown what drives this chronic inflammatory state, but it has been speculated to induce some of the organ dysfunction seen across different organs as we age. A counterpart to inflammaging is “Immunosenescence”, where there is a decline in the immune function leading to an impaired immune response. Both these entities are thought to be interconnected. A previous study performed transcriptional profiling of aged human kidneys using microarray and noted increased expression of immune genes in the aged kidney [85]. They identified 447 genes that are specifically and differentially expressed in the aged kidney. Of those, 257 were upregulated and 190 were downregulated, thus providing evidence of the changes in the immune landscape of the aging kidney. Perhaps genes that are repressed are important for the repair response and their downregulation at baseline might be a contributor for failed repair. Further preclinical studies are needed to dissect the roles of these immune changes and their effects in the reparative response.
Conclusion
Science and medicine have made great advances in preventing and treating disease and this has led to an increase in life expectancy and in turn gradually change the make-up of the world population with an observed increase in the elderly segment. One area that has been underappreciated is that elderly patients are at increased risk of development of AKI and this places them at risk for poor outcomes, like death or progression to CKD and potential need for long-term dialysis.
Increasing awareness of preventable risk factors, such as avoidance of nephrotoxic medications or proper medication dosing, along with close monitoring of kidney function during the hospital course are some of the current steps that can be taken. Further studies are needed to identify therapies that can improve some of the intrinsic kidney changes that can predispose to AKI or therapies to enhance the repair response or decrease the risk of progression to CKD.
Acknowledgement
I want to thank Dr. Benjamin Humphreys for critical reading of the manuscript. I apologize to all authors whose work could not be cited due to space restriction. This work was supported by the NIH/NIDDK 1K08DK122124-01A1.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA (2018) The Population 65 Years and Older in the United States: 2016 American Community Survey Reports. ACS-38 (U.S. Census Bureau, Washington, DC: ) [Google Scholar]
- 2.Hommos MS, Glassock RJ, Rule AD (2017) Structural and Functional Changes in Human Kidneys with Healthy Aging. J Am Soc Nephrol 28:2838–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Sullivan ED, Hughes J, Ferenbach DA (2017) Renal Aging: Causes and Consequences. J Am Soc Nephrol 28:407–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt R, Melk A (2017) Molecular mechanisms of renal aging. Kidney Int 92:569–579 [DOI] [PubMed] [Google Scholar]
- 5.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ (2009) Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20:223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabbian F, Savrie C, De Giorgi A, Cappadona R, Di Simone E, Boari B, Storari A, Gallerani M, Manfredini R (2019) Acute Kidney Injury and In-Hospital Mortality: A Retrospective Analysis of a Nationwide Administrative Database of Elderly Subjects in Italy. J Clin Med 8:1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee H, Jang KS, Park JM, Kang JS, Hwang NK, Kim IY, Song SH, Seong EY, Lee DW, Lee SB, Kwak IS (2016) Short- and Long-Term Mortality Rates of Elderly Acute Kidney Injury Patients Who Underwent Continuous Renal Replacement Therapy. PLoS One 11:e0167067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, Hung KY (2015) The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep 5:13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusaka J, Koga H, Hagiwara S, Hasegawa A, Kudo K, Noguchi T (2012) Age-dependent responses to renal ischemia-reperfusion injury. J Surg Res 172:153–158 [DOI] [PubMed] [Google Scholar]
- 10.Diao C, Wang L, Liu H, Du Y, Liu X (2019) Aged kidneys are refractory to autophagy activation in a rat model of renal ischemia-reperfusion injury. Clin Interv Aging 14:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin H, Zhang Y, Ding Q, Wang SS, Rastogi P, Dai DF, Lu D, Purvis M, Cao C, Wang A, Liu D, Ren C, Elhadi S, Hu MC, Chai Y, Zepeda-Orozco D, Campisi J, Attanasio M (2019) Epithelial innate immunity mediates tubular cell senescence after kidney injury. JCI Insight 4:e125490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, Cerdá J, Chawla LS (2018) Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14:607–625 [DOI] [PubMed] [Google Scholar]
- 13.Pavkov ME, Harding JL, Burrows NR (2018) Trends in Hospitalizations for Acute Kidney Injury - United States, 2000–2014. MMWR Morb Mortal Wkly Rep 67:289–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honoré PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, Kellum JA (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41:1411–1423 [DOI] [PubMed] [Google Scholar]
- 15.Druml W, Lenz K, Laggner AN (2015) Our paper 20 years later: from acute renal failure to acute kidney injury--the metamorphosis of a syndrome. Intensive Care Med 41:1941–1949 [DOI] [PubMed] [Google Scholar]
- 16.Turney JH, Marshall DH, Brownjohn AM, Ellis CM, Parsons FM (1990) The evolution of acute renal failure, 1956–1988. Q J Med 74:83–104 [PubMed] [Google Scholar]
- 17.Feest TG, Round A, Hamad S (1993) Incidence of severe acute renal failure in adults: results of a community based study. BMJ 306:481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V (2019) US Renal Data System 2018 Annual Data Report Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 73(3 Suppl 1):A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosner MH (2013) Acute kidney injury in the elderly. Clin Geriatr Med 29:565–578 [DOI] [PubMed] [Google Scholar]
- 20.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR (2008) Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis 52:262–271 [DOI] [PubMed] [Google Scholar]
- 21.Merideth MA, Gordon LB, Clauss S, Sachdev V, Smith AC, Perry MB, Brewer CC, Zalewski C, Kim HJ, Solomon B, Brooks BP, Gerber LH, Turner ML, Domingo DL, Hart TC, Graf J, Reynolds JC, Gropman A, Yanovski JA, Gerhard-Herman M, Collins FS, Nabel EG, Cannon RO 3rd, Gahl WA, Introne WJ (2008) Phenotype and course of Hutchinson-Gilford progeria syndrome. N Engl J Med 358:592–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delahunt B, Stehbens WE, Gilbert-Barness E, Shozawa T, Ruger BM (2000) Progeria kidney has abnormal mesangial collagen distribution. Pediatr Nephrol 15:279–285 [DOI] [PubMed] [Google Scholar]
- 23.Dorado B, Pløen GG, Barettino A, Macías A, Gonzalo P, Andrés-Manzano MJ, González-Gómez C, Galán-Arriola C, Alfonso JM, Lobo M, López-Martín GJ, Molina A, Sánchez-Sánchez R, Gadea J, Sánchez-González J, Liu Y, Callesen H, Filgueiras-Rama D, Ibáñez B, Sørensen CB, Andrés V (2019) Generation and characterization of a novel knockin minipig model of Hutchinson-Gilford progeria syndrome. Cell Discov 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention (2019) Chronic Kidney Disease in the United States, 2019. Atlanta, GA: US Department of Health and Human Services. [Google Scholar]
- 25.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, Brown WW, Burrows NR, Jurkovitz CT, McFarlane SI, Norris KC, Shlipak M, Whaley-Connell AT, Chen SC, Bakris GL, McCullough PA (2010) Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55(3 Suppl 2):S23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruilope LM, Lahera V, Rodicio JL, Carlos Romero J (1994) Are renal hemodynamics a key factor in the development and maintenance of arterial hypertension in humans? Hypertension 23:3–9 [DOI] [PubMed] [Google Scholar]
- 27.Pinto E (2007) Blood pressure and ageing. Postgrad Med J 83:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D (1997) Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 96:308–315 [DOI] [PubMed] [Google Scholar]
- 29.Franklin SS, Jacobs MJ, Wong ND, L’Italien GJ, Lapuerta P (2001) Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 37:869–874 [DOI] [PubMed] [Google Scholar]
- 30.Chirinos JA, Segers P, Hughes T, Townsend R (2019) Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 74:1237–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto J, Ito S (2011) Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension 58:839–846 [DOI] [PubMed] [Google Scholar]
- 32.Kamide K, Kawano Y (2005) [Age related hemodynamic changes in the elderly]. Nihon Rinsho 63:969–972 [PubMed] [Google Scholar]
- 33.Jerkic M, Vojvodic S, Lopez-Novoa JM (2001) The mechanism of increased renal susceptibility to toxic substances in the elderly. Part I. The role of increased vasoconstriction. Int Urol Nephrol 32:539–547 [DOI] [PubMed] [Google Scholar]
- 34.Chang YP, Huang SK, Tao P, Chien CW (2012) A population-based study on the association between acute renal failure (ARF) and the duration of polypharmacy. BMC Nephrol 13:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Bonventre JV, Parrish AR (2014) The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci 15:15358–15376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips PA, Hodsman GP, Johnston CI (1991) Neuroendocrine mechanisms and cardiovascular homeostasis in the elderly. Cardiovasc Drugs Ther 4 Suppl 6:1209–1213 [DOI] [PubMed] [Google Scholar]
- 37.Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J (2018) A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis 9:143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cronin RE (2010) Contrast-induced nephropathy: pathogenesis and prevention. Pediatr Nephrol 25:191–204 [DOI] [PubMed] [Google Scholar]
- 39.Palli E, Makris D, Papanikolaou J, Garoufalis G, Zakynthinos E (2014) Contrast-induced nephropathy in aged critically ill patients. Oxid Med Cell Longev 2014:756469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodson JA, Hajduk A, Curtis J, Geda M, Krumholz HM, Song X, Tsang S, Blaum C, Miller P, Parikh CR, Chaudhry SI (2019) Acute Kidney Injury Among Older Patients Undergoing Coronary Angiography for Acute Myocardial Infarction: The SILVER-AMI Study. Am J Med 132:e817–e826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long DA, Mu W, Price KL, Johnson RJ (2005) Blood vessels and the aging kidney. Nephron Exp Nephrol 101:e95–99 [DOI] [PubMed] [Google Scholar]
- 42.Aalami OO, Fang TD, Song HM, Nacamuli RP (2003) Physiological features of aging persons. Arch Surg 138:1068–1076 [DOI] [PubMed] [Google Scholar]
- 43.Kremers WK, Denic A, Lieske JC, Alexander MP, Kaushik V, Elsherbiny HE, Chakkera HA, Poggio ED, Rule AD (2015) Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: the Aging Kidney Anatomy study. Nephrol Dial Transplant 30:2034–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiggins JE (2012) Aging in the glomerulus. J Gerontol A Biol Sci Med Sci 67:1358–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Floege J, Hackmann B, Kliem V, Kriz W, Alpers CE, Johnson RJ, Kühn KW, Koch KM, Brunkhorst R (1997) Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int 51:230–243 [DOI] [PubMed] [Google Scholar]
- 46.Kasiske BL (1987) Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 31:1153–1159 [DOI] [PubMed] [Google Scholar]
- 47.Uesugi N, Shimazu Y, Kikuchi K, Nagata M (2016) Age-Related Renal Microvascular Changes: Evaluation by Three-Dimensional Digital Imaging of the Human Renal Microcirculation Using Virtual Microscopy. Int J Mol Sci 17:1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schelling JR (2016) Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol 31:693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S, Cleveland RP, Koch CJ, Schelling JR (1999) Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Lab Invest 79:1089–1099 [PubMed] [Google Scholar]
- 50.Afsar B, Afsar RE, Dagel T, Kaya E, Erus S, Ortiz A, Covic A, Kanbay M (2018) Capillary rarefaction from the kidney point of view. Clin Kidney J 11:295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luckey AE, Parsa CJ (2003) Fluid and electrolytes in the aged. Arch Surg 138:1055–1060 [DOI] [PubMed] [Google Scholar]
- 52.Tamma G, Goswami N, Reichmuth J, De Santo NG, Valenti G (2015) Aquaporins, vasopressin, and aging: current perspectives. Endocrinology 156:777–788 [DOI] [PubMed] [Google Scholar]
- 53.Perazella MA, Mahnensmith RL (1997) Hyperkalemia in the elderly: drugs exacerbate impaired potassium homeostasis. J Gen Intern Med 12:646–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlanger LE, Bailey JL, Sands JM (2010) Electrolytes in the aging. Adv Chronic Kidney Dis 17:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlanger L (2009) Kidney Senescence. Online Curricula: Geriatric Nephrology: American Society of Nephrology. Available from https://www.asn-online.org [Google Scholar]
- 56.Fliser D, Zeier M, Nowack R, Ritz E (1993) Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 3:1371–1377 [DOI] [PubMed] [Google Scholar]
- 57.Fliser D, Franek E, Joest M, Block S, Mutschler E, Ritz E (1997) Renal function in the elderly: impact of hypertension and cardiac function. Kidney Int 51:1196–1204 [DOI] [PubMed] [Google Scholar]
- 58.Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, Merrill JP (1974) Senescence and the renal vasculature in normal man. Circ Res 34:309–316 [DOI] [PubMed] [Google Scholar]
- 59.Sharma A, Mucino MJ, Ronco C (2014) Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract 127:94–100 [DOI] [PubMed] [Google Scholar]
- 60.Palsson R, Waikar SS (2018) Renal Functional Reserve Revisited. Adv Chronic Kidney Dis 25:e1–e8 [DOI] [PubMed] [Google Scholar]
- 61.De Moor B, Vanwalleghem JF, Swennen Q, Stas KJ, Meijers BKI (2018) Haemodynamic or metabolic stimulation tests to reveal the renal functional response: requiem or revival? Clin Kidney J 11:623–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swedko PJ, Clark HD, Paramsothy K, Akbari A (2003) Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 163:356–360 [DOI] [PubMed] [Google Scholar]
- 63.Raman M, Middleton RJ, Kalra PA, Green D (2017) Estimating renal function in old people: an in-depth review. Int Urol Nephrol 49:1979–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scarr D, Bjornstad P, Lovblom LE, Lovshin JA, Boulet G, Lytvyn Y, Farooqi MA, Lai V, Orszag A, Weisman A, Keenan HA, Brent MH, Paul N, Bril V, Cherney DZI, Perkins BA (2019) Estimating GFR by Serum Creatinine, Cystatin C, and beta2-Microglobulin in Older Adults: Results From the Canadian Study of Longevity in Type 1 Diabetes. Kidney Int Rep 4:786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ (2001) Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 37:601–611 [DOI] [PubMed] [Google Scholar]
- 66.Kramann R, Tanaka M, Humphreys BD (2014) Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol 25:1924–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mansour SG, Zhang WR, Moledina DG, Coca SG, Jia Y, Thiessen-Philbrook H, McArthur E, Inoue K, Koyner JL, Shlipak MG, Wilson FP, Garg AX, Ishibe S, Parikh CR; TRIBE-AKI Consortium (2019) The Association of Angiogenesis Markers With Acute Kidney Injury and Mortality After Cardiac Surgery. Am J Kidney Dis 74:36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291 [DOI] [PubMed] [Google Scholar]
- 69.Humphreys BD, Czerniak S, DiRocco DP, Hasnain W, Cheema R, Bonventre JV (2011) Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A 108:9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang-Panesso M, Kadyrov FF, Lalli M, Wu H, Ikeda S, Kefaloyianni E, Abdelmageed MM, Herrlich A, Kobayashi A, Humphreys BD (2019) FOXM1 drives proximal tubule proliferation during repair from acute ischemic kidney injury. J Clin Invest 129:5501–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt R, Marlier A, Cantley LG (2008) Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 19:2375–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114:1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Braun H, Schmidt BM, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross ML, Serrano M, Schmitt R, Melk A (2012) Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23:1467–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burton DG, Krizhanovsky V (2014) Physiological and pathological consequences of cellular senescence. Cell Mol Life Sci 71:4373–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munoz-Espin D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15:482–496 [DOI] [PubMed] [Google Scholar]
- 76.Childs BG, Durik M, Baker DJ, van Deursen JM (2015) Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med 21:1424–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM (2017) Cellular senescence in renal ageing and disease. Nat Rev Nephrol 13:77–89 [DOI] [PubMed] [Google Scholar]
- 79.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM (2016) Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530:184–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Melk A, Schmidt BM, Vongwiwatana A, Rayner DC, Halloran PF (2005) Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5:1375–1382 [DOI] [PubMed] [Google Scholar]
- 81.Ferlicot S, Durrbach A, Ba N, Desvaux D, Bedossa P, Paradis V (2003) The role of replicative senescence in chronic allograft nephropathy. Hum Pathol 34:924–928 [DOI] [PubMed] [Google Scholar]
- 82.Oberhuber R, Ge X, Tullius SG (2012) Donor age-specific injury and immune responses. Am J Transplant 12:38–42 [DOI] [PubMed] [Google Scholar]
- 83.Zhao H, Alam A, Soo AP, George AJT, Ma D (2018) Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 28:31–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14:576–590 [DOI] [PubMed] [Google Scholar]
- 85.Rodwell GE, Sonu R, Zahn JM, Lund J, Wilhelmy J, Wang L, Xiao W, Mindrinos M, Crane E, Segal E, Myers BD, Brooks JD, Davis RW, Higgins J, Owen AB, Kim SK (2004) A transcriptional profile of aging in the human kidney. PLoS Biol 2:e427. [DOI] [PMC free article] [PubMed] [Google Scholar]