Abstract
Rational & Objective:
The clearance of protein-bound solutes by the proximal tubules is an innate kidney mechanism for removing putative uremic toxins that could exert cardiovascular toxicity in humans. However, potential associations between impaired kidney clearances of secretory solutes and cardiovascular events among patients with chronic kidney disease (CKD) remains uncertain.
Study Design:
A multicenter, prospective, cohort study.
Setting & Participants:
We evaluated 3,407 participants from the Chronic Renal Insufficiency Cohort (CRIC) study.
Exposures:
Baseline kidney clearances of eight secretory solutes. We measured concentrations of secretory solutes in plasma and paired 24-hour urine specimens using liquid chromatography tandem mass spectrometry (LC-MS/MS).
Outcomes:
Incident heart failure, myocardial infarction, and stroke events.
Analytical Approach:
We used Cox regression to evaluate associations of baseline secretory solute clearances with incident study outcomes adjusting for estimated GFR (eGFR) and other confounders.
Results:
Participants were characterized by a mean age of 56 years; 45% women; 41% Black; and a median eGFR of 43 mL/min/1.73m2. Lower 24-hour kidney clearances of secretory solutes were associated with incident heart failure and myocardial infarction, but not incident stroke, over long-term follow-up after controlling for demographics and traditional risk factors. However, these associations were attenuated and not statistically significant after adjustment for eGFR.
Limitations:
The exclusion of patients with severely reduced eGFR at baseline; measurement variability in secretory solutes clearances.
Conclusions:
In a national cohort study of CKD, we found no clinically or statistically relevant associations between the kidney clearances of endogenous secretory solutes and incident heart failure, myocardial infarction, and stroke after adjustment for eGFR. These findings suggest that tubular secretory clearance provides little additional information about the development of cardiovascular disease events beyond glomerular measures of GFR and albuminuria among patients with mild-to-moderate CKD.
Keywords: secretory solutes clearances, proximal tubule, chronic kidney disease, heart failure, stroke, myocardial infarction
Plain language summary
The kidneys eliminate retained toxins by two mechanisms: glomerular filtration and tubular secretion. Yet, current assessment of kidney function is based primarily on measures of glomerular filtration. We developed a novel procedure to measure kidney tubular secretory clearance. We estimated this kidney function in a national cohort study of patients with chronic kidney disease (CKD). We found that lower kidney clearances of secretory solutes were associated with greater risks of incident heart failure and myocardial infarction, but not stroke, over long-term follow-up. However, these associations were removed by adjustment for the estimated glomerular filtration rate. These findings suggest that tubular secretory clearance provides little additional information about the development of cardiovascular disease beyond glomerular measures in mild-to-moderate CKD.
INTRODUCTION
The presence and severity of chronic kidney disease (CKD), assessed by estimated glomerular filtration rate (GFR), are associated with greatly increased risk of heart failure and atherosclerotic cardiovascular disease.1, 2 The excess cardiovascular risk associated with CKD has been attributed partly to a greater burden of traditional atherosclerotic risk factors, such as diabetes and hypertension, but also to metabolic disturbances arising from kidney disease itself, including activation of pro-inflammatory and oxidative stress pathways and promotion of vascular and soft tissue calcification.3–5
In addition to filtering freely circulating solutes, the kidneys directly extract retained substances from the circulation via tubular secretion. Transporters on the basolateral surface of proximal tubular epithelial cells, including organic anion transporters (OAT) 1 and 3 and the organic cation transporter (OCT) 2, shuttle solutes from the post-glomerular capillaries into the cell.6, 7 These solutes are subsequently extruded into the urine by active transporters located on the luminal cell surface, primarily members of the ATP-binding cassette transporter superfamily.8 Tubular secretory clearance is an innate kidney mechanism for eliminating protein-bound solutes and drugs that cannot be efficiently filtered due to size and charge selectivity of the glomerular basement membrane. Many secretory solutes that derive from dietary sources are putative uremic toxins associated with wide-ranging clinical and metabolic disturbances, described by the European Uremic Toxin Work Group.9 For example, indoxyl sulfate and p-cresol sulfate induce endothelial cell damage, stimulate vascular smooth muscle cell proliferation, and activate profibrotic cardiac pathways in animal models.10–12 Kynurenic acid, a protein-bound metabolite of tryptophan metabolism, impairs cardiac mitochondrial coupling and ATP synthesis.13
We hypothesized that lower native kidney clearances of endogenous secretory solutes would be associated with excess cardiovascular risk in CKD beyond that of GFR. To test this hypothesis, we measured baseline 24-hour kidney clearances of eight endogenous solutes suspected to be eliminated primarily by tubular secretion in 3,407 persons from a national cohort study of CKD. We evaluated associations of these kidney clearances with incident heart failure, myocardial infarction, and stroke over follow-up after controlling for estimated GFR, albuminuria, and other cardiovascular risk factors.
METHODS
Data source and study population
The Chronic Renal Insufficiency Cohort (CRIC) Study is a multicenter prospective cohort study that recruited 3,939 patients with chronic kidney disease (CKD) from 2003 to 2007.14 The CRIC study excluded persons with an estimated glomerular filtration rate (GFR) < 20 mL/min/1.73m2 at baseline, a history of kidney transplantation, polycystic kidney disease, multiple myeloma, pregnancy, HIV infection, cirrhosis, severe heart failure, and those receiving active immunosuppression. For the current study, we excluded 532 CRIC study participants who did not have an available 24-hour urine and plasma sample at baseline for the measurement of secretory solutes. We then restricted analyses of incident heart failure events to persons without prevalent heart failure at baseline (N=3,072, Supplemental Figure 1); analyses of incident myocardial infarction to persons without prevalent myocardial infarction (N=3,128); and analyses of incident stroke to persons without prevalent stroke (N=3,077). The institutional review boards at all CRIC sites approved the study protocol. All participants provided written informed consent.
Measurements of secretory solute clearance
We measured the baseline kidney clearance of eight endogenous solutes suspected to be eliminated primarily by proximal tubular secretion: pyridoxic acid, isovalerylglycine, tiglylglycine, kynurenic acid, cinnamoylglycine, xanthosine, indoxyl sulfate, and p-cresol sulfate. We previously selected these organic solutes based on literature review indicating specificity for OAT1/3 transporters in the proximal tubules, an increase in circulating levels in rodent models following OAT1/3 knockout, a high degree of protein binding, low diurnal variation in plasma, and/or higher kidney clearances than GFR or creatinine clearance.15–17
We measured total (not free) plasma and urine concentration of secretory solutes using solid phase extraction and targeted liquid chromatography tandem mass spectrometry (LC-MS/MS).18, 19 A complete description of the method and the validation of the method is presented in Supplemental Methods. Plasma samples underwent protein precipitation prior to solid phase extraction. Data were normalized to labeled purified compounds (internal standards) added to each well. A single point calibration method was used to account for potential drift by measuring five replicates of calibrators during each run (pooled human serum and urine). We determined absolute concentrations of secretory solutes in the external calibrators by quantitative nuclear magnetic resonance (NMR) and standard addition of purified compounds. Intra- and inter-assay coefficients of variation for these secretory solutes range from 3.4% to 14.7% in plasma concentrations and from 4.5% to 10.1% in urine. Biologic variability in these solutes and their urinary clearances are presented in Table S1.
We calculated the 24-hour kidney clearance of each secretory solute as:
In this equation, UX represents the concentration of the secretory solute in the 24-hour urine sample, V represents the corresponding urine volume in mL per minute, and PX represents the concentration of the solute in plasma.
Measurement of outcomes
Individual primary outcomes of this study are incident heart failure, myocardial infarction, and stroke. The CRIC study identified hospitalizations via telephone calls alternating every six months with in-clinic visits.20 Study personnel retrieved medical records with codes relevant to study outcomes for centralized adjudicated review. Two study physicians reviewed all possible heart failure, myocardial infarction, and stroke events for classification. Definite or probable heart failure events were defined using Framingham and ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) criteria based on clinical symptoms, radiographic evidence of pulmonary congestion, physical examination findings, and echocardiographic imaging, if available.21, 22 Definite or probable myocardial infarction events were determined based on characteristic symptoms, cardiac biomarkers, and electrocardiographic data.23 Definite stroke events were defined based on the sudden onset of neurologic symptoms supported by imaging findings. Probable stroke events were determined as sudden or rapid onset of one major or two minor neurologic signs or symptoms lasting for more than 24 hours or until the patient died but with no evidence of hemorrhage or infarction on computed tomography or magnetic resonance imaging performed within 24 hours of the onset of symptoms.24 Secondary outcomes included all heart failure events (incident and recurrent), and ischemic and hemorrhagic strokes assessed separately.
Measurements of covariates
Participant self-reported sociodemographic characteristics and medical histories at baseline. Medication use was determined using the inventory method.14, 25 GFR was estimated using an equation developed in 1,433 CRIC participants who completed 125-Iothalamate GFR (iGFR) clearance studies26, which includes terms for (serum creatinine)−0.598, (serum cystatin C)−0.636, (age)−0.034, (female)−0.184, and (Black race)0.043. Serum creatinine concentrations were measured using an enzyme-based assay with values traceable to isotope dilution mass spectrometry (Hitachi Vitros 950 AT). Serum cystatin C concentrations were measured using a Siemens BNII instrument and longitudinal control materials were used to correct for drift over time when using different calibrator and reagent lots.27 24-hour urine albumin was measured on the Siemens Immulite.26 The mean of the latter two out of three seated blood pressure measurements was used for analysis.28 Triglyceride and high-density lipoprotein were measured by spectrophotometry, and low-density lipoprotein by β quantification after separation by ultracentrifugation.29 Hemoglobin was measured at each CRIC clinical center.30 The CRIC study defined diabetes by a fasting glucose concentration ≥126 mg/dL, a non-fasting glucose ≥ 200 mg/dL, or the use of antidiabetic medication. Plasma glucose was measured on the Hitachi Vitros 950 AT.31
Statistical analyses
We evaluated the kidney clearances of each secretory solute individually and in combination using a scaled summary score. To create the summary score, we first standardized the kidney clearances to a common 0–100 scale by computing their natural logarithm, subtracting the minimum value in the distribution, and dividing by the range of values in the distribution:
where ln(clearance) is the kidney clearance of each secretory solutes after log-transformation, min(ln(clearance)) is the minimum value in the distribution, and range(ln(clearance)) is the difference between the maximum and minimum values in the distribution. We then calculated the summary secretion score as the mean of the eight standardized clearances (Figure S2).32 We computed univariate Pearson correlations between log-transformed secretory clearances and log-transformed eGFR, and among the individual log-transformed secretory clearances.
We used Cox proportional hazards regression to estimate associations of secretory solute clearances at baseline with the time to each incident cardiovascular event. For analyses of incident events, follow-up time began at the baseline exam, when secretory clearances were measured, and was continued until either the first occurrence of the event of interest or the data were censored due to death, withdrawal, loss to follow-up, or the end of the follow-up period (May 2014), whichever occurred first.33, 34 We built nested models to control for potential confounding characteristics. Model 1 adjusted for age, race, and sex. Model 2 additionally adjusted for CRIC clinical site, educational attainment, smoking status, body mass index, systolic blood pressure, blood triglyceride levels, history of diabetes, history of cardiovascular disease (except for the outcome being evaluated in the model), 24-hour urinary albumin excretion, and the use of angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers, loop diuretics, and beta-blockers. Model 3 additionally adjusted for eGFR based on the CRIC study creatinine and cystatin C equation. All adjustment covariates are from the baseline examination. We used analogous Poisson regression models to evaluate associations between secretory solute clearances and the rate of all heart failure events (incident and recurrent). For this analysis, we did not exclude participants with prevalent heart failure at baseline. In sensitivity analyses, we evaluated associations with ischemic stroke and hemorrhagic stroke separately. We repeated our analyses stratified by Black vs. non-Black race, baseline diabetes status, and baseline categories of eGFR. To address competing risk by death, we used the subdistribution proportional hazard model developed by Fine and Grey to evaluate associations with the subdistribution hazard ratio of cardiovascular events.35 In order to detect potential threshold effect, we used cubic splines to plot the hazard ratio of cardiovascular outcomes versus the summary secretion score. We then tested the association between plasma concentrations of secretory solutes and each incident cardiovascular event. We tested proportional hazards assumption of Cox models using scaled Schoenfeld residuals. We detected no evidence of violation of the proportional hazards assumption. We used the Hommel method to correct for multiple comparisons (nine comparisons in Tables 3–5).36 We used complete-cases analysis, as covariates were missing in less than 1% of study participants. Analyses were performed using Stata/IC 14.2 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX) and RStudio 3.6.3 (R Core Team 2017, Vienna, Austria).
Table 3.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRd | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Pyridoxic acid | 1.34 | 1.23–1.46 | < 0.001* | 1.19 | 1.08–1.31 | < 0.001* | 1.08 | 0.96–1.21 | 0.2 |
| Isovalerylglycine | 1.44 | 1.32–1.58 | < 0.001* | 1.21 | 1.09–1.35 | < 0.001* | 1.13 | 1.01–1.26 | 0.04 |
| Tiglylglycine | 1.36 | 1.25–1.47 | < 0.001* | 1.21 | 1.10–1.33 | < 0.001* | 1.11 | 0.99–1.23 | 0.07 |
| Kynurenic acid | 1.41 | 1.27–1.57 | < 0.001* | 1.20 | 1.06–1.35 | 0.004* | 1.03 | 0.89–1.19 | 0.7 |
| Xanthosine | 1.25 | 1.15–1.35 | < 0.001* | 1.14 | 1.05–1.24 | 0.001* | 1.08 | 0.99–1.17 | 0.08 |
| Cinnamoylglycine | 1.14 | 1.07–1.23 | < 0.001* | 1.09 | 1.01–1.17 | 0.03 | 1.03 | 0.95–1.11 | 0.5 |
| Indoxyl sulfate | 1.36 | 1.23–1.50 | < 0.001* | 1.18 | 1.05–1.32 | 0.005* | 1.04 | 0.92–1.20 | 0.5 |
| p-cresol sulfate | 1.20 | 1.11–1.30 | < 0.001* | 1.08 | 0.98–1.18 | 0.1 | 0.97 | 0.87–1.08 | 0.5 |
| Summary score | 1.61 | 1.42–1.82 | < 0.001* | 1.30 | 1.13–1.50 | < 0.001* | 1.10 | 0.92–1.30 | 0.3 |
Results from Cox proportional hazard regression. There were 439 incident heart failure events.
Model 1 adjusted for age, race, and sex. Model 2 additionally adjusted for clinical sites, educational attainment, smoking status, history of cardiovascular disease, diabetes, body mass index, systolic blood pressure, triglyceride, 24-hour urinary albumin excretion, and ACEi/ARB, loop diuretics, and beta-blockers. Model 3 additionally adjusted for estimated GFR.
denotes statistical significance after correction for multiple comparisons using the Hommel method.
Hazard ratio expressed per 50% lower secretory solute clearance or per 10 units lower summary secretion score
Table 5.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRd | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Pyridoxic acid | 1.28 | 1.08–1.51 | 0.005* | 1.17 | 0.96–1.42 | 0.1 | 1.17 | 0.94–1.46 | 0.2 |
| Isovalerylglycine | 1.20 | 1.01–1.44 | 0.04 | 1.07 | 0.87–1.30 | 0.5 | 1.04 | 0.84–1.30 | 0.7 |
| Tiglylglycine | 1.18 | 1.00–1.40 | 0.05 | 1.06 | 0.88–1.28 | 0.6 | 1.03 | 0.83–1.29 | 0.8 |
| Kynurenic acid | 1.15 | 0.93–1.42 | 0.2 | 0.99 | 0.78–1.26 | 0.9 | 0.93 | 0.69–1.24 | 0.6 |
| Xanthosine | 1.03 | 0.89–1.21 | 0.7 | 1.01 | 0.86–1.18 | 0.9 | 0.98 | 0.83–1.16 | 0.9 |
| Cinnamoylglycine | 1.04 | 0.91–1.19 | 0.6 | 1.00 | 0.87–1.15 | 0.9 | 0.98 | 0.84–1.14 | 0.8 |
| Indoxyl sulfate | 0.99 | 0.81–1.20 | 0.9 | 0.84 | 0.67–1.05 | 0.1 | 0.79 | 0.62–1.02 | 0.07 |
| p-cresol sulfate | 1.00 | 0.85–1.19 | 0.9 | 0.92 | 0.77–1.11 | 0.4 | 0.87 | 0.71–1.08 | 0.2 |
| Summary score | 1.20 | 0.94–1.54 | 0.2 | 1.01 | 0.76–1.35 | 0.9 | 0.94 | 0.66–1.33 | 0.7 |
Results from Cox proportional hazard regression. There were 123 incident stroke events.
Model 1 adjusted for age, race, and sex. Model 2 additionally adjusted for clinical sites, educational attainment, smoking status, history of cardiovascular disease, diabetes, body mass index, systolic blood pressure, triglyceride, 24-hour urinary albumin excretion, and ACEi/ARB, loop diuretics, and beta-blockers. Model 3 additionally adjusted for estimated GFR.
denotes statistical significance after correction for multiple comparisons using the Hommel method.
Hazard ratio expressed per 50% lower secretory solute clearance or per 10 units lower summary secretion score
RESULTS
Study population and characteristics
This ancillary study included 3,407 participants from the Chronic Renal Insufficiency Cohort (CRIC) Study at baseline. Their mean age was 56 years; 45% were women; 41% were Black; 12% were Hispanic; and the median eGFR was 43 mL/min/1.73m2 (IQR: 32–55 mL/min/1.73m2). Among the eight secretory solutes evaluated, pyridoxic acid exhibited the highest 24-hour kidney clearance (median: 456 mL/min, Table S2) and p-cresol sulfate the lowest (median: 10 mL/min). The kidney clearances of six of the eight secretory solutes were higher than eGFR. We observed moderate correlations between eGFR and the clearances of the individual secretory solutes, ranging from 0.40 for cinnamoylglycine to 0.61 for kynurenic acid. Participants with higher summary secretion scores, defined by the scaled average of the individual solute clearances, had higher eGFRs, were relatively younger, and were more likely to be male and non-Black compared to participants with lower secretion scores (Table 1). Participants with higher summary secretion scores also had a lower baseline prevalence of cardiovascular disease, lower systolic blood pressure, lower 24-hour urine albumin excretion, lower blood triglycerides, and were less likely to use beta-blockers and loop diuretics.
Table 1.
| Summary secretion score | ||||
|---|---|---|---|---|
| Characteristics | Quartile 1 (Lowest) | Quartile 2 | Quartile 3 | Quartile 4 (Highest) |
| N = 851 | N = 852 | N = 853 | N = 851 | |
| Summary secretion score | 27 – 53 | 53 – 58 | 58 – 63 | 63 – 78 |
| eGFRCRIC, mL/min/L73m2, b | 29 (24, 38) | 38 (31, 47) | 47 (40, 56) | 59 (49, 69) |
| Age, years | 59 ± 11 | 58 ± 11 | 58 ± 11 | 56 ± 10 |
| Female | 449 (53) | 428 (50) | 345 (40) | 307 (36) |
| Black | 406 (48) | 359 (42) | 323 (38) | 319 (37) |
| Hispanic | 141 (17) | 109 (13) | 95 (11) | 60 (7) |
| Body mass index, kg/m2 | 31 ± 8 | 32 ± 8 | 32 ± 7 | 33 ± 8 |
| Education categories (1–4) | ||||
| Less than high school | 251 (29) | 193 (23) | 140 (16) | 95 (11) |
| High school graduate | 185 (22) | 170 (20) | 149 (17) | 143 (17) |
| Some college | 244 (29) | 241 (28) | 253 (30) | 245 (29) |
| College graduate or higher | 171 (20) | 248 (29) | 311 (36) | 367 (43) |
| Current smoker | 131 (15) | 108 (13) | 100 (12) | 96 (11) |
| History of Diabetes | 407 (48) | 438 (51) | 419 (49) | 368 (43) |
| History of cardiovascular disease | 346 (41) | 309 (36) | 280 (33) | 198 (23) |
| History of peripheral vascular disease | 83 (10) | 66 (8) | 38 (4) | 41 (5) |
| Systolic blood pressure, mmHg | 134 ± 25 | 128 ± 22 | 125± 20 | 125 ± 20 |
| Diastolic blood pressure, mmHg | 72 ± 14 | 71 ± 13 | 71 ± 12 | 72 ± 12 |
| Lab measurements | ||||
| 24-hour urine albumin, mg/24hc | 192 (27, 890) | 69 (13, 586) | 47 (9, 453) | 24 (7, 261) |
| Triglyceride, mg/dL | 165 ± 126 | 161 ± 127 | 160 ± 108 | 141 ± 102 |
| LDL, mg/dL | 102 ± 37 | 101 ± 35 | 102 ± 36 | 106 ± 32 |
| HDL, mg/dL | 47 ± 16 | 48 ± 16 | 47 ± 16 | 48 ± 15 |
| Hemoglobin, g/dL | 12.0 ± 1.7 | 12.4 ± 1.7 | 12.8 ± 1.8 | 13.2 ± 1.7 |
| Medications | ||||
| Statin | 450 (53) | 494 (58) | 485 (57) | 438 (52) |
| ACEi / ARB | 525 (62) | 625 (73) | 619 (73) | 545 (64) |
| Beta Blocker | 488 (58) | 464 (55) | 414 (49) | 320 (38) |
| Loop diuretic | 437 (52) | 365 (43) | 272 (32) | 201 (24) |
| Thiazide diuretic | 179 (21) | 260 (31) | 281 (33) | 244 (29) |
For continuous variable: mean ± SD; for categorical variables: N(%).
Conversion factors for units: triglyceride in mg/dL to mmol/L, ×0.01129; LDL and HDL in mg/dL to mmol/L, ×0.02586.
Median (Interquartile range)
Associations of kidney clearances of secretory solutes with heart failure
Over a median follow-up of 8.0 years, 439 participants developed a first definite or probable heart failure event. The unadjusted incidence of heart failure was 1.2 events per 100 person-years among participants in the highest quartile of the summary secretion score and 3.2 events per 100 person-years among participants in the lowest quartile (Table 2). After adjustment for age, race, sex (Model 1), and other risk factors (Model 2), lower kidney clearances of most secretory solutes, and a lower summary secretion score, were associated with greater risks of incident heart failure (Table 3). However, these associations were substantially attenuated by further adjustment for eGFR (Model 3), with only isovalerylglycine remaining significant. Similar results were observed for all heart failure events (incident and recurrent) and for the subdistribution hazard ratio of heart failure (Tables S3 and S4). The fully adjusted association between the summary secretion score and incident heart failure was statistically similar across categories of baseline eGFR, race, and diabetes status (Figure 1, p for interaction: 0.2, 0.9, and 0.8, respectively).
Table 2.
| Summary secretion score | ||||||
|---|---|---|---|---|---|---|
| All | Quartile 1 (Lowest) | Quartile 2 | Quartile 3 | Quartile 4 (Highest) | ||
| Summary secretion sore | 27 – 53 | 53 – 58 | 58 – 63 | 63 – 78 | ||
| Heart failure | No. of events | 439 | 154 | 126 | 89 | 70 |
| (N = 3,072)c | Incidence rate | 2.0 | 3.2 | 2.4 | 1.6 | 1.2 |
| Myocardial infarction | No. of events | 310 | 99 | 84 | 78 | 49 |
| (N = 3,128)c | Incidence rate | 1.2 | 1.8 | 1.4 | 1.2 | 0.7 |
| Stroke | No. of events | 123 | 32 | 33 | 32 | 26 |
| (N = 3,077)c | Incidence rate | 0.5 | 0.6 | 0.5 | 0.5 | 0.4 |
Median follow-up time of heart failure, myocardial infarction, and stroke was 8.0, 9.2 and 9.3 years, respectively.
Incidence rates shown as numbers of events per 100 person-years, calculated from unadjusted Poisson regression.
Participants with prevalent heart failure, myocardial infarction, or stroke at baseline were excluded from the corresponding analysis of each outcome.
Figure 1. Association of summary secretion score with incident heart failure (i), myocardial infarction (ii), and stroke (iii) events by subgroups a, b, c, d.
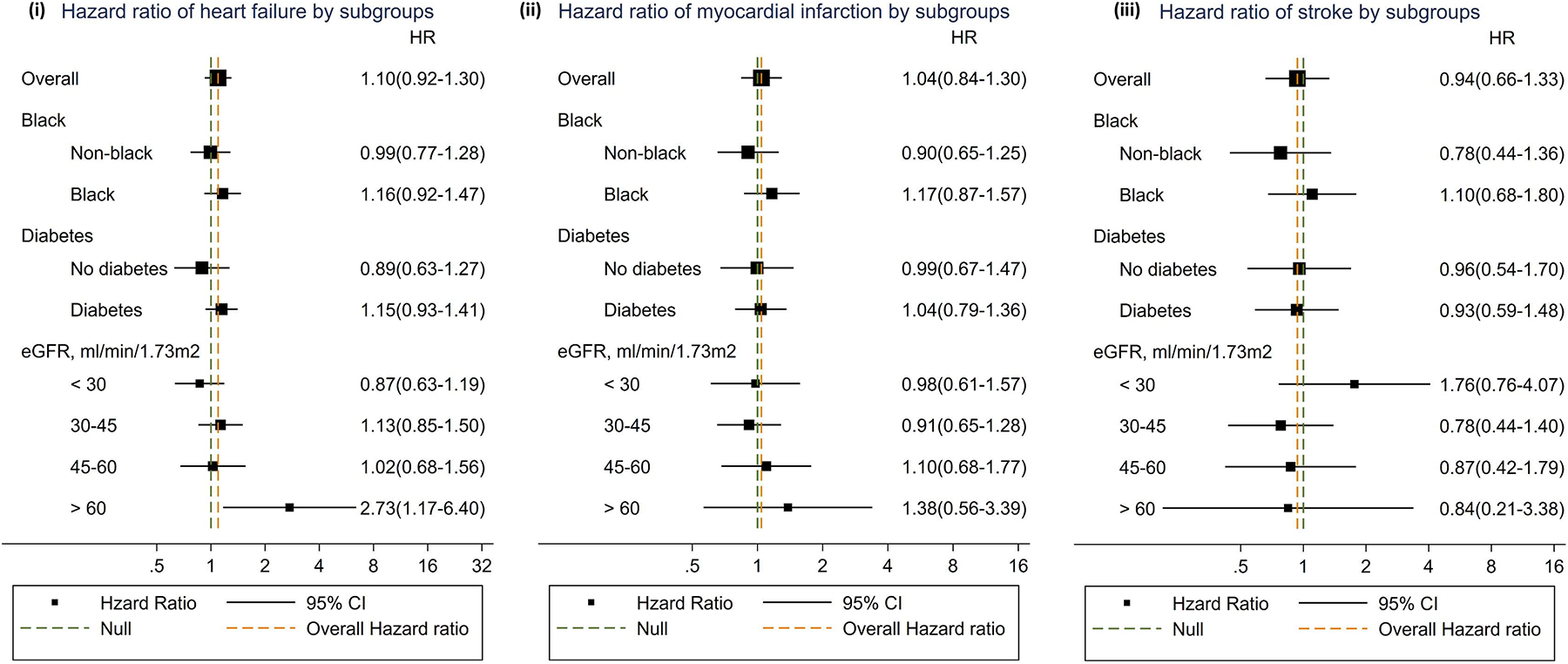
a Solid square: hazard ratio; solid line: 95% confidence interval; green dashed line: null; orange dashed line: overall hazard ratio.
b Results from Cox proportional hazard regression.
c Model adjusted for age, race, sex, clinical sites, educational attainment, smoking status, history of cardiovascular disease, diabetes, body mass index, systolic blood pressure, triglyceride, 24-hour urinary albumin excretion, ACEi/ARB, loop diuretics, beta-blockers, and estimated GFR.
d Hazard ratio expressed per 10 units lower summary secretion score.
Associations of kidney clearances of secretory solutes with myocardial infarction
There were 310 incident myocardial infarction events over a median follow-up of 9.2 years. Unadjusted incidence rates of myocardial infarction were higher among participants who had lower summary secretion scores (Table 2). The kidney clearances of three secretory solutes were associated with myocardial infarction in the demographic and health characteristics adjusted Model 2 (Table 4). However, again these associations largely disappeared after further adjustment for eGFR.
Table 4.
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRd | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Pyridoxic acid | 1.26 | 1.13–1.39 | < 0.001* | 1.13 | 1.00–1.27 | 0.05 | 1.03 | 0.90–1.18 | 0.7 |
| Isovalerylglycine | 1.32 | 1.18–1.48 | < 0.001* | 1.11 | 0.97–1.26 | 0.1 | 1.03 | 0.90–1.18 | 0.7 |
| Tiglylglycine | 1.31 | 1.18–1.46 | < 0.001* | 1.16 | 1.03–1.32 | 0.02 | 1.08 | 0.93–1.24 | 0.3 |
| Kynurenic acid | 1.36 | 1.19–1.54 | < 0.001* | 1.14 | 0.99–1.32 | 0.08 | 1.01 | 0.86–1.20 | 0.9 |
| Xanthosine | 1.25 | 1.14–1.37 | < 0.001* | 1.15 | 1.05–1.26 | 0.003* | 1.10 | 1.00–1.21 | 0.05 |
| Cinnamoylglycine | 1.06 | 0.97–1.16 | 0.2 | 1.01 | 0.93–1.11 | 0.8 | 0.96 | 0.87–1.06 | 0.4 |
| Indoxyl sulfate | 1.18 | 1.04–1.33 | 0.009* | 0.99 | 0.86–1.14 | 0.9 | 0.85 | 0.73–1.00 | 0.05 |
| p-cresol sulfate | 1.15 | 1.04–1.28 | 0.006* | 1.03 | 0.91–1.15 | 0.7 | 0.93 | 0.82–1.06 | 0.3 |
| Summary score | 1.48 | 1.27–1.72 | < 0.001* | 1.22 | 1.02–1.45 | 0.03 | 1.04 | 0.84–1.30 | 0.7 |
Results from Cox proportional hazard regression. There were 310 incident myocardial infarction events.
Model 1 adjusted for age, race, and sex. Model 2 additionally adjusted for clinical sites, educational attainment, smoking status, history of cardiovascular disease, diabetes, body mass index, systolic blood pressure, triglyceride, ACEi/ARB, loop diuretics, beta-blockers, and 24-hour urinary albumin excretion. Model 3 additionally adjusted for estimated GFR.
denotes statistical significance after correction for multiple comparisons using the Hommel method.
Hazard ratio expressed per 50% lower secretory solute clearance or per 10 units lower summary secretion score
Associations of kidney clearances of secretory solutes with stroke
One hundred and twenty-three incident stroke events occurred over a median follow-up of 9.3 years. No appreciable associations of the individual secretory solute clearances with incident stroke were observed after adjustment for demographics and health characteristics (Model 2; Table 5), nor for the subdistribution hazard ratio of stroke (Table S3). Null results also were observed when ischemic stroke and hemorrhagic stroke were analyzed separately (Table S5).
No threshold effect was detected in the association between the summary secretion score and cardiovascular outcomes (Figure S3). In models evaluating plasma concentrations of the secretory solutes alone, rather than their respective kidney clearances, none of the solutes under evaluation were associated with incident heart failure, myocardial infarction, or stroke after adjustment for eGFR and other confounding characteristics (Table S6).
DISCUSSION
In a national prospective cohort study of CKD, we observed lower 24-hour kidney clearances of secretory solutes to be associated with incident heart failure and myocardial infarction, but not incident stroke, over long-term follow-up after controlling for demographic characteristics and traditional risk factors. However, these associations were largely removed by adjustment for eGFR measured contemporaneously with the secretory solute clearances. Results were similar across categories of race, diabetes status, and eGFR and in models that considered the competing risk of death. Moreover, plasma concentrations of secretory solutes were not associated to any appreciable degree with the cardiovascular outcomes of interest after adjustment. Although methodological limitations of this study preclude definitive conclusions, these findings do not provide support for a causal role of retained secretory solutes on cardiovascular outcomes in nondialyzed persons with an eGFR >20 mL/min/1.73m2.
Several of the solutes included in this study are uremic toxins that demonstrate cardiovascular toxicity in experimental models. Indoxyl sulfate and p-cresol sulfate stimulate the production of reactive oxygen species (ROS), as well as the activity of NADH/NADPH oxidase and glutathione peroxidase, which in turn, induce oxidative stress.37–39 Oxidative stress, and downstream inflammation play critical roles in the development of atherosclerosis, including dyslipidemia, endothelial dysfunction, initial plaque formation and progression, and plaque rupture.39–41 Uremic toxins may also impair vascular endothelial cell functioning. Both indoxyl sulfate and p-cresol sulfate, when tested at uremic concentrations, were found to reduce the proliferation and repair of endothelial cells in vitro.41 Higher concentrations of indoxyl sulfate further inhibit the migration and tube formation of endothelial cells, possibly through the depletion of nitric oxide availability.42, 43 Indoxyl sulfate and kynurenic acid also act as agonists of the transcription factor aryl hydrocarbon receptor (AhR), which mediates a prothrombotic and proatherosclerotic phenotype of endothelial cells.44–47 In addition, animal studies have found higher blood indoxyl sulfate concentration associated with the development of diastolic dysfunction by promoting cardiac hypertrophy and fibrosis.12 Finally, uremic toxins may also increase the sympathetic nervous activity among patients with CKD, aggravating hypertension by stimulating presympathetic neurons in the rostral ventrolateral medulla, an important brain region that regulates blood pressure.48 These experimental studies provide biological plausibility for the hypothesis that diminished kidney clearances of these secretory solutes are associated with cardiovascular outcomes, particularly in the setting of advanced CKD.
Previous human studies have reported associations between plasma concentrations of uremic toxins and cardiovascular events among patients receiving maintenance hemodialysis. For example, cohort studies in dialysis populations have reported associations of indoxyl sulfate, p-cresol, and p-cresol sulfate with incident heart failure, cardiovascular death, and a composite outcome of cardiovascular death, myocardial infarction, myocardial ischemia, ischemic stroke, and new onset of peripheral vascular disease.49–51 Among patients with mild-moderate CKD, some studies have reported associations of indoxyl sulfate and p-cresol sulfate concentrations with cardiovascular events; however, these studies did not control for estimated GFR in their analyses.52, 53 In the current study, adjustment for estimated GFR markedly diminished the associations of secretory solute clearances with incident cardiovascular events.
Our findings suggest that the kidney clearances of the measured solutes, and circulating concentrations of these solutes, are negligibly associated with cardiovascular outcomes for a given level of GFR. It is possible that tubular secretory clearance and GFR are too closely linked within an individual to reliably distinguish their cardiovascular impacts among stable outpatients with CKD. However, we previously observed associations of these secretory clearances with CKD progression and mortality after adjustment for GFR in this cohort, including adjustment for directly measured GFR determined by iothalamate clearance.32, 54 It is also possible that the burden of secretory solutes in our study population was insufficiently high to impact the selected cardiovascular outcomes above and beyond GFR. Another possible explanation is that only the unbound portion of these solutes, which is eliminated by glomerular filtration, exerts cardiovascular toxicity. This hypothesis is supported by previous studies in which total p-cresol and p-cresol sulfate concentrations were less strongly associated with cardiovascular events compared with their free counterparts, in spite of a high correlation between these two variables.53 Finally, the cardiovascular outcomes under study have heterogeneous etiologies, such that compromised tubular secretory clearance could contribute to just a small fraction of their development.
We also previously reported an independent association between lower secretory clearances and all-cause mortality in the CRIC cohort.32 This raises a question about the underlying mechanism of the association seen with death, as in the current study we found no association between lower secretory clearances and cardiovascular events, which is the leading cause of death in patients with CKD.55, 56 Taken together, this evidence may suggest potential associations between compromised proximal tubular secretion and other major causes of death in CKD, such as cancer and infections. Previous studies have linked the accumulation of uremic toxins with cancer and infection; however, the relationship between lower kidney clearances of secretory solutes and these outcomes remains unknown, warranting further investigations.37, 38, 57, 58
Important strengths of this study include accurate and precise laboratory measurements of secretory solutes using LC-MS/MS assays that were developed for this purpose, evaluation of 24-hour kidney clearances of these solutes to reduce dependency on their production, and physician adjudicated cardiovascular outcomes over long-term follow-up. Studies were conducted in a large, nationally representative prospective cohort study of patients with CKD using standardized procedures to measure potential confounding characteristics, including eGFR. Several important limitations should be considered. First, the kidney clearances of secretory solutes are subject to variability due to biologic flucutation in plasma concentrations and imprecision of timed urine collections. Errors in measuring sectetory clearances may have contributed to null associations with the cardiovascular outcomes under investigation. Decades of work have refined estimation of GFR whereas measurements of secretory solute clearance are less well established. Second, the CRIC study excluded persons with severely reduced eGFR at baseline (<20 mL/min/1.73m2), precluding investigation of the highest circulating concentrations of secretory solutes. Third, we excluded patients with a prior history of heart failure, myocardial infarction, and stroke at baseline to investigate associations with the initial development of these outcomes. However, subclinical cardiovascular disease is highly prevalent in CKD, suggesting that the mechanistic processes contributing to the study outcomes may have already been underway.
In summary, we found no clinically or statistically relevant association between the kidney clearances of eight endogenous secretory solutes and incident heart failure, myocardial infarction, and stroke after adjustment for eGFR in a national cohort study of CKD. These findings suggest that tubular secretory clearance provides little additional information about the development of cardiovascular disease beyond GFR among patients with mild-to-moderate CKD. Further studies are needed to elucidate why the cardiovascular toxicity of uremic toxins seen in experimental models does not translate into cardiovascular risk in human studies and to explore potential non-cardiovascular mechanisms by which these toxins are associated with all-cause mortality.
Supplementary Material
Table S1. Variability of secretory solutes and their respective urinary clearances.
Table S2. Kidney clearances and protein binding of secretory solutes.
Table S3. Associations between secretory solute clearances and subdistribution hazard ratio of cardiovascular events.
Table S4. Associations between secretory solute clearances and all heart failure events.
Table S5. Associations of secretory solute clearances with ischemic stroke and hemorrhagic stroke.
Table S6. Associations of plasma concentrations of secretory solutes with incident heart failure, myocardial infarction, and stroke.
Table S7. Associations of model covariates with incident heart failure, myocardial infarction, and stroke.
Figure S1. Participant flow diagram.
Figure S2. Distribution of the summary secretion score.
Figure S3. Association of summary secretion score with incident heart failure (i), myocardial infarction (ii), and stroke (iii).
Support:
This work was supported by NIH R01 DK107931. Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences(NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433,University of Illinois at Chicago CTSAUL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CT SI UL1 RR-024131. All funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRIC Study Investigators: Aside from authors Feldman, Go, and Lash, the CRIC Study Investigators comprise Lawrence J. Appel, MD, MPH, Jiang He, MD, PhD, Panduranga S. Rao, MD, Mahboob Rahman, MD, and Raymond R. Townsend, MD.
Financial Disclosure: The authors declare that they have no relevant financial interests.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. New England Journal of Medicine. 2004;351(13): 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. New England Journal of Medicine. 2005;352(20): 2049–2060. [DOI] [PubMed] [Google Scholar]
- 3.Shlipak MG, Fried LF, Cushman M, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. Jama. 2005;293(14): 1737–1745. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz U, Buzello M, Ritz E, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrology Dialysis Transplantation. 2000;15(2): 218–223. [DOI] [PubMed] [Google Scholar]
- 5.Tomiyama C, Higa A, Dalboni MA, et al. The impact of traditional and non-traditional risk factors on coronary calcification in pre-dialysis patients. Nephrology Dialysis Transplantation. 2006;21(9): 2464–2471. [DOI] [PubMed] [Google Scholar]
- 6.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharmaceutical research. 2007;24(7): 1227–1251. [DOI] [PubMed] [Google Scholar]
- 7.Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. American Journal of Physiology-Renal Physiology. 2008;294(4): F867–F873. [DOI] [PubMed] [Google Scholar]
- 8.Yacovino LL, Aleksunes LM. Endocrine and metabolic regulation of renal drug transporters. Journal of biochemical and molecular toxicology. 2012;26(10): 407–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duranton F, Cohen G, De Smet R, et al. Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23(7): 1258–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii H, Nishijima F, Goto S, et al. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol Dial Transplant. 2009;24(7): 2089–2095. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69(10): 1780–1785. [DOI] [PubMed] [Google Scholar]
- 12.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? European heart journal. 2010;31(14): 1771–1779. [DOI] [PubMed] [Google Scholar]
- 13.Baran H, Staniek K, Kepplinger B, Gille L, Stolze K, Nohl H. Kynurenic acid influences the respiratory parameters of rat heart mitochondria. Pharmacology. 2001;62(2): 119–123. [DOI] [PubMed] [Google Scholar]
- 14.Feldman HI, Appel LJ, Chertow GM, et al. The chronic renal insufficiency cohort (CRIC) study: design and methods. Journal of the American Society of Nephrology. 2003;14(suppl 2): S148–S153. [DOI] [PubMed] [Google Scholar]
- 15.Bush KT, Wu W, Lun C, Nigam SK. The drug transporter OAT3 (SLC22A8) and endogenous metabolite communication via the gut–liver–kidney axis. Journal of Biological Chemistry. 2017;292(38): 15789–15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee EP, Clish CB, Ghorbani A, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. Journal of the American Society of Nephrology. 2013;24(8): 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney international. 2013;84(3): 585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Zelnick LR, Chen Y, et al. Alterations of Proximal Tubular Secretion in Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol. 2019;15(1): 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Zelnick LR, Hoofnagle AN, et al. Differences in proximal tubular solute clearance across common etiologies of chronic kidney disease. Nephrol Dial Transplant. 2019;35(11): 1916–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bansal N, Anderson AH, Yang W, et al. High-sensitivity troponin T and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and risk of incident heart failure in patients with CKD: the Chronic Renal Insufficiency Cohort (CRIC) Study. Journal of the American Society of Nephrology. 2015;26(4): 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. New England Journal of Medicine. 1971;285(26): 1441–1446. [DOI] [PubMed] [Google Scholar]
- 22.Einhorn PT, Davis BR, Massie BM, et al. The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) heart failure validation study: diagnosis and prognosis. American heart journal. 2007;153(1): 42–53. [DOI] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Journal of the American College of Cardiology. 2007;50(22): 2173–2195. [DOI] [PubMed] [Google Scholar]
- 24.Liu KD, Yang W, Go AS, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. American Journal of Kidney Diseases. 2015;65(2): 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clinical Journal of the American Society of Nephrology. 2009;4(8): 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AH, Yang W, Hsu C-y, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. American Journal of Kidney Diseases. 2012;60(2): 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu C-y, Propert K, Xie D, et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. Journal of the American Society of Nephrology. 2011;22(10): 1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radulescu V, Goyfman M, Mohler ER III, Gao YL, Budoff MJ, Investigators CS. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: Results from CRIC study. Atherosclerosis. 2015;242(1): 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman M, Yang W, Akkina S, et al. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clinical Journal of the American Society of Nephrology. 2014;9(0): 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeffer MA, Burdmann EA, Chen C-Y, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine. 2009;361(21): 2019–2032. [DOI] [PubMed] [Google Scholar]
- 31.Bundy JD, Chen J, Yang W, et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: The CRIC study. Atherosclerosis. 2018;271: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Zelnick LR, Wang K, et al. Kidney Clearance of Secretory Solutes Is Associated with Progression of CKD: The CRIC Study. Journal of the American Society of Nephrology. 2020;31(4): 817–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bajaj A, Xie D, Cedillo-Couvert E, et al. Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. American Journal of Kidney Diseases. 2019;73(6): 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bansal N, Zelnick L, Go A, et al. Cardiac Biomarkers and Risk of Incident Heart Failure in Chronic Kidney Disease: The CRIC (Chronic Renal Insufficiency Cohort) Study. Journal of the American Heart Association. 2019;8(21): e012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999;94(446): 496–509. [Google Scholar]
- 36.Hommel G. A stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;75(2): 383–386. [Google Scholar]
- 37.Dou L, Cerini C, Brunet P, et al. P-cresol, a uremic toxin, decreases endothelial cell response to inflammatory cytokines. Kidney international. 2002;62(6): 1999–2009. [DOI] [PubMed] [Google Scholar]
- 38.Faure V, Cerini C, Paul P, Berland Y, Dignat-George F, Brunet P. The uremic solute p-cresol decreases leukocyte transendothelial migration in vitro. International immunology. 2006;18(10): 1453–1459. [DOI] [PubMed] [Google Scholar]
- 39.Dou L, Jourde-Chiche N, Faure V, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. Journal of Thrombosis and Haemostasis. 2007;5(6): 1302–1308. [DOI] [PubMed] [Google Scholar]
- 40.Vaziri ND, Zhao Y-Y, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrology Dialysis Transplantation. 2016;31(5): 737–746. [DOI] [PubMed] [Google Scholar]
- 41.Moradi H, Sica DA, Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. American journal of nephrology. 2013;38(2): 136–148. [DOI] [PubMed] [Google Scholar]
- 42.Kharait S, Haddad DJ, Springer ML. Nitric oxide counters the inhibitory effects of uremic toxin indoxyl sulfate on endothelial cells by governing ERK MAP kinase and myosin light chain activation. Biochemical and biophysical research communications. 2011;409(4): 758–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Addi T, Dou L, Burtey S. Tryptophan-derived uremic toxins and thrombosis in chronic kidney disease. Toxins. 2018;10(10): 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gondouin B, Cerini C, Dou L, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney international. 2013;84(4): 733–744. [DOI] [PubMed] [Google Scholar]
- 45.Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins. 2014;6(3): 934–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu C-C, Lu Y-C, Chiu C-A, et al. Levels of indoxyl sulfate are associated with severity of coronary atherosclerosis. Clinical and Investigative Medicine. 2013: E42–E49. [DOI] [PubMed] [Google Scholar]
- 47.DiNatale BC, Murray IA, Schroeder JC, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicological Sciences. 2010;115(1): 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oshima N, Onimaru H, Matsubara H, et al. Uric acid, indoxyl sulfate, and methylguanidine activate bulbospinal neurons in the RVLM via their specific transporters and by producing oxidative stress. Neuroscience. 2015;304: 133–145. [DOI] [PubMed] [Google Scholar]
- 49.Wu I-W, Hsu K-H, Hsu H-J, et al. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrology Dialysis Transplantation. 2012;27(3): 1169–1175. [DOI] [PubMed] [Google Scholar]
- 50.Meijers B, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney international. 2008;73(10): 1174–1180. [DOI] [PubMed] [Google Scholar]
- 51.Cao X-S, Chen J, Zou J-Z, et al. Association of indoxyl sulfate with heart failure among patients on hemodialysis. Clinical Journal of the American Society of Nephrology. 2015;10(1): 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barreto FC, Barreto DV, Liabeuf S, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clinical Journal of the American Society of Nephrology. 2009;4(10): 1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meijers BK, Claes K, Bammens B, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clinical journal of the american society of nephrology. 2010;5(7): 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suchy-Dicey AM, Laha T, Hoofnagle A, et al. Tubular secretion in CKD. Journal of the American Society of Nephrology. 2016;27(7): 2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson S, James M, Wiebe N, et al. Cause of death in patients with reduced kidney function. Journal of the American Society of Nephrology. 2015;26(10): 2504–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. The Lancet. 2013;382(9889): 339–352. [DOI] [PubMed] [Google Scholar]
- 57.Russo P. End stage and chronic kidney disease: associations with renal cancer. Frontiers in oncology. 2012;2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen G, Hörl WH. Immune dysfunction in uremia—an update. Toxins. 2012;4(11): 962–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variability of secretory solutes and their respective urinary clearances.
Table S2. Kidney clearances and protein binding of secretory solutes.
Table S3. Associations between secretory solute clearances and subdistribution hazard ratio of cardiovascular events.
Table S4. Associations between secretory solute clearances and all heart failure events.
Table S5. Associations of secretory solute clearances with ischemic stroke and hemorrhagic stroke.
Table S6. Associations of plasma concentrations of secretory solutes with incident heart failure, myocardial infarction, and stroke.
Table S7. Associations of model covariates with incident heart failure, myocardial infarction, and stroke.
Figure S1. Participant flow diagram.
Figure S2. Distribution of the summary secretion score.
Figure S3. Association of summary secretion score with incident heart failure (i), myocardial infarction (ii), and stroke (iii).


