Abstract
An important challenge in mental health research is to translate findings from cognitive neuroscience and neuroimaging research into effective treatments that target the neurobiological alterations involved in psychiatric symptoms. To address this challenge, in this review we propose a heuristic neurocircuit-based taxonomy to guide the treatment of obsessive-compulsive disorder (OCD). We do this by integrating information from several sources. First, we provide case vignettes in which patients with OCD describe their symptoms and discuss different clinical profiles in the phenotypic expression of the condition. Second, we link variations in these clinical profiles to underlying neurocircuit dysfunctions, drawing on findings from neuropsychological and neuroimaging studies in OCD. Third, we consider behavioral, pharmacological and neuromodulatory treatments that could target those specific neurocircuit dysfunctions. Finally, we suggest methods of testing this neurocircuit-based taxonomy as well as important limitations to this approach that should be considered in future research.
“Essentially all models are wrong, but some are useful”
George Box
Introduction
Mental health researchers face the conceptual challenge of developing new frameworks to understand and classify mental disorders and translating this knowledge into effective treatments. Current diagnostic classification systems [1–2] are effective in ensuring communication of diagnoses among clinicians but are largely “etiologically agnostic” when determining the biological nature of psychopathology [3]. High levels of psychiatric comorbidity and heterogeneity, fluid symptom trajectories, cross-diagnostic overlap in genetic and neurobiological factors, and the limited efficacy of many psychiatric treatments restrict our understanding of the neurobiology of mental illness based on categorical diagnoses [3–6]. An alternative approach is to exploit the new knowledge gained from advances in neuroimaging concerning the brain circuits involved in psychiatric symptoms to build a neurocircuit-based taxonomy for understanding and treating mental disorders. Indeed, clusters of individuals with neural network alterations have been identified in depression [7–8], psychotic disorders [9] and attention-deficit/hyperactivity disorder (ADHD [10]). Distinct neurocircuit alterations could help explain the heterogeneity of mental disorders, and targeted interventions focused on specific circuit dysfunctions could improve treatment efficacy and guide clinical practice.
Obsessive-compulsive disorder (OCD) may be particularly amenable to this approach since it represents one of the better investigated neurocircuit-based models [11]. OCD has traditionally been associated with dysfunctional cortico-striatal-thalamo-cortical (CSTC) circuits [12–13], although it is now recognized that alterations in other circuits (fronto-limbic, fronto-parietal, cerebellar) also play a role [14]. Abnormalities in these different neurocircuits likely interact with each other to generate the complex OCD phenotype [13–14]. Therefore, individuals with OCD may have different degrees and patterns of alterations in these neurocircuits, leading to the phenotypic heterogeneity. Finally, there are already several initiatives identifying trans-diagnostic aspects of the clinical phenomenology of obsessive-compulsive symptoms based on their association with broader concepts, such as compulsivity and anxiety [14–16].
In this review, we expand on previous models of the neurocircuitry involved in OCD [12–13, 16] to propose a heuristic neurocircuit-based taxonomy which could be used to guide the treatment of the disorder in future. We do so by firstly providing case vignettes in which patients describe their symptoms, covering much of the phenotypic expression of OCD. Second, we present hypothetical associations between the phenomenology of OCD symptoms and underlying neurocircuit dysfunctions, drawing on findings from neuropsychological and neuroimaging studies in OCD. Third, we consider treatments that could target those specific neurocircuit dysfunctions to improve therapeutic response. Fourth, we suggest ways in which this neurocircuit-based taxonomy could be tested in future research to support or refute our hypothesized links between aspects of clinical phenomenology, specific neurocircuit alterations and treatment methods. Finally, we discuss limitations to the neurocircuit-based approach, in particular the challenge involved in attempting to map different clinical profiles that overlap in individual patients, onto discrete neurocircuits, which are interconnected and affect each other.
OCD and implicated neurocircuits
OCD has a lifetime prevalence of 1–3% worldwide [17]. The disease course is often chronic [18] and associated with significant functional impairments, financial costs and increased mortality [19–20]. OCD is characterized by compulsions (repetitive, ritualized behaviors or mental acts) that are often performed in response to obsessions (recurrent, intrusive, unwanted, distressing, time-consuming thoughts) that generate fear and anxiety (with or without autonomic symptoms) or doubts/uncertainty. At the same time, there is phenotypic heterogeneity. For example, the content of obsessions and expression of compulsions can vary dramatically between individuals, ranging from concerns about contamination or harm to preoccupation with symmetry or taboo thoughts [21]. In addition, some patients do not have obsessions but perform their rituals to relieve or achieve specific tactile, visual or auditory sensations, a sense of completeness or a just-right feeling (i.e., sensory phenomena [22]). Repetitive behaviors originally undertaken to neutralize obsessions may, over time, become habits that are elicited by stimuli rather than by intrusive thoughts [23]. Some patients carry out compulsive behaviors because they feel rewarding and almost pleasurable, rather than to elicit a sense of relief [24]. Finally, higher-order cognitive alterations, such as executive dysfunction, may trigger or perpetuate compulsive behaviors for some patients [25].
Attempts to subtype OCD into phenotypes based on clinical factors that vary across individuals, including age-at-onset, symptom dimension, degree of insight into the rationality of symptoms, comorbid psychiatric conditions, course of illness and response to treatment, have been conducted [22, 26–31]. However, it remains unclear how these different phenotypic subtypes are associated with specific pathophysiological mechanisms or their usefulness in guiding treatment selection [21]. Therefore, in this review we take a different approach to characterizing OCD phenotypes and identifying treatment targets. Specifically, we link variations in the clinical presentation of the disorder (Table 1) to neurocognitive dysfunctions in five neurocircuits that have previously been implicated in OCD (Figure 1, Table 2) [12–14, 16, 32] and discuss how treatments that more specifically target these circuits (Table 3) might benefit patients. Only a few previous attempts have been made to relate alterations in these circuits to particular clinical profiles and treatment approaches [e.g. 11, 33–34]. To our knowledge, no published work has incorporated patients’ subjective reports of their experiences of symptoms or considered specific treatment strategies directed at a range of clinical profiles and neurocircuit alterations. It is important to note that in the following sections we present a simplified account of the functions of the five neurocircuits for the purposes of synthesizing a vast literature and providing coherent and testable hypotheses of links between specific clinical profiles and neurobiological alterations in each neurocircuit. However, we emphasize in advance that the functions of these neurocircuits are considerably more complex and not limited to the neurocognitive alterations we describe here. Likewise, the neurocircuits are highly interactive and not as segregated as they may appear in the following sections. We highlight some of these complexities as we discuss each circuit and consider them in more depth in the Limitations section at the end of the article.
Table 1.
Case vignettes: translated reports from patients describing different clinical profiles of OCD that could reflect abnormalities in specific neurocircuits
| OCD Clinical Profile | Case vignette | Predominant neurocircuit involved |
|---|---|---|
| Dysregulated fear | Case vignette 1: A 13-year-old female without previous history of obsessive-compulsive symptoms (OCS). She describes the hardest period of her OCD as follows: | Fronto-limbic |
| “I thought I was pregnant. This made me feel really bad and fearful. It was a thought that used to come into my head all the time. This feeling started the day after I went to a party and kissed, for the first time, my boyfriend on the mouth. [The thought started] for now reason and I was suddenly feeling very bad about it – scared and distressed that I was pregnant. I wanted to lie in bed all day, I didn’t want to go to school, even though I knew I could feel better, but I didn’t feel like doing anything. When I had these thoughts [that I was pregnant] I felt unwell: chest pain, sometimes shortness of breath, my heart pounding and feeling sick, sometimes nauseated. To alleviate that discomfort I used to ask my mother or my best friend all the time if I was pregnant. I asked them to reassure me that I was not [pregnant] and then I used to feel a little better, but later everything started again. And when I thought I was going to get better, it got worse.” | ||
| Two years later she reported feeling fine without OCS. When asked about what made her feel she was pregnant she responded: | ||
| “I don’t know, I felt so bad that I thought it was true, I felt anxious, difficulty breathing, chest tightness, sweat, cold hands, I didn’t feel like eating. So I thought: If I wasn’t [pregnant] I wouldn’t be feeling like this.” | ||
| Intolerance of Uncertainty (IU) | Case vignette 2: An 18-year-old male with a history of panic attacks and generalized anxiety disorder describes an overlap of OCS (characterized by somatic obsessions) and IU: | Fronto-limbic |
| “Control for me is the essential basis for everything. I seek control in everything I do, otherwise I don’t do it. Either I have control or I don’t do it…. Because if I have control, there is no uncertainty, which is what I’m so afraid of, because if I have control I’ll be prepared, and it’s something that makes me more comfortable. For instance, I have the concern that I can feel dizzy or unwell when I leave home. What if I have not eaten enough or my body needs more while I am out? What if there is something wrong with my stomach and I get nauseated? Therefore, every time I leave home, I have to take some meals with me and specific medicines to make sure I have them all if I feel unwell. | ||
| Sensory phenomena (SP) | Case vignette 3: A 15-year-old male who also has Tourette syndrome reports: | Sensorimotor |
| “I felt my hands, like there is always some dirty thing stuck onto them bothering me. To relieve it, I wash them over and over again. It is this tactile sensation in my hands that drives me to wash them. I do not feel any type of fear or disgust associated to it”. | ||
| Altered habit-formation and impaired inhibition | Case vignette 4: A 28-year-old female, whose OCS started when she was 13 years old, describes her checking rituals: | Sensorimotor and Ventral cognitive |
| “At work I felt a need to check everything every time I was leaving, as I’m the last to leave. I checked if the windows were closed, if my computer was off, if the printers were off, if the fan was off… My concern was that something could happen, such as a short circuit, or that something could be left on and catch fire. I had a fear about it.” | Ventral cognitive | |
| “But then, over time, it became an automatic behavior, whenever I left I checked everything and no longer thought so much about the fears. I knew the consequences, it was clear to me what would happen if I didn’t do it [check everything], but I didn’t think so much, I did things automatically. Now I’ve been trying to avoid it [checking everything]… at first I was worried about what would happen, but then it became something a feeling that something was missing, that I forgot something, sometimes I checked if my backpack was with me when I was close to home because it seemed like I forgot something, something was missing, something was incomplete. I was no longer afraid, but it was a sense of incompleteness.” | Sensorimotor | |
| Altered reward responsiveness | Case vignette 5: An 18-year-old male (the same patient as in vignette 2) with somatic obsessions and intrusive thoughts about not being prepared with all he might need when leaving the house presents with altered reward responsiveness related to his OCS: | Ventral reward |
| “Just as it could potentially give me pleasure, it may potentially not give me pleasure, it could make me feel bad, so I prefer not to take that risk. Faced with new situations I prefer not to risk; I duck out and I assume in my head that I do not want that or that I wouldn’t like to do it. I feel like staying home is wonderful in this moment, my dream is to stay home because it is a safe haven. I don’t need to plan to stay home, I don’t need to be careful, I don’t need to check schedules, I don’t need to carry medicines with me, because I’m home and I have everything I need there, so I don’t have these worries and this psychological pressure. In this sense, staying at home and not having the uncomfortable feeling I experience when I leave the house is more pleasurable for me than doing something that could potentially be fun.” |
Figure 1. Five circuits involved in OCD.
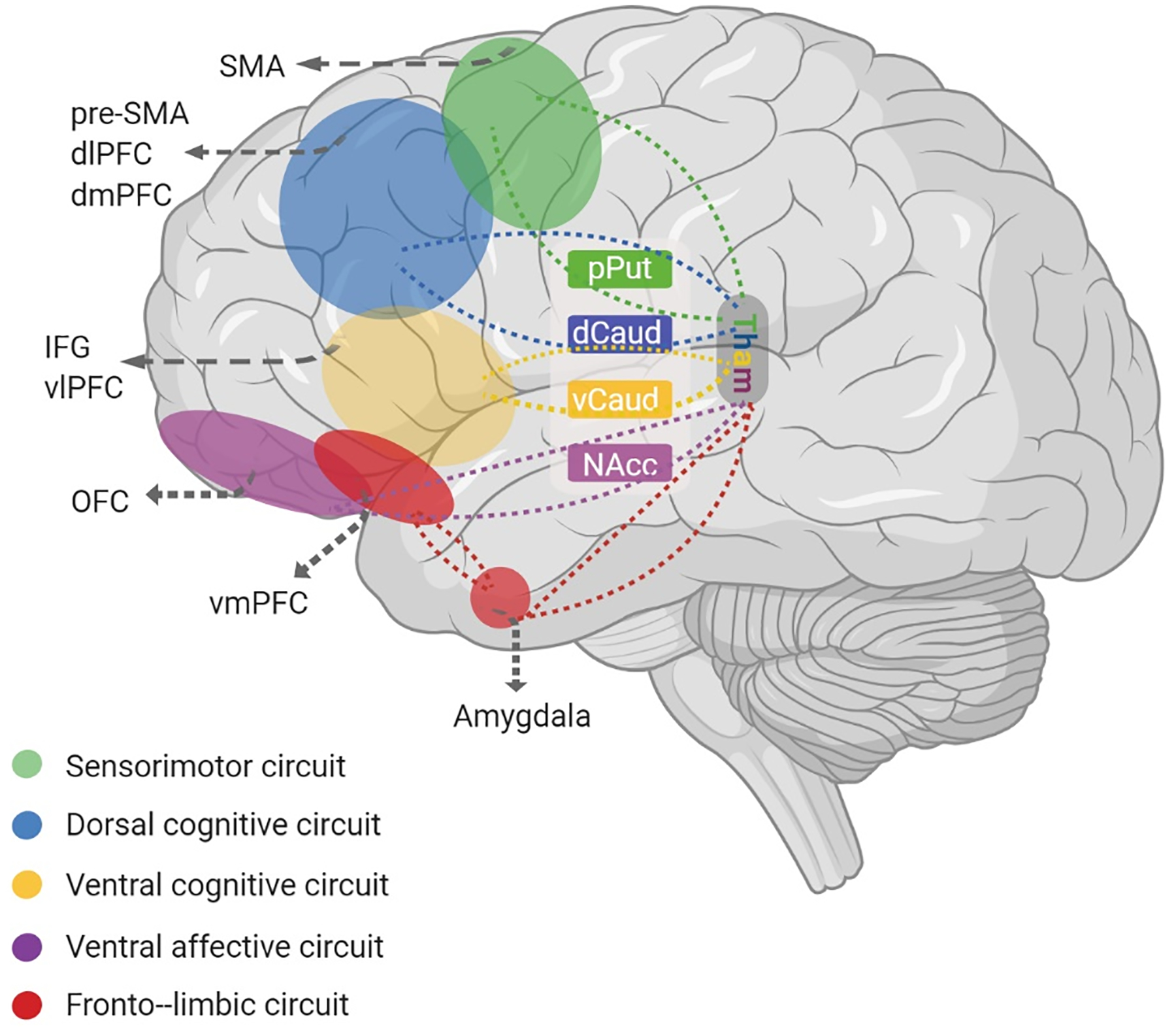
according to van den Heuvel et al. (2016) [16]. The fronto-limbic circuit (red) includes the amygdala and ventromedial prefrontal cortex (vmPFC) and is involved in producing emotional responses, such as fear and anxiety. The sensorimotor circuit (green) includes the supplementary motor area (SMA), putamen and thalamus and is involved in producing and controlling motor behavior and the integration of sensory information. The ventral cognitive circuit (yellow) includes the inferior frontal gyrus (IFG), ventrolateral prefrontal cortex (vlPFC), ventral caudate and thalamus and is involved in self-regulatory behavioral control. The ventral affective circuit (purple) includes the orbitofrontal cortex, nucleus accumbens (NAcc) and thalamus and is involved in processing and responding to reward. The dorsal cognitive circuit (blue) includes the dorsolateral prefrontal cortex (dlPFC), dorsomedial prefrontal cortex (dmPFC), dorsal caudate and thalamus and is involved in executive functions (e.g. working memory, planning) and emotion regulation.
Table 2.
Neurocognitive functions of the five neurocircuits that may represent OCD clinical profiles and examples of experimental tasks and instruments that have been used to measure these functions
| Neurocircuit principally involved | Neurocognitive dysfunction / potential OCD clinical profile | Methods of assessment: examples of experimental tasks and instruments |
|---|---|---|
| Fronto-limbic | Dysregulated fear: excessive and/or poorly controlled responses to fear-provoking stimuli |
Fear/symptom provocation tasks [39, 60]: Participants are presented with stimuli that provoke OCD symptoms (e.g. pictures of electrical appliances/doors to provoke checking, dirty toilet to provoke contamination/washing), aversive/threatening stimuli that are unrelated to OCD symptoms but provoke general fear responses (e.g. pictures of mutilated bodies, spiders), and neutral stimuli. Some versions of this task also include emotional regulation conditions in which participants attempt to reduce their emotional reactions, e.g. using cognitive reappraisal. Fear conditioning/fear extinction tasks [36, 40–41]: Participants are presented with two “conditioned” stimuli (“CS”, e.g. yellow and blue squares), one of which (“CS+”) is paired with an aversive “unconditioned” stimulus (“US”, e.g. a scream) and the other (“CS−”) is not. Repeated presentations of these stimuli result in learning of the association between the CS+ and US and a fear response develops to the CS+ (fear acquisition), as measured by the skin conductance responses (SCR). Subsequently, the CS+ and CS− are presented alone, without the aversive US, resulting in extinction of the learned fear response to the CS+. |
| Fronto-limbic | Intolerance of Uncertainty (IU): the tendency to perceive and interpret uncertain or ambiguous situations as negative or threatening, a reduced ability to cope with uncertainty or to respond with impulsive or avoidance behaviours, and an excessive need to establish certainty |
Intolerance of Uncertainty Scale (IUS) [193]: The IUS is a 27-item self-report rating scale in which participants rate the extent to which uncertainty is perceived to be unacceptable and reflects badly on a person and causes frustration, stress and the inability to act. Items are rated on a 5-point Likert scale ranging from 1 (not at all characteristic of me) to 5 (entirely characteristic of me). Higher scores reflect greater IU. Uncertainty paradigms [46–47]: These tasks involve the presentation of positive, negative and neutral stimuli preceded by informative or uninformative cues creating certain or uncertain anticipation conditions [46] or decision-making under varying levels of uncertainty [47]. |
| Sensorimotor | Sensory phenomena (SP): aversive or uncomfortable sensations or perceptions that drive repetitive behaviors, the sensation or perception (tactile, auditory, or visual domains) that things are “not-just right”, “incompleteness” symptoms, where a repetitive behavior is performed until the patient feels it is finished or “complete”, or tactile sensations of feeling dirty that drive washing and cleaning compulsions (rather than fear of illness or disease). |
Sensory Phenomena Scale (SPS) [75]: The SPS is a semi-structured interview containing a checklist composed of examples of different types of SP preceding or occurring at the same time as repetitive behaviors. The checklist includes items describing physical sensations, “not just right” sensations, incompleteness, general energy or inner tension build-up, and urges. Total severity (of all SP endorsed) is measured through ratings of frequency, distress, and interference, with a total score ranging from zero (no SP) to 15 (extreme SP). Not-Just-Right Experiences Questionnaire (NJREQ) [194]: a self-report scale containing 19 items that assess feelings of things being “not just right” and sensations of incompleteness, e.g. “I have had the sensation after getting dressed that parts of my clothes did not feel just right”. Items are rated for their presence/absence, distress, rumination, urge to respond and feeling of responsibility over the past month. Higher NJREQ scores reflect greater not-just-right experiences. Incompleteness subscale of the Obsessive-Compulsive Core Dimensions Questionnaire (OC-CDQ) [77]: a self-report scale that contains 10 items that assess feelings of incompleteness and behaviors associated with these e.g. “I must do things in a certain way or they will not feel right”. Higher Incompleteness subscale scores reflect greater feelings of incompleteness. Body focused videos task [87, 105]: Participants view “body-focused” videos depicting repeated movements and/or sensory stimulation of the body (e.g. a brush stroking a hand, a person’s throat while swallowing) designed to elicit activation in sensorimotor circuitry and control videos (e.g. the tip of a brush moving across a table, a ball sliding through a tube). Urges-for-action tasks [80–85]: These tasks involve measuring neural activity during “urges-for-action”, i.e. sensations that drive an individual to perform a behavior, such as the urge to swallow, scratch, or blink. |
| Sensorimotor | Altered habit formation: habits are inflexible behaviors that are carried out largely unconsciously and are insensitive to motivation and consequences. Excessive habit-formation due to excessive sensorimotor circuit activity and/or an over-reliance on the habit-learning system at the expense of the goal-directed system may underlie some forms of compulsions (e.g. checking) in OCD. | Habit-learning tasks [88–91]: these tasks involve participants learning, by trial-and-error, associations between stimuli (e.g. colored shapes) and simple motor responses (e.g. left- or right-hand button presses) with valenced feedback or rewards to reinforce the correct stimulus-response associations. After the stimulus-response associations have been learned, tests are administered to assess the degree of habit-learning. These tests include “slips-of-action” in which some stimulus-response associations are no longer rewarded (“outcome-devaluation”) and should therefore no longer be produced. Habit-learning is inferred when the participant continues to make the motor responses for devalued associations, since this indicates the behavior is insensitive to rewards. |
| Ventral cognitive | Impaired response inhibition: impaired ability to withhold inappropriate or unwanted behaviors | Go/Nogo task, Stop Signal Task (SST) [119]: The Go/Nogo task requires participants to make rapid motor responses (usually a button-press) to frequently presented “Go” stimuli and to withhold those motor responses to infrequent “Nogo” stimuli. The requirement to respond frequently and rapidly to Go stimuli builds up a prepotent (automatic) response tendency, which is difficult to withhold on Nogo trials requiring the engagement of inhibitory control circuitry. The SST similarly involves producing rapid motor responses to frequent Go stimuli, unless those stimuli are followed by a “stop signal” (usually an auditory tone) indicating that the response should be “stopped”; similarly to Nogo trials, Stop trials require the engagement of inhibitory control to prevent oneself producing the motor response. |
| Ventral affective | Altered reward responsiveness: alterations in the ability to anticipate, represent and respond to rewards, such blunted sensitivity to rewards and over-generalization of punishment |
Monetary Incentive Task [137]: Participants are presented with three cues (e.g. different colored squares) which predict either a monetary gain (reward), monetary loss (punishment), or neutral outcome (no monetary gain or loss) if they make a response to an upcoming target within a certain time-frame. Gambling tasks e.g. the Iowa Gambling Test [146]: Gambling tasks such as the IGT require the participant to play a game in which they make choices from different options, such as choosing cards from different decks in the IGT, to win as much money or as many points as possible. Choices from different options (e.g. decks of cards) are associated with different probabilities of winning and losing money/points and with different values of rewards and punishments. The participant must learn which decks are safe and which are risky, and use that information to guide their decision making. Reward learning tasks [143, 149]: Participants are required to learn new behaviors, such as associations between stimuli and outcomes or stimuli and responses, by trial-and-error using reward and punishment information provided as feedback after each trial. |
| Dorsal cognitive | Executive dysfunction: impairments in functions that are necessary for effective goal-directed behavior, including working memory (the ability to hold and manipulate information) and planning (the ability to organise thoughts and behaviors) |
N-back working memory task [121]: The N-back task has different versions and, while some use letters, others use the spatial location of geometric figures as stimuli. In this task, participants see a sequence of stimuli and they have to remember if the current stimulus is equal to the previous one (N = 1), two (N=2), three (N=3) or N trials ago. The participant has to respond with a delay, accordingly to which condition is being evaluated at the moment: a higher N means higher difficulty and higher loads of working memory. Tower of London (ToL) and Tower of Hanoi (ToH) planning tasks [121]: These tasks evaluate visuospatial planning; the participant has to solve a problem using a minimal number of steps (movements) to rearrange a pattern accordingly to a model. In the ToL, the disks differ by color while in the ToH they are of different sizes. |
| Dorsal cognitive | Impaired emotion regulation: difficulty in exerting top-down control over emotional responses | Regulation of responses to OCD-provocation tasks [39, 60]: Participants are asked to alter their emotional responses to OCD-provoking stimuli by methods such as cognitive re-appraisal (changing their evaluation of the stimulus or changing their thoughts and emotions) and distraction (think of something other than the stimulus). |
Table 3.
Treatment options for OCD
| Treatment | Description | Neurocircuits/clinical profiles affected |
|---|---|---|
| Behavioral interventions | ||
| Cognitive behavioral therapy (CBT) | CBT is a first-line treatment for children and adults with OCD and comprises two components: exposure and response prevention (ERP) and cognitive reappraisal. ERP involves gradual and prolonged exposure to fear-provoking stimuli combined with instructions to abstain from the compulsive behavior. Cognitive reappraisal involves the patient learning to change their thoughts and emotions concerning symptoms. | The ERP aspect of CBT is likely to be most helpful for individuals with dysregulated fear, targeting over-active fronto-limbic circuitry (amygdala, vmPFC) during the experience of OCD symptoms. The cognitive reappraisal aspect of CBT engages dorsal cognitive circuitry to down-regulate over-active fronto-limbic activity and may be useful for individuals with dysregulated fear (fronto-limbic circuit dysfunction) and impaired emotion regulation (dorsal cognitive circuit dysfunction). |
| Habit-reversal training | Habit-reversal training is currently used for treatment of tics in Tourette syndrome (TS). Patients are trained to become aware of the sensory sensations that precede tics (premonitory urges) and to make competing, voluntary movements that prevent tic production. | Habit-reversal training could be used to address compulsive habits in OCD, for instance those that are preceded by sensory phenomena (sensorimotor system dysfunction). |
| Pharmacotherapy | ||
| Selective serotonin reuptake inhibitors (SSRIs) | SSRIs are the first-line pharmacological treatment for OCD based on their evidence of efficacy, tolerability, safety and absence of abuse potential. As a rule, higher doses of SSRIs are used for OCD than for other anxiety disorders or major depression. | SSRIs may be particularly appropriate for individuals with dysregulated fear and intolerance of uncertainty symptom expressions (fronto-limbic circuit dysfunction) but may also be helpful for individuals with altered responsiveness to rewards (ventral affective circuit dysfunction). |
| Pharmacotherapy in treatment-resistant OCD | ||
| Antipsychotics and methylphenidate | Antipsychotic agents have shown efficacy as add-ons for patients who do not show symptom improvements with SSRIs (including clomipramine), especially haloperidol, risperidone, aripiprazole, olanzapine and quetiapine [175]. Symptom improvements with augmentation with methylphenidate has also been found in treatment-resistant patients. | Because antipsychotic medications and methylphenidate regulate dopaminergic signalling, the use of such medications may be particularly helpful for individuals with impaired response inhibition (ventral cognitive circuit dysfunction), altered responsiveness to rewards (ventral affective circuit dysfunction) and executive dysfunction (dorsal cognitive circuit dysfunction). |
| Neuromodulation and neurosurgery | ||
| Deep Brain Stimulation (DBS) | Deep brain stimulation is a neurosurgical intervention for OCD patients refractory to multiple conventional treatments. An implantable neurostimulator is connected to electrodes that are inserted in a brain region related to the pathophysiology of OCD. Different targets have been studied so far, such as the anterior limb of the internal capsule (ALIC) and ventral capsule/ventral striatum (VC/VS), white matter bundles immediately above the accumbens, the subthalamic nucleus (STN), the bed nucleus of stria terminalis (BNST), and the inferior thalamic peduncle. The supero-lateral branch of the medial forebrain bundle (slMFB) and the anteromedial globus pallidus internus (GPi) are currently under investigation as targets as well. | DBS has applications for improving dysfunctions in fronto-limbic circuitry (BNST target for intolerance of uncertainty), ventral cognitive circuitry (STN target for response inhibition) and ventral affective circuitry (NAcc target for altered reward responsiveness). Note, however, that stimulation of the STN, the entire ALIC or its most ventral portions (as in NAcc DBS) might act on a common white matter network connecting frontal regions to the STN [70] and further tractography studies are needed to investigate the specificity of these targets to individual neurocircuits. The ALIC may be an effective target for dysfunctions in several of the neurocircuits depending on where it is targeted and the connecting white matter networks affected by stimulation [70–71, 132]. |
| Transcranial Magnetic Stimulation (TMS) | Repetitive TMS (rTMS) can be either excitatory or inhibitory and modulates neuronal activity via electric currents that are induced by a magnetic coil positioned over the head. The excitatory and inhibitory effects of rTMS are hypothesized to be similar to long-term potentiation (LTP) and long-term depression (LTD), respectively. LTP and LTD are two mechanisms of synaptic plasticity that lead to synaptic strengthening (LTP) or weakening (LTD) (i.e. an increase or decrease in synaptic efficiency). rTMS is considered to be excitatory or inhibitory when applying high-frequency (≥ 10Hz) or low-frequency (< 1Hz) protocols, respectively [195]. Widely used targets include the (pre-SMA) and the dlPFC. | rTMS targeting the SMA could be useful for addressing sensorimotor dysfunctions while rTMS targeted at the dlPFC would have applications in improving top-down control of emotional reactions (dysregulated fear symptom expression, fronto-limbic circuit) and planning and working memory problems (dorsal cognitive circuit dysfunctions). Deep TMS targeting the ACC could potentially address problems with reward alterations involved in hyperactive monitoring of errors (ventral affective circuit dysfunction). |
| Transcranial Direct Current Stimulation (tDCS) | tDCS involves the application of a weak current to the scalp, with only a fraction of the current entering the brain. Anodal tDCS is usually facilitatory whereas cathodal tDCS induces inhibitory effects [195]. | tDCS targeted at the SMA could be useful in improving sensorimotor atypicalities caused by disturbances in the sensorimotor circuit (sensory phenomena), while tDCS targeted at the OFC may help to improve fear extinction and hyperactive fear responses involved in the dysregulated fear symptom expression (fronto-limbic circuit). |
| Neurofeedback | Neurofeedback involves learning to modulate neural activity through real-time monitoring of one’s current brain state (self-regulation of mind-brain). This self-regulatory neuromodulation can be done using EEG, functional near-infrared spectroscopy (fNIRS) and fMRI. | Neurofeedback could be targeted at key regions within any of the five circuits discussed here as implicated in OCD. EEG and fNIRS based neurofeedback would be appropriate only for regions close to the surface of the head (e.g. SMA, dlPFC) due to their inability to measure deep brain structures, while fMRI-based neurofeedback could be used for both surface and deep brain structures. |
| Executive Function training | Cognitive training programs target specific cognitive functions, such as organization/planning and working memory, with daily training. | Cognitive training could be targeted at a variety of neurocognitive alterations implicated in OCD, including executive dysfunction (dorsal cognitive circuit), dysregulation fear and intolerance of uncertainty (fronto-limbic circuit) and response inhibition (ventral cognitive circuit). |
Fronto-limbic circuit
Fronto-limbic dysfunctions in OCD
The fronto-limbic circuit includes subcortical and cortical brain regions involved in generating (amygdala) and evaluating (ventromedial prefrontal cortex, vmPFC) emotional responses (Figure 2) [16, 35] amongst other functions. The circuit also connects to cortical regions involved in top-down behavioral control, including dorsolateral and dorsomedial prefrontal (dlPFC/dmPFC) regions of the dorsal cognitive circuit (Figure 1), for emotion regulation [16, 35]. Dysfunctional activity in the fronto-limbic circuit during emotional processing is a robust finding in OCD [36]. Below, we consider evidence for two specific neurocognitive alterations associated with fronto-limbic dysfunction that may be particularly relevant to OCD.
Figure 2. The fronto-limbic circuit.
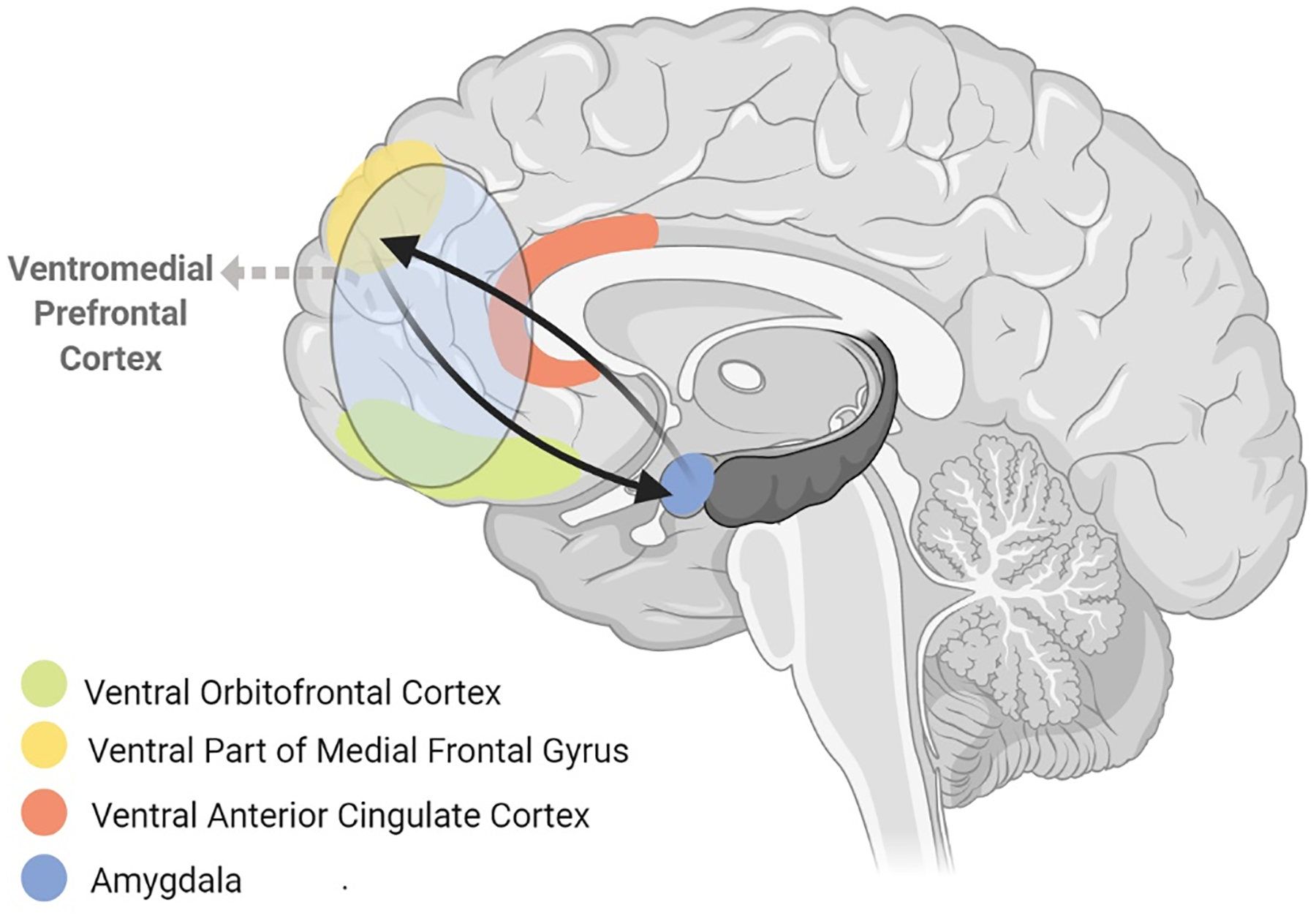
includes the amygdala and ventromedial prefrontal cortex (vmPFC, consisting of ventral orbitofrontal cortex, ventral anterior cingulate cortex and the ventral part of medial frontal gyrus). These regions are structurally and functionally connected with each other to form a network that generates emotional responses (amygdala and NAcc) and evaluates whether those responses are appropriate or require regulation (vmPFC). The fronto-limbic circuit is connected with the hippocampus and regions from other circuits that are involved in top-down behavioral control, including the dorsolateral prefrontal cortex (dlPFC) from the dorsal cognitive circuit, and can recruit these regions to dampen fronto-limbic activity thereby facilitating emotional regulation.
First, dysregulated fear, i.e. excessive and/or poorly controlled fear responses mediated by fronto-limbic circuitry, has been proposed as a key mechanism in OCD [37] (Table 2 gives a full explanation of neurocognitive functions and their associated neurocircuits, as well as examples of experimental tasks and instruments that have been used to measure these functions). Clinically, some patients report that their symptoms are triggered or maintained by feelings of fear. For example, in Case vignette 1 (Table 1), what seemed to trigger or perpetuate the patient’s obsessive belief that she was pregnant was the physiological fear response to the thought that she could be pregnant (I must be pregnant, or I wouldn’t be feeling like this). Thus, in some patients, dysregulated fear responses to intrusive thoughts mediated by fronto-limbic circuitry could be the starting point that turns ordinary thoughts into obsessions. In support, previous studies investigating the content of patients’ descriptions of their OCD symptoms have revealed that physiological changes associated with fear and anxiety, such as a racing heart, play a key role in triggering a variety of obsessions and compulsions [38]. Difficulties with top-down cognitive appraisal, such as interpreting one’s own thoughts and feelings as evidence that an unlikely event occurred, likely also play a role in perpetuating obsessive symptoms. Consistent with these clinical observations, neuroimaging studies have reported increased amygdala activity, reduced dorsal prefrontal activity and reduced prefrontal-amygdala functional connectivity during the provocation and cognitive reappraisal of stimuli that provoke OCD symptoms and increase physiological arousal [36, 39]. Together, these findings suggest that hyperactive limbic fear responses and insufficient recruitment of dorsal prefrontal top-down control are involved in the production and/or maintenance of fear in the context of OCD triggers. There is also evidence that individuals with OCD have difficulty extinguishing learned fear responses [37, 40–42], likely due to impaired “safety-signaling” functions of the vmPFC [40], which may further contribute to the maintenance of fear-related OCD symptoms.
Second, intolerance of uncertainty (IU, Table 2) may also be involved in the OCD phenotype. IU is the tendency to perceive and interpret uncertain situations as negative or threatening; it is associated with reduced ability to cope and impulsive or avoidant responses to uncertainty, and an excessive need to establish certainty [43]. Many patients with OCD report IU when describing their symptoms. For example, the patient in Case vignette 2 (Table 1) reports being afraid of uncertainty, which drives a need for control related to his somatic obsessions. Studies investigating the content of patients’ experiences of symptoms have also identified that “a need for certainty” is a key theme involved in reassurance-seeking symptoms of OCD [44] and other studies have shown that that higher IU trait scores predict more severe OCD symptoms [43, 45]. Importantly, neuroimaging studies have reported atypically increased vmPFC and amygdala activity during the anticipation of uncertain threat [46] and during uncertainty in decision-making [47] in OCD patients, suggestive of fronto-limbic hyper-responsivity to uncertainty in OCD. Interestingly, IU has been shown to predict impaired fear extinction in non-psychiatric volunteers [48], suggesting these fronto-limbic dysfunctions may be related to one another.
It should be noted that these regions of the fronto-limbic circuit involved in dysregulated fear and IU also form networks with structures of the other five neurocircuits to participate in other neurocognitive functions. For example, the amygdala and vmPFC are also involved in reward processing along with regions of the ventral affective circuit [49]. Since altered reward processing is also implicated in OCD (discussed further in the Ventral Affective Circuit section, below), it will be important for future research to investigate how fear and uncertainty-related functional alterations in the fronto-limbic circuit affect reward processing mechanisms in the ventral affective circuit. The interactive nature of these circuits should also be kept in mind when considering neurocircuit-specific treatment strategies for the fronto-limbic circuit, discussed next.
Treatments targeting fronto-limbic dysfunctions in OCD
Several treatments for OCD may target fronto-limbic dysfunction (summarized in Table 3). For example, cognitive behavioral therapy (CBT) is an evidence-based form of psychotherapy for OCD that typically includes exposure and response prevention (ERP) with different formats varying in the degree of and procedures for training in cognitive reappraisal [50]. ERP seems to be mediated, at least in part, by modulation of amygdala-vmPFC connectivity, while cognitive reappraisal appears to dampen fronto-limbic activity via the engagement of top-down dorsal prefrontal regions [35–36, 39, 51]. CBT may therefore target fronto-limbic dysfunction directly via ERP or indirectly via cognitive reappraisal. Neuroimaging studies indicate that better CBT response in OCD is predicted by hyperactive fronto-limbic responses to OCD-provoking stimuli and weaker amygdala-prefrontal functional connectivity [52–53]. These findings lead to the hypothesis that patients who show fronto-limbic dysfunction associated with dysregulated fear may benefit most from CBT. Although not explicitly addressed in treatment, CBT/ERP has been shown to reduce IU in OCD and reductions in IU occurred prior to, or concurrent with, reductions in OCD symptoms [54–55].
Concerning pharmacological therapies, there is evidence that selective serotonin reuptake inhibitors (SSRIs), the first-line pharmacological treatment for OCD [13], reduce fronto-limbic responses to threatening stimuli in non-psychiatric volunteers [56, but see 57] and are associated with lower limbic response during emotion processing and symptom provocation in OCD [36]. Further, stronger functional connectivity between the serotonergic raphe nuclei and temporal and limbic regions including the amygdala predicts better response to SSRIs in OCD [58]. Thus, SSRIs may improve OCD symptoms in part by reducing excessive limbic activity. SSRIs can also reduce IU [59], though the mechanism underlying this improvement has not been studied.
Neuromodulation techniques (Table 3) may offer a more direct method of targeting fronto-limbic dysfunction. A sham-controlled study with OCD patients showed that high-frequency repetitive transcranial magnetic stimulation (rTMS) of the left dlPFC reduced both fear distress ratings and neural activity in the amygdala and other visual emotion processing areas and altered functional connectivity between the amygdala and dmPFC during a fear/OCD provocation task [60]. Another sham-controlled study in patients with generalized anxiety disorder showed that low-frequency (inhibitory) rTMS of the right dlPFC normalized atypical dlPFC-amygdala functional connectivity that was induced by uncertainty during a gambling task designed to elicit IU [61]. The finding that both high [60] and low [61] frequency rTMS resulted in improved fronto-limbic circuit function may seem contradictory since these protocols are hypothesized to have opposite effects on neural plasticity (see Table 3). Yet, this is consistent with findings from a recent systematic review and meta-analysis [62] of 18 randomized controlled trials (RCTs) evaluating the efficacy of rTMS for OCD, which showed that low-frequency and high-frequency rTMS were both superior to sham in improving OCD symptoms. Finally, a recently published, large (n=99) multicenter randomized clinical trial showed that deep TMS targeting mPFC and the anterior cingulate cortex, associated with individualized symptom provocation, was superior to sham in improving OCD symptoms [63].
Another series of studies has reported that fMRI-based neurofeedback targeted at orbitofrontal regions in which activity was correlated with contamination anxiety in individuals without psychiatric diagnoses but with high trait scores of contamination obsessions and washing compulsions resulted in significantly reduced connectivity between the orbitofrontal regions and other limbic areas, including the amygdala, as well as significantly increased connectivity in prefrontal regions associated with emotion regulation, including right lateral PFC [64]. Importantly, self-report anxiety ratings while viewing contamination-provoking stimuli were also significantly reduced several days following fMRI-neurofeedback [64]. Furthermore, this fMRI-neurofeedback protocol resulted in a 20% decrease in OCD symptom severity in a small sample (n=5) of patients with contamination-related OCD [65]. Although not tested in patients with OCD, fMRI-neurofeedback targeting regulation of amygdala activity was effective in reducing amygdala activity and increasing amygdala-vmPFC functional connectivity in non-psychiatric participants [66]. Further investigation of non-invasive neuromodulatory interventions targeting fronto-limbic circuitry is warranted in OCD.
Studies with treatment-resistant OCD patients indicate that invasive neuromodulation techniques, such as deep brain stimulation (DBS), may improve fronto-limbic circuit function. For instance, one study examining DBS of the ventral anterior limb of the internal capsule (ALIC) in refractory OCD patients reported improvements in OCD symptoms and co-occurring depression and anxiety as well as changes in functional connectivity between the amygdala and vmPFC [67]. Other work has indicated that the bed nucleus of the stria terminalis (BNST), a center of integration for limbic information that is implicated in processing uncertain threat [68], is a potentially efficacious target for OCD symptom improvements [69]. However, other research on DBS in OCD (and other psychiatric disorders) has highlighted that the efficacy of DBS in reducing symptoms may depend less on the gray or white matter stimulation target and more on the white matter networks affected by the stimulation [70–71] (Table 3). Indeed, a recent analysis examining tractography correlates of OCD symptom improvement following DBS targeted at different stimulation sites, including the ALIC, indicated that successful reduction of symptoms was associated with connectivity of all stimulation sites to a common fiber bundle [70]. Thus, an important step for future research is to identify the optimal white matter networks associated with different cortical neurocircuit dysfunctions.
Sensorimotor circuit
Sensorimotor dysfunctions in OCD
The sensorimotor circuit includes cortical and subcortical regions involved in the generation and control of motor behaviors and integration of sensory information (Figure 3) [16, 72]. Although perhaps the most well-known examples of OCD symptoms involve fear of harmful events with compulsions that resemble reactions to potential threat, a significant proportion of patients (60–70%) also experience sensory phenomena (SP) consisting of aversive or uncomfortable sensations or perceptions that drive repetitive behaviors (Table 2) [73–76]. SP are prevalent in patients with ordering, arranging, counting and repeating compulsions, which are frequently performed until the patient achieves the sensation or perception that things are “just right” (“not-just-right” experiences) [73, 75] or “complete” [77]. These phenomena additionally manifest as tactile sensations of feeling dirty that drive washing and cleaning compulsions, rather than fear of illness or disease [73, 75]. For instance, the patient in Case vignette 3 reports a tactile sensation of having dirty hands, which prompts excessive hand washing (Table 1). Similarly, content analyses have revealed a key theme in the content of contamination obsessions to be sensations of feeling dirty under the skin, which lead to washing rituals [78]. SP are also prevalent in Tourette syndrome (TS) [22, 73], in which they manifest as premonitory urges or “sensory tics” [79] in a specific body part prior to a tic.
Figure 3. The sensorimotor circuit.
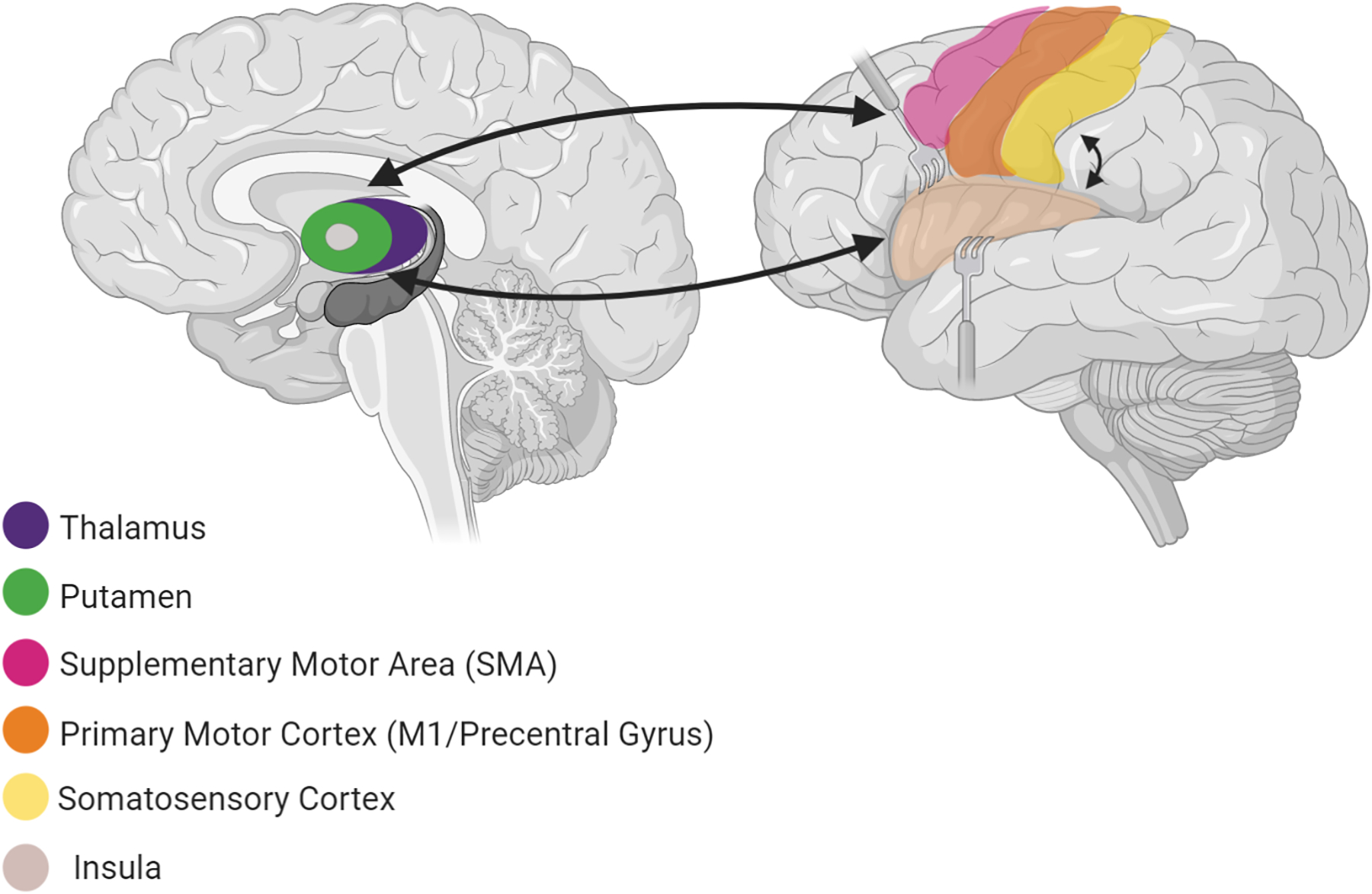
includes cortical and subcortical regions involved in the generation and control of motor behaviours (primary motor cortex - precentral gyrus, supplementary motor area, putamen, globus pallidus and thalamus) and the integration of sensory information (postcentral gyri, secondary somatosensory cortex, insula).
In terms of the neural mechanisms contributing to SP, an analogy has been drawn between “urges-for-action” (Table 2), which may be similar to urges leading to repetitive behaviors in OCD and TS [80–81]. Neuroimaging studies report activation in the insula, parietal somatosensory regions and motor/premotor regions to be involved in the build-up and suppression of urges-for-action [80–82], with a common region of mid-posterior insula and cingulate motor area (CMA) active during urges-for-action in non-psychiatric participants and the urge to tic in TS [81, see also 83–84]. A recent study examining the urge to blink in OCD found that patients exhibited increased activity in mid and anterior insula and CMA during eyeblink suppression [85], supporting the notion that the urges-for-action network is also dysfunctional in OCD.
Two neuroimaging studies [86–87] have directly examined the neural correlates of SP in OCD. Subirà and colleagues [86] reported larger gray matter volumes in a sensorimotor area encompassing postcentral gyrus, the posterior extent of precentral gyrus and paracentral lobule in individuals with OCD and SP (OCD+SP) compared to those with OCD without SP (OCD-SP) and non-psychiatric controls. OCD+SP (but not OCD-SP) had larger volumes of posterior putamen, pallidum, and thalamus than controls. Sensorimotor volume increases were not correlated with SP severity, but group differences remained after controlling for medication and comorbid conditions, including TS. Brown et al. [87] examined neural activity while OCD patients viewed “body-focused” videos (described in Table 2) designed to elicit activation in sensorimotor circuitry. Greater SP severity was correlated with greater activity in mid-posterior insula and, at a lower statistical threshold, bilateral postcentral gyri, orbitofrontal cortex (OFC), and lateral PFC. Activation in these areas remained correlated with SP severity after controlling for medication status, comorbidity and overall OC symptom severity, suggesting that these effects were specific to SP.
Another function relevant to OCD that relies on efficient function of the sensorimotor circuit is habit formation. Habits are rigid, largely non-conscious and automatic behaviors that are performed regardless of motivation and outcome (Table 2) [12]. The formation and execution of habits is mediated by motor portions of the sensorimotor circuit (putamen and premotor/motor cortex) [88–89]. In OCD, it has been proposed that compulsions may reflect maladaptive sensorimotor circuit-mediated habitual behaviors that, over time, become decoupled from the initial obsessive thoughts [12, 90]. Consistent with this hypothesis, some patients report habit-like compulsive behaviors. The patient in Case vignette 4 (Table 1), for example, reports a checking ritual that was initially associated with obsessive beliefs about harm. With multiple repetitions over time, her obsessive worries became less important and her checking compulsions became more automatic and were performed to relieve feelings of incompleteness. This subjective feeling could be the result of changes in the stimulus-response associations produced by the excessive repetitions involving the checking behavior. Thus, over the years, increased sensorimotor circuit activity involved in habit-formation may have played a role in the transition from goal-directed (in this case, repetitive behaviors performed to relieve specific fears/worries) to habitual behaviors (here, repetitive behaviors to relieve feelings of incompleteness). In support of this hypothesis, studies examining the content of compulsions have reported that patients rate some of their compulsions as highly habit-like, i.e. they are felt to be automatic behaviors that are performed largely without conscious awareness, they have a long history of repetition over the patient’s life, and they form part of the patient’s daily routines [24]. Moreover, longer duration of illness predicted higher habit-ratings, in line with the idea that habit-like compulsions develop slowly over time [24]. Similarly, content analyses have revealed that some patients initially perform compulsions to elicit a sense of relief and that even though their compulsive rituals lose the ability to provide relief over time, the patients continue to perform the behaviors [38]. Further support for this hypothesis comes from experimental studies, which report excessive habit formation and/or an over-reliance on habitual behavior at the expense of flexible goal-directed behavior on experimental tasks in OCD [90–91], as well as in other disorders characterized by compulsive behavior including TS [92–93].
It is important to note that while sensory phenomena and habit formation are linked to similar circuitry, there is no direct evidence linking these features of OCD to each other (i.e. no studies have reported that the transition from goal-directed to habitual behavior is more common in patients with prominent sensory phenomena than those with fears or negative thoughts). Indeed, despite the overlap in somatosensory and motor cortex, there are some distinctions between the neurocircuitry, with sensory phenomena additionally associated with the insula and habit formation involving subcortical in addition to cortical sensorimotor areas. The link between sensory phenomena and habit formation has yet to be fully elucidated in the literature; neuroimaging studies examining each process individually suggest that they exhibit both overlapping and distinct circuitry and behavioral features. It should also be highlighted that regions involved in these sensorimotor circuit alterations also play important roles in the neurocognitive functions of other neurocircuits. The insula, for example, participates in emotional processing with the amygdala and other fronto-limbic structures and, like those regions, also shows hyperactivity during emotional processing in OCD [36]. In addition, the sensorimotor circuit functions considered here also engage regions and functions of the other neurocircuits. For instance, in its early stages, habit-formation relies on dopaminergic reward signaling in the ventral affective circuit [94].
Treatments targeting sensorimotor dysfunction in OCD
Sensory phenomena, although highly distressing [95], associated with increased obsessive-compulsive and tic severity [73, 75, 95] and reduced quality of life [95], are infrequently considered as outcomes in treatment trials [96]. However, a recent study reported reductions in not-just-right experiences alongside reduced contamination obsessions in OCD patients following ERP [97]. Furthermore, habit-reversal training (Table 3), an effective behavioral therapy for tic symptoms used in TS, targets sensory phenomena and has been shown to reduce premonitory urges as well as tic symptom severity in adults with TS [98–99]. Habit-reversal therapy works by breaking tic “habits” by training patients to recognize the sensory urges that precede tics and to perform a less obtrusive and more voluntary behavior in place of the tic. A neuroimaging study reported significantly reduced activity in the putamen following habit-reversal training in patients with TS, suggesting that this form of therapy works (at least in part) by normalizing atypically increased sensorimotor circuit activity associated with excessive habit formation [100]. Habit-reversal therapy is similar to the response prevention aspect of ERP (Table 3) in that both aim to break the association between sensation(s) (sensory phenomena/obsessions/feelings of fear/anxiety) and repetitive, compulsive behaviors (compulsions/tics) [101]. However, the awareness training component of habit-reversal therapy may be particularly helpful for OCD patients in whom symptoms have become habit-like; for these patients, additional training may be needed to recognize the sensations that precede their habit-like compulsions. Consistent with this suggestion, habit-reversal training was reported to be effective in reducing OCD symptom severity in a patient with treatment-refractory OCD [102]. Further work testing the efficacy of including awareness training as an additional component of ERP in OCD patients with these types of compulsive symptoms will be important in future research.
The first-line pharmacological therapy for OCD (SSRIs) has been shown to decrease resting-state activity of the anterior insula in depression [103], suggesting that this form of treatment could be modulating circuitry related to SP. Single high doses of ondansetron, a 5-HT3 receptor antagonist FDA approved for severe nausea and vomiting [104], reduce activation in the insula, precentral and postcentral gyri, and cingulate cortex [105]. Interestingly, several smaller pilot trials showed that repeated dosing of ondansetron over multiple weeks reduced overall symptom severity in OCD [106–107] and TS [108], with potential utility as an augmentation strategy in SSRI-resistant OCD [106]. However, a larger Phase 2 trial using adjunctive very low dose ondansetron in 168 OCD patients failed to find a significant Y-BOCS reduction at the end of 12 weeks [109]. These discrepant findings might indicate that the low dose in the Phase 2 trial was not sufficient to engage relevant neural circuitry; alternatively, perhaps ondansetron is effective only for sensory phenomena, but their prevalence was not reported in this or prior trials. Addressing this issue, a double-blind placebo-controlled mechanistic trial testing the effects of repeated high-dose ondansetron on SP and neural circuitry is currently underway in OCD (NCT03239210).
Non-invasive neuromodulation approaches (Table 3) can also be used to target SP circuitry. Open-label studies have shown efficacy in reducing OCD and tic symptom severity using low frequency (inhibitory) rTMS to the SMA [110–111 but see 112], though these studies did not examine effects on SP specifically. Based on findings from rTMS and DBS studies in OCD, Senço et al. [113] proposed using tDCS with the cathode over pre-SMA and anode over an extra-cephalic region, a montage that has shown some efficacy in reducing OCD symptom severity [114]. One limitation of standard rTMS approaches is their relatively low depth, which corresponds to targeting mostly cortical structures. To overcome this issue, novel rTMS coils (H-coils) have been designed [115], which generate deeper electromagnetic fields at the same motor threshold intensity [116]. A study in non-psychiatric volunteers showed that H-coil TMS targeting the insula significantly modulated metabolic activity in connection regions, including the sensorimotor striatum [117]. Interestingly, the insula is associated with addiction behavior and clinical trials have shown that H-coil TMS of the insula in substance use disorders alleviated symptoms [118]. Taken together, these findings suggest that H-coil TMS can modulate the insula and might therefore be useful in improving SP in OCD.
Ventral cognitive circuit
Ventral cognitive dysfunctions in OCD
The ventral cognitive circuit includes prefrontal and striatal regions involved in self-regulatory behaviors (Figure 4) [16]. One ventral cognitive function that has been proposed as fundamentally involved in OCD is response inhibition, which is the ability to withhold inappropriate behaviors. Response inhibition is mediated in part by the inferior frontal gyrus (IFG) and the subthalamic nucleus (STN) (Table 2) [16, 119], although other regions beyond the ventral cognitive circuit, such as the inferior parietal lobule and insula, are also involved [120]. The persistence of maladaptive, repetitive thoughts and behaviors in OCD, despite awareness that these are excessive, unreasonable and have negative consequences, is suggestive of impairment inhibition. Such deficits might explain (partly at least) both the expression of obsessions and the consequent compulsive behaviors. For instance, the patient in Case vignette 4 (Table 1) describes an excessive checking ritual that she feels driven to perform (despite consciously knowing it is excessive) before leaving the office at the end of the work day, which is tied to her obsessive belief that something bad might happen (an electrical short circuit might cause a fire). Impairments in inhibition may, in part, have underpinned the expression of this obsessive-compulsive ritual at this point; specifically, the patient was unable to prevent herself from performing the checking rituals (failed response inhibition to repetitive behaviors). In support, content analyses have indicated that the ability to ignore obsessive thoughts and withhold associated compulsive behaviors (i.e. use inhibition effectively) is a key theme in patients’ experiences of symptoms [38].
Figure 4. The ventral cognitive circuit.
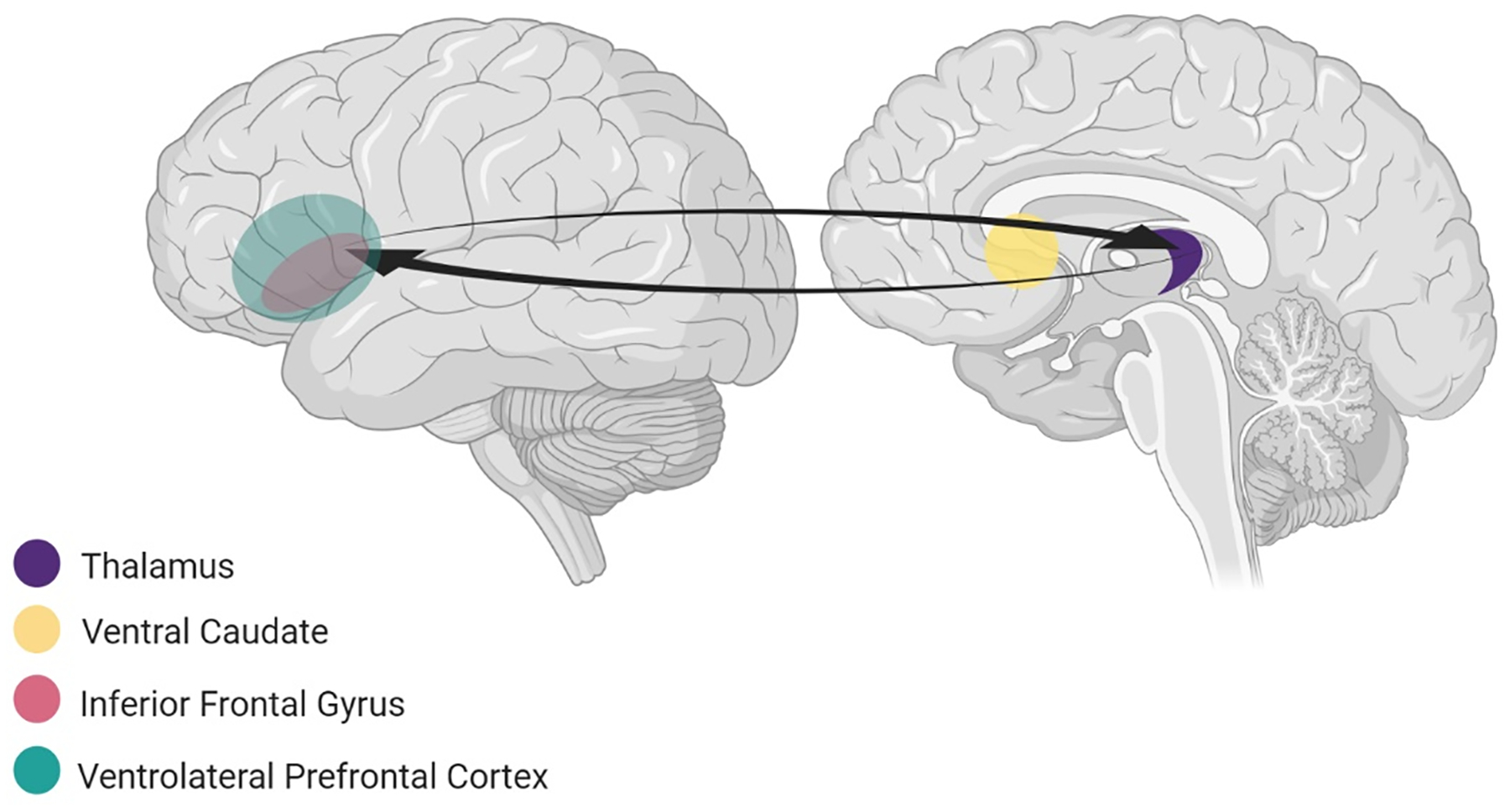
includes prefrontal (inferior frontal gyrus, ventrolateral prefrontal cortex) and subcortical regions (ventral caudate and thalamus) involved in self-regulatory functions. The inferior frontal gyrus (IFG), particularly in the right hemisphere, and ventral caudate together act as a “braking system”, which implements response inhibition, the ability to withhold inappropriate responses.
Consistent with these clinical observations, meta-analyses have reported consistent impairments in response inhibition performance [121] and neuroimaging studies have reported reduced IFG and/or caudate activity during response inhibition [122–123] in OCD. Still, not all studies report impairments in response inhibition in individuals with OCD [124–126]. A meta-analysis comparing neuropsychological performance between OCD patients with predominant washing versus checking symptoms reported significantly better response inhibition in washers than checkers [127], suggesting that the presence of response inhibition deficits may depend on the dimension of obsessive-compulsive symptoms.
It should be noted that the regions of the ventral cognitive circuit also interact with the other neurocircuits and play a role in their functions. For instance, the STN is involved in the control of emotional and motivational behaviors via its connections with fronto-limbic and ventral affective circuits, respectively [128]. The IFG participates along with regions of the dorsal cognitive circuit to form a network that mediates working memory [129]. Thus, targeting the ventral cognitive circuit for response inhibition impairments in OCD, discussed next, is likely to affect the function of other neurocircuits.
Treatments targeting ventral cognitive dysfunction in OCD
Support for the utility of targeting neural circuitry underlying response inhibition in treatment for OCD was recently provided by Rappel and colleagues [130]. Recordings of oscillatory theta activity, a robust neurophysiological marker of inhibitory control processes [131], were taken from electrodes implanted in the STN for DBS treatment in patients with treatment-refractory OCD. STN theta oscillations were found to be smaller during failed inhibition trials of a Go/Nogo task and during OCD-symptom provocation prior to DBS stimulation. This pattern is consistent with the literature reporting that theta oscillations increase during successful response inhibition and decrease during inhibitory failures [131] and suggests that similar response inhibition failures were involved in the experience of OCD symptoms during provocation. Fluctuations in OCD symptom severity measured over 1-year post-DBS-activation were accompanied by and correlated with alterations in the level of theta oscillations; decreases in symptom severity co-occurred with increases in theta oscillations and vice versa [130]. This finding suggests that better function in neural mechanisms implicated in response inhibition are involved in OCD symptom improvement in (some) treatment-refractory patients. Furthermore, this study indicates that stimulation of the STN is an effective treatment approach for some individuals with OCD. Another recent study also indicated that ventral cognitive circuit function can be improved by ventral capsular/ventral striatal (VC/VS) DBS targeting the ALIC [132]. Widge et al. [132] found that stimulation at this target resulted in significantly greater theta oscillations in prefrontal regions, including the IFG, during an inhibition task in patients with OCD and/or depression. These findings are consistent with recent connectomic analyses suggesting that a specific white matter network connecting frontal regions to the STN was associated with clinical response in ALIC, STN and other DBS techniques [70]. Future work should consider whether this form of treatment is mainly effective for those patients with response inhibition deficits.
In terms of non-invasive neuromodulatory treatments, fMRI-based neurofeedback training to increase activity in the IFG during response inhibition has been successful in ADHD [133–134]. Post-training, adolescents with ADHD showed reduced symptom severity as well as increased IFG activity and stronger IFG-striatal functional connectivity during a (non-trained) response inhibition task and neural activity changes were correlated with symptom improvements [133–134]. A similar approach may be helpful for OCD patients with response inhibition impairments.
Ventral affective circuit
Ventral affective dysfunctions in OCD
The ventral affective circuit includes the OFC, the ventral striatum (particularly the nucleus accumbens, NAcc) and the thalamus (Figure 5) [16, 135] and is involved in reward-related processes. One process relevant for OCD is reward responsiveness, which refers to the ability to anticipate, represent and respond to rewards (Table 2) and is largely mediated by dopaminergic signaling within the ventral affective circuit. In OCD, compulsions are suggested to function either as rewards or as a means to avoid punishments because they successfully (if maladaptively) diminish some of the anxiety associated with obsessions [136–137]. Reduced sensitivity to rewards and/or exaggerated anticipation of punishments have been proposed to underpin these reward responsiveness alterations [136–137] and can be observed in the clinical phenotype of OCD. For instance, the patient in Case vignette 5 (Table 1) describes a preference for staying at home rather than trying new things; his sensitivity to the potential rewards of trying new things appears to be blunted or at least overshadowed by exaggerated anticipation of potential punishment (feeling bad). Similarly, previous studies have reported clinically significant levels of anhedonia, conceptualized as the inability to experience pleasure and reduced sensitivity to rewards, in OCD patients, which predicted OCD symptom severity independently of depression symptoms [138–139]. Previous work has also indicated that a proportion of patients with OCD report feelings of reward and positive affect following the completion of compulsive behaviors [24, 38].
Figure 5. The ventral affective circuit.
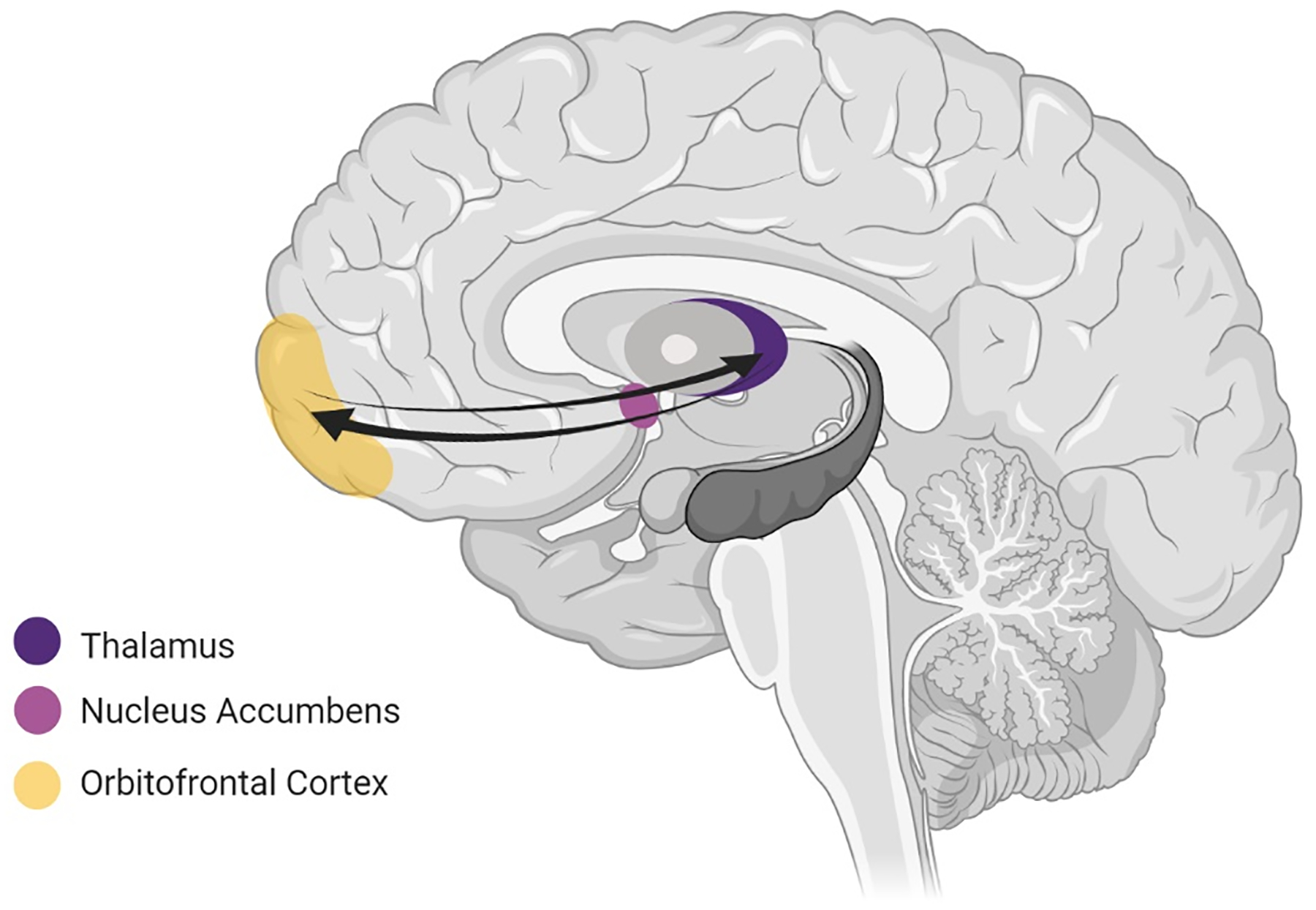
includes the orbitofrontal cortex (OFC), ventral striatum (particularly the nucleus accumbens, NAcc) and the thalamus. This circuit is crucially involved in reward functions which are largely mediated by dopaminergic signaling.
In support of these clinical observations, altered function of the ventral affective circuit has been reported in OCD and linked to OCD symptoms. For instance, increased functional connectivity between the NAcc and other reward circuit regions, including the OFC, during rest has been reported in OCD and this increased connectivity correlated with OCD symptom severity [140–141]. Similarly, studies examining neural activity during experimental paradigms that engage the reward circuit (see Table 2) have reported altered functional connectivity between the NAcc and/or OFC and other reward-related regions during the anticipation of reward and punishment in OCD [141–142]. Several studies have also reported underactivation of the NAcc and OFC during anticipation of reward (but not during anticipation of punishment) and during reward-based decision-making [136, 143–145] as well as poorer performance on reward-based decision-making and reward-learning tasks [146–147]. Impaired generalization of reward but not punishment has also been found in OCD [148]. These findings support the proposal that individuals with OCD have lowered sensitivity to rewards. Yet, findings from other studies support the view that individuals with OCD show enhanced sensitivity to, or an aversion of, punishment, as indicated by increased learning from punishment on reward learning tasks [149], less risky decision-making on gambling tasks [150] and greater reward-circuit activity during anticipation and/or receipt of punishment but not reward [137, 151–152]. Altered dopaminergic signals during reward learning have also been reported in OCD [153].
It is important to highlight that the NAcc plays a crucial role in the functions of several of the other neurocircuits. In particular, dopaminergic reward signals from the NAcc are integrated with activity in the sensorimotor circuit to facilitate the learning of associations between stimuli and responses in the early stages of habit-learning [94]. The NAcc also participates in processing and control of negative emotion along with fronto-limbic regions [154]. Further, regions from other neurocircuits participate in the reward functions of the ventral affective circuit, including the amygdala and vmPFC from the fronto-limbic circuit [49] and the STN from the ventral cognitive circuit [128]. The anterior cingulate cortex (ACC), which participates in emotional processing functions of the fronto-limbic circuit [36], is also critically involved in processing reward signals relayed from the NAcc [135] and has been found to be hyperactive during reward tasks in OCD [155].
Treatments targeting ventral affective dysfunction in OCD
Several treatment options may target reward mechanisms in OCD (Table 3). First, patients on SSRIs have been found to perform better on reward-learning tasks than patients off-SSRIs [156], indicating that these medications may be beneficial for improving reward-related alterations in OCD. This is consistent with increasing recognition of the importance of serotonin signaling in reward function [157]. Dopamine-acting drugs such as methylphenidate (Table 3) are effective in normalizing atypical reward-related performance and neural activity in ADHD [158] and may be an effective augmentative strategy for some OCD treatment resistant patients [155]. A recent study similarly reported altered neural activity in the reward-circuit during a reward-learning task in OCD following acute administrations of the dopamine-regulating drugs pramipexole and amisulpride [155].
Neuromodulation of the NAcc with DBS has been shown to significantly improve symptoms, reduce aberrant NAcc activity and restore altered fronto-striatal connectivity in treatment-resistant OCD [159]. Based on this finding, the authors [159] suggested that, for some patients, dysregulated reward circuit activity interferes with the functioning of other (i.e. fronto-striatal) circuits, and contributes to OCD symptoms, and that this can be effectively reversed with neuromodulation. While originally developed for reward alterations in depression, DBS targeting the medial forebrain bundle (white matter tracts connecting regions of the reward system including the NAcc) also improves symptoms in treatment-resistant OCD [160]. The approach of these studies targeting white matter fiber bundles is in line with recent findings showing the importance of targeting white matter networks rather than discrete cortical targets [70–71].
In terms of non-invasive neuromodulatory treatments, fMRI-based neurofeedback has successfully been used in non-psychiatric samples to train individuals to increase NAcc activity, which also elicited feelings of positive arousal [161]. Future research testing whether fMRI-based neurofeedback training of the NAcc could benefit OCD patients who show altered reward-related functions will be an important direction in the search for novel OCD treatment targets.
Dorsal cognitive circuit
Dorsal cognitive dysfunctions in OCD
The dorsal cognitive circuit (Figure 6) underpins executive functions required for effective goal-directed behavior, including planning, working memory and top-down control of emotional, motivational and sensorimotor processes [16] (Table 2). These dorsal cognitive regions connect to inferior parietal regions, forming the frontoparietal network [162–163], which is discussed further in the Limitations section below. Given the central and generic nature of the functions of this circuit, we do not propose particular dorsal cognitive dysfunctions to be related to specific OCD symptom profiles (no vignettes were included). Instead, dysfunction in this circuit may contribute to impairments in OCD in a more general manner. For instance, working memory and planning appear to be impaired in OCD [121, 164] and, even when patients do not present behavioral deficits, they can show altered brain activity, including underactive dlPFC and weaker dlPFC-striatal functional connectivity [165–166]. Experimental studies have reported impaired memory functions to correlate with OCD symptom dimensions, including pathological doubt [166] and compulsive checking [167]. It is possible that these executive dysfunctions exacerbate obsessive-compulsive symptoms. Indeed, studies examining the content of patients’ symptom experiences have suggested that difficulties with attention and memory play a key role in aggravating OCD symptoms; for example, some patients report that failures of these functions during compulsive rituals prevent them achieving a sense of completeness and/or they lead to the patient having to engage in further repetitions of the ritual [38]. As discussed in relation to the fronto-limbic circuit, difficulties recruiting dorsal prefrontal regions for efficient emotion regulation has been reported during cognitive reappraisal of OCD-provoking stimuli in OCD [39, 60] and may contribute to the persistence of fear-related obsessive-compulsive symptoms. Treatments targeted at dorsal cognitive circuitry may therefore help with top-down control of the emotional response to symptoms in OCD.
Figure 6. The dorsal cognitive circuit.
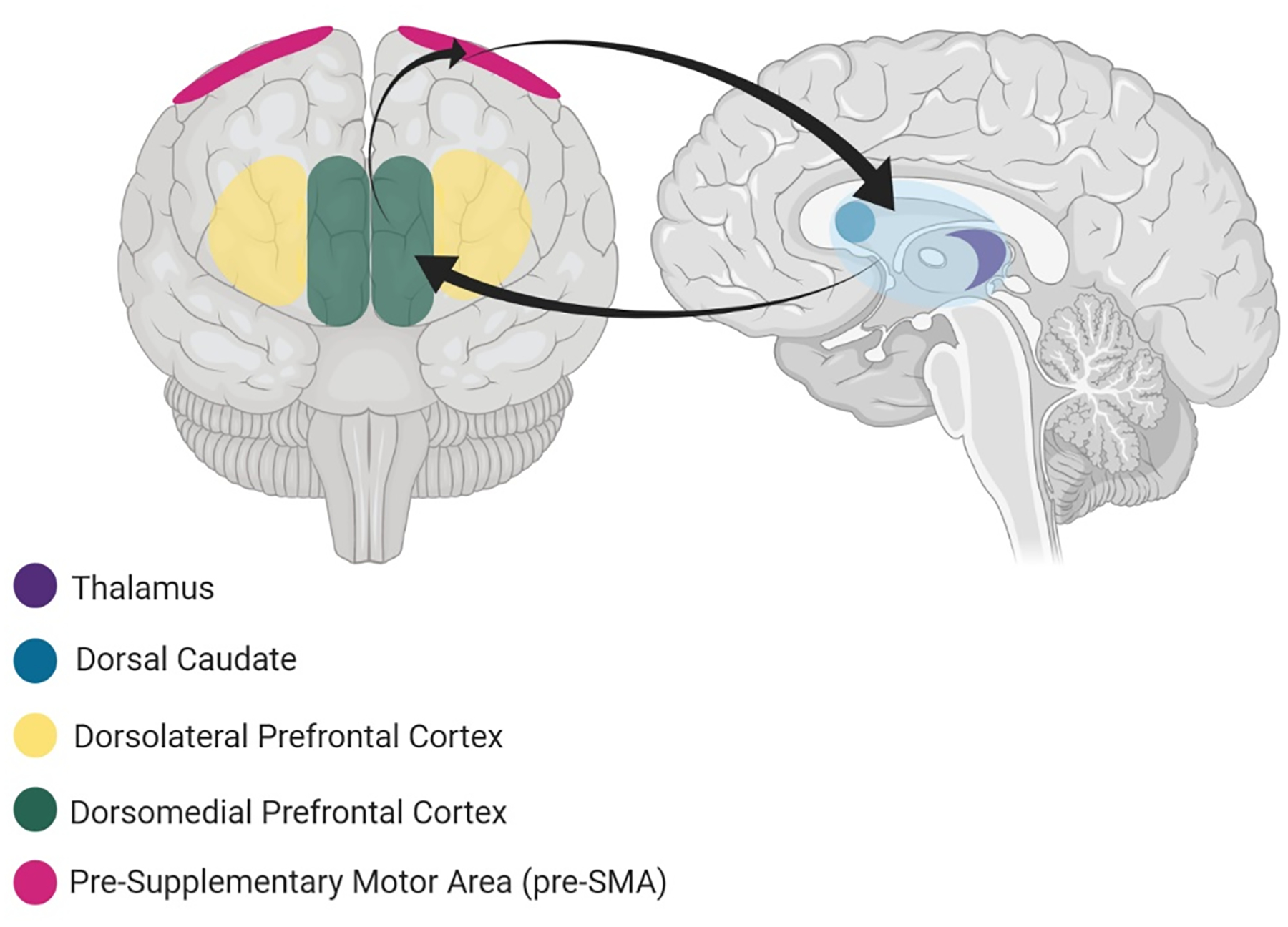
involves the pre-supplementary motor area (pre-SMA), dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), dorsal caudate and thalamus and handles executive functions such as working memory and planning/organization and emotion regulation.
Treatments targeting dorsal-cognitive dysfunction in OCD
The cognitive reappraisal component of CBT (Table 3) trains patients to change their thoughts and emotional responses to OCD symptoms and is known to engage dorsal prefrontal regions [35–36, 39, 51]. Cognitive reappraisal is therefore likely to be useful for patients presenting with emotion regulation deficits mediated by dorsal cognitive circuitry. Cognitive training programs for OCD targeting executive functions including organizational strategies and planning (see Table 3) have been tried in a small number of studies [168–171]. The programs were found to improve the executive functions targeted [168–171] and, in three studies, OCD symptoms [169–171]. Preliminary findings suggest that methylphenidate augmentation may be beneficial in treatment-resistant OCD [172]. Further, some patients with OCD who report their symptoms to be exacerbated by executive function problems also note that their symptoms have improved during treatment with methylphenidate [38]. Methylphenidate increases central dopamine and norepinephrine activity, which improves executive and attentional functioning and emotion regulation [173], in addition to influencing reward mechanisms as reported above. However, there are some case reports that methylphenidate can worsen OCD symptoms [174] and for some patients antipsychotic medications that reduce dopamine levels are effective [175] (Table 3). These findings are in line with other research indicating that optimal dopamine levels for executive functions follow an inverted U-shaped curve and depend on the individual’s baseline levels [176]. Non-invasive neuromodulatory approaches (rTMS, tDCS) stimulating dorsal cognitive regions including the dlPFC and pre-SMA show efficacy in reducing OCD symptoms [62, 177–179] even in treatment-resistant cases [178–179]. It should be noted, however, that the pre-SMA target is likely to affect activity in several circuits (sensorimotor, ventral cognitive) and not the dorsal cognitive circuit alone.
Testing the neurocircuit-based taxonomy to guide OCD treatment
The circuit-based approach to OCD presented here is speculative at this point but provides hypotheses to be tested in future research. For instance, the following strategies could be employed based on the neurocircuits involved: a) increase dorsal PFC recruitment to down-regulate fronto-limbic activity to improve fear/anxiety regulation, and modulate amygdala and vmPFC function directly to restore typical emotional responses; b) reduce atypically increased sensorimotor circuit activity to ameliorate sensory phenomena and excessive habit formation; c) increase IFG activity to improve inhibitory control of compulsive and habitual behaviors; d) regulate NAcc activity to achieve typical responsiveness to rewards; and e) restore typical dorsal PFC function to re-establish effective executive functioning.
These hypotheses could be tested in a number of ways. Re-analysis of existing datasets from treatment trials in OCD (pooled across individual studies, if necessary) that have included neuroimaging and/or neurocognitive measures could be conducted to examine whether the different neurocognitive dysfunctions and neurocircuit alterations discussed here predict better or worse response to our suggested neurocircuit-specific treatment options (e.g. do patients with fronto-limbic and ventral affective circuit dysfunctions respond better to SSRIs than those without?). For new studies, an important step will be to confirm the presence of the clinical profiles we propose. This could be achieved by content analyses of interviews in which patients describe their symptoms to confirm that dysregulated fear-like emotions, difficulties coping with uncertainty, sensory phenomena, a transition from obsession-driven compulsions to ritualistic habit-like behaviors, reward alterations and executive dysfunctions are valid themes under which patients can be grouped. Next, patients could be stratified based on their clinical profiles (e.g. sensory phenomena, dysregulated fear, excessive habit-formation), performance on neurocognitive tasks and underlying neural activity patterns in different neurocircuits and randomized to different treatment methods (e.g. dlPFC or pre-SMA tDCS/rTMS, amygdala fMRI-based neurofeedback, habit-reversal training) to assess whether treatments tailored to specific neurocircuits are more effective.
Limitations
In this paper we report dysfunctions in distinct neurocircuits, which we propose may underlie different clinical profiles in OCD. Yet, the complex phenomenology of the condition likely reflects widespread abnormalities across multiple brain regions and circuits encompassing a variety of emotional, cognitive, and behavioral domains, like most psychiatric disorders [5, 7]. A single mechanism is therefore unlikely to explain such a heterogeneous disorder, and the different clinical profiles of OCD we present here will overlap, interact, and co-occur in individual patients. Likewise, CSTC circuits are interconnected rather than fully segregated and activity (or dysfunction) in one circuit will affect the integrity of the others [13]. Moreover, OCD symptoms wax and wane and change in type over the lifespan. Consequently, a patient may have different circuit abnormalities at different stages of development. Such developmental variations have not been captured in previous neuroimaging studies, which have mostly been cross-sectional rather than longitudinal. The stage and chronicity of disease and effects of past and current treatments on brain neuroplasticity have also not been adequately addressed in neuroimaging work [16].
Furthermore, although CSTC circuits have been most extensively studied and related to the neurobiology of OCD, other structures also appear to be relevant. For example, recent work highlights the importance of dysfunction in connectional “hub” regions in a range of disorders [180–183]. “Hubs” are regions characterized by particularly dense connections with other brain regions and as such are crucial for the efficient function of neurocircuits [184]. Hub regions include, among others, the inferior parietal lobule, which is strongly connected with frontal and subcortical regions and critically involved in mediating attention, particularly attentional bias towards disease-relevant stimuli (such as threatening images), and may represent a key transdiagnostic region of dysfunction and a treatment target for OCD and anxiety disorders [180–181]. Indeed, a large multisite consortium study (ENIGMA, [185]) revealed thinner inferior parietal cortex in OCD patients compared to non-psychiatric volunteers [186]. Other hub regions include the cingulum, anterior insula, anterior cingulate cortex (ACC) and dorsal striatum [182–184], which are involved in several of the circuits discussed here. These hub regions appear to be involved in many self-regulatory control functions, including cognitive reappraisal and extinction of learned fear, and as such may be key targets in different forms of interventions for OCD and other disorders [182, 184]. Finally, the fronto-parietal network, strongly connected to the dorsal and ventral cognitive circuits, is also likely to be important in OCD [14, 162–163, 187]. This network is involved in coordinating attention, cognitive control and working memory [162–163, 188] and has been implicated in other disorders including depression and schizophrenia [7, 189]. These considerations are important since many previous neuroimaging studies in OCD have used seed-based analysis of fronto-striatal circuits, despite the potential for abnormalities in other regions [187].
Regarding non-invasive neuromodulatory strategies, although they represent an interesting approach to explore and modulate circuits, it should be underscored that the current montage of some techniques such as tDCS and rTMS has low spatial focality and several circuits are likely to be modulated. Moreover, tDCS and rTMS still present large inter- and intra-individual variability in response [190]. Concerning DBS, although this intervention is more focal, it is also costly and used only in refractory, severe cases that likely involve several neurocircuits or a very severe disruption in a specific circuit. Furthermore, in this review we focused mainly on the gray or white matter target of DBS in suggesting neurocircuit-specific treatment strategies, but recent evidence suggests that the white matter network to which each target connects may be more important and that several discrete DBS targets may converge on a single network to improve OCD symptoms [70–71, 132].
Finally, given the multiple mechanisms underlying psychiatric symptoms and the many considerations relevant to treatment decisions (which require a complex assessment of a range of factors, including understanding the function of symptoms, their social context, the risks versus benefits of treatment and patient access and preferences for treatment), a neurocircuit approach as we propose here may not be any simpler or more scientifically tractable than DSM defined disorders or other constructs. For example, SSRIs, whose effects impact on several of these circuits, at high doses are a remarkably useful intervention in OCD across different phenotypes and easier for a clinician to remember than having to target several different putative clinical profiles of the condition [191]. There is also the issue of how to identify clinical profiles and underlying neurocircuit alterations in individual patients, which would require the development of validated assessment protocols with established sensitivity and specificity.
Concluding remarks
We present a heuristic model of how to integrate and transform information generated by the vast array of recent neuroscience studies into guided principles toward treatment of OCD patients. Although a neurocircuit-based taxonomy for OCD is still in its nascent stage, knowledge accumulated in the last few decades regarding different neurocognitive functions relevant to OCD and their underlying neural mechanisms can provide insights into possible disease mechanisms and potential novel neurocircuit-based treatment targets. Given that the current diagnostic approach [1–2] of defining mental disorders by groups of symptoms does not demonstrate robust and reliable circuitry abnormalities across individuals diagnosed with the same disorder, treatments targeting specific circuits open the possibility of addressing their underlying symptomatology rather than the disorder as a whole [192]. If specific alterations in circuits governing functions like fear, subjective sensory experiences, habitual behaviors, response inhibition, reward and executive function define particular clinical profiles of OCD, then therapies targeting specific circuits and corresponding clinical profiles could be developed. Since OCD phenotypes involve overlapping circuits, we speculate that successful treatment of OCD will necessitate addressing multiple circuits, according to the clinical profile. Given that some of these clinical profiles are seen in other disorders (e.g., sensory phenomena in tic disorders), once specific circuit-based treatment approaches are better established they could also be used to treat these clinical profiles across diagnoses rather than within a single categorical diagnosis [192].
Acknowledgements
We are very grateful to the patients who shared experiences of their symptoms and agreed for these to be included in this article. This work was supported by a postdoctoral fellowship awarded to Elizabeth Shephard from the São Paulo Research Foundation (18/22396-7) and research grants from the São Paulo Research Foundation (2014/50917-0) and CNPq (465550/2014-2) awarded to Euripedes C Miguel as part of the National Institute of Developmental Psychiatry for Children and Adolescents (INDP).
Footnotes
Conflicts of Interests
The authors Elizabeth Shephard, Emily R. Stern, Odile A. van den Heuvel, Daniel L.C. Costa, Priscilla B.G. Godoy, Marcelo C. Batistuzzo, Antonio C. Lopes, Marcelo Q. Hoexter, Christine Lochner, and Euripedes C. Miguel have no conflicts of interests to declare. Roseli G. Shavitt has received consulting honoraria from Lundbeck and a travel grant from LIBBS. Andre R Brunoni has received funding from the São Paulo Research Foundation (2017/50223–6, 2018/10861–7), the Brazilian National Council of Scientific Development productivity support (PQ-1B) and the University of São Paulo Medical School productivity support (PIPA-A), and is the Chief Medical Advisor of Flow Neuroscience (Malmö, Sweden) and has a small equity in this company. Janardhan Reddy Y.C. has received support from the various Government of India funding agencies for research on OCD, including the Department of Biotechnology (DBT), Department of Science and Technology (DST), and the Indian Council of Medical Research (ICMR); he is also currently involved in an NIMH multicenter international study on OCD. Dan J. Stein has received research grants and/or consultancy honoraria from Lundbeck and Servier. H. Blair Simpson has received research funds for a multi-site industry sponsored clinical trial from Biohaven Inc., royalties from Cambridge University Press and UpToDate Inc., and a stipend from the American Medical Association for serving as an Associate Editor for JAMA Psychiatry.
References
- [1].American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Association; 2013. [Google Scholar]
- [2].World Health Organization. The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. World Health Organization; 1993. [Google Scholar]
- [3].Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC medicine 2013; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cross-Disorder Group of the Psychiatric Genomics Consortium. (2013). Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet, 381(9875), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].van den Heuvel MP, Sporns O. (2019). A cross-disorder connectome landscape of brain dysconnectivity. Nature reviews neuroscience, 20(7), 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang T, Zhang X, Li A, Zhu M, Liu S, Qin W et al. (2017). Polygenic risk for five psychiatric disorders and cross-disorder and disorder-specific neural connectivity in two independent populations. NeuroImage: Clinical, 14, 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 2016; 3:472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wager TD, Woo CW. Imaging biomarkers and biotypes for depression. Nat Med. 2017. January 6;23(1):16–17. doi: 10.1038/nm.4264. [DOI] [PubMed] [Google Scholar]
- [9].Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am J Psychiatry. 2016. April 1;173(4):373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barth B, Mayer-Carius K, Strehl U, Kelava A, Häußinger FB, Fallgatter AJ, et al. Identification of neurophysiological biotypes in attention deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2018. November;72(11):836–848. [DOI] [PubMed] [Google Scholar]
- [11].Dougherty DD, Brennan BP, Stewart SE, Wilhel S, Widge AS, Rauch SL. Neuroscientifically informed formulation and treatment planning for patients with obsessive-compulsive disorder: a review. JAMA Psychiatry 2018; 75:1081–1087. [DOI] [PubMed] [Google Scholar]
- [12].Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron 2000; 28:343–347. [DOI] [PubMed] [Google Scholar]
- [13].Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends cogn sci 2012; 16:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive–compulsive disorder. Nat rev dis primers 2019; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gillan CM, Fineberg NA, Robbins TW. A trans-diagnostic perspective on obsessive-compulsive disorder. Psychol Med 2017; 47:1528–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van den Heuvel OA, van Wingend G, Soriano-Mase C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol 2016; 26:810–827. [DOI] [PubMed] [Google Scholar]
- [17].Torres AR, Prince MJ, Bebbington PE, Bhugra D, Brugha TS, Farrell M, et al. Obsessive-compulsive disorder: prevalence, comorbidity, impact, and help-seeking in the British National Psychiatric Morbidity survey of 2000. Am J Psychiatry 2006; 163:1978–1985. [DOI] [PubMed] [Google Scholar]
- [18].Bloch MH, Green C, Kichuk SA, Dombrowski PA, Wasylink S, Billingslea E, et al. Long-term outcome in adults with obsessive-compulsive disorder. Depress anxiety 2013; 30:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hollander E, Stein DJ, Kwon JH, Rowland C, Wong CM, Broatch J, et al. Psychosocial Function and Economic Costs of Obsessive-Compulsive Disorder. CNS Spectrums 1997; 2:16–25. [Google Scholar]
- [20].Fernández de la Cruz L, Rydell M, Runeson B, D’Onofrio BM, Brander G, Rück C, et al. Suicide in obsessive-compulsive disorder: a population-based study of 36 788 Swedish patients. Mol Psychiatry 2017; 22:1626–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, et al. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol psychiatry 2005; 10. [DOI] [PubMed] [Google Scholar]
- [22].Miguel EC, Prado HS, Rauch SL, Coffey BJ, Baer L, Savage CR, et al. Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry 2000; 61: 150–156. [DOI] [PubMed] [Google Scholar]
- [23].Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry 2011; 168:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ferreira GM, Yücel M, Dawson A, Lorenzetti V, Fontenelle LF. Investigating the role of anticipatory reward and habit strength in obsessive-compulsive disorder. CNS Spectr 2017; 22:295–304. [DOI] [PubMed] [Google Scholar]
- [25].Endrass T, Klawohn J, Schuster F, Kathmann N. Overactive performance monitoring in obsessive-compulsive disorder: ERP evidence from correct and erroneous reactions. Neuropsychologia 2008; 46:1877–87. [DOI] [PubMed] [Google Scholar]
- [26].de Avila RCS, do Nascimento LG, de Moura Porto RL, Fontenelle L, Miguel Filho EC, Brakoulias V, et al. Level of Insight in Patients With Obsessive–Compulsive Disorder: An Exploratory Comparative Study Between Patients With “Good Insight” and “Poor Insight”. Front psychiatry 2019; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mataix-Cols D, Rauch SL, Manzo PA, Jenike MA, Baer L. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999; 156:1409–1416. [DOI] [PubMed] [Google Scholar]
- [28].Miguel EC, Baer L, Coffey BJ, Rauch SL, Savage CR, O’Sullivan RL, et al. Phenomenological differences appearing with repetitive behaviours in obsessive-compulsive disorder and Gilles de la Tourette’s syndrome. Br J Psychiatry 1997; 170: 140–145. [DOI] [PubMed] [Google Scholar]
- [29].do Rosario-Campos MC, Leckman JF, Mercadante MT, Shavitt RG, Prado HDS, Sada P, et al. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry 2001; 158: 1899–1903. [DOI] [PubMed] [Google Scholar]
- [30].Shavitt RG, Belotto C, Curi M, Hounie AG, Rosário-Campos MC, Diniz JB, et al. Clinical features associated with treatment response in obsessive-compulsive disorder. Comprehensive Psychiatry 2006; 47: 276–281. [DOI] [PubMed] [Google Scholar]
- [31].Lochner C, Hemmings SM, Kinnear CJ, Niehaus DJ, Nel DG, Corfield VA, et al. Cluster analysis of obsessive-compulsive spectrum disorders in patients with obsessive-compulsive disorder: clinical and genetic correlates. Compr Psychiatry. 2005; 46:14–9. [DOI] [PubMed] [Google Scholar]
- [32].Pauls DL, Abramovitch A, Rauch SL, Geller DA. Obsessive–compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci 2014; 15: 410–424. [DOI] [PubMed] [Google Scholar]
- [33].Mataix-Cols D, van den Heuvel OA. Common and distinct neural correlates of obsessive-compulsive and related disorders. Psychiatric Clinics 2006; 29:391–410. [DOI] [PubMed] [Google Scholar]
- [34].Figee M, Luigies J, Goudriaan A, Willuhn I, Denys D. Neurocognitive basis of compulsivity. 2019. In Fontenelle L & Yücel M (Eds) A Transdiagnostic Approach to Obsessions, Compulsions and Related Phenomena (pp. 61–73). Cambridge, UK: Cambridge University Press. [Google Scholar]
- [35].Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation - an ALE meta-analysis and MACM analysis. Neuroimage 2014; 87:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Thorsen AL, Hagland P, Radua J, Mataix-Cols D, Kvale G, Hansen B, et al. Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, et al. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry 2013; 70:608–618. [DOI] [PubMed] [Google Scholar]
- [38].Van Schalkwyk GI, Bhalla IP, Griepp M, Kelmendi B, Davidson L, Pittenger C. Toward understanding the heterogeneity in obsessive-compulsive disorder: Evidence from narratives in adult patients. Australian & New Zealand Journal of Psychiatry 2016; 50:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paul S, Beucke JC, Kaufmann C, Mersov A, Heinzel S, Kathmann N, et al. Amygdala–prefrontal connectivity during appraisal of symptom-related stimuli in obsessive–compulsive disorder. Psychol Med 2019; 49:278–286. [DOI] [PubMed] [Google Scholar]
- [40].Apergis-Schoute AM, Gillan CM, Fineberg NA, Fernandez-Egea E, Sahakian BJ, Robbins TW. Neural basis of impaired safety signaling in Obsessive Compulsive Disorder. PNAS 2017; 114:3216–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McLaughlin NC, Strong D, Abrantes A, Garnaat S, Cerny A, O’Connell C, et al. Extinction retention and fear renewal in a lifetime obsessive–compulsive disorder sample. Behavioural Brain Research 2015; 280:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fyer AJ, Schneier FR, Simpson HB, Choo TH, Tacopina S, Kimeldorf MB, et al. Heterogeneity in Fear Processing across and within Anxiety, Eating, and Compulsive Disorders. Journal of Affective Disorders 2020; 271:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Jacoby RJ, Abramowitz JS. Intolerance of Uncertainty in OCD. Obsessive-compulsive Disorder: Phenomenology, Pathophysiology, and Treatment 2017; 12:171. [Google Scholar]
- [44].Kobori O, Salkovskis PM, Read J, Lounes N, Wong V. A qualitative study of the investigation of reassurance seeking in obsessive–compulsive disorder. Journal of Obsessive-Compulsive and Related Disorders 2012; 1:25–32. [Google Scholar]
- [45].Gentes EL, Ruscio AM. A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive-compulsive disorder. Clin Psychol Rev 2011; 31:923–933. [DOI] [PubMed] [Google Scholar]
- [46].Weidt S, Lutz J, Rufer M, Delsignore A, Jakob NJ, Herwig U, et al. Common and differential alterations of general emotion processing in obsessive-compulsive and social anxiety disorder. Psychol Med 2016; 46:1427–1436. [DOI] [PubMed] [Google Scholar]
- [47].Stern ER, Welsh RC, Gonzalez R, Fitzgerald KD, Abelson JL, Taylor SF. Subjective uncertainty and limbic hyperactivation in obsessive‐compulsive disorder. Human Brain Mapping 2013; 34:1956–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Morriss J, Christakou A, van Reekum CM. Intolerance of uncertainty predicts fear extinction in amygdala-ventromedial prefrontal cortical circuitry. Biol Mood Anxiety Disord 2015; 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience & Biobehavioral Reviews 2013; 37(4):681–96. [DOI] [PubMed] [Google Scholar]
- [50].Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-Compulsive Disorder: Advances in Diagnosis and Treatment. JAMA 2017; 317:1358–1367. [DOI] [PubMed] [Google Scholar]
- [51].Buhle JT, Silvers AJ, Wager TD, Lopez R, Onyemekwu C, Kober H. Cognitive Reappraisal of Emotion: A meta-analysis of human neuroimaging studies. Cereb Cortex 2014; 24:2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Olatunji BO, Ferreira-Garcia R, Caseras X, Fullana MA, Wooderson S, Speckens A, et al. Predicting response to cognitive behavioral therapy in contamination-based obsessive–compulsive disorder from functional magnetic resonance imaging. Psychol Med 2014; 44:2125–2137. [DOI] [PubMed] [Google Scholar]
- [53].Fullana MA, Zhu X, Alonso P, Cardoner N, Real E, López-Solà C, et al. Basolateral amygdala–ventromedial prefrontal cortex connectivity predicts cognitive behavioural therapy outcome in adults with obsessive–compulsive disorder. Journal of psychiatry & neuroscience: JPN. 2017;42(6):378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Belloch A, Cabedo E, Carrió C, Fernández-Alvarez H, García F, Larsson C. Group versus individual cognitive treatment for obsessive-compulsive disorder: Changes in non-OCD symptoms and cognitions at post-treatment and one-year follow-up. Psychiatry Res 2011; 187:174–179. [DOI] [PubMed] [Google Scholar]
- [55].Overton SM, Menzies RG. Cognitive Change During Treatment of Compulsive Checking. Behav Change 2005; 22:172–184. [Google Scholar]
- [56].Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacol 2008; 196: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Selvaraj S, Walker C, Arnone D, Cao B, Faulkner P, Cowen PJ, et al. Effect of citalopram on emotion processing in humans: a combined 5-HT 1A [11 C] CUMI-101 PET and functional MRI study. Neuropsychopharmacology 2018; 43:655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kim M, Kwak S, Yoon YB, Kwak YB, Kim T, Cho KI, et al. Functional connectivity of the raphe nucleus as a predictor of the response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. Neuropsychopharmacology 2019; 44:2073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry 2009; 166: 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].De Wit SJ, Van der Werf YD, Mataix-Cols D, Trujillo JP, Van Oppen P, Veltman DJ, et al. Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychological Medicine 2015; 45:3059–3073. [DOI] [PubMed] [Google Scholar]
- [61].Assaf M, Rabany L, Zertuche L, Bragdon L, Tolin D, Goethe J, et al. Neural functional architecture and modulation during decision making under uncertainty in individuals with generalized anxiety disorder. Brain and Behavior 2018; 8:e01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of obsessive-compulsive disorder (OCD). Psychiatric Quarterly 2018; 89(3):645–65. [DOI] [PubMed] [Google Scholar]
- [63].Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. American Journal of Psychiatry 2019; 176(11):931–8. [DOI] [PubMed] [Google Scholar]
- [64].Scheinost D, Stoica T, Saksa J, Papademetris X, Constable RT, Pittenger C, et al. Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Translational Psychiatry 2013; 3(4):e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Scheinost D, Stoica T, Wasylink S, Gruner P, Saksa J, Pittenger C, et al. Resting state functional connectivity predicts neurofeedback response. Frontiers in Behavioral Neuroscience 2014; 8:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Paret C, Ruf M, Gerchen MF, Kluetsch R, Demirakca T, Jungkuntz M, et al. fMRI neurofeedback of amygdala response to aversive stimuli enhances prefrontal–limbic brain connectivity. Neuroimage 2016; 125:182–188. [DOI] [PubMed] [Google Scholar]
- [67].Fridgeirsson EA, Figee M, Luigjes J, van den Munckhof P, Schuurman PR, van Wingen G, et al. Deep brain stimulation modulates directional limbic connectivity in obsessive-compulsive disorder. Brain 2020; 143(5):1603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].LeDoux JE, Pine DS. Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 2016; 173:1083–1093. [DOI] [PubMed] [Google Scholar]
- [69].Luyten L, Hendrickx S, Raymaekers S, Gabriëls L, Nuttin B. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry 2016; 21:1272–1280. [DOI] [PubMed] [Google Scholar]
- [70].Li N, Baldermann JC, Kibleur A, Treu S, Akram H, Elias GJ, et al. A unified connectomic target for deep brain stimulation in obsessive-compulsive disorder. Nature Communications 2020; 11(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Liebrand LC, Caan MW, Schuurman PR, van den Munckhof P, Figee M, Denys D, et al. Individual white matter bundle trajectories are associated with deep brain stimulation response in obsessive-compulsive disorder. Brain Stimulation 2019; 12(2):353–60. [DOI] [PubMed] [Google Scholar]
- [72].Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 2010; 214:519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ferrão YA, Shavitt RG, Prado H, Fontenelle LF, Malavazzi DM, de Mathis MA, et al. Sensory phenomena associated with repetitive behaviors in obsessive-compulsive disorder: an exploratory study of 1001 patients. Psychiatry Res 2012; 197:253–258. [DOI] [PubMed] [Google Scholar]
- [74].Lee JC, Prado HS, Diniz JB, Borcato S, da Silva CB, Hounie AG, Miguel EC, Leckman JF, do Rosário MC. Perfectionism and sensory phenomena: phenotypic components of obsessive-compulsive disorder. Compr Psychiatry. 2009; 50:431–436. [DOI] [PubMed] [Google Scholar]
- [75].Rosario MC, Prado HS, Borcato S, Diniz JB, Shavitt RG, Hounie AG. Validation of the University of São Paulo Sensory Phenomena Scale: initial psychometric properties. CNS Spectr. 2009. June; 14:315–323. [DOI] [PubMed] [Google Scholar]
- [76].Shavitt RG, de Mathis MA, Oki F, Ferrao YA, Fontenelle LF, Torres AR, et al. Phenomenology of OCD: lessons from a large multicenter study and implications for ICD-11. J Psychiatr Res 2014; 57:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Summerfeldt LJ, Kloosterman PH, Antony MM, Swinson RP. Examining an obsessive-compulsive core dimensions model: Structural validity of harm avoidance and incompleteness. JOCRD 2014; 3:83–94. [Google Scholar]
- [78].Coughtrey AE, Shafran R, Lee M, Rachman SJ. It’s the feeling inside my head: A qualitative analysis of mental contamination in obsessive-compulsive disorder. Behavioural and Cognitive Psychotherapy 2012; 40(2):163–73. [DOI] [PubMed] [Google Scholar]
- [79].Reese HE, Scahill L, Peterson AL, Crowe K, Woods DW, Piacentini J, et al. The premonitory urge to tic: measurement, characteristics, and correlates in older adolescents and adults. Behav Ther 2014; 45:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Berman BD, Horovitz SG, Morel B, Hallett M. Neural correlates of blink suppression and the buildup of a natural bodily urge. Neuroimage 2012; 59:1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Jackson SR, Parkinson A, Kim SY, Schüermann M, Eickhoff SB. On the functional anatomy of the urge-for-action. Cogn Neurosci 2011; 2:227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mazzone SB, Cole LJ, Ando A, Egan GF, Farrell MJ et al. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 2011; 31:2948–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Bohlhalter S, Goldfine A, Matteson S, Garraux G, Hanakawa T, Kansaku K, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(8):2029–37. [DOI] [PubMed] [Google Scholar]
- [84].Neuner I, Werner CJ, Arrubla J, Stöcker T, Ehlen C, Wegener HP, et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Frontiers in Human Neuroscience. 2014; 8:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Stern ER, Brown C, Ludlow M, Shahab R, Collins K, Lieval A, et al. The buildup of an urge in obsessive–compulsive disorder: Behavioral and neuroimaging correlates. Human Brain Mapping. 2020; 41:1611–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Subirà M, Sato JR, Alonso P, do Rosário MC, Segalàs C, Batistuzzo MC, et al. Brain structural correlates of sensory phenomena in patients with obsessive–compulsive disorder. J Psychiatry Neurosci 2015; 40: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Brown C, Shahab R, Collins K, Fleysher L, Goodman WK, Burdick KE, et al. Functional neural mechanisms of sensory phenomena in obsessive-compulsive disorder. J Psychiatr Res 2019; 109: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tricomi E, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur. J. Neurosci 2009; 29:2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].De Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J. Neurosci 2012; 32:12066–12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gillan CM, Robbins TW. Goal-directed learning and obsessive-compulsive disorder. Philos Trans R Soc Lond B Biol Sci 2014; 369:20130475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gillan CM, Apergis-Schoute AM, Morein-Zamir S, Urcelay GP, Sule A, Fineberg NA, et al. Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry. 2015; 172:284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Delorme C, Salvador A, Valabregue R, Roze E, Palminteri S, Vidailhet M, et al. Enhanced habit formation in Gilles de la Tourette syndrome. Brain 2016; 139:605–15. [DOI] [PubMed] [Google Scholar]
- [93].Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND. Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. Elife 2016; 5:e11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience 2006; 7(6):464–76. [DOI] [PubMed] [Google Scholar]
- [95].Prado HS, Rosário MC, Lee J, Hounie AG, Shavitt RG, Miguel EC. Sensory phenomena in obsessive-compulsive disorder and tic disorders: a review of the literature. CNS Spectr 2008; 13:425–432. [DOI] [PubMed] [Google Scholar]
- [96].Cavanna AE, Black KJ, Hallett M, Voon V. Neurobiology of the premonitory urge in Tourette’s syndrome: pathophysiology and treatment implications. The Journal of Neuropsychiatry and Clinical Neurosciences 2017; 29(2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mathes BM, Kennedy GA, Wilver NL, Carlton CN, Cougle JR. A multi-method analysis of incompleteness in behavioral treatment of contamination-based OCD. Behaviour Research and Therapy 2019; 114:1–6. [DOI] [PubMed] [Google Scholar]
- [98].Houghton DC, Capriotti MR, Scahill LD, Wilhelm S, Peterson AL, Walkup JT, et al. Investigating habituation to premonitory urges in behavior therapy for tic disorders. Behavior Therapy 2017; 48(6):834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].McGuire JF, Piacentini J, Brennan EA, Lewin AB, Murphy TK, Small BJ, et al. A meta-analysis of behavior therapy for Tourette syndrome. Journal of Psychiatric Research 2014; 50:106–12. [DOI] [PubMed] [Google Scholar]
- [100].Deckersbach T, Chou T, Britton JC, Carlson LE, Reese HE, Siev J, et al. Neural correlates of behavior therapy for Tourette׳ s disorder. Psychiatry Research: Neuroimaging 2014; 224:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Van de Griendt JM, Verdellen CW, Van Dijk MK, Verbraak MJ. Behavioural treatment of tics: habit reversal and exposure with response prevention. Neuroscience & Biobehavioral Reviews. 2013; 37:1172–7. [DOI] [PubMed] [Google Scholar]
- [102].Dillenburger K Habit reversal training as a treatment for refractory OCD–A case study. European Journal of Behavior Analysis 2006; 7:67–75. [Google Scholar]
- [103].Chau DT, Fogelman P, Nordanskog P, Drevets WC, Hamilton JP. Distinct neural-functional effects of treatments with selective serotonin reuptake inhibitors, electroconvulsive therapy, and transcranial magnetic stimulation and their relations to regional brain function in major depression: a meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2017; 2:318–26. [DOI] [PubMed] [Google Scholar]
- [104].Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev 2001; 7:199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Stern ER, Shahab R, Grimaldi SJ, Leibu E, Murrough JW, Fleysher L. High-dose ondansetron reduces activation of interoceptive and sensorimotor brain regions. Neuropsychopharmacology 2019; 44:390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Heidari M, Zarei M, Hosseini SM, Taghvaei R, Maleki H, Tabrizi M. Ondansetron or placebo in the augmentation of fluvoxamine response over 8 weeks in obsessive-compulsive disorder. Int Clin Psychopharmacol 2014; 29:344–350. [DOI] [PubMed] [Google Scholar]
- [107].Pallanti S, Bernardi S, Antonini S, Singh N, Hollander E. Ondansetron augmentation in patients with obsessive-compulsive disorder who are inadequate responders to serotonin reuptake inhibitors: improvement with treatment and worsening following discontinuation. Eur Neuropsychopharmacol 2014; 24:375–380. [DOI] [PubMed] [Google Scholar]
- [108].Toren P, Weizman A, Ratner S, Cohen D, Laor N. Ondansetron treatment in Tourette’s disorder: a 3-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2005; 66:499–503. [DOI] [PubMed] [Google Scholar]
- [109].Transcept Pharmaceuticals I. Transcept Pharmaceuticals Announces that a Phase 2 Clinical Trial of TO-2061 as Adjunctive Therapy for Obsessive Compulsive Disorder Did Not Meet Primary Endpoint. 2012; http://ir.transcept.com/releasedetail.cfm?ReleaseID=728327. Accessed October 4, 2014.
- [110].Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH. Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol 2010; 13:217–227. [DOI] [PubMed] [Google Scholar]
- [111].Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci 2013; 20: 257–262. [DOI] [PubMed] [Google Scholar]
- [112].Landeros-Weisenberger A, Mantovani A, Motlagh MG, de Alvarenga PG, Katsovich L, Leckman JF, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimul 2015; 8:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Senço NM, Huang Y, D’Urso G, Parra LC, Bikson M, Mantovani A, et al. Transcranial direct current stimulation in obsessive–compulsive disorder: emerging clinical evidence and considerations for optimal montage of electrodes. Expert Rev Med Devices 2015; 12:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].D’Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A. Transcranial direct current stimulation for obsessive-compulsive disorder: A randomized, controlled, partial crossover trial. Depress Anxiety 2016; 33:1132–1140. [DOI] [PubMed] [Google Scholar]
- [115].Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clinical Neurophysiology 2014; 125(6):1202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zibman S, Pell GS, Barnea-Ygael N, Roth Y, Zangen A. Application of transcranial magnetic stimulation for major depression: coil design and neuroanatomical variability considerations. European Neuropsychopharmacology 2019; In Press. [DOI] [PubMed] [Google Scholar]
- [117].Malik S, Jacobs M, Cho SS, Boileau I, Blumberger D, Heilig M, et al. Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [11 C] PHNO-PET pilot trial in healthy humans. Brain Imaging and Behavior 2018; 12(5):1306–17. [DOI] [PubMed] [Google Scholar]
- [118].Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biological Psychiatry 2014; 76(9):742–9. [DOI] [PubMed] [Google Scholar]
- [119].Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 2014; 18:177–185. [DOI] [PubMed] [Google Scholar]
- [120].Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 2011; 56(3):1655–65. [DOI] [PubMed] [Google Scholar]
- [121].Abramovitch A, Abramowitz JS, Mittelman A. The neuropsychology of adult obsessive–compulsive disorder: a meta-analysis. Clinical psychology review 2013; 33:1163–71. [DOI] [PubMed] [Google Scholar]
- [122].De Wit SJ, de Vries FE, van der Werf YD, Cath DC, Heslenfeld DJ, Veltman EM, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. American Journal of Psychiatry 2012; 169:1100–8. [DOI] [PubMed] [Google Scholar]
- [123].Kang DH, Jang JH, Han JY, Kim JH, Jung WH, Choi JS, et al. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2013; 40:340–6. [DOI] [PubMed] [Google Scholar]
- [124].Rasmussen J, Siev J, Abramovitch A, Wilhelm S. Scrupulosity and contamination OCD are not associated with deficits in response inhibition. J Behav Ther Exp Psychiatry 2016; 50:120–6. [DOI] [PubMed] [Google Scholar]
- [125].Silveira VP, Frydman I, Fontenelle LF, Mattos P, de Oliveira-Souza R, Moll J, et al. Exploring response inhibition and error monitoring in obsessive-compulsive disorder. Journal of Psychiatric Research 2020; 126:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kalanthroff E, Teichert T, Wheaton MG, Kimeldorf MB, Linkovski O, Ahmari SE, et al. The role of response inhibition in medicated and unmedicated obsessive‐compulsive disorder patients: evidence from the stop‐signal task. Depression and Anxiety 2017; 34:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Leopold R, Backenstrass M. Neuropsychological differences between obsessive-compulsive washers and checkers: a systematic review and meta-analysis. J Anxiety Disord 2015; 30:48–58. [DOI] [PubMed] [Google Scholar]
- [128].van Wijk BC, Alkemade A, Forstmann BU. Functional segregation and integration within the human subthalamic nucleus from a micro-and meso-level perspective. Cortex 2020; 131:103–113. [DOI] [PubMed] [Google Scholar]
- [129].Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, et al. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 2012; 60(1):830–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Rappel P, Marmor O, Bick AS, Arkadir D, Linetsky E, Castrioto A, et al. Subthalamic theta activity: a novel human subcortical biomarker for obsessive compulsive disorder. Transl Psychiatry. 2018; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Cavanagh JF, Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 2014; 18:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Widge AS, Zorowitz S, Basu I, Paulk AC, Cash SS, Eskandar EN, et al. Deep brain stimulation of the internal capsule enhances human cognitive control and prefrontal cortex function. Nature Communications 2019; 10(1):1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Alegria AA, Wulff M, Brinson H, Barker GJ, Norman LJ, Brandeis D, et al. Real‐time f MRI neurofeedback in adolescents with attention deficit hyperactivity disorder. Human brain mapping 2017; 38:3190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rubia K, Criaud M, Wulff M, Alegria A, Brinson H, Barker G, et al. Functional connectivity changes associated with fMRI neurofeedback of right inferior frontal cortex in adolescents with ADHD. NeuroImage 2019; 188:43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, et al. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry 2011; 69:867–74. [DOI] [PubMed] [Google Scholar]
- [137].Kaufmann C, Beucke JC, Preuße F, Endrass T, Schlagenhauf F, Heinz A, et al. Medial prefrontal brain activation to anticipated reward and loss in obsessive–compulsive disorder. Neuroimage Clin 2013; 2:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Abramovitch A, Pizzagalli DA, Reuman L, Wilhelm S. Anhedonia in obsessive-compulsive disorder: Beyond comorbid depression. Psychiatry Research 2014; 216(2):223–9. [DOI] [PubMed] [Google Scholar]
- [139].Pushkarskaya H, Sobowale K, Henick D, Tolin DF, Anticevic A, Pearlson GD, et al. Contrasting contributions of anhedonia to obsessive-compulsive, hoarding, and post-traumatic stress disorders. Journal of Psychiatric Research 2019; 109:202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Xie C, Ma L, Jiang N, Huang R, Li L, Gong L, et al. Imbalanced functional link between reward circuits and the cognitive control system in patients with obsessive-compulsive disorder. Brain imaging and behavior 2017; 11:1099–109. [DOI] [PubMed] [Google Scholar]
- [141].Jung WH, Kang D-H, Kim E, Shin KS, Jang JH, Kwon JS. Abnormal corticostriatal-limbic functional connectivity in obsessive–compulsive disorder during reward processing and resting-state. Neuroimage Clin 2013; 3:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Koch K, Reeß TJ, Rus OG, Gürsel DA, Wagner G, Berberich G, et al. Increased default mode network connectivity in obsessive–compulsive disorder during reward processing. Frontiers in psychiatry 2018; 9:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Admon R, Bleich-Cohen M, Weizmant R, Poyurovsky M, Faragian S, Hendler T. Functional and structural neural indices of risk aversion in obsessive–compulsive disorder (OCD). Psychiatry Research: Neuroimaging 2012; 203:207–13. [DOI] [PubMed] [Google Scholar]
- [144].Norman LJ, Carlisi CO, Christakou A, Murphy CM, Chantiluke K, Giampietro V, et al. Frontostriatal dysfunction during decision making in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2018; 3:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Moreira PS, Macoveanu J, Marques P, Coelho A, Magalhaes R, Siebner HR, et al. Altered response to risky decisions and reward in patients with obsessive--compulsive disorder. Journal of Psychiatry and Neuroscience 2020; 45:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Grassi G, Pallanti S, Righi L, Figee M, Mantione M, Denys D, et al. Think twice: Impulsivity and decision making in obsessive–compulsive disorder. Journal of Behavioral Addictions 2015; 4:263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Grassi G, Makris N, Pallanti S. Addicted to compulsion: assessing three core dimensions of addiction across obsessive-compulsive disorder and gambling disorder. CNS spectrums 2019; 20:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Rouhani N, Wimmer GE, Schneier FR, Fyer AJ, Shohamy D, Simpson HB. Impaired generalization of reward but not loss in obsessive–compulsive disorder. Depression and Anxiety 2019; 36:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Voon V, Baek K, Enander J, Worbe Y, Morris LS, Harrison NA, et al. Motivation and value influences in the relative balance of goal-directed and habitual behaviours in obsessive-compulsive disorder. Translational Psychiatry 2015; 5:e670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Sip KE, Gonzalez R, Taylor SF, Stern ER. increased loss aversion in Unmedicated Patients with Obsessive–compulsive Disorder. Frontiers in Psychiatry 2018; 8:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Jung WH, Kang DH, Han JY, Jang JH, Gu BM, Choi JS, et al. Aberrant ventral striatal responses during incentive processing in unmedicated patients with obsessive–compulsive disorder. Acta Psychiatrica Scandinavica 2011; 123:376–86. [DOI] [PubMed] [Google Scholar]
- [152].Choi JS, Shin YC, Jung WH, Jang JH, Kang DH, Choi CH, et al. Altered brain activity during reward anticipation in pathological gambling and obsessive-compulsive disorder. PLoS One 2012; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hauser TU, Iannaccone R, Dolan RJ, Ball J, Hättenschwiler J, Drechsler R, et al. Increased fronto-striatal reward prediction errors moderate decision making in obsessive–compulsive disorder. Psychological Medicine 2017; 47:1246–1258. [DOI] [PubMed] [Google Scholar]
- [154].Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry 2005; 57(3):210–9. [DOI] [PubMed] [Google Scholar]
- [155].Murray GK, Knolle F, Ersche KD, Craig KJ, Abbott S, Shabbir SS, et al. Dopaminergic drug treatment remediates exaggerated cingulate prediction error responses in obsessive-compulsive disorder. Psychopharmacology 2019; 236:2325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Palminteri S, Clair A-H, Mallet L, Pessiglione M. Similar improvement of reward and punishment learning by serotonin reuptake inhibitors in obsessive-compulsive disorder. Biol Psychiatry 2012; 72:244–250. [DOI] [PubMed] [Google Scholar]
- [157].Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience 2010; 166:1023–1035. [DOI] [PubMed] [Google Scholar]
- [158].Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009; 57:640–52. [DOI] [PubMed] [Google Scholar]
- [159].Figee M, Luigjes J, Smolders R, Valencia-Alfonso CD, van Wingen G, de Kwaasteniet B, et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci 2013; 16:386–387. [DOI] [PubMed] [Google Scholar]
- [160].Coenen VA, Schlaepfer TE, Goll P, Reinacher PC, Voderholzer U, Tebartz van Elst L, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr 2017; 22:282–289. [DOI] [PubMed] [Google Scholar]
- [161].Greer SM, Trujillo AJ, Glover GH, Knutson B. Control of nucleus accumbens activity with neurofeedback. Neuroimage 2014; 96:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Marek S, Dosenbach NU. The frontoparietal network: function, electrophysiology, and importance of individual precision mapping. Dialogues in Clinical Neuroscience 2018; 20:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Vossel S, Geng JJ, Fink GR. Dorsal and ventral attention systems: distinct neural circuits but collaborative roles. The Neuroscientist 2014; 20:150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Snyder HR, Kaiser RH, Warren SL, Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: A meta-analysis. Clinical Psychological Science 2015; 3:301–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Vaghi MM, Vértes PE, Kitzbichler MG, Apergis-Schoute AM, van der Flier FE, Fineberg NA, et al. Specific frontostriatal circuits for impaired cognitive flexibility and goal-directed planning in obsessive-compulsive disorder: evidence from resting-state functional connectivity. Biol Psychiatry 2017; 81:708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Olson CA, Hale LR, Hamilton N, Powell JN, Martin LE, Savage CR. Altered source memory retrieval is associated with pathological doubt in obsessive–compulsive disorder. Behavioural Brain Research, 2016; 296:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Jaafari N, Frasca M, Rigalleau F, Rachid F, Gil R, Olié JP, et al. Forgetting what you have checked: A link between working memory impairment and checking behaviors in obsessive-compulsive disorder. European Psychiatry 2013; 28:87–93. [DOI] [PubMed] [Google Scholar]
- [168].Buhlmann U, Deckersbach T, Engelhard I, Cook LM, Rauch SL, Kathmann N, et al. Cognitive retraining for organizational impairment in obsessive-compulsive disorder. Psychiatry Res 2006; 144:109–116. [DOI] [PubMed] [Google Scholar]
- [169].Park HS, Shin YW, Ha TH, Shin MS, Kim YY, Lee YH, et al. Effect of cognitive training focusing on organizational strategies in patients with obsessive‐compulsive disorder. Psychiatry and Clinical Neurosciences 2006; 60:718–26. [DOI] [PubMed] [Google Scholar]
- [170].Kashyap H, Reddy P, Mandadi S, Narayanaswamy JC, Sudhir PM, Reddy YJ. Cognitive training for neurocognitive and functional impairments in obsessive compulsive disorder: A case report. Journal of Obsessive-Compulsive and Related Disorders 2019; 23:100480. [Google Scholar]
- [171].Kalanthroff E, Steinman SA, Schmidt AB, Campeas R, Simpson HB. Piloting a personalized computerized inhibitory training program for individuals with obsessive-compulsive disorder. Psychotherapy and Psychosomatics 2018; 87:52–5. [DOI] [PubMed] [Google Scholar]
- [172].Zheng H, Jia F, Han H, Wang S, Guo G, Quan D. Combined fluvoxamine and extended-release methylphenidate improved treatment response compared to fluvoxamine alone in patients with treatment-refractory obsessive-compulsive disorder: A randomized double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2019; 29:397–404. [DOI] [PubMed] [Google Scholar]
- [173].Faraone SV. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci Biobehav Rev 2018; 87:255–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Woolley JB, Heyman I. Dexamphetamine for obsessive-compulsive disorder. American Journal of Psychiatry 2003; 160(1):183. [DOI] [PubMed] [Google Scholar]
- [175].Bloch M, Landeros-Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF. A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Molecular Psychiatry 2006; 11(7):622–32. [DOI] [PubMed] [Google Scholar]
- [176].Cools R, D’Esposito M. Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry 2011; 69(12):e113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Zhou DD, Wang W, Wang GM, Li DQ, Kuang L. An updated meta-analysis: short-term therapeutic effects of repeated transcranial magnetic stimulation in treating obsessive-compulsive disorder. Journal of Affective Disorders 2017; 215:187–196. [DOI] [PubMed] [Google Scholar]
- [178].D’Urso G, Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A. Transcranial direct current stimulation for obsessive–compulsive disorder: a randomized, controlled, partial crossover trial. Depression and Anxiety 2016; 33:1132–40. [DOI] [PubMed] [Google Scholar]
- [179].Gowda SM, Narayanaswamy JC, Hazari N, Bose A, Chhabra H, Balachander S, et al. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: A randomized, double blinded, sham controlled trial. Brain stimulation 2019; 12:922–9. [DOI] [PubMed] [Google Scholar]
- [180].Choi EY, Tanimura Y, Vage PR, Yates EH, Haber SN. Convergence of prefrontal and parietal anatomical projections in a connectional hub in the striatum. Neuroimage 2017; 146:821–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Picó-Pérez M, Radua J, Steward T, Menchón JM, Soriano-Mas C. Emotion regulation in mood and anxiety disorders: a meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79:96–104. [DOI] [PubMed] [Google Scholar]
- [182].Picó-Pérez M, Alemany-Navarro M, Dunsmoor JE, Radua J, Albajes-Eizagirre A, Vervliet B, et al. Common and distinct neural correlates of fear extinction and cognitive reappraisal: A meta-analysis of fMRI studies. Neurosci Biobehav Rev 2019; 104:102–115. [DOI] [PubMed] [Google Scholar]
- [183].Tang W, Jbabdi S, Zhu Z, Cottaar M, Grisot G, Lehman JF, et al. A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. Elife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Haber SN, Tang W, Choi EY, Yendiki A, Liu H, Jbabdi S, Versace A, et al. Circuits, networks, and neuropsychiatric disease: Transitioning from anatomy to imaging. Biological Psychiatry 2020; 87:318–27. [DOI] [PubMed] [Google Scholar]
- [185].van den Heuvel OA, Boedhoe P, Bertolin S, Bruin WB, Francks C, Ivanov I, et al. ENIGMA-OCD Working Group. An overview of the first 5 years of the ENIGMA obsessive–compulsive disorder working group: The power of worldwide collaboration. Human Brain Mapping 2020. Available online ahead of print: 10.1002/hbm.24972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Boedhoe P, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. ENIGMA OCD Working Group. Cortical abnormalities associated with pediatric and adult obsessive-compulsive disorder: findings from the ENIGMA Obsessive-Compulsive Disorder Working Group. American Journal of Psychiatry 2018; 175:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PloS one 2012; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Scolari M, Seidl-Rathkopf KN, Kastner S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr opin behav sci 2015; 1:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM, MacDonald III AW, et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia 2015; 73:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Bikson M, Brunoni AR, Charvet LE, Clark VP, Cohen LG, Deng ZD, et al. Rigor and reproducibility in research with transcranial electrical stimulation: An NIMH-sponsored workshop. Brain stimul 2018; 11:465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Stein DJ. An integrative approach to psychiatric diagnosis and research. World Psychiatry 2014; 13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Dougherty DD. Will deep brain stimulation help move precision medicine to the clinic in psychiatry? Biol Psychiatry 2019; 85:706–707. [DOI] [PubMed] [Google Scholar]
- [193].Freeston MH, Rhéaume J, Letarte H, Dugas MJ, Ladouceur R. Why do people worry? Person Individ Diff 1994; 17:791–802. [Google Scholar]
- [194].Coles ME, Heimberg RG, Frost RO, Steketee G. Not just right experiences and obsessive-compulsive features: experimental and self-monitoring perspectives. Behav Res Ther 2005; 43:153–167. [DOI] [PubMed] [Google Scholar]
- [195].Brunoni AR, Sampaio-Junior B, Moffa AH, Aparício LV, Gordon P, Klein I, et al. Noninvasive brain stimulation in psychiatric disorders: a primer. Braz J Psychiatry 2019; 41:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


