Abstract
Over 30 million Americans are diagnosed with diabetes and this number is only expected to increase. There are various causes that induce complications with diabetes, including oxidative stress. In oxidative stress, lipid peroxidation-derived reactive carbonyl species such as 4-hydroxy-2-nonenal (4-HNE) is shown to cause damage in organs that leads to diabetic complications. We provided evidence to show that 4-HNE or/and 4-HNE-protein adducts are elevated in various organ systems of diabetic patients and animal models. We then discussed the advantages and disadvantages of different methodologies used for the detection of 4-HNE in diabetic tissues. We also discussed how novel approaches such as electrochemistry and nanotechnology can be used for monitoring 4-HNE levels in biological systems in real-time. Thus, this review enlightens the involvement of 4-HNE in the pathogenesis of diabetes and its complications and efficient methods to identify it. Furthermore, the article presents that 4-HNE can be developed as a biomarker for end-organ damage in diabetes such as diabetic cardiac complications.
Keywords: Diabetes, diabetic cardiac complications, reactive carbonyl species, 4-hydroxy-2-nonenal (4-HNE), biomarker, electrochemistry
Introduction
Diabetes mellitus (DM) is a multifactorial disease. Based on the type, DM has different etiopathogenesis. However, their pathogenesis convenes at oxidative stress despite having different etiologies. Since DM leads to complications and ultimately end-organ damage, oxidative stress became an integral part of the pathogenesis of diabetic complications [1]. Oxidative stress is, again, a complex process. The generation of free radicals a.k.a. reactive oxygen species (ROS) initiates oxidative stress. Despite having a short half-life, ROS start the lipid peroxidation of biological membranes and thus produce reactive carbonyl species (RCS) such as 4-hydroxy-2-nonenal (4-HNE) to further continue the oxidative stress process. Thus, RCS are usually regarded as secondary and longer-lasting products of oxidative stress [2]. For instance, in long-standing diabetes when diabetic complications ensue, apart from lipid peroxidation, nonenzymatic (glycation and amino acid oxidation) and enzymatic (polyol pathway and glucose oxidation) reactions also generate RCS [3]. RCS exert either biological/physiological or cytotoxic and genotoxic properties based on their concentration at the tissue or the systemic level.
4-HNE, a common cellular reactive aldehyde, is getting more attention in diabetic research pertaining to oxidative stress as they are implicated in pathogenesis. Increased levels of 4-HNE can be detected in serum, plasma, blood, urine, cells, and tissues after diabetes by different methods either directly or indirectly [4,5]; however, each method and sample types have their own challenges and merits. Generally, few notable challenges in measuring reactive aldehydes in biological samples are their volatility, polarity, and instability [5] besides the compounding chemical and enzymatic reactions that can pose challenges in pre-analytical and analytical phases [6]. In addition, 4-HNE with its electro-philicity can covalently modify nucleophilic macromolecules like proteins and DNA a.k.a. 4-HNE adduct formation [7]. However, 4-HNE protein adducts can be degraded by proteolysis, and thus measurements of the levels of these adducts at a single time point could underestimate their formation [8]. The merits are 4-HNE like RCS having a relatively longer half-life [9] making them a better indicator of oxidative stress in pathology compared to ROS [10]. Since 4-HNE is highly reactive and forms adducts with macromolecules, their levels can be measured either in both free form and as an adduct [11]. Although there are merits and demerits in measuring them in free and adducted forms, they can be taped into serving as reliable biomarkers at least for certain specific pathologies, such as diabetes and its complications, in which 4-HNE generation is induced via different mechanisms other than lipid peroxidation. Thus, measuring the accurate concentration of 4-HNE and its adducts in the disease process can help in determining their role in pathogenesis, and thus we can tap into the potential of developing 4-HNE like RCS as a reliable biomarker and device a method to assess them.
This review will cover how 4-HNE is pertinent in diabetes through oxidative stress and lipid peroxidation. It will also provide proof of how oxidative stress negatively impacts multiple organ systems of diabetes patients from pathophysiological changes to diseases, by the contribution from 4-HNE. Finally, it will analyze various approaches to detect 4-HNE levels and which method is ideal. A deep understanding of 4-HNE can impact the perception of diabetes and its complications, and thus can pave to salient research and clinical applications. Thus, in this review, we describe types of diabetes and their complications, free 4-HNE levels and 4-HNE adducts in diabetic complications of different body systems, currently available methodologies to detect 4-HNE levels in the biological samples and finally novel approaches for detecting 4-HNE levels in biological samples including samples from diabetic complications.
Diabetes and its complications
Americans of 34.2 million—just over 1 in 10—have diabetes, according to a recent study by the Center for Disease Control [12]. In the following years, diabetes patients will increase by 165%, from 11 million in 2000 to 29 million in 2050 [13]. DM is primarily characterized by hyperglycemia. Diabetes exists in various forms, including type 1 diabetes (insulin-dependent), type 2 diabetes (non-insulin-dependent), gestational diabetes, cystic fibrosis-related diabetes, and monogenic diabetes [14].
Diabetic complications that occur due to macrovascular and microvascular damage in the eye, kidney, and heart include diabetic retinopathy, diabetic nephropathy, diabetic cardiomyopathy, and a variety of clinical neuropathies [15].
Diabetes can cause adverse complications in different organ systems (Figure 1). In the cardiovascular system, diabetes can lead to heart diseases, peripheral vascular disease (PVD), stroke, coronary artery disease (CAD), and atherosclerosis. Diabetes also affects the heart muscle, causing both systolic and diastolic heart failure [16]. Diabetes can also impact the nervous system by inducing complications such as hyperglycemia-induced metabolic derangements, neurochemical alterations, serum lipid changes, and vascular coagulation, and thrombotic abnormalities [17]. Neurological disease associated with diabetes includes myotonic muscular dystrophy [18]. Diabetes is also associated with causing chronic hepatitis C, non-alcoholic fatty liver disease, cirrhosis, acute liver failure, and hepatocellular carcinoma in the liver [19]. Complications in the kidney due to diabetes include diabetic nephropathy, kidney failure, diabetic kidney disease, and microalbuminuria [20,21]. In the eyes, diabetes can cause diabetic macular edema (DME), neovascular glaucoma, and retinal detachment [22]. In the skeletal system, diabetes can result in decreased bone mineral density, increased fracture risk, and varying bone metabolism [23]. Demyelination, axon cylinder loss in the posterior columns, the peripheral nerves, and mild abnormalities in the blood vessels of the spinal cord can cause diabetic neuropathy [24].
Figure 1.
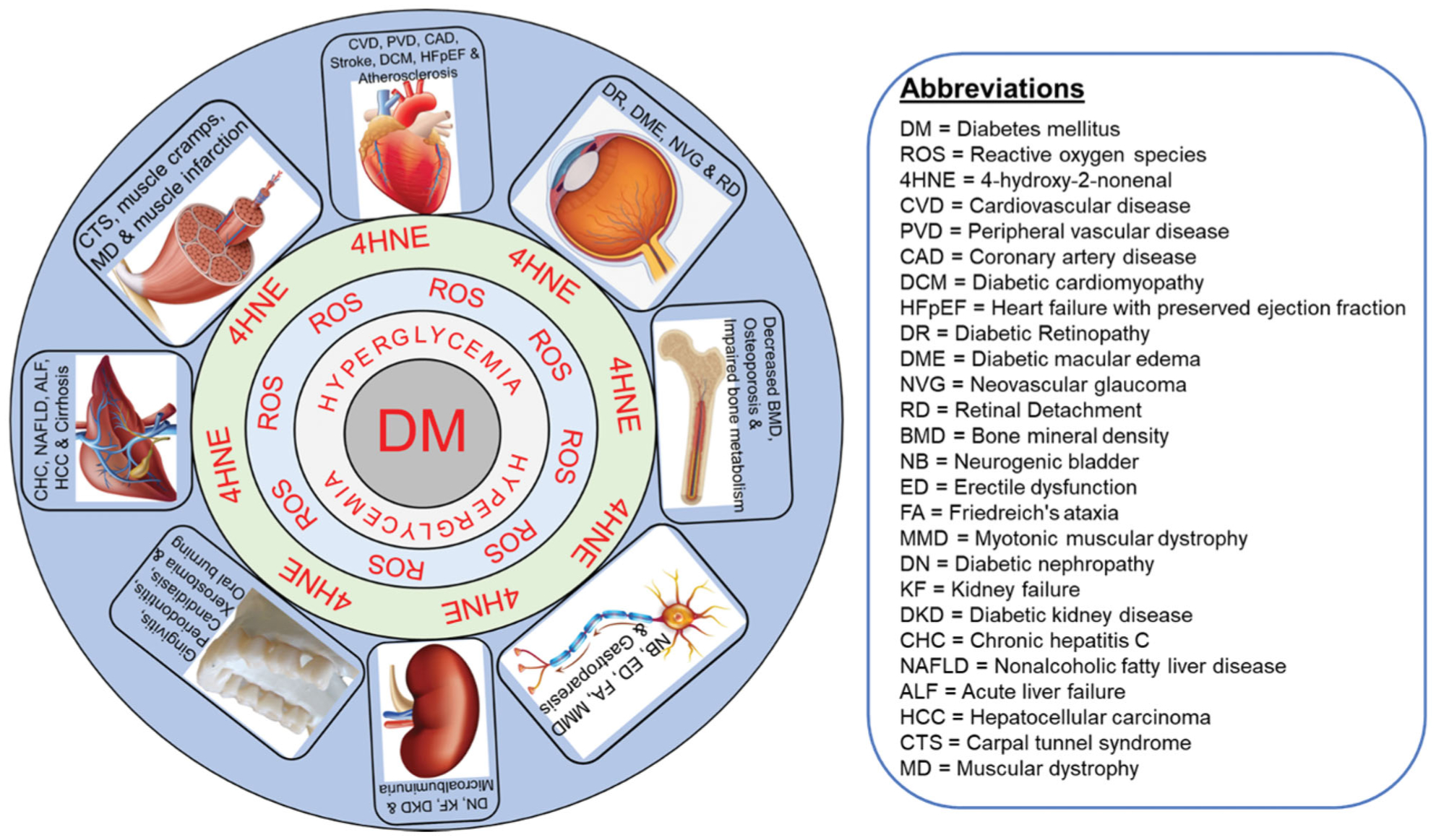
Depiction of diabetes mellitus (DM)-mediated 4-hydroxy-2-nonenal (4-HNE) affecting multiple organs systems. DM mediated hyperglycemia generates reactive oxygen species (ROS) which in turn produces 4HNE which forms adducts with multiple organs including cardiovascular, muscle, gastrointestinal, dental, renal, nervous, bone and eye and cause multiple complications as mentioned in each organ systems. ALF: acute liver failure; BMD: bone mineral density; CAD: coronary artery disease; CHC: chronic hepatitis C; CTS: carpal tunnel syndrome; CVD: cardiovascular disease; DCM: diabetic cardiomyopathy; DKD: diabetic kidney disease; DME: diabetic macular edema; DN: diabetic nephropathy; DR: diabetic retinopathy; ED: erectile dysfunction; FA: Friedreich’s ataxia; HCC: hepatocellular carcinoma; HFpEF: heart failure with preserved ejection fraction; KF: kidney failure; MD: muscular dystrophy; MMD: myotonic muscular dystrophy; NAFLD: non-alcoholic fatty liver disease; NB: neurogenic bladder; NVG: neovascular glaucoma; PVD: peripheral vascular disease; RD: retinal Detachment.
The plethora of complications in multiple systems during diabetes poses a big health challenge. The unique anatomical, physiological, and biochemical components of each tissue contribute to its specific disease; however, there could be some common stress to the tissues that are brought by diabetes including hyperglycemia, low insulin effect (either low insulin in type-1 DM and insulin resistance in type-2 DM) and oxidative stress.
Oxidative stress and RCS
Oxidative stress is an imbalance between the ROS and their secondary effects and antioxidant defenses which can cause tissue damage [25]. Sources of oxidative stress in diabetes include ROS produced by auto-oxidation reactions of sugars and sugar adducts to protein and by autoxidation of unsaturated lipids in plasma and membrane protein. The stress can amplify the damage in a continuous cycle leading to tissue damage, cell death, and metabolic stress [26]. The damage, in turn, can lead to several pathophysiological changes in the body and cause severe complications in different organ systems. According to a study by Giacco & Brownlee, oxidative stress can cause extreme damage to the microvasculature and cardiovascular system [27]. While ROS attacks a variety of substances, one of the main compounds they target are lipids which lead to lipid peroxidation. Lipid peroxidation occurs when oxidants such as ROS attack lipids containing carbon-carbon double bonds [11]. Lipid peroxidation products include majorly hydroperoxides and then RCS. RCS can diffuse across cell membranes and thus modify proteins throughout the cell and modify cell signaling pathways [11]. RCS include saturated aldehydes, unsaturated aldehydes, 4-hydroxy-2-alkenals, and dicarbonyls. Among these RCS, in this review, we focus on 4-HNE, a cellular α, β, 4-hydroxy-2-alkenal. There is an increase in aldehyde levels after diabetes because of a variety of reasons, including exposure of cells to 4-hydroxyalkenals levels, prolonged generation of 4-hydroxyalkenals that attack cells that lack an effective aldehyde neutralization system, and modified redox signaling [28]. Another study found the carbonyl pathway might stimulate oxidative stress in diabetic tissues resulting in the devastation of the retina and aortic tissue [28].
As oxidative stress can recommence due to a continuous cycle, RCS levels will also increase because of its correlation with levels of oxidative stress. Increased levels of aldehydes and carbonyls can lead to pathophysiological changes such as tissue damage, increased inflammation, and alterations of gene expression [28]. Since the levels of RCS correlate with the severity of organ damage in diabetes, they can be used as a biomarker to identify the extent of complications. 4-HNE, a cellular reactive aldehyde, is one type of such compound that is used for this purpose. Although 4-HNE is normally present in all cells, its elevated levels are often taken as markers of oxidative stress [29] therefore increased levels of 4-HNE and its adducts can signify the severity of diabetic complications.
4-HNE levels and adduct formation in diabetes: relevance to types, gender, and age
As mentioned, levels of 4-HNE may correspond with the degree of complications in DM. Therefore, 4-HNE levels are higher in diabetic conditions compared to normal controls in preclinical and clinical samples. Numerous studies testing type 1 and type 2 diabetic samples of various tissues for 4-HNE levels support this notion [30,31]. Two separate studies compared 4-HNE levels in type 1 diabetic and control mice using their tissue and plasma [30,31]. 4-HNE levels were about 2.5 folds higher in the testicular tissue in type 1 diabetic mice compared to the controls [30]. Furthermore, type 1 diabetic rats had a range of 6.7–7.5 ng/mg of protein 4-HNE in their plasma and the non-diabetic control rats had a range of 0.001–0.005 ng/mg of protein 4-HNE in their plasma [31]. These research studies confirm that there is a correlation between 4-HNE and diabetes.
Similar studies performed in type 2 diabetic conditions echo the above results. For example, Toyokuni et al. found that the serum of type 2 diabetes patients had significantly higher levels of 4-HNE-modified albumin (736.1 ± 34.2 pmol/ml) than the non-diabetics (611.4 ± 39.1 pmol/ml) [32]. In another clinical study, the role of oxidative stress in diabetic nephropathy was discovered by noting significantly higher 4-HNE levels in plasma and lymphocytes from patients with nephropathy secondary to type 2 diabetes than in control subjects [33]. Also, Jaganjac et al. observed that 4-HNE in adipose tissue correlated with the level of severity of type 2 diabetes [34]. When studying obese type 2 diabetic patients, they observed increased levels of 4-HNE-protein adducts in the blood of diabetic obese men [34]. Zinc can protect against oxidative stress in diabetes-induced pathogenic changes and it was shown that there was an inverse correlation between zinc levels and 4-HNE levels. It also indicated that male rats have a slight increase in 4-HNE levels compared to female rats, but only by a smaller margin [35]. In another study, pulmonary toxicity induced with cellulose nanocrystals (CNC) increased 4-HNE levels in the lungs significantly higher in females compared to males [36]. Pertaining to diabetes, RCS was significantly increased in the skeletal muscle of high-fat diet-fed female rats compared to male rats [37]; however, there was no gender difference was observed in liver tissue from mice which were fed with a trans-fatty acid diet [38]. Thus, more focused studies are needed to define the gender role in 4-HNE metabolism.
In terms of age, there was a higher level of 4-HNE-modified proteins in the pancreatic beta-cells of aged diabetic rats than in the controls which was correlated with increase in fibrosis of the pancreatic islets [39]. Furthermore, when comparing 4-HNE levels in the serum of two different age groups diagnosed with diabetes, Altura et al. found that the 23- to 38-year old age group had a 4-HNE level range of 0.22 ± 0.04 μmol. In contrast, the 77- to 86-year old range group had a 4-HNE level range of 0.73 ± 0.06 μmol [40]. Both studies share a consensus that an older age shows higher ranges of 4-HNE levels. Therefore, the data verify age is a variable that can affect 4-HNE levels. There is a consistent pattern of increase in 4-HNE levels in diabetic patients compared to non-diabetic patients with increased age.
Hyperglycemia-mediated oxidative stress in both type-1 and type-2 diabetes increases the tissue 4-HNE-levels. Compared to gender, age may be a factor in impacting 4-HNE metabolism in various organ systems in diabetes. Since type 2 diabetes (90% diabetics have type-2 diabetes) and its complications occur after middle age, increased 4-HNE within older age signifies our interest to focus on 4-HNE as a biomarker.
4-HNE levels and adduct formation in various diabetic complications
In the following section, we provided evidence for increased free 4-HNE and 4-HNE adduct levels in the diabetic complications of various organ systems (Figure 1) to give an idea of how multiple organ systems are affected by diabetes.
Cardiovascular system
Diabetic cardiomyopathy comprises structural and functional abnormalities of the myocardium in diabetic conditions without hypertension or CAD [41]. Oxidative stress in the heart can cause pathophysiological changes such as metabolic derangements, impairments in excitation-contraction coupling, loss of normal micro-vasculature, and remodeling of the extracellular matrix involved in contractile dysfunction [41]. There is a positive correlation for the level of 4-HNE in the cardiac tissue in diabetic cardiomyopathy. For instance, 4-HNE levels were increased in the myocardium of diabetic rats with an increase of 0.6 nM compared to healthy rats [41]. Furthermore, when studying the heart tissue of type 2 diabetic rats compared to normal rats, the tissue had an increased formation of 4-HNE adducts compared to the heart tissue of the normal rats [42]. The data proves that there is a statistically significant difference in 4-HNE levels in diabetic cardiomyopathy [42]. The human ventricular myocyte contains high amounts of mitochondria which increases myocardial oxidative capacity and is a constant source of free radical generation and 4-HNE production [43]. In our lab, we have also found increased 4-HNE adducts in diabetic rat and mouse hearts from both type-1 and type-2 diabetes and it can contribute to diabetic heart damage [44–47]. Thus, it can be summarized that diabetes-mediated oxidative stress-induced 4-HNE contributes to cellular dysfunction of different types of cardiac cells to culminate in to diabetic cardiac complications such as diabetic cardiomyopathy and heart failure such as heart failure with preserved ejection fraction (Figure 2).
Figure 2.
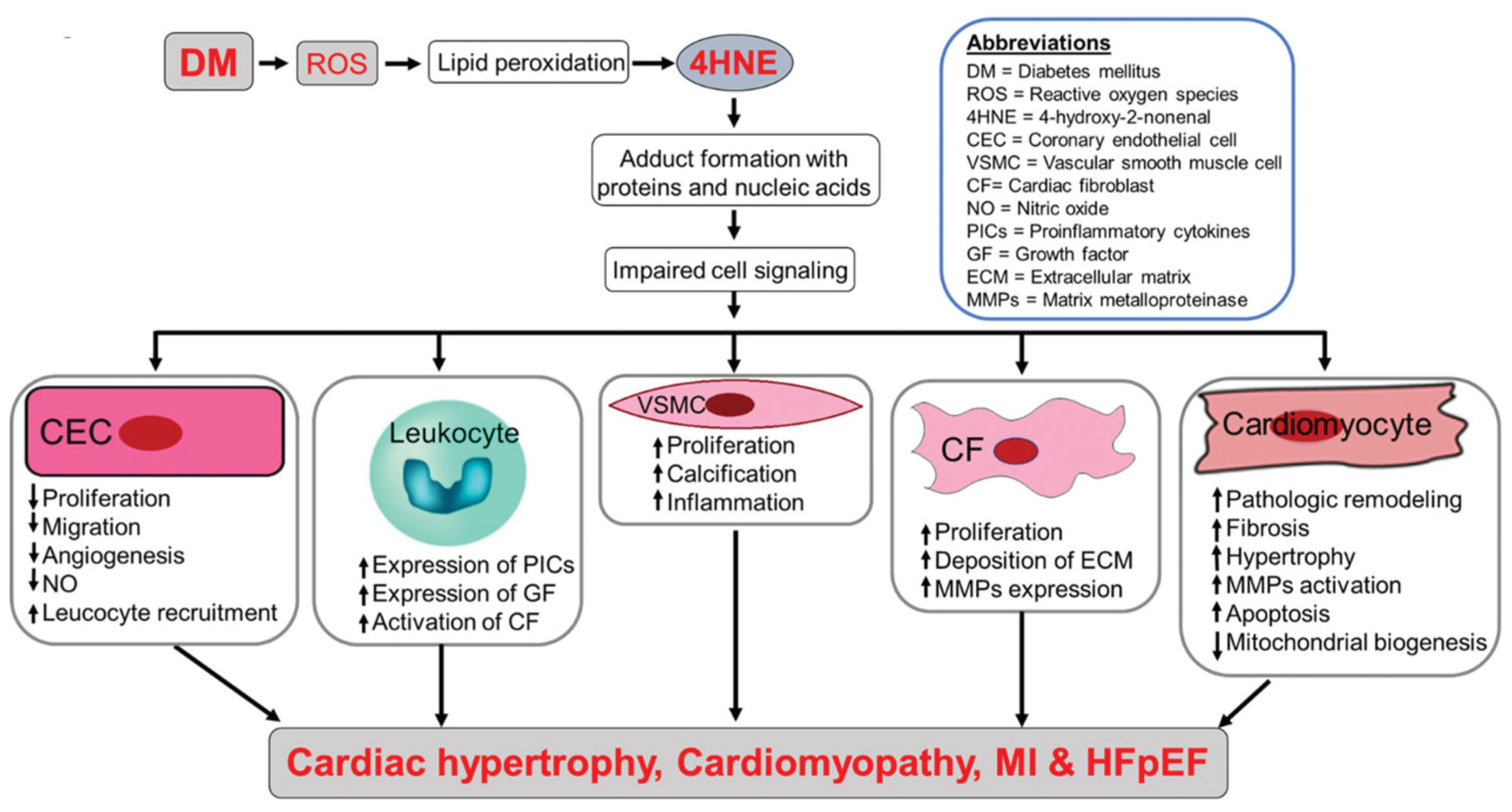
Possible mechanism of diabetes mellitus (DM)-mediated 4-HNE-induced cardiac complications. DM-mediated 4-HNE forms adducts with proteins and nucleic acids which affects the cell signaling of multiple cell types in the heart. Thus, it leads to cellular dysfunction and ultimately cardiac complications such as cardiac hypertrophy, cardiomyopathy, myocardial infarction, and heart failure with preserved ejection fraction. 4-HNE: 4-hydroxy-2-nonenal; CEC: coronary endothelial cell; CF = cardiac fibroblast; ECM: extracellular matrix; GF: growth factor; MMPs: matrix metalloproteinase; NO: nitric oxide; PICs: proinflammatory cytokines; ROS: reactive oxygen species; VSMC: vascular smooth muscle cell.
Nervous system
Diabetic neuropathy is the damage done to the nervous system as a result of long-standing diabetes. Complications of diabetic neuropathy can eventually cause paralysis of the bladder, erectile dysfunction, and gastroparesis. In diabetic neuropathy, there was an increase in 4-HNE adduct formation by ~1.3-fold in human Schwann cells which was treated in 30 mmol/l glucose compared with those treated in 5.5 mmol/l glucose [48]. When examining the axons of streptozotocin (STZ) induced diabetic rats, they had an increase in 4-HNE level (50 μm), while the control group had a 4-HNE level of 35 μm [49].
Gastrointestinal system including the liver
The gastrointestinal (GIT) system is one of the major organ systems in which oxidative stress can negatively impact its function and structure. Zhang et al. found that 4-HNE accumulation in the liver of diabetic mice was increased two times more than the liver of control mice [50]. Furthermore, the intestines of diabetic animals exhibited increased 4-HNE protein adducts which were > 3.2-fold compared with the healthy control group [51]. In another study investigating whether hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, researchers measured the levels of 4-HNE-modified proteins and found 4-HNE-modified proteins in the pancreatic beta-cells of GK rats were higher than in the control Wistar rats which were non-diabetic [39].
Skeletal system
DM has been associated with increased bone fracture rates, impaired bone regeneration, delayed bone healing, and depressed osteogenesis [52]. Pathophysiological changes to bone structure and function by oxidative stress can lead to grave complications in diabetics. Li et al. reported that alveolar bone loss and apoptosis in cells are associated with an increase in 4-HNE levels in type-1 diabetes [53]. In another study, in analyzing bone marrow-derived mesenchymal cells of STZ-injected diabetic rats, it was found that the staining intensity of the 4-HNE positive area was significantly increased in diabetic rats compared to control rats [54].
Muscle
Diabetes affects the muscular systems causing muscle cramps, muscle infarction, and carpal tunnel syndrome [55]. 4-HNE levels are elevated in the muscle samples from diabetic groups compared to samples of non-diabetic controls. 4-HNE protein adducts and intramyocellular lipid content are significantly increased in the skeletal muscle of T2DM patients compared to control subjects. The lipid content in 4-HNE protein adducts was increased by 1.6-fold in T2DM compared with control adults [56]. 4-HNE concentrations were increased by almost 4 μm in the cytosol of the gastrocnemius muscle of STZ-induced diabetic rats versus control rats [57].
Eye
Diabetic retinopathy is the most common diabetic eye disease and a leading cause of blindness in American adults. It is caused by changes in the blood vessels of the retina. In some people with diabetic retinopathy, blood vessels may swell and leak fluid. In other people, abnormal new blood vessels grow on the surface of the retina. One of the causes of the pathophysiological changes in the structure and function of the eye is due to oxidative stress. Several studies have verified that oxidative stress in the form of lipid peroxidation is increased in the eye of diabetic patients compared to non-diabetic patients. For example, Ali et al. reported that 4-HNE adducts were the most covalently altered protein residues and lead to functional impairments. The study found a 1.4-fold increase of 4-HNE in human retinal homogeneity of diabetic subjects compared with non-diabetic controls [58]. Also, elevated levels of 4-HNE were reported in the diabetic retina compared to controls [59].
Dental system
The dental system can be negatively impacted due to complications caused by DM. Diseases such as gingivitis, periodontitis, candidiasis or thrush, xerostomia or dry mouth, and oral burning can be induced due to long-standing diabetes [60]. Studies have shown that levels of 4-HNE are increased in the dental tissue of diabetic subjects compared to non-diabetic controls. For instance, Pradeep et al. found the serum and gingival crevicular fluid had higher amounts of 4-HNE in the adducts in type 2 diabetic mellitus subjects compared with non-diabetic subjects [29]. In another study, 4-HNE levels were elevated in diabetic rats with periodontitis compared to the control group, by 8-folds [53] citing augmented lipid peroxidation in the dental system in diabetes.
In summary, we provided evidence where DM is linked to having increased levels of 4-HNE in multiple organ systems. 4-HNE has been suggested to impair mitochondrial function and cellular energetics as indicated by the substantial reduction in mitochondrial membrane potential, oxygen consumption, and depletion of the reserve capacity. The products of lipid peroxidation can further aggravate mitochondrial damage. This would result in a cycle of damage and amplification of oxidative stress-related pathophysiological damage. This would finally cause organ damage and grave complications. Thus, analyzing 4-HNE levels helps not only to identify the degree of oxidative stress in the organ system but also its role in pathophysiology. However, there are many ways to analyze 4-HNE. Each method serves a different purpose for each experiment with its own merits and demerits. Thus, having a thorough understanding of the methods will lead to accurate and optimal results for a specific disease and purpose. Thus, in the following sections, we describe various methodologies available to measure 4-HNE in biological samples.
Current methodologies to detect 4-HNE levels in biological samples
In this section, we provided evidence for each method by which 4-HNE or 4-HNE adducts were measured in diabetic tissues and cells with diabetic stress and other biological samples (summarized in Table 1). We also analyze their advantages and disadvantages as a method to measure 4-HNE levels (Figure 3).
Table 1.
Summary of 4-HNE levels in different samples measured by various methods.
| Animal model | Tissue/sample types | Methods of 4-HNE detection | 4-HNE/4-HNE adduct levels | References |
|---|---|---|---|---|
| Rats | Blood Plasma | HPLC | 5–75 mmol/l | [61] |
| Rats | Feces | 0.10–0.15 μM | [62] | |
| CCI4 treated F344 rats | Liver DNA | ~37-fold increase in 4-HNE adducts vs. WT | [63] | |
| Obese mice | Epididymal adipose tissue | MS/MS | ~2–3-fold increase in 4-HNE adducts | [64] |
| Rat | Plasma | GC-MS | 2.5–250 nmol/L | [65] |
| Spontaneously hypertensive rats | Blood/plasma | ~1.8-fold increase in 4-HNE adducts vs. control | [66] | |
| CCI4 treated rats | Plasma | ~0.2–183.7 ng/ml | [67] | |
| Zucker obese rats | Urines | LC-MS/MS | 0.01–0.075 nM/ml | [68] |
| Mice | Liver | 107 ± 7 – 240 ± 8pmol/100mg tissue weight | [69] | |
| Obese patients | Blood | Immunodetection (ELISA) | ~1.75-fold increases 4-HNE adducts vs. normal subjects | [70] |
| Diabetic mice | Heart | Immunodetection (Western blot) | ~3-fold increase vs. control | [46] |
CCl4: carbon tetrachloride; HPLC: high-performance liquid chromatography; MS: mass spectrometry; GC: gas chromatography; LC: liquid chromatography; and ELISA: enzyme linked immunosorbent assay.
Figure 3.
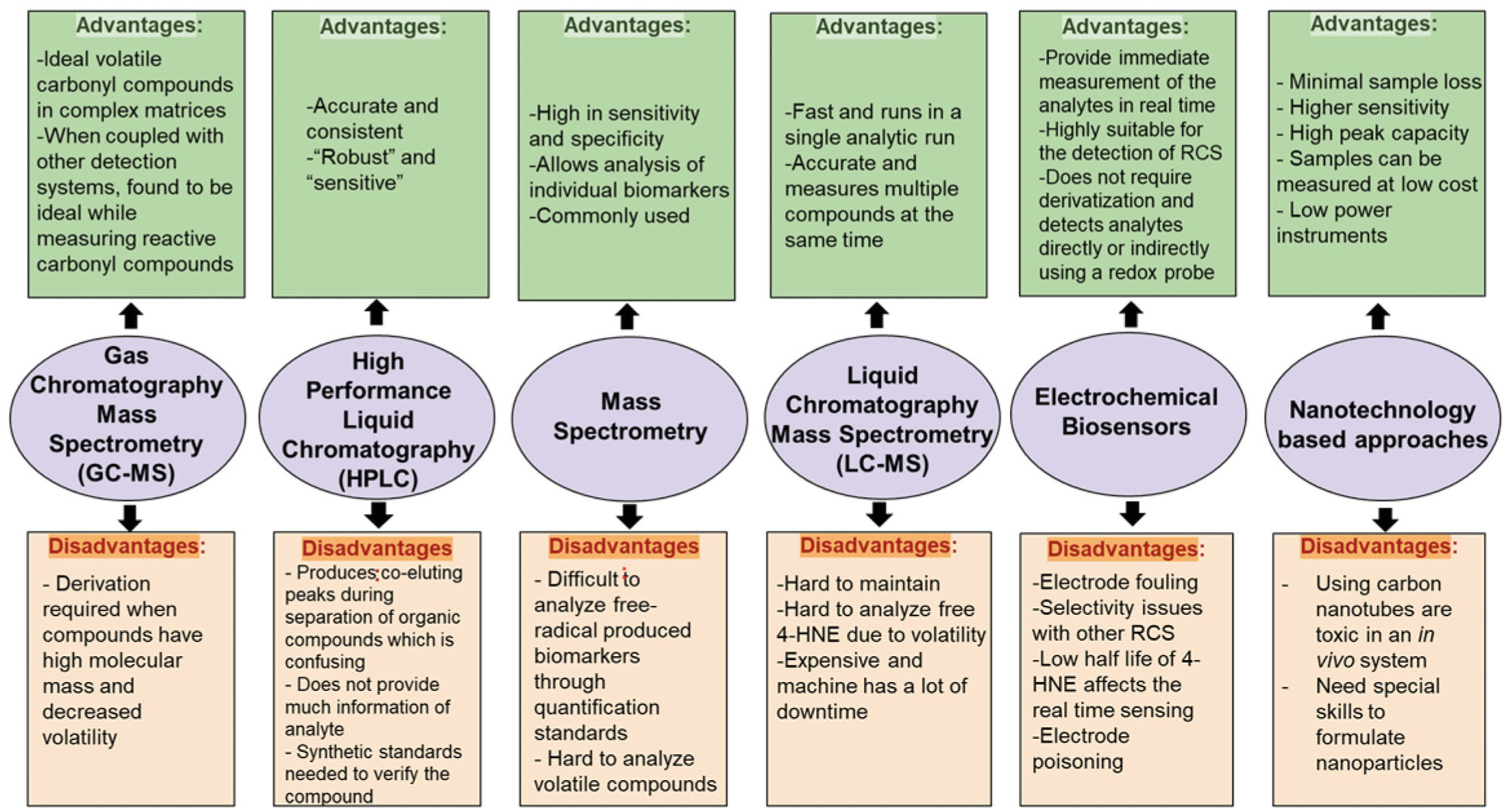
Summary of advantages and disadvantages of each approach to measure 4-HNE levels. The summary of each analytical method that was used in measuring 4-HNE from biological samples were enlisted with their advantages and disadvantages.
High-performance liquid chromatography
High-performance liquid chromatography (HPLC) employs derivatization of 4-HNE to detect 4-HNE levels and determine their connection with pathophysiology [5]. Xiao et al. opted to use the HPLC method to determine 4-HNE levels in diabetic cataractogenesis using diabetic rats [71]. By using 2,4-Dinitrophenylhydrazine as a derivatizing agent for 4-HNE, Korytar et al. also used HPLC and found a linear range of 5–75 μmol/l in the plasma samples they used [61]. HPLC was considered to have good “robustness” and “sensitivity” by Dator et al. [5]. However, HPLC is used for quantitative analysis but not good for qualitative analysis, i.e. identify any structural information of the compound being analyzed. Thus, HPLC often needs synthetic standards to verify and recognize the compound. This could be a drawback in understanding the structural information and not having access to many resources makes HPLC a less efficient approach. Also, the approach constructs co-eluting peaks during the separation of organic compounds which can create confusion when identifying and analyzing the organic compound [5].
Gas chromatography-mass spectrometry
Gas chromatography-mass spectrometry (GC/MS) is one of the main techniques to identify 4-HNE levels in free form. The approach is used to identify organic compounds in exhaled breath and to analyze volatile carbonyls with a low molecular weight. Some of these carbonyls can be derived biomarkers and are identified through exhaled breath. Since it is coupled with mass spectrometry, this method can directly analyze volatile carbonyl compounds in complex biological substances [5]. GC/MS method was adopted as the primary way to detect 4-HNE levels in human and rat plasma by derivatizing 4-HNE with pentafluorobenzyl hydroxylamine–HCl and by trimethylsilylation to trimethylsilyl ethers [65]. Zelzer et al. analyzed OR-pentafluorobenzyl (O-PFB) oxime-trimethylsilyl, a 4-HNE derivative that is not volatile. Since free 4-HNE is extremely reactive and found in low amounts in biological substances, derivatization is done to increase sensitivity for detection. Zelzer et al. justified their choice of using GC/MS for 4-HNE detection by stating that GC/MS was the ideal approach to analyze unstable aldehydes as their results showed consistent linearity and the accuracy of the data is between 99% and 104% [65]. GC/MS was regarded as the ideal tool to measure reactive carbonyl compounds in complicated biological substances. However, if the organic compound has a high molecular weight and decreased volatility, then derivatization is required [5]. Since 4-HNE has a relatively large molecular mass, derivatization is most probably required, which can be a potential drawback.
Mass spectrometry
Mass spectroscopy (MS) is used to detect 4-HNE adduction on the amino acids of proteins. Especially, nucleophilic groups containing amino acids, histidine, lysine, and cysteines form Michael adducts with 4-HNE. 4-HNE-modified lysines have also been characterized in both Schiff base and 2-pentylpyrrole forms [72]. The mass of 4-HNE is 156.225 g mol−1. If there is an increase in the mass of a protein with ~156, it can be identified by MS.A Michael addition to lysine or histidine residue results in a mass shift of 156 Da. However, there are differences in mass with 4-HNE adductions, for instance, in Schiff base with lysine residues with the combined loss of water showing 138 Da and 4-HNE-lysine 2-pentylpyrrole adduct formed from the double dehydration of a Michael adduct which results in a mass increase of 120 [72].
Isom et al. showed that 4-HNE-adducted cytochrome c was shown with increases in masses with four different modified protein states. The molecular weight of unmodified cytochrome c is 12,361.96 Da (m/z 12,365) and when 4-HNE forming four covalent Michael additions the masses increased to m/z 12,522, 12,680, 12,838, and 12,994 [73]. By using MS, adipocyte fatty acid-binding protein, a protein implicated in the regulation of insulin resistance, was found to be 4-HNE modified in obese mice [64]. The amount of 4-HNE adducts measured by matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) MS was utilized to evaluate the degree of oxidative stress in liver samples of type-2 diabetic obese patients [74]. In another research study, Siddiqui et al. used MS for the quantification and characterization of 4-HNE in cultured PC-12 cells [75]. In a recent study, our group identified 4-HNE adduction sites in mitochondrial DNA repair enzyme 8-oxoguanine glycosylase-1 (OGG-1) by incubating 4-HNE with OGG-1 recombinant enzyme [76]. This approach has high levels of sensitivity and specificity which is useful when evaluating individual analytes making it a successful method [65]. However, there are limitations in the method of studying high reactivity as the compounds are constantly coupled with other proteins or volatile compounds. Therefore, it is not as effective when measuring 4-HNE alone i.e. free 4-HNE [61]. MS measures intact protein and then sequence peptides following fairly standard proteomics approaches. These methods need for in-gel or in-solution digestion and proteolytic enzymatic digestion. Sample running and preparation require more time. Data analysis is also tedious and very time-consuming. There are also problems with false positive IDs of compounds.
Liquid chromatography-mass spectrometry
Liquid chromatography-mass spectrometry (LC-MS) is one of the main methods to detect free 4-HNE and protein-bound 4-HNE adducts including histidine modifications. Unlike GC/MS, LC/MS does not require derivatization to detect HNE or any other reactive compound [77]. However, due to the high reactivity of 4-HNE, it is usually detected as an adduct (i.e. attached to other biomolecules) [77]. The next level in LC-MS is having MS/MS i.e. the combination of two mass analyzers in one mass spec instrument. The first MS filters for the precursor ion followed by a fragmentation of the precursor ion with high energy such as using e.g. nitrogen gas. A second mass analyzer is then filtering for the product ions, generated by the fragmentation. This is usually done in a triple quadrupole MS (QQQ) or the quadrupole time-of-flight (QTOF) mass spectrometer. This pairing results in accurate mass measurement, the ability to carry out fragmentation experiments, and high-quality quantitation. The advantage of MS/MS is the increased sensitivity (in the QQQ, due to reduction of noise) and gain more structural information on the analyte (QTOF) based on the fragmentation pattern. MRM mode (multiple reaction monitoring mode) scanning for both precursor and product ion increases the specificity in addition to enhanced sensitivity. MS/MS advantage exists when two mass analyzers work in tandem and because of this LC-MS/MS is also mentioned as tandem mass spectrometry. Using LC-MS/MS, 4-HNE adducts to histidine, and histidine-containing peptides were found in the urine samples of Zucker obese rats [68].
LC-MS is one of the latest technologies, having high precision and efficiency. Its fast scanning speeds allow multiple organic compounds to be measured in a single round. However, the instrument is bulky and expensive and requires constant maintenance and operation by skilled professionals, and it also has down-times making it harder to measure multiple compounds in a short time frame.
Immunodetection
In the routine research laboratory setting, using the aforementioned instrumental techniques may be difficult. Thus many labs including ours use immunodetection methods.
Immunodetection uses antibodies raised against 4-HNE protein adducts to detect the corresponding 4-HNE adduct levels in biological systems. While immunodetection cannot be used for detecting the free 4-HNE levels, as 4-HNE can readily form adducts with proteins, this approach is easier and feasible for studies employing samples from in vitro, ex vivo, and in vivo model systems. This analytical principle is applied in common enzyme-linked immunosorbent assay (ELISA), co-immunoprecipitation, and immunoblot/Western blot and immunostaining (both immunofluorescence and immunohistochemistry). A plethora of sources including tissues, plasma/serum, blood, urine, cells, and recombinant proteins were used in the aforementioned methods. 4-HNE levels were measured in plasma/serum/blood/tissue lysates using monoclonal anti-4-hydroxynonenal antibody and anti-HNE-His antibody with ELISA [70], as Weber et al. found increased 4-HNE-protein adducts in plasma from diabetic patients [70]. Immunoblotting is performed in tissue and cell lysates. For instance, we showed increased 4-HNE adducts using polyclonal antibodies against chemically reduced amino acid-4-HNE adducts in cardiac tissue sections from STZ-induced type-1 diabetic rats [46] and high-fat diet and low-dose STZ mediated type-2 diabetic mice [44]. We also immunoprecipitated using polyclonal antibodies against chemically reduced amino acid-4-HNE adducts and then immunoblotted using aldehyde dehydrogenase 2 (ALDH2) to show the ALDH2 protein is 4-HNE adducted. Next, we also employed both immunofluorescence staining [76] and immunohisto-chemistry [46] using polyclonal antibodies against chemically reduced amino acid-4-HNE adducts for evaluating increased 4-HNE protein adducts in cardiac sections from diabetic animals. Calabrese et al. used a Western blot to identify 4-HNE adduct levels in plasma samples and lymphocyte pellets using polyclonal antibodies [33]. In another study, the immunohistochemical staining technique was employed to identify 4-HNE by using anti-hydroxynonenal-lysine antibodies on the tissue samples of the aortic arch, valves, and coronary arteries [78]. An estimated 6–8% of the proteins are 4-HNE modified in adipose proteins in obesity and insulin resistance [64]. The immunodetection methods are qualitative or semiquantitative and they have the advantages of low cost, relatively crude, and robust way to evaluate 4-HNE levels in the protein adducts form. Accurate measuring of free 4-HNE during pathological conditions in real-time is not possible by these methods.
Novel and emerging technologies
Electrochemical biosensors
Chemical sensors and biosensors are defined as sensors that use chemical and/or biological molecules to quantitatively measure chemical or biomolecular targets with sufficient sensitivity and selectivity. The significance and driving force for developing sensors come from their ability to provide an immediate measurement of the analytes in real-time and/or in situ. In recent years, a number of electrochemical sensors have been increasingly used for the detection of aldehydes concentrations in human breath sample or environmental samples. Compared to other methods such as spectroscopy [79–81] and gas chromatography [82–84], electrochemical sensors possess several advantages such as real time measurements with low cost, simple, and miniaturized instrumentations. The electrochemical sensor is typically based on the direct oxidation of aldehyde compounds at an electrode to provide quantitative measurements. A variety of amperometric [85–88] and voltammetric methods [89,90] have been developed for the detection of aldehydes. These sensors are typically based on electrodes consisting noble metals and/or catalytic metal nanoparticles [85,91–95], carbon-based materials [88,90], and biological recognition elements [86,87] to facilitate the oxidation of aldehyde analytes. In addition to conventional aqueous electrolytes, aldehyde sensing is currently being studied in ionic liquid-based electrolytes that are more stable and have wider potential windows. For instance, Rosanna et al. studied the sensing of volatile aldehydes coming from lipid oxidation processes using a room temperature ionic liquid modified electrochemical microprobe [91]. An electrochemistry-based method was developed to detect gaseous aldehyde, acetaldehyde, in the ionic liquid 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide ([Bmpy] [NTf2]) by cyclic voltammetry and potential step methods, in conditions closely related to real world ambient situations, where oxygen and water co-exist [96].
Due to the significance of aldehydes in biochemical processes and their ability to serve as important biomarkers [97–100], there is a need for the development of efficient electrochemical methods to sense aldehydes in human breath and biological systems. A biosensor for analysis for breath ethanol and acetaldehyde with alcohol oxidase and aldehyde dehydrogenase was reported by Mitsubayashi et al. [87]. More recently, Obermeier et al. developed an electrochemical sensor system for breath analysis of aldehydes, carbon monoxide (CO) and nitric oxide (NO) [101]. Electrochemical sensors have also been developed for the quantification of carbonyl groups in oxidized proteins, which can be used for the analysis of oxidative stress at the protein level [89]. The challenges associated with real time sensing of aldehydes such as 4-HNE are: (1) aldehydes including 4-HNE are typically highly reactive and unstable at regular temperatures. (2) They also have higher oxidation potentials that could result in side-reactions and electrolyte decomposition, necessitating the need for the development of new electrocatalysts, electrochemical sensing mechanisms and more stable electrolytes. To overcome such challenges, Platinum (Pt)-based electrodes have been used for efficient electro-oxidation of aldehydes. However, the formation of poisonous chemisorbed CO on the electrode surface inhibits the reaction and sensor performance [102]. Efforts to minimize this electrode poisoning problem have centered around the development of new nano-fabrication techniques [92]and the addition of metal co-catalysts [94]. Pallidum (Pd)-based catalytic electrodes [92,93] can be used as substitutes for Pt since they have similar efficient electrocatalytic activity at lower cost. Finally, biofouling of the electrode surface is another major challenge to be addressed during the design of sensor electrodes for biological applications. A majority of strategies currently used to reduce fouling involve the modification of sensor electrodes with carbon-based materials, metallic nanomaterials, and polymers [103,104]. Alternatively, fouling can be minimized in conventional electrodes through the use of electrochemical activation techniques that remove absorbed material using physical/oxidative processes or modify the electrode surface chemistry [103,104].
Electrochemical sensors are suitable for either direct redox reaction of the RCS analyte or indirect reaction of a redox probe that can provide quantitative measurements of the RCS analyte. For example, the carbonyl group in RCS allows nucleophilic addition reaction. This is where a chemical compound with an electron-deficient (electrophilic) double or triple bond reacts with an electron-rich reactant (nucleophile) with disappearance of the double bond and creation of two new single bonds [105]. The molecular design and structure diversification directly determine the sensitivity and specificity of the electrochemical measurements [106]. As shown in Figure 4, the presence of three functional groups in 4-HNE are leading to carbonyl stress and can cause a significant amount of macromolecular damage in several diseases. Each of these functional groups when they chemically get modified such as oxidation, electron transfer reaction occurs which can be measured galvanically. The high reactivity of 4-HNE makes a viable electrochemistry approach for its detection. To detect 4-HNE optically a derivatization reagent, cyclohexane-1,3-dione (CHD) is used. CHD is not naturally fluorescent by itself, but it can easily react with aliphatic aminocarbonyls to which 4-HNE form a fluorescent adduct that is relatively stable [107]. Electrochemical sensors typically do not require derivation and can detect the analytes directly or indirectly using a redox probe.
Figure 4.
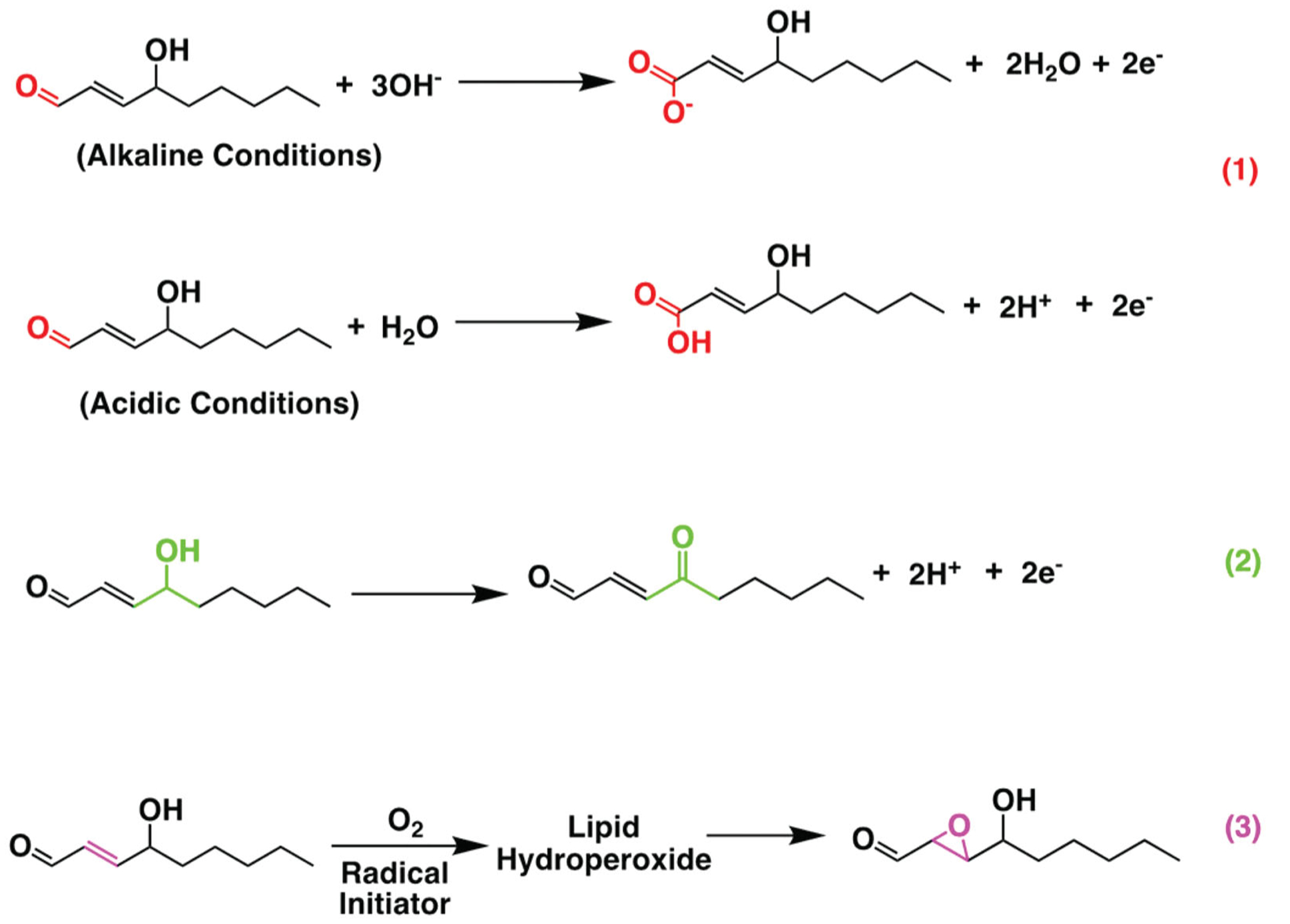
Schematic figure of electrochemical reaction of 4-HNE oxidation. Electrochemical reaction pathways of oxidation of 4-HNE that can take place at three positions: the aldehyde to form salt of carboxyl acid in alkaline condition and carboxylic acid in acidic condition (1). The OH group to form C = O group (2) and the double bond to form epoxides (3).
In a recent study, a novel electrochemical approach [108] was developed utilizing the extreme sensitivity of metal electrocatalysts in nanoscales. Briefly, the redox reactions of analyte or the adsorption of the analyte was measured using a metallic catalytic Pd/Gold (Au) surface that is extremely sensitive to the surface adsorption of the molecules as well as the oxidation and reduction of the analyte. This method is especially powerful allowing the synergistic integrating of two different metallic catalysts, into a surface structure on nanoscale so that their unique surface electrochemistry enables an innovative analytical method for highly sensitive and selective detection of reactive species which can be adapted for both direct and indirect detection of RCS as well [108].
Nanotechnology-based approaches
Recently, nanomaterials such as metallic nanoparticles, quantum dots, and carbon nanotubes have been used to develop nanotechnology-based rapid diagnostic tests. This diagnostic approach has potential to further develop monitoring technologies and point-of-care diagnostics. One nanotechnology-based method to detect 4-HNE levels is quantum dots. In the whole nanotechnology field, nanoparticles are used as markers for biomolecules due to the extensive advantages compared to traditional dyes. Quantum dots, which are man-made semiconductor nanocrystals, are an example of these nanoparticles [109]. Two other types of methods used to detect carbonyl-containing compounds such as 4-HNE are the nano-ESI (electrospray ionization) and the nano-UPLC (ultra-performance liquid chromatography). Nano-ESI takes a liquid sample to do mass spectroscopy in order to detect 4-HNE levels [110]. Nano-UPLC differs from the regular UPLC procedure and has many benefits including minimal sample loss, a higher sensitivity, and a high peak capacity [110].
Ideal approaches
While all above mentioned methods can be useful in determining either free 4-HNE levels or 4-HNE adducts in biological samples, LC-MS/MS seems to fare better. It can detect 4-HNE levels accurately without derivatization, thus it minimizes handling time. This can be especially useful as 4-HNE is a volatile compound and needs to be handled quickly in order to get accurate measurements. LC-MS can accurately detect 4-HNE levels in a single run saving time and resources. Novel approaches based on electrochemistry and nanotechnology bring more value and potential as they can be used with relatively less samples and be measured in situ and real-time as well in miniaturized platforms with low cost and low power instruments. Especially, combining both electrochemistry with nanoscale approaches as we recently employed [108] is opening the field to explore innovative and efficient methods to detect free 4-HNE like RCS even in low quantity in real-time during the pathogenesis. It is noteworthy that even though 4-HNE quickly forms adducts nucleophilic components of macromolecules; however, there may be highly concentrated free 4-HNE present during the pathogenesis. Though most studies project 4-HNE adducts are irreversible, however some reports indicate an equilibrium exists between free 4-HNE and adducted 4-HNE. Thus, measuring this low amount free 4-HNE by using miniaturized biosensors can be useful in clinical laboratory settings besides research purposes.
Conclusion
In conclusion, 4-HNE-mediated oxidative stress due to diabetes can cause major pathophysiological changes in multiple organ systems. Those pathophysiological changes then lead to complications and which affect the quality of life of the diabetic patients. For instance, diabetic nephropathy affects the kidney and requires dialysis, peripheral artery diseases lead to amputation extremities, retinopathy leads to cataract formation and blindness, and finally diabetic heart diseases such as heart failure can lead to ~65% of deaths in diabetic patients. Thus, 4-HNE should be a candidate for biomarker in diabetic complications. Next, developing a biosensor to monitor the 4-HNE levels precisely during the disease pathogenesis is an exciting opportunity for new collaborations between various fields, such as biology, electrochemistry, engineering/technology, and medicine. By using such a multidisciplinary approach, inventing a novel biosensor that can use 4-HNE as a biomarker will be useful in the diagnosis, management, and treatment of diabetes and its complications.
Funding
SSP is supported by a grant from the National Heart, Lung, and Blood Institute [grant no. 1R01HL139877-01A1] and an internal grant from Henry Ford Health System [grant no. A10249].
Footnotes
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- [1].Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34(5):575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gianazza E, Brioschi M, Fernandez AM, et al. Lipoxidation in cardiovascular diseases. Redox Biol. 2019;23:101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vistoli G, De Maddis D, Cipak A, et al. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic Res. 2013;47(Suppl 1):3–27. [DOI] [PubMed] [Google Scholar]
- [4].Ito F, Sono Y, Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants (Basel). 2019;8(3):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dator RP, Solivio MJ, Villalta PW, et al. Bioanalytical and mass spectrometric methods for aldehyde profiling in biological fluids. Toxics. 2019;7(2):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tsikas D Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30. [DOI] [PubMed] [Google Scholar]
- [7].Hall SE, Aitken RJ, Nixon B, et al. Electrophilic aldehyde products of lipid peroxidation selectively adduct to heat shock protein 90 and arylsulfatase A in stallion spermatozoa. Biol Reprod. 2017;96(1): 107–121. [DOI] [PubMed] [Google Scholar]
- [8].Griendling KK, Touyz RM, Zweier JL, et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circ Res. 2016; 119(5):e39–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Semchyshyn HM. Reactive carbonyl species in vivo: generation and dual biological effects. Sci World J. 2014;2014:417842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singh M, Kapoor A, Bhatnagar A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem Biol Interact. 2015;234:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Centers for Disease Control and Prevention. National Diabetes Statistics Report. Atlanta (GA): Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2020. [Google Scholar]
- [13].Boyle JP, Honeycutt AA, Narayan KM, et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24(11):1936–1940. [DOI] [PubMed] [Google Scholar]
- [14].American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1): S14–S31. [DOI] [PubMed] [Google Scholar]
- [15].Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102(12): 4343–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pechlivani N, Ajjan RA. Thrombosis and vascular inflammation in diabetes: mechanisms and potential therapeutic targets. Front Cardiovasc Med. 2018;5(1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Takeshima K, Ariyasu H, Ishibashi T, et al. Myotonic dystrophy type 1 with diabetes mellitus, mixed hypogonadism and adrenal insufficiency. Endocrinol Diabetes Metab Case Rep. 2018;2018:17–0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li X, Gao Y, Xu H, et al. Diabetes mellitus is a significant risk factor for the development of liver cirrhosis in chronic hepatitis C patients. Sci Rep. 2017;7(1): 9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gheith O, Farouk N, Nampoory N, et al. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2016;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- [22].Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roy B Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J Diabetes. 2013;4(4):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Selvarajah D, Wilkinson ID, Davies J, et al. Central nervous system involvement in diabetic neuropathy. Curr Diab Rep. 2011;11(4):310–322. [DOI] [PubMed] [Google Scholar]
- [25].Pizzino G, Irrera N, Cucinotta M, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017:8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tangvarasittichai S Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jaganjac M, Tirosh O, Cohen G, et al. Reactive aldehydes-second messengers of free radicals in diabetes mellitus. Free Radic Res. 2013;47(Suppl 1):39–48. [DOI] [PubMed] [Google Scholar]
- [29].Pradeep AR, Agarwal E, Bajaj P, et al. 4-Hydroxy-2-nonenal, an oxidative stress marker in crevicular fluid and serum in type 2 diabetes with chronic periodontitis. Contemp Clin Dent. 2013;4(3):281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhao Y, Song W, Wang Z, et al. Resveratrol attenuates testicular apoptosis in type 1 diabetic mice: role of Akt-mediated Nrf2 activation and p62-dependent Keap1 degradation. Redox Biol. 2018;14:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Knaś M, Maciejczyk M, Daniszewska I, et al. Oxidative damage to the salivary glands of rats with streptozotocin-induced diabetes-temporal study: oxidative stress and diabetic salivary glands. J Diabetes Res. 2016;2016:4583742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Toyokuni S, Yamada S, Kashima M, et al. Serum 4-hydroxy-2-nonenal-modified albumin is elevated in patients with type 2 diabetes mellitus. Antioxid Redox Signal. 2000;2(4):681–685. [DOI] [PubMed] [Google Scholar]
- [33].Calabrese V, Mancuso C, Sapienza M, et al. Oxidative stress and cellular stress response in diabetic nephropathy. Cell Stress Chaper. 2007;12(4):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jaganjac M, Almuraikhy S, Al-Khelaifi F, et al. Combined metformin and insulin treatment reverses metabolically impaired omental adipogenesis and accumulation of 4-hydroxynonenal in obese diabetic patients. Redox Biol. 2017;12:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miao X, Wang Y, Sun J, et al. Zinc protects against diabetes-induced pathogenic changes in the aorta: roles of metallothionein and nuclear factor (erythroid-derived 2)-like 2. Cardiovasc Diabetol. 2013;12: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shvedova AA, Kisin ER, Yanamala N, et al. Gender differences in murine pulmonary responses elicited by cellulose nanocrystals. Part Fibre Toxicol. 2016;13(1): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gomez-Perez Y, Amengual-Cladera E, Catala-Niell A, et al. Gender dimorphism in high-fat-diet-induced insulin resistance in skeletal muscle of aged rats. Cell Physiol Biochem. 2008;22(5–6):539–548. [DOI] [PubMed] [Google Scholar]
- [38].Bloomer SA, Wellen KE, Henderson GC. Sexual dimorphism in the hepatic protein response to a moderate trans fat diet in senescence-accelerated mice. Lipids Health Dis. 2017;16(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ihara Y, Toyokuni S, Uchida K, et al. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999; 48(4):927–932. [DOI] [PubMed] [Google Scholar]
- [40].Altura BM, Carella A, Gebrewold A, et al. Why there is an increased risk of cardiac failure, widening of pulse pressure and hemorrhagic stroke in type 2 diabetics over age 60: roles of unrecognized hypomagnesemia and epigenetics coupled with increased levels of ceramides, cytokines, ROS, 4-HNE and platelet activating factor. J Clin Case Stud. 2020;5(2):10. [Google Scholar]
- [41].Zhao MX, Zhou B, Ling L, et al. Salusin-β contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 2017;8(3):e2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Liu M, Verma N, Peng X, et al. Hyperamylinemia increases IL-1β synthesis in the heart via peroxidative sarcolemmal injury. Diabetes. 2016;65(9):2772–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Csala M, Kardon T, Legeza B, et al. On the role of 4-hydroxynonenal in health and disease. Biochim Biophys Acta. 2015;1852(5):826–838. [DOI] [PubMed] [Google Scholar]
- [44].Mali VR, Ning R, Chen J, et al. Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp Biol Med (Maywood). 2014; 239(5):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Deshpande M, Mali VR, Pan G, et al. Increased 4-hydroxy-2-nonenal-induced proteasome dysfunction is correlated with cardiac damage in streptozotocin-injected rats with isoproterenol infusion. Cell Biochem Funct. 2016;34(5):334–342. [DOI] [PubMed] [Google Scholar]
- [46].Mali VR, Pan G, Deshpande M, et al. Cardiac mitochondrial respiratory dysfunction and tissue damage in chronic hyperglycemia correlate with reduced aldehyde dehydrogenase-2 activity. PLoS One. 2016; 11(10):e0163158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pan G, Deshpande M, Pang H, et al. Precision medicine approach: empagliflozin for diabetic cardiomyopathy in mice with aldehyde dehydrogenase (ALDH) 2*2 mutation, a specific genetic mutation in millions of East Asians. Eur J Pharmacol. 2018;839: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Obrosova IG, Drel VR, Pacher P, et al. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54(12):3435–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Akude E, Zherebitskaya E, Roy Chowdhury SK, et al. 4-Hydroxy-2-nonenal induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy. Neurotox Res. 2010;17(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zhang C, Lu X, Tan Y, et al. Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS One. 2012;7(12):e49257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Barman S, Srinivasan K. Attenuation of oxidative stress and cardioprotective effects of zinc supplementation in experimental diabetic rats. Br J Nutr. 2017;117(3):335–350. [DOI] [PubMed] [Google Scholar]
- [52].Bacevic M, Brkovic B, Albert A, et al. Does oxidative stress play a role in altered characteristics of diabetic bone? A systematic review. Calcif Tissue Int. 2017; 101(6):553–563. [DOI] [PubMed] [Google Scholar]
- [53].Li X, Sun X, Zhang X, et al. Enhanced oxidative damage and Nrf2 downregulation contribute to the aggravation of periodontitis by diabetes mellitus. Oxid Med Cell Longev. 2018;2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kubota K, Nakano M, Kobayashi E, et al. An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS One. 2018;13(9): e0204252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wyatt LH, Ferrance RJ. The musculoskeletal effects of diabetes mellitus. J Can Chiropr Assoc. 2006;50(1): 43–50. [PMC free article] [PubMed] [Google Scholar]
- [56].Ingram KH, Hill H, Moellering DR, et al. Skeletal muscle lipid peroxidation and insulin resistance in humans. J Clin Endocrinol Metab. 2012;97(7): E1182–E1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Aragno M, Mastrocola R, Catalano MG, et al. Oxidative stress impairs skeletal muscle repair in diabetic rats. Diabetes. 2004;53(4):1082–1088. [DOI] [PubMed] [Google Scholar]
- [58].Ali TK, Matragoon S, Pillai BA, et al. Peroxynitrite mediates retinal neurodegeneration by inhibiting nerve growth factor survival signaling in experimental and human diabetes. Diabetes. 2008;57(4): 889–898. [DOI] [PubMed] [Google Scholar]
- [59].Shu XS, Zhu H, Huang X, et al. Loss of beta-catenin via activated GSK3beta causes diabetic retinal neurodegeneration by instigating a vicious cycle of oxidative stress-driven mitochondrial impairment. Aging (Albany NY) 2020;12(13):13437–13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Al-Maskari AY, Al-Maskari MY, Al-Sudairy S. Oral manifestations and complications of diabetes mellitus: a review. Sultan Qaboos Univ Med J. 2011;11(2): 179–186. [PMC free article] [PubMed] [Google Scholar]
- [61].Korytar P, Sivonova M, Maruniakova A, et al. Influence of 2,5-dihydroxybenzylidene aminoguani-dine on lipid oxidative damage and on antioxidant levels in model diabetes mellitus. Pharmazie. 2003; 58(10):733–737. [PubMed] [Google Scholar]
- [62].Chevolleau S, Noguer-Meireles MH, Jouanin I, et al. Development and validation of an ultra high performance liquid chromatography-electrospray tandem mass spectrometry method using selective derivatisation, for the quantification of two reactive aldehydes produced by lipid peroxidation, HNE (4-hydroxy-2(E)-nonenal) and HHE (4-hydroxy-2(E)-hexenal) in faecal water. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1083:171–179. [DOI] [PubMed] [Google Scholar]
- [63].Chung FL, Nath RG, Ocando J, et al. Deoxyguanosine adducts of t-4-hydroxy-2-nonenal are endogenous DNA lesions in rodents and humans: detection and potential sources. Cancer Res. 2000;60(6):1507–1511. [PubMed] [Google Scholar]
- [64].Grimsrud PA, Picklo MJ Sr., Griffin TJ, et al. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6(4):624–637. [DOI] [PubMed] [Google Scholar]
- [65].Zelzer S, Mangge H, Oberreither R, et al. Oxidative stress: determination of 4-hydroxy-2-nonenal by gas chromatography/mass spectrometry in human and rat plasma. Free Radic Res. 2015;49(10):1233–1238. [DOI] [PubMed] [Google Scholar]
- [66].Asselin C, Bouchard B, Tardif JC, et al. Circulating 4-hydroxynonenal-protein thioether adducts assessed by gas chromatography-mass spectrometry are increased with disease progression and aging in spontaneously hypertensive rats. Free Radic Biol Med. 2006;41(1):97–105. [DOI] [PubMed] [Google Scholar]
- [67].Kim Y, Kim D, Hwang J, et al. Determination of 4-hydroxynonenal in rat plasma by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2002;16(12):1238–1242. [DOI] [PubMed] [Google Scholar]
- [68].Orioli M, Aldini G, Benfatto MC, et al. HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Anal Chem. 2007;79(23):9174–9184. [DOI] [PubMed] [Google Scholar]
- [69].Warnke MM, Wanigasekara E, Singhal SS, et al. The determination of glutathione-4-hydroxynonenal (GSHNE), E-4-hydroxynonenal (HNE), and E-1-hydroxynon-2-en-4-one (HNO) in mouse liver tissue by LCESI-MS. Anal Bioanal Chem. 2008;392(7–8): 1325–1333. [DOI] [PubMed] [Google Scholar]
- [70].Weber D, Milkovic L, Bennett SJ, et al. Measurement of HNE-protein adducts in human plasma and serum by ELISA-Comparison of two primary antibodies. Redox Biol. 2013;1:226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Xiao TL, Shoeb M, Ansari NH. Metabolism and detoxification of the lipid derived aldehyde, 4-Hydroxynonenal in diabetic cataractogenesis in rat. Zhonghua Yan Ke Za Zhi. 2009;45(3):248–253. [PubMed] [Google Scholar]
- [72].Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23(4):281–305. [DOI] [PubMed] [Google Scholar]
- [73].Isom AL, Barnes S, Wilson L, et al. Modification of cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom. 2004;15(8):1136–1147. [DOI] [PubMed] [Google Scholar]
- [74].Valle A, Catalan V, Rodriguez A, et al. Identification of liver proteins altered by type 2 diabetes mellitus in obese subjects. Liver Int. 2012;32(6):951–961. [DOI] [PubMed] [Google Scholar]
- [75].Siddiqui MA, Kashyap M, Khanna V, et al. Metabolism of 4-hydroxy trans 2-nonenal (HNE) in cultured pc-12 cells. ANS. 2008;15(3):60–68. [Google Scholar]
- [76].Pan G, Deshpande M, Pang H, et al. 4-Hydroxy-2-nonenal attenuates 8-oxoguanine DNA glycosylase 1 activity. J Cell Biochem. 2020;121(12):4887–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Spickett CM. The lipid peroxidation product 4-hydroxy-2-nonenal: advances in chemistry and analysis. Redox Biol. 2013;1:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sima A, Stancu C, Starodub O, et al. Immunodetection of modified lipoproteins in plasma and arterial walls of patients with coronary heart disease. Rom J Intern Med. 1997;35(1–4):29–38. [PubMed] [Google Scholar]
- [79].Nanto H, Yokoi Y, Mukai T, et al. Novel gas sensor using polymer-film-coated quartz resonator for environmental monitoring. Mater Sci Eng C. 2000; 12(1–2):43–48. [Google Scholar]
- [80].Yang P, Lau C, Liang JY, et al. Zeolite-based cataluminescence sensor for the selective detection of acetaldehyde. Luminescence. 2007;22(5):473–479. [DOI] [PubMed] [Google Scholar]
- [81].Xiaoan Cao ZZ, Zhang X. A novel gaseous acetaldehyde sensor utilizing cataluminescence on nanosized BaCO3. Sens Actuators B. 2004;99(1):6. [Google Scholar]
- [82].Bicanic D, Persijn S, Taylor A, et al. Detection of ethanol and acetaldehyde released from cabbage seeds of different quality: laser photoacoustic spectroscopy versus FTIR and headspace gas chromatography. Rev Sci Instrum. 2003;74(1):4. [Google Scholar]
- [83].Ohata HO, Otsuka M, Ohmori S. Determination of acetaldehyde in biological samples by gas chromatography with electron-capture detection. J Chromatogr B Biomed Sci Appl. 1997;693(2):9. [DOI] [PubMed] [Google Scholar]
- [84].Li Z, Jacobus LK, Wuelfing WP, et al. Detection and quantification of low-molecular-weight aldehydes in pharmaceutical excipients by headspace gas chromatography. J Chromatogr A. 2006;1104(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- [85].Jacquinot Pweh A, Hauser C, Müller P, et al. Amperometric detection of gaseous ethanol and acetaldehyde at low concentrations on an Au–Nafion electrode. Analyst. 1999;124(6):7. [Google Scholar]
- [86].Avramescu AN, Noguer T, Avramescu M, et al. Screen-printed biosensors for the control of wine quality based on lactate and acetaldehyde determination. Anal Chim Acta. 2002;458(1):11. [Google Scholar]
- [87].Mitsubayashi KM, Matsunaga H, Nishio G, et al. Biosniffer sticks for breath analysis after drinking. Sens Actuators B Chem. 2005;108(1):5. [Google Scholar]
- [88].Titoiu AM, Lapauw M, Necula-Petrareanu G, et al. Carbon nanofiber and meldola blue based electrochemical sensor for NADH: application to the detection of benzaldehyde. Electroanalysis. 2018;30(11): 2676–2688. [Google Scholar]
- [89].Enache TA, Matei E, Diculescu VC. Electrochemical sensor for carbonyl groups in oxidized proteins. Anal Chem. 2019;91(3):1920–1927. [DOI] [PubMed] [Google Scholar]
- [90].Shereema RM, Nambiar SR, Shankar SS, et al. CeO2–MWCNT nanocomposite based electrochemical sensor for acetaldehyde. Anal Methods. 2015;7(12):7. [Google Scholar]
- [91].Toniolo R, Dossi N, Bortolomeazzi R, et al. Volatile aldehydes sensing in headspace using a room temperature ionic liquid-modified electrochemical microprobe. Talanta. 2019;197:522–529. [DOI] [PubMed] [Google Scholar]
- [92].Zhang YZ, Cai M, Chen Z, et al. A novel electrochemical sensor for formaldehyde based on palladium nanowire arrays electrode in alkaline media. Electrochim Acta. 2012;68(6):172–177. [Google Scholar]
- [93].Safavi AM, Maleki N, Farjami F, et al. Electrocatalytic oxidation of formaldehyde on palladium nanoparticles electrodeposited on carbon ionic liquid composite electrode. J Electroanal Chem. 2009;626(1):5. [Google Scholar]
- [94].Raoof J-BH, Ojani R, Aghajani S. Fabrication of bimetallic Cu/Pd particles modified carbon nanotube paste electrode and its use towards formaldehyde electro-oxidation. J Mol Liq. 2015;204(6):106–111. [Google Scholar]
- [95].Yan R-W, Jin B-K. Study of the electrochemical oxidation mechanism of formaldehyde on gold electrode in alkaline solution. Chin Chem Lett. 2013;24(2): 159–162. [Google Scholar]
- [96].Chi X, Tang Y, Zeng X. Electrode reactions coupled with chemical reactions of oxygen, water and acetaldehyde in an ionic liquid: new approaches for sensing volatile organic compounds. Electrochim Acta. 2016;216:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Reinheckel T, Noack H, Lorenz S, et al. Comparison of protein oxidation and aldehyde formation during oxidative stress in isolated mitochondria. Free Radic Res. 1998;29(4):297–305. [DOI] [PubMed] [Google Scholar]
- [98].Kinter M Analytical technologies for lipid oxidation products analysis. J Chromatogr B Biomed Sci Appl. 1995;671(1):14. [DOI] [PubMed] [Google Scholar]
- [99].Esterbauer HS, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991; 11(1):81–128. [DOI] [PubMed] [Google Scholar]
- [100].Poli DG, Corradi M, Acampa M, et al. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME–GC/MS. J Chromatogr B. 2010;878(27):2643–2651. [DOI] [PubMed] [Google Scholar]
- [101].Obermeier J, Trefz P, Wex K, et al. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J Breath Res. 2015;9(1):016008. [DOI] [PubMed] [Google Scholar]
- [102].Leung LWH, Weaver MJ. Influence of adsorbed carbon monoxide on electrocatalytic oxidation of simple organic molecules at platinum and palladium electrodes in acidic solution: a survey using real-time FTIR spectroscopy. Langmuir. 1990;6(2):323–333. [Google Scholar]
- [103].Liu N, Xu Z, Morrin A, et al. Low fouling strategies for electrochemical biosensors targeting disease biomarkers. Anal Methods. 2019;11(6):702–711. [Google Scholar]
- [104].Hanssen BL, Siraj S, Wong DKY. Recent strategies to minimise fouling in electrochemical detection systems. Rev Anal Chem. 2016;35(1):1–28. [Google Scholar]
- [105].Peng H, Yu Q, Wang S, et al. Molecular design strategies for electrochemical behavior of aromatic carbonyl compounds in organic and aqueous electrolytes. Adv Sci (Weinh). 2019;6(17):1900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bondue CJ, Koper MTM. Electrochemical reduction of the carbonyl functional group: the importance of adsorption geometry, molecular structure, and electrode surface structure. J Am Chem Soc. 2019; 141(30):12071–12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vidal N, Cavaille JP, Graziani F, et al. High throughput assay for evaluation of reactive carbonyl scavenging capacity. Redox Biol. 2014;2:590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ju J, Liu X, Yu JJ, et al. Electrochemistry at bimetallic Pd/Au thin film surfaces for selective detection of reactive oxygen species and reactive nitrogen species. Anal Chem. 2020;92(9):6538–6547. [DOI] [PubMed] [Google Scholar]
- [109].Lyberopoulou A, Efstathopoulos EP, Gazouli M. Nanotechnology-based rapid diagnostic tests. In: Saxena SK, editor. Proof and Concepts in Rapid Diagnostic Tests and Technologies. Rijeka: IntechOpen; 2016. p. 91–105. [Google Scholar]
- [110].Augustyniak E, Adam A, Wojdyla K, et al. Validation of protein carbonyl measurement: a multi-centre study. Redox Biol. 2015;4:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]


