Abstract
Pregnant women may be at higher risk of severe complications associated with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which may lead to obstetrical complications. We performed a case control study comparing pregnant women with severe coronavirus disease 19 (cases) to pregnant women with a milder form (controls) enrolled in the COVI-Preg international registry cohort between March 24 and July 26, 2020. Risk factors for severity, obstetrical and immediate neonatal outcomes were assessed. A total of 926 pregnant women with a positive test for SARS-CoV-2 were included, among which 92 (9.9%) presented with severe COVID-19 disease. Risk factors for severe maternal outcomes were pulmonary comorbidities [aOR 4.3, 95% CI 1.9–9.5], hypertensive disorders [aOR 2.7, 95% CI 1.0–7.0] and diabetes [aOR2.2, 95% CI 1.1–4.5]. Pregnant women with severe maternal outcomes were at higher risk of caesarean section [70.7% (n = 53/75)], preterm delivery [62.7% (n = 32/51)] and newborns requiring admission to the neonatal intensive care unit [41.3% (n = 31/75)]. In this study, several risk factors for developing severe complications of SARS-CoV-2 infection among pregnant women were identified including pulmonary comorbidities, hypertensive disorders and diabetes. Obstetrical and neonatal outcomes appear to be influenced by the severity of maternal disease.
Subject terms: Risk factors, Infection, Clinical microbiology, Reproductive signs and symptoms, Respiratory signs and symptoms, Viral infection
Introduction
Altered immunity, reduced respiratory capacity, vascular and hemodynamic changes put pregnant women at higher risk of complications, while specific harm to the exposed fetus/newborn may be observed. Although, early reports from the SARS-CoV-2 epidemic1 suggested that the clinical course for infected pregnant women was similar to the general population, more recent data suggest a higher risk of severe outcomes in pregnant women compared to the general population at an equivalent age, with severe outcomes observed in 8 to 11%2–6. In the general population, preexisting health conditions, namely pulmonary pathologies, hypertension and diabetes have been associated with severe outcomes7,8. Information on the impact of these determinants on the maternal disease evolution and other risk factors specific to pregnancy is still fragmented, although evidence suggest that they might contribute to the severity of the disease6,9. Furthermore, fetal/newborn risks still need to be better assessed as vertical transmission of the virus and placental infection appears to be possible with newborns potentially demonstrating related symptoms10–13, while a significantly higher rate of preterm deliveries (25–30%) among women with Coronavirus disease 19 (COVID-19) has been reported3,4.
Information on specific risks among pregnant women are urgently needed to provide evidence-based guidelines for the management of this vulnerable population. To accomplish this, we developed an international web registry14 in March 2020, to promote a structured collection of data regarding pregnant women and their fetuses exposed to SARS-CoV-2. Using this dataset, we performed a case–control study to assess the risk of severe maternal outcomes and associated risk factors as well as a description of pregnancy/neonatal outcomes stratified for the severity of the disease among pregnant women with a confirmed SARS-CoV-2 infection.
Materials and methods
Study setting and population
The patients enrolled in this study are part of the COVI-Preg international registry investigating the consequences of SARS-Cov-2 infection during pregnancy14. All pregnant women tested for SARS-CoV-2 infection at any stage of gestation were eligible for inclusion in this multicenter study except those < 18 years of age as well as individuals declining to consent or not able to consent for themselves. Informed oral or written consent was obtained for all participants. Deidentified data were prospectively recorded by each center (Table S1) using the REDCap (Research Electronic Data Capture) electronic data capture tool15,16. Quality checks were performed as described in the Supplementary Materials. Using this dataset, we performed a case control study among pregnant women with a confirmed SARS-CoV-2 infection.
The study was approved by both the Swiss Ethical Board (CER-VD-2020-00548) and the local ethics boards at each participating center. The study was conducted from March 24th to July 26th, 2020. All methods were carried out in accordance with relevant guidelines and regulations in the manuscript.
Inclusion criteria and SARS-CoV-2 status
Pregnant women were tested for SARS-CoV-2 either because of a suspected infection due to ongoing symptoms compatible with COVID-19 or an history of potential exposure or through routine systematic screening instituted during the pandemic in some hospitals depending on local capacities and guidelines. Maternal testing was performed using a nasopharyngeal RT-PCR for SARS-CoV-2 swab test. Pregnant women with a positive RT- PCR test result at any stage during pregnancy irrespective of clinical signs and symptoms were considered as having a confirmed infection and included in the present study. Pregnant women with a SARS-CoV-2 negative test and no other positive test result during the entire follow-up period were excluded.
Case and control definition
Pregnant women with severe adverse outcomes, defined as any of the following: (1) the need for advanced oxygen support (i.e. high flow cannula, non-invasive ventilation through CPAP or mechanical ventilation), (2) admission to the intensive care unit (ICU) and (3) maternal death, were classified as cases. The control group included pregnant women with either mild adverse outcomes, defined as maternal hospitalization requiring oxygen supplementation, or no adverse outcomes, defined as outpatient management or hospitalization not requiring oxygen supplementation.
Identification of risk factors for severe adverse maternal outcome
Pregnant women with severe adverse outcomes (cases) were compared to pregnant women with mild or no adverse outcomes (controls). The effect of maternal characteristics known to be risk factors7,8,17 for SARS-CoV-2 severe adverse outcomes in the general population were tested (i.e. maternal age > 35 years old, obesity defined as a BMI > 30, hypertensive disorders, pre-and gestational diabetes, preexisting pulmonary, cardiovascular, renal, or oncologic disease and immunosuppression), as well as pregnancy related risk factors such as nulliparity (dichotomized as yes/no), ethnicity (defined as Caucasian yes/no), multiple pregnancy, gestational age at infection (dichotomized as < or > 20 WG)9.
Secondary outcomes: absolute risk (%) of obstetrical outcomes and neonatal outcomes
For completed pregnancies (i.e. pregnancy ending in either fetal loss > 14 WG or livebirth, obstetrical outcomes (pregnancy outcome, GA at delivery, mode of delivery) and neonatal outcomes (neonatal death, neonatal admission to the ICU (NICU), birthweight and rates of suspected perinatal SARS-CoV-2) were assessed. For multiple gestations (n = 26), the analysis considered the whole pregnancy. Fetal loss was defined as a spontaneous antepartum fetal death > 14 WG (i.e. late miscarriage (14–24 WG) and stillbirth (fetal demise > 24 WG). Suspected perinatal SARS-CoV-2 transmission was defined as a positive RT-PCR result performed at birth.
Statistical analysis
We performed a multivariate analysis to estimate odds ratios (OR) with 95% CIs adjusting for risk factors of COVID-19 severity (i.e. maternal age, BMI, pre- and gestational hypertensive disorders (including pre-eclampsia), pre-and gestational diabetes, pre-existent pulmonary comorbidities, other pre-gestational comorbidities (cardiovascular, renal, oncological diseases and immunosuppression), and gestational risk factors of severe maternal outcomes (ethnicity, parity, pregnancy conditions (threatened preterm labor, placenta previa, placental malfunction and PPROM) and exposure after 20WG) and accounting for missing values as described in the supplementary material.
Statistical analyses were performed using Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). A P value less than 0.05 was considered as statistically significant.
Results
Between March 24 and July 26, 2020, 1079 pregnant women tested for SARS-CoV-2 were enrolled in the registry among which 926 had a confirmed SARS-CoV-2 infection (Fig. 1). Socio-demographic characteristics are presented in Table 1. A third of the women were asymptomatic (31.9% n = 295/926), while cough (40.4%, n = 374/926), fever (32.4%, n = 300/926) and anosmia/ageusia (17.8%, n = 165/926) were the most reported symptoms. 9.9% (n = 92/926) experienced severe maternal outcomes, including 7.3% (n = 68/926) requiring advanced oxygen support and 4.0% (n = 37/926) requiring ICU admission; 6 maternal deaths were recorded (0.6%) (Table 2).
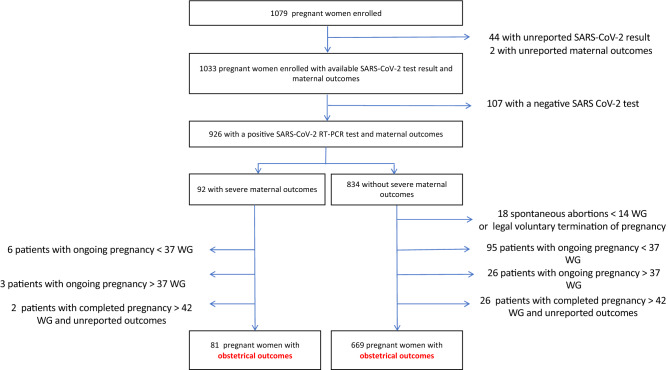
Figure 1.
Flow chart. The COVI-Preg international registry was launched in March 2020. To date, 120 centers from 16 countries have contributed patients (supplementary Table 1). All pregnant women tested for SARS-CoV-2 infection at any stage of gestation were eligible for inclusion in this multicenter study except those < 18 years of age as well as individuals declining to consent or not able to consent for themselves. Deidentified data were prospectively recorded by each center using the REDCap (Research Electronic Data Capture) electronic data capture tool15,16. At inclusion (i.e. at the time of SARS-CoV-2 screening), the following data were recorded: socio-demographic characteristics, obstetrical history and information on SARS-CoV-2 exposure. Pregnancies were monitored as clinically indicated according to local protocols. After inclusion, the following data were collected: results of maternal testing (SARS-CoV-2 and/or other infectious pathogens), COVID-19 history, maternal, pregnancy and neonatal outcomes. Data were analyzed using Stata 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2WG, weeks ‘gestation.
Table 1.
Description of the population (sociodemographic characteristics).
| Socio-demographic factors | Pregnant women with a confirmed SARS-CoV-2 infection (n = 926) |
|---|---|
| Maternal age | |
| Median—y.o. (IQR) | 32 (28–36) |
| Age > 35 y.o.– no (%) | 272 (29.4) |
| Unknown | 5 (0.5) |
| Ethnicity—no (%) | |
| Caucasian | 494 (53.4) |
| Hispanic or Latin-American | 217 (23.4) |
| Afro-American | 117 (12.6) |
| Asian or Pacific Islands | 30 (3.2) |
| Other | 44 (4.8) |
| Unknown | 24 (2.6) |
| Region of residence—no (%) | |
| North America | 27 (2.9) |
| South and Central America | 249 (26.9) |
| Europe | 490 (52.9) |
| Middle East | 17 (1.8) |
| Central Asia | 3 (0.3) |
| South East Asia | 6 (0.6) |
| Africa | 26 (2.8) |
| Unknown | 108 (11.6) |
| Previous pregnancies—no (%) | |
| Nulliparous | 346 (37.4) |
| Multiparous | 568 (61.3) |
| Multiparous ≥ 3 | 102 (11.0) |
| Previous cesarean sections > 1 | 135 (14.6) |
| Unknown | 12 (1.3) |
| Previous adverse pregnancy outcomes—no (%) | |
| Stillbirths | 18 (1.9) |
| Unknown | 163 (17.6) |
| Maternal comorbidities | |
| Any maternal comorbidities—no (%) | 170 (18.4) |
| Pulmonary comorbidities | 35 (3.8) |
| Cardiac comorbidities | 14 (1.5) |
| Hypertension | 19 (2.1) |
| Pregestational diabetes | 12 (1.3) |
| Immunosuppression | 4 (0.4) |
| Thyroid dysfunction | 34 (3.7) |
| Oncologic comorbidities | 9 (1.0) |
| Hematologic comorbidities | 17 (1.8) |
| Auto-immune diseases | 4 (0.4) |
| Other (neurological, urological, digestive, orthopedic) | 85 (9.2) |
| Unknown | 4 (0.4) |
| Maternal BMI | |
| Median (IQR) | 26 (23–30) |
| BMI > 30—no (%) | 208 (22.5) |
| BMI > 35—no (%) | 81 (8.8) |
| Unknown—no (%) | 122 (13.2) |
| Any drugs | 63 (6.8) |
| Cigarettes | 61 (6.6) |
| Alcohol | 5 (0.5) |
| Unknown | 17 (1.8) |
| Current pregnancy—no (%) | |
| Multiple pregnancy | 24 (2.6) |
| Ongoing pregnancy conditions | |
| Any | 114 (12.3) |
| Pre-eclampsia | 10 (1.1) |
| Gestational diabetes | 45 (4.9) |
| IUGR | 7 (0.8) |
| Abnormal fetal doppler | 1 (0.1) |
| Macrosomia | 6 (0.7) |
| Threatening preterm labor | 5 (0.5) |
| Placenta previa | 2 (0.2) |
| PPROM | 5 (0.5) |
| Other | 46 (5.0) |
| Unknown | 33 (3.6) |
| Fetal malformation | 18 (1.9) |
| Risk of DS | |
| High risk > 1/1000 | 24 (2.6) |
| Unknown | 341 (36.8) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; y.o., years old; IQR, interquartile range; BMI, body mass index; PPROM, preterm premature rupture of the membranes; IUGR, intrauterine growth restriction; DS, Down syndrome; WG, weeks’ gestation.
Table 2.
Description of the population (COVID-19 history).
| COVID-19 history | Pregnant women with a confirmed SARS-CoV-2 infection (n = 926) |
|---|---|
| Timing of exposure—no (%) | |
| < 20 WG | 89 (9.6) |
| Median GA at exposure WG (IQR) | 12 (9–16) |
| > 20 WG | 826 (89.2) |
| Median GA at exposure WG (IQR) | 38 (34–40) |
| Unknown | 11 (1.2) |
| Clinical manifestation—no (%) | |
| Asymptomatic | 295 (31.9) |
| Fever | 300 (32.4) |
| Cough | 374 (40.4) |
| Dyspnea | 146 (15.8) |
| Sore throat | 83 (9.0) |
| Myalgia | 148 (16.0) |
| Fatigue | 191 (20.6) |
| Headache | 121 (13.1) |
| Nausea/vomiting | 48 (5.2) |
| Anosmia/ageusia | 165 (17.8) |
| Other | 81 (8.8) |
| Maternal outcomes—no (%) | |
| No adverse outcomes | 828 (89.4) |
| Mild adverse outcomes | 6 (0.6) |
| Severe adverse outcomes | 92 (9.9) |
| Maternal deaths | 6 (0.6) |
| Admission to ICU | 37 (4.0) |
| Advanced oxygen support | 68 (7.3) |
First trimester was defined from 1 to 13 6/7 weeks’ gestation (WG), second trimester from 14 0/7 to 27 6/7 WG and third trimester from 28 WG. For symptomatic patients, trimester of exposure was defined as the gestational age (GA) at onset of symptoms. For asymptomatic patients, the trimester of exposure was defined as the GA at SARS-CoV-2 testing.
For symptomatic patients, the trimester of exposure was defined as the gestational age (GA) at onset of symptoms. For asymptomatic patients, the trimester of exposure was defined as the GA at SARS-CoV-2 testing.
IQR, interquartile range; ICU, Intensive Care Unit; WG, weeks’ gestation.
Risk factors for severe maternal outcomes among positive pregnant women
In a univariate analysis pulmonary comorbidities [crude OR 3.9, 95% CI 1.6–8.9], hypertensive disorders [crude OR 3.5, 95% CI 1.2–9.1], diabetes [crude OR 2.6, 95% CI 1.2–5.3] and BMI > 30 [crude OR 1.7, 95% CI 1.1–2.9] were significantly associated with an increased risk of severe maternal outcomes (Table 3). In a multivariate analysis adjusting for risk factors of COVID-19 severity, gestational risk factors of severe maternal outcomes, and accounting for missing values through multiple imputation, pulmonary comorbidities [aOR 4.3, 95% CI 1.9–9.5], hypertensive disorders [aOR 2.7, 95% CI 1.0–7.0] and diabetes [2.2, 95% CI 1.1–4.5] remained significantly associated, while BMI > 30 did not retain significance [aOR 1.3, 95% CI 0.8–2.2]. When adjusting for COVID-19 risk factors only, similar results were obtained (Table 3). Common pregnancy related risk factors were not associated with severe maternal outcomes (i.e. nulliparity, ethnicity, multiple pregnancy, gestational age at infection).
Table 3.
Risk factors for severe adverse maternal outcomes among pregnant women with a positive SARS-CoV-2 test.
| Maternal outcomes | Pregnant women with a POSITIVE test result for SARS-CoV-2 | ORa | 95%CI | p value | aORb | 95%CI | p value | aORc | 95%CI | p value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe adverse maternal outcomes n = 92 |
No/mild adverse maternal outcomes n = 834 |
||||||||||||
| n (%) | 95% CI | n (%) | 95% CI | ||||||||||
| Maternal age | |||||||||||||
| Age > 35 y.o | 28 (30.4) | 21.3–40.9 | 244 (29.3) | 26.2–32.5 | 1.0 | 0.6–1.7 | 0.9042 | 1.1 | 0.7–1.8 | 0.708 | 1.1 | 0.7–1.7 | 0.755 |
| Unknown | 0 (0.0) | n.a. | 5 (0.6) | 0.2–1.4 | |||||||||
| Ethnicity | |||||||||||||
| Caucasian | 41 (44.6) | 34.2–55.3 | 453 (54.3) | 50.9–57.7 | 0.7 | 0.4–1.1 | 0.0926 | 0.7 | 0.5–1.2 | 0.214 | |||
| Unknown | 3 (3.3) | 0.7–9.2 | 21 (2.5) | 1.6–3.8 | |||||||||
| Previous pregnancies | |||||||||||||
| Nulliparous—no (%) | 29 (31.5) | 22.2–42.0 | 317 (38.0) | 34.7–41-4 | 0.8 | 0.5–1.2 | 0.2564 | 0.8 | 0.5–1.3 | 0.412 | |||
| Unknown | 1 (1.1) | 0.0–5.9 | 11 (1.3) | 0.7–2.3 | |||||||||
| Maternal comorbidities gestational/pre-gestational | |||||||||||||
| Pre-gestational comorbidities | 19 (20.7) | 12.9–35.7 | 123 (14.8) | 12.4–17.3 | |||||||||
| Pulmonary comorbidities | 10 (10.9) | 5.3–19.1 | 25 (3.0) | 1.9–4.4 | 3.9 | 1.6–8.9 | 0.0013 | 4.3 | 1.9–9.5 | 0.000 | 4.0 | 1.8–8.9 | 0.001 |
| Any other | 6 (6.5) | 2.6–13.7 | 40 (4.8) | 0.7–6.5 | 1.4 | 0.5–3.4 | 0.4473 | 0.9 | 0.3–2.4 | 0.841 | 0.9 | 0.4–2.4 | 0.891 |
| Cardiac comorbidities | 3 (3.3) | 0.7–9.2 | 11 (1.3) | 0.7–2.3 | |||||||||
| Renal diseases | 2 (2.2) | 0.3–7.6 | 4 (0.5) | 0.1–1.2 | |||||||||
| Immunosuppression | 1 (1.1) | 0.0–5.9 | 3 (0.4) | 0.1–1.0 | |||||||||
| Oncologic comorbidities | 1 (1.1) | 0.0–5.9 | 8 (1.0) | 0.4–1.9 | |||||||||
| Hematologic comorbidities | 2 (2.2) | 0.2–7.6 | 15 (1.8) | 1.0–2.9 | |||||||||
| Auto-immune diseases | 1 (1.1) | 0.0–5.9 | 3 (0.4) | 0.1–1.0 | |||||||||
| Gestational comorbidities | 9 (9.8) | 4.6–17.8 | 71 (8.5) | 6.7–10.6 | 1.2 | 0.5–2.5 | 0.6949 | 1.2 | 0.6–2.6 | 0.592 | |||
| Multiple pregnancy | 2 (2.2) | 0.2–7.6 | 22 (2.6) | 1.7–4.0 | |||||||||
| Other | 8 (8.7) | 3.8–16.4 | 54 (6.5) | 4.9–8.4 | |||||||||
| Hypertensive disorders | 7 (7.6) | 3.1–15.1 | 19 (2.3) | 1.4–3.5 | 3.5 | 1.2–9.1 | 0.0103 | 2.7 | 1.0–7.0 | 0.044 | 2.7 | 1.0–7.1 | 0.042 |
| Pre-gestational | 4 (4.3) | 1.2–10.8 | 15 (1.8) | 1.0–2.9 | |||||||||
| Gestational /Pre-eclampsia | 4 (4.3) | 1.2–10.8 | 6 (0.7) | 0.3–1.6 | |||||||||
| Diabetes | 12 (13.0) | 6.9–21.7 | 45 (5.4) | 4.0–7.2 | 2.6 | 1.2–5.3 | 0.0094 | 2.2 | 1.1–4.5 | 0.036 | 2.2 | 1.1–4.5 | 0.034 |
| Pregestational | 4 (4.3) | 1.2–10.8 | 8 (1.0) | 0.4–1.9 | |||||||||
| Gestational | 8 (8.7) | 3.8–16.4 | 37 (4.4) | 3.1–6.1 | |||||||||
| Unknown | 0 (0.0) | n.a. | 2 (0.2) | 0.0–0.9 | |||||||||
| Maternal BMI | |||||||||||||
| BMI > 30 | 28 (30.4) | 21.3–40.9 | 180 (21.6) | 18.8–24.5 | 1.7 | 1.1–2.9 | 0.0220 | 1.3 | 0.8–2.2 | 0.351 | 1.4 | 0.8–2.4 | 0.201 |
| BMI > 35 | 15 (16.3) | 9.4–25.5 | 66 (7.9) | 6.2–10.0 | |||||||||
| Unknown | 12 (13.0) | 6.9–21.7 | 110 (13.2) | 11.0–15.7 | |||||||||
| COVID-19 exposure | |||||||||||||
| Timing of exposure | |||||||||||||
| > 20 weeks gestation | 84 (91.3) | 83.6–96.2 | 742 (89.0) | 86.6–91.0 | 1.1 | 0.5–2.8 | 0.8538 | 1.4 | 0.7–3.2 | 0.356 | |||
| Unknown | 0 (0.0) | n.a. | 11 (1.3) | 0.7–2.3 | |||||||||
The effect of maternal characteristics known to be risk factors7,8,17 were tested (i.e. maternal age > 35 year old, obesity defined as a BMI > 30, hypertensive disorders (including pre-eclampsia), pre-and gestational diabetes, pre-existent pulmonary, cardiovascular, renal, oncologic diseases and immunosuppression), as well as pregnancy related risk factors such as pregnancy conditions (threatened preterm labor, placenta previa, placental malfunction and preterm premature rupture of the membrane (PPROM) (dichotomized as yes/no))), nulliparity (dichotomized as yes/no), ethnicity (defined as Caucasian yes/no), multiple pregnancy, age of pregnancy at infection (dichotomized as < or > 20 WG)9.
In bold are presented significant results.
SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; OR, odds ratio; aOR, adjuster odds ratio; y.o.; years old; BMI, Body Mass Index; n.a., non-applicable.
aORs were calculated without missing values.
bAdjusted for specific COVID-19 risk factors (maternal age, pulmonary comorbidities, hypertensive disorders, diabetes mellitus, maternal BMI and other maternal comorbidities with a low prevalence in the cohort), specific pregnancy risk factors (ethnicity, parity, other pregnancy conditions (placenta previa, preterm premature rupture of the membrane , preterm labor, IUGR ) and timing of exposure.
cAdjusted for specific COVID-19 risk factors only (maternal age, pulmonary comorbidities, hypertensive disorders, diabetes mellitus, maternal BMI and other maternal comorbidities with a low prevalence in the cohort).
Secondary outcomes
Absolute risk of pregnancy, obstetrical and neonatal outcomes
No differences were observed in terms of livebirth rate among positive women with severe adverse outcomes (i.e. cases) compared to women with no or mild adverse outcomes (i.e. controls) [absolute rate 92.6% (n = 75/81) compared to 98.1% (n = 656/669)] (Table 4), although a trend toward poorer obstetrical outcomes was observed among women with severe adverse outcomes [absolute rate of fetal loss > 14 WG 7.4% (n = 6/81) compared to 1.9% (n = 13/669)]. An increased risk of caesarean section was observed among patients with severe adverse outcomes [absolute caesarean sections rate 70.7% (n = 53/75) compared to 30.9% (n = 203/656)]. Similarly, women with severe maternal outcomes were at increased risk of preterm delivery < 37WG [absolute risk 62.7% (n = 32/51) compared to 36.3% (78/215)] and < 34 WG [absolute risk 51.9% (n = 14/27) compared to 20.5% (24/117)], most of which were iatrogenic [81.3% (n = 26/32) and 85.7% (n = 12/14), respectively]. Newborns born to mothers with severe adverse pregnancy outcomes were more frequently admitted to NICU [absolute risk 41.3% (n = 31/75) compared to 11.6% (n = 76/656)]. The most frequent reasons for admission were prematurity [71.0% (n = 22/31)] and respiratory distress [48.5% (n = 15/31)] (Table 4). A positive SARS-CoV-2 test at birth was observed in 2.9% of neonates (n = 11/384).) The rates of suspected perinatal transmission and reduced birthweight were similar between newborns born to mothers with severe outcomes compared to those with no or mild outcomes.
Table 4.
Obstetrical and neonatal outcomes depending on maternal severity among women with a positive SARS-CoV-2 test.
| Obstetrical/neonatal outcomes | Pregnant women with a positive test result for SARS-CoV-2 | |||
|---|---|---|---|---|
| Severe adverse maternal outcomes n = 81 |
No/mild adverse maternal outcomes n = 669 |
|||
| n (%) | 95% CI | n (%) | 95% CI | |
| Pregnancy outcomes > 14 WG | ||||
| Livebirth | 75 (92.6) | 84.6–97.2 | 656 (98.1) | 96.7–99.0 |
| Fetal loss > 14 WG | 6 (7.4) | 2.8–15.4 | 13 (1.9) | 1.0–3.3 |
| Termination of pregnancy | 1 (1.2) | 0.0–6.7 | 2 (0.3) | 0.0–1.1 |
| Obstetrical outcomes among livebirth | 75 | 656 | ||
| GA at delivery (Weeks gestation) | ||||
| Median GA (IQR) | 37 (34–38) | 39 (38–40) | ||
| Unknown GA at delivery | 0 (0.0) | n.a. | 2 (25.8) | 15.1–41.0 |
| Obstetrical management | ||||
| All vaginal deliveries | 22 (29.3) | 19.4–41.0 | 447 (68.1) | 64.4–71.7 |
| Vaginal delivery after spontaneous onset of labour | 10 (45.5) | 24.4–67.8 | 280 (62.6) | 58.0–67.1 |
| Vaginal delivery after induction of labour | 12 (54.5) | 32.2–75.6 | 167 (37.4) | 32.9–42.0 |
| Caesarean sections—no (%) | 53 (70.7) | 59.0–80.6 | 203 (30.9) | 27.4–34.6 |
| Elective caesarean sections—no (%) | 21 (39.6) | 26.5–54.0 | 85 (41.9) | 35.0–49.0 |
| Emergency pre-labor caesarean sections—no (%) | 12 (22.6) | 12.3–36.2 | 16 (7.9) | 4.6–12.5 |
| In labour caesarean sections after induction | 12 (22.6) | 12.3–36.2 | 52 (25.6) | 19.8–32.2 |
| In labour caesarean sections after spontaneous | 8 (15.1) | 6.7–27.6 | 50 (24.6) | 18.9–31.2 |
| Unknown | 0 (0.0) | n.a. | 6 (0.9) | 0.3–2.0 |
| Preterm birth among pregnancy with exposure < 37 WG | 51 | 215 | ||
| All preterm birth < 37 WG—no (%) | 32 (62.7) | 48.1–75.9 | 78 (36.3) | 29.8–43.1 |
| Latrogenic birth among preterm birth—no (%) | 26 (81.3) | 63.6–92.8 | 49 (62.8) | 51.1–73.5 |
| Unknown—no (%) | 0 (0.0) | n.a. | 1 (1.3) | 0.0–6.9 |
| Unknown GA at delivery | 0 (0.0) | n.a. | 1 (0.5) | 0.1–2.6 |
| Preterm birth among pregnancy with exposure < 34WG | 27 | 117 | ||
| All preterm birth < 34 WG—no (%) | 14 (51.9) | 31.9–71.3 | 24 (20.5) | 13.6–29.0 |
| Latrogenic birth among preterm birth—no (%) | 12 (85.7) | 57.2–98.2 | 14 (58.3) | 36.6–77.9 |
| Unknown—no (%) | 0 (0.0) | n.a. | 0 (0.0) | n.a. |
| Unknown GA at delivery | 0 (0.0) | n.a. | 1 (0.9) | 0.0–4.7 |
| Neonatal outcomes among livebirths | 75 | 656 | ||
| Neonatal death | 0 (0.0) | n.a. | 1 (0.2) | 0.0–0.8 |
| NICU admission—no (%) | ||||
| All NICU admission | 31 (41.3) | 30.1–53.4 | 76 (11.6) | 9.2–14.3 |
| Prematurity | 22 (71.0) | 52.0–85.8 | 32 (42.1) | 30.9–54.0 |
| Respiratory distress | 15 (48.4) | 30.2–66.9 | 18 (23.7) | 14.7–34.8 |
| Sepsis | 0 (0.0) | n.a. | 5 (6.6) | 2.2–14.7 |
| Cardiovascular complications | 0 (0.0) | n.a. | 0 (0.0) | n.a. |
| Hypoglycemia | 0 (0.0) | n.a. | 10 (13.2) | 6.5–22.9 |
| Hyperbilirubinemia | 1 (3.2) | 0.1–16.7 | 9 (11.8) | 5.6–21.3 |
| Coagulopathy | 0 (0.0) | n.a. | 0 (0.0) | n.a. |
| Neurologic complications | 0 (0.0) | n.a. | 2 (2.6) | 0.3–9.2 |
| Other | 3 (9.7) | 2.0–25.8 | 19 (25.0) | 15.7–36.3 |
| Unknown | 5 (6.7) | 2.2–14.9 | 47 (7.2) | 5.3–9.4 |
| SARS-CoV-2 perinatal transmission rates | ||||
| Total of SARS-CoV-2 test at birth—no (%) | 44 (58.7) | 46.7–69.9 | 340 (51.8) | 47.8–55.7 |
| Suspected SARS CoV-2 perinatal transmission (positive RT-PCR at birth)—no (%) | 2 (4.5) | 0.6–15.5 | 9 (2.6) | 1.2–5.0 |
| Birthweight | ||||
| Birthweight < P10—no (%) | 1 (1.3) | 0.0–7.2 | 39 (5.9) | 4.3–8.0 |
| Unknown | 5 (6.7) | 2.2–14.9 | 12 (1.8) | 0.9–3.2 |
Obstetrical and neonatal outcomes among positive women were assessed based on the severity of maternal disease through a case control study comparing positive women with severe adverse maternal outcomes (cases) to positive women with no or mild adverse maternal outcomes (control).
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CI, confidence interval; WG, weeks ‘gestation; GA, gestational age; NICU, Neonatal Intensive Care Unit; n.a., non-applicable.
Discussion
In this study, we present the largest cohort of pregnant women tested for SARS-Cov-2 worldwide and the first analysis of primary data stratified by the severity of maternal disease, allowing us to identify specific risk factors associated with adverse maternal outcomes.
Severe adverse outcomes, defined by maternal death, admission to ICU and/or advanced oxygen support were observed in 9.9% of cases. Pulmonary comorbidities, hypertensive disorders and diabetes mellitus were significantly associated with an increased risk of severe maternal outcomes, while usual pregnancy related risk factors were not. No difference in the livebirth rate was observed between pregnant women with severe adverse outcomes and patients with an uncomplicated course. Nevertheless, a significant increased risk of caesarean section, preterm birth and neonatal admission to the intensive care unit was observed, highlighting that obstetrical and neonatal outcomes are influenced by the severity of maternal disease.
The rate of severe disease observed here is similar to what has been previously reported in other large cohorts3–5 and summarized in a recent meta-analysis6,where the risk of severe disease among pregnant women with COVID-19 was estimated to be 13% (95%CI 6–21%). Importantly, this risk of severe maternal complications appears significantly higher when compared to a non-pregnant population at an equivalent age, with an increased odds of ICU admission or mechanical ventilation up to 1.6 (95%CI 1.3–2.0) and 1.9 (95%CI 1.4–2.6) respectively6.
Risk factors for severe maternal disease appear to be similar to what has been previously described in the general population, namely pulmonary pathologies, hypertension and diabetes7,8. Congruently, in their meta-analysis, Allotey et al. observed an increased risk of severe disease among pregnant women > 35 y.o., those with chronic hypertension, pre-existing diabetes, or body mass index > 306. Interestingly, in our study, after adjustment, obesity was not independently associated with an increased risk of severe adverse outcomes. This could be explained by the fact that overweight patients often suffer from hypertension and diabetes (metabolic syndrome), which could act as the predominant causal factors. Both are associated with macro- and micro-vascular complications, and endothelial dysfunction has been suggested as a major pathophysiological mechanism associated with COVID-19 severity18,19. In pregnancy, endothelial change is a well-known mechanism of obstetrical complications, such as gestational hypertension, HELLP (Hemolysis, elevated liver enzymes, low platelets) and pre-eclampsia20, and may contribute to the increased risk of COVID-19 complications. In our study, we did not observe any association with maternal age. This could be explained by the low number of patients > 35 y.o. included. Similarly, ethnicity (non-Caucasian versus Caucasian) was not associated with poorer outcomes, unlike previously described21.
We observed a 2.9% rate of positive test among newborns born to mothers with a positive SARS-CoV-2 test. The clinical relevance of this finding remains unclear, as, at the time of the study, we were lacking comprehensive data regarding COVID-related symptoms or COVID-suspected symptoms among newborns, repeated testing and long-term follow-up. Perinatal transmission of SARS-CoV-2 has been reported by others, both in case of vaginal and cesarean sections, and was associated in some cases with neonatal symptoms1,4,22. In all reported cases, the possibility of postnatal infection through contacts with parents or medical personal remains difficult to exclude1,4. Alternatively, transplacental transmission has been suspected in few cases, where specific IgM were detected among newborns23,24. Nevertheless, perinatal/vertical transmission appear to be rare and mainly associated with good neonatal outcomes1,4,23,24.
Our study has several limitations. First, we present here the outcomes among pregnant women with a confirmed SARS-CoV-2 infection and therefore only observational conclusions can be drawn regarding the absolute risks of severe disease and adverse obstetrical/neonatal outcomes, as a control group of negative patients was not included. Nevertheless, this was beyond the scope of the present study, whose first aim was to identify specific risk factors.
Second heterogeneities exist between participating centers in the testing of pregnant women. While some centers performed routine systematic screening of presenting women independently of compatible symptoms, other only tested symptomatic pregnant women. This could have led to a selections bias of more severe symptomatic COVID-19 cases. If a symptomatic SARS-CoV-2 infection is associated with poorer maternal, obstetrical and neonatal outcomes, this selection bias may have resulted in an overestimation of the absolute risk of adverse outcomes. However, the rate of asymptomatic infections among included positive women of 31.9% (n = 295/926) is quite similar to the rate of asymptomatic infection described in the general population, estimated to range around 40–45%25,26 and suggests a low impact of this potential bias. Similarly, patients admitted with severe disease were very likely systematically tested for SARS-CoV-2, which may have led to a possible overestimation of the actual rate of severe adverse outcome among positive patients. Follow-up analysis, including patients with ongoing pregnancies with an uncomplicated course based on systematic screening will help assess the exact risk in a more general population of pregnant women.
Third, most patients were included during the 3rd trimester of gestation, with the majority included close to delivery, while 130 pregnancies were still ongoing at the time of analysis. Although, we did not observe any impact of the gestational age (i.e. > 20 WG) on the severity of maternal disease, this could be related to a lack of statistical power. Pregnancy-related vascular complications only occur after 20 WG, which would suggest an increased risk of maternal complications in cases of maternal infection at a later stage of the pregnancy, as observed by others9. In our cohort, severe maternal outcomes were also observed in women exposed at < 20 WG, with an overall similar risk (n = 8/89, 9.0%) to what was described in the whole cohort. Therefore, caution should also be taken with pregnant women infected in early pregnancy.
Although our data regarding obstetrical outcomes are reassuring, definite conclusions cannot be drawn. Infections occurring at an earlier stage of gestation may be associated with poorer obstetrical outcomes. Viral particles have been detected within the placentas of women infected earlier during pregnancy10,12,13,27. Although placental infection seems rare, it has been associated with evidence of malperfusion28–30, which is known to be associated with reduced fetal growth and intra-uterine fetal death. Of note, Khalil et al. have shown an increase in the number of stillbirths during the epidemic peak, without being able to determine whether this is a direct effect of the virus31. At the time of analysis, pregnancies < 37WG that were exposed during the 1st and 2nd-trimesters were still ongoing (Fig. 1), suggesting an uncomplicated course. Subsequent analysis, including those patients, are needed to better define obstetrical and neonatal outcomes.
In conclusion, pregnant women, particularly those with associated comorbidities, seem to be at higher risk of severe complications of SARS-CoV-2 infection. Obstetrical and neonatal outcomes appear to be influenced by the severity of maternal disease; complications include caesarean sections, neonatal prematurity and neonatal admission to the intensive care unit. Further studies are needed to assess maternal and neonatal outcomes for cases of earlier exposure.
Supplementary Information
Acknowledgements
We thank all patients, midwives and nurses involved in this project for their contribution and for providing crucial help in data management, data entry and ethical procedures, especially Mrs Karine Lepigeon and Caroline Lombard (Lausanne University Hospital), Véronique Othenin-Girard, Marika Santagata and Monia Moreau (Geneva University Hospital).
Author contributions
M.V., G.F., O.M.P., L.P., D.B. and A.P. conceived and designed COVI-Preg. All authors (n = 129) provided cases in COVI-Preg. M.V. and A.P. performed the statistical analysis. M.V., G.F., L.P., D.B. and A.P. interpreted the results, did the literature review and wrote the first draft. All authors provided critical inputs to the paper, reviewed and approved the final version.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Manon Vouga, Guillaume Favre, Oscar Martinez-Perez, Leo Pomar, David Baud and Alice Panchaud.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92357-y.
References
- 1.Yu N, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: A retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collin J, Byström E, Carnahan A, Ahrne M. Public Health Agency of Sweden’s Brief Report: Pregnant and postpartum women with severe acute respiratory syndrome coronavirus 2 infection in intensive care in Sweden. Acta Obstet. Gynecol. Scand. 2020;99:819–822. doi: 10.1111/aogs.13901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knight M, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: National population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martínez-Perez O, et al. Association between mode of delivery among pregnant women with COVID-19 and maternal and neonatal outcomes in Spain. JAMA. 2020 doi: 10.1001/jama.2020.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivanti AJ, et al. Retrospective description of pregnant women infected with severe acute respiratory syndrome coronavirus 2, France. Emerg. Infect. Dis. 2020;26:2069. doi: 10.3201/eid2609.202144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allotey J, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrilli CM, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badr DA, et al. Are clinical outcomes worse for pregnant women ≥ 20 weeks’ gestation infected with COVID-19? A multicenter case-control study with propensity score matching. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sisman J, et al. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr. Infect. Dis. J. 2020 doi: 10.1097/INF.0000000000002815. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baud D, et al. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA. 2020 doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Algarroba GN, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panchaud A, et al. An international registry for emergent pathogens and pregnancy. Lancet. 2020;395:1483–1484. doi: 10.1016/S0140-6736(20)30981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12-March 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteil V, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varga Z, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts JM, et al. Preeclampsia: An endothelial cell disorder. Int. J. Gynecol. Obstet. 1990;32:299–299. doi: 10.1016/0020-7292(90)90402-7. [DOI] [Google Scholar]
- 21.Sze S, et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, et al. A case report of neonatal COVID-19 infection in China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong L, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng H, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020 doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann. Intern. Med. 2020 doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavezzo E, et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature. 2020 doi: 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- 27.Penfield CA, et al. Detection of SARS-COV-2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020;100133:438. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prabhu M, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: A prospective cohort study. BJOG. 2020 doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shanes ED, et al. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosier H, et al. SARS-CoV-2 infection of the placenta. J. Clin. Investig. 2020 doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil A, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020 doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.