Abstract
Tracheobronchial amyloidosis, manifested by amyloid deposits limited specifically to tracheal and bronchial tissue, is a rare manifestation with only a few hundred published cases. Patients classically present with symptoms related to fixed upper airway obstruction caused by tracheal stenosis. Clinical symptoms are non-specific and include hoarseness, dyspnea, cough, stridor, hemoptysis, and dysphagia, which are similar to those caused by more common airway disorders, often leading to incorrect, missed, and delayed diagnosis. The wide-spread use of computerized tomography (CT) imaging has the potential of dramatically advancing the early diagnosis of tracheobronchial amyloidosis. We present a case of a patient with chronic and progressive hoarseness, diagnosed with tracheobronchial amyloidosis, with a focus on unusually clear and precise CT soft tissue neck imaging. CT imaging demonstrated nodular circumferential raised mass-like thickening involving the long-segment posterior wall of the distal trachea. The wall thickening also extended into the proximal left main stem bronchi, but spared the distal bronchial tree. This resulted in moderate (approximately 50%) narrowing of the tracheal lumen, which explained the patient's hoarseness. Routine CT imaging of patients with chronic and progressive respiratory symptoms, including cough, hoarseness, and dyspnea, is recommended. Tracheobronchial amyloidosis is an uncommon disease, but it may become more commonly recognized with broader use of more effective CT imaging protocols.
Keywords: Tracheobronchial amyloidosis, Tracheal stenosis
Abbreviations: CT, Computerized tomography; TBA, Tracheobronchial amyloidosis
Background
Amyloidosis is a rare disease associated with an abnormal extracellular deposition of autologous proteins, or amyloids, that can build up in a wide variety of organs producing functional damage and can be fatal. Amyloidosis can be systemic or localized in one organ with little or no spread, with very different prognosis and treatment implications. It is estimated that about 50% of amyloidosis cases are localized in the respiratory system and are categorized in 3 forms: (1) tracheobronchial amyloidosis, (2) nodular parenchymal amyloidosis, and (3) diffuse parenchymal or alveolar septal amyloidosis [1], [2], [3], [4], [5], [6].
The diagnosis of respiratory amyloidosis is challenging and often missed since the clinical symptoms, when present, are non-specific and extremely common. Many patients with respiratory amyloidosis are asymptomatic. Symptoms may include chronic wheezing, dyspnea, cough, and dysphonia, that are more often associated with disorders such as asthma, chronic obstructive pulmonary disease (COPD), malignancy, tuberculosis, and other lung diseases.
The gold standard for diagnosing amyloidosis is a tissue biopsy using congo red stain. Bronchoscopy is typically necessary to diagnose respiratory amyloidosis [1]. However, bronchoscopy is an invasive procedure and, therefore, not always considered early in the assessment process, especially for asymptomatic and relatively mild-to-moderate respiratory symptoms [3]. Furthermore, the identification of amyloidosis through biopsy alone may not clarify the specific form and severity, which can be critical for determining optimal treatment.
The wide-spread use of computerized tomography (CT) imaging has the potential of dramatically advancing the early diagnosis and classification of the form and severity of respiratory amyloidosis [2,4,6]. Since CT is less invasive and can both identify and localize abnormal growths, it can be used earlier in the assessment process and, at times, discover incidental abnormal growths in asymptomatic patients, which then can be referred for bronchoscopic biopsies with congo red stain for definitive diagnosis. Given the limited research on these rare disorders, studies are needed to identify essential CT imaging features of each form of respiratory amyloidosis. We present a case of a patient with tracheobronchial amyloidosis with a focus on unusually clear and precise CT imaging.
Case report
A 57-year-old man with a history of hypertension, type II diabetes, Crohn's disease, and a 20-year history of smoking (1-pack daily, terminated at age 42), presented to the clinic for evaluation of hoarseness. Four years earlier, the patient complained of hoarseness and was diagnosed with laryngeal amyloidosis following suspension microlaryngoscopy with biopsy of the right glottic and subglottic lesion, causing approximately 50% airway obstruction. Surgical debridement of the vocal process lesion was attempted, but electrocardiogram abnormalities precluded completion. Following the procedure, the patient's voice improved slightly but not his breathing.
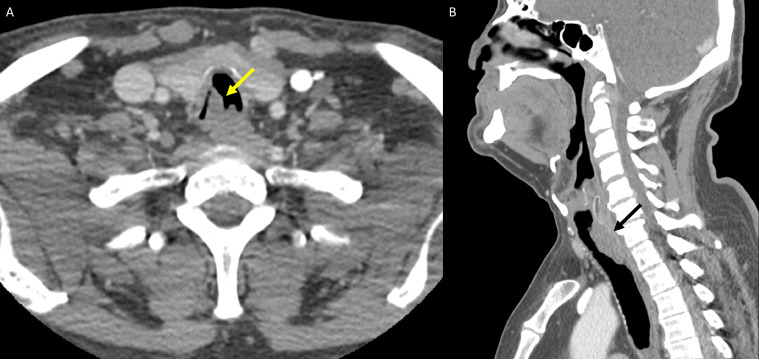
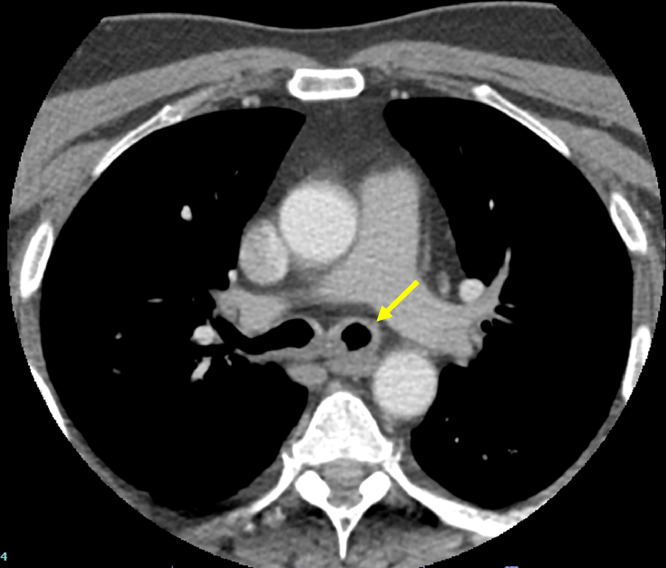
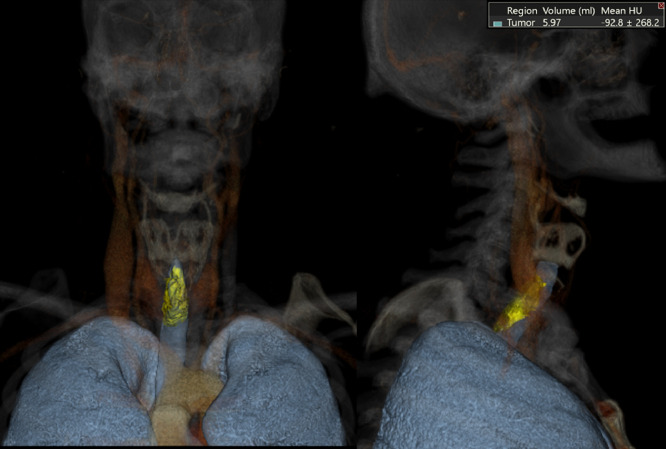
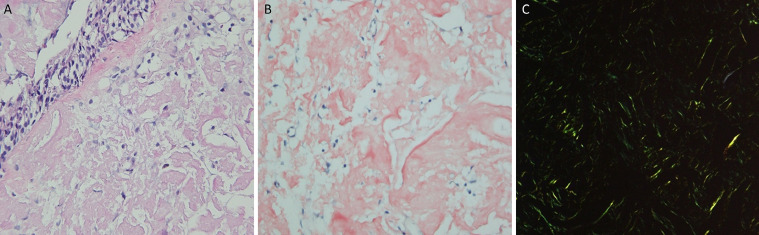
At this consultation, the patient reported progressive exacerbation of hoarseness since surgery four years earlier, but he denied any associated dyspnea, weight loss, dysphagia or hemoptysis. On examination, he was afebrile, and vitals were stable. Respiratory, HEENT (head, eyes, ears, nose, and throat) and cardiac exams were normal. Initial laboratory examinations, including Complete Blood Count, Comprehensive Metabolic Panel, and Thyroid Function Test were negative. CT soft tissue neck with intravenous contrast was ordered to assess the patient's dysphonia. Axial images of the neck from the base of the skull to the level of the pulmonary arteries were obtained. Multiplanar images with bone, lung, and soft tissue windows were reviewed. CT soft tissue neck demonstrated nodular circumferential raised mass-like thickening involving the long-segment posterior wall of distal trachea, beginning at the lower posterior cricoid cartilage level and extending into the region of thoracic inlet, measuring 1.8 × 1.5 x. 3.8 cm (Figs. 1A and B). The wall thickening also extended into the proximal left main stem bronchi (Fig. 2) but spared the distal bronchial tree. CT 3-dimensional reconstruction demonstrated the circumferential, nodular soft tissue thickening involving the posterior membrane extending into the bilateral main stem bronchi (Fig. 3). This wall thickening resulted in moderate (approximately 50%) narrowing of the tracheal lumen, which explained the patient's hoarseness. No other significant findings were detected from the neck CT. The bilateral lung apices were clear. The patient was referred to Otolaryngology for further diagnostic workup of suspected tracheobronchial amyloidosis, and treatment, if indicated. Congo red stain was performed on specimen from bronchoscopy biopsy, showing apple-green birefringence on polarization consistent with amyloid, confirming a diagnosis of tracheobronchial amyloidosis (Figs. 4A-C).
Fig. 1.
CT neck with contrast demonstrates a nodular mass-like thickening involving the posterior (membranous) wall of trachea (Fig. 1A and B, yellow and black arrows), with relative sparing of the anterior (cartilaginous) wall.
Fig. 2.
CT neck with intravenous contrast demonstrates proximal left main-stem bronchus wall thickening (yellow arrow).
Fig. 3.
3D reconstruction images demonstrate nodular soft tissue mass arising from the membranous trachea.
Fig. 4.
Amyloid deposition in the lamina propria with no involvement of the overlying pseudostratified ciliated respiratory epithelium (H&E 400x) (Fig. 4A). Congo red stain highlighting pink amorphous amyloid deposit in the lamina propria with no involvement of the overlying epithelium (400x) (Fig. 4B). Amyloid is shown here demonstrating apple-green birefringence with polarized light with Congo red (200x) (Fig. 4C).
Discussion
Tracheobronchial amyloidosis, manifested by amyloid deposits limited specifically to tracheal and bronchial tissue, is a rare disorder with only a few hundred published cases [7], [8], [9], [10], [11], [12], [13], [14], [15]. Patients classically present with non-specific symptoms related to fixed upper airway obstruction caused by tracheal stenosis [9]. Clinical symptoms include hoarseness, dyspnea, cough, stridor, hemoptysis, and dysphagia, which are similar to those caused by more common airway disorders, often leading to incorrect, missed, and delayed diagnosis [2,4,14]. While definitive diagnosis of tracheobronchial amyloidosis typically depends on bronchoscopy biopsy with congo red stain [13], [14], [15], narrowing of the major airways often limits inspection of the tracheobronchial tree using bronchoscopy [8]. There are also a variety of complications of bronchoscopy and biopsy [3].
CT imaging is less invasive than bronchoscopy and is recommended for the initial diagnostic assessment as well as episodically over time to monitor the course of the disease process [4,8]. Therefore, it is important to determine the fundamental CT imaging features of tracheobronchial amyloidosis. CT imaging has been found to reveal mucosal thickening, with or without calcification, of the trachea, main bronchus, lobar and segmental bronchi, together or in part with luminal narrowing [2,4,8,14]. As a result of this mucosal thickening, CT imaging in tracheobronchial amyloidosis typically shows narrowing of the airway, producing more than one-third tracheal or bronchial stenosis [2,4,8], as seen in our patient. Occasionally, the disease may manifest as a raised, tumor-like mass of amyloid material (as in our case), and mimic malignancy [2,12,14]. The key to differentiate this from other etiologies is the predominant involvement of the posterior wall. The ability of CT imaging to visually map the airway passages and identify extraluminal manifestations of tracheobronchial amyloidosis makes it the preferred imaging modality to assess the severity and extent of the disease process [8]. However, definitive diagnosis is made through histopathological study of the affected area.
In nearly all the published cases, there was no evidence of disease outside the tracheobronchial system, nor was the localized condition associated with systemic amyloidosis or pulmonary parenchymal involvement [7], [8], [9],13]. Once the diagnosis of tracheobronchial amyloidosis is determined through CT imaging and bronchoscopy biopsy, the condition is nearly always localized without systemic spread, and generally has better prognosis than systemic amyloidosis [3,10]. However, since a few rare cases of systemic spread have been reported in the literature [7], screening for symptoms of systemic amyloidosis should be performed on any patient with confirmed tracheobronchial amyloidosis. While there is no known "cure" for tracheobronchial amyloidosis, treatment can be effective in reducing symptoms at least temporarily, especially with primary distal airway involvement, though the amyloids may return over time, requiring recurrent interventions over the years [14], as reflected in our patient's history. Unfortunately, more serious cases, particularly those with proximal airway involvement, may be fatal [8]. No systematic research to determine specific risk factors and mortality rates has yet been published.
Diagnosis of tracheobronchial amyloidosis early in the course of disease is important for several reasons. First, misdiagnosis can lead to ineffective treatments, such as steroids or antibiotics for other respiratory diseases. The literature reports many patients who were misdiagnosed and treated inappropriately for years [12]. Second, once diagnosed accurately, long-term prognosis is fairly good for tracheobronchial amyloidosis, as long as effective treatments are provided early in the disease process, such as focused radiation and bronchoscopy-guided debridement and ablation of the amyloid growths [8]. Tracheobronchial amyloidosis tends to recur. Ongoing careful medical surveillance and monitoring of the patient's symptoms with regular CT imaging is important to identify increased size and thickening of amyloid regions. Doing so is important and facilitates appropriate interventions, as were done for our patient.
Public health education and physician awareness about tracheobronchial amyloidosis is essential since non-specific respiratory symptoms, such as chronic coughing, hoarseness, and dyspnea, are common and can be ignored by patients and providers for many years, leading to serious progression of the disease process, which can be fatal [8,9]. This disease can be an unusual diagnostic challenge even for otolaryngologists and pulmonologists given the common non-specific respiratory symptomatology [10,14]. Routine screening CT soft tissue neck imaging of patients with chronic and progressive respiratory symptoms, including cough, hoarseness, and dyspnea, is recommended. Tracheobronchial amyloidosis is an uncommon disease, but it may become more commonly recognized with broader use of more effective CT imaging protocols.
Patient consent statement
Informed written consent was obtained from the patient for publication of the Case Report and all imaging studies. Consent form on record.
Declaration of Competing Interest
All authors do not have any relevant disclosures.
Footnotes
Submission declaration and verification: The authors have not published, posted, or submitted any related papers from this same study.
Acknowledgments: None.
Authors would like to thank Ms. Emily Gay, RT(R) and Ms. Stephanie Willis, RT(C) for their assistance with CT image acquisition and post-processing. This study was supported by Resident Managed Peer Mentoring Program at West Virginia University.
References
- 1.Cordier JF, Loire R, Brune J. Amyloidosis of the lower respiratory tract. Clinical and pathologic features in a series of 21 patients. Chest. 1986;90(6):827–831. doi: 10.1378/chest.90.6.827. [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, Im JG, Song KS, Lee KS, Kim SJ, Kim JS. Localized amyloidosis of the respiratory system: CT features. J Comput Assist Tomogr. 1999;23(4):627–631. doi: 10.1097/00004728-199907000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Milani P, Basset M, Russo F, Foli A, Palladini G, Merlini G. The lung in amyloidosis. Eur Respir Rev. 2017;26(145) doi: 10.1183/16000617.0046-2017. Published 2017 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandelik SC, Heussel CP, Kauczor HU, Röcken C, Huber L, Basset M. CT features in amyloidosis of the respiratory system - Comprehensive analysis in a tertiary referral center cohort. Eur J Radiol. 2020;129 doi: 10.1016/j.ejrad.2020.109123. [DOI] [PubMed] [Google Scholar]
- 5.Yamada M, Takayanagi N, Yamakawa H, Ishiguro T, Baba T, Shimizu Y. Amyloidosis of the respiratory system: 16 patients with amyloidosis initially diagnosed ante mortem by pulmonologists. ERJ Open Res. 2020;6(3) doi: 10.1183/23120541.00313-2019. 00313-2019. Published 2020 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polo-Nieto JF, Quiroga-Dussan MDP, Castañeda-González JP, Fierro-Rodríguez DM, Durán-Acuña R, Carrillo-Bayona JA. Perilymphatic micronodular pattern as a manifestation of pulmonary amyloidosis on high-resolution computed tomography. Radiol Case Rep. 2021;16(4):850–854. doi: 10.1016/j.radcr.2021.01.027. Published 2021 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capizzi SA, Betancourt E, Prakash UB. Tracheobronchial amyloidosis. Mayo Clin Proc. 2000;75(11):1148–1152. doi: 10.4065/75.11.1148. [DOI] [PubMed] [Google Scholar]
- 8.O'Regan A, Fenlon HM, Beamis JF, Jr, Steele MP, Skinner M, Berk JL. Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine (Baltimore) 2000;79(2):69–79. doi: 10.1097/00005792-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Ding L, Li W, Wang K, Chen Y, Xu H, Wang H. Primary tracheobronchial amyloidosis in China: analysis of 64 cases and a review of literature. J Huazhong Univ Sci Technolog Med Sci. 2010;30(5):599–603. doi: 10.1007/s11596-010-0549-7. [DOI] [PubMed] [Google Scholar]
- 10.Birkeland AC, McHugh JB, Spector ME. Tracheobronchial amyloidosis: a case report and review of the literature. J Case Rep Med. 2014;3 doi: 10.4303/jcrm/235859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jivraj K, Elliot T, MacEachern PR. Tracheobronchial amyloidosis. Can Respir J. 2014;21(5):272. doi: 10.1155/2014/624615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanrıverdi E, Özgül MA, Uzun O, Gül Ş, Çörtük M, Yaşar Z. Tracheobronchial amyloidosis mimicking tracheal tumor. Case Rep Med. 2016;2016 doi: 10.1155/2016/1084063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu X, He B, Wang G, He B, Wang L, Chen Q. Bronchoscopic diagnosis and treatment of primary tracheobronchial amyloidosis: a retrospective analysis from China. Biomed Res Int. 2017;2017 doi: 10.1155/2017/3425812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mangla L, Vadala R, Kadli SK, Prajapat D, Talwar D. Tracheobronchial amyloidosis: an uncommon disease with a common presentation. Respirol Case Rep. 2020;8(7):e00630. doi: 10.1002/rcr2.630. Published 2020 Aug 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuong Vu L, Minh Duc N, Tra My TT, Ba Tung N, Phuong Thuy LT, Minh Thong P. Laryngotracheobronchial amyloidosis: a case report. Respir Med Case Rep. 2021;32 doi: 10.1016/j.rmcr.2021.10137. Published 2021 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]