Abstract
Indigenous chicken breeds are considered to be more disease tolerant than exotic chicken breeds especially for the bacterial diseases. Nicobari and Vanaraja chicken were evaluated for the survivability/mortality patterns and host immune response after experimental infection with P. multocida A1 isolate. The birds were inoculated with 1.9 × 105 CFU/mL through intraperitoneal (I/P) and intranasal (I/N) routes at 2 different age groups viz., 12 wk and 18 wk. Symptoms, mortality rates, lesions in dead birds were observed; Serum from surviving birds of different groups from both breeds were collected at 5, 14, 21, 28, 35 and 42nd d and specific antibody titers were measured by indirect ELISA. At 12 wk of age, the mortality rates were 100% and 16% in birds inoculated by I/P and I/N routes respectively in Vanaraja birds; whereas the mortality rates were 50% and 16% I/P and I/N routes respectively in Nicobari birds. At 18 wk of age the mortality rates were 16% and 50% for I/P routes in Nicobari and Vanaraja birds respectively. The mortality rates were 16% for I/N route in both Nicobari and Vanaraja birds. Lesions such as necrotic foci on liver, congestion in the liver were observed in dead birds. Serum titers were significantly (P < 0.05) higher in surviving Nicobari birds inoculated through I/P route followed by I/N route. The peak titers were reached on 14th d postinfection and declined thereafter. However, no significant difference was found in I/N route of inoculation between 2 breeds. Nicobari chicken breed showed significantly higher survivability and longer mean death time than Vanaraja germplasm to experimental Pasteuralla infection at both the ages however the survivability rate in both breeds improved at later ages.
Key words: fowl cholera, disease resistance, Nicobari chicken
INTRODUCTION
Disease resistance is a polygenic trait and influenced by many environmental factors. Differences in disease susceptibility/resistance exists among different chicken breeds and strains (Zekarias et al., 2002). Native chicken breeds are considered genetically distinct and more disease tolerant than exotic chicken breeds which are under extensive selection pressure for production traits. Exploration of native chicken germplasms and their immune competence has been focused in recent years worldwide. Further, their scope in developing improved genetic stocks for disease tolerance also has been emphasized. Better immune competence in Indian native chicken breeds has been indicated earlier by higher complement activity, higher serum lysozyme level, and antibody response (Haunshi and Sharma, 2002; Baelmans et al., 2005). However, detailed studies on resistance to specific diseases are lacking.
Nicobari chicken breed is one of the 22 Indian native chicken breeds and is an endemic breed of Nicobar Islands of India. Nicobari breed is small to medium sized bird with characteristic short legs. This breed is well adapted to hot and humid island climate with good egg production capacity in the backyard free range farming system. Better survivability and disease resistance were also observed (Rai and Ahlawat, 1995). There are 3 strains of Nicobari fowl, namely, Brown, Black, and White Nicobari fowl. The brown Nicobari has been shown to have higher genetic similarity by RAPD polymorphisms study than other 2 strains. Although there are few evidences for the better immunocompetence of Nicobari chicken breed, detailed investigations on disease tolerance/resistance/susceptibility to any specific pathogen are lacking. Vanaraja is a dual-purpose chicken variety developed from exotic breeds adapted to Indian climatic conditions for rearing in low input backyard system. Vanaraja is most popular and well adapted to different regions of India. Exploration of disease resistance/susceptibility pattern in native chicken breed would provide better understanding on genetic and immunological basis of disease resistance as well as for better exploiting the native germplasms in breeding program for the development of immunocompetent varieties or crosses. Hence, in this study, we investigated the mortality percentage, pattern and host antibody response to Pasteurella multocida A:1 through experimental infection model.
MATERIALS AND METHODS
The study was carried out at the experimental poultry farm of Indian Council of Agricultural Research-Directorate of Poultry Research (ICAR-DPR), Hyderabad. India. The study was approved by the Institute Animal Ethics committee (IAEC/DPR/2017/7).
Birds
Straight run day old chicks of Nicobari (40) and Vanaraja (40) were obtained from experimental hatchery of ICAR-DPR. Nicobari chicks were hatched from fertile eggs from brown Nicobari parents through random mating. Vanaraja chicks were hatched from fertile eggs from parent lines through artificial insemination. Birds were reared in battery brooders from day-old until the start of experiment by providing standard Soya and maize based diet and potable water ad libitum. The birds were vaccinated against Marek's disease Newcastle disease, infectious bursal disease, fowl pox, and infectious bronchitis.
Pasteurella multocida A:1 isolate
The P. multocida A:1 isolate maintained at avian health lab of ICAR-DPR was used in this study. It was originally isolated from fowl cholera outbreak of colored broiler breeder flock. The isolate was confirmed by colony morphology and by Pasteurella specific PCR. The virulence of the isolate was tested by inoculating in day-old chicks and reisolation from heart blood swab. The inoculums were prepared as described by standard procedure. Single colony of virulent isolate from brain heart infusion (BHI) agar was inoculated into BHI broth and was incubated aerobically at 37°C for 24 h (HiMedia labs, Mumbai, India; Cat # M210). The size of inoculums was determined by the plate-spread method and contained 1.9 × 105 colony-forming units (CFU)/mL of broth.
Experimental Inoculation
The experimental inoculation study was carried out in 2 stages viz., trial 1 at 12 wk of age and trial 2 at 18 wk of age. For each trial, Nicobari (n = 18) and Vanaraja (n = 18) birds were divided into 3 groups (n = 6/group). One group of each breed was inoculated with 1 mL of BHI broth through intraperitoneal (I/P) route and the other group was inoculated through intranasal route (I/N). The third group of birds was kept as uninoculated control. Trial 2 was also carried out with similar groups at the age of 18 wk. The birds were kept in different chicken isolators during the experiment following the guidelines. Birds were given feed and water ad libitum.
Morbidity, Mortality, and Histopathology
The inoculated and control birds were observed for clinical signs and for any mortality. The dead birds were removed from isolator following the standard procedure and postmortem examination was performed. Lesions in different organs including liver, heart, spleen, lungs, and wattles were observed. Swabs were taken from liver and heart for bacteriological isolation and examination by standard procedure. Organs showing lesions were collected and were fixed in 10% neutral buffered formalin. Sections were made from fixed and paraffin embedded liver and spleen tissue and stained with hematoxylin and eosin (H&E) staining. The slides were examined under light microscopy under high magnification (40× and 100×) for histopathological changes.
Serum Antibody Response
Blood samples were collected form surviving birds of all groups on 5, 14, 21, 28, 35- and 42-d postinoculation from wing vein. Serum was separated by standard procedure and stored at -20°C until further use. Sera collected on different intervals were tested for Pasteurella multocida specific antibodies by indirect ELISA using commercially available kit (IDEXX, Hoofddorp, The Netherlands; Cat # 99-09251) following manufacturer's instructions. The OD values were converted into titers using their software.
Statistical Analysis
The antibody titers were expressed as mean titers ±SEM. The mean ELISA titers of different groups and control were compared and analyzed by one-way analysis of variance (ANOVA) to test for significance at overall effect. Mean values were compared by Duncan's Posthoc test to identify significant difference among groups. P values at <0.05 were considered as significant.
RESULTS AND DISCUSSION
Mortality Pattern Upon Experimental Challenge
The mortality pattern, percentage, and mean death time are presented in Table 1. At 12 wk of age, the mortality rates were 100% and 16% in Vanaraja birds inoculated by I/P and I/N routes respectively; whereas the mortality rates were 50% and 16% in Nicobari birds inoculated through I/P and I/N routes respectively. Mean death time was 29.3 h and 30.4 h for I/P route in Nicobari and Vanaraja birds respectively. Mean death time for I/N route was 27 d and 18 d in Nicobari and Vanaraja birds respectively. At 18 wk of age the mortality rates were 16% and 50% for I/P routes in Nicobari and Vanaraja birds respectively. The mortality rates were 16% for I/N route in both Nicobari and Vanaraja birds. No mortality was observed in control birds. Nicobari chicken breed showed significantly higher survivability and longer mean death time than Vanaraja germplasm to experimental Pasteuralla infection at both the ages however the survivability rate in both breeds improved at later ages.
Table 1.
Mortality pattern during the experimental inoculation with P. multocida A:1 in Nicobari and Vanaraja chicken.
| Parameters | Nicobari |
Vanaraja |
||||
|---|---|---|---|---|---|---|
| I/P | I/N | Control | I/P | I/N | Control | |
| Trial 1: 12 wk | ||||||
| N | 6 | 6 | 6 | 6 | 6 | 6 |
| No. of birds died/total challenged | 3/6 | 1/6 | 0/6 | 6/6 | 1/6 | 0/6 |
| Percentage of mortality | 50% | 16% | - | 100% | 16% | - |
| Mean death time | 29.3 h | 27 d | - | 30.4 h | 18 d | - |
| Trial 2: 18 wk | ||||||
| N | 6 | 6 | 6 | 6 | 6 | 6 |
| No. of birds died/total challenged | 1/6 | 1/6 | 0/6 | 3/6 | 1/6 | 0/6 |
| Percentage of mortality | 16% | 16% | - | 50% | 16% | - |
| Mean death time | 26 h | 31 d | - | 35.4 h | 19 d | - |
Abbreviations: I/P, intraperitoneal; I/N, intranasal.
The degree of susceptibility to P. multocida infection is variable among different and types of birds and different age groups (Rhodes and Rimler, 1989). Generally, it is observed that chicks and young growers are less susceptible to fowl cholera than older birds (Heddleston, 1962). Heddleston and Watko (1965) showed that 9- to 16-week-old chickens were less susceptible than 52-week-old New Hampshire chickens to Pasteurella experimental infection. In contrary to these, the present study, showed high degree of susceptibility at 12 wk of age in both Nicobari and Vanaraja in comparison to 18 wk of age. However, Mbuthia et al. (2008) demonstrated that 12 wk chickens were pronouncedly expressed the fowl cholera signs than 16-week-old chicken upon experimental infection. Hence, the present findings reiterate that different age groups have variable susceptibility to P. multocida as reported previously (Heddleston, 1962; Heddleston and Watko, 1965; Rhoades and Rimler, 1989; Mbuthia et al., 2008). The observed difference in age susceptibility between this study and earlier reports could be attributed primarily to the genetic makeup of chicken under study and stain of P. multocida used for challenge experiment.
Clinical Signs, Lesions, and Histopathology
The clinical signs manifested during experimental infection included depression, fever, rales, coughing and predominantly diarrhea in most of the birds. Generally, Serotype A causes acute form of fowl cholera (Rhoades and Rimler, 1989). The signs were observed between 1 and 6 d postinfection in surviving birds and were similar to earlier reports (Mbuthia et al., 2008). Similar to previous reports significant weight losses were observed in surviving infected birds. Lesions observed in challenged birds included necrotic foci, microabscess, lymphocytic infiltrations and hemorrhages in liver and spleen, similar to earlier observations.
Serum Antibody Response
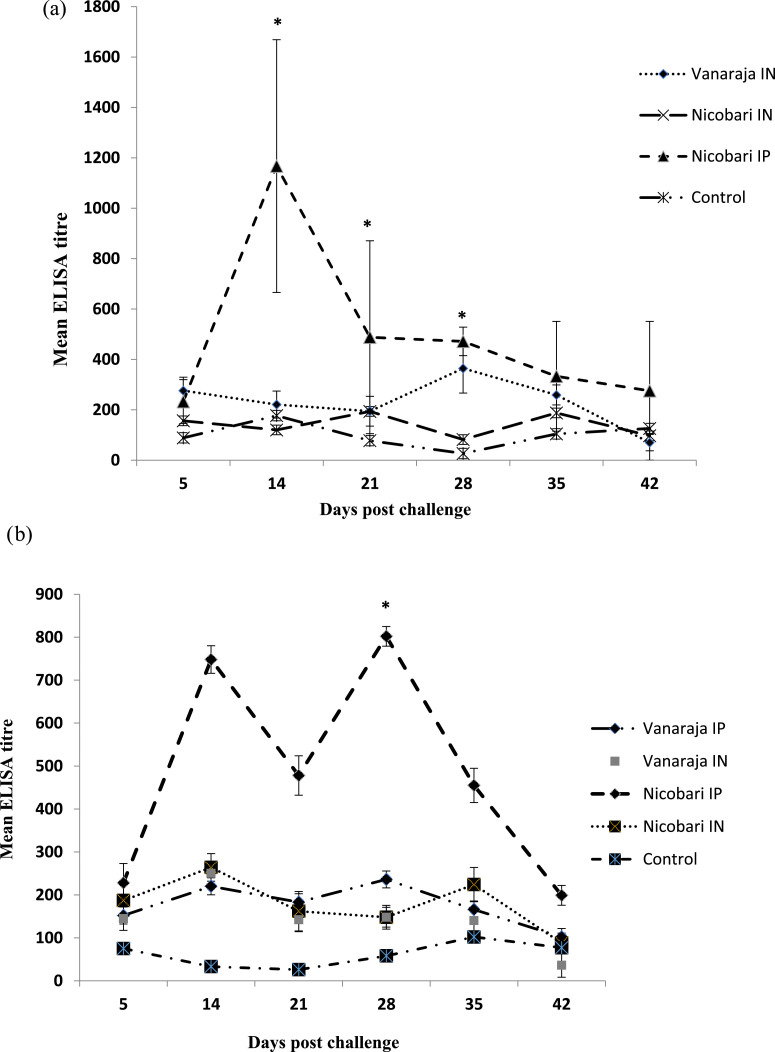
The serum antibody response measured by indirect ELISA in surviving birds during post challenge is presented in Figure 1. Serum titers were significantly (P < 0.05) higher in surviving Nicobari birds inoculated through I/P route followed by I/N route. The peak titers were reached on 14th d postinfection and declined thereafter. However, no significant difference was found in I/N route of inoculation between 2 breeds.
Figure 1.
Serum antibody titers postchallenge. (A) Trial 1 at 12 wk of age. (B) Trial 2 at 18 wk of age.
In the present study, birds were sero-negative for P. multocida specific antibodies before the challenge experiment, hence the differences in susceptibility observed in Nicobari breed might be attributed by innate immunity. Innate immunity is first line of defense by host in fighting any invading pathogen. Toll-like receptors (TLRs), complement, are the major components of innate immunity. Polymorphisms in TLR genes are extensively studied in recent years for their role in disease resistance. Allelic variation in TLR4 gene was earlier demonstrated to be linked with increased susceptibility to Salmonella typhimurium infection. In our previous study higher basal transcript expression levels were onbserved in Indian native chicken breeds including Aseel and Kadaknath compared to WL. Very recently our group reported the higher polymorphisms in TLR genes of Nicobari breed in comparison to Ghagus and WL (Haunshi et al., 2018). However, their role in imparting resistance to any specific pathogen needs to be investigated. Natural antibodies (NAb) are present in the host prior to infection and play a major role in clearing the pathogens before the development of adaptive immunity. Higher NAb levels in layers were associated with better survivability (Sun et al., 2011).
To the best of our knowledge, this is first report on tolerance/ susceptibility pattern of Indian native chicken breed and backyard chicken variety to any specific pathogen. The observed relative tolerance/ resistance of Nicobari chicken may be attributed to MHC haplotype, NRAMP1, or PAMPs etc. The lesser susceptibility of Nicobari chicken breed to P. multocida infection and more potent serum antibody response to infection, both together implies greater immunocompetence of Nicobari chicken to Pasteurella pathogen.
Acknowledgments
ACKNOWLEDGMENTS
The authors gratefully acknowledge Indian Council of Agricultural Research- Directorate of Poultry Research, Hyderabad, India for financial support.
DISCLOSURES
Authors declare that there is no conflict of interest.
REFERENCES
- Baelmans R., Parmentier H.K., Nieuwland M.G., Dorny P., Demey F., Berkvens D. Haemolytic complement activity and humoral immune responses to sheep red blood cells in indigenous chickens and in eight German Dahlem Red chicken lines with different combinations of major genes (dwarf, naked neck and frizzled) of tropical interest. Trop. Anim. Health Prod. 2005;37:173–186. doi: 10.1023/b:trop.0000049274.28640.d7. [DOI] [PubMed] [Google Scholar]
- Haunshi S., Burramsetty A.K., Ramasamy K., Chatterjee R.N. Polymorphisms in pattern recognition receptor genes of indigenous and White Leghorn breeds of chicken. Arch. Anim. Breed. 2018;61:441–449. doi: 10.5194/aab-61-441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haunshi S., Sharma D. Immunocompetence in native and exotic chicken populations and their crosses developed for rural farming. Indian J. Poult. Sci. 2002;37:10–15. [Google Scholar]
- Heddleston K.L., Watko L.P. Fowl cholera: comparison of serologic and immunologic responses of chickens and turkeys. Avian Dis. 1965;9:367–376. [PubMed] [Google Scholar]
- Heddleston K.L. Studies on pasteurellosis. V. Two immunogenic types of Pasteurella multocida associated with fowl cholera. Avian Dis. 1962;6:315–321. [Google Scholar]
- Mbuthia P.G., Njagi L.W., Nyaga P.N., Bebora L.C., Minga U., Kamundia J., Olsen J.E. Pasteurella multocida in scavenging family chickens and ducks: carrier status, age susceptibility and transmission between species. Avian Pathol. 2008;37:51–57. doi: 10.1080/03079450701784891. [DOI] [PubMed] [Google Scholar]
- Rai R.B., Ahlawat S.P.S. Evaluation of disease resistance characteristics of Nicobari fowl. Ind. Vet. J. 1995;72:354–357. [Google Scholar]
- Rhoades, K. R., R. B. Rimler. 1989. Fowl cholera. Pages 95–113 in Pasteurella and Pasteurellosis. C. Adlam and J. M. Rutter, eds. London. Academic Press, London, UK.
- Sun Y, Parmentier H.K., Frankena K., van der Poel J.J. Natural antibody isotypes titers as predictors of survival in purebred laying hens. Poult. Sci. 2011;90:2263–2274. doi: 10.3382/ps.2011-01613. [DOI] [PubMed] [Google Scholar]
- Zekarias B., Ter Huurne A.A., Landman W.J., Rebel J.M., Pol J.M., Gruys E. Immunological basis of differences in disease resistance in the chicken. Vet. Res. 2002;33:109–125. doi: 10.1051/vetres:2002001. [DOI] [PubMed] [Google Scholar]