Abstract
The aim is to optimize the dimethylacetamide (DMA) straw freezing technology of Black silkies rooster semen through the handy patent equipment, screening the formula of freezing basic extender and optimizing the DMA addition method, and then by comparing the fertility of DMA straw frozen semen with the pellet frozen semen. After the DMA straw freezing technology is optimized, it is extended to the Youxian Partridge drake semen. The result showed that the frozen sperm motility of Lake and Ravie (LR) group is 64%, the fertility 49.57% and the hatchability 91.52%, all of which are superior to those of FEB, Beltsville Poultry Semen Extender (BPSE) and Lake (P < 0.05). The sperm motility of adding DMA stock solution is 59%, which is superior to adding DMA directly into diluted semen (P > 0.05). The fertility and hatchability of DMA straw group are 77.61% and 92.30%, respectively, and it is significantly higher than those in the pellet group (P < 0.01; P < 0.05). The fresh drake sperm motility of induction collection method is 71%, the massage collection method 61% and the frozen drake sperm motility of induction 33% while the massage 19%. The fertility of frozen drake semen group is 85.93%, while that of the fresh semen group is 88.17%. The frozen drake semen fertility of the highest batch is 93.8%. In conclusion, the world's advanced fertility of frozen semen can be obtained both in the chicken and drake through the optimized DMA straw freezing technology and the method of screening freeze-resistant individuals.
Key words: semen freezing, chicken, drake, DMA, fertility
INTRODUCTION
Due to the sensitivity and instability of poultry semen to external conditions and the nonrepeatability and instability of high fertility of poultry semen after thawing, to a certain extent, it limits the development of poultry semen freezing technology and leads to the technology inapplicable to commercial production and genetic information and resource protection (Mphaphathi et al., 2012; Zaniboni et al., 2014; Mosca et al., 2016; Mphaphathi et al., 2016). In order to stabilize and improve the fertility of poultry frozen semen, researchers try to optimize this technology by improving cryoprotectant, poultry semen extender and freezing rate etc. (Kenji et al., 2010; Blanco et al., 2012; Long et al., 2014; Mosca et al., 2016; Deori et al., 2020; Gangwar et al., 2020). Among the cryoprotectants, DMA and glycerol are the most effective cryoprotectants for chicken semen freezing (Blesbois and Brillard, 2007; Long et al., 2010; Rakha et al., 2017). However, glycerol has an antagonistic effect on the fertilization of hen, even if 1 to 2% content will lead to unsatisfactory fertility. Therefore, glycerol must be removed by adding extender and centrifugation before artificial insemination (Tselutin et al., 1999; Blanco et al., 2008; Moc´e et al., 2010). But the complex centrifugation process can cause irreversible physical damage to sperm, while DMA as a cryoprotectant, reduces physical damage to sperm without centrifugation (Tselutin et al., 1999). However, the fertility of DMA straw is far less than glycerol straw (Long et al., 2014; Abouelezz et al., 2015). Many reports have compared the fertility of frozen semen between glycerol straw and DMA pellet, and concluded that the frozen semen of DMA pellet has a higher fertility, but that of glycerol straw is more convenient to preserve (Tselint et al., 1999; Blesbois and Brillard, 2007). However, it was also reported that semen freezing process was affected mainly the cryoprotectant types and the interaction between semen and freezing packaging form (Rakha et al., 2016). In addition to cryoprotectants, freezing basic extender is also a key factor affecting fertility. FEB (Tselutin et al., 1995), Lake, BPSE and LR are the most commonly used extenders based on freezing at present (Abouelezz et al., 2015; Rakha et al., 2016). However, high sperm motility and fertility of Lake and BPSE extenders cannot be obtained (Han et al., 2005; Rakha et al., 2018) and most embryo deaths occur in the combination of Lake extender with DMA or glycerol (Long et al., 2014). LR extenders are more stable than other extenders, but the fertility is not high when the DMA straw is used in combination (Tselutin et al., 1995; Santiago et al., 2011; Long et al., 2014). As a result, there is no unified basic extender for poultry semen freezing at present (Abouelezz et al., 2015). The freezing rate and freezing equipment are in the same situation. The freezing methods are mainly divided into spray method (controlling the liquid nitrogen vapor into the sample freezing container, automatically) (Tselutin et al., 1995; Santiago et al., 2011) and distance method (controlling the distance of the sample from liquid nitrogen surface, manually) (Purdy et al., 2009; Iaffaldano et al., 2016; Fattach et al., 2017). But the equipment of spray method is expensive and the liquid nitrogen consumption is high. After using, it is necessary to wait for the temperature of the freezing chamber returned to 4°C before starting the next freezing. Therefore, it is not suitable for the daily research work where the number of straws is small and the frequency of use is high while the distance method, especially in a multi-step freezing method, lack standard equipment. Based on these problems, this experiment further improves the DMA straw freezing technology by screening the basic semen extender, optimizing the DMA preparation method and using self-made portable patent freezing equipment. Then, the optimized DMA straw freezing technology is promoted to drake semen freezing and provides a simple and practical technology for the popularizations and development of poultry semen freezing technology.
MATERIAL AND METHODS
Experimental Animals
The roosters and drakes were housed in individual cages, and 1 to 3 females were housed in 1 cage at the farms in Hunan Institute of Animal and Veterinary Science. Their breeding followed the specification for healthy poultry production (GB/T32148-2015).
Experimental Facility
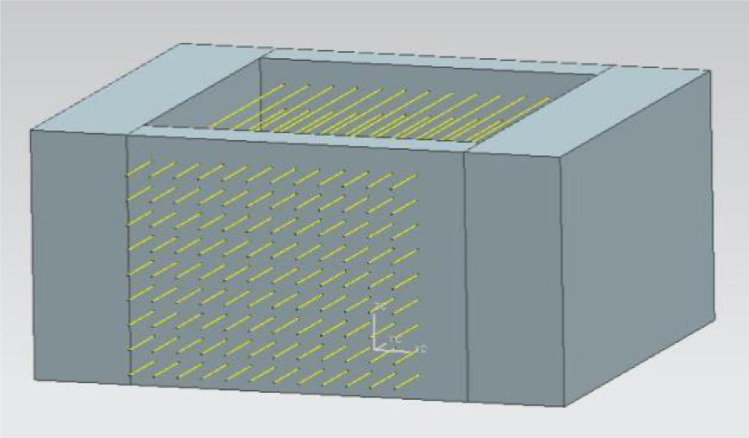
A cheap and convenient small equipment was invented, with the structure of a perimeter length (21 cm) x width (17 cm) x height (10 cm) and a 9-layer x 12-hole foam frame. Each adjacent hole was 1 cm apart, and the thickness of the foam frame body was 2 cm (Figure 1, China patent ZL201210105884.8, certification issued in 2013).
Figure 1.
The patent equipment for straw semen freezing.
Experimental Design
The study consisted of 4 experiments and only experiment 2 assessed the sperm motility, while the other experiments assessed sperm motility, fertility and hatchability. Experiment 1 was the screening of freezing semen extender by pooled semen and pellet. Four different extenders were FEB (F), BPSE (B), Lake (L) and LR (Table 1). The birds were 80 Isa hens (30 weeks), 80 White Leghorn hens (35 weeks) and 90 Black Silkies roosters (17–18 weeks) and this experiment was repeated 3 times in different dates, 3 parallel samples in each time and 3 pellets in each parallel sample (repetitions (n) = 27).
Table 1.
The composition of 4 extenders.
| Formulas |
||||
|---|---|---|---|---|
| Ingredients | F | B | L | LR |
| Distilled water/mL | 100.00 | 100.00 | 100.00 | 100.00 |
| D - fructose (g) | 0.80 | 0.87 | 0.80 | - |
| D-glucose monohydrate (g) | - | - | - | 0.80 |
| Potassium acetate (g) | 0.50 | - | 0.50 | 0.50 |
| Sodium citrate (g) | - | 0.06 | - | - |
| Magnesium acetate tetrahydrate (g) | - | - | 0.07 | 0.08 |
| Glutathiose (g) | - | - | 0.05 | - |
| Sodium acetate trihydrate (g) | - | 0.43 | - | - |
| L-Sodium glutamate (g) | 2.12 | 0.50 | 1.92 | 1.92 |
| KH2PO4 (g) | - | 1.27 | - | - |
| PVP (g) | 0.30 | - | 0.30 | 0.30 |
| Protamine sulfate salt (g) | 0.03 | - | - | - |
| TES (g) | - | 0.40 | - | - |
| BES (g) | - | - | 0.10 | - |
| pH | 7.2∼7.5 | 7.2∼7.5 | 7.0∼7.2 | 7.0∼7.2 |
| Osmotic pressure (mOsm/kg) | 260∼380 | 260∼380 | 340∼350 | 340∼350 |
Abbreviations: F, FEB; B, BPSE; L, Lake; LR, Lake and Ravie; KH2PO4, potassium hydrogen phosphate anhydrous; PVP, potassium phosphate dibasic; TES, trihydroxyamino ethane sulfonic acid; BES, N, N-BIS(2-hydroxyethyl) -2-aminoethylate.
This is based on the extender formulation of Abouelezz et al. (2015) with some modifications.
Experiment 2 was the comparison of different DMA addition methods. This experiment adopted the single chicken semen and pellet. 10 Black Silkies roosters and 3 Xiangfei native roosters in 17 to 18 weeks of age were used as the experimental birds. There were 3 samples of Black Silkies roosters each time and the experiments were repeated 5 times (n = 15). For Xiangfei native roosters, there were 3 samples in each time and the experiments were repeated 3 times (n = 9).
Experiment 3 was the comparison of the effect on frozen semen in DMA pellet and straw and adopted the single chicken semen and optimized DMA addition method. 9 Black Silkies roosters and 9 Xiangfei native roosters in 17 to 18 weeks of age were used as the experiment birds (the repetitions are shown in Table 2).
Table 2.
The result of White Leghorn hens inseminated by DMA straw and pellet from Black Silkies rooster.
| Semen | Repetitions (n) | The incubated eggs | The fertile eggs | The hatched eggs | Fertility (%) | Hatchability (%) |
|---|---|---|---|---|---|---|
| DMA pellet | 9 | 72 | 47 | 39 | 65.27 ± 19.59A | 82.97 ± 10.86b |
| DMA straw | 9 | 67 | 52 | 48 | 77.61 ± 05.05B | 92.30 ± 07.57a |
| Fresh semen | 9 | 89 | 84 | 80 | 94.38 ± 06.83C | 95.23 ± 09.60a |
A-C a,bIn the same column, different capital letters mean significant difference (P < 0.01), different lowercase letters mean significant difference (P < 0.05), and the same letters mean no significant differences (P > 0.05).
Experiment 4 was promoted the optimized DMA straw freezing method to drake semen freezing and adopted the single drake semen and straw. Experimental animals (24 weeks of age): 12 Youxian Partridge drakes were selected from 43 birds and 30 Linwu female ducks were selected from 121 birds (the repetitions are shown in Table 3).
Table 3.
Sperm motility before and after freezing using different collected methods.
| Induction |
Massage |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sperm motility (%) |
Sperm motility (%) |
||||||||
| Drake No. | Repetitions (n) | Before freezing | After freezing | Decrease rate (%) | Drake No. | repetitions (n) | Before freezing | After freezing | Decrease rate (%) |
| 1 | 10 | 70 ± 0.18ab | 41 ± 0.13a | 46 ± 0.11 | 4 | 6 | 53 ± 0.17b | 14 ± 0.05b | 72 ± 0.10 |
| 2 | 8 | 71 ± 0.15ab | 39 ± 0.09a | 45 ± 0.08 | 8 | 7 | 64 ± 0.12ab | 28 ± 0.19a | 55 ± 0.30 |
| 5 | 7 | 68 ± 0.10b | 18 ± 0.09b | 67 ± 0.18 | 9 | 5 | 71 ± 0.15a | 17 ± 0.08ab | 77 ± 0.07 |
| 6 | 5 | 80 ± 0.07a | 33 ± 0.07a | 58 ± 0.12 | 11 | 7 | 59 ± 0.15ab | 19 ± 0.16ab | 67 ± 0.23 |
| 7 | 7 | 69 ± 0.16ab | 29 ± 0.21ab | 61 ± 0.22 | 13 | 6 | 63 ± 0.08ab | 16 ± 0.07ab | 74 ± 0.17 |
| 10 | 10 | 74 ± 0.13ab | 31 ± 0.08ab | 58 ± 0.13 | - | - | - | - | - |
| 15 | 7 | 74 ± 0.13ab | 37 ± 0.14a | 49 ± 0.20 | - | - | - | - | - |
| Average | 71 ± 0.16 | 33 ± 0.14 | 54 ± 0.16 | Average | 61 ± 0.14 | 19 ± 0.13 | 68 ± 0.21 | ||
a,bThe different lowercase letters in the same column mean significant differences (P < 0.05), and the same lowercase letters mean no significant differences (P > 0.05).
Semen Collection and Preparation
The graduated centrifuge tube with extender was preheated by body heating before semen collection. After 2-week semen collection training period, chicken semen was routinely collected every 48 h with 5 mL centrifuge tubes over the study period using the technique described by Burrows and Quinn (1935). Drake semen was collected every 72 h by a cup and collected into 5 mL graduated centrifuge tube and the collecting method could be divided into induction and massage. First, the drakes should be selected by female ducks whether it was suitable for the induction method, and for the unsuitable drakes, the massage method was used for semen collection. Induction method: the female duck's legs should be tied, and then put it into the drake cage. When the drake was stood on the female duck and bit its feather of neck, the ejaculation cup should be against the female duck's cloaca, and waiting for the drake ejaculation. Massage method: the semen collector person sat on the bench and fixed the drake, then massaged the drake from waist to tail about 30 times until the drake had a genital erection. Then the ejaculation cup was against to the genital and the semen collector person gently squeezed the genital to collect semen.
After each collection, excepting experiment 1, the chicken semen should be immediately diluted 1:1 (v: v) with LR extender. For the drake semen, the massage collecting method was diluted 1:2(v/v) with LR, and that of the induction method was 1:4(v/v). After that, all the semen cooled in a small foam box filled with ice and was then transported to the laboratory for assessment. The semen was cooled and kept in a thermostat at 5°C for 30 min.
In experiment 1, about 8 mL semen was collected and pooled. Each pooled semen sample should be divided into 4 portions, approximately 2 mL each, and immediately diluted 1:1 (v/v) with the FEB, BPSE, Lake and LR, respectively.
Assessment of Sperm Variables
Sperm motility was assessed by dropping 20 μL diluted semen sample on a glass slide preheated to 32°C and then detected by a video thermostat microscope (BH-2, Shangrao Tiance Optical Instrument Co., Ltd.). The sperm motility score was divided into 9 levels (Table 4). Rooster diluted sperm motility of 70% or above was qualified (50% or above for drake semen), while the frozen sperm motility was 60% or above (30% or above for drake semen).
Table 4.
Assessment of sperm variables.
| The percentage of motility (%) | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|---|---|---|---|---|---|---|---|---|
| The number of motile spermatozoa | Less | Little | Portion | Many | Many | Most | Most | Almost all | All |
| The sperm of rectilinear/progressive movement | Tail movements | Tail movements | Only circular sperm movements | Less RM | Little RM | Large RM | Large RM | Large and strong RM | Large and strong RM |
| The rate of movement | Barely | Slow | Slow | Fluent | Fast | Fast | Faster | Faster | Faster |
| The scale of sperm movement | No | Less | Little | Little | Large | Large | Larger | Larger | Larger |
Note: This is based on the assessment of sperm variables of Abouelezz et al. (2015) with some modifications.
Abbreviation: RM, rectilinear movement.
Addition of Cryoprotectant
DMA stock solution (12% and 18%) was made by adding DMA to basic extender and stored at -20°C. And the 6% DMA (final concentration) was used as the cryoprotectant. Both DMA and DMA stock solution were precooled at 5°C and added to diluted semen at the same temperature. After addition, all the samples were equilibrated in a thermostat at 5°C for 10 min. In experiment 1, DMA was added directly to diluted semen. In experiment 2, the diluted semen was divided into 2 groups. Group D (Direct) was added DMA directly into diluted semen (6% final concentration); group I (Indirect) was added 18% DMA stock solution into diluted semen with 0.5:1(stock solution: diluted semen). In experiment 3, DMA addition method was the same as the group I in experiment 2. During the equilibration, the same sample was divided into 2 equal samples and one of that was made into straw, the other was made into pellet. In experiment 4, 12% DMA stock solution was added 1:1(v/v) to diluted semen.
Freezing Procedure of Pellet and Straw
The pellet freezing method was applied in experiment 1, experiment 2 and a part of experiment 3. Liquid nitrogen was prepared 2 min in advance and the precooled glass dropper was used to absorb the diluted semen. Then the glass dropper with diluted semen was placed parallelly to the top of liquid nitrogen and plunged drop-by-drop (about 50 μL per drop) slowly and uniformly into liquid nitrogen. After the semen was frozen for 10 min and formed the pellets, they were collected and stored in a micromesh cup in liquid nitrogen for use.
The straw freezing method was applied in experiment 4 and a part of experiment 3. Liquid nitrogen was poured into the patented device (foam container) 3 min in advance and the liquid nitrogen depth was 7 cm. For the straw freezing method, after equilibration, the samples were loaded into 0.25 mL French straws (IMV Casuko Co, Ltd.) and then inserted into the patent frame (the straws of experiment 3 were inserted into the second and the third layers of the patented frame (Figure 1), and experiment 4 was the second layer). The patent device with straws was placed on the liquid nitrogen surface for 7 min to freeze and then the straws were plunged into liquid nitrogen (at -196°C) until thawing. The specific operation of straw was as follows: At 5°C, the end of the straw without cotton plug was inserted into the diluted semen, and the semen was sucked to the volume of 8 of 10 of the straw, and about 1 cm of air must be left in the middle of the straw. The end of straw containing semen without cotton was inserted into the HKF powder (France IMV Casuko Co, Ltd.) for sealing.
Thawing Procedure
For thawing, the pellets were placed in a beaker with 5 mL and then waggled the beaker in constant temperature water bath (HH-S1, Jintan, China) at 60°C. When the pellets were dissolved to one third, the beaker was placed in room temperature water until the pellets were completely thawed. The straws were thawed for 5 s in a constant temperature water bath at 40°C in experiment 3 while 10 s in experiment 4. In experiment 1, the frozen semen pellets of FEB, BPSE, Lake and LR groups were thawed and mixed respectively within each group. In experiment 3, the frozen semen in the straw and the pellet group were performed as the above methods and mixed within the same group. After mixing, all the semens were immediately used for insemination.
Artificial Insemination
All chicken artificial insemination (AI) experiments were performed between 16:00 and 17:00. In experiment 1, 80 hens of Isa and 80 hens of White Leghorn were randomly divided into 4 groups (20 hens of Isa and 20 hens of White Leghorn in each group). The hens were given superficial AI (2–3 cm depth) and AI volume was 0.2 mL/time. In experiment 3, 60 hens of White Leghorn were randomly divided into 3 groups (20 hens/group). The hens were given a deep AI (4 cm depth) and AI volume was 0.3 mL/time. In experiments 1 and 3, inseminating 2 times in succession for the first time, after that, once every 72 h. The fertile eggs were collected from the 3rd d after the first AI and stopped collection from the 6th d after the last AI. The fertile eggs were brought back to the laboratory for disinfection with 70% alcohol spray, and the fertile eggs collected every 3 d were hatched in a batch. The incubation conditions: 38.5°C and 65% to 70% humidity in the 1 to 18th d, 37.5°C and 70% to 75% humidity in the 19 to 21st d, each batch of fertilized eggs was candled for evaluating fertility on the 7th d after entering the incubator. In total, there were 4 times of AI and 4 batches of incubation in experiment 1 and 3 times of AI and 3 batches of incubation in experiment 3.
In the experiment 4, 30 Linwu female ducks were randomly divided into 2 groups, and deep AI (4–5 cm depth) was performed between 9:00 and 10:00. Experimental group: the frozen semen was mixed and the AI volume was 0.2 mL/time. Control group: the fresh semen was collected for AI and the AI volume was 0.05 mL/time. After the first AI, the second AI was performed in both groups at an interval of 48 h. After the second AI, the later AI was performed every 96 h. The fertile eggs were collected on the 2nd d after the first AI and ended the collection on the 14th d. The fertilized eggs collected every 3 d were hatched in one batch. There were 4 times AI and 3 batches of incubation. The fertilized eggs were hatched in the temperature of 37.5°C and the humidity of 60%.
Statistical Analysis
Data were analyzed through ANOVA of SPSS 20.0. Analysis of variance on fresh/frozen sperm motility, fertility (fertile eggs/incubated eggs × 100), and hatchability (hatched eggs/fertile eggs × 100) was performed using the General Linear Model (GLM) and the mean of data were compared using Duncan's Multiple Range Test (DMRT). The statistical significance was set at P < 0.05. Before the statistical analysis, all percentage data were normalized with an arcsine transformation and data were represented as mean ± standard error of mean (SEM).
RESULTS
Experiment 1
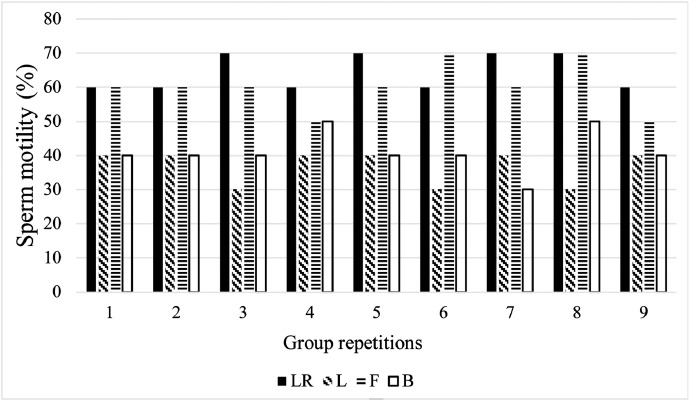
As shown in Figure 2, in each extender group, the differences in the motility of parallel samples were relatively stable. The frozen sperm motility of FEB and LR Group were 60% to 70%, while Lake and BPSE Group were 30% to 40%. There was no significant difference of frozen sperm motility between LR Group (64%) and FEB Group (61%) (P > 0.05), but the frozen sperm motility of LR and FEB Group were significantly higher than that of BPSE Group (41%) and Lake Group (37%) (P < 0.05).
Figure 2.
The frozen sperm motility with different diluents of Black Silkies rooster. Mean value(%) = 64 ± 0.003, 61 ± 0.004, 41 ± 0.004, 37 ± 0.003 for LR, F, B, and L, respectively. Abbreviations: LR, Lake and Ravie; F, FEB; B, BPSE; L, Lake.
As shown in Table 5, the fertility was basically consistent with the frozen sperm motility, and they were positively correlated. When the chicken was Isa hen, there were significant differences among the 4 groups in both fertility and hatchability (P < 0.05). The fertility and hatchability of LR group were 49.57% and 91.52%, respectively, significantly higher than that of FEB (43.33%, 89.79%), BPSE (25.21%, 76.66%) and Lake group (10.81%, 75.00%) (P < 0.05). When the bird was replaced to White Leghorn hen, there were significant differences for the LR group compared to the other 3 groups in both fertility and hatchability (P < 0.05). However, there was no significant difference between BPSE and Lake group in both fertility and hatchability (P > 0.05). The fertility and hatchability for LR was 49.57% and 91.52%; for FEB, 43.33% and 89.79%; for BPSE, 25.21% and 76.6%; and for Lake, 15.23% and 81.25%. Under the same conditions, the fertility of 4 groups of Isa hens was higher than that of White Leghorn hens.
Table 5.
The fertility and hatchability of frozen semen with 4 different extenders on Isa hens and White Leghorns hens.
| Hens | Extenders | Motility (%) | The incubated eggs | The fertile eggs | The hatched eggs | Fertility (%) | Hatchability (%) |
|---|---|---|---|---|---|---|---|
| Isa | F | 61 ± 0.02a | 113 | 49 | 44 | 43.33 ± 10.25b | 89.79 ± 5.35b |
| B | 41 ± 0.02b | 119 | 30 | 23 | 25.21 ± 6.8c | 76.66 ± 7.96c | |
| L | 37 ± 0.02b | 74 | 8 | 6 | 10.81 ± 2.23d | 75.00 ± 3.44d | |
| LR | 64 ± 0.01a | 119 | 59 | 54 | 49.57 ± 5.78a | 91.52 ± 9.83a | |
| White | F | 61 ± 0.02a | 123 | 28 | 24 | 22.76 ± 5.91b | 85.71 ± 5.34b |
| Leghorns | B | 41 ± 0.02b | 128 | 20 | 16 | 15.60 ± 1.22c | 80.00 ± 2.31c |
| L | 37 ± 0.02b | 105 | 16 | 13 | 15.23 ± 11.25c | 81.25 ± 3.41c | |
| LR | 64 ± 0.01a | 130 | 48 | 44 | 36.92 ± 5.7a | 91.66 ± 5.33a |
a-dIn the same column and breeds, different lowercase letters meant significant difference (P < 0.05); the same lowercase letters meant no significant difference (P > 0.05).
The repetitions (n) of motility assessment = 27. The repetitions of fertility and hatchability assessment = 12. Abbreviations: F, FEB; B, BPSE; L, Lake; LR, Lake and Ravie.
Experiment 2
As shown in Table 6, when the experimental birds were Black Silkies roosters, the frozen sperm motility of Group I was the highest (59%), higher than that of Group D (51%), but there was no significant difference between the 2 groups (P > 0.05). When Xiangfei native rooster was used for experiment, the frozen sperm motility of Group I was 42%, significantly higher than that of Group D 37% (P < 0.05). Between the 2 breeds, the frozen sperm motility of Black Silkies rooster Group I (59%) was higher than that of Xiangfei native rooster Group I (42%) (P > 0.05), and significantly higher than that of Xiangfei native rooster Group D (37%) (P < 0.05). Furthermore, there was no significant difference between Black Silkies rooster Group D (51%) and Xiangfei native rooster Group I (42%) (P > 0.05), but Black Silkies rooster Group D was significantly higher than that of Xiangfei native rooster Group D (37%) (P < 0.05).
Table 6.
The motility of frozen semen with different DMA adding method.
| Roosters | Repetitions (n) | Fresh semen motility (%) | Collected semen volume (mL) | FSM of group D (%) | FSM of group I (%) |
|---|---|---|---|---|---|
| Black Silkies | 15 | 80 ± 0.02 | 0.63 ± 0.18 | 51 ± 0.13ab | 59 ± 0.13a |
| Xiangfei | 9 | 72 ± 0.06 | 0.50 ± 0.00 | 37 ± 0.15c | 42 ± 0.23ab |
Note: Black Silkies rooster is an indigenous breed from China. Xiangfei native rooster is an indigenous breed from Hunan Province, China.
a-cIn the same column and row, different lowercase letters meant significant difference (P < 0.05); the same lowercase letters meant no significant difference (P > 0.05).
Abbreviation: FSM, frozen sperm motility.
Experiment 3
As shown in Table 7, the frozen sperm motility of DMA straw group in Black Silkies rooster was 54% higher than that of pellet group 53%; the frozen sperm motility of DMA straw group in Xiangfei native rooster was 51%, higher than that of pellet group 49%, and there was no significant difference between the straw and pellet group in different breeds (P > 0.05).
Table 7.
The sperm motility of different roosters before and after freezing with DMA straw and pellet.
| Repetitions | Sperm motility (%) |
|||||
|---|---|---|---|---|---|---|
| Roosters | (n) | Average volume of semen collection (mL) | Fresh | Pellet | Straw | |
| Black Silkies | 9 | 0.76 ± 0.17 | 78 ± 0.03 | 53 ± 0.13a | 54 ± 0.09a | |
| Xiangfei | 9 | 0.61 ± 0.27 | 72 ± 0.06 | 49 ± 0.15a | 51 ± 0.13a | |
aIn the same row, the same lowercase letters meant no significant difference (P > 0.05).
As shown in Table 2, the fertility in the fresh semen group was 94.38%, and the hatchability was 95.23%, which indicated that the experimental hens were healthy. The fertility and hatchability of DMA straw group were 77.61% and 92.38% respectively, which were significantly higher than that of pellet group (65.27%, 82.97%) (P < 0.01, P < 0.05).
Experiment 4
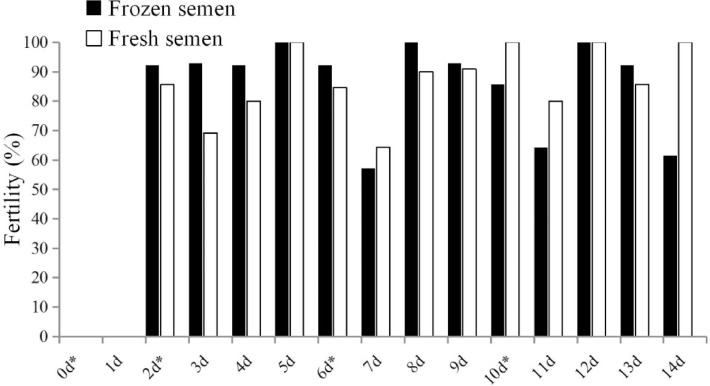
As shown in Table 3, in the induction group, the fresh sperm motility was 71%, the frozen sperm motility 33% and the loss of frozen sperm motility (motility of fresh semen minus motility of frozen semen) 54%. In the massage group, the fresh sperm motility was 61%, the frozen sperm motility 19%, and the loss of frozen sperm motility 68%. In terms of the fertility (Figure 3), the highest (93.8%) fertility of frozen semen group was in the first batch (2–6 d), while that in the fresh semen group was (92.3%) in the third batch (11–14 d). In terms of 100% fertility, it appeared 3 times in the frozen semen group 2 to 3 d after the AI which were in the 5th (11/11), 8th (13/13) and 12th d (12/12). There were 4 times in the fresh semen group 2 to 4 d after the AI that were in the 5th (14/14), 10th (9/9), 12th (12/12), and 14th d (7/7). As for the fertility, except the last day, the tendency of the frozen semen group was the same as that of the fresh group. However, the fertility of the fresh semen group was more stable than that of the frozen semen group. After AI of 2nd, 6th and 10th d, the entire fertility decreased on the first day. The fertility dropped to 64.3% at most, but increased again on the second day. The lowest fertility tendency was found in frozen semen group on the 7th, 11th, and 14th d, which decreased to 52.7% at the lowest level. The average fertility and hatchability of the frozen semen group were similar to those of the fresh semen group. After the third AI, the eggs production in the fresh semen group decreased, leading to the total number of eggs less than frozen group (Table 3). According to Figure 3, it could be predicted that if the intervals of AI were shortened, the fertility would be higher. The drake's age in the fresh semen was much older than that of frozen semen group and it might be the reason why the hatchability of the frozen group was slightly higher than that of the fresh semen group. The lowest fertility in both groups occurred on the 7th d (Figure 3).
Figure 3.
The relationship between fertility and insemination time of Youxian Partridge drake fresh and frozen semen. * = the day of artificial insemination.
DISCUSSION
In the freezing technology of semen, pH, osmotic pressure, buffer capacity and nutrients of extender are the key factors in the formula design. Lake and Mcindoe (1959) found that the pH value range of chicken semen extender was 6.8 and 7.1; Li (1992) concluded that high fertility was obtained when pH value at 5.97 to 8.33. Nabi et al. (2016) speculated that the increase of osmotic pressure in extender could produce abundant superoxide anions leading to the apoptosis of sperm during the freezing or thawing procedure. The osmotic pressure range of chicken semen extender was 330 to 420 mOsm/kg, and the other was 278 to 434 mOsm/kg (Nabi et al., 2016). It could be seen that the range of osmotic pressure and pH in extender was generally wide. Therefore, the main reason for the difference between the 4 extenders should come from other factors. And the range of pH and osmotic pressure of extender in this experiment were similar to those reported by Li (1992) and Nabi et al. (2016), in which the pH and osmotic pressure of FEB and BPSE was 7.2 to 7.5 and 260 to 380 mOsm/kg respectively and the pH and the osmotic pressure of Lake and LR (the best freezing effect extender) was 7.0 to 7.2 and 340 to 350 mOsm/kg, respectively.
The complex composition of semen leads to the complexity of developing the composition of semen extender. Extender mainly contains saccharide (glucose, fructose, etc.) to supply energy for sperm, buffer (sodium citrate, TES, BES, etc.) to maintain sperm homeostasis and conducive to sperm survival, isotonic solution (sodium glutamate, etc.) to maintain sperm osmotic pressure and antimicrobial substances. The favorable effect ranking in this paper was LR > FEB > BPSE > Lake. And there was a phenomenon that the more the solute composition there was, the worse effect would be on extender. We speculated that there may be several causes. First, semen freezing and semen cold storage were entirely different processes for sperm metabolism. The detection index of general extender formula study was the quality change of sperm stored in vitro for a certain time. However, the whole semen freezing process from collection to the end of freezing was pretty short (within 2 h) and performed in a low temperature (about 5°C) (Miranda et al., 2018). Therefore, in the freezing process, sperm metabolism was slow and the consumption of nutrients was less, which was not enough to reflect the quality of the extender, and the components with advantages to sperm during cold storage were not suitable for freezing. Second, for the similarity of 4 extender components, LR performed the best so it was taken as the standard, the similarity of components (the proportion of similar compositions in extender) was FEB (60%), Lake (57.1%) and BPSE (16.7%) respectively. The FEB was the most relevant, and the effect of extender quality was second. But Lake, with a similarity of 57.1% had a negative effect on sperm motility. Thus, the unique composition from Lake, BES (buffer) and glutathione (antioxidant) might be the main reasons affecting the effect on the extender. The -SH radical group on glutathione (GSH) could protect the highly unsaturated fatty acids on the sperm cell membrane from the damage of oxidation reaction and end products of peroxide reaction. However, it was likely that sperm itself could prevent sperm oxidation under the conditions of freezing at extremely low temperature, so it was likely that GSH couldn't play its advantages under freezing conditions, but increased the solute components in the extender, resulting in antagonism between GSH and other components in the extender.
The difference solute components between LR and FEB were glucose and protamine sulfate salt. Glucose, as a nonpermeable cryoprotectant, not only provides energy but also protects sperm by the direct reaction to sperm membrane. Studies showed that the frequency of using fructose in semen cold storage was much higher than that of glucose because the decomposition rate of fructose was slowly and the production of acidic substances was less, which caused no serious damage to semen (Sexton et al., 1980; Tselutin et al., 1995; Zaniboni et al., 2014). Glucose could directly decompose itself to provide energy for sperm, while fructose needed to be transformed into glucose, then provided energy. Therefore, we speculated that rapid supply of glucose could make sperm absorb enough energy to resist cell damage during freezing. This may be the reason why fructose tended to be used in cold storage extenders and glucose in freezing extenders. At the same time, the antimicrobial action of protamine sulfate salt also failed to play a role and instead increased its extender solute with being frozen at -196°C in a short time. Based on the above analysis, we claimed that in the following study, chicken semen freezing extender should focus on the field of freezing physical damage rather than the biochemical and biological processes that affected sperm metabolism.
Adding DMA directly to diluted semen was the traditional way to protect diluent semen during freezing. (Tselutin et al., 1995; Zaniboni et al., 2014; Mosca et al., 2016). But in this study, we were the first to add DMA into the extender for generating DMA stock solution and then add the DMA stock solution into diluted semen. According to the above results, the DMA-adding method of stock solution greatly simplified the operation steps of semen freezing technology, reduced the deviation of DMA preparation and made it more convenient for popularization and application. Besides, the direct contact of high concentration DMA to sperm was avoided, which may reduce the toxic effect of DMA on sperm to a certain extent and improve the motility of frozen sperm.
In terms of chicken semen, Tselutin et al. (1999) obtained fertility of 92.7% by using Lake extender, 6% DMA pellet method. For the glycerol straw spray method, the fertility was 53.7%–63.9% and the 2-step freezing procedure was: drop from 5°C to -35°C (-7°C/min), and then from -35°C to -140°C (-20°C/ min). Kowalczyk and Łukaszewicz (2015) used EK extender, DMF, straw and distance method (place the straw on the polystyrene container and stainless-steel bracket 2 cm away from the liquid nitrogen surface for fumigations) to freeze the Capercaillie semen. The fertility reached 84.4%, and hatchability reached 70.7%. However, there were only 3 experimental hens and 32 fertile eggs; thus, the data could not reflect the reliability. Chuaychu et al. (2017) added cyclodextrin CLC and DMF to the extender (LR) and freezing chicken semen by distance method (place the straw 11 cm away from liquid nitrogen surface (-35°C) and maintained 12 min, then moved it 3 cm away from the liquid nitrogen surface (-135°C) and maintained 5 min). The addition of 1 mg/mL cyclodextrin group obtained 91.9% fertility. However, when this scheme was repeated, it was found that the procedure of preparing cyclodextrin was complicated, and the effect was far less than that of our methods in this study.
The early studies reported that DMA was suitable for pellets, while glycerol was suitable for straws (Tselutin et al., 1999; Blesbois and Brillard, 2007). Blesbois and Brillard (2007) reported that the hatchability of DMA straw semen was much lower than that of pellets. It was because of the harmful interaction between DMA and straw plastics. Besides, this negative effect was more harmful to the species with low fertility. As a result, it recommended that the straws be used with glycerol and DMA with pellets (Tselutin et al., 1999). However, it has also been recently reported that there were no significant differences between the 2 methods (Long et al., 2014). For the first time, the results of this study have shown that the effects of DMA straw frozen semen were superior to DMA pellets. The average fertility of DMA straws was 77.61%, while that of DMA pellets was only 65.24%. And both the fertility and hatchability had a significant difference between the 2 methods. This result overturned the previously study that DMA pellets were superior to DMA straws. Thus, the optimized DMA straw method in this paper was not only superior to DMA pellet in all aspects but also may be used as the primary technology of poultry semen freezing in the future.
Liu et al. (2005) once frozen drake and chicken semen in pellet and straws respectively at the same time, by using 11% glycerol. It was found that the frozen drake sperm motility was higher than chicken sperm motility. The results of this study also shown that the fertility of drake semen was higher than that of chicken even with the same method. Poultry sperms were similar in appearance and size; they were both rod-like and long-tailed. Therefore, it can be inferred that the same technology may be applied to other poultry. But the length of chicken sperm (90–100 μm) was much longer than that of drake sperm (56.3–71.9 μm) and the result also shown that drake semen has better freezing resistance than chicken. We inferred that it may be attributed to the fact that longer sperm in total length has worse freezing resistance.
In summary, for the first time, the DMA stock extender is used as poultry semen cryoprotectant, which simplified the semen freezing operation and reduced the DMA toxicity to the poultry semen. This study screened basic extender formula, improved the freezing devices and used single poultry semen to screen the individual with higher freezing resistance. These improvements and innovations may pave the way for the more convenient and advanced straw semen freezing technology, both in chicken and drake.
ACKNOWLEDGMENTS
The authors express their gratitude to all the staff of the funding supported institutions (see Funding) and would like to appreciate the valuable suggestions from Mr. Chuang Li and Jingyang Yuan, which improved the manuscript substantially.
The research was funded by the Department of Science, Technology and Education, Ministry of Agriculture and Rural Affairs of the People's Republic of China (13200397); the International Cooperation in Science and Technology of China (2013DFG31810); Hunan Engineering Research Center of Poultry Production Safety.
DISCLOSURES
The authors declare that they have no conflicts of interest to this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with this work submitted.
REFERENCES
- Abouelezz F.M., Castano C., Toledano-Diaz A., Esteso M.C., Lopez-Sebastian A., Campo J.L., Santiago-Moreno J. Effect of the interaction between cryoprotectant concentration and cryopreservation method on frozen/thawed chicken sperm variables. Reprod. Domest. Anim. 2015;50:135–141. doi: 10.1111/rda.12464. [DOI] [PubMed] [Google Scholar]
- Abouelezz F.M.K., Castaño C., Toledano-Díaz, A., Esteso M.C., López-Sebastián A., Dávila S.G., Gil M.G., Cuenca O.T., Campo J.L., Blesbois E., Santiago-Moreno J. Successful use of artificial insemination in the production of red-legged partridges (alectoris rufa) Eur J Wildlife Res. 2015;61:645–647. [Google Scholar]
- Blanco J.M., Long J.A., Gee G., Donoghue A.M., Wildt D.E. Osmotic tolerance of avian spermatozoa: Influence of time, temperature, cryoprotectant and membrane ion pump function on sperm viability. Cryobiology. 2008;56:8–14. doi: 10.1016/j.cryobiol.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Blanco J.M., Long J.A., Gee G., Wildt D.E., Donoghue A.M. Comparative cryopreservation of avian spermatozoa: Effects of freezing and thawing rates on turkey and sandhill crane sperm cryosurvival. Anim. Reprod. Sci. 2012;131:1–8. doi: 10.1016/j.anireprosci.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Blesbois E., Brillard J.P. Specific features of in vivo and in vitro sperm storage in birds. Animal. 2007;1:1472–1481. doi: 10.1017/S175173110700081X. [DOI] [PubMed] [Google Scholar]
- Burrows W.H., Quinn J.P. A method of obtaining spermatozoa from the domestic fowl. Poultry Sci. 1935;14:251–253. [Google Scholar]
- Chuaychu-Noo N., Thananurak P., Chankitisakul V., Vongpralub T. Supplementing rooster sperm with cholesterol-loaded-cyclodextrin improves fertility after cryopreservation. Cryobiology. 2017;74:8–12. doi: 10.1016/j.cryobiol.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Deori S., Johannisson A., Morrell J. Single layer centrifugation with 20% or 30% porcicoll separates the majority of spermatozoa from a sample without adversely affecting sperm quality. Reprod. Domest. Anim. 2020;55:1337–1342. doi: 10.1111/rda.13779. [DOI] [PubMed] [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V., Najafi A. L-carnitine in rooster semen cryopreservation: Flow cytometric, biochemical and motion findings for frozen-thawed sperm. Cryobiology. 2017;74:148–153. doi: 10.1016/j.cryobiol.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Gangwar C., Kharche S.D., Mishra A.K., Saraswat S., Kumar N., Sikarwar A.K. Effect of diluent sugars on capacitation status and acrosome reaction of spermatozoa in buck semen at refrigerated temperature. Trop. Anim. Health Prod. 2020;52:3409–3415. doi: 10.1007/s11250-020-02374-8. [DOI] [PubMed] [Google Scholar]
- Han X.F., Niu Z.Y., Liu F.Z., Yang C.S. Effects of diluents, cryoprotectants, equilibration time and thawing temperature on cryopreservation of duck semen. J. Poult. Sci. 2005;4:197–-201. [Google Scholar]
- Iaffaldano N., Iorio M.D., Miranda M., Zaniboni L., Manchisi A., Cerolini S. Cryopreserving turkey semen in straws and nitrogen vapour using dmso or dma: Effects of cryoprotectant concentration, freezing rate and thawing rate on post-thaw semen quality. Br. Poult. Sci. 2016:264–270. doi: 10.1080/00071668.2016.1148261. [DOI] [PubMed] [Google Scholar]
- Kenji S., Toshiaki T., Mariko T., Tatsuya N., Takayuki I., Mitsuru N., Atsushi T., Yasuhiro N. A method for cryopreserving semen from yakido roosters using n-methylacetamide as a cryoprotective agent. J. Poult. Sci. 2010;47:297–301. [Google Scholar]
- Kowalczyk A., Lukaszewicz E. Simple and effective methods of freezing capercaillie (tetrao urogallus l.) semen. PLoS One. 2015;10: doi: 10.1371/journal.pone.0116797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake P.E., Mc I.W. The glutamic acid and creatine content of cock seminal plasma. Biochem. J. 1959;71:303–306. doi: 10.1042/bj0710303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. The Ph of chicken semen diluent [In Chinese] Animal Husbandry and Veterinary Medicine. 1992;024:117–118. [Google Scholar]
- Liu J., Zheng L., Zhang L. The relationship between the viability and acrosome breakage rate of chicken sperm after cryopreservation. J. Huazhong Agric. Univ. 2005;24:380–382. [ In Chinese] [Google Scholar]
- Long J.A., Bongalhardo D.C., Pelaez J., Saxena S., Settar P., O'Sullivan N.P., Fulton J.E. Rooster semen cryopreservation: Effect of pedigree line and male age on postthaw sperm function. Poult. Sci. 2010;89:966–973. doi: 10.3382/ps.2009-00227. [DOI] [PubMed] [Google Scholar]
- Long J.A., Purdy P.H., Zuidberg K., Hiemstra S.J., Velleman S.G., Woelders H. Cryopreservation of turkey semen: Effect of breeding line and freezing method on post-thaw sperm quality, fertilization, and hatching. Cryobiology. 2014;68:371–378. doi: 10.1016/j.cryobiol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Miranda M., Kulikova B., Vasicek J., Olexikova L., Iaffaldano N., Chrenek P. Effect of cryoprotectants and thawing temperatures on chicken sperm quality. Reprod. Domest. Anim. 2018;53:93–100. doi: 10.1111/rda.13070. [DOI] [PubMed] [Google Scholar]
- Mocé E., Grasseau I., Blesbois E. Cryoprotectant and freezing-process alter the ability of chicken sperm to acrosome react. Anim. Reprod. Sci. 2010;122:359–366. doi: 10.1016/j.anireprosci.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Mosca F., Madeddu M., Sayed A.A., Zaniboni L., Iaffaldano N., Cerolini S. Data on the positive synergic action of dimethylacetamide and trehalose on quality of cryopreserved chicken sperm. Data Brief. 2016;9:1118–1121. doi: 10.1016/j.dib.2016.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mphaphathi M.L., Luseba D., Sutherland B., Nedambale T.L. Comparison of slow freezing and vitrification methods for venda cockerel's spermatozoa. J. Anim. Sci. 2012;02:204–210. [Google Scholar]
- Mphaphathi M.L., Seshoka M.M., Luseba D., Sutherland B., Nedambale T.L. The characterisation and cryopreservation of venda chicken semen. Asian. Pac. J. Reprod. 2016;5:132–139. [Google Scholar]
- Nabi M.M., Kohram H., Zhandi M., Mehrabani-Yeganeh H., Sharideh H., Zare-Shahaneh A., Esmaili V. Comparative evaluation of nabi and beltsville extenders for cryopreservation of rooster semen. Cryobiology. 2016;72:47–52. doi: 10.1016/j.cryobiol.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Purdy P.H., Song Y., Silversides F.G., Blackburn H.D. Evaluation of glycerol removal techniques, cryoprotectants, and insemination methods for cryopreserving rooster sperm with implications of regeneration of breed or line or both. Poult. Sci. 2009;88:2184–2191. doi: 10.3382/ps.2008-00402. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Akhter S., Hussain I., Blesbois E. Cryopreservation of indian red jungle fowl (gallus gallus murghi) semen. Anim. Reprod. Sci. 2016;174:45–55. doi: 10.1016/j.anireprosci.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Akhter S., Zafar Z., Naseer A., Hussain I., Blesbois E., Santiago-Moreno J. Use of dimethylsulfoxide for semen cryopreservation in indian red jungle fowl (gallus gallus murghi) Theriogenology. 2018;122:61–67. doi: 10.1016/j.theriogenology.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Akhter S., Zafar Z., Naseer A., Hussain I., Santiago-Moreno J., Blesbois E. Dimethyleacetamide improves the cryosurvivability of indian red jungle fowl (Gallus gallus murghi) sperm. Theriogenology. 2017;103:83–89. doi: 10.1016/j.theriogenology.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Rakha B.A., Ansari M.S., Hussain I., Anwar M., Akhter S., Blesbois E.2016. Comparison of extenders for liquid storage of indian red jungle fowl (Gallus gallus murghi) spermatozoa. Avian. Biol. Res. 2016;9:207–212. [Google Scholar]
- Santiago-Moreno J., Castano C., Toledano-Diaz A., Coloma M.A., Lopez-Sebastian A., Prieto M.T., Campo J.L. Semen cryopreservation for the creation of a spanish poultry breeds cryobank: Optimization of freezing rate and equilibration time. Poult. Sci. 2011;90:2047–2053. doi: 10.3382/ps.2011-01355. [DOI] [PubMed] [Google Scholar]
- Sexton T.J., Jacobs L.A., McDaniel G.R. A new poultry semen extender. 4. Effect of antibacterials in control of bacterial contamination in chicken semen. Poult. Sci. 1980;59:274–281. doi: 10.3382/ps.0590274. [DOI] [PubMed] [Google Scholar]
- Tselutin K., Seigneurin F., Blesbois E. Comparison of cryoprotectants and methods of cryopreservation of fowl spermatozoa. Poult. Sci. 1999;78:586–590. doi: 10.1093/ps/78.4.586. [DOI] [PubMed] [Google Scholar]
- Tselutin K., Narubina L., Mavrodina T., Tur B. Cryopreservation of poultry semen. Br. Poult. Sci. 1995;36:805–811. doi: 10.1080/00071669508417825. [DOI] [PubMed] [Google Scholar]
- Zaniboni L., Cassinelli C., Mangiagalli M.G., Gliozzi T.M., Cerolini S. Pellet cryopreservation for chicken semen: Effects of sperm working concentration, cryoprotectant concentration, and equilibration time during in vitro processing. Theriogenology. 2014;82:251–258. doi: 10.1016/j.theriogenology.2014.04.007. [DOI] [PubMed] [Google Scholar]