Abstract
Antifreeze proteins (AFP) have the potential for improving sperm cryopreservation. We have applied Type III antifreeze protein (AFP3) on the cryopreservation of spermatozoa from broiler breeder roosters, aiming to enhance post-thawing quality and fertility. Semen was extended at 37°C in Lake's extender containing AFP3 at 0.01, 0.1, 1, 5, and 10 µg/mL (no AFP3 as control). Post-thawing sperm assessment included sperm motility (CASA), morphology, membrane functionality by hypoosmotic swelling test (HOST), lipoperoxidation as malondialdehyde (MDA) production, and sperm viability, early apoptosis (phosphatidylserine exposure as annexin V-positive staining in viable spermatozoa), and mitochondrial activity by flow cytometry. Fertility was assessed after artificial insemination (30 hens/treatment). Total and progressive motility, membrane functionality, and mitochondrial activity increased in 0.1 and 1 µg/mL AFP, compared to control and other concentrations, whereas apoptosis was significantly lower. VAP, VSL, and viability were significantly higher for 1 µg/mL AFP3 than with the other treatments except for 0.1 µg/mL (which was not always significantly different from the control or other concentrations), and with abnormal forms being significantly lower. The proportion of fertilized and hatched eggs was also higher for 1 µg/mL AFP3, with 0.1 µg/mL also showing significantly higher results than the control, and no differences with other concentrations). In conclusion, 1 µg/mL AFP3 could improve the post-thawing results of rooster spermatozoa frozen in Lake's extender. According to our results, concentrations between 1 and 0.1 µg/mL could be similarly efficient.
Key words: type III antifreeze protein; rooster, spermatozoon; cryopreservation
INTRODUCTION
Conserving genetic resources is critical for preserving the genetic diversity of economically relevant or endangered animals (Thélie et al., 2019). The impact of reproductive technologies in the poultry industry is already essential in some fields and spreading quickly (Purdy et al., 2009). Therefore, it is imperative to develop optimized protocols for improving artificial insemination efficiency, an important breeding technology for domestic species. In this regard, the primary efforts are dedicated to sperm cryopreservation, as it is the most effective method of preserving and applying avian spermatozoa (Donoghue and Wishart, 2000; Santiago-Moreno et al., 2011).
The avian spermatozoon have a relatively lower surface area to volume ratio and a thinner tail than the mammalian one. Therefore, they are more susceptible to chemical, thermal, and mechanical stresses during freezing (Donoghue and Wishart, 2000; Long, 2006). Freezing and thawing lead to irreversible damage to the sperm mitochondria and acrosome, and consequently, physiological processes (Long, 2006). Besides, during cryopreservation, sperm may lose ATP owing to energy metabolism, and glycoproteins or glycolipids required for transport and maturation (Long, 2006).
Many studies have investigated methods for decreasing sperm damage by proposing different extenders and freezing protocols. Whereas cryopreservation is the best method for long-term preservation of biological material, it involves complex processes, dealing with cooling rates, sample volume, and cryoprotectants choice (e.g., dimethyl sulfoxide DMSO or glycerol), all relevant in the success of the cryopreservation process (Baust et al., 2017). Some reports have shown that the cryodamage induced by freezing and thawing can be minimized by adding lipoproteins or optimizing the cooling rate and cryoprotectant (Wu et al., 2013). Corcuera et al. (2007) highlighted the importance of nonpermeable and permeable cryoprotectants, a combination that is commonly used. According to these authors, nonpermeable cryoprotectants, such as sugars, protect the cell membrane from volume changes upon freezing and thawing. In contrast, permeable cryoprotectants, such as glycerol, effectively reduce the intracellular water freezing point and consequently intracellular ice crystal formation (Silva et al., 2015).
In the search for more efficient cryopreservation protocols, some authors have proposed the use of antifreeze proteins (AFP). Different organisms produce antifreeze proteins and glycoproteins (AFGP) to survive in cold conditions (Crevel et al., 2002; Robles et al., 2019). These proteins prevent the growth of ice crystals in cold temperatures, therefore protecting the cells and tissues. Also, some organisms might allow ice formation, with AFP inhibiting ice recrystallization during thawing (Hasan et al., 2018). AFP can also protect the structural integrity of the cell membrane (Eskandari et al., 2020). One of them, Type III AFP (AFP3), is a globular AFP of 7 kDa present in members of the subclass Zoarcoidei, a group of polar fish (Antson et al., 2001; Salvay et al., 2010). There are some reports that AFP3 could be effective for freezing spermatozoa. For instance, 1 µg/mL AFP3 improved the post-thawing quality of Japanese white rabbit spermatozoa (Nishijima et al., 2014), and the progressive motility and plasma membrane integrity of cryopreserved buffalo spermatozoa improved using 0.1 µg/mL AFP3. In contrast, higher concentrations were inefficient (Qadeer et al., 2015).
Whereas AFPs have been tested for cryopreservation of spermatozoa of some mammal and fish species (Qadeer et al., 2014; Xin et al., 2018b; Correia et al., 2021), there are no studies on poultry. We aim to improve rooster sperm cryopreservation, therefore increasing fertility rates with thawed semen. Since AFP3 seems promising in other species, we hypothesize that its use as a supplement could enhance the results of current cryopreservation protocols. It could be an incremental step in the poultry industry, and if successful, its use might be applied to other commercial species and used within protection programs for endangered avian species.
The objective of this study was to determine the effect of the antifreeze protein AFP3 in both the post-thawing quality and fertility of rooster spermatozoa by evaluating a range of concentrations in the semen extender.
MATERIALS AND METHODS
Chemicals and Reagents
Chemicals were purchased from Merck (Darmstadt, Germany) unless otherwise specified.
Animal Management and Semen Samples
The experimental semen samples were obtained from 15 Ross 308 breeder roosters (age = 30 wk). Previously, the Animal Welfare Committee of the Department of Animal Science, University of Tabriz, reviewed and approved the experimental plan. Semen was collected twice a week by abdominal massage (Najafi et al., 2020a). The samples were transferred to the laboratory in a water bath (37°C) within 5 min after ejaculation and assessed by routine quality check. Samples were accepted for the study following criteria: >80% total motility, >3 × 109 sperm cells/mL concentration, and ≥90% normal morphology. The semen samples from the 15 roosters were mixed to eliminate the individual male effect. For the experiment, 7 ejaculates were collected (twice a week), pooling them in each collection.
Cryopreservation Procedure
We tested 6 experimental treatments, in which the semen extender was supplemented with different levels of AFP3: 0.01, 0.1, 1, 5, and 10 µg/mL, and control (no AFP3). The semen pool was split and carefully diluted at 37°C with Lake extender (3.74 g/L glycine, 0.7 g/L magnesium acetate, 5 g/L potassium citrate, 19.2 g/L sodium glutamate, 8 g/L D-fructose and 3 g/L polyvinylpyrrolidone) supplemented with each of the AFP3 concentrations (control with no supplementation). Glycerol was added to the base extender at 3.8% (v/v). The 6 aliquots of extended semen were gradually cooled at 4°C for 3 h. The samples were then loaded into 0.25 mL straws (IMV, L'Aigle, France) (final concentration of 100 × 106 spermatozoa/mL), sealing with polyvinyl alcohol. The straws were frozen in liquid nitrogen vapors, 7 min at 4 cm above the liquid nitrogen, and finally plunged into liquid nitrogen. After 4 wk of storage, the samples were taken from LN2 and thawed in a water bath at 37°C for 30 s for analysis and AI.
Sperm Motility
Sperm motility and velocity parameters were determined by computer-aided sperm analysis (CASA) on samples prediluted at 20 × 106 mL with PBS and 37°C. The sample was loaded into prewarmed Leja chambers (depth 20 µm; Leja Products, Luzernestraat B.V., Holland) and evaluated by a CEROS v. 12.3 system (Hamilton-Thorne Biosciences, Beverly, MA). At least 200 spermatozoa in 5 microscopic fields were assessed at a magnification of × 10. The evaluated parameters were: Total and progressive motility (%), linearity (LIN, VSL/VCL, %), the amplitude of the lateral head displacement (ALH, µm), straightness (STR, VSL/VAP, %), curvilinear velocity (VCL, µm/s), average path velocity (VAP, µm/s), and straight-line velocity (VSL, µm/s).
Morphology
For evaluating abnormal forms, spermatozoa were first fixed with Hancock solution Najafi et al. (2020b) (Hancock's solution consisted of 426 mM sodium, 21.4 mM formalin (37%), 304.29 mM Na2HPO4, and 99.42 mM K2HPO4). Fifteen microliters of the semen sample were mixed with 300 µL of Hancock solution. One drop of the mixture was observed at × 400 phase contrast (Labomed LX400; Labomed Inc., Culver City, CA) by slide and coverslip. At least 200 sperm were assessed and the number of abnormal spermatozoa was recorded.
Hypo-Osmotic Swelling Test (HOST)
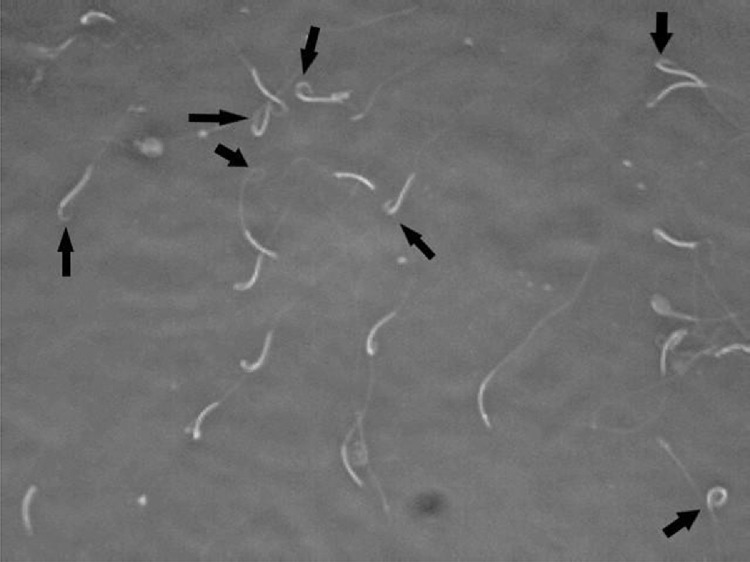
Hypo-osmotic swelling test (HOS) was selected to examine membrane functionally (Mehdipour et al., 2020b). Ten microliters of semen were added to 100 μL of hypoosmotic solution (100 mOsm/kg; sodium citrate 1.9 mM, fructose 5.0 mM), mixed incubated at 37°C for 30 min. A drop of the mixture was observed at × 400 phase contrast (Labomed LX400; Labomed Inc., Culver City, CA) by slide and coverslip. Two hundred spermatozoa were counted in 5 microscopic fields. For recording the membrane functionally of the cells, the percentages of the cells having swollen and curled tails were defined as sperm with a functional plasma membrane (Figure1).
Figure 1.
Examples of spermatozoa as evaluated for the hypo-osmotic swelling (HOS) test for membrane functionality assessment. The swollen spermatozoa are shown by arrow.
Flow Cytometer Configuration
A FACSCalibur (Becton Dickinson, San Jose, CA) with an argon-ion laser at 488 nm was used to conduct the flow cytometry analysis. A forward/side-scatter gate gated sperm cells from debris. Green fluorescence (Rhodamine 123 and Annexin V-FITC) was detected using a band-pass filter (530/30 nm) at the FL-1 detector, and red fluorescence (PI) was detected using a long-pass filter (610 nm) at the FL-3 detector. Acquisition and data analysis were carried out using the CellQuest software v. 3.3 (Becton Dickinson, San Jose, CA). At least 10,000 spermatozoa per sample were measured.
Assessment of Phosphatidylserine Externalization by Annexin V
The determination of phosphatidylserine (PS) externalization was carried out by annexin V binding (Mehdipour et al., 2020a) with the Phosphatidylserine Detection Kit (Immune Quality Products (IQP), Groningen, The Netherlands). The semen sample was diluted in a calcium solution at 1 × 106 mL, adding 10 µL of Annexin V-FITC (AV) solution (stock solution at 0.01 mg/mL). The mixture was incubated at room temperature (25°C) in the dark for 15 min. Before analysis, 10 µL of PI (1 mg/mL stock) was added as a viability counterstain, incubating for 10 min more. Samples were examined by flow cytometry, evaluating the populations of alive (AV–PI–), apoptotic (AV+PI–), and dead spermatozoa (PI+).
Mitochondrial Activity Assessment
The percentage of sperm with active mitochondria was examined by rhodamine-123 (R123) and PI following Mehdipour et al. (2020a). Three hundred microliter of diluted semen were mixed with R123 (10 μL at 0.01 mg/mL; Thermo Fisher Scientific, Waltham, MA) and PI (10 μL at 1 mg/mL), incubating at 37°C for 15 min in the dark. R123+/PI– spermatozoa were considered viable with active mitochondria.
In Vivo Fertility Assessment
The frozen-thawed spermatozoa were tested for fertility by inseminating layer hens. A total of 180 hens were randomly allocated to 6 groups of 30 hens for each of the experimental treatments. The hens were inseminated with the contents of one straw each (0.25 mL semen/hen, 100 × 106 spermatozoa/hen), all at the same hour of the day, 15:00. The egg collection was carried out up to 5 d after the last artificial insemination. For each group, 200 eggs were considered for incubation within 2 wk. After egg collection, they were set on turning trays and then disinfected for 15 min. Next, the eggs were placed in a standard incubator for 18 d at 37.7°C. For the last 3 d of incubation, the eggs were moved to the hatcher. The fertility evaluation was performed by candling the eggs on d 7. The hatchability was evaluated after 21 d of incubation.
Statistical Analysis
Sperm quality data were analyzed in the R statistical environment by linear mixed-effects models. The AFP3 treatments factor was considered as a fixed effect, with the replicate as the grouping factor of the random part of the model. Unless otherwise indicated, the results are expressed as mean±95%CI. The fertility data (fertilized and hatched eggs and hatchability as hatched vs. fertilized) were analyzed by general linear models as binomial, logit function link. The signification threshold was established at P ≤ 0.05.
RESULTS
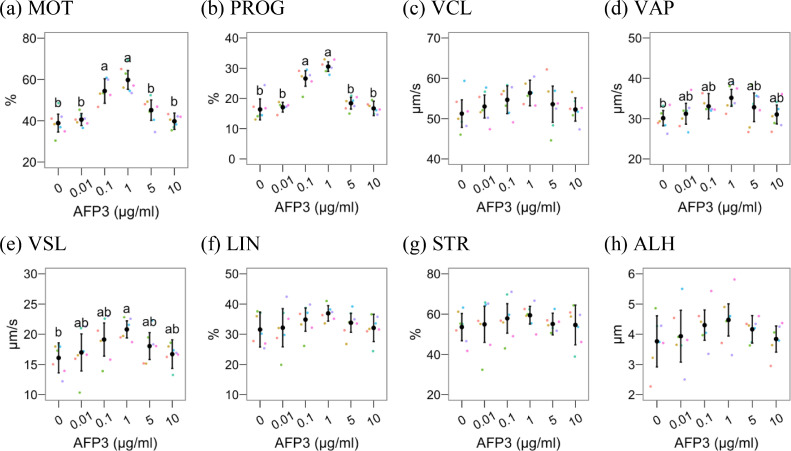
Figure 2, Figure 3 show the results of AFP3 supplementation on post-thawing quality. Considering CASA results (Figure 2), total and progressive motility (Figures 2A and 2B) were higher (P < 0.05) at 0.1 and 1 µg/mL AFP3 compared to control and the other groups. VAP and VSL (Figures 2D and 2E) were significantly higher in 1 µg/mL AFP3 compared to the control (P < 0.05), whereas the other concentrations significantly differed neither with the control nor 1 µg/mL. The AFP3 supplementation did not significantly affect the other motility parameters, including VCL, LIN, STR, and ALH.
Figure 2.
Results for CASA parameters after cryopreserving rooster semen with different Type III AFP concentrations. The figures show mean ± 95% CI for control (0 µg/mL) and Type III AFP treatments, with observations as dots. Treatments with different Latin lowercase letters differ P ≤ 0.05. Abbreviations: ALH, mean amplitude of the lateral head displacement (µm); BCF, mean of the beat cross frequency (Hz); LIN, linearity (%); STR, straightness (%); VAP, average path velocity (µm/s); VCL, curvilinear velocity (µm/s); VSL, straight-line velocity (µm/s); WOB, Wobble (%);.
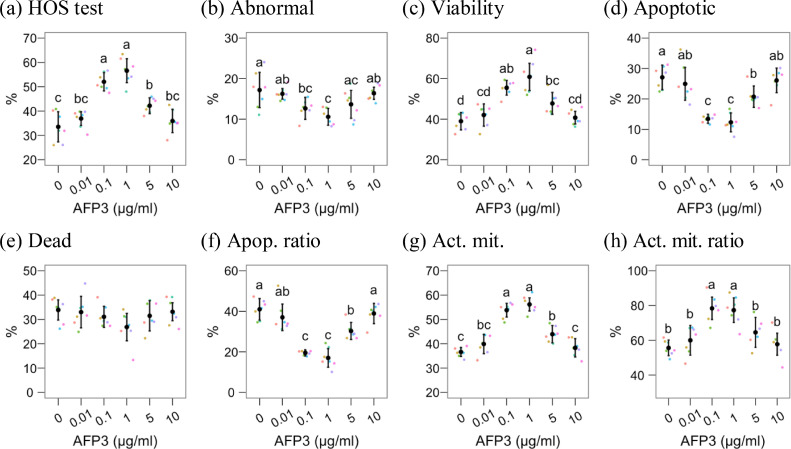
Figure 3.
Results for HOS test, abnormal forms, and physiological parameters assessed by flow cytometry after cryopreserving rooster semen with different Type III AFP concentrations. The figures show mean ± 95% CI for control (0 µg/mL) and Type III AFP treatments, with observations as dots. Treatments with different Latin lowercase letters differ P ≤ 0.05. The ratio refers to the proportion of positive spermatozoa within the viable (PI–) population. Abbreviations: Act. mit., active mitochondria; Apop. ratio, apoptotic ratio.
Membrane functionality (HOST, Figure 3A), active mitochondria, and active mitochondria ratio (Figures 3G and 3H) were significantly higher in 0.1 and 1 µg/mL AFP3, compared to the control and the other treatments. These treatments also yielded the lowest proportion of apoptotic spermatozoa (Figure 3D) and the apoptotic ratio (Figure 3F), comparing with the other treatments and the control (P < 0.05).
The 1 µg/mL AFP3 treatment also significantly achieved the lowest proportion of abnormal forms (Figure 3B) and the highest proportion of viable spermatozoa (Figure 3C), compared to the control and any other treatment except 0.1 µg/mL. This treatment showed a lower performance for these parameters, significantly different from the control, but not significantly different from 0.01, 5, and 10 µg/mL for abnormal forms and 5 µg/mL for viability.
The reproductive performance of the semen samples is presented in Table 1. Samples supplemented with 1 µg/mL AFP3 achieved the highest results for the 3 parameters (fertilized eggs, hatched eggs, and hatchability), significantly higher than the control and any other concentration, except 0.1 µg/mL for the number of fertilized eggs and hatched eggs. The 0.1 µg/mL AFP3 achieved similar results and was significantly superior to the control. However, it was not significantly different from the other concentrations for fertilized eggs and only significantly higher than 10 µg/mL for hatched eggs. None of the other concentrations were significantly different from the control.
Table 1.
Fertility results after cryopreserving rooster semen with different Type III AFP concentrations.
| Treatments (µg/mL Type III AFP) | Fertilized eggs | Hatched eggs | Hatched eggs ratio (hatched/fertilized, %) |
|---|---|---|---|
| 0 (control) | 84 (42)c | 49 (24.5)c | 58.3 |
| 0.01 | 87 (44)bc | 53 (27)bc | 62.1 |
| 0.1 | 114 (57)ab | 79 (39.5)ab | 69.3 |
| 1 | 123 (61.5)a | 93 (46.5)a | 75.6 |
| 5 | 90 (45)bc | 58 (29)bc | 64.4 |
| 10 | 86 (43)bc | 51 (25.5)c | 59.3 |
Each experimental group contained 200 eggs initially (n = 200). Numbers are absolute counts of eggs, with percentages (ratio respect to the initial egg count) between parentheses, except for the hatched eggs ratio. a-cDifferent superscripts within the same column indicate significant differences among groups (P < 0.05).
DISCUSSION
This study considered the effects of Type III AFP (AFP3) on rooster sperm cryopreservation, focusing on post-thaw quality and in vivo fertility. Whereas AFPs have been tested in some mammals and fish species for improving sperm cryopreservation, their use on bird species is still novel. Several conditions during the freezing process have been proposed as the fundamental causes of low sperm quality after thawing, including abrupt changes of temperature, ice formation, and osmotic stress (Robles et al., 2019). This is why AFPs have been applied as cryoprotectants in the semen extender to mitigate this damage (Nishijima et al., 2014), and therefore they are promising for improving sperm cryopreservation in avian species.
Our results showed that AFP3 improved sperm motility and other characteristics at 0.1 and 1 µg/mL. We hypothesize that the protective effect of AFP3 on the plasma membrane integrity can be due to its ice recrystallization inhibition. AFPs bind to tiny ice crystals and prevent the formation of large and lethal ice crystals and possibly their recrystallization in critical phases of freezing and thawing. AFPs have some significant properties that could explain their protective roles, including thermal hysteresis and ice recrystallization inhibition and their ability to interact with biological membranes. The supplementation with AFPs might also enable a lower freezing temperature, and these proteins could associate with the cell plasma membranes, protecting them from cryoinjury (Robles et al., 2019). Xin et al. (2018a) confirmed that the plasma membrane integrity of frozen-thawed sterlet sperm was greatly enhanced by antifreeze proteins (AFP I and AFP III).
In our experiment, we found that AFP III not only preserved membrane integrity and functionality but also decreased phosphatidylserine externalization and lipid peroxidation. The sperm plasma membrane contains many polyunsaturated fatty acids (PUFAs), with roles in membrane fluidity and permeability (Lee et al., 2020). One of the adverse effects of the freezing-thawing process is the lowering in the relative content of PUFAs, partly because of lipid peroxidation (Cerolini et al., 2001). This reduction in PUFAs and the concomitant enhancement in membrane saturation reduces membrane fluidity and weakens its structural integrity (Chakrabarty et al., 2007). AFPs might protect the plasma membrane not only by preventing direct ice damage but also by interacting with the phospholipids, preventing the peroxidation of PUFAs and the increase in the saturated fatty acid proportion (Beirao et al., 2012). Whereas we need specific analyses to confirm this hypothesis (evaluation of the lipidic content), the improvement of membrane status in the present study could be attributed to these effects. Other studies have also reported positive effects of AFPs and AFGP, and specifically of AFP3, on sperm membranes, as in human (Zandiyeh et al., 2020).
Moreover, part of the AFP3 effects on sperm motility could be its interaction with ion channels, stabilizing transmembrane electrolyte gradients. Indeed, other authors showed protective or stimulating effects on sperm motility. Shaliutina-Kolesova et al. (2019) demonstrated that supplementing the extender with Type I and III AFP (0.1 μg/mL, 1 μg/mL, and 10 μg/mL) had positive effects on the post-thawing motility and velocity of common carp, especially with AFP3. Similar results have been obtained in sturgeon at 10 µM (Abed-Elmdoust et al., 2017) and buffalo at 0.1 µg/mL (Qadeer et al., 2014). However, Correia et al. (2021) showed that ram semen samples diluted with AFP III had a lower percentage of motile spermatozoa, while AFP I enhanced the percentage of sperm with plasma membrane integrity. This inconsistency is probably related to the species differences and maybe interactions with the freezing protocols.
In the current study, AFP3 also improved mitochondrial activity. Cryopreservation also causes mitochondrial malfunction and oxidative stress, and part of the preventive effect on lipid peroxidation could be due to mitoprotection (either directly or by preventing ice damage in the midpiece). Mitochondrial damage reduces available ATP (Park and Pang, 2021), and electron leaking from the electron transport chain increases the production of reactive oxygen species (ROS) and other oxidative molecules (Chianese and Pierantoni, 2021). In the same line, ROS and mitochondrial damage could lead to the activation of cellular apoptosis. The lower ratio of apoptotic membrane changes (externalization of phosphatidylserine) in our study with 0.1 and 1 µg/mL AFP3 could have resulted from a mitoprotective effect. This hypothesis is supported by studies in other cell types showing similar results on reducing apoptotic cell death (Amir et al., 2005). Future studies assessing mitochondrial status, ROS production, and the ultrastructure of the membranes and mitochondria by electron microscopy could determine the specific mechanism of AFP3 mitochondrial protection and other results obtained in the present work.
The present study was designed with the practical aim of improving the results of AI with rooster cryopreserved spermatozoa. Therefore, we included an AI trial with the AFP3 treatments. Our results followed the quality analyses, with 1 µg/mL of AFP III leading to a higher ratio of fertilized and hatched eggs. In this regard, few studies have considered the effects of AFPs on sperm fertility, and the results are not conclusive. For instance, Qadeer et al. (2016) obtained a higher in vitro cleavage ratio for Nili-Ravi buffalo (Bubalus bubalis) spermatozoa after in vitro insemination with spermatozoa frozen with DAFPs (recombinant AFP from the beetle Dendroides canadensis). However, neither in vitro nor in vivo results were significant in that study. However, in fish, Pacific abalone spermatozoa frozen with 10 µg/mL AFP3 showed a significant improvement in fertilization and hatching rates (Hossen et al., 2021).
However, the comparison with birds regarding sperm fertility is complex due to the differences in mating and reproductive tracts. Birds have sperm storage tubules (SSTs) in the female reproductive tract, and sperm entering these sites can persist for extended periods (Sasanami et al., 2013), with differences among species. This mechanism enables a female to lay fertilized eggs after single artificial insemination for a prolonged time. Sperm motility and viability are critical for transport into SSTs (Froman, 2003), and the greater motility and protection of the plasma membrane of spermatozoa by AFP III might improve the entry and storage in SSTs and provide greater survival during the storage time. Therefore, we can hypothesize that the higher sperm quality (also, better mitochondrial status and lower apoptotic features) could enable rooster thawed spermatozoa for improved colonization and lifespan in the female reproductive tract, resulting in a higher ratio of fertilized and ultimately hatched eggs.
CONCLUSIONS
AFP III in Ross 308 breeder roosters may benefit sperm cryopreservation, considering post-thawing sperm quality and fertility parameters. Specifically, we have shown that a 1 µg/mL AFP III was the most appropriate concentration, with an optimal concentration potentially between 0.1 and 1 µg/mL.
Acknowledgments
ACKNOWLEDGMENTS
This research was supported by a research grant from the University of Tabriz (number 5782).
DISCLOSURES
None of the authors have any conflict of interest to declare.
REFERENCES
- Abed-Elmdoust A., Farahmand H., Mojazi-Amiri B., Rafiee G., Rahimi R. Novel droplet vitrification combined with fish antifreeze protein type III enhances cryoprotection of semen in wild endangered Persian sturgeon Acipenser persicus (Borodin, 1897) Aquac. Res. 2015;46:2392–2397. [Google Scholar]
- Amir G., Rubinsky B., Basheer S.Y., Horowitz L., Jonathan L., Feinberg M.S., Smolinsky A.K., Lavee J. Improved viability and reduced apoptosis in sub-zero 21-hour preservation of transplanted rat hearts using antifreeze proteins. J. Hear. lung Transplant. 2005;24:1915–1929. doi: 10.1016/j.healun.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Antson A.A., Smith D.J., Roper D.I., Lewis S., Caves L.S.D., Verma C.S., Buckley S.L., Lillford P.J., Hubbard R.E. Understanding the mechanism of ice binding by type III antifreeze proteins. J. Mol. Biol. 2001;305:875–889. doi: 10.1006/jmbi.2000.4336. [DOI] [PubMed] [Google Scholar]
- Baust J.M., Campbell L.H., Harbell J.W. Best practices for cryopreserving, thawing, recovering, and assessing cells. Vitr. Cell. Dev. Biol. 2017;53:855–871. doi: 10.1007/s11626-017-0201-y. [DOI] [PubMed] [Google Scholar]
- Beirao J., Zilli L., Vilella S., Cabrita E., Schiavone R., Herraez M.P. Improving sperm cryopreservation with antifreeze proteins: effect on gilthead seabream (Sparus aurata) plasma membrane lipids. Biol. Reprod. 2012;86:59. doi: 10.1095/biolreprod.111.093401. [DOI] [PubMed] [Google Scholar]
- Cerolini S., Maldjian A., Pizzi F., Gliozzi T.M. Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction. 2001;121:395–401. [PubMed] [Google Scholar]
- Chakrabarty J., Banerjee D., Pal D., De J., Ghosh A., Majumder G.C. Shedding off specific lipid constituents from sperm cell membrane during cryopreservation. Cryobiology. 2007;54:27–35. doi: 10.1016/j.cryobiol.2006.10.191. [DOI] [PubMed] [Google Scholar]
- Chianese R., Pierantoni R. Mitochondrial reactive oxygen species (ROS) production alters sperm quality. Antioxidants. 2021;10:92. doi: 10.3390/antiox10010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcuera B.D., Marigorta P., Sagüés A., Saiz-Cidoncha F., Pérez-Gutiérrez J.F. Effect of lactose and glycerol on the motility, normal apical ridge, chromatin condensation and chromatin stability of frozen boar spermatozoa. Theriogenology. 2007;67:1150–1157. doi: 10.1016/j.theriogenology.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Correia L.F.L., Espirito-Santo C.G., Braga R.F., Carvalho-de-Paula C.J., da Silva A.A., Brandao F.Z., Freitas V.J.F., Ungerfeld R., Souza-Fabjan J.M.G. Addition of antifreeze protein type I or III to extenders for ram sperm cryopreservation. Cryobiology. 2021;98:194–200. doi: 10.1016/j.cryobiol.2020.11.001. [DOI] [PubMed] [Google Scholar]
- Crevel R.W.R., Fedyk J.K., Spurgeon M.J. Antifreeze proteins: characteristics, occurrence and human exposure. Food Chem. Toxicol. 2002;40:899–903. doi: 10.1016/s0278-6915(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Donoghue A.M., Wishart G.J. Storage of poultry semen. Anim. Reprod. Sci. 2000;62:213–232. doi: 10.1016/s0378-4320(00)00160-3. [DOI] [PubMed] [Google Scholar]
- Eskandari A., Leow T.C., Rahman M.B.A., Oslan S.N. Antifreeze proteins and their practical utilization in industry. Med. Agricul. Biomol. 2020;10:1649. doi: 10.3390/biom10121649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froman D. Deduction of a model for sperm storage in the oviduct of the domestic fowl (Gallus domesticus) Biol. Reprod. 2003;69:248–253. doi: 10.1095/biolreprod.102.013482. [DOI] [PubMed] [Google Scholar]
- Hasan M., Fayter A.E.R., Gibson M.I. Ice recrystallization inhibiting polymers enable glycerol-free cryopreservation of microorganisms. Biomacromolecules. 2018;19:3371–3376. doi: 10.1021/acs.biomac.8b00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen S., Sharker M.R., Cho Y., Sukhan Z.P., Kho K.H. Effects of antifreeze protein III on sperm cryopreservation of pacific abalone, haliotis discus hannai. Int. J. Mol. Sci. 2021;22:3917. doi: 10.3390/ijms22083917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kim Y., Kang B.H., Yun Y.S., Park C. The relationship between acrosome reaction and polyunsaturated fatty acid composition in boar sperm. Reprod. Domest. Anim. 2020;55:624–631. doi: 10.1111/rda.13661. [DOI] [PubMed] [Google Scholar]
- Long J.A. Avian semen cryopreservation: what are the biological challenges? Poult. Sci. 2006;85:232–236. doi: 10.1093/ps/85.2.232. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Kia H.D., Martínez-Pastor F. Poloxamer 188 exerts a cryoprotective effect on rooster sperm and allows decreasing glycerol concentration in the freezing extender. Poult. Sci. 2020;99:6212–6220. doi: 10.1016/j.psj.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh Kia H., Najafi A., Mohammadi H., Alvarez-Rodriguez M. Effect of crocin and naringenin supplementation in cryopreservation medium on post-thaw rooster sperm quality and expression of apoptosis associated genes. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi A., Kia H.D., Mehdipour M., Hamishehkar H., Álvarez-Rodríguez M. Effect of quercetin loaded liposomes or nanostructured lipid carrier (NLC) on post-thawed sperm quality and fertility of rooster sperm. Theriogenology. 2020;152:122–128. doi: 10.1016/j.theriogenology.2020.04.033. [DOI] [PubMed] [Google Scholar]
- Najafi D., Taheri R.A., Najafi A., Shamsollahi M., Alvarez-Rodriguez M. Effect of astaxanthin nanoparticles in protecting the post-thawing quality of rooster sperm challenged by cadmium administration. Poult. Sci. 2020;99:1678–1686. doi: 10.1016/j.psj.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijima K., Tanaka M., Sakai Y., Koshimoto C., Morimoto M., Watanabe T., Fan J., Kitajima S. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology. 2014;69:22–25. doi: 10.1016/j.cryobiol.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Park Y.-J., Pang M.-G. Mitochondrial functionality in male fertility: from spermatogenesis to fertilization. Antioxidants. 2021;10:98. doi: 10.3390/antiox10010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy P.H., Song Y., Silversides F.G., Blackburn H.D. Evaluation of glycerol removal techniques, cryoprotectants, and insemination methods for cryopreserving rooster sperm with implications of regeneration of breed or line or both. Poult. Sci. 2009;88:2184–2191. doi: 10.3382/ps.2008-00402. [DOI] [PubMed] [Google Scholar]
- Qadeer S., Khan M.A., Ansari M.S., Rakha B.A., Ejaz R., Husna A.U., Ashiq M., Iqbal R., Ullah N., Akhter S. Evaluation of antifreeze protein III for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2014;148:26–31. doi: 10.1016/j.anireprosci.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Qadeer S., Khan M.A., Ansari M.S., Rakha B.A., Ejaz R., Iqbal R., Younis M., Ullah N., DeVries A.L., Akhter S. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015;157:56–62. doi: 10.1016/j.anireprosci.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Qadeer S., Khan M.A., Shahzad Q., Azam A., Ansari M.S., Rakha B.A., Ejaz R., Husna A.U., Duman J.G., Akhter S. Efficiency of beetle (Dendroides canadensis) recombinant antifreeze protein for buffalo semen freezability and fertility. Theriogenology. 2016;86:1662–1669. doi: 10.1016/j.theriogenology.2016.05.028. [DOI] [PubMed] [Google Scholar]
- Robles V., Valcarce D.G, Riesco M.F. The use of antifreeze proteins in the cryopreservation of gametes and embryos. Biomolecules. 2019;9:181. doi: 10.3390/biom9050181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvay A.G., Gabel F., Pucci B., Santos J., Howard E.I., Ebel C. Structure and interactions of fish type III antifreeze protein in solution. Biophys. J. 2010;99:609–618. doi: 10.1016/j.bpj.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Moreno J., Castaño C., Toledano-Díaz A., Coloma M., López-Sebastián A., Prieto M., Campo J. Semen cryopreservation for the creation of a Spanish poultry breeds cryobank: optimization of freezing rate and equilibration time. Poult. Sci. 2011;90:2047–2053. doi: 10.3382/ps.2011-01355. [DOI] [PubMed] [Google Scholar]
- Sasanami T., Matsuzaki M., Mizushima S., Hiyama G. Sperm storage in the female reproductive tract in birds. J. Reprod. Dev. 2013;59:334–338. doi: 10.1262/jrd.2013-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaliutina-Kolesova A., Dietrich M., Xian M., Nian R. Seminal plasma transferrin effects on cryopreserved common carp Cyprinus carpio sperm and comparison with bovine serum albumin and antifreeze proteins. Anim. Reprod. Sci. 2019;204:125–130. doi: 10.1016/j.anireprosci.2019.03.013. [DOI] [PubMed] [Google Scholar]
- Silva C.G., Cunha E.R., Blume G.R., Malaquias J.V, Bao S.N., Martins C.F. Cryopreservation of boar sperm comparing different cryoprotectants associated in media based on powdered coconut water, lactose and trehalose. Cryobiology. 2015;70:90–94. doi: 10.1016/j.cryobiol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Thélie A., Bailliard A., Seigneurin F., Zerjal T., Tixier-Boichard M., Blesbois E. Chicken semen cryopreservation and use for the restoration of rare genetic resources. Poult. Sci. 2019;98:447–455. doi: 10.3382/ps/pey360. [DOI] [PubMed] [Google Scholar]
- Wu T., Cheng F., Chen I., Yang C., Tsai M., Chang M., Wang J., Wu J. The combinatorial effect of different equex STM paste concentrations, cryoprotectants and the straw-freezing methods on the post-thaw boar semen quality. Reprod. Domest. Anim. 2013;48:53–58. doi: 10.1111/j.1439-0531.2012.02022.x. [DOI] [PubMed] [Google Scholar]
- Xin M., Sterba J., Shaliutina-Kolesova A., Dzyuba B., Lieskovska J., Boryshpolets S., Siddique M.A.M., Kholodnyy V., Lebeda I., Linhart O. Protective role of antifreeze proteins on sterlet (Acipenser ruthenus) sperm during cryopreservation. Fish Physiol. Biochem. 2018;44:1527–1533. doi: 10.1007/s10695-018-0538-5. [DOI] [PubMed] [Google Scholar]
- Xin M., Tuckova V., Rodina M., Kholodnyy V., Dadras H., Boryshpolets S., Shaliutina-Kolesova A., Linhart O. Effects of antifreeze proteins on cryopreserved sterlet (Acipenser ruthenus) sperm motility variables and fertilization capacity. Animal Reprod. Sci. 2018;196:143–149. doi: 10.1016/j.anireprosci.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Zandiyeh S., Shahverdi A., Ebrahimi B., Sabbaghian M. A novel approach for human sperm cryopreservation with AFPIII. Reprod. Biol. 2020;20:169–174. doi: 10.1016/j.repbio.2020.03.006. [DOI] [PubMed] [Google Scholar]