Abstract
Coccidioidomycosis is a common fungal infection in people living with HIV-1, particularly in southwest regions of the United States where the Coccidioides sp. is endemic, but rates of infection have significantly declined in the era of potent combination antiretroviral therapy (cART). Natural coccidioidomycosis also occurs in outdoor-housed macaques residing in the southwestern states that are utilized in biomedical research. Here, we report on a recrudescent case of previously treated, naturally occurring coccidioidomycosis in a pigtail macaque that was experimentally infected with simian immunodeficiency virus (SIV) and virally suppressed on cART. Coccidioides IgG antibody titer became detectable before discontinuation of cART, but symptomatic coccidioidomycosis developed subsequent to cART withdrawal. This animal was screened and treated in accordance with the guidelines for the prevention and treatment of coccidioidomycosis, suggesting that macaques with a history of coccidioidomycosis should be excluded from enrollment in HIV studies. Continual monitoring for known endemic pathogens based on the colony of origin is also recommended for animals utilized for HIV/AIDS research.
Keywords: coccidioidomycosis, pigtail macaque, combination antiretroviral therapy (cART), simian immunodeficiency virus (SIV)
Coccidioidomycosis is caused by a soil-dwelling fungus and is highly endemic in the southwest regions of the United States. Most human coccidioidomycosis cases result in pneumonia, and account for up to 30% of community-acquired pneumonia in endemic areas.1,2 Symptomatic coccidioidomycosis is more common in people living with HIV (PLWH) if there is progression to AIDS, productive HIV viral replication, and/or depletion of CD4 T lymphocyte counts below 250 cells/μL of blood.2,3 However, potent combination antiretroviral therapy (cART) has significantly reduced the incidence of coccidioidomycosis in PLWH.3
Coccidioidomycosis has also been documented in outdoor-housed macaques utilized for biomedical research in the southwestern states.4 The Washington National Primate Research Center's (WaNPRC) breeding colony in Mesa, Arizona, which houses pigtail macaques (PTM) (Macaca nemestrina) in a mixed indoor/outdoor setting, reported a prevalence of 12% in 2017 (personal communication C.M.M.). Notably, the PTM model of HIV has gained popularity because aspects of simian immunodeficiency virus (SIV) infection in this species more closely resemble that of humans infected with HIV-1, compared with other species of macaques.5 To date, coccidioidomycosis in SIV-infected macaques has not been described. Here, we report recurrence of previously treated, naturally occurring coccidioidomycosis in a PTM that was experimentally infected with SIV and virally suppressed on cART. Coccidioidomycosis IgG titer became detectable before discontinuation of cART, but symptomatic coccidioidomycosis developed subsequent to cART withdrawal.
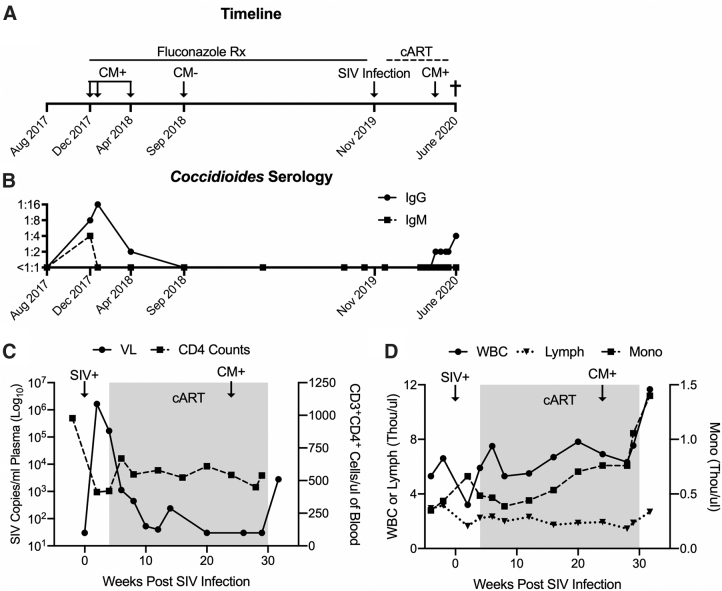
A 5-year-old intact male PTM was enrolled in a study evaluating a novel hepatitis B virus vaccine for use in PLWH. All experimental procedures performed on this macaque were approved by the University of Washington IACUC. In December 2017, 2 years before study enrollment, the macaque presented with coughing while at the WaNPRC Arizona colony. A diagnosis of coccidioidomycosis was based upon positive IgM (1:4) and IgG (1:8) antibody titers (ProtaTek Reference Lab, Mesa, AZ). As per standard protocol at the WaNPRC, and in accordance with the prevention and treatment guidelines for coccidioidomycosis,2 long-term fluconazole therapy (10 mg/kg once daily; Zydus Pharmaceuticals, Pennington, NJ) was initiated until the animal was confirmed to have at least three negative IgG/IgM antibody titers over a 1-year period. Coughing resolved within 1 month of treatment, and follow-up titers indicated decreasing IgG and IgM antibody titers that were undetectable by September 2018, with a final negative titer in September 2019 (Fig. 1A, B).
FIG. 1.
Re-emergence of naturally acquired coccidioidomycosis (CM) in an SIV virally-suppressed, immunologically intact PTM. (A) Time line of PTM indicating transient detection of natural CM, fluconazole treatment (Rx), and experimental SIVmac239M infection with subsequent cART. CM recrudescence led to clinically required euthanasia in June 2020. (B) IgG and IgM Coccidioides antibody titers measured by endpoint ELISA in blood plasma. (C) Plasma SIV VL measured by RT-qPCR (limit of detection is 30 copies/mL of plasma), and CD3+CD4+ cell counts measured via flow cytometry. (D) Clinically relevant CBC analytes following SIV infection; WBC, lymphocytes (Lymph), and monocytes (Mono) measured in whole blood by Beckman Coulter® AC*T™ 5 Diff Hematology Analyzer. (C–D) Shaded regions indicate period of cART. cART, combination antiretroviral therapy; CM, coccidioidomycosis; ELISA, enzyme-linked immunosorbent assay; PTM, pigtail macaque; RT-qPCR, real-time qualitative polymerase chain reaction; SIV, simian immunodeficiency virus; VL, viral load; WBC, white blood cell.
Three months before study enrollment, the macaque was relocated to the WaNPRC Seattle site, an AAALAC international-accredited institution, and maintained in a strictly indoor setting. The macaque was intravenously inoculated with 10,000 infectious units of SIVmac239M6 in November 2019. Daily cART consisting of 5.1 mg/kg tenofovir disoproxil fumarate (Gilead Sciences, Foster City, CA), 2.5 mg/kg dolutegravir (ViiV Healthcare, Research Triangle Park, NC), and 30 mg/kg emtricitabine (Gilead Sciences) was administered subcutaneously from 4 to 30 weeks post-SIV infection (Fig. 1A). Blood was collected before SIV infection and every other week thereafter for complete blood count (CBC), subset analysis, biochemistry, or viral load evaluation.
During acute SIV infection, baseline CD4 counts (978 cells/μL) declined to as low as 412 cells/μL and peak SIV viremia reached 6.2 Log10 copies/mL of plasma. During the period of cART, CD4 counts remained stable (449–669 cells/μL) and plasma viremia was <30 copies/mL by 10 weeks on cART (Fig. 1C). CBC analyses were unremarkable during acute SIV and cART (Fig. 1D). Approximately 2 weeks after cART withdrawal, the macaque presented with dyspnea marked by open-mouth breathing and audible wheezing, and was sedated with intramuscular ketamine (10 mg/kg; Covetrus, Dublin, OH) for evaluation. Oxygen saturation was 99% on flow-by oxygen, but rapidly decreased to 85% on room air. The macaque was administered intravenous midazolam (0.5 mg/kg; Heritage Pharmaceuticals, Eatontown, NJ) and intubated to facilitate ventilation, but the animal's respiration could not be stabilized. Due to grave prognosis, the animal was euthanized with intravenous Nembutal (160 mg/kg; Virbac AH, Westlake, TX) and necropsied.
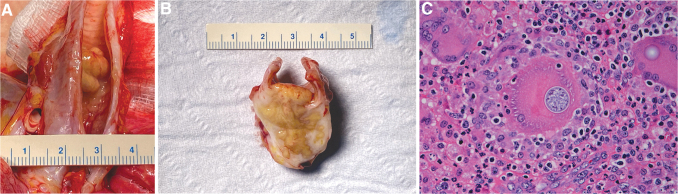
Necropsy revealed a 1–1.5 cm3 white and brown multinodular firm mass located in the tracheal lumen approximately 1 cm cranial to the bifurcation (Fig. 2A, B). This mass was contiguous with a 2–3 cm3 similar mass located ventral to the trachea, with extension through and direct connection to the luminal tracheal mass. On section, the lesion consisted of coalescing brown-tan nodules exuding moderate amounts of viscous material, and with white firm reticulation. Representative tissues and organs were fixed in 10% formalin, embedded in paraffin, sectioned, and then hematoxylin and eosin stained.
FIG. 2.
Coccidioides sp.-associated tracheal lesion gross pathology and histology. (A) Portions of trachea, lung, heart, and associated structures. Trachea was opened dorsally revealing in the distal third large, coalescing, firm, tan-brown nodules that in aggregate form an approximately 1–1.5 cm3 luminal mass with partial tracheal obstruction. (B) Cross section through the approximate middle of the tracheal mass revealing that the majority of the mass is ventral to the trachea and was histologically associated with a mostly effaced lymph node. The mass was firm, approximately 2–3 cm3, was mostly tan-brown, and contained moderate amounts of viscous exudate. The white reticulation represents mature and reactive fibrous tissue. The mass protrudes through the tracheal wall and the luminal portion again measures approximately 1 cm wide and extends approximately 6–7 mm dorsally into the tracheal lumen. (C) Hematoxylin and eosin-stained section of formalin-fixed, paraffin-embedded tracheal mass. The section reveals abundant mixed inflammation: neutrophils, lymphocytes, and macrophages. Multiple multinucleated macrophages (“giant cells”) are present, one of which contains a fungal spherule (center of figure) and another that contains a portion of a spherule capsule (upper right of figure). The largest central spherule measures 31 to 33 μm for reference, and contains developing endospores and a thin refractile capsule. More mature spherules (not shown) had numerous endospores approximately 2–5 μm. Size and morphology are diagnostic for Coccidioides sp.
Sections of trachea and associated structures (jugular veins, carotid arteries, paratracheal lymph nodes) had effacing inflammation apparently arising from a paratracheal lymph node that was sclerosing and pyogranulomatous with pyogranulomas, large numbers of giant cells, and moderate numbers of fungal spherules consistent with Coccidioides sp: spherules ranged from 10 to 40 μm with a thin refractile capsule and contained few to numerous 2–5 μm endospores (Fig. 2C). Transmural inflammation extended into the tracheal lumen with ulceration and partial occlusion. Where not ulcerated, the tracheal epithelium was attenuated to hyperplastic and had submucosal granulomatous and eosinophilic inflammation. There also were regional vasculitis and cellulitis. Fite's acid fast stain was negative.
Lungs had moderate multifocal areas with peribronchial granulomatous and eosinophilic inflammation with giant cells and tract formation, multiple moderately large areas of effacing, similar alveolar inflammation, and with occasional extension of inflammation extending into bronchioles. Other tissues and organs were unremarkable besides moderate inflammatory bowel disease unrelated to coccidioidomycosis. Interpretations were chronic, occlusive, recrudescent tracheal and peritracheal coccidioidomycosis arising from a quiescent residual infection of a paratracheal lymph node. The pneumonia was subacute and subsequent to aspiration of exudate and fungal organisms, and likely also had a secondary bacterial component.
Cryopreserved plasma retroactively submitted for IgM and IgG Coccidioides titers indicated a low but detectable IgG titer in April 2020, approximately 8 weeks before discontinuation of cART (Fig. 1A), which continued to increase thereafter despite stable CD4 counts (400–700 cells/μL) and undetectable SIV viral load (Fig. 1C). CD4 counts were not evaluated at euthanasia, however, CD3+CD4+ T cells in blood mononuclear cells (19.4%) by flow cytometry were consistent with levels in 5 other SIV-infected PTMs enrolled in the same study at the same time point (18.8 ± 6.05) (data not shown), suggesting that further CD4 decline did not occur in this animal after cART withdrawal. Increases in white blood cell and monocyte counts (Fig. 1D) and globulin levels (4.6 g/dL) (data not shown) corresponded to resurgence of detectable Coccidioides IgG titer, however, only after cART withdrawal (Fig. 1D).
Since the advent of potent cART, the incidence of symptomatic coccidioidomycosis in PLWH has significantly declined,3 and case reports of symptomatic coccidioidomycosis are primarily associated with therapeutic noncompliance.7–9 For PLWH in regions with endemic Coccidioides, one to two serologic tests per year are recommended, and treatment is only implemented with positive serology and until CD4 counts are >250 cells/μL.2 Similarly, this macaque was screened for coccidioidomycosis at least twice per year and CD4 counts remained above 250 cells/μL during the course of the experimental study. Recurrence of coccidioidomycosis following antifungal therapy is rare in humans and often due to exposure to other Coccidioides sp. strains.10 However, because Coccidioides fungal spores are transmitted by inhalation from the environment, rather than between animals and humans,1,4,11 and as this macaque was strictly housed indoors in Seattle, which is nonendemic for the fungus, exposure to another strain is unlikely. CD4 T helper cells, including Th1 and Th17 subsets, are important for Coccidioides control.12 The SIV infection may have impaired Th1 and Th17 responses in this animal and likely reduced its ability to keep the fungal infection suppressed and contributed to recrudescence of the initial infection.
To reduce confounding variables in HIV/AIDS research, specific pathogen-free (SPF) macaque colonies that are absent of simian retrovirus type D (SRV-D), simian T lymphotropic virus-1 (STLV-1), SIV, and macacine herpesvirus-1 (McHV-1) are necessary.13–15 However, screening for other pathogens [e.g., rhesus rhadinovirus (RRV), cytomegalovirus (CMV), lymphocryptovirus (LCV), and simian foamy virus (SFV)] may also be recommended. Furthermore, naturally transmitted pathogens endemic to specific regions of the United States, such as Chagas disease (Trypanosoma cruzi) and histoplasmosis (Histoplasma sp.), have been reported to recur in the macaque species enrolled in HIV/AIDS research.16–18 Coccidioidomycosis has been documented in other nonhuman primate (NHP) species used for HIV/AIDS research, including rhesus macaques (Macaca mulatta) and sooty mangabeys (Cercocebus atys).4,19,20 To our knowledge, this is the first case report documenting recrudescence of naturally occurring coccidioidomycosis in an NHP enrolled in HIV/AIDS-related research. This case report emphasizes the importance of coccidioidomycosis screening in all NHPs planned for enrollment into HIV/AIDS research when there is potential for fungal exposure, particularly NHPs originating from outdoor colonies in endemic areas. In addition, NHPs with a history of coccidioidomycosis, regardless of successful antifungal treatment and negative follow-up Coccidioides titers, should be excluded from enrollment in SIV/SHIV studies.
Acknowledgments
The authors thank Solomon Wangari, Naoto Iwayama, Chul Ahrens, and William Garrison for outstanding NHP support, and Gilead Sciences and Viiv Healthcare for providing cART. They thank Brandon Keele for providing the SIVmac239M. They also thank the University of Washington Virology & Immunology Core, Sandra Dross, Bei Ming, and Jessica Li for plasma viral load assays and CBC subset analysis, the University of Washington Department of Laboratory Medicine for serum chemistries, and Amirah Ullah and Erin Broderick for technical support. This study was supported by grants from the National Institutes of Health (NIH), the National Institute of Allergy and Infectious Diseases (R56 AI141494 to DHF), and the National Institute of Mental Health (K01 MH1235258 to MAO). The WaNPRC is supported by grant P51 OD010425 from the NIH Office of Research Infrastructure Programs.
Authors' Contributions
Methodology: K.A.G., R.D.M., T.B.L., B.B., A.B., D.A.J., C.M.M., and M.A.O. Data analysis: K.A.G., R.D.M., T.B.L., B.B., A.B., and M.A.O. Writing—original draft preparation: K.A.G. and M.A.O. Writing—review and editing: K.A.G., R.D.M., T.B.L., B.B., A.B., D.A.J., C.M.M., D.H.F., and M.A.O.
Author Disclosure Statement
The authors declare no conflicts of interest.
Funding Information
This work was funded by the National Institutes of Health (NIH) grants R56 AI141494 and P51 OD010425. M.A.O. was supported by NIH K01 MH1235258.
References
- 1. Valley Fever Statistics | Coccidioidomycosis | Types of Fungal Diseases | Fungal | CDC [Internet]: [cited September 2, 2020]. Available at https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html (2020), accessed July17, 2020
- 2. Panel on Opportunistic Infections in Adults and Adolescents with HIV: Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: Recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. [Internet]. [cited 2020 Aug 31]. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf, accessed July17, 2020
- 3. Masannat FY, Ampel NM: Coccidioidomycosis in patients with HIV-1 infection in the era of potent antiretroviral therapy. Clin Infect Dis 2010;50:1–7 [DOI] [PubMed] [Google Scholar]
- 4. Koistinen K, Mullaney L, Bell T, et al. : Coccidioidomycosis in nonhuman primates: Pathologic and clinical findings. Vet Pathol 2018;55:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klatt NR, Canary LA, Vanderford TH, et al. : Dynamics of simian immunodeficiency virus SIVmac239 infection in pigtail macaques. J Virol 2012;86:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fennessey CM, Pinkevych M, Immonen TT, et al. : Genetically-barcoded SIV facilitates enumeration of rebound variants and estimation of reactivation rates in nonhuman primates following interruption of suppressive antiretroviral therapy. PLoS Pathog 2017;13:e1006359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mu A, Shein TT, Jayachandran P, Paul S: Immune reconstitution inflammatory syndrome in patients with AIDS and disseminated coccidioidomycosis: A case series and review of the literature. J Int Assoc Provid AIDS Care 2017;16:540–545 [DOI] [PubMed] [Google Scholar]
- 8. D'Avino A, Di Giambenedetto S, Fabbiani M, Farina S: Coccidioidomycosis of cervical lymph nodes in an HIV-infected patient with immunologic reconstitution on potent HAART: A rare observation in a nonendemic area. Diagn Microbiol Infect Dis 2012;72:185–187 [DOI] [PubMed] [Google Scholar]
- 9. Mathew G, Smedema M, Wheat LJ, Goldman M: Relapse of coccidioidomycosis despite immune reconstitution after fluconazole secondary prophylaxis in a patient with AIDS. Mycoses 2003;46:42–44 [DOI] [PubMed] [Google Scholar]
- 10. Cox RA, Magee DM: Coccidioidomycosis: Host response and vaccine development. Clin Microbiol Rev 2004;17:804–839, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown J, Benedict K, Park BJ, Thompson GR: Coccidioidomycosis: Epidemiology. Clin Epidemiol 2013;5:185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castro IS de, Gordon SN, Liu J, et al. : Expression of CD40L by the ALVAC-simian immunodeficiency virus vector abrogates T cell responses in Macaques. J Virol [Internet]. 2020;94:e01933–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yee JL, Vanderford TH, Didier ES, et al. : Specific pathogen free macaque colonies: A review of principles and recent advances for viral testing and colony management. J Med Primatol 2016;45:55–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morton WR, Agy MB, Capuano SV, Grant RF: Specific pathogen-free macaques: Definition, history, and current production. ILAR J 2008;49:137–144 [DOI] [PubMed] [Google Scholar]
- 15. Mansfield K: Specific pathogen-free rhesus macaques. In: International Perspectives: The Future of Nonhuman Primate Resources [Internet]. National Academies Press, 2003 [cited November 9, 2020]. Available at https://www.ncbi.nlm.nih.gov/books/NBK221769/ [PubMed]
- 16. Dickerson MF, Astorga NG, Astorga NR, Lewis AD: Chagas disease in 2 geriatric rhesus macaques (Macaca mulatta) housed in the Pacific Northwest. Comp Med 2014;64:323–328 [PMC free article] [PubMed] [Google Scholar]
- 17. Kunz E, Mätz-Rensing K, Stolte N, Hamilton PB, Kaup F-J: Reactivation of a Trypanosoma cruzi infection in a rhesus monkey (Macaca mulatta) experimentally infected with SIV. Vet Pathol 2002;39:721–725 [DOI] [PubMed] [Google Scholar]
- 18. Baskin GB: Disseminated histoplasmosis in a SIV-infected rhesus monkey. J Med Primatol 1991;20:251–253 [PubMed] [Google Scholar]
- 19. Kundu MC, Ringenberg MA, d'Epagnier DL, Haag HL, Maguire S: Coccidioidomycosis in an indoor-housed rhesus macaque (Macaca mulatta). Comp Med 2017;67:452–455 [PMC free article] [PubMed] [Google Scholar]
- 20. Pappagianis D, Vanderlip J, May B: Coccidioidomycosis naturally acquired by a monkey, Cercocebus atys, in Davis, California. Sabouraudia 1973;11:52–55 [DOI] [PubMed] [Google Scholar]