Abstract
Impairments in physical function and increased systemic levels of inflammation have been observed in middle-aged and older persons with HIV (PWH). We previously demonstrated that in older persons, associations between gut microbiota and inflammation differed by HIV serostatus. To determine whether relationships between the gut microbiome and physical function measurements would also be distinct between older persons with and without HIV, we reanalyzed existing gut microbiome and short chain fatty acid (SCFA) data in conjunction with previously collected measurements of physical function and body composition from the same cohorts of older (51–74 years), nonfrail PWH receiving effective antiretroviral therapy (N = 14) and age-balanced uninfected controls (N = 22). Associations between relative abundance (RA) of the most abundant bacterial taxa or stool SCFA levels with physical function and body composition were tested using HIV-adjusted linear regression models. In older PWH, but not in controls, greater RA of Alistipes, Escherichia, Prevotella, Megasphaera, and Subdoligranulum were associated with reduced lower extremity muscle function, decreased lean mass, or lower Short Physical Performance Battery (SPPB) scores. Conversely, greater RA of Dorea, Coprococcus, and Phascolarctobacterium in older PWH were associated with better muscle function, lean mass, and SPPB scores. Higher levels of the SCFA butyrate associated with increased grip strength in both PWH and controls. Our findings indicate that in older PWH, both negative and positive associations exist between stool microbiota abundance and physical function. Different relationships were observed in older uninfected persons, suggesting features of a unique gut–physical function axis in PWH.
Keywords: HIV, aging, physical function, microbiome, inflammation
Introduction
With advances in treatment, nearly half of people with HIV (PWH) in the United States are now 50 years or older.1 Despite effective antiretroviral therapy (ART), impairments in physical function have been reported across several cohorts of middle-aged and older PWH.2–10 These impairments in physical function among ART-treated PWH have been linked to systemic inflammation [e.g., levels of IL-6, soluble TNF receptors (sTNFR)].11–14 Understanding the factors that promote inflammation in older PWH will allow the development of targeted therapies for PWH who may experience unique aging and physical function impairments.
Multiple factors contribute to systemic inflammation and immune activation in PWH with early and sustained disruption of gut homeostasis considered a major contributor.15–17 Disruption of the epithelial barrier allows for a greater translocation of bacteria or bacterial products from the lumen, into the underlying tissue and into the systemic circulation and this process, termed microbial translocation, has been linked to inflammation and immune activation.18 Alterations in stool or mucosa-associated enteric bacterial communities, generally characterized by higher abundances of gut commensal bacteria with inflammatory potential and lower abundances of immune-regulatory bacteria, including those capable of producing the short chain fatty acid (SCFA) butyrate, have also been observed (reviewed in Refs.19,20). Furthermore, these dysbiotic profiles have been linked to systemic inflammation, microbial translocation19–22 and recently to metabolic syndrome,21 and various inflammatory comorbidity events.22
In addition to the contribution from inflammation, emerging evidence supports an association between the gut microbiome and physical function/frailty in aging23–30 with many of these features similar to the dysbiotic profiles observed in PWH. In a recent study investigating the combined effect of age and HIV infection on the stool microbiome of nonfrail, older PWH and uninfected controls, we observed that HIV and age were independently associated with distinct changes in stool bacterial communities.31 Furthermore, associations between the stool microbiome (focused on the 25 most abundant genera) and inflammatory systemic biomarkers differed by HIV serostatus. To further investigate the link that the microbiome may have on physical function, we reanalyzed the previously acquired microbiome and SCFA datasets in conjunction with previously collected physical function and body composition measurements.31–33 We hypothesized that the stool microbiome would be associated with physical function and, given the HIV serostatus differences between inflammation and the most abundant genera observed in our prior work, we further hypothesized that the association between these genera and physical function would also differ among older adults with and without HIV.
Materials and Methods
Study participants
Previously acquired stool 16S rRNA sequencing data, SCFA (acetate, butyrate, propionate) levels and physical function and body composition measurements were used for the analyses in this study.31–33 These data were obtained from 8 study participants with HIV and 22 study participants without HIV (controls) recruited from the “Exercise in Healthy Aging” study (Clinical Trials No. NCT02404792)32,33 and 6 additional PWH recruited from the “Assessing Tenofovir Pharmacology in Older HIV-infected Individuals Receiving Tenofovir-based Antiretroviral Therapy” study (Clinical Trials No. NCT02304263).34 All participants were between 50 and 75 years of age. PWH were on ART for ≥2 years with HIV-1 RNA <200 copies/mL and a CD4 count of >200 cells/μL. Key exclusion criteria included active diarrhea, antibiotic use within the last 2 weeks, active hepatitis C infection, diabetes requiring insulin, body mass index (BMI) <20 or >40 kg/m2, and use of corticosteroids or other immunomodulators. All participants provided written, informed consent; both clinical trials were approved by the Colorado Multiple Institutional Review Board.
Outcome measures
Physical function and body mass measurements have been previously detailed.32,35 Briefly, functional outcomes included continuous time (s) to complete a 400-m walk, climb a flight of 10 stairs, or to complete 10 repeat chair rises. The Short Physical Performance Battery (SPPB) test was scored as previously described36 with 0–4 points for each of: five repeat chair rises, 4-m walk, and progressive balance tests of up to 10 s. Hand grip strength was assessed as the average of three dominant measurements (kg) using a Jamar Dynamometer (Lafayette Instruments, Lafayette, IN). Additional muscle strength measurements included bench press, leg press and lateral press enumerated as the maximal weight (kg) lifted one time using correct lifting form through the full range of motion and normalized to lean body mass. Muscle mass was estimated with measurements (kg) of lean body mass (LBM), and appendicular lean mass (ALM), as fat-free, bone-free mass, quantified by whole-body dual-energy X-ray absorptiometry (DXA) scans, using either the Hologic Discovery W (Apex 4.0.1) or Horizon W (Apex 5.6.05) instrument (Hologic, Inc., Bedford MA). Both measurements were height adjusted.
Microbiome analysis
Stool bacterial profiles were generated in a previously completed study by broad-range amplification and sequencing of 16S rRNA genes.31,37,38 In brief, stool samples were self-collected and immediately stored in the study participants' home freezer until transfer to a −80°C freezer within 3 days. All samples were processed within 6 months. DNA was isolated using the QIAamp PowerFecal Kit (Qiagen, Carlsbad, CA) and 16S rRNA gene amplicons were generated using oligonucleotide primers targeting the V3V4 variable region. Illumina paired-end sequencing was performed on the MiSeq platform (Illumina, San Diego, CA). Paired-end reads were quality filtered, demultiplexed, merged, and classified using SINA(1.3.0-r23838),39,40 as previously described.31,41–45 Operational taxonomic units were produced by clustering with identical taxonomic assignments.31 Explicet (v2.8.2)46 was used for organization, analysis (i.e., alpha-diversity calculations), and visualization of microbiome data.31 Stool microbiome profiling was successful in all samples (median of 115,568 sequences/sample, median Good coverage of 99.97%).31 The effect of HIV serostatus and age on the stool microbial communities of the study participants were extensively reported.31
Stool SCFA extraction and analysis
Stool SCFA levels were measured in the previously completed study using gas chromatography, as previously described.31,47,48 Seven grams of stool was taken from disparate sections of each sample and purified water was added (1:5, w/v) before homogenization by vortexing. The homogenate was centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant (400 μL) was then mixed with 100 μL of internal standard solution containing 50 mM 4-methyl-valeric acid, 5% meta-phosphoric acid, and 1.56 mg/mL of copper sulfate. The samples were centrifuged again at 10,000 rpm for 10 min at 4°C. Supernatant (4 μL) was injected into a gas chromatography equipped with a fused silica capillary column (Supelco No: 40369-03A; Nukon™, Bellefonte, PA) and a flame ionization detector (GC-FID 7890A; Agilent Technologies, Inc., Santa Clara, CA). The gas chromatography conditions were as follows: injector temperature at 230°C; initial oven temperature at 100°C; and temperature increase of 8°C/min to 200°C with a hold for 3 min at final temperature. Helium was used as a carrier gas at 0.75 mL/min. SCFA quantification was assessed through measurements of peak areas for acetate, propionate, and butyrate relative to 4-methyl valeric acid. Acetate, propionate, and butyrate (Fisher Scientific, Hampton, NH) were used as external standards. Levels were expressed as mmol/g.
Statistical analyses
Participant characteristics were summarized using frequencies (%) for categorical variables and mean [standard deviation (SD)] for continuous variables. Fisher's exact and two-sample t-tests were used to compare differences between PWH and uninfected controls. Relative abundances (RA) of bacterial taxa were calculated as the number of sequences for a specific taxa standardized to the total number of sequences for the study participant. Linear regression models provided estimates of associations between stool microbiota abundance or SCFAs with physical function or body mass measurements. HIV status and an interaction term between HIV and either stool microbiota abundance or SCFAs was included regardless of significance. Mean estimates with 95% confidence intervals were reported. Two-sided tests are reported assuming a 0.05 significance level. Analyses were conducted in SAS v9.4 (Carey, NC). Outcomes were considered complementary and reported without adjustment for multiple comparisons.49
Results
Study participant demographics
The 14 PWH and 22 controls were similar by age, race, ethnicity, BMI, smoking status, and alcohol consumption (Table 1). All except one study participant identified as male. Physical function measurements (Table 2) were not significantly different between PWH and controls, although PWH had slower 400-m walk times and lower SPPB scores (p = .05 and p = .08, respectively). LBM and ALM were not significantly different (Table 2).
Table 1.
Study Participant Characteristics
| Characteristic | Controls (N = 22) | PWH (N = 14) | p |
|---|---|---|---|
| Male sex | 21 (95) | 14 (100) | 1.0 |
| Age, years | 59.50 (6.62) | 60.79 (7.71) | .61 |
| Race | .33 | ||
| White | 17 (77) | 10 (71) | |
| Black/African American | 2 (9) | 4 (29) | |
| Asian | 1 (5) | 0 | |
| More than one race | 2 (9) | 0 | |
| Hispanic/Latino Ethnicity | 3 (14) | 2 (14) | 1.0 |
| HIV-1 RNA (<200 copies/mL) | NA | 14 (100) | |
| CD4 count (cells/μL) | ND | 680.69 (281.06) | |
| Sexual orientation | <.001 | ||
| MSM | 6 (27) | 13 (93) | |
| Heterosexual | 16 (73) | 1 (7) | |
| BMI | 27.81 (4.29) | 27.46 (4.34) | .82 |
| Current tobacco smoker | 2 (9) | 3 (21) | .36 |
| Alcohol intake >2 drinks/day | 2 (9) | 1 (7) | 1.0 |
Values are shown frequency (%) or mean (SD).
BMI, body mass index; MSM, men who have sex with men; PWH, people with HIV; NA, not applicable; ND, not determined; SD, standard deviation.
Table 2.
Physical Function and Body Mass Measurements
| Controls (N = 22) | PWH (N = 14) | p | |
|---|---|---|---|
| Bench press, kg | 0.95 (0.20) | 0.99 (0.15) | .53 |
| Lateral press, kg | 1.35 (0.23) | 1.36 (0.17) | .83 |
| Leg press, kg | 2.45 (0.55) | 2.39 (0.34) | .72 |
| Hand grip strength, kg | 35.70 (8.86) | 36.36 (7.91) | .82 |
| 10-time chair rise time, s | 17.46 (4.26) | 20.69 (6.70) | .12 |
| Stair climb time, s | 3.53 (0.63) | 3.71 (0.87) | .58 |
| 400-m walk, s | 226.16 (27.03) | 241.52 (17.16) | .05 |
| SPPB score | 11.81 (0.40) | 11.29 (0.99) | .08 |
| Lean body mass/height2, kg/m2 | 19.33 (1.63) | 19.94 (2.17) | .38 |
| Appendicular lean body mass/height2, kg/m2 | 8.74 (0.83) | 8.83 (1.18) | .80 |
Values are shown as mean (SD). Bench, lateral, and leg press measurements were normalized to lean body mass (kg).
SPPB, short physical performance battery.
Stool microbiota associations with physical function and body composition in PWH
We previously reported that (1) measurements of alpha-diversity (richness, diversity, and evenness) were similar between PWH and controls; (2) PERMANOVA tests of beta-diversity showed minimal overall composition differences in microbiota between PWH and controls at the phylum, family, or genus levels; and (3) negative binomial regression models demonstrated HIV and age-specific effects at the family and genus levels.31 Potential relationships between physical function/body composition and stool microbiota in PWH and in controls, were explored using linear regression models; to minimize the number of statistical tests, we focused on the top 5 most abundant phyla, top 10 most abundant families, and top 25 most abundant genera (Supplementary Table S1 and Supplementary Fig. S1). Of these taxa, we previously observed no significant differences between controls and PWH at the phylum level and only significantly lower RA of Lachnospiraceae (family) and Alistipes (genus) in PWH versus controls.31 Phylum and family taxa were detected in all persons, except for Fusobacteria which was detected in 77% of controls. Genera were represented in at least 80% of PWH or controls with the following exceptions—Fusobacterium: 77% of controls, 64% of PWH; Megasphaera: 77% of controls; Succinivibrio: 50% of controls, 71% of PWH; and Megamonas: 55% of controls, 71% of PWH.
At the phylum level (Supplementary Table S2), greater RA of Proteobacteria associated with lower SPPB scores, slower chair rise and stair climb times, and less ALM. Increasing RA of Enterobacteriaceae, a family within Proteobacteria, also significantly associated with lower SPPB and a slower stair climb (Supplementary Table S3). Bacteroidetes associated with a longer time to complete the 400 m walk in PWH with no associations between 400 m walk and abundance of bacteria within other phylum, family, or genus taxa noted. No other significant associations between the top 5 most abundant phylum and physical function or body composition measurements in PWH were observed (Supplementary Table S2). Additional significant associations between greater abundance of bacteria at the family taxa and function (Supplementary Table S3) included Lachnospiraceae, which associated with a faster chair rise time, Bacteroidaceae with lower SPPB and less ALM, and Veillonellaceae with slower stair climb.
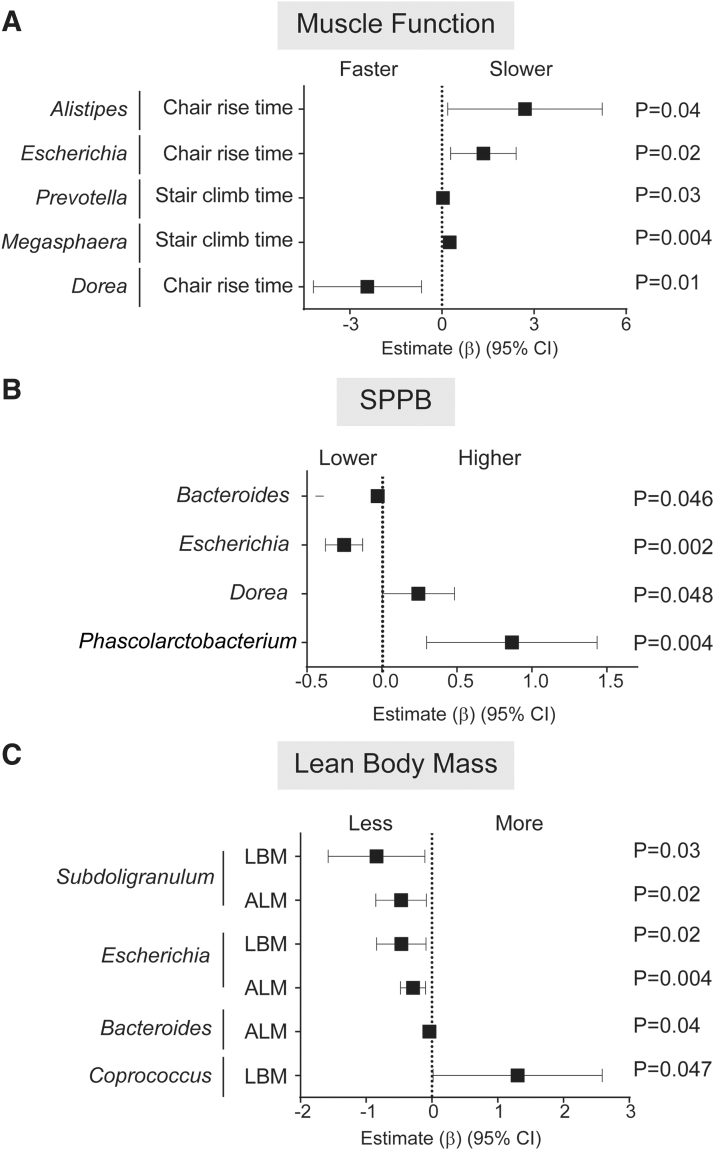
The 25 most abundant genera belonged to the phyla Actinobacteria, Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria and included the majority of genera reported to contain butyrate-producing bacteria (Supplementary Table S1).50,51 Given that Butyrivibrio fibrisolvens is a known butyrate-producing bacterium,50,51 the genus Butyrivibrio (Firmicutes) was also included. Among PWH, greater RA of numerous genera, including Alistipes, Escherichia, Prevotella, and Megasphaera were associated with reduced lower extremity muscle function, as indicated by slower time to complete 10 chair rises or stair climbs (Fig. 1A and Supplementary Table S4). Greater RA of Escherichia and Bacteroides were associated with a lower SPPB performance (Fig. 1B and Supplementary Table S4), less ALM, and less LBM (Escherichia only) (Fig. 1C and Supplementary Table S4), while greater Subdoligranulum RA was significantly associated with less ALM and LBM (Fig. 1C and Supplementary Table S4). Conversely, higher RA of Dorea was associated with faster chair rise time (Fig. 1A and Supplementary Table S4) and higher SPPB scores (Fig. 1B and Supplementary Table S4), Phascolarctobacterium with higher SPPB scores (Fig. 1B and Supplementary Table S4), and Coprococcus with greater LBM (Fig. 1C and Supplementary Table S4). In some instances, similar relationships with function were observed at the phylum or family level to which these genera belong (e.g., Escherichia of the Enterobacteriaceae family and Proteobacteria phylum; Bacteroides and Bacteroidaceae family) suggesting it was the RA at the genus level driving the observed association at the higher taxonomic classifications. No other significant associations between genera and function were observed (Supplementary Table S4).
FIG. 1.
Associations between stool microbiota abundance with physical function or body mass measurements in PWH. Linear regression models with interaction terms between HIV and stool microbiota abundance were conducted to determine estimates for physical function or body mass change for each 1% increase in relative abundance of each genera. Significant associations between microbiota and (A) muscle function, (B) SPPB, and (C) lean body mass in PWH study participants. Forest plots were created to display the estimate (β) and 95% CI for associations between increasing abundance of genera with (A) muscle function based on time (s) to complete 10 chair rises or climb 1 flight of stairs, (B) SPPB scores, and (C) estimates of LBM or ALM normalized for height. Estimates >1 indicate associations between increasing abundance of specific microbiota and reduced muscle function, higher SPPB scores, or greater body mass. ALM, appendicular lean mass; CI, confidence intervals; LBM, lean body mass; PWH, people with HIV; SPPB, Short Physical Performance Battery.
Stool microbiota and associations with physical function and body composition in controls
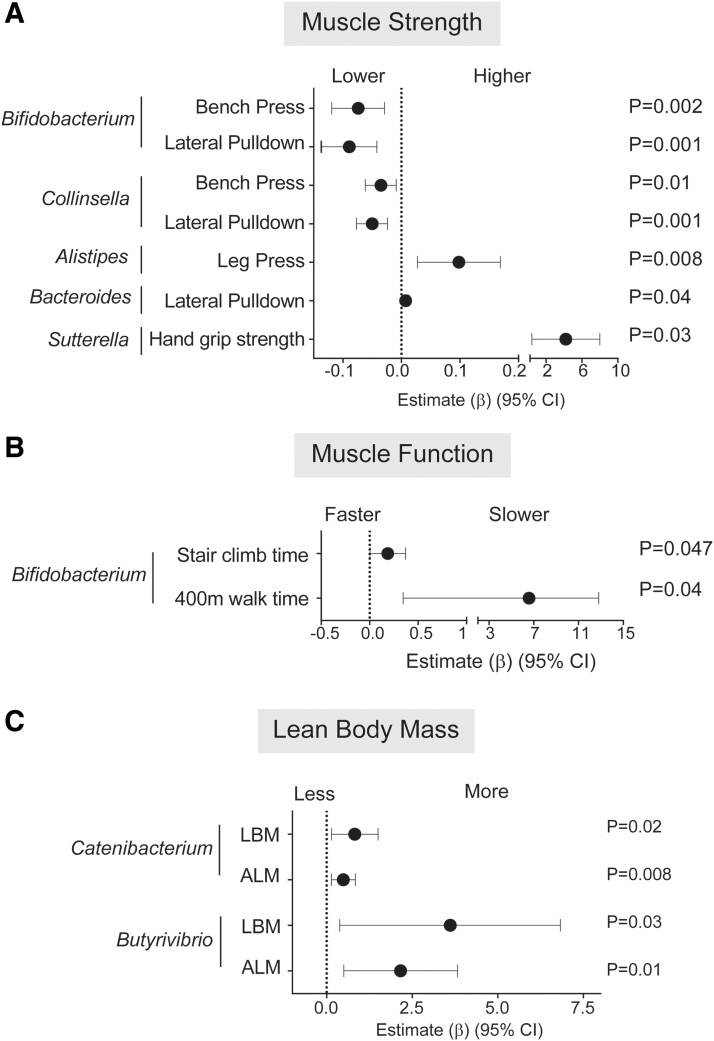
In older persons without HIV, greater RA of Bacteroidetes was significantly associated with greater strength on bench press and lateral pulldown (Supplementary Table S2), the latter of which was also observed at the family (Bacteroidaceae; Supplementary Table S3) and genus (Bacteroides; Fig. 2A and Supplementary Table S4) levels. Greater RA of Actinobacteria significantly associated with lower muscle strength (bench press, lateral pulldown) (Supplementary Table S2). This association was in keeping with the significant associations observed for Coriobacteriaceae, a family in the Actinobacteria phylum (Supplementary Table S3), for Collinsella, a genus in the Coriobacteriaceae family and for Bifidobacterium (Actinobacteria phylum) (Fig. 2A and Supplementary Table S4). Alistipes, Bacteroides, and Sutterella abundance were also associated with greater muscle strength (Fig. 2A and Supplementary Table S4); observations reflected at the higher taxa levels of Rikenellaceae (family containing Alistipes) and Bacteroidaceae (family containing Bacteroides) (Supplementary Table S3). Additional significant findings between genera and function included greater abundance of Bifidobacteria associated with a longer time to complete a stair climb or 400-m walk (Fig, 2B and Supplementary Table S4). Greater RA of Catenibacterium and Butyrivibrio were associated with higher estimates of LBM and ALM (Fig. 2C and Supplementary Table S4). Greater abundance of Erysipelotrichaceae, the family to which Catenibacterium belongs, was also significantly associated with higher lean mass measurements (Supplementary Table S3).
FIG. 2.
Associations between stool microbiota abundance with physical function or body mass measurements in Controls. Linear regression models with interaction terms between HIV and stool microbiota abundance were conducted to determine estimates for physical function or body mass change for each 1% increase in relative abundance of each genera. Significant associations between microbiota and (A) muscle strength, (B) muscle function, and (C) lean body mass in control study participants. Forest plots were created to display the estimate (β) and 95% CI for associations between increasing abundance of genera with (A) muscle strength measured as maximal weight (kg) of one complete bench press, lateral pulldown (normalized by LBM) or hand grip strength (kg), (B) muscle function based on time (s) to climb one flight of stairs or complete a 400 m walk, and (C) estimates of LBM or ALM normalized for height. Estimates >1 indicate associations between increasing abundance of specific microbiota and reduced muscle function, greater muscle strength, or greater body mass.
Stool butyrate levels associate with greater handgrip strength
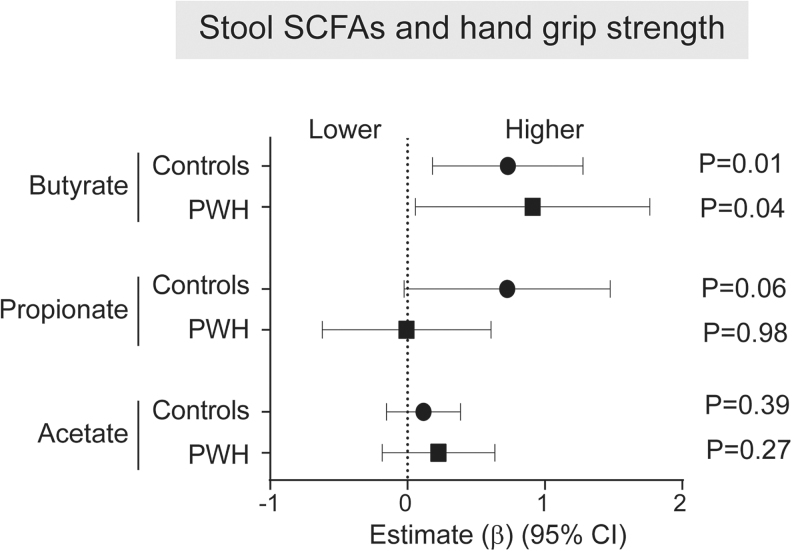
No statistically significant differences in stool butyrate, propionate, or acetate between PWH [mean level (mmol/g) (SD): butyrate 7.3 (5.0), propionate 14.0 (7.6), acetate 42.0 (11.7)] and controls [butyrate 7.7 (6.1), propionate 10.2 (4.9), acetate 37.7 (14.1)] were observed [mean (SD): butyrate PWH 7.3], as previously reported.31 Relationships between stool butyrate, propionate, and acetate levels with physical function and body mass measurements were similarly explored using linear regression models (Supplementary Table S5). In both controls and PWH, increasing levels of butyrate were significantly associated with greater grip strength (Fig. 3); propionate levels were also associated with increasing grip strength in controls but failed to reach statistical significance (p = .06). No other associations between butyrate, propionate, or acetate with physical function or body mass measurements were significant in either group.
FIG. 3.
Associations between stool SCFAs and hand grip strength. Linear regression models with interaction terms between HIV and levels of stool SCFAs were conducted to determine estimates for physical function or body mass change for each 1 unit increase in SCFA levels. A Forest Plot was created to display the estimate (β) and 95% CI for associations between increasing levels of stool butyrate, propionate, or acetate and hand grip strength. Estimates >1 indicate associations between increasing SCFA levels and greater hand grip strength. SCFAs, short chain fatty acids.
Discussion
To the best of our knowledge, this study is the first to investigate potential relationships between stool commensal microbiota and physical function in older, nonfrail PWH. We observed several positive and negative correlations between individual genera and poor physical function in older PWH that were not found in uninfected older adults, suggesting a unique gut microbiome–physical function axis in older PWH.
In older PWH, greater abundance of Dorea, Phascolarctobacterium, and Coprococcus was associated with better physical function and greater LBM. Phascolarctobacterium and Coprococcus include species known to produce the SCFAs propionate or butyrate, respectively.51 These SCFAs have reported roles in skeletal muscle metabolism and in preventing muscle atrophy or altering muscle fiber composition (reviewed in Frampton et al.52), which may, in part, explain the relationship to physical function. Although we observed positive relationships between certain genera and physical function or LBM, we noted that a greater number of genera had inverse associations with physical function in older PWH. While many of these genera correlated with a single measure of poor physical function or lower body mass, greater abundance of Escherichia was associated with reduced muscle function (slower chair rise time, lower SPPB score) and lower total body and ALM. We have previously shown in this cohort that Escherichia abundance was positively correlated with inflammatory biomarkers, most notably sTNFR.31 Additional mechanistic studies demonstrating the link among Escherichia, inflammation, and reduced physical function measurements are warranted. In this exploratory study, Prevotella associated with slower stair climb time, a measure of leg muscle power. Recent studies have demonstrated that Prevotella was enriched in men who have sex with men (MSM) likely due to sexual practices, including receptive anal intercourse.21,22,53–56 Because the majority (93%) of our PWH cohort were MSM versus 27% in controls, the association of Prevotella with leg muscle power may not be related to HIV infection per se.
Despite finding no significant differences in physical function and body composition between older PWH and controls, the associations between gut microbiota and the various physical function measurements noted in older PWH were not observed in similarly aged controls, suggesting that HIV infection combined with age confers a unique gut microbiome–physical function axis compared with the effects of age alone. Indeed, in controls, abundance of certain gut microbial taxa was associated with measures of maximal muscle strength but not physical function. Intriguingly, in our cohort of generally healthy, older persons, greater abundance of Bifidobacterium was associated with lower muscle mass and strength. This observation is in marked contrast to the well-described health benefits of certain Bifidobacterium spp.57 and improvement in grip strength following a 12-week administration of Bifidobacterium longum into aged mice.58 However, although rare, cases of Bifidobacterium bacteremia, particularly in immunocompromised people, have been reported59,60 suggesting certain species may have inflammatory potential.
The association between stool butyrate and greater handgrip strength in both PWH and uninfected controls suggest the previously reported mechanistic features linking butyrate to muscle strength are related more to aging52,61–64 and may not be additionally impacted by HIV infection. Indeed, we previously reported similar levels of stool butyrate in these same study participants.31 Butyrate has multiple beneficial roles in the maintenance of gut immune homeostasis65 and, in rodent models, impacts muscle metabolism and function.52 Butyrate levels are typically highest in the gut lumen and primarily utilized in gut tissue, resulting in low systemic levels.52 It is tempting to speculate that greater butyrate levels, through maintenance of epithelial barrier function and/or anti-inflammatory functions in the intestinal mucosa, lead to a healthier gut, limited systemic inflammation, and greater grip strength.
Our study strengths include the objective measures of physical function and DXA-derived measures of lean tissue mass. However, several limitations are acknowledged and our results should be considered exploratory. This study performed retrospective analysis of previously acquired microbiome and physical function/body composition datasets and is limited by small sample sizes and the ability to adjust for multiple confounders that may influence the microbiome, including dietary intake, prebiotic/probiotic use, and a lack of matching for MSM status and that current physical activity was only recorded in one of the two parent studies. Study participants were relatively high functioning, and only one woman was included in the study further limiting generalizability. The associations between gut microbiota and physical function observed in our study should be repeated in a larger cohort of women and men with appropriate matching for sexual practices and across a wider range of physical function. Finally, the cross-sectional study design precludes conclusions about causation.
Conclusion
This exploratory study is the first to demonstrate a potential gut microbiome–physical function axis in older, nonfrail PWH. Our current observations demonstrating that features of this gut microbiome–physical function axis differ between older adults with and without HIV provide further support to our previously reported concept that a “one bug fits all” approach to microbiome-based therapeutics is not likely relevant for older PWH.31 Future studies should investigate targeted therapies appropriate for this population of people who may experience aging, dysbiosis, and physical function impairments differently than those without HIV.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge all the study participants and study staff. They also acknowledge and thank Diana Ir for assistance with 16S rRNA gene sequencing, Miranda Kroehl and Rachel Johnson for microbiome analyses, and Sharon Seifert for assistance with study participant recruitment.
Author Disclosure Statement
K.M.E. has received consult payments from Gilead Sciences and ViiV (paid to the University of Colorado). The remaining authors have no conflicts of interest to disclose.
Funding Information
This work was supported by the National Institute of Aging of the National Institutes of Health [K23AG050260] to Kristine M. Erlandson, T32 AG279-15 to Jay Liu, the Gilead Sciences Research Scholars Program in HIV (to Kristine M. Erlandson), and the NCATS Colorado CTSA grant number UL1TR002535 to Kristine M. Erlandson. Cara C. Wilson, Daniel N. Frank, and Charles E. Robertson were supported in part by the GI and Liver Innate Immune Program (GALIIP) of the University of Colorado Anschutz Medical Campus.
Supplementary Material
References
- 1. Centers for Disease Control and Prevention: HIV Surveillance Report. Available at: www.cdc.gov/hiv/library/reports/hiv-surveillance.html (2018), accessed April22, 2021
- 2. Branas F, Jimenez Z, Sanchez-Conde M, et al. : Frailty and physical function in older HIV-infected adults. Age Ageing 2017;46:522–526 [DOI] [PubMed] [Google Scholar]
- 3. Crane HM, Miller ME, Pierce J, et al. : Physical functioning among patients aging with human immunodeficiency virus (HIV) versus HIV uninfected: Feasibility of using the short physical performance battery in clinical care of people living with HIV aged 50 or older. Open Forum Infect Dis 2019;6:ofz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erlandson KM, Allshouse AA, Jankowski CM, et al. : Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials 2012;13:324–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erlandson KM, Allshouse AA, Jankowski CM, MaWhinney S, Kohrt WM, Campbell TB: Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr 2013;63:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Erlandson KM, Allshouse AA, Rapaport E, et al. : Physical function impairment of older, HIV-infected adults is associated with cytomegalovirus immunoglobulin response. AIDS Res Hum Retroviruses 2015;31:905–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoury AL, Morey MC, Wong TC, et al. : Diminished physical function in older HIV-infected adults in the Southeastern U.S. despite successful antiretroviral therapy. PLoS One 2017;12:e0179874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrack JA, Althoff KN, Jacobson LP, et al. : Accelerated longitudinal gait speed decline in HIV-infected older men. J Acquir Immune Defic Syndr 2015;70:370–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schrack JA, Jacobson LP, Althoff KN, et al. : Effect of HIV-infection and cumulative viral load on age-related decline in grip strength. AIDS 2016;30:2645–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Umbleja T, Brown TT, Overton ET, et al. : Physical function impairment and frailty in middle-aged people living with human immunodeficiency virus in the REPRIEVE trial ancillary study PREPARE. J Infect Dis 2020;222(Suppl 1):S52–S62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baranoski AS, Harris A, Michaels D, et al. : Relationship between poor physical function, inflammatory markers, and comorbidities in HIV-infected women on antiretroviral therapy. J Womens Health (Larchmt) 2014;23:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford KW, Li X, Xu X, et al. : Lipodystrophy and inflammation predict later grip strength in HIV-infected men: The MACS Body Composition substudy. AIDS Res Hum Retroviruses 2013;29:1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erlandson KM, Allshouse AA, Jankowski CM, et al. : Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013;208:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fukui SM, Piggott DA, Erlandson KM: Inflammation strikes again: Frailty and HIV. Curr HIV/AIDS Rep 2018;15:20–29 [DOI] [PubMed] [Google Scholar]
- 15. Burgener A, McGowan I, Klatt NR: HIV and mucosal barrier interactions: Consequences for transmission and pathogenesis. Curr Opinion Immunol 2015;36:22–30 [DOI] [PubMed] [Google Scholar]
- 16. Tincati C, Douek DC, Marchetti G: Gut barrier structure, mucosal immunity and intestinal microbiota in the pathogenesis and treatment of HIV infection. AIDS Res Ther 2016;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Veazey RS: Intestinal CD4 Depletion in HIV/SIV infection. Curr Immunol Rev 2019;15:76–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zevin AS, McKinnon L, Burgener A, Klatt NR: Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS 2016;11:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillon SM, Wilson CC: What is the collective effect of aging and HIV on the gut microbiome? Curr Opin HIV AIDS 2020;15:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vujkovic-Cvijin I, Somsouk M: HIV and the gut microbiota: Composition, consequences, and avenues for amelioration. Curr HIV/AIDS Rep 2019;16:204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gelpi M, Vestad B, Hansen SH, et al. : Impact of human immunodeficiency virus-related gut microbiota alterations on metabolic comorbid conditions. Clin Infect Dis 2020;71:e359–e367 [DOI] [PubMed] [Google Scholar]
- 22. Vujkovic-Cvijin I, Sortino O, Verheij E, et al. : HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat Commun 2020;11:2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Claesson MJ, Jeffery IB, Conde S, et al. : Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–184 [DOI] [PubMed] [Google Scholar]
- 24. Fielding RA, Reeves AR, Jasuja R, Liu C, Barrett BB, Lustgarten MS: Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp Gerontol 2019;127:110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haran JP, Bucci V, Dutta P, Ward D, McCormick B: The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol 2018;67:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson MA, Jeffery IB, Beaumont M, et al. : Signatures of early frailty in the gut microbiota. Genome Med 2016;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maffei VJ, Kim S, Blanchard ET, et al. : Biological aging and the human gut microbiota. J Gerontol A Biol Sci Med Sci 2017;72:1474–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ticinesi A, Milani C, Lauretani F, et al. : Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep 2017;7:11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW: Fecal microbiota composition and frailty. Appl Environ Microbiol 2005;71:6438–6442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang L, Liao J, Chen Q, et al. : Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res 2020;32:2001–2011 [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Johnson R, Dillon S, et al. : Among older adults, age-related changes in the stool microbiome differ by HIV-1 serostatus. EBioMedicine 2019;40:583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erlandson KM, MaWhinney S, Wilson M, et al. : Physical function improvements with moderate or high-intensity exercise among older adults with or without HIV infection. AIDS 2018;32:2317–2326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jankowski CM, Mawhinney S, Wilson MP, et al. : Body composition changes in response to moderate- or high-intensity exercise among older adults with or without HIV infection. J Acquir Immune Defic Syndr 2020;85:340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abdo M, Coyle RP, Seifert SM, et al. : Associations between tenofovir diphosphate in dried blood spots, impaired physical function, and fracture risk. Open Forum Infect Dis 2021;8:ofaa577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Erlandson KM, Kitch D, Tierney C, et al. : Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013;27:2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guralnik JM, Simonsick EM, Ferrucci L, et al. : A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 37. He T, Xu C, Krampe N, et al. : High-fat diet exacerbates SIV pathogenesis and accelerates disease progression. J Clin Invest 2019;129:5474–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pandrea I, Xu C, Stock JL, et al. : Antibiotic and antiinflammatory therapy transiently reduces inflammation and hypercoagulation in acutely SIV-infected pigtailed macaques. PLoS Pathog 2016;12:e1005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pruesse E, Peplies J, Glockner FO: SINA: Accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 2012;28:1823–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Quast C, Pruesse E, Yilmaz P, et al. : The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res 2013;41:D590–D596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brumbaugh DE, Arruda J, Robbins K, et al. : Mode of delivery determines neonatal pharyngeal bacterial composition and early intestinal colonization. J Pediatr Gastroenterol Nutr 2016;63:320–328 [DOI] [PubMed] [Google Scholar]
- 42. Dillon SM, Lee EJ, Kotter CV, et al. : An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol 2014;7:983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuhn KA, Schulz HM, Regner EH, et al. : Bacteroidales recruit IL-6-producing intraepithelial lymphocytes in the colon to promote barrier integrity. Mucosal Immunol 2018;11:357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nadkarni MA, Martin FE, Jacques NA, Hunter N: Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 2002;148(Pt 1):257–266 [DOI] [PubMed] [Google Scholar]
- 45. Nycz BT, Dominguez SR, Friedman D, et al. : Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patients. PLoS One 2018;13:e0191232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robertson CE, Harris JK, Wagner BD, et al. : Explicet: Graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics 2013;29:3100–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bishehsari F, Engen PA, Preite NZ, et al. : Dietary fiber treatment corrects the composition of gut microbiota, promotes SCFA production, and suppresses colon carcinogenesis. Genes (Basel) 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tuncil YE, Nakatsu CH, Kazem AE, Arioglu-Tuncil S, Martens EC, Hamaker BR: Delayed utilization of some fast-fermenting soluble dietary fibers by human gut microbiota when presented in a mixture. J Funct Foods 2017;32:347–357 [Google Scholar]
- 49. Rothman KJ: No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46 [PubMed] [Google Scholar]
- 50. Louis P, Flint HJ: Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 2009;294:1–8 [DOI] [PubMed] [Google Scholar]
- 51. Louis P, Flint HJ: Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017;19:29–41 [DOI] [PubMed] [Google Scholar]
- 52. Frampton J, Murphy K, Frost G, Chambers E: Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab 2020;2:840–848 [DOI] [PubMed] [Google Scholar]
- 53. Armstrong AJS, Shaffer M, Nusbacher NM, et al. : An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome 2018;6:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kelley CF, Kraft CS, de Man TJ, et al. : The rectal mucosa and condomless receptive anal intercourse in HIV-negative MSM: Implications for HIV transmission and prevention. Mucosal Immunol 2017;10:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Noguera-Julian M, Rocafort M, Guillén Y, et al. : Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 2016;5:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tuddenham S, Koay WL, Sears C: HIV, sexual orientation, and gut microbiome interactions. Dig Dis Sci 2020;65:800–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gareau MG, Sherman PM, Walker WA: Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 2010;7:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ni Y, Yang X, Zheng L, et al. : Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res 2019;63:e1900603. [DOI] [PubMed] [Google Scholar]
- 59. Esaiassen E, Hjerde E, Cavanagh JP, Simonsen GS, Klingenberg C; Norwegian Study Group on Invasive Bifidobacterial Infections: Bifidobacterium Bacteremia: Clinical characteristics and a genomic approach to assess pathogenicity. J Clin Microbiol 2017;55:2234–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weber E, Reynaud Q, Suy F, et al. : Bifidobacterium species bacteremia: Risk factors in adults and infants. Clin Infect Dis 2015;61:482–484 [DOI] [PubMed] [Google Scholar]
- 61. Gao Z, Yin J, Zhang J, et al. : Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009;58:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lahiri S, Kim H, Garcia-Perez I, et al. : The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med 2019;11:eaan5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Walsh ME, Bhattacharya A, Sataranatarajan K, et al. : The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015;14:957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bohannon RW: Grip strength: An indispensable biomarker for older adults. Clin Interv Aging 2019;14:1681–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Parada Venegas D, De la Fuente MK, Landskron G, et al. : Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.