Abstract
Significance: Nonhealing wounds have been the subject of decades of basic and clinical research. Despite new knowledge about the biology of impaired wound healing, little progress has been made in treating chronic wounds, leaving patients with few therapeutic options. Diabetic ulcers are a particularly common form of nonhealing wound.
Recent Advances: Recently, investigation of therapeutic nucleic acids (TNAs), including plasmid DNA, small interfering RNA, microRNA mimics, anti-microRNA oligonucleotides, messenger RNA, and antisense oligonucleotides, has created a new treatment strategy for chronic wounds. TNAs can modulate the wound toward a prohealing environment by targeting gene pathways associated with inflammation, proteases, cell motility, angiogenesis, epithelialization, and oxidative stress. A variety of delivery systems have been investigated for TNAs, including dendrimers, lipid nanoparticles (NPs), polymeric micelles, polyplexes, metal NPs, and hydrogels. This review summarizes recent developments in TNA delivery for therapeutic targets associated with chronic wounds, with an emphasis on diabetic ulcers.
Critical Issues: Translational potential of TNAs remains a key challenge; we highlight some drug delivery approaches for TNAs that may hold promise. We also describe current commercial efforts to locally deliver nucleic acids to modulate the wound environment.
Future Directions: Localized nucleic acid delivery holds promise for the treatment of nonhealing chronic wounds. Future efforts to improve targeting of these nucleic acid therapies in the wound with both spatial and temporal control through drug delivery systems will be crucial to successful clinical translation.
Keywords: chronic wound, drug delivery, gene therapy, nucleic acids, wound healing, biomaterials

Paula T. Hammond, PhD
Scope and Significance
Nonhealing wounds pose a particularly significant challenge to clinicians, and few advances have been made in the last few decades. Diabetic ulcers are one particularly common source of nonhealing wounds. The increasing prevalence of diabetes and its associated complications throughout the world render strategies to improve ulcer healing even more imperative.
Translational Relevance
The interplay between wound healing biology and drug delivery enables the delivery of therapeutic nucleic acids (TNAs) that can modulate the wound environment to be prohealing. This work specifically couples wound healing, inflammation, angiogenesis, and RNA biology with biomaterials, drug delivery, and gene therapy research.
Clinical Relevance
Strategies to deliver TNAs that enable healing of chronic wounds have been explored in preclinical studies, clinical trials, and commercial ventures. They represent the potential future of wound care. This review is relevant to those who care for chronic wounds.
Mechanism of Wound Healing
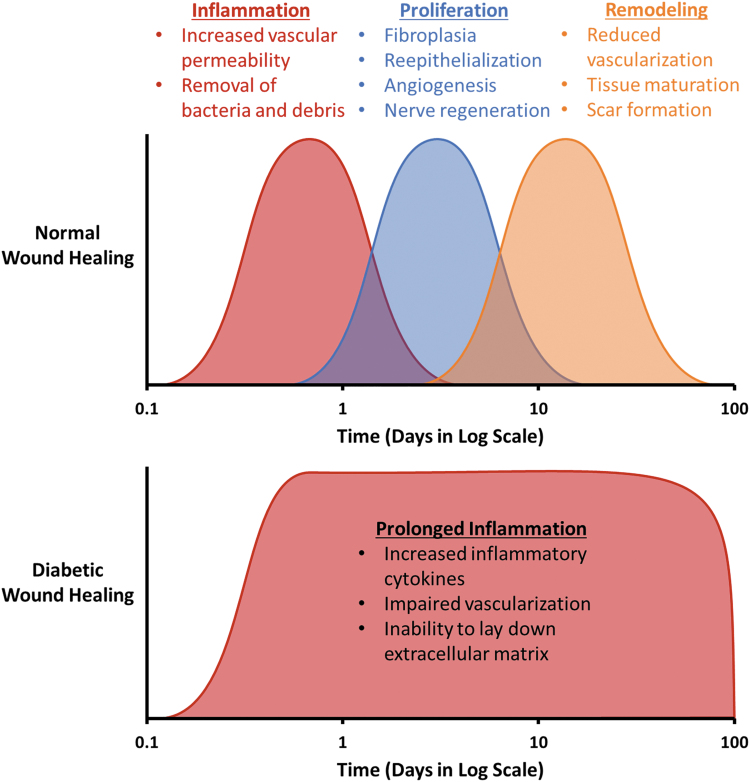
Wound healing is an intricate network of interactions between multiple cell types, growth factors, and cytokines. The course of wound healing has classically been organized into three overlapping stages: inflammation, proliferation, and remodeling (Fig. 1). The role of the inflammatory phase is to contain damage and eliminate pathogens. Inflammation typically lasts for a few days, but may persist up to 2 weeks.1 Release of the neuropeptide substance P from the peripheral nerves in a wound leads to increased vascular permeability, and various chemoattractants are released, allowing an influx of neutrophils and monocytes.2,3 Throughout the course of the inflammatory phase, monocytes mature into macrophages.4 Macrophages of the inflammatory phenotype (M1) work to remove bacteria and debris.5 Once the wound is free of foreign material, macrophages polarize toward the anti-inflammatory (M2) state to resolve the inflammatory phase.6 The proliferative phase is marked by fibroplasia with fibrous tissue deposition, reepithelialization, angiogenesis, and nerve regeneration.7 Proliferation starts ∼2–3 days after injury.1 Fibroblasts migrate into the wound area to initiate production of collagen.8 Keratinocytes begin to proliferate, covering the wound and initiating wound closure.9 Vascularization is initiated as proangiogenic factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor 2 (FGF-2), and platelet-derived growth factor (PDGF), are released.10 Schwann cells work to regrow injured nerves.11 The proliferative stage typically lasts up to a few weeks. The remodeling phase begins 2–3 weeks after injury and can last for months. During the remodeling phase, vascularization is reduced and collagenases break down granulation tissue. Collagen type I is synthesized as collagen type III degrades.12 The wound tissue matures and is restored to a functional state.
Figure 1.
Healthy wound healing can be broken into three overlapping phases. In diabetic wound healing, it is believed that a chronic inflammatory state drives poor healing. Color images are available online.
TNAs are used in various ways to locally restore the wound to this normal healing process. Most of the examples discussed in this review are based on improving the healing of diabetic ulcers; however, venous ulcers, pressure ulcers, burns, and myocardial infarction also have unique needs that can be addressed with TNAs, and we also present some examples of treating these other forms of chronic wounds.
Chronic Diabetic Wounds
The prevalence of diabetes is estimated at about 12–14% in the United States and has increased over the past few decades.13,14 Throughout the world, incidence of this disease is estimated to grow 55% by 2035 compared to 2013.15 This highly prevalent disease is associated with many serious complications, including diabetic ulcers, which are nonhealing wounds that often occur in extremities. Chronic wounds are estimated to cost Medicare $28.1–96.8 billion each year, with diabetic foot ulcers costing about $18.7 billion.16,17 Despite efforts to prophylactically prevent ulcers, the lifetime incidence is about 15%.18,19
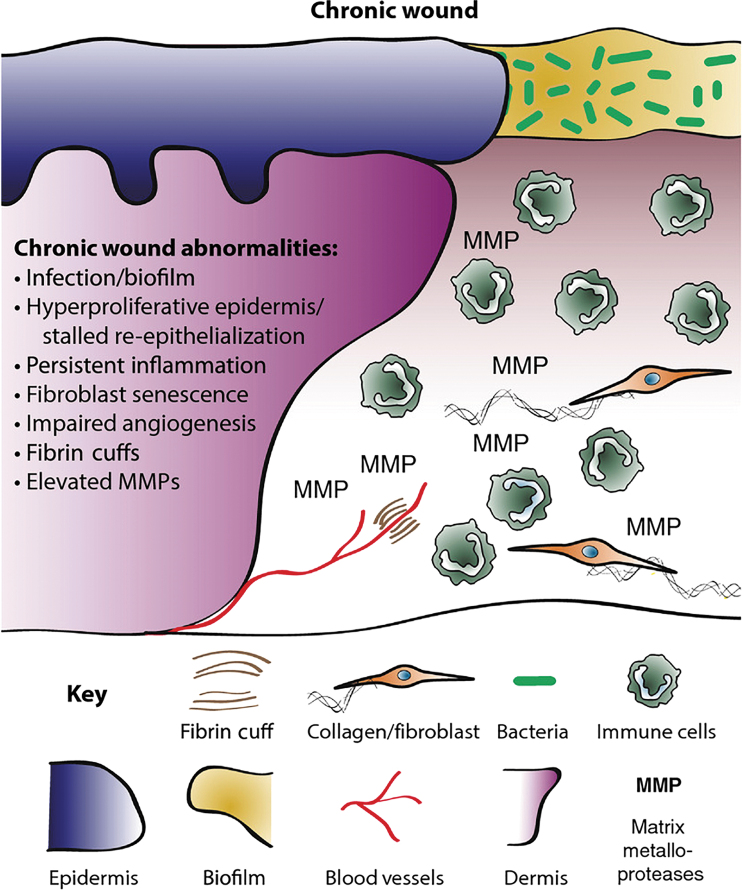
A detailed mechanism of diabetic ulcers is outside the scope of this review, but we refer readers to reviews by Martin and Nunan20 or Davis et al.20,21 A diagram highlighting the key issues leading to chronic wounds is presented in Fig. 2. In general, diabetic ulcers are believed to form when excess glucose damages the ends of nerve fibers, causing neuropathy, and when advanced glycation end products deposit in the endothelium of the microvasculature, causing reduced blood flow distally. The wound becomes unable to progress from the inflammatory to the proliferative stage of wound healing (Fig. 1). There is increased inflammatory cell infiltrate, especially neutrophils, and proinflammatory cytokines.18,20,21 This overly inflammatory environment is coupled with impaired neovascularization, which decreases granulation tissue formation.20 Keratinocytes overproliferate at the wound edge, but decreased migration prevents their transport into the core of the wound. In addition, it has been shown that peripheral nerve damage itself can impair healing, as neuropeptides, such as substance P, have been implicated in chronic wound healing.22
Figure 2.
Many interconnected mechanisms cause poor wound healing in diabetics. Current research suggests that local delivery of TNAs can intervene at almost all stages. Reproduced from Martin and Nunan under a CC-BY 4.0 license.20 TNA, therapeutic nucleic acid. Color images are available online.
Growth factors and cytokines important for wound resolution, such as transforming growth factor (TGF-β), PDGF, FGF-2, and epidermal growth factor (EGF), have a reduced presence in chronic wounds (Table 1).23 This is contrasted with the overexpression of matrix metalloproteinases (MMPs), which cause destruction of deposited extracellular matrix (ECM).24 Loss of tissue inhibitors of metalloproteinase expression exacerbates MMP-mediated ECM destruction.25,26 These growth factors and cytokines can be targets for TNA drug delivery systems targeting chronic wounds.
Table 1.
Several factors are commonly thought to be dysregulated in chronic diabetic wounds
| Factor | Function | Level in Chronic Wounds |
|---|---|---|
| TGFβ | Fibrosis, inflammatory resolution | ⇩ |
| FGF2 | Epithelialization, angiogenesis, tissue remodeling | ⇩ |
| PDGF | Angiogenesis, mesenchyme-derived cell proliferation and migration | ⇩ |
| EGF | Epithelialization | ⇩ |
| VEGF | Angiogenesis | ⇩ |
| CTGF | Scar formation, fibroblast stimulation, ECM deposition | ⇩ |
| IL1 | Proinflammatory | ⇧ |
| IL6 | Proinflammatory | ⇧ |
| TNFα | Proinflammatory | ⇧ |
| MMPs | Protease | ⇧ |
| TIMPs | Antiprotease | ⇩ |
| Neutrophil elastase | Antimicrobial, fibronectin degradation | ⇧ |
Adapted from Barrientos et al. with permission from John Wiley and Sons.23
CTGF, connective tissue growth factor; ECM, extracellular matrix; TGFβ, transforming growth factor β; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; IL, interleukin; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; TNFα, tumor necrosis factor α.
Patients with diabetic ulcers are left with few therapeutic options. Treatment typically focuses on offloading (redistributing load away from the wound), covering the wound, maintaining a moist environment, and administering antibiotics as needed to prevent infection and biofilm formation. Despite these efforts, many patients require amputation. Recent global estimates suggest that about 21–29% of patients with nonhealing diabetic ulcers undergo amputation.17,27–29 There is an urgent need for the development of new therapeutic approaches to facilitate healing of diabetic ulcers, and despite decades of research, little clinical progress has been made.
Advantages of Therapeutic Nucleic Acids
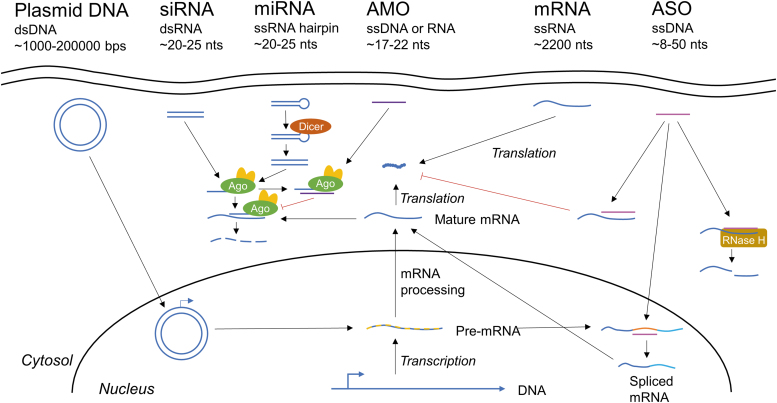
TNAs have many advantages over small molecules or protein therapeutics. TNAs include plasmid DNA, small interfering RNA (siRNA) oligonucleotides, antisense oligonucleotides (ASOs), microRNA (miRNA) mimics, anti-miRNA oligonucleotides (AMO), and messenger RNA (mRNA). Figure 3 demonstrates the mechanism of each of these therapies. The advantages of TNAs include the following: (1) nucleic acids can temporarily or permanently correct the biology underlying the disease process at the gene level, (2) nucleic acids may have a more durable effect, as they can regulate protein expression for long time periods without being susceptible to rapid clearance or degradation as is the case with proteins, (3) Watson–Crick base pairing of nucleic acids allow greater specificity than many conventional small molecule drugs, (4) nucleic acids can address previously “undruggable” targets, such as transcription factors, (5) delivery of nucleic acids can be modular, as changing the sequence of the bases has little effect on interaction with a drug delivery carrier, (6) software exists to design the TNA sequence, and (7) the reduction in price of whole-genome sequencing has provided an efficient method to identify the target mutation and develop TNAs. The power of TNAs was recently highlighted in a study where whole-genome sequencing was used to identify a mutation causing neurodegenerative disease in a pediatric patient. Within a year, a custom ASO was developed, verified, and administered.30 Overall, the modularity of TNAs means that they hold great promise for a diverse range of disease processes, including wound healing complications.
Figure 3.
TNAs act in diverse ways to regulate gene expression levels. Plasmid DNA is taken up into the nucleus, where it can be directly transcribed into pre-mRNA. siRNA acts through RNAi to downregulate gene expression. The siRNA binds with Ago2 as part of the RISC, complexing with the targeted mRNA and degrading it. miRNA can control multiple genes at once, and works similarly to siRNA, but is a single-stranded RNA hairpin that must be cleaved into a double-stranded RNA by Dicer. It is worth noting that miRNA mimics are double-stranded RNA to mimic a mature miRNA that has already been processed by Dicer. AMOs bind to miRNA loaded with Ago, blocking mRNA degradation. Delivered mRNA can be directly translated into protein. ASOs work by three mechanisms: (1) binding mRNA and blocking transcription, (2) modifying mRNA splicing, and (3) inducing RNase H-mediated mRNA cleavage. Ago2, argonaute 2; AMO/anti-miR, anti-microRNA oligonucleotide; ASO, antisense oligonucleotide; RNAi, RNA interference; siRNA, small interfering RNA; miRNA, micro RNA; RISC, RNA-induced silencing complex; mRNA, messenger RNA. Color images are available online.
Recently, there has been excitement about Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs)-based gene editing systems, which enable manipulation of the genome sequence in vitro and in vivo.31 Nonviral drug delivery systems have been developed for CRISPR32; however, the permanent nature of CRISPR gene editing renders it unsuitable for the dynamic, transient stages of the wound healing process. As such, little research has emerged on using CRISPR for wound healing applications, and it will not be covered in this review. One exception to this may be CRISPRi, which uses a catalytically dead Cas9 endonuclease to transiently silence rather than edit genes.33
Despite the many advantages of TNAs and many decades of research into them, TNAs are just now starting in clinical trials and gaining United States Food and Drug Administration (FDA) approval for a host of systemic diseases ranging from high cholesterol to liver amyloidosis.34,35 TNAs for wound healing have also entered clinical trials (see “Commercial ventures” section).
One of the biggest hurdles to improving TNA efficacy is effective delivery. Key barriers to delivery of TNAs include the following: targeting to the lesion of interest, avoiding innate immune system activation, and promoting cell uptake and endosomal escape.36 In addition, less stable nucleic acids, such as single stranded mRNA, rely on their drug delivery carrier to prevent degradation by RNases. Drug delivery carriers can address many of these issues, enabling realization of nucleic acid treatment for chronic wound healing.
Drug Delivery Approaches
Many cellular and acellular biomaterial approaches, such as acellular scaffolds, stem cells, and cell-derived exosomes, have been described for treating chronic wounds37–40; however, herein, we focus on the challenges of delivering TNAs. A wide variety of materials have been used preclinically to overcome barriers to TNA delivery. In this review, we focus on nonviral approaches, which are less or nonimmunogenic, can incorporate larger payloads, are easier to manufacture, and lack the safety issues of viruses. Much of this is because unlike viruses, nonviral approaches are nonintegrating, thus reducing the risk of insertional mutagenesis and carcinogenicity. Nonviral approaches also tend to be more modular and enable controlled release of the TNA through a drug delivery system.41–43
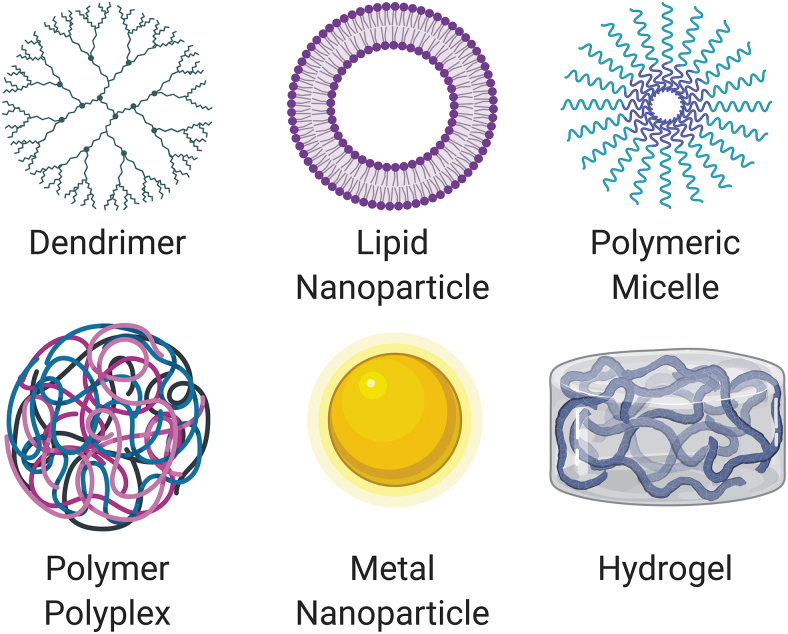
General categories of synthetic materials for drug delivery carriers include dendrimers, lipid nanoparticles (NPs), polymeric micelles, polyplexes, metal NPs, and hydrogels (Fig. 4). Combinations of these categories exist as well to leverage the unique properties that each provides. Normally, these delivery carriers have polycationic moieties that foster ionic complexation with the negatively charged phosphate backbone of TNAs and aid endosomal escape following cellular uptake.44–47 To date, most in vitro delivery of TNAs is performed with cationic lipids, which are amphiphiles with a polycationic head group and hydrophobic tail groups. While cationic lipids were initially thought to be too toxic for in vivo application, certain lipids have been developed for safe low-dose usage in vivo.48 The first siRNA therapy to gain FDA approval, Patisiran, is delivered with a lipid NP targeted to the liver.49
Figure 4.
General classes of synthetic materials used for TNA drug delivery include dendrimers, lipid nanoparticles, polymeric micelles, polymeric polyplexes, metal nanoparticles, and hydrogels. Combinations of these classes of materials exist. Created with Biorender.com. Color images are available online.
Delivery to the wound environment has its own advantages and challenges.38 One of the major advantages is that the dermal location allows localized delivery strategies with placement directly on the wound, preventing the need for active targeting. In addition, the ability to place the therapeutic directly onto the wound reduces the diffusion distance from the scaffold or bandage to the cells of the wound, reduces potential for systemic side effects, and can increase accumulation of the therapeutic in the targeted wound cells. This local delivery is in contrast to systemic delivery for which the carrier must circulate stably in the bloodstream and accumulate in the targeted tissue of interest.
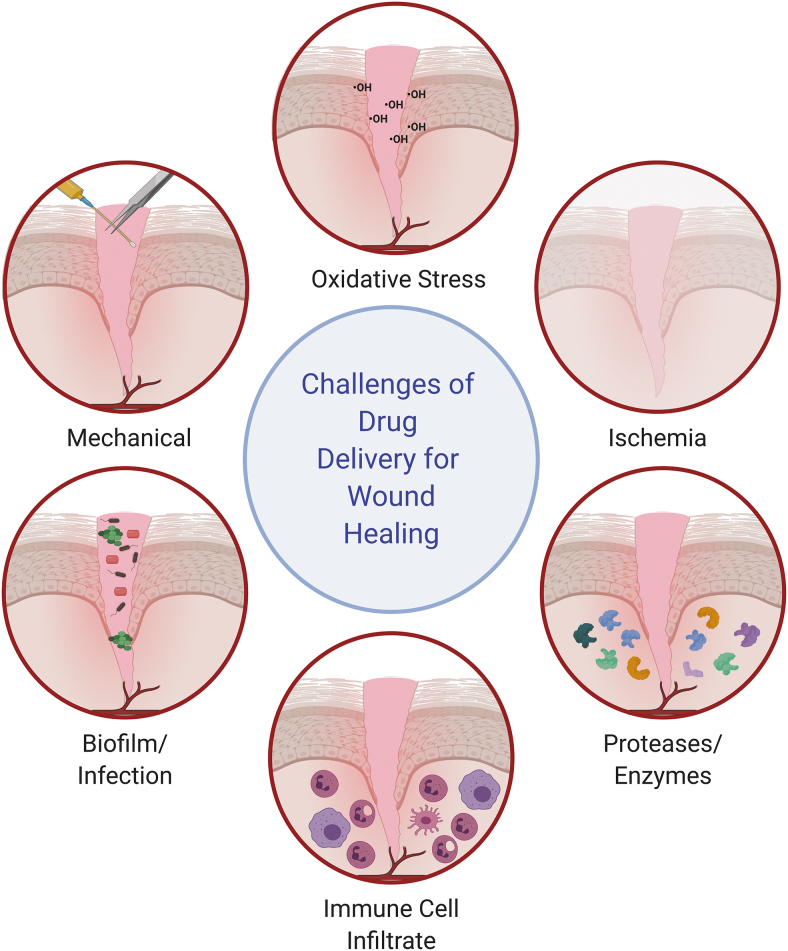
Despite these advantages, there are also unique challenges of the wound environment, described by Whittam et al.50 in five parts: (1) mechanical, as the openness of the delivered therapeutics to the outside world creates challenges in retaining them with dressing changes, debridements, or normal movement; (2) oxidative stress, as high levels of free radicals can damage therapeutics, their drug delivery carriers, or the cells to which they are targeted; (3) enzymatic, as high levels of proteases can degrade any drug delivery system and reduce levels of endogenous growth factors (some of these enzymes, such as MMPs, are upregulated in the chronic wound,51 posing even greater challenges for chronic wound treatment); (4) hypoxia and ischemia, as low levels of perfusion reduces cell viability and creates edema, which can slow transport kinetics of drug delivery systems and lower local pH conditions; and (5) infected/necrotic tissue, as this can impact drug transport in the wound tissue and influence which cells uptake the therapeutics.50 Biofilms may prevent TNA diffusion to the targeted wound cells and can prolong the chronic wound state through excess inflammation.52 The large immune cell infiltrate can also challenge drug delivery, in addition to the five challenges described by Whittam et al.,50 as immune cells can phagocytose the TNAs, modulating delivery to the target cells. These six challenges are highlighted in Fig. 5.
Figure 5.
Challenges of drug delivery in the wound healing environment include oxidative stress, ischemia, high levels of proteases and enzymes, a high immune cell infiltrate, biofilms/infection, and mechanical stresses to the wound. Concept adapted from Whittam et al.50 Created with Biorender.com. Color images are available online.
Preclinical Approaches
While much work has been done in this field for over a decade, this review will predominantly focus on literature from the last few years. The approaches are summarized in Table 2, organized by overall target strategy and TNA class.
Table 2.
A summary of preclinical studies using local delivery of therapeutic nucleic acids to modulate wound healing, ordered by addressed challenge
| Addressed Challenge | Target | TNA | Drug Delivery System Classification | Other Features of System | Release Kinetics | In Vivo Model Type | In Vivo Healing Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Epithelialization/cell migration | miR-210 | AMO | Lipid nanoparticles | Antimicrobial peptide-modified lipid nanoparticles | NR | Bi-pedicle flap wound in mice | • Increased wound closure over 7 days • Increased reepithelialization |
Ghatak et al.105 |
| Epithelialization/cell migration | miR-107 | AMO | Lipid nanoparticles | Keratinocyte-targeting peptide grafted on lipid nanoparticles | NR | Burn wound in mice | • Increased wound closure over 24 days • Reduced transepidermal water loss |
Li et al.106 |
| Epithelialization/cell migration | miR-378a-5p | AMO | Metal nanoparticles | Gold nanoparticles decorated with thiol-modified AMO | NR | Full-thickness wound in mice | • Increased wound closure over 6 days | Li et al.107 |
| Epithelialization/cell migration | Cx43 | ASO | Hydrogel | Topical application with Pluronic F-127 (PEG-PPG-PEG) gel | NR | Full-thickness wound in streptozocin-induced diabetic rats | • Increased wound closure over 5 days | Wang et al.124 |
| Epithelialization/cell migration | Cx43 | ASO | Hydrogel-polymer nanoparticle hybrids | Collagen scaffold decorated with PCL or PLGA nanoparticles containing ASO | Release over 7 days | Full-thickness wound in rats | • Increased wound closure over 5 days • Increased reepithelialization • Decreased granulation tissue long-term |
Gilmartin et al.125 |
| Epithelialization/cell migration | Smad3 | ASO | Hydrogel | Hydrogel made of chitosan and sodium alginate | Release over 24 h | Full-thickness wound in mice | • Increased wound closure over 15 days | Hong et al.126 |
| Epithelialization/cell migration | KGF | Plasmid DNA | Hydrogel | Electrospun mesh of poly(l-lactide) and PCL with layered PEI and DNA | Release over 1 week | Full-thickness wound in mice | • Increased wound closure over 7 days • Increased epidermal thickness |
Kobsa et al.97 |
| Epithelialization/cell migration | FL2 | siRNA | Polymer nanoparticles | Siloxane nanoparticles | NR | Full-thickness or burn wound in mice | • Increased wound closure at 6 or 7 days • Increased reepithelialization • Increased collagen |
Charafeddine et al.62 |
| Epithelialization/cell migration | FL2 | siRNA | Micelle-nanoparticle hybrid | Collagen microparticles in a surfactant polymer dressing | NR | Full-thickness or burn wound in mice | • Increased wound closure at 4 days • Decreased epidermal thickness • Increased reepithelialization • Increased collagen • Increased vascularization |
O'Rourke et al.64 |
| Epithelialization/cell migration | FliI | siRNA | Metal nanoparticles | Intradermal injection of thermally hydrocarbonized porous silicon nanoparticles coated with chitosan | 80% release after 35 h | Full-thickness wound in mice | • Increased wound closure at 7 days | Turner et al.65 |
| Excess proteases | MMP-9 | siRNA | Hydrogel | Layer by layer onto a Tegaderm bandage | Sustained release over 2 weeks | Full-thickness wound in db/db mice | • Increased wound closure at 1 and 2 weeks • Increased tissue thickness • Increased vascularization • Increased collagen |
Castleberry et al.55 |
| Excess proteases | MMP-9 | siRNA | Polymer-dendrimer hybrid | Intradermal injection of βCD-PAMAM star shaped polymer | NR | Full-thickness wound in streptozocin-induced diabetic rats | • Increased wound closure at 7 days | Li et al.58 |
| Excess proteases | MMP-9 | siRNA | Polymer-dendrimer polyplex hybrid | Intradermal injection of βCD-PAMAM star shaped polymer | NR | Full-thickness wound in streptozocin-induced diabetic rats | • Increased wound closure at 7 days • Increased collagen • Decreased neutrophil infiltration |
Li et al.57 |
| Excess proteases | MMP-9 | siRNA | Polymer-dendrimer hybrid | Bacterial cellulose grafted with cationic side chain or dendrimer | Release over about 1 week | Full-thickness wound in streptozocin-induced diabetic rats | • Increased wound closure at 7 days | Li et al.59 |
| Excess proteases | MMP-2 | siRNA | Nanofibrous mesh-polymer hybrid | PEI grafted onto nanofibrous mesh with MMP-responsive element | Only in presence of MMPs | Burn wound in streptozocin-induced diabetic mice | • Increased wound closure at 7 days • Increased keratin 5 and 14 expression • Increased collagen |
Kim and Yoo60 |
| Excess proteases | MMP-2 | siRNA | Hydrogel | Four-arm PEG with MMP-responsive element | Only in presence of MMPs | Burn wound in streptozocin-induced diabetic mice | • Increased wound closure at 14 days • Increased keratin 5 and 14 expression |
Kim et al.61 |
| Hypoxia | miR-615-5p | AMO | N/A | Intradermal injection around wound edge | N/A | Full-thickness wound in db/db mice | • Increased wound closure over 9 days • Increased granulation tissue thickness • Increased vascularization |
Icli et al.108 |
| Hypoxia | miR-135a-3p | AMO | N/A | Intradermal injection around wound edge | N/A | Full-thickness wound in db/db mice | • Increased wound closure over 9 days • Increased granulation tissue thickness • Increased vascularization |
Icli et al.109 |
| Hypoxia | miR-26a | AMO | N/A | Intradermal injection around wound edge | N/A | Full-thickness wound in db/db mice | • Increased wound closure over 9 days • Increased granulation tissue thickness • Increased vascularization |
Icli et al.111 |
| Hypoxia | miR-92a | AMO | N/A | Intradermal injection around wound edge | N/A | Full-thickness wound in db/db mice and porcine model | • Increased reepithelialization • Increased granulation tissue thickness • Increased vascularization |
Gallant-Behm et al.112 |
| Hypoxia | miR-92a | AMO | N/A | Intradermal injection around wound edge | N/A | Full-thickness wound in db/db mice | • Increased reepithelialization • Increased granulation tissue area • Increased vascularization |
Lucas et al.113 |
| Hypoxia | miR-184b | miRNA mimic | Hydrogel | Topical application of pluronic F-127 (PEG-PPG-PEG) gel | NR | Full-thickness wound in mice | • Increased wound closure over 7 days • Increased vascularization |
Miscianinov et al.115 |
| Hypoxia | VEGF | Plasmid DNA | N/A | Sonoporation | N/A | Full-thickness wound in streptozocin-induced diabetic mice | • Increased wound perfusion at 2 and 6 days | Ko et al.84 |
| Hypoxia | VEGF | Plasmid DNA | Dendrimer | Generation 4 PAMAM dendrimer | NR | Full-thickness wound in streptozocin-induced diabetic mice | • Increased wound closure over 12 days | Kwon et al.85 |
| Hypoxia | VEGF | Plasmid DNA | Polymer polyplex | Epidermal stem cell-loaded gelatin scaffold with βCD-PEI polyplexes | NR | Full-thickness wound in rats | • Increased wound closure over 14 days • Increased vascularization |
Peng et al.86 |
| Hypoxia | VEGF | Plasmid DNA | Polymer scaffold | Porous collagen-chitosan/silicone membrane | Release over about 2 weeks | Burn injury in porcine model | • Increased vascularization • Increased tissue tensile strength |
Guo et al.87 |
| Hypoxia | VEGF | Plasmid DNA | Polymer nanoparticles | PEI grafted to PLGA nanoparticles | Release over about a month | Full-thickness wound in streptozocin-induced diabetic rats | • Increased wound closure over 16 days • Increased vascularization • Increased granulation tissue • Increased collagen |
Shi et al.88 |
| Hypoxia | VEGF | Plasmid DNA | Hydrogel with polymer polyplexes | Hydrogel containing modified hyaluronic acid, dextran, and βCD containing polyplexes with PEI | NR | Burn wound in rats | • Increased wound closure over 21 days • Increased vascularization • Reduced inflammation |
Wang et al.90 |
| Hypoxia | VEGF | Plasmid DNA | Metal nanoparticles | Antimicrobial peptides grafted on gold nanoparticles | NR | Full-thickness wound in streptozocin-induced diabetic mice | • Increased wound closure over 10 days • Increased granulation tissue thickness • Increased epidermal thickness • Increased vascularization |
Wang et al.89 |
| Hypoxia | Angiogenin | Plasmid DNA | Hydrogel | Electrospun mesh made of PLGA, PEI-caboxymethyl chitosan, cellulose nanocrystals | About 2 weeks | Burn wound in rats | • Increased wound closure over 28 days • Increased vascularization • Increased collagen |
Mo et al.91 |
| Hypoxia | HIF-1α | Plasmid DNA | Hydrogel with polymer polyplexes | Fibrin matrix with PEG-PLK-peptide polymer polyplexes | Over 1 week | Full-thickness wound in streptozocin-induced diabetic rats | • Increased wound closure at 7 days • Increased vascularization |
Thiersch et al.92 |
| Hypoxia | PHD2 | siRNA | Polymer scaffold-micelle hybrid | Porous poly(thioketal-urethane) ROS-sensitive scaffold loaded with siRNA-containing diblock copolymer micelles | Release within 2 days in presence of ROS | Full-thickness wound in streptozocin-induced diabetic rats | • Increased vascularization • Increased granulation tissue |
Martin et al.76 |
| Inflammation | miR-223 | miRNA mimic | Polymer nanoparticle-hydrogel hybrid | HA-PEI or HA-PEG nanoparticles in a gelatin matrix applied topically | Release over 2 days | Full-thickness wound in mice | • Increased wound closure over 12 days • Increased vascularization • Increased collagen thickness • Increased M2 polarization of macrophages |
Saleh et al.116 |
| Inflammation | miR-129-2-3p | miRNA mimic | Hydrogel | Topical application of Pluronic F-127 (PEG-PPG-PEG) gel | NR | Full-thickness wound in db/db mice | • Increased wound closure over 21 days • Reduced neutrophil infiltration |
Umehara et al.117 |
| Inflammation | miR-497 | miRNA mimic | Polymer polyplexes | PEI polyplexes with intradermal injection | NR | Full-thickness wound in streptozocin-induced diabetic mice | • Increased wound closure over 14 days • Reduced proinflammatory cytokines |
Ban et al.118 |
| Inflammation | miR-146a | miRNA mimic | Metal nanoparticles | Cerium oxide nanoparticles | NR | Full-thickness wound in db/db mice and streptozocin-induced diabetic porcine model | • Increased wound closure at 7 days in mice and over 14 days in swine • Reduced inflammation • Increased vascularization |
Zgheib et al.120 |
| Inflammation | miR-146a | miRNA mimic | Metal nanoparticle-hydrogel hybrid | Cerium oxide nanoparticles embedded in a cryogel | Release over about a month | Full-thickness wound in db/db mice | • Increased wound closure over 20 days • Increased tissue strength • Decreased proinflammatory gene expression • Increased collagen gene expression |
Sener et al.121 |
| Inflammation | IL-10 | Plasmid DNA | Polymer nanoparticles | Collagen-silica nanoparticles containing PEI polymer polyplexes | NR | N/A | NR | Wang et al.96 |
| Inflammation | IRF5 | siRNA | Lipid nanoparticles | Intravenous injection of lipid nanoparticles | NR | Full-thickness wound in mice | • Increased wound closure over 12 days • Decreased monocytes and neutrophils • Lower proinflammatory cytokines • Reduced protease activity |
Courties et al.66 |
| Inflammation | TNFα | siRNA | Lipid nanoparticles | LNPs pipetted onto wound | NR | Full-thickness wound in db/db mice | • Increased wound closure over 13 days | Kasiewicz and Whitehead68 |
| Inflammation | ERK1 | siRNA | Lipid nanoparticle-hydrogel hybrid | Chitosan-coated lipid nanoparticles or cationic lipid nanoparticles embedded in collagen matrix | Release after about 24 h | N/A | NR | Tezgel et al.70 |
| Oxidant environment | Keap1 | siRNA | Lipid nanoparticles | Cationic lipid nanoparticles with a supercharged coiled-coil protein | Release over 7 days | Full-thickness wound in db/db mice | • Increased wound closure over 30 days • Increased vascularization • Increased rekeratinization • Increased granulation tissue formation |
Rabbani et al.79 |
| Oxidant environment | XDH | siRNA | Lipid nanoparticle-hydrogel hybrid | Lipid nanoparticles embedded in agarose gel | NR | Full-thickness wound in db/db mice | • Increased wound closure over 30 days | Weinstein et al.81 |
AMO, anti-microRNA oligonucleotide; ASO, antisense oligonucleotide; PEG, polyethylene glycol; PCL, poly(caprolactone); PLGA, poly(lactic-co-glycolic acid); KGF, keratinocyte growth factor; PEI, polyethyleneimine; FL2, fidgetin-like 2; FliI, flightless I; siRNA, small interfering RNA; MMP, matrix metalloproteinase; PAMAM, polyamidoamine; VEGF, vascular endothelial growth factor; HIF, hypoxia inducible factor; PHD2, prolyl hydroxylase domain 2; PLK, poly-L-lysine; PPG, polypropylene glycol; ROS, reactive oxygen species; IL, interleukin; IRF5, interferon regulatory factor 5; TNFα, tumor necrosis factor α; ERK1, extracellular signal-regulated kinase 1; NR, not reported; N/A, not applicable; HA, hyaluronic acid.
siRNA approaches
Regulation of gene expression through siRNA delivery has been one of the most common TNA approaches for chronic wounds. This strategy involves identifying a gene transcript that is overexpressed in diabetic chronic wounds and using a corresponding siRNA to target it for knockdown. Discovery of viable targets is rooted in mechanism-based basic biological research. For many genes, siRNAs are already available, but for others, computational approaches can help find amenable sequences for gene silencing. Herein, we describe different siRNA genes that have been targeted based on the underlying biology of chronic wound healing and strategies for their local delivery to the wound bed.
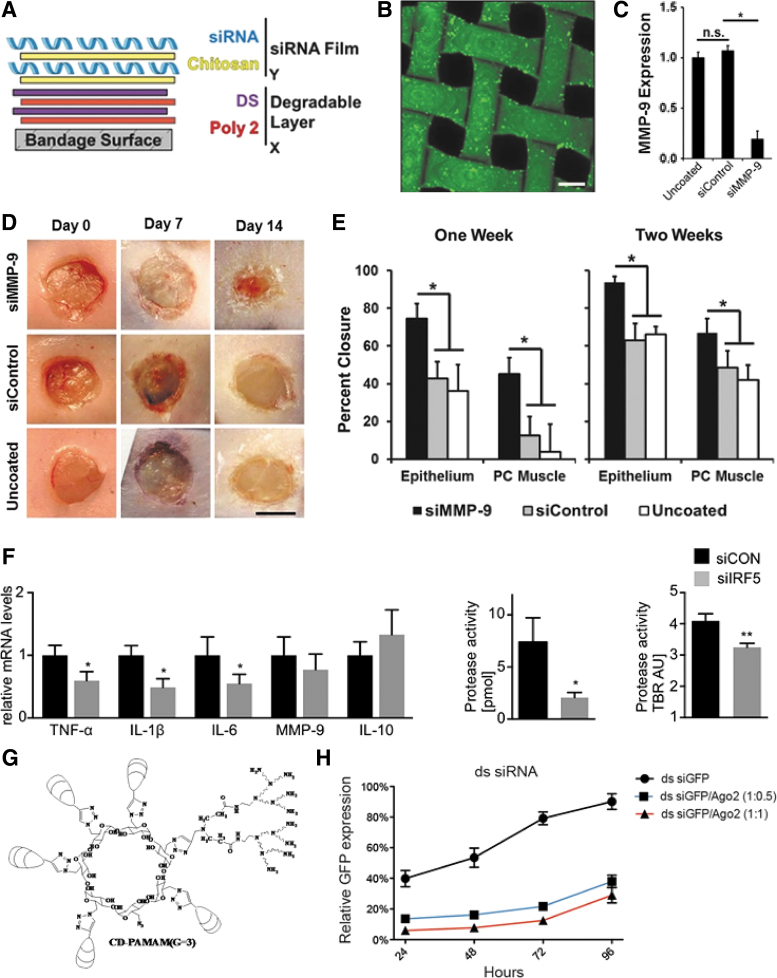
One target of interest is MMPs. Excess MMP activity can degrade the ECM that migrating keratinocytes depend on, leading to impaired wound reepithelialization.51 The Hammond Laboratory has demonstrated the application of layer-by-layer (LbL) technology, whereby alternatively charged polyions, such as siRNA, and polycationic transfection polymers, are conformally self-assembled onto a Tegaderm bandage surface, to deliver siRNA against MMP-9 (Fig. 6A, B).53 This work employed poly(β-aminoester) polymers to enable transfection, as this class of polymers has shown extensive efficacy in gene delivery.54 This system achieved sustained delivery of siRNA to the diabetic ulcer wound bed for 2 weeks. Knockdown of MMP-9 in diabetic (db/db) mice with full-thickness excisional wounds increased tissue thickness, percent wound closure, angiogenesis, overall collagen, and the ratio of collagen I:III, all signifying enhanced wound healing (Fig. 6C–E).55 β-Cyclodextrin grafted with the polycation dendrimer polyamidoamine (PAMAM, Fig. 6G) has also been used to deliver siRNA. With this system, a small molecule drug, methotrexate, releases after about 12 h, but release kinetics for siRNA are not reported.56 Li et al. used this platform to deliver siRNA against MMP-9, thus enhancing wound closure rate.57,58 siRNA against MMP-9 has recently been delivered using a bacterial cellulose modified with polycations, such as PAMAM and 3-(dimethylamino)-1-propylamine. The bacterial cellulose provided a degradable scaffold for the sustained release of siRNA over the course of about 1 week.59
Figure 6.
siRNA approaches enhance diabetic ulcer healing. (A) Scheme of LbL bandage coating with alternating layers of polycation and polyanions. (B) The thin film assembly produces uniform loading of a fluorescently labeled siRNA onto the Tegaderm. (C) In vivo MMP-9 expression at 2 weeks. (D) Representative gross images of the wound at 1 and 2 weeks. (E) Quantification of epithelium and PC muscle closure at 1 and 2 weeks postinjury. (A–E) Reproduced from Castleberry et al. with permission from John Wiley and Sons.55 (F) Excisional wounds were created in mice and injections with siIRF5 or a control siRNA were dosed daily for 4 days. Change in the levels of inflammatory markers and proteases after wound treatment with siIRF5 was measured. Reproduced from Courties et al. with permission from Elsevier.66 (G) Structure of a β-cyclodextrin-graft-gen3-PAMAM carrier for siRNA delivery. Reproduced from Li et al. with permissions from American Chemical Society.57 (H) Double-stranded (ds) RNA was co-delivered into GFP-expressing 293T cells at different ratios of RNA to Ago2 protein. Flow cytometry was used to quantify GFP expression levels in the cells as a function of time. Reproduced from Li et al. with permission from the National Academy of Sciences.83 *p < 0.05, **p < 0.01. LbL, layer-by-layer; PC, panniculus carnosus; MMP, matrix metalloproteinase; ns, not significant; TBR, target to background ratio; IRF5, interferon regulatory factor 5; PAMAM, polyamidoamine; GFP, green fluorescent protein. Color images are available online.
Kim and Yoo went beyond sustained release to show environmentally responsive release of siRNA against MMP-2. By incorporating an MMP-cleavable linker between their polyethyleneimine (PEI) transfection polymer and their electrospun nanofibrous mesh, they could control the release of siRNA as a function of the wound's instantaneous MMP-2 levels. Electrospinning applies a high voltage to a polymer pushed through a syringe and needle to create very thin nanofibers that assemble into a mesh. They showed the ability to limit the diffusion of siRNA from the nanofibrous mesh in the absence of MMP-mediated linker cleavage, only releasing drug when MMP-2 is present; however, in vivo siRNA release kinetics were not elucidated in this study.60 These authors later used a similar approach, where they conjugated the siRNAs with a polyethylene glycol (PEGylated) MMP-cleavable linker to create a four-arm, locally responsive gene delivery nanocarrier. In vivo, the nanocarrier dissociated in the MMP-rich environment of dorsal wounds in diabetic mice. The wounds treated with the four-arm siRNA carrier showed higher complete closure rates and increased keratinization.61
Other efforts have focused on delivering siRNA to alter cell motility into the wound. Charafeddine et al. looked at siRNA to alter microtubule cytoskeleton and cell motility by targeting fidgetin-like 2 (FL2).62 FL2 stimulates microtubule depolymerization and promotes cell migration. Although the link between these two functions is not completely elucidated, microtubules are suggested to control cell movement by the assembly of integrin-based focal adhesion complexes.63 siRNA against FL2 was loaded into siloxane-based NPs and delivered to full-thickness and thermal burn wounds. The siRNA-loaded NPs enabled significantly increased healing rates, increased collagen organization in the wound bed, and increased reepithelialization. Although this study did not report release kinetics of the siRNA from their NPs, the biological effect was more pronounced when dosed on both days 0 and 2 versus just on day 0, suggesting that a sustained delivery method may enhance efficacy.62 The authors recently followed up with a second study where they encapsulated the siRNA against FL2 in single emulsion collagen microparticles and embedded these into a nonionic amphiphilic micelle-based polymer dressing; however, the siRNA release kinetics were not described.64 While these articles did not focus on chronic wounds, they successfully delivered siRNA targeting a novel gene, FL2, to stimulate migration of keratinocytes and fibroblasts into the wound.
Turner et al.65 targeted the gene Flightless I (FliI), which also plays a role in cytoskeletal remodeling following injury and cell migration. siRNA against FliI was loaded into thermally hydrocarbonized porous silicon NPs, where thermal hydrocarbonization prevents oxidation of the reactive surface in aqueous solution. They coated these NPs with chitosan to enhance the polycationic charge density necessary for efficient transfection. The chitosan coating also slowed the release of siRNA from the NPs, releasing 80% of the siRNA after about 35 h. The authors tested their design in mice by intradermal injection to the wound bed and found improved wound healing.65
Others have attempted to modulate the chronic inflammatory state of the wound bed by altering macrophage phenotype. Courties et al.66 used lipidoid NPs to deliver siRNA against interferon regulatory factor 5 (IRF5), a macrophage transcription factor that upregulates M1 inflammatory macrophage phenotype. The authors hypothesized that they could polarize macrophages toward the M2 phenotype, priming them for tissue regeneration (Fig. 6F). This study highlights a key advantage of siRNA therapy: the ability to target transcription factors. Of note, they also showed this approach to improve healing after a myocardial infarction.66 Kasiewicz and Whitehead used lipidoid NPs to deliver siRNAs that silence tumor necrosis factor α (TNFα), a proinflammatory cytokine, and monocyte chemoattractant protein 1 (MCP-1).67 They recently showed the efficacy of their system in vivo.68 RNA interference (RNAi) of collapsin response mediator protein-2 has been investigated as a method to induce the M2 macrophage phenotype, decreasing inflammation and fibrosis after myocardial infarction.69 We believe that a similar approach could work in a chronic dermal wound and warrants further studies. Recently, Tezgel et al.70 demonstrated delivery of siRNA against extracellular signal regulated kinase (ERK) 1, which has been implicated in diverse cell signaling processes, including those related to initiating an inflammatory response.71 They used nanostructured lipid carriers to deliver the siRNA, and had controlled release, although not for more than about 24 h.70 In vivo evaluation of their delivery strategy is needed to better understand its translational potential. In addition, the many diverse downstream effects of the ERK-based signaling pathways cause concern about off-target effects from its inhibition.
Others have used localized delivery of siRNA to target the hypoxic environment of wounds. Prolyl hydroxylase domain 2 (PHD2) has become an attractive target for drug delivery to enhance healing of diabetic ulcers.72–74 PHD2 is a protein that causes hypoxia-inducible factor (HIF) hydroxylation and degradation in normoxia, thereby decreasing proangiogenic factors.75 The Duvall Laboratory looked at controlling the release of siRNA to the wound bed using scaffolds that degrade with a rate controlled by the local concentration of reactive oxygen species (ROS). Martin et al. made a polymer that would cleave upon the production of local ROS to release siRNA against PHD2 in the course of about 2 days. Further honing the sensitivity of the system to release with lower ROS concentrations will be critical to its translational potential.76 This environmentally responsive system expanded upon previous work that used pH-responsive block copolymer NPs in a polyurethane scaffold for delivery of siRNA against PHD2.77,78
The highly oxidative environment of the wound has also received attention as a target for local TNA delivery to the chronic wound. Rabbani et al.79 discovered that targeting Keap1, which represses nrf2—a central regulator of the oxidoreductive state of the cell, could enhance wound healing in a diabetic model.80 To locally deliver this siRNA, they synthesized a lipoproteoplex that combined a cationic lipid with a supercharged coiled-coil protein. The coiled-coil protein was designed such that arginine residues were at solvent-exposed sites and could facilitate ionic interactions with the phosphate group of RNA. The addition of the coiled-coil protein enhanced transfection and nucleic acid complexation. It is unclear what effect the coiled-coil structure itself has on nucleic acid transfection versus the polyarginine cationic polymer, but it may enable formation of a hydrophobic core that can be used to co-deliver TNAs with small-molecule drugs.79 Weinstein et al.81 focused on siRNA delivery to knockdown xanthine dehydrogenase, which led to decreased xanthine oxidase activity, and thus reduced ROS levels. The authors delivered their siRNA through complexation with a commercial transfection reagent and incorporation into agarose. Release kinetics from the agarose gel are not reported. They had to reapply this mixture once a week, likely due to the fact that their strategy may not have been a controlled release method.81
Two relatively new technologies have the potential to greatly enhance the effects of siRNA-based therapy. Although not used directly for targeting chronic wounds to the best of our knowledge, nanoneedles present a novel strategy for delivering DNA and siRNA without the need for transfection reagents. Using this method, which is facilitated by the penetration of nanoneedles through tissues and cell membranes, a currently uncharacterized mechanism leads to the drugs interfacing directly with the cytosol of the target cells. By eliminating the challenge of endosomal escape, this approach has great potential to radically change TNA delivery.82 Some of our laboratory's recent work on codelivery of siRNA with argonaute 2 (Ago2), the protein that mediates siRNA-based mRNA degradation, has the ability to drastically improve the efficacy of siRNA-based therapies. We have shown that co-delivery of self-assembled Ago2 with siRNA leads to more potent and durable gene knockdown than siRNA alone (Fig. 6H)83; such approaches could be adapted for direct local delivery to wounds.
Plasmid approaches
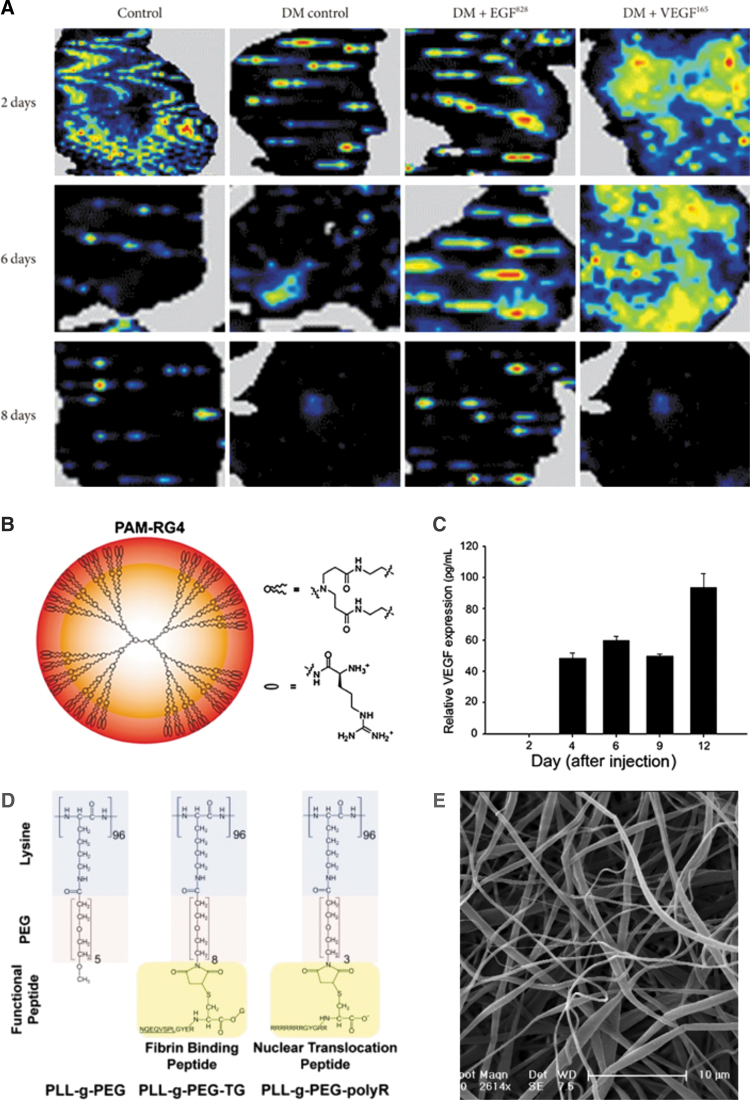
While approaches to plasmid delivery for wound healing have been diverse, the targets have mostly focused on angiogenesis stimulators, with gene delivery of VEGF being particularly well studied. One group tried to tackle the short half-life of growth factors by delivering plasmids for EGF and VEGF. They found that diabetes decreased blood flow in the wound, but local delivery of VEGF plasmids rescued blood flow by promoting angiogenesis. This trend held for days 2 and 6, but not day 8 postinjury (Fig. 7A). The study employed sonoporation to facilitate plasmid uptake; however, sonoporation is not a clinically viable TNA delivery strategy due to limitations in impacting large areas of tissue effectively and potential damage to surrounding tissue.84 Kwon et al., delivered minicircle plasmid DNA for VEGF using an arginine-grafted PAMAM dendrimer (Fig. 7B), enabling expression for nearly 2 weeks (Fig. 7C).85 Peng et al. encapsulated their plasmid DNA for VEGF in a β-cyclodextrin-graft-PEI NP and then loaded these NPs into an epidermal stem cell-coated gelatin matrix, which could be applied directly to the wound as a dressing.86 Guo et al. incorporated VEGF plasmid DNA into a porous collagen–chitosan/silicone membrane and applied it to a burn wound healing model. Their scaffold allowed for sustained release of the plasmid DNA over about 2 weeks, with 50% released in the first 5 days.87 Shi et al. used PEI-modified poly(lactic-co-glycolic acid) (PLGA) nanospheres to sustain delivery of plasmid DNA for VEGF and PDGF-B, over the course of 4 weeks.88 Wang et al.89 delivered plasmid DNA for VEGF and accompanied it with a strategy to treat bacterial wound infection. They grafted an antimicrobial peptide onto gold NPs and used the cationic charge of the antimicrobial peptide to mediate gene delivery.89 Finally, VEGF plasmid was recently combined with the anti-inflammatory resveratrol in a hydrogel made of hyaluronic acid (HA), dextran, and β-cyclodextrin, to aid the healing of burn wounds by promoting neovascularization. While release of the plasmid from the hydrogel was not reported, the resveratrol released over the course of about 20 h.90
Figure 7.
Examples of plasmid delivery to wounds. (A) Sonoporation was used to transfect plasmid for EGF or VEGF into mice wounds. Laser doppler imaging of blood flow in a normal wound, diabetic wound, and diabetic wound with treatment was recorded. From Ko et al. under a CC-BY NC License.84 (B) Structure of an arginine-modified generation four PAMAM dendrimer used for delivery of plasmid DNA. (C) Sustained relative VEGF expression after cationic dendrimer-mediated transfection of the VEGF microcircle plasmid into a mouse wound. (B, C) Reproduced from Kwon et al. with permission from John Wiley and Sons.85 (D) Structure of poly(l-lysine)-g-PEG polymers with binding and targeting peptide functionalization for plasmid delivery. Reproduced from Thiersch et al. with permission from Elsevier.92 (E) Electrospun poly(l-lactide) and poly(caprolactone) fibers for delivery of plasmid DNA to the wound bed. Reproduced from Kobsa et al. with permission from Elsevier.97 EGF, epidermal growth factor; VEGF, vascular endothelial growth factor; PEG, polyethylene glycol. Color images are available online.
Delivering plasmid DNA for angiogenesis-associated genes other than VEGF has also been explored. In particular, Mo et al. developed a dressing made of electrospun polymer nanofibers that released a plasmid DNA for angiogenin, a protein implicated in angiogenesis that has a half-life in serum on the order of hours, over 2 weeks. They combined their plasmid delivery with the anti-inflammatory small molecule curcumin to further enhance wound healing and saw that angiogenin increased neovascularization in a burn model.91 It remains unclear whether angiogenin gene delivery enhances angiogenesis more than the well-studied VEGF gene delivery. Thiersch et al. delivered components of the normal oxygen sensing system to boost angiogenesis. They delivered plasmid for HIF-1α, which is part of the oxygen-sensing system of cells and provides signaling to induce VEGF and PDGF production.92 Under normoxia, HIF-1α is constitutively expressed, but under hypoxic conditions, its expression can be upregulated.93 Their delivery strategy employed PEG grafted onto poly(l-lysine). They functionalized the ends of these grafted polymers with a fibrin binding peptide or a cellular uptake peptide (Fig. 7D). The polymer-DNA constructs were deposited into a fibrin matrix for sustained delivery; about two-thirds of the plasmid DNA was released after 1 week. The authors demonstrated that they could increase downstream angiogenesis genes, such as VEGF, and thus improve vascularization of chronic wounds.92
One group explored plasmid delivery to control the prolonged inflammatory state of chronic wounds. They delivered plasmids encoding interleukin-10 (IL-10), an anti-inflammatory cytokine, using a PEI–DNA complex associated with silica NPs.94 IL-10 protein has a half-life on the order of a few hours when intravenously administered, highlighting the need for TNA delivery if prolonged action is desired.95 Release kinetics of the plasmid were not reported, but the concentration of IL-10 reached a maximum by about day 5. This approach decreased the overall inflammatory state of the wounds, as indicated by reduced expression of transcripts for TNFα and IL-1β. Studies on the efficacy of this strategy to enable healing of chronic wounds are needed.96
Finally, researchers have used plasmid DNA to enhance epithelialization. The Saltzman group used electrospinning of poly(l-lactide) or poly(caprolactone) (PCL) to produce nanofiber scaffolds (Fig. 7E). LbL assembly deposited DNA and PEI onto the surface for effective transfection of plasmids encoding keratinocyte growth factor (also known as FGF7). The scaffolds showed about 10% burst release with another 5% of the total loaded plasmid releasing over the next week. This strategy enabled enhanced healing through increased epithelialization, keratinocyte proliferation, and granulation tissue formation.97
Anti-miRNA oligonucleotides approaches
While much is known about protein-coding gene expression levels in wounds, less is known about non-protein-coding gene expression levels, such as miRNAs.98–100 New studies are elucidating the function of putative miRNAs empirically found in screens to be associated with poor wound healing.101 Recent studies have explored localized delivery of oligonucleotides that inhibit miRNA (AMOs) or mimic miRNAs. miRNA-based therapeutics have added complexity, and added potential, in that they can control the expression of multiple genes at once. One estimate is that a single miRNA can regulate expression of about 100 mRNAs.102 This unique property of miRNAs can help address the limitation of drugging individual protein-coding genes in a gene network, where a separate sequence is needed for each gene.103 A recent review from the Day Lab discusses polymeric approaches to delivering AMOs and miRNA mimics in greater detail, although not specifically for wound healing applications.104
Researchers have delivered AMOs to wounds that increase reepithelialization. Ghatak et al. showed NP-based delivery of an AMO against miR-210, a miRNA that persists in the edges of wounds and promotes cell survival over tissue repair in hypoxic conditions. Their lipid NPs were functionalized with gramicidin, a short, ionophoric, natural antibiotic peptide, to improve endosomal escape and stability in cutaneous applications. Their anti-miR delivery constructs showed increased wound healing rates and increased wound reepithelialization (Fig. 8A).105 This group also functionalized their pH-responsive lipid nanocarriers with a keratinocyte-targeting small peptide sequence to deliver anti-miR-107 for burn wounds. This strategy enhanced keratinocyte proliferation and migration.106 AMOs have also been used to decrease levels of miR-378a-5p, thus increasing vimentin and β3 integrin expression and modulating levels of cell migration, fibroblast differentiation, and angiogenesis. After showing the biology behind the knockdown of miR-378a-5p and its effects, they adsorbed it onto 10 nm gold NPs and delivered it to full-thickness wounds.107
Figure 8.
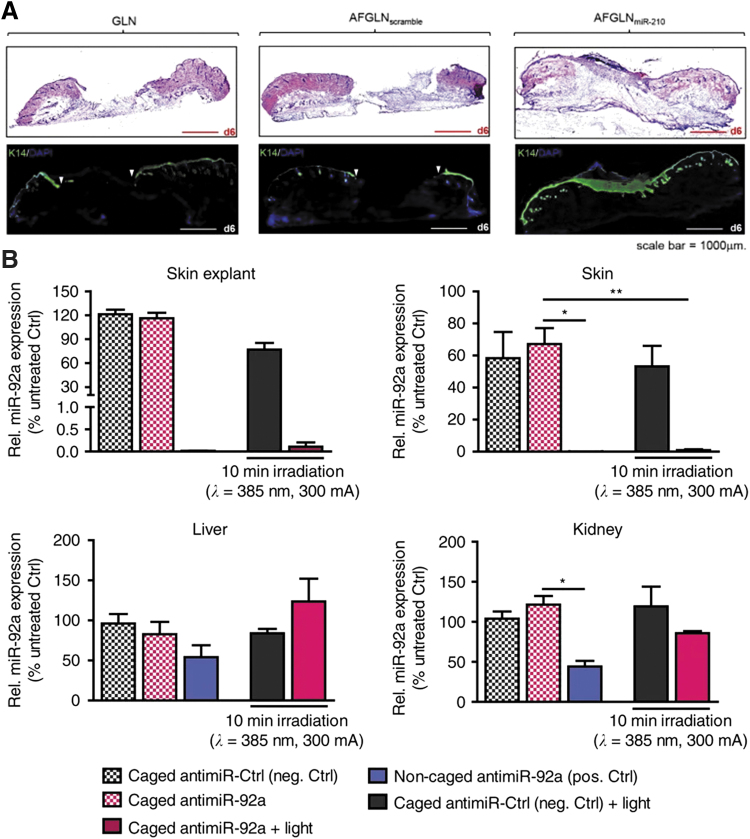
Examples of AMO local delivery to chronic wounds. (A) Increased epithelialization with anti-miR210-loaded lipid nanoparticles. Reproduced from Ghatak et al. with permission from Elsevier.105 (B) miR-92a expression levels after intradermal delivery of light-controlled caged anti-miR-92a in two specific and two nonspecific organ systems. From Lucas et al. under a CC-BY 4.0 license.113 *p < 0.05, **p < 0.01. GLN, gramicidin lipid nanoparticles; AFGLN, antihypoxamir functionalized gramicidin lipid nanoparticles. Color images are available online.
The Feinberg group has shown numerous applications of AMOs to increase angiogenesis in chronic diabetic wounds. Many of the miRNA pathways that they target are based on increasing VEGF through divergent pathways. For example, they showed improved wound healing with local injection of inhibitors against miR-615-5p, a miRNA that is part of the VEGF–AKT–eNOS signaling pathway and is upregulated roughly twofold in diabetic skin compared to control skin. No drug delivery carrier was used.108 They have similarly found that targeting of miR-135a-3p likewise modulates VEGF response, but through the VEGF-HIP1-p38K signaling axis. As with the laboratory's previous article, no drug delivery carrier was used. In vivo, they found that there is a threefold increase in CD31 positivity, a measure of angiogenesis using their approach.109 Finally, they showed that inhibition of miR-26a can also induce angiogenesis in diabetic wounds through SMAD1 signaling.110,111
Anti-miR-92a has also been investigated as a method to promote angiogenesis after myocardial infarction, limb ischemia, vascular injury, bone fracture, and cutaneous wounds. Anti-miR-92a outperformed recombinant human VEGF and PDGF-BB in terms of angiogenesis, and it exhibited reepithelialization and granulation tissue formation roughly comparable to the recombinant growth factors. The AMO was delivered using intradermal injection, but this proof of principle study suggests that drug delivery strategies may enhance the effects even further.112 The Dimmeler and coworkers'113 laboratories inquired whether they could use light to trigger anti-miR-92a AMO activity for enhanced spatial specificity and to reduce the possibility of off-target effects from AMO that gets absorbed into the bloodstream. The light-activatable caged AMOs function by blocking duplex formation with the miRNA in the caged state, thereby inhibiting the AMO's blocking effect. Blue light from a light-emitting-diode breaks this photo-labile bond, enabling duplex formation. The authors show that with their light-activatable AMO system, they can locally inhibit miR-92a without inhibiting nonirradiated skin (Fig. 8B).113 This technology is an exciting approach to control local TNA delivery, particularly for shallow or accessible wounds for which blue light penetration is possible.
miRNA mimics
Although many are using AMOs to control the level of miRNA and thus regulation of transcription, others have created mimics of endogenous miRNA to enable upregulation rather than downregulation. miRNA mimics are computationally designed and chemically synthesized, but function similar to miRNAs implicated in a certain biological pathway. They have increased stability over endogenous miRNAs. In addition, they are double stranded and can thus load directly into the RNA-induced silencing complex, immediately exerting action without a need for Dicer enzyme processing. Yet because of their synthetic nature, some have cautioned that miRNA mimics may have potent off-target effects on gene regulation.114
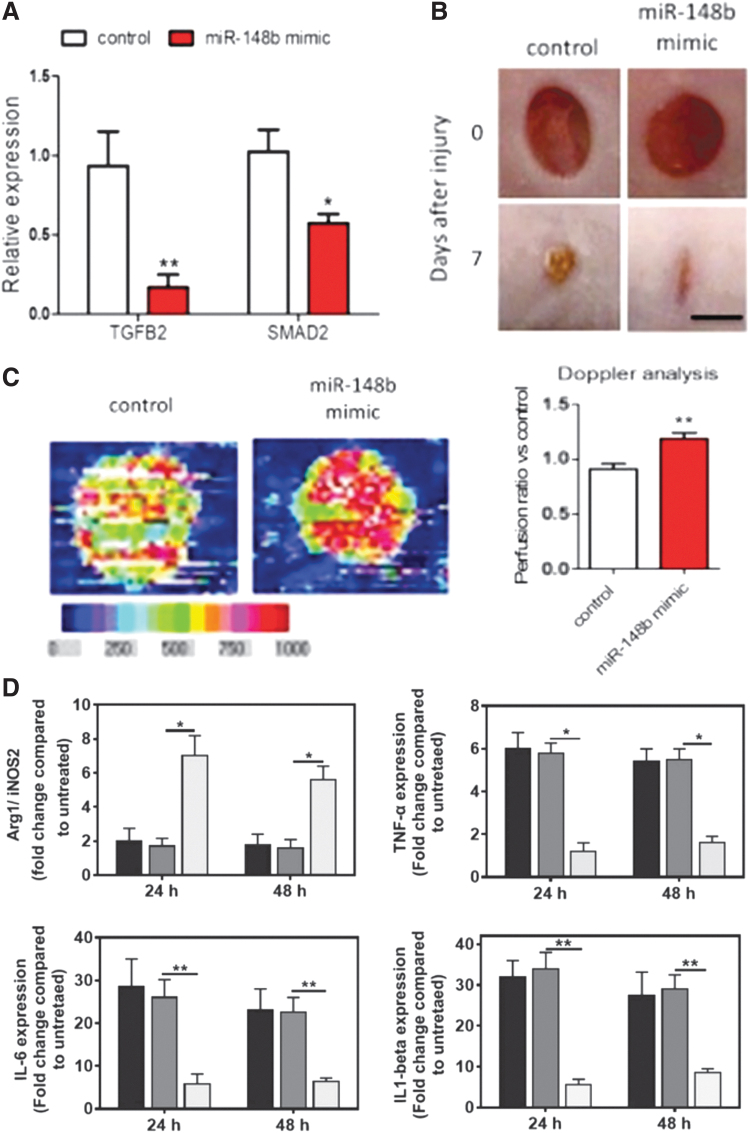
Miscianinov et al. recently created a mimic of miR-148b and showed that it increases endothelial cell migration, proliferation, and thus angiogenesis (Fig. 9A–C). Their transfection relied on release from a Pluronic F-127 gel.115
Figure 9.
Examples of miRNA mimics delivered to the chronic wound. (A) Expression of TGFβ2 and SMAD2, a mediator of TGFβ signaling, following miR-148b mimic transfection in human umbilical vein endothelial cells using lipidoid nanoparticles. (B) Wounds were created in mice and dosed with miR-148b mimic treatment 3 days postwounding and every other day for 7 days. Gross images of healing show that those with the miR-148b mimic heal quicker than those without it. (C) Doppler analysis of exemplary wound with and without miR-148b mimic dosing shows increased blood flow in the treated wounds. (A–C) From Miscianinov et al. with reuse permitted by CC-BY license.115 (D) miR-223 embedded in the hydrogel was placed into a transwell plate with J774A.1 macrophages (dark gray = untreated, medium gray = scrambled control miRNA, and light gray = miR-223). miR-223 mimic polarizes toward an anti-inflammatory environment in vitro in culture with macrophages, as demonstrated by Arg1/iNOS2, TNFα, IL-6, and IL-1β expression. From Saleh et al. with permission from John Wiley and Sons.116 *p < 0.05, **p < 0.01. TNFα, tumor necrosis factor α; IL, interleukin; TGF, transforming growth factor. Color images are available online.
Others have leveraged delivery of miRNA mimics to regulate immune cell phenotype. Saleh et al. controlled macrophage phenotype, as others have done with siRNA. They delivered a miR-223 mimic, as miR-223 has been shown to polarize macrophages toward the M2 phenotype. To stabilize the miRNA mimics, they were loaded into HA-g-PEI or HA-g-PEG NPs and embedded in an adhesive gelatin-based hydrogel. Release was on the order of one to multiple days, depending on the loading concentration. They demonstrated increased in vitro macrophage M2 polarization with their system (Fig. 9D). In vivo, increased vascularity was also noted.116 Umehara et al. targeted a less commonly addressed chronic wound pathway, aiming to restore neutrophil function in chronic wounds. They found that miR-129-2-3p is downregulated in the neutrophils of diabetic mice compared to control mice. The miRNA mimic was applied to wounds in pluronic gel, resulting in accelerated wound healing.117 Ban et al. recently demonstrated intradermal injection of a mimic for the anti-inflammatory miRNA, miR-497, which has been implicated in modulating the NF-κB inflammatory signaling cascade. They delivered their miRNA mimic by injecting polyplexes of the nucleic acid with PEI into diabetic ulcers.118 The Krebs and Liechty laboratories have particularly focused on mimics of miRNA-146a, as this miRNA has been shown to regulate the inflammatory environment in diabetic wounds. It is thought to act through targeting key adapter molecules in the NF-κB inflammatory signaling pathway, thus reducing the expression of proinflammatory cytokines, such as IL-6.119,120 The Liechty group originally delivered the miRNA-146a mimics by conjugating them to cerium oxide nanoparticles (CNPs). The CNPs themselves scavenge ROS, which might help reduce the overall inflammatory environment. The mice were given just 1 treatment at the time of wounding, and the release kinetics were not reported in this study. At 7 days, the miRNA-conjugated CNPs reduced wound area significantly compared to the miRNA mimic alone, emphasizing the importance of a suitable delivery carrier of TNAs.120 These miRNA-146a-CNPs were then loaded into a zwitterionic hydrogel to provide sustained release over nearly a month with about 50% of the CNPs released in the first few days. In vivo, the miRNA-146a-CNPs released from the hydrogel induced complete wound closure at 14 days compared to about 20 days for mice treated with the hydrogel alone.121
Antisense oligonucleotides
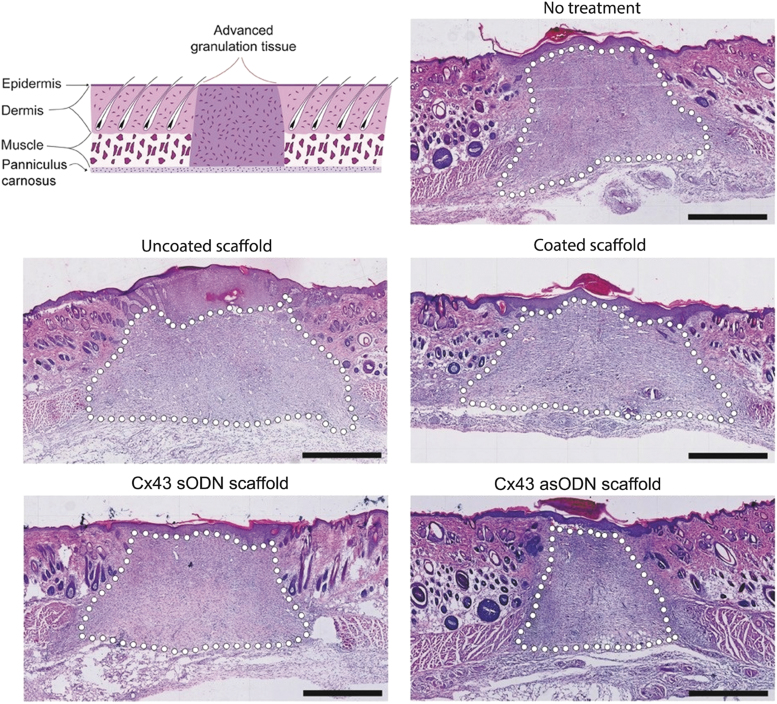
The last major class of TNAs leveraged for localized delivery is ASOs, which work through three distinct pathways to control gene expression (Fig. 3).122 Becker and coworkers investigated sustained delivery of an ASO against Cx43, which is a gap junction protein. They had previously shown that this gene is downregulated at the wound edges to allow for cell migration, but it is upregulated in diabetic wounds.123 An ASO against Cx43 restored balance of Cx43 levels and thus increased wound reepithelialization.124 To sustain release, they used collagen scaffolds coated with ASOs in PLGA or PCL, sustaining release over 7 days, and showed promising results of controlled delivery of ASOs to target Cx43 for enhanced wound healing. In particular, they showed that the wounds were smaller when locally treated with this ASO and had less granulation tissue in the long term, suggesting that the wounds progressed through the stages of healing (Fig. 10).125 Hong et al. targeted Smad3, part of intracellular TGF-β1 signaling, using an ASO impregnated in a chitosan/alginate complex to sustain release over 24 h and improve wound healing.126 While it may seem paradoxical to downregulate a downstream mediator of TGF-β, a signal commonly believed to promote healing, others have also shown the positive effects of knocking out Smad3 in the wound healing process.127
Figure 10.
An ASO against Cx43 was embedded into a collagen scaffold and placed on full-thickness wounds in a rat model. Scaffolds contained a polymer coating or no polymer coating. They also contained an ASO against Cx43 (asODN) or a nonfunctional control ASO (sODN). Hematoxylin and eosin histology shows long-term healing of wounds with or without ASO for Cx43. Dotted lines demarcate granulation tissue. Reproduced from Gilmartin et al. with permission from John Wiley and Sons.125 Color images are available online.
Commercial ventures
Given the excitement for local nucleic acid delivery in wound healing applications, numerous commercial ventures have been pursued. We suspect that given the high volume of literature on TNA delivery for wound healing over the last few years, more companies will form around this exciting area.
Sirnaomics is a company focusing on siRNA delivery for a host of diseases, including cancer and fibrosis.128 They focus on the use of histidine-lysine (HK) polypeptides as drug delivery carriers for their siRNA therapeutics.129 Their product STP705, which is meant for hypertrophic scar reduction, is currently in Phase II clinical trial in China. STP705 also underwent a joint Phase I/II clinical trial in the United States starting in 2017, but it is unclear what the results of the trial are at this time.130 Sirnaomics was recently granted a patent on using siRNA against TGF-β1, Cox-2, and Hoxb13 for scarless wound healing.131
SomaGenics is working on nucleic acid technologies to speed wound healing by targeting the hypoxia response pathway.132 Recently, they described a system that combined short small hairpin RNA, a form of RNAi, and AMOs to target chronic wounds. The targets examined were PHD2 and miR-210, respectively. They worked with LayerBio to create electrostatically assembled thin films on bandages containing the TNAs to sustain delivery over time.133 It is unclear where SomaGenics stands in terms of plans for clinical trials, but preclinical results have been promising.
miRagen is a company founded in 2006, aiming to bring miRNA and AMO drugs to the clinic.134 They have been working on an AMO against miR-92a (named MRG-110), and in 2018, they showed that it had preclinical efficacy in inducing angiogenesis in acute and chronic wounds.112 At the 2019 Oligonucleotide Therapeutics Society Annual Meeting, miRagen showed that MRG-110 is generally safe and well tolerated in a pair of Phase I clinical trials that investigated both intradermal and intravenous dosing. It was also shown that the drug could increase angiogenesis and periwound perfusion.135,136 Of note, miRagen has also demonstrated that the same drug is effective in a porcine model of heart failure, and their pipeline seems to suggest that trials of MRG-110 for cardiac perfusion are underway.
Delivery of plasmid DNA for hepatocyte growth factor (HGF) to increase angiogenesis in diabetic foot ulcers is currently being developed by Helixmith. They are using plasmid to express a combination of two HGF isoforms, HGF-728 and HGF-723. It is believed that these growth factors activate c-Met, inducing neovascularization, while reducing fibrosis and inflammation.137 Currently, their delivery strategy is intramuscular injection of naked DNA. Their product, Engensis, or VM202, has shown no serious adverse outcome and is currently in Phase III trials in the United States for diabetic ulcers and other indications.137–139 Engensis has also shown promise for myocardial infarction,140 diabetic peripheral neuropathy,141,142 and amyotrophic lateral sclerosis.137
Moderna and AstraZeneca are collaborating on AZD8601, a chemically modified mRNA for VEGF-A. Preclinical studies showed that AZD8601 increases vascularization and tissue oxygenation of the diabetic wound bed, thus inducing reepithelialization and accelerating wound healing.143 A Phase I clinical trial was recently completed in patients with diabetes. They showed that the drug is generally well tolerated, with minor injection-site reactions being the only adverse outcome. They also showed some potential for efficacy. Specifically, they showed that the mRNA increases VEGF-A expression in the wounds for about 1 day, with a peak 5.5–7.5 h after injection. They also demonstrated a twofold enhancement of basal blood flow in wounds treated with AZD8601, a trend that continued for 7 days postinjection.144 This study shows promise for the local delivery of VEGF-A mRNA in diabetic wounds. Of note, AstraZeneca and Moderna have also demonstrated efficacy of VEGF-A mRNA to increase heart function after myocardial infarction, which is another disease healing state that may benefit from localized delivery of TNAs targeting angiogenesis.145,146
Ocunexus (originally CoDa Therapeutics) developed Nexagon, which is an ASO against Cx43. It is based on work in the Becker and Green laboratories that demonstrated accelerated wound healing, reduced inflammation, and smaller scarring by inhibiting Cx43, a gap junction channel.147,148 It is unclear what the status of this drug is for treating skin wound healing, as a Phase I trial has no posted results and a later Phase I/II trial was terminated for business reasons.149,150 The company seems to have since shifted their focus on using Nexagon to enable corneal wound healing in nonhealing corneal epithelial defects, and they are collaborating with Eyevance Pharmaceuticals. A Phase II clinical trial is ongoing.151
The Future of Nucleic Acid Delivery for Wound Healing
Chronic wounds are a critical global issue expected to grow more prevalent in the near future. Despite the past few decades of research, few new options have become clinically available to treat chronic wounds. New strategies are needed, and nonviral delivery of TNAs may hold the answer. This review summarizes recent work in delivering TNAs to the chronic wound. Several drug delivery platforms and therapeutic targets have been explored and the commercial interest in drug delivery of TNAs for wound healing has been summarized.
We believe the following drug delivery system characteristics are integral to shifting the paradigm of treatment for chronic wounds and eventually other healing complications:
Wound dressings that incorporate sustained TNA delivery systems are likely more translational than injected approaches, as they can be pared to conform to the wound geometry and cover all parts of the wound, decreasing diffusion distance of the therapy.
Systems will need to release specific TNAs for each part of the dynamic wound healing process, suggesting a staged release process may be optimal. Stimuli-responsive systems have the potential to address this need by only releasing the TNAs when the wound environment proves amenable.
Given that many factors are implicated in the poor healing of wounds, a combinatorial approach may prove the most effective at promoting healing in the chronic wound. For example, TNAs may be combined to synergistically promote angiogenesis, induce M2 macrophage phenotype, and promote cell migration and reepithelialization, all from a single bandage. Timing the release of each of these components will be critical.
New co-delivery strategies to boost the action of the native TNA mechanism, such as co-delivery of siRNA and Ago2, may enhance the traditional low efficiency of TNA delivery. Novel systems for nonviral delivery, such as nanoneedles, also hold potential.
miRNA-based therapies hold much potential for modulating multiple genes at once, but further understanding of affected genes will be crucial before clinical translation. We present some examples where both the biology and therapeutic potential are understood.
Given the abundance of research on the regulation of genes in chronic and other wound healing disorders, nucleic acid approaches provide the most effective means of translating this knowledge into therapies that have large potential impact. Furthermore, recent advances in enhancing nucleic acid vector stability and in the manufacture of RNA and DNA constructs make such therapeutic approaches much more accessible with regard to cost, manufacturing feasibility, and product availability. The engineering of delivery systems that enable these therapeutics to reach the appropriate cells and enable controlled and appropriately staged release will yield exciting new treatments for chronic wounds and other healing disorders in the upcoming decade.
Take Home Messages
Increased understanding of the expression profiles of coding and noncoding nucleic acids implicated in chronic wounds has enabled TNA-based approaches to address wound healing.
Localized delivery of TNAs holds much promise for promoting the healing of chronic wounds by modulating inflammation, ECM degradation, cell motility, angiogenesis, epithelialization, and oxidative stress.
Drug delivery to the chronic wound has unique challenges, given oxidative stress, ischemia, high levels of proteases and enzymes, immune cell infiltrate, mechanical stresses, and biofilm/infection in the wound microenvironment.
Given the dynamic process of wound healing, strategies to deliver the appropriate TNA at the optimal time to the correct cell type will be critical to ensuring maximal effectiveness in correcting the underlying biology. Controlled release strategies and those incorporating stimuli-responsive release may help address these challenges.
Drug delivery carriers used for TNAs include dendrimers, lipid NPs, polymeric micelles, polymeric polyplexes, metal NPs, and hydrogels. Intrinsic properties of the drug delivery carrier system can impact biological efficacy of TNAs. The drug carriers can also protect TNAs from challenges in delivery to wounds.
Translation of research on localized delivery of TNAs has yielded numerous clinical trials and companies, but has not yet produced any approved treatments. We are hopeful that current preclinical efforts will translate into successful clinical therapies.
Abbreviations and Acronyms
- AFGLN
antihypoxamir functionalized gramicidin lipid nanoparticles
- Ago2
argonaute 2
- AMO/anti-miR
anti-microRNA oligonucleotide
- ASO
antisense oligonucleotide
- CNP
cerium oxide nanoparticle
- CRISPR
clustered regularly interspaces short palindromic repeat
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ERK1
extracellular signal-regulated kinase 1
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- FL2
fidgetin-like 2
- FliI
flightless I
- GFP
green fluorescent protein.
- GLN
gramicidin lipid nanoparticles
- HA
hyaluronic acid
- HGF
hepatocyte growth factor
- HIF
hypoxia-inducible factor
- IL
interleukin
- IRF5
interferon regulatory factor 5
- KGF
keratinocyte growth factor
- LbL
layer-by-layer
- MCP-1
monocyte chemoattractant protein 1
- miRNA
microRNA
- MMP
matrix metalloproteinase
- mRNA
messenger RNA
- NP
nanoparticle
- PAMAM
polyamidoamine
- PC
panniculus carnosus
- PCL
poly(caprolactone)
- PDGF
platelet-derived growth factor
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- PHD2
prolyl hydroxylase domain 2
- PLGA
poly(lactic-co-glycolic acid)
- RISC
RNA-induced silencing complex
- RNAi
RNA interference
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- TBR
target to background ratio
- TGFβ
transforming growth factor β
- TIMP
tissue inhibitor of metalloproteinases
- TNA
therapeutic nucleic acid
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
The authors would like to thank John Martin, Sarah Almofty, MayLin Howard, Sheryl Wang, and Apoorv Shanker for critical reading of this article. Research was sponsored by the Army Research Office and was accomplished under Cooperative Agreement Number W911NF-18-2-0048. The authors also acknowledge funding from the NIH (1R01CA235375).
Author Disclosure and Ghostwriting
P.T.H. has funding to her laboratory in conjunction with SomaGenics, a company focused on developing therapeutic nucleic acids for wound healing applications. P.T.H. is on the Scientific Advisory Board of Moderna Therapeutics and the Boards of Alector and LayerBio. The authors have no other relevant disclosures. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Adam G. Berger, is a MD-PhD student in the Harvard-MIT MD-PhD program and the Harvard-MIT Health Sciences and Technology Program. He is currently a graduate student in the Hammond Lab. Jonathan J. Chou, is a PhD student in the Department of Chemical Engineering at MIT. He is a graduate student in the Hammond Lab. Paula T. Hammond, PhD, is the David H. Koch Chair Professor of Engineering at MIT and the Head of the Department of Chemical Engineering. She is a member of MIT's Koch Institute for Integrative Cancer Research and a founding member of the MIT Institute for Soldier Nanotechnology. Her work focuses on self-assembled materials that enable drug delivery for applications in wound healing, bone healing, cancer, avascular tissues, infections, and hemostasis.
References
- 1. Li J, Chen J, Kirsner R. Pathophysiology of acute wound healing. Clin Dermatol 2007;25:9–18 [DOI] [PubMed] [Google Scholar]
- 2. Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther 2002;302:839–845 [DOI] [PubMed] [Google Scholar]
- 3. Suvas S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol 2017;199:1543–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 2010;10:427–439 [DOI] [PubMed] [Google Scholar]
- 5. Weiss G, Schaible UE. Macrophage defense mechanisms against intracellular bacteria. Immunol Rev 2015;264:182–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care (New Rochelle) 2012;1:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cañedo-Dorantes L, Cañedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflamm 2019;2019:Article ID 3706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Greaves NS, Ashcroft KJ, Baguneid M, Bayat A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J Dermatol Sci 2013;72:206–217 [DOI] [PubMed] [Google Scholar]
- 9. Rousselle P, Braye F, Dayan G. Re-epithelialization of adult skin wounds: cellular mechanisms and therapeutic strategies. Adv Drug Deliv Rev 2019;146:344–365 [DOI] [PubMed] [Google Scholar]
- 10. Eelen G, de Zeeuw P, Simons M, Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ Res 2015;116:1231–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zochodne DW. The challenges and beauty of peripheral nerve regrowth. J Peripher Nerv Syst 2012;17:1–18 [DOI] [PubMed] [Google Scholar]
- 12. Olczyk P, Mencner Ł, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. BioMed Res Int 2014;2014:Article ID 747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–149 [DOI] [PubMed] [Google Scholar]
- 16. Nussbaum SR, Carter MJ, Fife CE, et al. . An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21:27–32 [DOI] [PubMed] [Google Scholar]
- 17. Sen CK. Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle) 2019;8:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dinh T, Tecilazich F, Kafanas A, et al. . Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012;61:2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293:217. [DOI] [PubMed] [Google Scholar]
- 20. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol 2015;173:370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davis FM, Kimball A, Boniakowski A, Gallagher K. Dysfunctional wound healing in diabetic foot ulcers: new crossroads. Curr Diab Rep 2018;18:2. [DOI] [PubMed] [Google Scholar]
- 22. Leal EC, Carvalho E, Tellechea A, et al. . Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol 2015;185:1638–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 24. Rayment EA, Upton Z, Shooter GK. Increased matrix metalloproteinase-9 (MMP-9) activity observed in chronic wound fluid is related to the clinical severity of the ulcer. Br J Dermatol 2008;158:951–961 [DOI] [PubMed] [Google Scholar]
- 25. Vaalamo M, Weckroth M, Puolakkainen P, et al. . Patterns of matrix metalloproteinase and TIMP-1 expression in chronic and normally healing human cutaneous wounds. Br J Dermatol 1996;135:52–59 [PubMed] [Google Scholar]
- 26. Vaalamo M, Leivo T, Saarialho-Kere U. Differential expression of tissue inhibitors of metalloproteinases (TIMP-1, -2, -3, and -4) in normal and aberrant wound healing. Hum Pathol 1999;30:795–802 [DOI] [PubMed] [Google Scholar]
- 27. Li X, Xiao T, Wang Y, et al. . Incidence, risk factors for amputation among patients with diabetic foot ulcer in a Chinese tertiary hospital. Diabetes Res Clin Pract 2011;93:26–30 [DOI] [PubMed] [Google Scholar]
- 28. Lipsky BA, Weigelt JA, Sun X, Johannes RS, Derby KG, Tabak YP. Developing and validating a risk score for lower-extremity amputation in patients hospitalized for a diabetic foot infection. Diabetes Care 2011;34:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rastogi A, Goyal G, Kesavan R, et al. . Long term outcomes after incident diabetic foot ulcer: multicenter large cohort prospective study (EDI-FOCUS investigators) epidemiology of diabetic foot complications study. Diabetes Res Clin Pract 2020;162:108113. [DOI] [PubMed] [Google Scholar]
- 30. Kim J, Hu C, Moufawad El Achkar C, et al. . Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med 2019;381:1644–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Barrangou R, Doudna JA. Applications of CRISPR technologies in research and beyond. Nat Biotechnol 2016;34:933–941 [DOI] [PubMed] [Google Scholar]
- 32. Glass Z, Lee M, Li Y, Xu Q. Engineering the delivery system for CRISPR-based genome editing. Trends Biotechnol 2018;36:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qi Lei S, Larson Matthew H, Gilbert Luke A, et al. . Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013;152:1173–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sridharan K, Gogtay NJ. Therapeutic nucleic acids: current clinical status. Br J Clin Pharmacol 2016;82:659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Catuogno S, Esposito CL, Condorelli G, de Franciscis V. Nucleic acids delivering nucleic acids. Adv Drug Deliv Rev 2018;134:79–93 [DOI] [PubMed] [Google Scholar]
- 36. Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature 2009;457:426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jo JI, Gao JQ, Tabata Y. Biomaterial-based delivery systems of nucleic acid for regenerative research and regenerative therapy. Regen Ther 2019;11:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saghazadeh S, Rinoldi C, Schot M, et al. . Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev 2018;127:138–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Das S, Baker AB. Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol 2016;4:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tartarini D, Mele E. Adult stem cell therapies for wound healing: biomaterials and computational models. Front Bioeng Biotechnol 2015;3:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet 2014;15:541–555 [DOI] [PubMed] [Google Scholar]
- 42. Baum C, von Kalle C, Staal FJT, et al. . Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther 2004;9:5–13 [DOI] [PubMed] [Google Scholar]
- 43. Picanco-Castro V, Pereira CG, Covas DT, Porto GS, Athanassiadou A, Figueiredo ML. Emerging patent landscape for non-viral vectors used for gene therapy. Nat Biotechnol 2020;38:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med 2005;7:657–663 [DOI] [PubMed] [Google Scholar]
- 45. Freeman EC, Weiland LM, Meng WS. Modeling the proton sponge hypothesis: examining proton sponge effectiveness for enhancing intracellular gene delivery through multiscale modeling. J Biomater Sci Polym Ed 2013;24:398–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Behr J-P. The proton sponge: a trick to enter cells the viruses did not exploit. Chim Int J Chem 1997;51:34–36 [Google Scholar]
- 47. Vermeulen LMP, Brans T, Samal SK, et al. . Endosomal size and membrane leakiness influence proton sponge-based rupture of endosomal vesicles. ACS Nano 2018;12:2332–2345 [DOI] [PubMed] [Google Scholar]
- 48. Love KT, Mahon KP, Levins CG, et al. . Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A 2010;107:1864–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. U.S. Food and Drug Administration. FDA approves first-of-its kind targeted RNA-based therapy to treat a rare disease. 2019. http://fda.gov/news-events/press-announcements/fda-approves-first-its-kind-targeted-rna-based-therapy-treat-rare-disease (last accessed October27, 2019)
- 50. Whittam AJ, Maan ZN, Duscher D, et al. . Challenges and opportunities in drug delivery for wound healing. Adv Wound Care 2016;5:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Caley MP, Martins VLC, O'Toole EA. Metalloproteinases and wound healing. Adv Wound Care (New Rochelle) 2015;4:225–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao G, Usui ML, Lippman SI, et al. . Biofilms and inflammation in chronic wounds. Adv Wound Care (New Rochelle) 2013;2:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Castleberry S, Wang M, Hammond PT. Nanolayered siRNA dressing for sustained localized knockdown. ACS Nano 2013;7:5251–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cordeiro RA, Serra A, Coelho JFJ, Faneca H. Poly(β-amino ester)-based gene delivery systems: from discovery to therapeutic applications. J Control Release 2019;310:155–187 [DOI] [PubMed] [Google Scholar]
- 55. Castleberry SA, Almquist BD, Li W, et al. . Self-assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Adv Mater 2016;28:1809–1817 [DOI] [PubMed] [Google Scholar]
- 56. Deng J, Li N, Mai K, Yang C, Yan L, Zhang L-M. Star-shaped polymers consisting of a β-cyclodextrin core and poly(amidoamine) dendron arms: binding and release studies with methotrexate and siRNA. J Mater Chem 2011;21:5273–5281 [Google Scholar]
- 57. Li N, Luo H-C, Ren M, et al. . Efficiency and safety of β-CD-(D3)7 as siRNA carrier for decreasing matrix metalloproteinase-9 expression and improving wound healing in diabetic rats. ACS Appl Mater Interfaces 2017;9:17417–17426 [DOI] [PubMed] [Google Scholar]
- 58. Li N, Luo H-C, Yang C, et al. . Cationic star-shaped polymer as an siRNA carrier for reducing MMP-9 expression in skin fibroblast cells and promoting wound healing in diabetic rats. Int J Nanomed 2014;9:3377–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li N, Yang L, Pan C, et al. . Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater 2020;102:298–314 [DOI] [PubMed] [Google Scholar]
- 60. Kim HS, Yoo HS. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther 2013;20:378–385 [DOI] [PubMed] [Google Scholar]
- 61. Kim HS, Son YJ, Yoo HS. Clustering siRNA conjugates for MMP-responsive therapeutics in chronic wounds of diabetic animals. Nanoscale 2016;8:13236–13244 [DOI] [PubMed] [Google Scholar]
- 62. Charafeddine RA, Makdisi J, Schairer D, et al. . Fidgetin-like 2: a microtubule-based regulator of wound healing. J Invest Dermatol 2015;135:2309–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stehbens S, Wittmann T. Targeting and transport: how microtubules control focal adhesion dynamics. J Cell Biol 2012;198:481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. O'Rourke BP, Kramer AH, Cao LL, et al. . Fidgetin-like 2 siRNA enhances the wound healing capability of a surfactant polymer dressing. Adv Wound Care (New Rochelle) 2019;8:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Turner CT, Hasanzadeh Kafshgari M, Melville E, et al. . Delivery of flightless I siRNA from porous silicon nanoparticles improves wound healing in mice. ACS Biomater Sci Eng 2016;2:2339–2346 [DOI] [PubMed] [Google Scholar]
- 66. Courties G, Heidt T, Sebas M, et al. . In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol 2014;63:1556–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kasiewicz LN, Whitehead KA. Silencing TNFα with lipidoid nanoparticles downregulates both TNFα and MCP-1 in an in vitro co-culture model of diabetic foot ulcers. Acta Biomater 2016;32:120–128 [DOI] [PubMed] [Google Scholar]
- 68. Kasiewicz LN, Whitehead KA. Lipid nanoparticles silence tumor necrosis factor α to improve wound healing in diabetic mice. Bioeng Transl Med 2019;4:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhou L-S, Zhao G-L, Liu Q, Jiang S-C, Wang Y, Zhang D-M. Silencing collapsin response mediator protein-2 reprograms macrophage phenotype and improves infarct healing in experimental myocardial infarction model. J Inflamm 2015;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tezgel Ö, DiStasio N, Laghezza-Masci V, et al. . Collagen scaffold-mediated delivery of NLC/siRNA as wound healing materials. J Drug Deliv Sci Technol 2020;55:101421 [Google Scholar]
- 71. Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—from molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 2005;1754:253–262 [DOI] [PubMed] [Google Scholar]
- 72. Wetterau M, George F, Weinstein A, et al. . Topical prolyl hydroxylase domain-2 silencing improves diabetic murine wound closure. Wound Repair Regen 2011;19:481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sarett SM, Kilchrist KV, Miteva M, Duvall CL. Conjugation of palmitic acid improves potency and longevity of siRNA delivered via endosomolytic polymer nanoparticles. J Biomed Mater Res A 2015;103:3107–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang X, Yan X, Cheng L, et al. . Wound healing improvement with PHD-2 silenced fibroblasts in diabetic mice. PLoS One 2013;8:e84548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Appelhoff RJ, Tian Y-M, Raval RR, et al. . Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J Biol Chem 2004;279:38458–38465 [DOI] [PubMed] [Google Scholar]
- 76. Martin JR, Nelson CE, Gupta MK, et al. . Local delivery of PHD2 siRNA from ROS-degradable scaffolds to promote diabetic wound healing. Adv Healthc Mater 2016;5:2751–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nelson CE, Gupta MK, Adolph EJ, Guelcher SA, Duvall CL. siRNA delivery from an injectable scaffold for wound therapy. Adv Wound Care (New Rochelle) 2012;2:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nelson CE, Kim AJ, Adolph EJ, et al. . Tunable delivery of siRNA from a biodegradable scaffold to promote angiogenesis in vivo. Adv Mater 2014;26:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rabbani PS, Zhou A, Borab ZM, et al. . Novel lipoproteoplex delivers Keap1 siRNA based gene therapy to accelerate diabetic wound healing. Biomaterials 2017;132:1–15 [DOI] [PubMed] [Google Scholar]
- 80. Soares MA, Cohen OD, Low YC, et al. . Restoration of Nrf2 signaling normalizes the regenerative niche. Diabetes 2016;65:633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Weinstein AL, Lalezarzadeh FD, Soares MA, Saadeh PB, Ceradini DJ. Normalizing dysfunctional purine metabolism accelerates diabetic wound healing. Wound Repair Regen 2015;23:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chiappini C, De Rosa E, Martinez JO, et al. . Biodegradable silicon nanoneedles delivering nucleic acids intracellularly induce localized in vivo neovascularization. Nat Mater 2015;14:532–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li J, Wu C, Wang W, et al. . Structurally modulated codelivery of siRNA and Argonaute 2 for enhanced RNA interference. Proc Natl Acad Sci U S A 2018;115:E2696–E2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ko J, Jun H, Chung H, et al. . Comparison of EGF with VEGF non-viral gene therapy for cutaneous wound healing of streptozotocin diabetic mice. Diabetes Metab J 2011;35:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kwon MJ, An S, Choi S, et al. . Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J Gene Med 2012;14:272–278 [DOI] [PubMed] [Google Scholar]
- 86. Peng L-H, Wei W, Qi X-T, et al. . Epidermal stem cells manipulated by pDNA-VEGF165/CYD-PEI nanoparticles loaded gelatin/β-TCP matrix as a therapeutic agent and gene delivery vehicle for wound healing. Mol Pharm 2013;10:3090–3102 [DOI] [PubMed] [Google Scholar]
- 87. Guo R, Xu S, Ma L, Huang A, Gao C. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen–chitosan dermal equivalents. Biomaterials 2011;32:1019–1031 [DOI] [PubMed] [Google Scholar]
- 88. Shi R, Lian W, Han S, et al. . Nanosphere-mediated co-delivery of VEGF-A and PDGF-B genes for accelerating diabetic foot ulcers healing in rats. Gene Ther 2018;25:425–438 [DOI] [PubMed] [Google Scholar]
- 89. Wang S, Yan C, Zhang X, et al. . Antimicrobial peptide modification enhances the gene delivery and bactericidal efficiency of gold nanoparticles for accelerating diabetic wound healing. Biomater Sci 2018;6:2757–2772 [DOI] [PubMed] [Google Scholar]
- 90. Wang P, Huang S, Hu Z, et al. . In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater 2019;100:191–201 [DOI] [PubMed] [Google Scholar]
- 91. Mo Y, Guo R, Zhang Y, Xue W, Cheng B, Zhang Y. Controlled dual delivery of angiogenin and curcumin by electrospun nanofibers for skin regeneration. Tissue Eng Part A 2017;23:597–608 [DOI] [PubMed] [Google Scholar]
- 92. Thiersch M, Rimann M, Panagiotopoulou V, et al. . The angiogenic response to PLL-g-PEG-mediated HIF-1α plasmid DNA delivery in healthy and diabetic rats. Biomaterials 2013;34:4173–4182 [DOI] [PubMed] [Google Scholar]
- 93. Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393–402 [DOI] [PubMed] [Google Scholar]
- 94. Wang X, Hélary C, Coradin T. Local and sustained gene delivery in silica-collagen nanocomposites. ACS Appl Mater Interfaces 2015;7:2503–2511 [DOI] [PubMed] [Google Scholar]
- 95. Huhn RD, Radwanski E, Gallo J, et al. . Pharmacodynamics of subcutaneous recombinant human interleukin-10 in healthy volunteers. Clin Pharmacol Ther 1997;62:171–180 [DOI] [PubMed] [Google Scholar]
- 96. Wang X, Coradin T, Hélary C. Modulating inflammation in a cutaneous chronic wound model by IL-10 released from collagen–silica nanocomposites via gene delivery. Biomater Sci 2018;6:398–406 [DOI] [PubMed] [Google Scholar]
- 97. Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell ALM, Kyriakides TR, Saltzman WM. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials 2013;34:3891–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Luan A, Hu MS, Leavitt T, et al. . Noncoding RNAs in wound healing: a new and vast frontier. Adv Wound Care (New Rochelle) 2018;7:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mulholland EJ, Dunne N, McCarthy HO. MicroRNA as therapeutic targets for chronic wound healing. Mol Ther Nucleic Acids 2017;8:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Banerjee J, Sen CK. microRNA and wound healing. Adv Exp Med Biol 2015;888:291–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ozdemir D, Feinberg MW. MicroRNAs in diabetic wound healing: pathophysiology and therapeutic opportunities. Trends Cardiovasc Med 2019;29:131–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lim LP, Lau NC, Garrett-Engele P, et al. . Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005;433:769–773 [DOI] [PubMed] [Google Scholar]
- 103. Hammond SM. MicroRNA therapeutics: a new niche for antisense nucleic acids. Trends Mol Med 2006;12:99–101 [DOI] [PubMed] [Google Scholar]
- 104. Kapadia CH, Luo B, Dang MN, Irvin-Choy ND, Valcourt DM, Day ES. Polymer nanocarriers for microRNA delivery. Int J Appl Polym Sci 2020;137:48651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ghatak S, Li J, Chan YC, et al. . AntihypoxamiR functionalized gramicidin lipid nanoparticles rescue against ischemic memory improving cutaneous wound healing. Nanomed Nanotechnol Biol Med 2016;12:1827–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li J, Ghatak S, El Masry MS, et al. . Topical lyophilized targeted lipid nanoparticles in the restoration of skin barrier function following burn wound. Mol Ther 2018;26:2178–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li H, Chang L, Du WW, et al. . Anti-microRNA-378a enhances wound healing process by upregulating integrin beta-3 and vimentin. Mol Ther 2014;22:1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Icli B, Wu W, Ozdemir D, et al. . MicroRNA-615-5p regulates angiogenesis and tissue repair by targeting AKT/eNOS (protein kinase B/endothelial nitric oxide synthase) signaling in endothelial cells. Arterioscler Thromb Vasc Biol 2019;39:1458–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Icli B, Wu W, Ozdemir D, et al. . MicroRNA-135a-3p regulates angiogenesis and tissue repair by targeting p38 signaling in endothelial cells. FASEB J 2019;33:5599–5614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Icli B, Wara AK, Moslehi J, et al. . MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res 2013;113:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Icli B, Nabzdyk CS, Lujan-Hernandez J, et al. . Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol 2016;91:151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gallant-Behm CL, Piper J, Dickinson BA, Dalby CM, Pestano LA, Jackson AL. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair Regen 2018;26:311–323 [DOI] [PubMed] [Google Scholar]
- 113. Lucas T, Schäfer F, Müller P, Eming SA, Heckel A, Dimmeler S. Light-inducible antimiR-92a as a therapeutic strategy to promote skin repair in healing-impaired diabetic mice. Nat Commun 2017;8:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jin HY, Gonzalez-Martin A, Miletic AV, et al. . Transfection of microRNA mimics should be used with caution. Front Genet 2015;6:340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Miscianinov V, Martello A, Rose L, et al. . MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther 2018;26:1996–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Saleh B, Dhaliwal HK, Portillo-Lara R, et al. . Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small 2019;15:1902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Umehara T, Mori R, Mace KA, et al. . Identification of specific miRNAs in neutrophils of type 2 diabetic mice: overexpression of miRNA-129-2-3p accelerates diabetic wound healing. Diabetes 2019;68:617–630 [DOI] [PubMed] [Google Scholar]
- 118. Ban E, Jeong S, Park M, et al. . Accelerated wound healing in diabetic mice by miRNA-497 and its anti-inflammatory activity. Biomed Pharmacother 2020;121:109613. [DOI] [PubMed] [Google Scholar]
- 119. Xu J, Wu W, Zhang L, et al. . The role of MicroRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes 2012;61:2906–2912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zgheib C, Hilton SA, Dewberry LC, et al. . Use of cerium oxide nanoparticles conjugated with microRNA-146a to correct the diabetic wound healing impairment. J Am Coll Surg 2019;228:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sener G, Hilton SA, Osmond MJ, et al. . Injectable, self-healable zwitterionic cryogels with sustained microRNA-cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater 2020;101:262–272 [DOI] [PubMed] [Google Scholar]
- 122. Dias N, Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther 2002;1:347–355 [PubMed] [Google Scholar]
- 123. Gilmartin DJ, Alexaline MM, Thrasivoulou C, Phillips ARJ, Jayasinghe SN, Becker DL. Integration of scaffolds into full-thickness skin wounds: the connexin response. Adv Healthc Mater 2013;2:1151–1160 [DOI] [PubMed] [Google Scholar]
- 124. Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes 2007;56:2809–2817 [DOI] [PubMed] [Google Scholar]
- 125. Gilmartin DJ, Soon A, Thrasivoulou C, Phillips ARJ, Jayasinghe SN, Becker DL. Sustained release of Cx43 antisense oligodeoxynucleotides from coated collagen scaffolds promotes wound healing. Adv Healthc Mater 2016;5:1786–1799 [DOI] [PubMed] [Google Scholar]
- 126. Hong H-J, Jin S-E, Park J-S, Ahn WS, Kim C-K. Accelerated wound healing by smad3 antisense oligonucleotides-impregnated chitosan/alginate polyelectrolyte complex. Biomaterials 2008;29:4831–4837 [DOI] [PubMed] [Google Scholar]
- 127. Ashcroft GS, Yang X, Glick AB, et al. . Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1999;1:260–266 [DOI] [PubMed] [Google Scholar]
- 128. Sirnaomics, Inc. Sirnaomics. 2019. https://sirnaomics.com/ (last accessed November26, 2019)
- 129. Leng Q, Scaria P, Zhu J, Ambulos N, Campbell P, Mixson AJ. Highly branched HK peptides are effective carriers of siRNA. J Gene Med 2005;7:977–986 [DOI] [PubMed] [Google Scholar]
- 130. Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med 2017;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lu PY, Li L, Simonenko V, Inventors, Sirnaomics, Inc., Assignee. Multi-targeted RNAi therapeutics for scarless wound healing of skin. US patent USRE46873E12018
- 132. Somagenics, Inc. Wound Healing Program. https://somagenics.com/therapeuticswoundhealing (last accessed October28, 2019)
- 133. Dallas A, Trotsyuk A, Ilves H, et al. . Acceleration of diabetic wound healing with PHD2- and miR-210-targeting oligonucleotides. Tissue Eng Part A 2018;25:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. miRagen Therapeutics, Inc. Development of MRG 110 and LNA AntimiR-Targeting miR 92a for use in Tissue-Repair and Regeneration. http://www.miragen.com/publications/2019/10/Development-of-MRG-110-an-LNA-AntimiR-Targeting-miR-92a-for-Use-in-Tissue-Repair-and-Regeneration.pdf (last accessed June9, 2020)
- 135. Development of MRG-110, an LNA-antimiR targeting miR-92a for use in tissue repair and regeneration. Oligonucleotide Therapeutics Society Annual Meeting. Munich, Germany, 2019 [Google Scholar]
- 136. U.S. National Library of Medicine. Safety, tolerability, pharmacokinetics, and pharmacodynamics of MRG-110 following intradermal injection in healthy volunteers. https://clinicaltrials.gov/ct2/show/NCT03603431 (last accessed November26, 2019)
- 137. Helixmith Co. Engensis. https://www.helixmith.com/engensis#first (last accessed June9, 2020)
- 138. Kibbe MR, Hirsch AT, Mendelsohn FO, et al. . Safety and efficacy of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with critical limb ischemia. Gene Ther 2016;23:306–312 [DOI] [PubMed] [Google Scholar]
- 139. U.S. National Library of Medicine. Safety and efficacy study of VM202 in the treatment of chronic non-healing foot ulcers. https://clinicaltrials.gov/ct2/show/NCT02563522 (last accessed December30, 2019)
- 140. Cho KR, Choi J-S, Hahn W, et al. . Therapeutic angiogenesis using naked DNA expressing two isoforms of the hepatocyte growth factor in a porcine acute myocardial infarction model. Eur J Cardiothorac Surg 2008;34:857–863 [DOI] [PubMed] [Google Scholar]
- 141. Ajroud-Driss S, Christiansen M, Allen JA, Kessler JA. Phase 1/2 open-label dose-escalation study of plasmid DNA expressing two isoforms of hepatocyte growth factor in patients with painful diabetic peripheral neuropathy. Mol Ther 2013;21:1279–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kessler JA, Smith AG, Cha B-S, et al. . Double-blind, placebo-controlled study of HGF gene therapy in diabetic neuropathy. Ann Clin Transl Neurol 2015;2:465–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Sun N, Ning B, Hansson KM, et al. . Modified VEGF-A mRNA induces sustained multifaceted microvascular response and accelerates diabetic wound healing. Sci Rep 2018;8:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Gan L-M, Lagerström-Fermér M, Carlsson LG, et al. . Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat Commun 2019;10:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Carlsson L, Clarke JC, Yen C, et al. . Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol Ther Methods Clin Dev 2018;9:330–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zangi L, Lui KO, Gise Av, et al. . Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol 2013;31:898–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Qiu C, Coutinho P, Frank S, et al. . Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 2003;13:1697–1703 [DOI] [PubMed] [Google Scholar]
- 148. Ocunexus Therapeutics, Inc. Nexagon. 2017. https://ocunexus.com/nexagon (last accessed March25, 2020)
- 149. U.S. National Library of Medicine. A study evaluating nexagon in the treatment of skin wounds. https://clinicaltrials.gov/ct2/show/NCT00736593 (last accessed March25, 2020)
- 150. U.S. National Library of Medicine. The ASCEND study: a study to investigate the safety and clinical effect of nexagon to treat slow healing diabetic foot ulcers. https://clinicaltrials.gov/ct2/show/NCT00820703 (last accessed March25, 2020)
- 151. U.S. National Library of Medicine. NEXAGON for the treatment of corneal persistent epithelial defects following severe ocular chemical and/or thermal injuries. https://clinicaltrials.gov/ct2/show/NCT04081103. (last accessed March25, 2020)