INTRODUCTION
The term sarcopenia, derived from the Greek words sarx for flesh and penia for loss, was first introduced by Rosenberg1 in 1989 to describe the loss of muscle mass that accompanies aging. As he describes, there is probably no decline in structure and function more dramatic than the decline in lean body mass or muscle mass over the decades of life.1 Beginning as early as the fourth decade of life, there is a linear decrease in muscle mass and strength that accompanies increasing age.2 This age-related decline has been the focus of gerontology research and the basis for understanding functional decline and disability for several decades. Sarcopenia in the older adult has been associated with functional impairments, disability, increased risk of falls and fractures, reduced health-related quality of life (HRQOL), and increased risk of death.2,3 As sarcopenia often results in disability with a loss in independence necessitating caregiver assistance and/or long-term care placement, it has been estimated to cost the US health system approximately $18.5 billion in US dollars per year.4
CONTEXT
Key Objective
What is sarcopenia and why is it important in older adults with cancer?
Knowledge Generated
Older adults with cancer are at increased risk of sarcopenia, and in turn, increased risk of chemotherapy toxicities, tumor progression, and mortality. Sarcopenia can be assessed using a variety of available methods and individuals with sarcopenia may benefit from targeted interventions consisting of exercise and/or nutrition.
Relevance
Detecting sarcopenia in the growing number of older adults with cancer can aid in the assessment of the risk and benefit ratio of cancer treatments and assist in targeting interventions. Still, many important knowledge gaps persist in our understanding of sarcopenia in oncology and more research is needed.
While muscle strength and mass decrease with age, body fat gradually increases up until the seventh decade of life.5 Despite declining resting metabolic rates and physical activity levels with age, food intake remains disproportionately high, leading to yearly positive energy balance and weight gain.6 Thus, the development of sarcopenia is often accompanied concurrently with a gradual increase in adiposity termed sarcopenic obesity. Given the rising rates of obesity in the United States and abroad,7 the prevalence of this debilitating geriatric syndrome is increasing with a synergistic risk from both sarcopenia and obesity.8
More recently, sarcopenia is of increasing interest in oncology because of its high prevalence and association with adverse outcomes.9-12 Although the use of computed tomography (CT) for analysis of body composition was pioneered by Steven Heymsfield as early as the 1980s, it was not until 2004 that Shen et al described the methodology for estimating total-body skeletal muscle and adipose tissue from a single abdominal CT cross-sectional image.13-15 This method was later adapted specifically for the use of quantifying body composition in oncology research using archived images acquired during routine care.16 Only a few short years later in 2007, Prado et al17 used these methodologies to describe body composition as an independent determinant of fluorouracil based chemotherapy toxicities. From these initial studies, there has been a flurry of publications across nearly every cancer type and stage examining the association of CT-based body composition metrics and cancer outcomes. As of February 2021, a PubMed search using the terms sarcopenia and cancer resulted in 1,821 hits. Although sarcopenia has been a focus of research for many years, no broadly accepted consensus definition of sarcopenia exists, in oncology or elsewhere. The definition of sarcopenia is probably one of the least agreed upon and most debated topics in body composition research.18 Sarcopenia was first defined as two standard deviations below the mean muscle mass of healthy younger adults by Baumgartner et al19 using dual-energy X-ray absorptiometry (DEXA). More recently, the definition of sarcopenia has broadened to not only involve low muscle mass from one or more methods, but also reduced strength and/or physical performance.20 Although there is consensus within the larger gerontology literature that the definition of sarcopenia should include muscle strength and function as well as muscle mass, cancer-associated sarcopenia research has relied more heavily on CT-determined muscle mass as a diagnostic criterion. Complicating this further, a variety of CT derived cut-points have been used to characterize what is normal muscle mass in patients with cancer.12 Part of the discrepancy in the definition of sarcopenia between the gerontologic and oncologic literature exists because of the different viewpoints and outcomes under consideration. Geriatrics views sarcopenia in the light of developing disability, whereas oncology has focused more on low muscle mass and its association with increased mortality and complications from cancer treatments. Regardless, the lack of a consensus definition of sarcopenia has limited the generalizability and comparison of study findings and overall progress in the field.
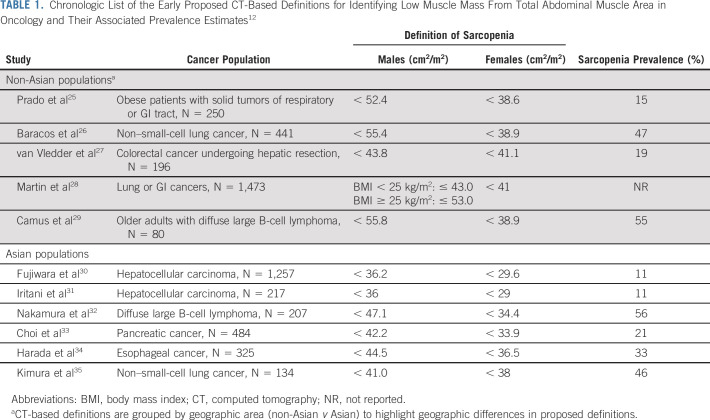
Given the variety of definitions applied, the prevalence of sarcopenia varies widely from 5%-89% based on the definition or cut-point applied and the population of interest.9,12 In recent reviews of the literature, 11 different definitions of sarcopenia using total abdominal muscle area were used to calculate prevalence and evaluate prognosis across the included studies (Table 1).9,12 As illustrated in Table 1, summary of the early proposed definitions of sarcopenia in oncology, the identified cut-point varies depending on the cancer population as well as the geographic area. Of particular interest is that while the prevalence of sarcopenia is higher in older adults, the phenomenon of low muscle mass is not limited to the older adult population in oncology. For example, in a recent study of more than 3,000 adults with early stage colorectal cancer, 58.3% of adults older than 70 years and 26.8% of adults younger than 60 years suffered from sarcopenia.21
TABLE 1.
Chronologic List of the Early Proposed CT-Based Definitions for Identifying Low Muscle Mass From Total Abdominal Muscle Area in Oncology and Their Associated Prevalence Estimates12
CAUSES OF SARCOPENIA
Sarcopenia can arise and progress because of normal physiologic aging and inactivity or, secondarily, as a consequence of chronic disease states like chronic obstructive pulmonary disease, AIDS, or cancer.1 Decreases in anabolic hormones, particularly testosterone, contribute significantly to age-related sarcopenia.22 Structurally, age-related sarcopenia is characterized by a decrease in the size of type II muscle fibers (also known as fast-twitch muscle fibers).23 Type II fiber atrophy is driven by several physiologic processes of aging, including loss of motor neurons, muscle denervation, neuromuscular junction instability, and decreases in muscle satellite cells responsible for growth, maintenance and repair of muscle.23,24 Aging also results in mitochondrial dysfunction characterized by reduced mitochondrial DNA and ATP production in skeletal muscle and the accumulation of intracellular reactive oxygen species.36 Muscle fiber atrophy is accompanied by the infiltration of adipose tissue, which produces a decrease in muscle quality.37
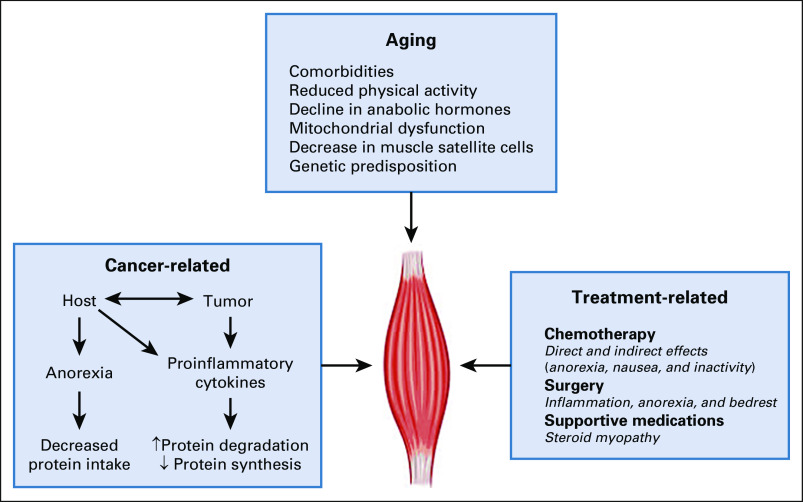
Cancer and its treatment can increase the risk of developing sarcopenia and exacerbate pre-existing muscle wasting in older adults.38 Cancer can potentiate many of the factors that contribute to age-related sarcopenia including anorexia, inactivity, and a proinflammatory state (Fig 1). In addition, many cancer treatments, particularly chemotherapeutic agents, can cause both indirect (anorexia, nausea, and fatigue) and direct damage to muscle tissue by pathways that upregulate proteasome activity, activate mitogen-activated protein kinase and extracellular regulated kinase signaling, and induce mitochondrial dysfunction irrespective of anorexia or nutrition.39-41
FIG 1.
Multifactorial causes of muscle loss in the older adult with cancer. ↑, increase; ↓, decrease.
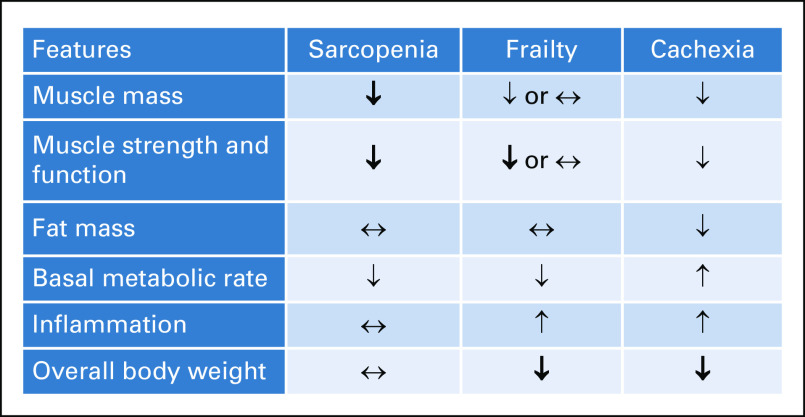
Sarcopenia has significant clinical overlap with cachexia, although the two are distinct wasting syndromes.42 Cachexia is diagnosed exclusively in disease states like cancer and is characterized as a syndrome of both weight and muscle loss with or without loss of fat mass (Fig 2).43 Like sarcopenia, cachexia is quite common in older adults with cancer and is accompanied by decreased muscle function and reduced physical performance.42,43 Although several physiologic derangements like low testosterone and mitochondrial dysfunction occur in both conditions, their pathophysiologies differ. Cachexia is driven by a procatabolic state that is influenced by tumor metabolism and by proinflammatory cytokines (ie, interleukin-6 and tumor necrosis factor) that are directly secreted by tumor tissue or produced by the host's immune response.44 These factors lead not only to protein and muscle degradation, but also to lipolysis.
FIG 2.
Comparisons of the similarities and distinctions between sarcopenia, cachexia, and frailty. The bolded arrows indicate these are part of the formal definition of that entity. ↑, increase; ↓, decrease; ↔, can be increased, decreased, or unchanged with no real effect.
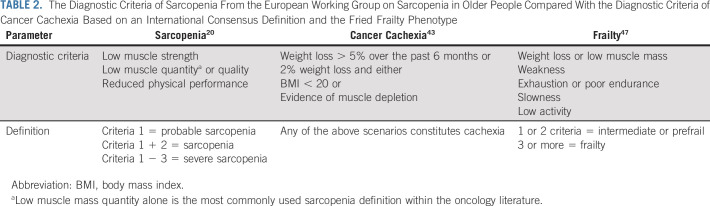
Frailty also has a close relationship and overlap with sarcopenia, but the two terms are certainly not interchangeable.45 Frailty is rarer and represents a state in which older adults are vulnerable under stress.46 A critical hallmark shared by sarcopenia and frailty is reduction in physical function; both conditions can be accompanied by a change in mass, although in frailty, it could be fat or lean mass.47 The frailty phenotype (three of five of the following: weakness, slowness, weight loss, fatigue, and decreased activity) described by Fried et al,47 however, encompasses a broader condition that can be influenced by other geriatric syndromes including sarcopenia, neurocognitive impairments, mood disorders, and socioeconomic factors. Frailty, sarcopenia, and cachexia have important areas of overlap and distinct differences with each other, with entity-associated adverse outcomes and vulnerability. Table 2 compares the common diagnostic criteria for sarcopenia, cancer cachexia, and frailty.20,43,47
TABLE 2.
The Diagnostic Criteria of Sarcopenia From the European Working Group on Sarcopenia in Older People Compared With the Diagnostic Criteria of Cancer Cachexia Based on an International Consensus Definition and the Fried Frailty Phenotype
METHODS FOR IDENTIFYING SARCOPENIA
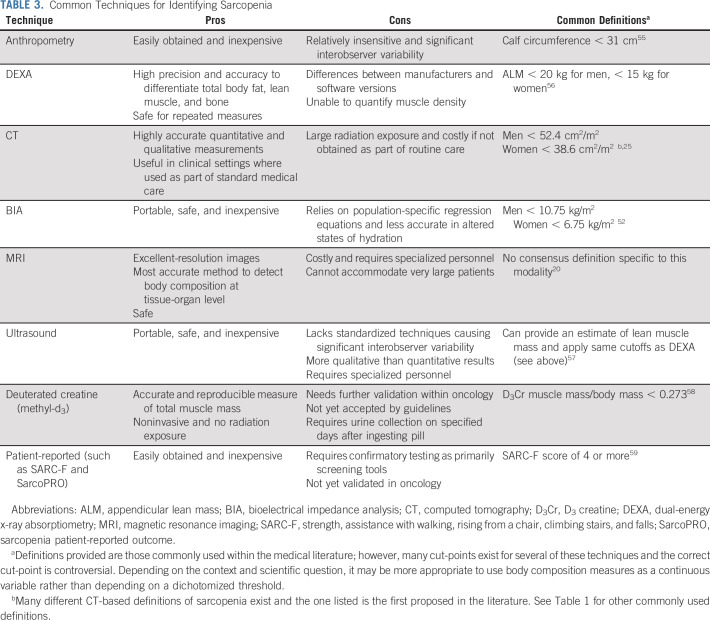
The growth and emergence of new imaging techniques have greatly affected the field of body composition research. Although body weight and body mass index are well-known and commonly used measures in clinical practice to gauge health and nutritional status, they are inaccurate, particularly in older patients, and unable to differentiate between various tissue compartments.18,48 Today, many different techniques and methodologies are available that can be used in the assessment of body composition ranging from simple anthropometric measures to more advanced magnetic resonance imaging (MRI) and even patient-reported measures. See Table 3 for a list of commonly used body composition techniques.
TABLE 3.
Common Techniques for Identifying Sarcopenia
One of the most commonly used imaging techniques within the nononcologic literature is DEXA.49 DEXA imaging is a very precise method for quantifying whole-body and regional composition with minimal radiation exposure. However, DEXA provides only an estimate of lean body mass or fat-free mass and is typically unable to decipher total body skeletal muscle mass nor differentiate types of adipose tissue.18 In addition, there is a variety of different hardware and software packages across manufacturers resulting in inconsistencies between machines, which limits comparability.
Within oncology, muscle mass is most often quantified by routine CT imaging, given their common use for cancer staging, disease monitoring, and cancer surveillance. Although CT is more costly and complex compared with many other measures, its routine use within many cancer types in oncology makes it very attractive for wide-scale use. Practical and precise measurement approaches have been developed to quantify body composition using CT images acquired during routine care.14,16 This approach commonly involves the use of a single cross-sectional slice to estimate whole-body muscle volume. The third lumbar vertebra landmark has been identified as the strongest correlate of total adiposity and muscle or fat-free mass when compared with MRI and DEXA.14,16 Given the abundance of CT imaging within oncology, the majority of the sarcopenia literature in cancer is derived from archived CT images. One additional advantage of using CT imaging is the ability to provide additional qualitative measurements of muscle and fat.50 Muscle radiodensity provides information regarding the composition of muscle otherwise only available from muscle biopsy. Attenuation of muscle is inversely related to muscle fat content and can be used as a surrogate measure of muscle quality.50 Fat infiltration in skeletal muscle, known as myosteatosis, is represented by lower muscle density and has been shown to correlate with frailty and physical functional impairments in older adults with cancer.45,51
Bioelectrical impedance analysis (BIA) is a low-cost, quick, and safe tool to estimate fat-free mass that can be performed at the bedside. BIA uses a small electrical signal sent through the body and measures resistance to provide estimations of total body water and fat-free mass.18 In a recent systematic review of 24 studies using BIA for the identification of sarcopenia in cancer, BIA was an accurate method for detecting sarcopenia and for evaluating associations with adverse outcomes.52 The primary drawback and barrier to the use of BIA in oncology is the lack of precision with fluctuations in hydration status and in the presence of edema.52 Nevertheless, if used carefully and in a standardized fashion, BIA represents an inexpensive and simple tool that does not require skilled personnel.
More novel and/or accurate techniques of assessing skeletal muscle mass have been developed over the past few years. Most notably, the use of the D3-creatine dilution method is a direct assessment of muscle mass that is gaining traction within the gerontologic literature.53,54 Although a promising method, D3-creatine dilution has not been reported on within oncology and warrants further use and validation at this time. In addition, patient-reported measures that ascertain impairments in strength and functioning are becoming increasingly common. In a recent study of strength, assistance with walking, rising from a chair, climbing stairs, and falls (SARC-F), a five-item questionnaire for rapid screening of sarcopenia, 33% of older adults with cancer screened positive, and positivity was associated with increased geriatric assessment impairments, reduced HRQOL, and inferior survival.59 This tool and other patient-reported sarcopenia screening tools are attractive, given the ease with which they could be incorporated into clinical care, but require confirmatory testing afterward and warrant more validation in oncology. Finally, although rarely incorporated in oncologic studies and not available retrospectively, measuring muscle strength and physical performance is important in understanding sarcopenia.60 Handgrip strength is a good and simple measure of muscle strength that correlates well with leg strength, poor mobility, and incident disability in activities of daily living (ADL).61 A wide range of techniques are available for assessing physical performance, including short physical performance battery, usual gait speed, stair climb power test, and 6-minute walk test.60 In older adult populations, physical performance assessments are often incorporated within geriatric assessments, which can be used to better understand sarcopenia and its consequences. As geriatric assessments are recommended as part of the routine care of older adults with cancer, this tool can be also be used to identify individuals at risk for sarcopenia.
The choice of measuring method of sarcopenia depends on several factors, including the specific purpose (clinical v research), the setting (inpatient v outpatient), and the cancer type (colorectal cancer v breast cancer, etc). There is no consensus regarding the best imaging modality to use to evaluate muscle mass at this time. In settings in which either CT, MRI, or DEXA imaging is used as part of routine care, these are preferred as they require no additional costs or radiation exposure. However, although these imaging modalities may be archived and accessible, muscle mass is not regularly measured or reported on radiology reports. As the literature and importance of sarcopenia grows, hopefully, reporting this information will become more commonplace. Of note, as the majority of the oncology literature is based on cross-sectional CT derived cut-points of muscle mass, this is the definition used in the majority of the cited literature on associations with outcomes. Which CT derived cut-point to use in defining sarcopenia is an area of contention and depends on many factors. For research studies, we suggest using muscle measures as a continuous variable (often stratified by sex) rather than using a cut-point as dichotomizing muscle measures assumes that everyone below and above the cut-point is the same risk, which is most often not true. For clinical use, it is more convenient to use a cut-point, and choosing one derived from a similar population in terms of geography, ethnicity, cancer type, and disease status is important.
SARCOPENIA AND ONCOLOGY OUTCOMES
In an umbrella review that included 30 meta-analyses of sarcopenia and adverse outcomes, sarcopenia was significantly associated with poorer prognosis across 12 cancer types: gastric, hepatocellular, urothelial, head and neck, hematologic malignancy, pancreatic, breast, colorectal, lung, esophageal, hematologic malignancies, and ovarian.62 All studies examined associations with overall survival or all-cause mortality. Sarcopenia increases risk of recurrence and cancer-related death in gastric, urothelial, colorectal, and hepatocellular cancer. Importantly, almost all (95% CI) of the meta-analyses included in the review demonstrated that sarcopenia was related to poorer prognosis with overall hazard ratios (HRs) ranging from 1.11 to 2.12.62 Greatest effects were seen in gastric, pancreatic, hepatocellular, and head and neck cancer. Similarly, in a recent meta-analysis of sarcopenia in malignant hematologic diseases, sarcopenia was an independent predictor of mortality disease (HR, 1.94).63 Only a few meta-analyses have specifically examined the association of sarcopenia with mortality in older adults, but those studies were not limited to patients with a cancer diagnosis. In those studies, sarcopenia was associated with an increased risk of mortality with the magnitudes of effect similar to those seen in the aforementioned cancer studies.64,65
In the same umbrella review, five of the meta-analyses examined sarcopenia and 16 postoperative outcomes in GI cancers (all), esophageal, gastric, or colorectal cancer.62 Sarcopenia was significantly related to one or more of major postoperative complications in each of those cancers. Where significant associations with sarcopenia and specific postoperative outcomes were found, effect sizes ranged from HRs of 1.35 to 6.24. Sarcopenia associated most strongly with postoperative pulmonary complications, infections, readmission rates, and length of hospitalization. Two additional systematic reviews, both in urologic cancers, had conflicting results. Sarcopenia was associated with a higher 90-day complication rate, whereas in the other, there was no association between sarcopenia and postoperative outcomes after urologic oncology surgery. In patients of age 65 years and older not restricted to cancer, low muscle mass was associated with increased length of hospital stay in endoscopic surgeries.66 No reviews have specially addressed sarcopenia and surgical outcomes among older adults with cancer. Furthermore, sarcopenia was associated with complications and increased length of hospital stay in patients undergoing autologous or allogenic hematopoietic cell transplantation.67,68
In a systematic review done in 2016, Gerard et al69 examined the association between body composition and chemotherapy or targeted therapy toxicity, which included 14 studies across the following cancer types: thyroid, colorectal, renal, hepatocellular, breast, esophageal, and ovarian cancer. Twelve of the 14 studies demonstrated higher chemotherapy toxicity associated with reduced lean mass or sarcopenia compared with normal or high lean mass. Of the various chemotherapy and targeted therapies studied, no significant association was reported between lean mass and chemotoxicity in studies of women receiving anthracyclines for breast and ovarian cancer. Evidence regarding the relationship between sarcopenia and chemotherapy toxicity specifically in older adults is lacking. Investigating this relationship in the geriatric oncology population is crucially important, given the known changes in loss of lean mass with age and the observation that approximately half of the older patients will present with severe toxicity.70,71
EXPLAINING SARCOPENIA AND ADVERSE OUTCOMES
Although many studies have demonstrated the association of sarcopenia with adverse outcomes in cancer, only a few explanations have been proposed to explain these findings. Dosing of the majority of cytotoxic chemotherapeutics is based solely on height and weight and does not take into consideration differences in volume of distribution, drug metabolism, and clearance. Commonly, with aging, there is a decline in muscle mass while there is an increase in fat tissue. These body-composition changes result in the change of pharmacokinetics of both hydrophilic drugs (increasing the volume of distribution and extend the half-life time of hydrophilic drugs) and hydrophobic drugs (decrease in volume of distribution and also in case of hypoalbuminemia, increase free fraction bound of protein-bound drugs).69,72 These body-composition changes result in the change of pharmacokinetics of both hydrophilic drugs (increasing the volume of distribution and extending the half-life) and hydrophobic drugs (decreasing the volume of distribution, and also in the case of hypoalbuminemia, increasing the free fraction of protein-bound drugs).72
Another proposed explanation is sarcopenia as a marker of an increased cancer-related inflammatory response. In a large-scale study of patients with colorectal cancer (n = 2,470) prediagnosis inflammation (measured as neutrophil-to-lymphocyte ratio) was associated with at-diagnosis sarcopenia. Sarcopenia combined with inflammation nearly doubled risk of death from colorectal cancer (HR, 2.43; 95% CI, 1.79 to 3.29).73 Chronic inflammation is characterized by increase in circulating proinflammatory cytokines, accompanied by elevated presence of dysfunctional T-regulatory cells and a T-cell–senescent phenotype. These changes decrease the immune response, resulting in a condition known as immune-senescence.74 A study by Arrieta et al75 evaluated the relationship of inflammatory markers and chemotherapy toxicity in patients with non–small-cell lung cancer who have received paclitaxel-cisplatin. Elevated inflammatory markers were associated with grade 3-4 toxicities as measured by platelet-lymphocyte ratio and anemia.
To date, there are limited prospective data on the added benefit of body composition beyond other known predictors of treatment toxicity and survival, such as geriatric assessment factors.70 Critical prospective studies, including dedicated pharmacokinetics studies, are missing and highly needed to fully understand the relationship of sarcopenia with adverse outcomes and to inform effective and targeted interventions.
SARCOPENIA IN SURVIVORSHIP
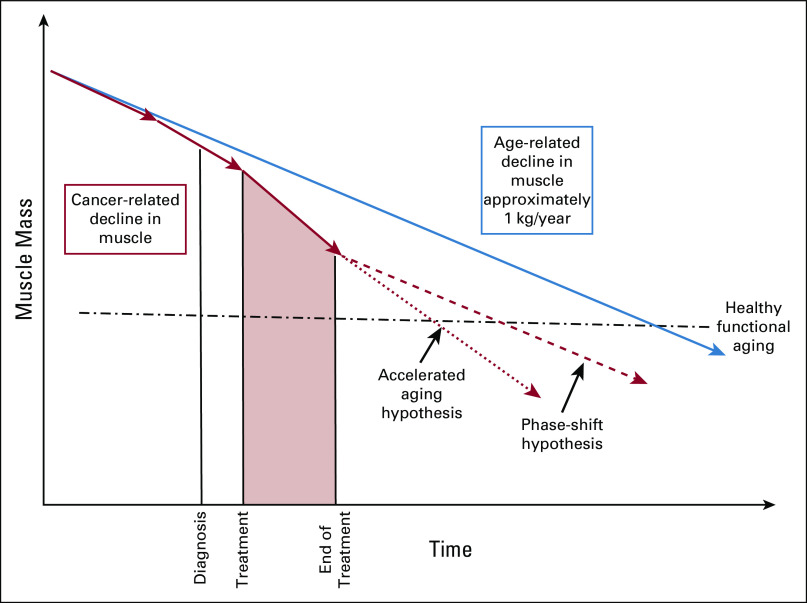
With the growing number of cancer survivors, there is increasing focus on the needs of cancer survivors, many of whom are older adults.76 Many older adults with cancer have limitations in physical function and impairments in ADL and instrumental ADLs.77,78 These impairments have been linked with sarcopenia and are likely related to worsening sarcopenia from a cancer diagnosis and cancer treatments. In a recent study of community-dwelling older adults, those diagnosed with cancer had an accelerated decline of appendicular lean muscle mass compared with noncancer and age-matched controls.38 Figure 3 represents the hypothetical declines in sarcopenia associated with a cancer diagnosis. There is likely some acceleration of sarcopenia related directly to the cancer itself that occurs before diagnosis, which is likely further exacerbated by antineoplastic therapies. In patients who complete curative intent cancer treatments and enter the survivorship phase, it remains unknown whether there is recovery, continued accelerated declines, or rather a phase shift of sarcopenia (Fig 3). Given the rising number of older adult cancer survivors and functional independence being a high-priority outcome for the older population, future studies are needed to better understand sarcopenia as a survivorship issue amendable to targeted intervention.
FIG 3.
Hypothetical graph of the changes in muscle mass in older adults before and after a cancer diagnosis in comparison to normal age-related declines.
INTERVENING ON SARCOPENIA
Because of a high prevalence of sarcopenia among older adults, and its association with adverse outcomes, a number of interventions are being investigated to combat sarcopenia and minimize such adverse events. These include physical exercise, nutritional supplementation, hormone replacement, and therapeutic agents promoting muscle mass. However, most studies have been performed among noncancer patients, and studies focused among patients with cancer are relatively limited.42,79
Exercise interventions incorporating resistance training have been shown to increase both muscle mass and physical function among adults with cancer. In a cohort of patients with metastatic prostate cancer receiving androgen deprivation therapy, where muscle loss is common, a combined resistance and aerobic exercise program helped combat and reverse the loss of muscle.80 Another study testing 12 weeks of resistance training versus usual care among 37 patients with prostate cancer receiving androgen deprivation therapy similarly reported improvement in lean mass, sarcopenia prevalence, muscle strength, and HRQOL.81 Another study among patients with breast cancer receiving adjuvant chemotherapy randomized resistance exercise training (n = 64), aerobic exercise training (n = 66), or usual care (n = 70) demonstrated that resistance exercise can reverse sarcopenia and improve quality of life.82 In a meta-analysis of 11 randomized controlled trials examining the impact of resistance training in cancer survivors of all ages, resistance training was shown to be associated with improvements in muscle function and body composition.83 Nonetheless, major barriers exist to implementing these interventions as many community-dwelling older adults lack access or motivation to partake in a rigorous exercise program and the optimal type, intensity, and timing of exercise interventions remain unknown.83,84
Malnutrition is highly prevalent among older adults with cancer and represents one of the mechanisms contributing to sarcopenia.85,86 Although the recommended protein requirement for older individuals (0.8 g/kg per day) has remained unchanged for decades, a higher protein intake may be needed to optimize muscle protein synthesis.87 Aside from physical activity status, total daily protein intake appears to be the most important determinant of skeletal muscle mass, and several studies have examined acute and chronic effects of nutritional supplementation among older adults.88 In a study of 61 patients undergoing cystectomy for bladder cancer randomly assigned to oral nutritional supplementation versus multivitamin supplementation, a reduced prevalence of sarcopenia and fewer adverse events or readmissions were seen favoring the oral nutrition group.89 Another study randomly assigned patients with colorectal cancer undergoing chemotherapy into whey protein supplementation versus placebo and reported improved sarcopenia indices and nutritional status favoring the intervention arm.90 However, in a large individual patient data meta-analysis of eight randomized trials incorporating 557 older adults studying the effect of protein or amino acid supplementation on muscle mass and strength, no significant positive effects of protein or amino acid supplementation on muscle mass or strength were seen.91 Combinations of exercise and nutritional supplementation may have an additive effect on optimizing muscle protein synthesis and reversing sarcopenia, and trials are ongoing to test the effectiveness of such multimodal interventions among older adults with cancer.92
Finally, several pharmacologic interventions for combating sarcopenia remain under investigation. Studied agents include antibodies against myostatin (eg, stamulumab and trevogrumab), activin receptor agonists (eg, bimagrumab), exercise mimetics (eg, PPARβ/δ agonist), and selective androgen receptor modulators (eg, enobosarm). However, a clear benefit from larger definitive phase III trials remains to be seen and as a result, currently no US Food and Drug Administration–approved therapeutic agents exist for treatment of sarcopenia.79
DISCUSSION
The growing field of body composition in cancer holds great promise in better assessing and treating older adults with cancer, yet the research of body composition and geriatric oncology has many unanswered questions. How is the mechanism or process of muscle loss different between aging patients with and without cancer? How should sarcopenia be defined in oncology? What factors and interventions modify the adverse trajectories of sarcopenia related to cancer and cancer treatments? How can we best treat and help to maintain muscle mass and function? The care of older adults with cancer is challenging with the dual focus to maintain the quality of life and functional independence as best as possible while at the same time maintaining treatment efficacy. The use of body composition in assessing older adults with cancer is a promising avenue to provide more personalized oncologic treatment that could ultimately improve outcomes.
Grant R. Williams
Honoraria: Carevive Systems, Cardinal Health
Richard F. Dunne
Consulting or Advisory Role: Exelixis
Smith Giri
Honoraria: CareVive, OncLive
Research Funding: Carevive Systems, Pack Health
Shlomit S. Shachar
Consulting or Advisory Role: Novartis, Roche, Pfizer, Lilly
Travel, Accommodations, Expenses: Pfizer, Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported in part by the National Cancer Institute of the National Institutes of Health (Grant No. K08CA234225, G.R.W.), the American Cancer Society (Grant No. ACS-18-162-59-IRG, G.R.W.) and the National Center for Advancing Translational Sciences of the National Institutes of Health (Grant No. KL2TR001999, R.F.D.).
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sarcopenia in the Older Adult With Cancer
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Grant R. Williams
Honoraria: Carevive Systems, Cardinal Health
Richard F. Dunne
Consulting or Advisory Role: Exelixis
Smith Giri
Honoraria: CareVive, OncLive
Research Funding: Carevive Systems, Pack Health
Shlomit S. Shachar
Consulting or Advisory Role: Novartis, Roche, Pfizer, Lilly
Travel, Accommodations, Expenses: Pfizer, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rosenberg IH.Sarcopenia: Origins and clinical relevance J Nutr 127990S–991S1997suppl 5 [DOI] [PubMed] [Google Scholar]
- 2.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives J Nutr Health Aging 12433–4502008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons J Gerontol A Biol Sci Med Sci 60324–3332005 [DOI] [PubMed] [Google Scholar]
- 4.Janssen I, Shepard DS, Katzmarzyk PT, et al. The healthcare costs of sarcopenia in the United States J Am Geriatr Soc 5280–852004 [DOI] [PubMed] [Google Scholar]
- 5.Heo M, Faith MS, Pietrobelli A, et al. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999-2004 Am J Clin Nutr 95594–6022012 [DOI] [PubMed] [Google Scholar]
- 6.Wilson MM, Morley JE.Invited review: Aging and energy balance J Appl Physiol 951728–17362003 [DOI] [PubMed] [Google Scholar]
- 7.Malik VS, Willett WC, Hu FB.Global obesity: Trends, risk factors and policy implications Nat Rev Endocrinol 913–272013 [DOI] [PubMed] [Google Scholar]
- 8.Batsis JA, Villareal DT.Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies Nat Rev Endocrinol 14513–5372018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shachar SS, Williams GR, Muss HB, et al. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review Eur J Cancer 5758–672016 [DOI] [PubMed] [Google Scholar]
- 10.Dunne RF, Roussel B, Culakova E, et al. Characterizing cancer cachexia in the geriatric oncology population J Geriatr Oncol 10415–4192019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kazemi-Bajestani SMR, Mazurak VC, Baracos V.Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes Semin Cell Dev Biol 542–102016 [DOI] [PubMed] [Google Scholar]
- 12.Rier HN, Jager A, Sleijfer S, et al. The prevalence and prognostic value of low muscle mass in cancer patients: A review of the literature Oncologist 211396–14092016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Wang Z, Baumgartner RN, et al. Human body composition: Advances in models and methods Annu Rev Nutr 17527–5581997 [DOI] [PubMed] [Google Scholar]
- 14.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image J Appl Physiol (1985) 972333–23382004 [DOI] [PubMed] [Google Scholar]
- 15.Heymsfield SB, Olafson RP, Kutner MH, et al. A radiographic method of quantifying protein-calorie undernutrition Am J Clin Nutr 32693–7021979 [DOI] [PubMed] [Google Scholar]
- 16.Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care Appl Physiol Nutr Metab 33997–10062008 [DOI] [PubMed] [Google Scholar]
- 17.Prado CM, Baracos VE, McCargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity Clin Cancer Res 133264–32682007 [DOI] [PubMed] [Google Scholar]
- 18.Prado CM, Heymsfield SB.Lean tissue imaging: A new era for nutritional assessment and intervention JPEN J Parenter Enteral Nutr 38940–9532014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico Am J Epidemiol 147755–7631998 [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis Age Ageing 4816–312019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS study) Cancer Epidemiol Biomarkers Prev 261008–10152017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin MJ, Jeon YK, Kim IJ.Testosterone and sarcopenia World J Mens Health 36192–1982018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdijk LB, Snijders T, Drost M, et al. Satellite cells in human skeletal muscle; from birth to old age Age (Dordr) 36545–5472014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson L, Degens H, Li M, et al. Sarcopenia: Aging-related loss of muscle mass and function Physiol Rev 99427–5112019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prado CMM, Liefers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study Lancet Oncol 9629–6352008 [DOI] [PubMed] [Google Scholar]
- 26.Baracos VE, Reiman T, Mourtzakis M, et al. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis Am J Clin Nutr 911133S–1137S2010 [DOI] [PubMed] [Google Scholar]
- 27.van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases Br J Surg 99550–5572012 [DOI] [PubMed] [Google Scholar]
- 28.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index J Clin Oncol 311539–15472013 [DOI] [PubMed] [Google Scholar]
- 29.Camus V, Lanic H, Kraut J, et al. Prognostic impact of fat tissue loss and cachexia assessed by computed tomography scan in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy Eur J Haematol 939–182014 [DOI] [PubMed] [Google Scholar]
- 30.Fujiwara N, Nakagawa H, Kudo Y, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma J Hepatol 63131–1402015 [DOI] [PubMed] [Google Scholar]
- 31.Iritani S, Imai K, Takai K, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma J Gastroenterol 50323–3322015 [DOI] [PubMed] [Google Scholar]
- 32.Nakamura N, Hara T, Shibata Y, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma Ann Hematol 942043–20532015 [DOI] [PubMed] [Google Scholar]
- 33.Choi Y, Oh DY, Kim TY, et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS One. 2015;10:e0139749. doi: 10.1371/journal.pone.0139749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada K, Ida S, Baba Y, et al. Prognostic and clinical impact of sarcopenia in esophageal squamous cell carcinoma Dis Esophagus 29627–6332016 [DOI] [PubMed] [Google Scholar]
- 35.Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer Support Care Cancer 231699–17082015 [DOI] [PubMed] [Google Scholar]
- 36.Boengler K, Kosiol M, Mayr M, et al. Mitochondria and ageing: Role in heart, skeletal muscle and adipose tissue J Cachexia Sarcopenia Muscle 8349–3692017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frontera WR, Zayas AR, Rodriguez N.Aging of human muscle: Understanding sarcopenia at the single muscle cell level Phys Med Rehabil Clin N Am 23201–207xiii2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams GR, Chen Y, Kenzik KM, et al. Assessment of sarcopenia measures, survival, and disability in older adults before and after diagnosis with cancer. JAMA Netw Open. 2020;3:e204783. doi: 10.1001/jamanetworkopen.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen JA, Splenser A, Guillory B, et al. Ghrelin prevents tumour- and cisplatin-induced muscle wasting: Characterization of multiple mechanisms involved J Cachexia Sarcopenia Muscle 6132–1432015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barreto R, Mandili G, Witzmann FA, et al. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016;7:472. doi: 10.3389/fphys.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression FASEB J 1839–512004 [DOI] [PubMed] [Google Scholar]
- 42.Dunne RF, Loh KP, Williams GR, et al. Cachexia and sarcopenia in older adults with cancer: A comprehensive review. Cancers (Basel) 2019;11:1861. doi: 10.3390/cancers11121861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: An international consensus Lancet Oncol 12489–4952011 [DOI] [PubMed] [Google Scholar]
- 44.Friesen DE, Baracos VE, Tuszynski JA. Modeling the energetic cost of cancer as a result of altered energy metabolism: Implications for cachexia. Theor Biol Med Model. 2015;12:17. doi: 10.1186/s12976-015-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams GR, Deal AM, Muss HB, et al. Frailty and skeletal muscle in older adults with cancer J Geriatr Oncol 968–732018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gingrich A, Volkert D, Kiesswetter E, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019;19:120. doi: 10.1186/s12877-019-1115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype J Gerontol A Biol Sci Med Sci 56M146–M1562001 [DOI] [PubMed] [Google Scholar]
- 48.Shachar SS, Williams GR.The obesity paradox in cancer-moving beyond BMI-response Cancer Epidemiol Biomarkers Prev 2613–162017 [DOI] [PubMed] [Google Scholar]
- 49.Shepherd JA, Ng BK, Sommer MJ, et al. Body composition by DXA Bone 104101–1052017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation Acta Physiol 210489–4972014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams GR, Deal AM, Muss HB, et al. Skeletal muscle measures and physical function in older adults with cancer: Sarcopenia or myopenia? Oncotarget 833658–336652017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aleixo GFP, Shachar SS, Nyrop KA, et al. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: A systematic review Oncologist 25170–1822020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evans WJ, Hellerstein M, Orwoll E, et al. D3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass J Cachexia Sarcopenia Muscle 1014–212019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clark RV, Walker AC, O'Connor-Semmes RL, et al. Total body skeletal muscle mass: Estimation by creatine (methyl-d3) dilution in humans J Appl Physiol 1161605–16132014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landi F, Onder G, Russo A, et al. Calf circumference, frailty and physical performance among older adults living in the community Clin Nutr 33539–5442014 [DOI] [PubMed] [Google Scholar]
- 56.Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates J Gerontol A Biol Sci Med Sci 69547–5582014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nijholt W, Scafoglieri A, Jager-Wittenaar H, et al. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review J Cachexia Sarcopenia Muscle 8702–7122017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cawthon PM, Orwoll ES, Peters KE, et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men J Gerontol A Biol Sci Med Sci 74844–8522019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams GR, Al-Obaidi M, Dai C, et al. SARC-F for screening of sarcopenia among older adults with cancer Cancer 1271469–14752021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on sarcopenia in older people Age Ageing 39412–4232010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia J Appl Physiol 951851–18602003 [DOI] [PubMed] [Google Scholar]
- 62.Xia L, Zhao R, Wan Q, et al. Sarcopenia and adverse health-related outcomes: An umbrella review of meta-analyses of observational studies Cancer Med 97964–79782020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surov A, Wienke A.Sarcopenia predicts overall survival in patients with malignant hematological diseases: A meta-analysis Clin Nutr 401155–11602021 [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Wang C, Dou Q, et al. Sarcopenia as a predictor of all-cause mortality among older nursing home residents: A systematic review and meta-analysis. BMJ Open. 2018;8:e021252. doi: 10.1136/bmjopen-2017-021252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu P, Hao Q, Hai S, et al. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: A systematic review and meta-analysis Maturitas 10316–222017 [DOI] [PubMed] [Google Scholar]
- 66.Hossain M, Yu D, Bikdeli B, et al. Sarcopenia and adverse post-surgical outcomes in geriatric patients: A scoping review J Frailty Aging 1063–692021 [DOI] [PubMed] [Google Scholar]
- 67.Armenian SH, Xiao M, Berano Teh J, et al. Impact of sarcopenia on adverse outcomes after allogeneic hematopoietic cell transplantation J Natl Cancer Inst 111837–8442019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Caram MV, Bellile EL, Englesbe MJ, et al. Sarcopenia is associated with autologous transplant-related outcomes in patients with lymphoma Leuk Lymphoma 562855–28622015 [DOI] [PubMed] [Google Scholar]
- 69.Gerard S, Brechemier D, Lefort A, et al. Body composition and anti-neoplastic treatment in adult and older subjects—A systematic review J Nutr Health Aging 20878–8882016 [DOI] [PubMed] [Google Scholar]
- 70.Williams GR, Rier HN, McDonald A, et al. Sarcopenia & aging in cancer J Geriatr Oncol 10374–3772019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer J Clin Oncol 322595–26032014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hopkins JJ, Sawyer MB.Interactions of lean soft-tissue and chemotherapy toxicities in patients receiving anti-cancer treatments Cancer Chemother Pharmacol 821–292018 [DOI] [PubMed] [Google Scholar]
- 73.Cespedes Feliciano EM, Kroenke CH, Meyerhardt JA, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the C SCANS study. JAMA Oncol. 2017;3:e172319. doi: 10.1001/jamaoncol.2017.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pascual-Fernández J, Fernández-Montero A, Córdova-Martínez A, et al. Molecular pathways and potential targets for intervention. Int J Mol Sci. 2020;21:8844. doi: 10.3390/ijms21228844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arrieta O, Michel Ortega RM, Villanueva-Rodríguez G, et al. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: A prospective study. BMC Cancer. 2010;10:50. doi: 10.1186/1471-2407-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bluethmann SM, Mariotto AB, Rowland JH.Anticipating the “Silver Tsunami”: Prevalence trajectories and comorbidity burden among older cancer survivors in the United States Cancer Epidemiol Biomarkers Prev 251029–10362016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nightingale G, Battisti NML, Loh KP, et al. Perspectives on functional status in older adults with cancer: An interprofessional report from the International Society of Geriatric Oncology (SIOG) nursing and allied health interest group and young SIOG J Geriatr Oncol 406830490–304922020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pergolotti M, Deal AM, Williams GR, et al. Activities, function, and health-related quality of life (HRQOL) of older adults with cancer J Geriatr Oncol 8249–2542017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwak JY, Kwon KS.Pharmacological interventions for treatment of sarcopenia: Current status of drug development for sarcopenia Ann Geriatr Med Res 2398–1042019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galvao DA, Taaffe DR, Spry N, et al. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: A randomized controlled trial J Clin Oncol 28340–3472010 [DOI] [PubMed] [Google Scholar]
- 81.Dawson JK, Dorff TB, Todd Schroeder E, et al. Impact of resistance training on body composition and metabolic syndrome variables during androgen deprivation therapy for prostate cancer: A pilot randomized controlled trial. BMC Cancer. 2018;18:368. doi: 10.1186/s12885-018-4306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adams SC, Segal RJ, McKenzie DC, et al. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial Breast Cancer Res Treat 158497–5072016 [DOI] [PubMed] [Google Scholar]
- 83.Strasser B, Steindorf K, Wiskemann J, et al. Impact of resistance training in cancer survivors: A meta-analysis Med Sci Sports Exerc 452080–20902013 [DOI] [PubMed] [Google Scholar]
- 84.Kilari D, Soto-Perez-de-Celis E, Mohile SG, et al. Designing exercise clinical trials for older adults with cancer: Recommendations from 2015 Cancer and Aging Research Group NCI U13 Meeting J Geriatr Oncol 7293–3042016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams GR, Al-Obaidi M, Dai C, et al. Association of malnutrition with geriatric assessment impairments and health-related quality of life among older adults with gastrointestinal malignancies Cancer 1265147–51552020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang X, Pang L, Sharma SV, et al. Prevalence and factors associated with malnutrition in older patients with cancer J Geriatr Oncol 10763–7692019 [DOI] [PubMed] [Google Scholar]
- 87.Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group Clin Nutr 33929–9362014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McKendry J, Currier BS, Lim C, et al. Nutritional supplements to support resistance exercise in countering the sarcopenia of aging. Nutrients. 2020;12:2057. doi: 10.3390/nu12072057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ritch CR, Cookson MS, Clark PE, et al. Perioperative oral nutrition supplementation reduces prevalence of sarcopenia following radical cystectomy: Results of a prospective randomized controlled trial J Urol 201470–4772019 [DOI] [PubMed] [Google Scholar]
- 90.Mazzuca F, Roberto M, Arrivi G, et al. Clinical impact of highly purified, whey proteins in patients affected with colorectal cancer undergoing chemotherapy: Preliminary results of a placebo-controlled study. Integr Cancer Ther. 2019;18:1534735419866920. doi: 10.1177/1534735419866920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tieland M, Franssen R, Dullemeijer C, et al. The impact of dietary protein or amino acid supplementation on muscle mass and strength in elderly people: Individual participant data and meta-analysis of RCT's J Nutr Health Aging 21994–10012017 [DOI] [PubMed] [Google Scholar]
- 92.Naito T, Mitsunaga S, Miura S, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer J Cachexia Sarcopenia Muscle 1073–832019 [DOI] [PMC free article] [PubMed] [Google Scholar]