Abstract
PURPOSE
To determine whether germline BRCA (gBRCA) pathogenic variants are associated with decreased ovarian reserve.
MATERIALS AND METHODS
An individual patient-level data meta-analysis was performed using five data sets on 828 evaluable women who were tested for gBRCA. Of those, 250 carried gBRCA, whereas 578 had tested negative and served as controls. Of the women with gBRCA, four centers studied those affected with breast cancer (n = 161) and one studied unaffected individuals (n = 89). The data were adjusted for the center, age, body mass index, smoking, and oral contraceptive pill use before the final analysis. Anti-Müllerian hormone (AMH) levels in affected women were drawn before presystemic therapy.
RESULTS
The mean age of women with versus without gBRCA1/2 (34.1 ± 4.9 v 34.3 ± 4.8 years; P = .48) and with gBRCA1 versus gBRCA2 (33.7 ± 4.9 v 34.6 ± 4.8 years; P = .16) was similar. After the adjustments, women with gBRCA1/2 had significantly lower AMH levels compared with controls (23% lower; 95% CI, 4 to 38; P = .02). When the adjusted analysis was limited to affected women (157 with gBRCA v 524 without, after exclusions), the difference persisted (25% lower; 95% CI, 9 to 38; P = .003). The serum AMH levels were lower in women with gBRCA1 (33% lower; 95% CI, 12 to 49; P = .004) but not gBRCA2 compared with controls (7% lower; 95% CI, 31% lower to 26% higher; P = .64).
CONCLUSION
Young women with gBRCA pathogenic variants, particularly those affected and with gBRCA1, have lower serum AMH levels compared with controls. They may need to be preferentially counseled about the possibility of shortened reproductive lifespan because of diminished ovarian reserve.
INTRODUCTION
BRCA1 and BRCA2 (BRCA1/2) play an essential role in double-strand DNA break (DSB) repair through recombination with undamaged, homologous DNA strands.1 Mutations in these genes are associated with increased susceptibility to breast and ovarian cancer.2 Starting with our first clinical and laboratory observations,3-7 a growing body of laboratory, translational, and clinical evidence has emerged within the last decade, indicating a role for BRCA and related DNA DSB repair genes in ovarian function and aging.6,7
CONTEXT
Key Objective
Deoxyribonucleic acid repair deficiency is emerging as a joint mechanism for breast cancer and reproductive aging. Recent studies showed that ovarian reserve may be lower in women with BRCA (gBRCA) pathogenic variants because of deoxyribonucleic acid repair deficiency. However, clinical studies using the most sensitive serum ovarian reserve marker anti-Müllerian hormone provided mixed results. Given the heterogeneity of the data from clinical studies, we performed an individual patient data meta-analysis to determine if gBRCA is associated with lower ovarian reserve.
Knowledge Generated
gBRCA is associated with diminished ovarian reserve, as determined by serum Anti-Müllerian hormone, and this association seems to be restricted to gBRCA1. This finding is firmer for affected women as this individual patient data meta-analysis predominantly studied those with breast cancer.
Relevance
Women with gBRCA may have shortened reproductive life span because of diminished ovarian reserve and should be proactively counseled for fertility preservation especially if faced with chemotherapy or delaying childbearing.
Anti-Müllerian hormone (AMH) is the best available serum marker of ovarian reserve. It is produced by granulosa cells of small antral and preantral follicles in the ovary and, by proportion, reflects the primordial follicle reserve.8 Serum AMH levels do not significantly fluctuate and can be measured at any point during the menstrual cycle. In contrast, the levels of indirect and less sensitive ovarian reserve markers such as follicle-stimulating hormone and E2 are highly dependent on the menstrual cycle day. One limitation for all ovarian reserve markers is that their levels can be affected by smoking, oral contraceptive use, and obesity.9 Several studies have used serum AMH to investigate whether germline BRCA (gBRCA) pathogenic variants are associated with diminished ovarian reserve. Although a majority of studies indicated diminished ovarian reserve in women with gBRCA1/2, some provided conflicting results.5-19 Several clinical studies including our own5 and transgenic mouse data indicated a stronger association of gBRCA1 with diminished ovarian reserve than with gBRCA2; however, one study found gBRCA2 but not the gBRCA1 to be associated with lower ovarian reserve.15 Several other studies did not detect lower serum AMH levels in women with gBRCA compared with controls.14,18,19
We recently performed a systematic review to investigate the role of gBRCA in ovarian aging.6 We found that the small sample size, lack of adjustment for important covariates (such as age, smoking, and oral contraceptive pill use), not accounting for differences between the gBRCA1 and gBRCA2 carriers, and inadequate statistical methods were among the major limitations of many studies investigating the association between gBRCA and serum AMH levels. To address these limitations and to provide more conclusive clinical assessment of this critical topic, we performed an individual patient-level data (IPD) analysis with studies that investigated serum AMH levels in women with gBRCA1/2.
Based on laboratory5 and clinical data,6 we hypothesized that AMH levels are lower in women with gBRCA1/2, especially in those carrying gBRCA1, compared with the individuals who tested negative for gBRCA1/2. To that end, we report the comparison of serum AMH levels in women with gBRCA1/2 compared with those who were found to be negative for mutations in the same genes.
MATERIALS AND METHODS
We searched for published articles in the PubMed database containing keywords, BRCA, BRCA1, BRCA2, mutations, BRCA pathogenic variants, ovarian reserve, and AMH in the English-language literature until December 2019. We found 12 original studies investigating the association between gBRCA1/2 and serum AMH levels, four of which included only affected women with breast cancer5,10,20,21 and one included both affected and unaffected18 (Data Supplement, online only). After the study was approved by the Institutional Review Board (TR21092018/025), invitation letters were sent to all corresponding authors of the published articles. Four centers declined to participate, and three did not respond. Of the seven nonparticipating centers, all studied unaffected women with the exception of one, which also included a small contingent of affected women. Five centers shared their IPD from their publications. In addition, Lambertini et al10 updated their data with additional cases. In their published manuscript, the numbers of women with and without gBRCA1/2 were 25 and 60, respectively. After updating their data, these numbers reached 50 and 85, respectively. As a result, a meta-analysis with five centers using IPD with some common key variables was conducted. Of those five centers, four (centers from New York, South Korea, Belgium, and France) studied women affected with breast cancer. One center studied unaffected women (Los Angeles, CA).
Data Collection and Inclusion and Exclusion Criteria
For all participants enrolled in each of the included studies, IPD that contained demographics, parity, smoking status, oral contraceptive pill use, the gBRCA1 and/or gBRCA2 testing status, breast cancer stage (if affected), and serum AMH levels were collected. In affected women, serum AMH was drawn before the initiation of chemotherapy.
The common inclusion criteria were age 18-45, premenopausal status, no prior or ongoing chemotherapy or pelvic surgery, no use of endocrine therapy, and having been tested for gBRCA1/2. Other than one center (Los Angeles, CA), all excluded women with irregular periods and history of polycystic ovarian diseases or other reproductive endocrine disorders.
AMH Assessment
Statistics
We summarized patient characteristics by gBRCA status using standard descriptive statistics—mean and standard deviation (SD) for continuous variables and frequency and proportion for categorical variables. We set 0.01 as the detection limit and used log10-transformed AMH data following our previously published approach22 and our examination of the AMH data for this IPD meta-analysis.
Data were analyzed using the statistical methods for multicenter studies or IPD meta-analysis with patient-level covariates and outcomes.23-25 The age-adjusted model was fit for five studies or centers individually, and sequentially adjusted models (from center and age only to center, age, smoking, oral contraceptive pill use, and body mass index [BMI]) for the combined sample. Smoking and oral contraceptive pill (OCP) use were categorized to three levels (Y, N, and missing) to avoid imputation and to use maximum sample size. Patients with missing BMI (as continuous variable) were not included when BMI was adjusted, ie, we did not impute missing continuous variables, including BMI. Fixed effects (FE) models were chosen as the primary method as explained in the Data Supplement.
The primary exposure of interest was gBRCA status (Y/N). In the secondary analysis, three levels of gBRCA type 1 versus gBRCA type 2 versus negative (as reference group) were considered. We analyzed data using SAS 9.4 (SAS Institute, Cary, NC). AMH differences in each study and pooled version were visualized in a forest plot.
RESULTS
General Description of the Study Population
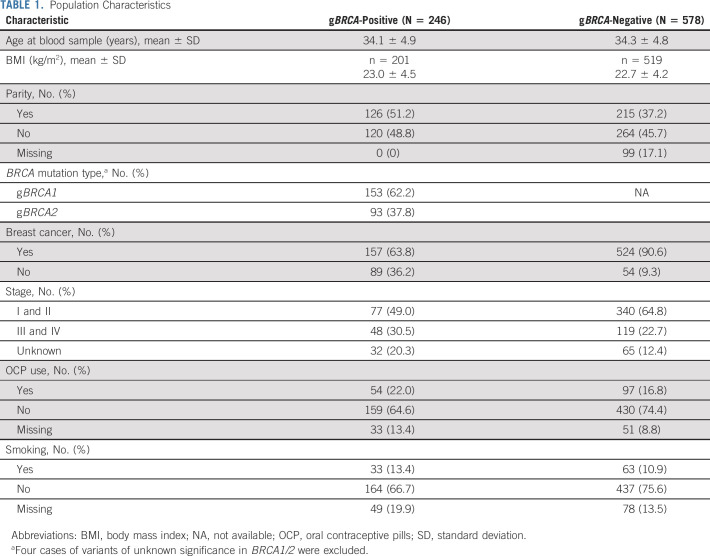
After excluding four women with variance of unknown significance in BRCA, a total of 824 of 828 women were eligible for the final analysis (Fig 1). Two hundred and forty-six women tested positive for gBRCA1/2, and 157 (78.5%) of those were affected with breast cancer. Eighty-nine women with gBRCA were unaffected. Of the 246 women with gBRCA, 153 (62.2%) were positive for gBRCA1, whereas 93 women (37.8%) for gBRCA2. Among the mutation negative controls (n = 578), 524 were affected with breast cancer.
FIG 1.
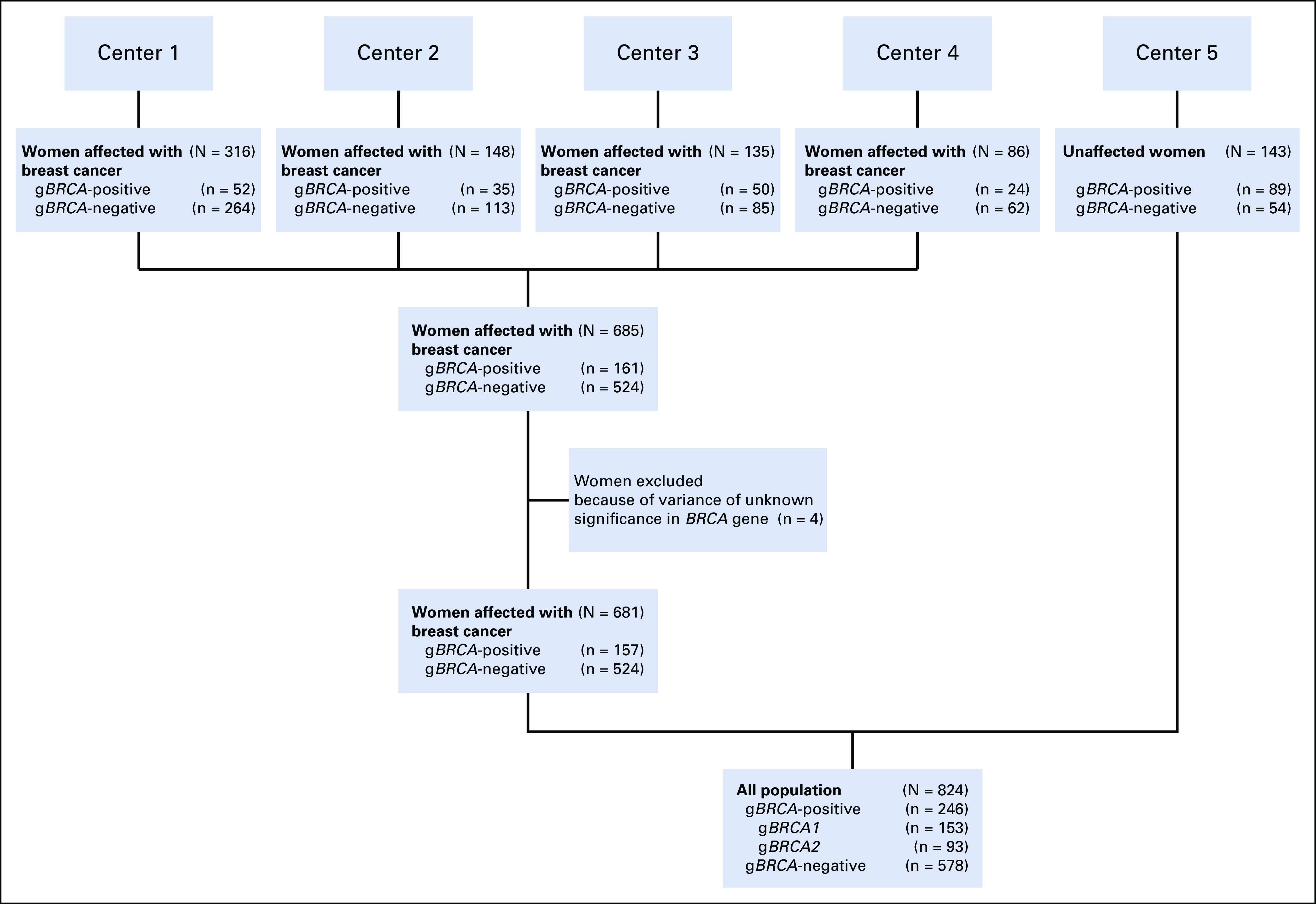
Study inclusion and exclusion flowchart.
Women with and without gBRCA had similar age at study inclusion compared with noncarrier controls (mean ± SD, 34.1 ± 4.9 v 34.3 ± 4.8 years, respectively; P = .48). The demographic characteristics of the entire or combined sample are summarized in Table 1.
TABLE 1.
Population Characteristics
Comparison of Serum AMH Levels in Women With and Without gBRCA1/2
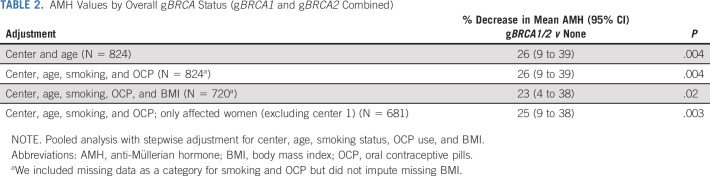
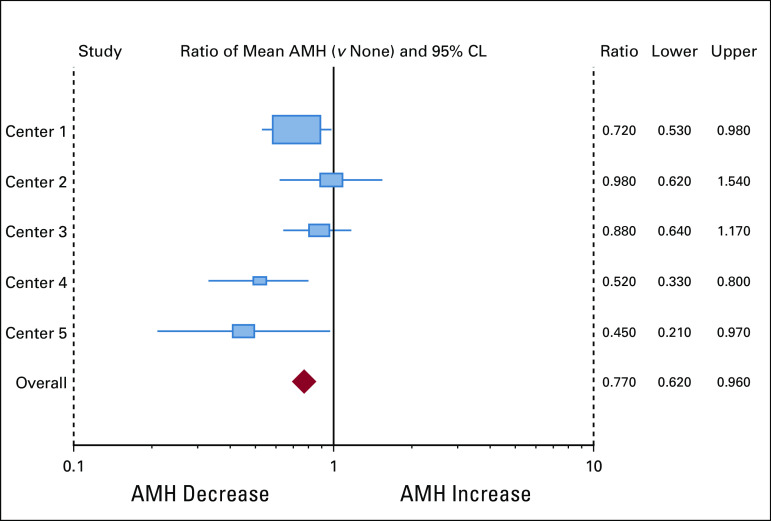
The mean AMH level in women with gBRCA1/2 was 2.04 ng/mL (SD = 2.0, median of 1.5, and geometric mean of 0.99), whereas it was 3.36 ng/mL (SD = 3.1, median of 2.5, and geometric mean of 1.96) in women without mutations. After adjusting for center, age, smoking status, and OCP use, women with gBRCA had significantly lower AMH levels compared with those without (26% lower [95% CI, 4 to 38]; P = .004). After the inclusion of BMI in the adjusted model, the sample size was reduced because of the missing data (from 824 to 720; Table 2), but qualitatively similar results were observed; for example, we found 23% decrease in AMH (95% CI, 4 to 38; P = .02) for gBRCA1/2 carriers versus noncarriers (Fig 2 and Table 2).
TABLE 2.
AMH Values by Overall gBRCA Status (gBRCA1 and gBRCA2 Combined)
FIG 2.
AMH values by BRCA. Forest plot analysis of individual results from five participating centers. One is null value of 0 difference or decrease. Individual centers were minimally adjusted, whereas overall data were adjusted for center, age, smoking, OCP, and BMI. AMH, anti-Müllerian hormone; BMI, body mass index; OCP, oral contraceptive pills.
Comparison of Serum AMH Levels in Women With gBRCA1 Versus gBRCA2 With Controls
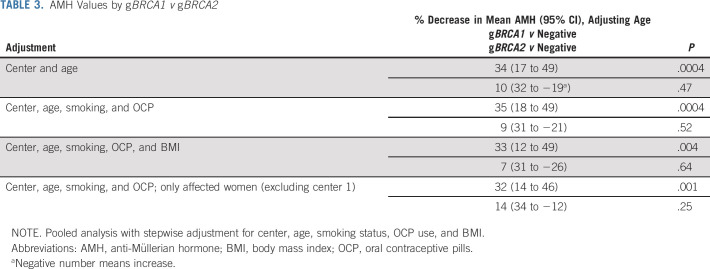
To further evaluate whether there was a difference in ovarian reserve according to type of gBRCA, we categorized women into those with gBRCA1, gBRCA2, and no mutations. The comparison among these three groups showed that the AMH levels were significantly lower in women with gBRCA1 compared with controls after adjusting for age, smoking, and OCP use with (33% lower; P = .004) or without (35% lower; P = .0004) adjustment for BMI (Table 3). A similar comparison of AMH levels between the women with gBRCA2 and controls did not reveal a difference; 7% lower (P = .64) and 9% lower (P = .52), respectively (Table 3).
TABLE 3.
AMH Values by gBRCA1 v gBRCA2
Comparison of Serum AMH Levels in Affected (Breast Cancer) Women With and Without gBRCA
It is possible that the BRCA dysfunction is more severe in affected women with mutations, and hence, the ovarian reserve may be more severely compromised in the same group. Therefore, we repeated our analysis by excluding the data from center 5 (n = 143), which studied unaffected women with and without gBRCA. Of the remaining 681 women with a new diagnosis of breast cancer, 91 had gBRCA1, 66 had gBRCA2, and 524 tested negative for gBRCA1/2 (Tables 2 and 3).
The mean age of 157 and 524 affected women with and without gBRCA1/2 was 33.3 ± 4.3 and 33.8 ± 4.5 years, respectively (P = .18). The mean AMH level was 2.54 ng/mL (SD = 2.3, median of 1.9, and geometric mean of 1.66) in affected women with gBRCA, whereas it was 3.59 ng/mL in women without gBRCA (SD = 3.2, median of 2.8, and geometric mean of 2.31). After adjusting for center, age, smoking status, BMI, and OCP use, affected women with gBRCA had significantly lower AMH levels compared with women without gBRCA (25% lower, 95% CI, 9 to 38; P = .003). Furthermore, after adjusting for center, age, smoking, and OCP use, AMH levels of women with gBRCA1 were lower compared with the controls (32% lower, 95% CI, 14% to 46% lower; P = .001). The serum AMH levels of affected women with gBRCA2 showed no significant difference in comparison with controls (14% lower, 95% CI, 34% lower to 12% higher; P = .25).
Secondary or Sensitivity Analysis With RE Versus FE
When we fitted a random effects (RE) model as a secondary or sensitivity analysis, our analysis also showed robust results. For example, when we estimated % decrease in mean AMH between gBRCA1/2 and none, adjusting center, and age only, our original analysis yielded 26 (95% CI, 9 to 39; P = .004), whereas a newly fitted RE model yielded 27 (15 to 37; P ≤ .0001). As another extreme case with only affected women, adjusting for center/age/smoking and OCP use, the estimated % decrease was 25 (9 to 38; P = .003) versus 25 (10 to 38; P = .002) for these two models, respectively. This sensitivity analysis shows that the FE model that we used for the primary analysis for our IPD data was slightly more conservative than the RE model.
DISCUSSION
We performed an IPD analysis from five centers to investigate the relationship between gBRCA and AMH levels. After adjusting for potential confounders, we found that women with gBRCA, specifically those affected and carrying gBRCA1, have lower serum AMH levels compared with women without gBRCA. To our knowledge, this is the first multicenter analysis and the largest study investigating the relationship between gBRCA and AMH levels in women with or at risk for breast cancer.
Oktay et al3 first reported low response to ovarian stimulation and subsequently lower serum AMH levels in women with breast cancer.5 This was followed by several studies supporting the finding of lower serum AMH in both affected and unaffected women with gBRCA,5,11,13,20 but others were unable to detect similar differences.14,18,19 We have recently reviewed the published evidence on the impact of gBRCA on ovarian aging and discussed the limitations and possible reasons for discrepancies among the studies.6 This individual patient level meta-analysis of published and updated data was performed to overcome the shortcomings of distinct studies. The current study confirmed the findings from most studies that particularly the presence of a gBRCA1 negatively affects the ovarian reserve of young women affected with or at risk for breast cancer.
Laboratory studies in human ovarian tissue have determined the potential mechanism of diminished oocyte reserve in women with gBRCA. BRCA1/2 are the members of the ataxia-telangiectasia mutated (ATM)–mediated DNA DSB repair pathway. Inadequate repair of DNA DSBs results in severe mutagenesis leading to carcinogenesis and tissue aging.26,27 The ATM-mediated DNA DSB repair pathway is charged with repair of this most lethal form of DNA damage, which is estimated to occur nearly a million times every day.26 The basic research from Dr Oktay's laboratory showed that gBRCA1 but not the gBRCA2 mutant mice have fewer primordial follicles that accumulate more DNA DSBs in their oocytes with age compared with the wild type mice. These mice also ovulate fewer oocytes and have lower litter size than the controls.5
The same team has also shown that the ovaries of women with gBRCA carry fewer primordial follicles, which are lost in an accelerated manner and accumulate more DNA DSBs with age compared with ovaries from controls.12 Oktay's laboratory also showed that gonadotoxic chemotherapy induces primordial follicle death and ovarian reserve loss by inducing DNA DSBs and apoptosis of oocytes and some oocytes may be able to repair themselves by activating the ATM pathway.28 In addition, recent longitudinal and laboratory data suggest that women with gBRCA may lose larger ovarian reserve after chemotherapy because of oocyte DNA repair deficiency.22,29,30 This may be a double whammy for affected women with gBRCA as their already lower ovarian reserve status is compounded by larger chemotherapy-induced loss, rendering them highly liable for infertility. However, further studies will be needed to confirm that women with gBRCA lose clinically significantly larger ovarian reserve after chemotherapy, compared with those without mutations.
In fact, BRCA1 and other ATM pathway genes and the age-induced decline in their function appear to be central in ovarian aging.5,6 BRCA1 has a more complex involvement in the ATM-mediated DNA DSB repair pathway than BRCA2. Although BRCA1 plays a role in damage sensing, homologous recombination repair, and checkpoint regulation (such as through CHEK2), the role of BRCA2 is limited to homologous recombination only. Moreover, the age-related decline in the BRCA1 function has been shown to occur earlier than the BRCA2 function in human oocytes.5 Although that decline appears to become prominent in the third decade of life in women with gBRCA1, the same may not happen until the fourth decade in the case of gBRCA2.5 Because women with gBRCA have one dysfunctional allele, age-induced decline in the function of the intact allele results in an acquired homozygocity with age.30 This then results in the accelerated accumulation of DNA DSBs in human oocytes, which triggers apoptotic death mechanisms of cell senescence, resulting in the accelerated reduction of ovarian reserve.5,6,22 Because the function of BRCA1 declines earlier in life than that of BRCA2, this may explain why ovarian reserve loss is more prominent in women with gBRCA1 compared with gBRCA2.
Considering the possibility that the affected women may have more severely accelerated ovarian aging, we analyzed our data with and without the inclusion of unaffected women, but this analysis did not alter our results. In our IPD analysis, only one center (Cedars Sinai Medical Center, Los Angeles, CA) studied unaffected women and found lower serum AMH level in those with gBRCA1. In this meta-analysis, we included all published studies in affected women, whereas the nonparticipating studies, except for one (Gunnala et al18 studied both affected and unaffected women), were performed among the unaffected (Data Supplement). In total, there have been six studies that assessed the relationship between gBRCA1/2 and AMH levels only in unaffected women. Although two studies were negative,14,19 four showed that there were lower AMH levels in women with gBRCA1/2,31 with only gBRCA113,32 or only gBRCA2.15 Therefore, although the preponderance of evidence also suggests lower serum AMH level in unaffected women compared with controls, further research is needed to determine the magnitude of serum AMH differences between affected and unaffected women and those with gBRCA1 versus gBRCA2. Therefore, our findings are on firmer ground with affected women with gBRCA.
There is other evidence supporting lower ovarian reserve in women with gBRCA. Several studies showed earlier menopausal age, particularly for those with gBRCA1.16,33 A large meta-analysis of genome-wide association analysis identified polymorphism in the BRCA1 as one of the key determinants of age at natural menopause.34
It is also possible that the differences we have reported here are underestimations as those most severely affected might have already had early risk reducing salpingo-oophorectomy and/or developed early breast or ovarian cancer or menopause and lose their reproductive function iatrogenically.35,36 These patients would then not be accounted in studies analyzing serum AMH.
Although there is no uniform normal range for AMH, in general, the mean value of 2.0 ng/mL in gBRCA in our IPD meta-analysis is well below the lower range of age-matched normal (2.9 ng/mL).37 Within that range, an average difference of 1.32 ng/mL is highly significant as it is 35% lower than the controls, which could translate into a shortening of reproductive life period by 10 years.38
Despite our repeated efforts, we could not obtain raw data from seven of 12 studies we identified, all involving unaffected woman. Because the data from the nonparticipating studies greatly varied in data format, availability, and/or quality, it was not possible to perform any reasonable meta-analysis or preliminary data processing or standardization as a sensitivity or secondary analysis. However, the five studies that were included represent > 80% of all published data on affected women. For this reason, our analysis is robust for the relationship between gBRCA and ovarian reserve in women who developed breast cancer. However, the nonparticipation of seven studies that nearly exclusively studied unaffected women does not allow us to reach a firm conclusion on the association of diminished ovarian reserve with gBRCA in unaffected carriers.
In conclusion, by IPD analysis from five centers, we showed that women with gBRCA have lower AMH levels compared with those without, and this appeared to be restricted to those with gBRCA1. Therefore, based on this IPD analysis and the supporting basic science and translational data,5,6,39 we recommend that especially the affected women with gBRCA1 should proactively receive reproductive and fertility preservation counseling if they are postponing childbearing to the third decade and beyond. This conclusion is firmer for affected women as our IPD meta-analysis predominantly studied those with breast cancer, but further original and meta-analytic studies are needed to determine if there is a difference between the ovarian reserve of affected and unaffected women with gBRCA and to understand the magnitude of ovarian reserve differences between women with gBRCA1 and gBRCA2.
Matteo Lambertini
Consulting or Advisory Role: Roche, Novartis, Lilly, AstraZeneca
Speakers' Bureau: Theramex, Takeda, Roche, Lilly, Novartis, Pfizer, Sandoz
Erica Wang
Consulting or Advisory Role: OncoPep
Research Funding: Merck
Florian Clatot
Honoraria: Merck Serono, Bristol-Myers Squibb, AstraZeneca
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, Roche, Merck Serono
Research Funding: AstraZeneca, Roche Diagnostics
Travel, Accommodations, Expenses: Merck Serono, Roche, Bristol-Myers Squibb
Beth Y. Karlan
Consulting or Advisory Role: Roche Pharma AG, Merck, Mercy BioAnalytics
Research Funding: VBL Therapeutics, AstraZeneca
Patents, Royalties, Other Intellectual Property: US and EU patent on gene signature
Other Relationship: Elsevier
Isabelle Demeestere
Consulting or Advisory Role: Roche
Research Funding: Roche Diagnostics
Travel, Accommodations, Expenses: Ferring
No other potential conflicts of interest were reported.
SUPPORT
Supported by RO1 HD053112 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Cancer Institute. H.B. was partly supported by the National Institutes of Health through grant UL1 TR001860.
AUTHOR CONTRIBUTIONS
Conception and design: Volkan Turan, Kutluk Oktay
Administrative support: Kutluk Oktay
Provision of study materials or patients: Matteo Lambertini, Erica Wang, Florian Clatot, Beth Y. Karlan, Isabelle Demeestere, Kutluk Oktay
Collection and assembly of data: Volkan Turan, Matteo Lambertini, Dong-Yun Lee, Erica Wang, Florian Clatot, Beth Y. Karlan, Isabelle Demeestere, Kutluk Oktay
Data analysis and interpretation: Volkan Turan, Heejung Bang, Kutluk Oktay
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Association of Germline BRCA Pathogenic Variants With Diminished Ovarian Reserve: A Meta-Analysis of Individual Patient-Level Data
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Matteo Lambertini
Consulting or Advisory Role: Roche, Novartis, Lilly, AstraZeneca
Speakers' Bureau: Theramex, Takeda, Roche, Lilly, Novartis, Pfizer, Sandoz
Erica Wang
Consulting or Advisory Role: OncoPep
Research Funding: Merck
Florian Clatot
Honoraria: Merck Serono, Bristol-Myers Squibb, AstraZeneca
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, Roche, Merck Serono
Research Funding: AstraZeneca, Roche Diagnostics
Travel, Accommodations, Expenses: Merck Serono, Roche, Bristol-Myers Squibb
Beth Y. Karlan
Consulting or Advisory Role: Roche Pharma AG, Merck, Mercy BioAnalytics
Research Funding: VBL Therapeutics, AstraZeneca
Patents, Royalties, Other Intellectual Property: US and EU patent on gene signature
Other Relationship: Elsevier
Isabelle Demeestere
Consulting or Advisory Role: Roche
Research Funding: Roche Diagnostics
Travel, Accommodations, Expenses: Ferring
No other potential conflicts of interest were reported.
REFERENCES
- 1.Venkitaraman AR.Beyond cancer genomics: After the end of the beginning Curr Opin Genet Dev 221–22012 [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck TR, Mitra N, Wan F, et al. The CIMBA Consortium Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer JAMA 3131347–13612015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oktay K, Kim JY, Barad D, et al. Association of BRCA1 mutations with occult primary ovarian insufficiency: A possible explanation for the link between infertility and breast/ovarian cancer risks J Clin Oncol 28240–2442010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turan V, Bedoschi G, Emirdar V, et al. Ovarian stimulation in patients with cancer: Impact of letrozole and BRCA mutations on fertility preservation cycle outcomes Reprod Sci 2526–322018 [DOI] [PubMed] [Google Scholar]
- 5.Titus S, Li F, Stobezki R, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5:172ra21. doi: 10.1126/scitranslmed.3004925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turan V, Oktay K.BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging Hum Reprod Update 2643–572020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambertini M, Goldrat O, Toss A, et al. Fertility and pregnancy issues in BRCA-mutated breast cancer patients Cancer Treat Rev 5961–702017 [DOI] [PubMed] [Google Scholar]
- 8.Tal R, Seifer DB.Ovarian reserve testing: A user's guide Am J Obstet Gynecol 217129–1402017 [DOI] [PubMed] [Google Scholar]
- 9.Ulrich ND, Marsh EE.Ovarian reserve testing: A review of the options, their applications, and their limitations Clin Obstet Gynecol 62228–2372019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambertini M, Goldrat O, Ferreira AR, et al. Reproductive potential and performance of fertility preservation strategies in BRCA-mutated breast cancer patients Ann Oncol 29237–2432018 [DOI] [PubMed] [Google Scholar]
- 11.Wang ET, Pisarska MD, Bresee C, et al. BRCA1 germline mutations may be associated with reduced ovarian reserve Fertil Steril 1021723–17282014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin W, Titus S, Moy F, et al. Ovarian aging in women with BRCA germline mutations J Clin Endocrinol Metab 1023839–38472017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giordano S, Garrett-Mayer E, Mittal N, et al. Association of BRCA1 mutations with impaired ovarian reserve: Connection between infertility and breast/ovarian cancer risk J Adolesc Young Adult Oncol 5337–3432016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Tilborg TC, Broekmans FJ, Pijpe A, et al. Do BRCA1/2 mutation carriers have an earlier onset of natural menopause? Menopause 23903–9102016 [DOI] [PubMed] [Google Scholar]
- 15.Johnson L, Sammel MD, Domchek S, et al. Anti-Müllerian hormone levels are lower in BRCA2 mutation carriers Fertil Steril 1071256–1265.e62017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rzepka-Gorska I, Tarnowski B, Chudecka-Głaz A, et al. Premature menopause in patients with BRCA1 gene mutation Breast Cancer Res Treat 10059–632006 [DOI] [PubMed] [Google Scholar]
- 17.Oktay K, Turan V, Titus S, et al. BRCA mutations, DNA repair deficiency and ovarian aging. Biol Reprod. 2015;93:67. doi: 10.1095/biolreprod.115.132290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunnala V, Fields J, Irani M, et al. BRCA carriers have similar reproductive potential at baseline to noncarriers: Comparisons in cancer and cancer-free cohorts undergoing fertility preservation Fertil Steril 111363–3712019 [DOI] [PubMed] [Google Scholar]
- 19.Michaelson-Cohen R, Mor P, Srebnik N, et al. BRCA mutation carriers do not have compromised ovarian reserve Int J Gynecol Cancer 24233–2372014 [DOI] [PubMed] [Google Scholar]
- 20.Son KA, Lee DY, Choi D. Association of BRCA mutations and anti-Müllerian hormone level in young breast cancer patients. Front Endocrinol (Lausanne) 2019;10:235. doi: 10.3389/fendo.2019.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambertini M, Olympios N, Lequesne J, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-Müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. 2019;9:575. doi: 10.3389/fonc.2019.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oktay K, Bedoschi G, Goldfarb S, et al. Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency Fertil Steril 1131251–1260.e12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome—when, why, and how? BMC Med Res Methodol. 2014;14:20. doi: 10.1186/1471-2288-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. ed 2. Volume 7. John Wiley & Sons; 2011. section 9.7. [Google Scholar]
- 25.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: Rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 26.Roos WP, Kaina B.DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis Cancer Lett 332237–2482013 [DOI] [PubMed] [Google Scholar]
- 27.Cohen IS, Bar C, Paz-Elizur T, et al. DNA lesion identity drives choice of damage tolerance pathway in murine cell chromosomes Nucleic Acids Res 431637–16452015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soleimani R, Heytens E, Darzynkiewicz Z, et al. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise Aging (Albany NY) 3782–7932011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oktay K, Moy F, Titus S, et al. Age-related decline in DNA repair function explains diminished ovarian reserve, earlier menopause, and possible oocyte vulnerability to chemotherapy in women with BRCA mutations J Clin Oncol 321093–10942014 [DOI] [PubMed] [Google Scholar]
- 30.Goldfarb SB, Turan V, Bedoschi G, et al. Impact of adjuvant chemotherapy or tamoxifen-alone on the ovarian reserve of young women with breast cancer Breast Cancer Res Treat 185165–1732021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Aharon I, Levi M, Margel D, et al. Premature ovarian aging in BRCA carriers: A prototype of systemic precocious aging? Oncotarget 915931–159412018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips KA, Collins IM, Milne RL, et al. Anti-Müllerian hormone serum concentrations of women with germline BRCA1 or BRCA2 mutations Hum Reprod 311126–11322016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finch A, Valentini A, Greenblatt E, et al. Frequency of premature menopause in women who carry a BRCA1 or BRCA2 mutation Fertil Steril 991724–17282013 [DOI] [PubMed] [Google Scholar]
- 34.Day FR, Ruth KS, Thompson DJ, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair Nat Genet 471294–13032015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin WT, Beattie M, Chen LM, et al. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California Cancer 1191652–16592013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santoro N. BRCA mutations and fertility: Do not push the envelope! Fertil Steril. 2013;99:1560. doi: 10.1016/j.fertnstert.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 37.Almog B, Shehata F, Suissa S, et al. Age-related normograms of serum antimullerian hormone levels in a population of infertile women: A multicenter study Fertil Steril 952359–23632011 [DOI] [PubMed] [Google Scholar]
- 38.Tehrani FR, Solaymani-Dodaran M, Tohidi M, et al. Modeling age at menopause using serum concentration of anti-mullerian hormone J Clin Endocrinol Metab 98729–7352013 [DOI] [PubMed] [Google Scholar]
- 39.Govindaraj V, Krishnagiri H, Chakraborty P, et al. Age-related changes in gene expression patterns of immature and aged rat primordial follicles Syst Biol Reprod Med 6337–482017 [DOI] [PubMed] [Google Scholar]