Abstract
PURPOSE
Predictive biomarkers to identify patients with human epidermal growth factor receptor 2 (HER2)–positive breast cancer who may benefit from targeted therapy alone are required. We hypothesized that early measurements of tumor maximum standardized uptake value corrected for lean body mass (SULmax) on 18F-labeled fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) would predict pathologic complete response (pCR) to pertuzumab and trastuzumab (PT).
PATIENTS AND METHODS
Patients with stage II or III, estrogen receptor–negative, HER2-positive breast cancer received four cycles of neoadjuvant PT. 18F-labeled fluorodeoxyglucose positron emission tomography-computed tomography was performed at baseline and 15 days after PT initiation (C1D15). Eighty evaluable patients were required to test the null hypothesis that the area under the curve of percent change in SULmax by C1D15 predicting pCR is ≤ 0.65, with a one-sided type I error rate of 10%.
RESULTS
Eighty-eight women were enrolled (83 evaluable), and 85% (75 of 88) completed all four cycles of PT. pCR after PT alone was 22%. Receiver operator characteristic analysis of percent change in SULmax by C1D15 yielded an area under the curve of 0.72 (80% CI, 0.64 to 0.80; one-sided P = .12), which did not reject the null hypothesis. However, between patients who obtained pCR and who did not, a significant difference in median percent reduction in SULmax by C1D15 was observed (63.8% v 41.8%; P = .004) and SULmax reduction ≥ 40% was more prevalent (83% v 52%; P = .03; positive predictive value, 31%). Participants not obtaining a 40% reduction in SULmax by C1D15 were unlikely to obtain pCR (negative predictive value, 91%).
CONCLUSION
Although the primary objective was not met, early changes in SULmax predict response to PT in estrogen receptor–negative and HER2-positive breast cancer. Once optimized, this quantitative imaging strategy may facilitate tailoring of therapy in this setting.
INTRODUCTION
Dual human epidermal growth factor receptor 2 (HER2)–directed therapy with pertuzumab and trastuzumab (PT) in combination with chemotherapy is more effective than trastuzumab and chemotherapy for patients with HER2-positive breast cancer.1 The NeoSPHERE and TRYPHAENA trials reported high pathologic complete response (pCR) rates with the dual approach, which led to the approval of neoadjuvant PT–based regimens in high-risk HER2-positive breast cancer.2,3 Indeed, pCR is an accepted primary end point in neoadjuvant clinical trials and can result in drug approval contingent on confirmatory results in the adjuvant setting.4 Data from several studies have suggested that a proportion of patients derive benefit from HER2-directed therapy alone and may not require chemotherapy. The NeoSPHERE and Adjuvant Dynamic Marker-Adjusted Personalized Therapy (ADAPT) trial investigators reported pCR rates of approximately 30% for patients with estrogen receptor (ER)–negative and HER2-positive breast cancer who received neoadjuvant PT without chemotherapy.2,5 Thus, the development of predictive biomarkers that can identify a subgroup of patients with HER2-positive breast cancer who may be treated with HER2-directed therapy alone and potentially spared chemotherapy is of clinical and scientific interest.
CONTEXT
Key Objective
The primary objective of TBCRC026 was to correlate baseline and early percentage change (by C1D15) in maximum standardized uptake value corrected for lean body mass (SULmax) (breast) on fluorodeoxyglucose (FDG)-positron emission tomography-computed tomography with pathologic complete response to neoadjuvant pertuzumab and trastuzumab (without chemotherapy) in estrogen receptor–negative and human epidermal growth factor receptor 2–positive breast cancer.
Knowledge Generated
Although the primary objective did not meet the predefined statistical boundaries, the data indicate that early changes in SULmax on FDG-PET predict response to four cycles of pertuzumab and trastuzumab in this setting, ie, an absence of 40% decline in SULmax by C1D15 is associated with a lower likelihood of achieving pathologic complete response (negative predictive value, 91%).
Relevance
The TBCRC026 results suggest that optimized FDG-positron emission tomography-computed tomography quantitative imaging–based biomarkers may facilitate intensification and de-intensification of neoadjuvant therapy pending further evaluation.
Changes in 18F-labeled fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET)-computed tomography (CT) as early as a few weeks after commencement of neoadjuvant therapy have promise as a predictive biomarker in early breast cancer.6,7 In the prospective Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimization (NeoALLTO) trial, changes in standardized uptake value (SUV) on FDG-PET-CT were assessed as a predictor of response to neoadjuvant HER2-directed therapy.8 Patients were randomly assigned to a 6-week biologic window of HER2-directed therapy alone (lapatinib alone, trastuzumab alone, or lapatinib with trastuzumab) followed by the addition of chemotherapy before surgery.9 Metabolic changes in breast tumors were detected as early as 2 weeks after commencing HER2-directed therapy, and pCR rates were found to be twice as high in patients who were designated as responders compared with nonresponders by FDG-PET-CT findings.10 Because chemotherapy was added after HER2-directed therapy in this trial, concluding that early change in SUV would predict pCR to HER2-directed therapy alone is not possible.
We hypothesized that early changes in maximum SUV corrected for lean body mass (SULmax) on FDG-PET-CT would correlate with pCR in patients with ER-negative, HER2-positive breast cancer who receive neoadjuvant PT, without chemotherapy. To test this hypothesis, we performed a multicenter phase II study in which women with stage II or III, ER-negative and HER2-positive breast cancer received 12 weeks of neoadjuvant PT and incorporated serial FDG-PET-CT imaging and blood and tumor biopsy collection.
Since the original publication of this manuscript,11 we identified errors in the pathologic response data entry, predominantly because of misclassification of ypT1a/microinvasive disease as pCR. Reanalysis resulted in the inability to reject the null hypothesis of the original study design (the actions taken to address these errors can be found in the Appendix, online only). The original publication has been retracted,12 and this updated manuscript provides the corrected results.
PATIENTS AND METHODS
Eligibility
Eligible women were 18 years of age or older with untreated histologically proven infiltrating carcinoma of the breast and clinical stage T2-4(a-c), any N, and M0 disease (American Joint Committee on Cancer 7th edition staging). Tumors must have ER ≤ 10% and be HER2-positive13 by local pathology review. An Eastern Cooperative Oncology Group performance status 0-1, a left ventricular ejection fraction ≥ 50%, and adequate organ function were required. Patients agreed to baseline and follow-up FDG-PET-CT and study-specific procedures with no minimum SULmax of primary breast cancer required for eligibility. Patients also signed a written informed consent approved by the institutional review boards of the participating institutions.
Study Design
Participants received 12 weeks of neoadjuvant PT (Appendix Fig A1, online only). Pertuzumab (supplied by Genentech, South San Francisco, CA) was administered every 3 weeks (840 mg loading dose and then 420 mg), and trastuzumab every 3 weeks (8 mg/kg loading dose and then 6 mg/kg), both intravenously. Dose modification was not permitted.
FDG-PET-CT was performed before research tumor biopsies, at baseline, and at day 15 after commencement of therapy (C1D15). Tumor tissue was obtained from the surgical specimen on the day of surgery for research purposes. Plasma samples were obtained at baseline and C1D15, post-treatment or presurgery, and postsurgery (Appendix Fig A1). Common Terminology Criteria for Adverse Events (version 4.0) were used to grade toxicity.
Neoadjuvant HER2-directed therapy and chemotherapy, given after study treatment with PT and before definitive surgery, was allowed per physician discretion if incomplete response or disease progression is observed (Appendix Fig A1). Taxane-based chemotherapy with PT was recommended. Tumor biopsy to confirm the presence of residual disease histologically (ie, no pCR) was required before additional chemotherapy to ensure data available for primary end point. Axillary evaluation and type of breast surgery performed were per surgeon discretion. Postoperative systemic therapy and radiation per standard of care were recommended.
18F-FDG PET-CT
Participants underwent FDG-PET-CT at baseline and C1D15 (3-day window). Approval of FDG-PET-CT facilities included provision of study manual to sites, review of representative clinical scans, and phantom images. After a 60-minute uptake phase following intravenous FDG injection, a combined FDG-PET-CT scan was obtained from midskull to midfemur in general conformance with the Uniform Protocols for Imaging in Clinical Trials FDG-PET-CT and Radiological Society of North America Quantitative Imaging Biomarkers Alliance profiles.14 Scans were transmitted digitally, and central review and quantitation were performed by readers blinded to clinical data.6 Measurements were acquired by placing an all-encompassing spherical volume of interest over the target primary breast cancer tissue and recording SULmax, with care taken not to include adjacent normal FDG-avid tissue. SULmax was collected because it is more consistent than SUV in normal tissues from patient to patient, being less weight dependent.15 SULmax of primary breast cancer is reported in this analysis (primary end point).
Statistical Considerations
The primary objective was to correlate baseline and early percentage change (by C1D15) in SULmax on FDG-PET-CT of the primary breast cancer with pCR after four cycles of neoadjuvant PT. pCR was defined as no viable invasive cancer in breast and axilla by local pathology review. All other patients were classified as non-pCR, including those with histologically confirmed residual disease after 12 weeks of PT or clinical progression on PT. An early stopping rule was in place to suspend or terminate the study if the proportion of clinical progression exceeded 10%.
To be evaluable for the primary analysis, both baseline and C1D15 FDG-PET-CT scan was performed, SULmax data were collected, and pCR status after PT was evaluated. The study was powered to test the null hypothesis that the area under the receiver operating characteristic (ROC) curve of percent of change in SULmax at C1D15, a measure used to quantify the overall predictive power of the biomarker, was ≤ 0.65. Eighty evaluable patients with a projected pCR rate of 25% would have an 81% or greater power to detect a true area under the curve (AUC) of 0.80 against the null hypothesis (an AUC ≤ 0.65 at a one-sided type I error rate of 0.10). The AUC measures how well the biomarker discriminates between responders (those obtaining pCR) and nonresponders (no pCR), with a greater AUC indicating higher diagnostic accuracy. The 80% CIs for AUC were reported to align with the one-sided primary hypothesis test at 10% type 1 error and obtained by means of U-Statistics theory.16 A sample size of up to 88 patients was planned to account for nonevaluable cases. Safety analysis included patients who received at least one dose of any study drug and was based on the frequency of adverse events (AEs).
Continuous variables were listed by means, standard deviations, and medians and ranges and were compared between patients who obtained pCR and those who did not using Wilcoxon rank-sum tests. Binary outcomes were listed by proportions and binomial exact CIs and compared using Fisher's exact tests. ROCs with SULmax parameters as the predictor were generated with the goal of identifying the SULmax cut point that maximized the sum of sensitivity and specificity. We correlated baseline, C1D15, and percent reduction in SULmax with pCR using logistic regressions. We evaluated positive predictive value and negative predictive value (NPV) for predicting pCR with selected cutoffs.
All other statistical tests, unless otherwise stated, were two-sided and considered statistically significant at P < .05. The analyses were carried out using R version 3.4.2 software packages.17 The research Protocol (online only) and article were written by the authors and reviewed by the pharmaceutical funders; the funders had no access to the study database and were not involved in the study analysis or interpretation of results.
RESULTS
Patient Characteristics
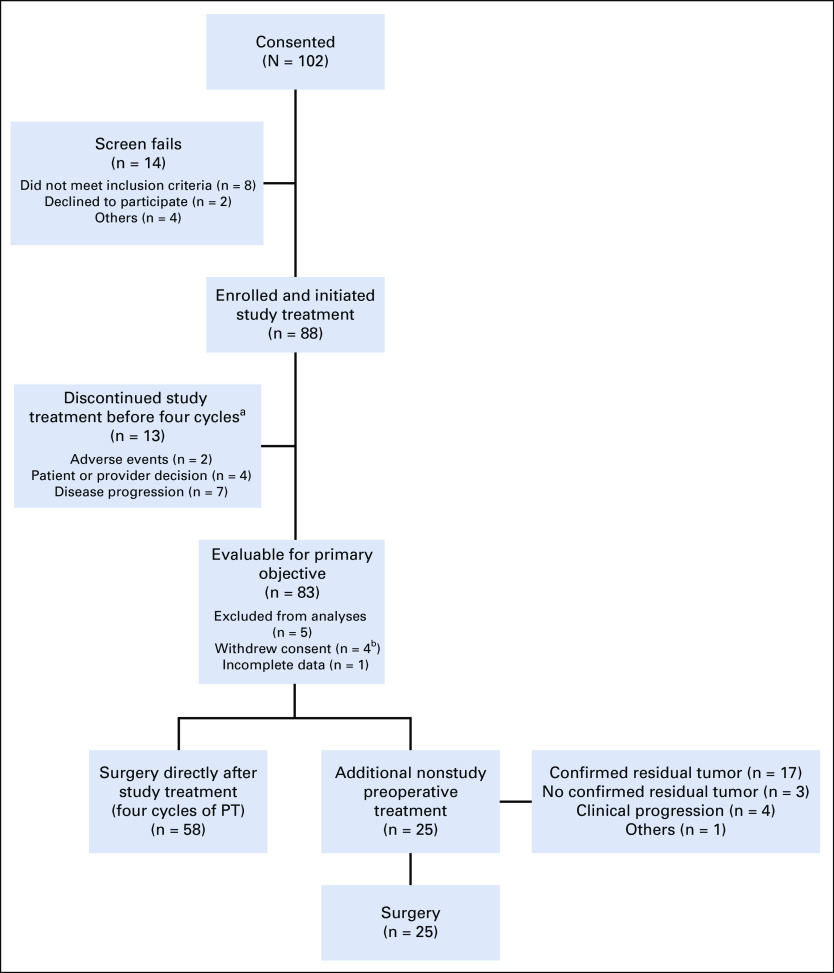
From January 2014 to August 2017, 88 women enrolled in the study across nine sites. The median age was 58 years (range, 29-82 years), and the median tumor size was 3.7 cm (range, 2-15 cm) by standard imaging (Table 1). Of these women, 83 were evaluable for the primary analysis (Fig 1). All four cycles of PT were completed in 85% of cases (75 of 88), and all 83 evaluable patients underwent the primary surgery. Twenty-five patients (28%) received neoadjuvant nonstudy therapy (Fig 1) and were classified as not obtaining pCR in the intention-to-treat (ITT) analysis. Of note, 22 of these 25 patients met the definition of histologically confirmed residual disease after 12 weeks of PT and/or clinical progression on study therapy. Additional nonstudy neoadjuvant therapy included taxane and carboplatin-based (n = 17), and taxane-based (n = 7) therapy with PT, and one patient received a fifth cycle of PT. Two patients also received anthracycline-based neoadjuvant chemotherapy.
TABLE 1.
Patient Characteristics
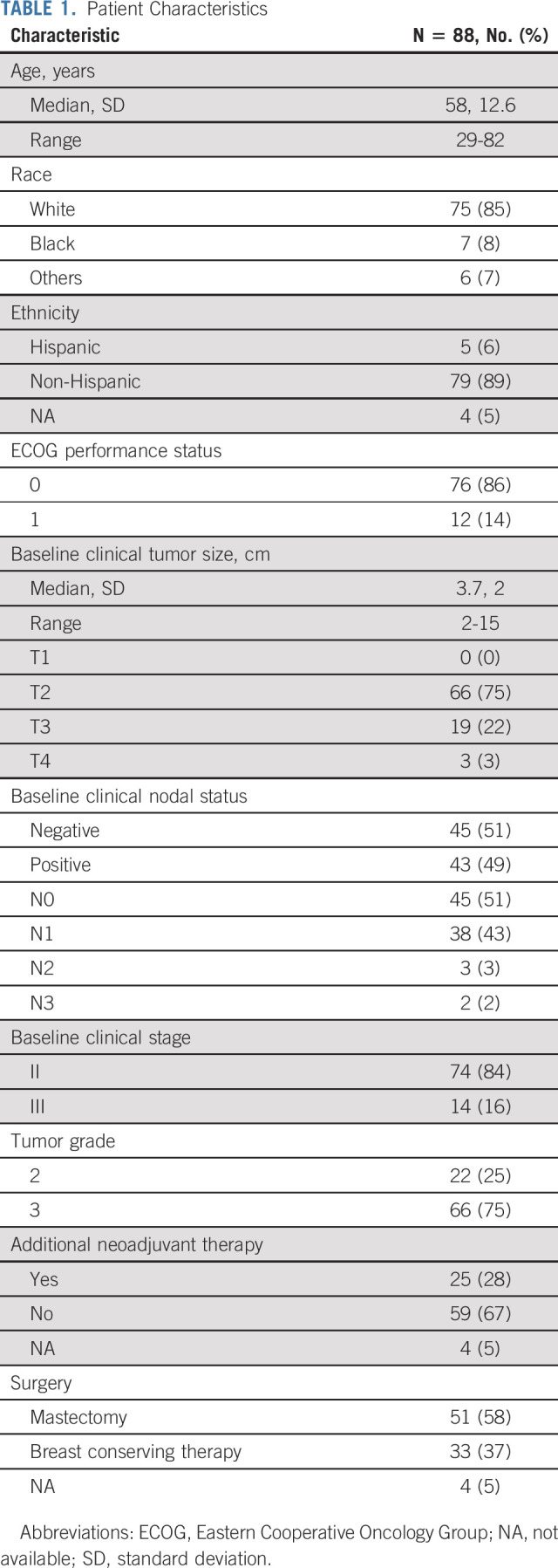
FIG 1.
Study flow diagram. Study treatment = PT. aAll patients remained evaluable. bReasons for withdrawal of consent include one participant refused further participation after C1D1, one participant refused further participation after C2D1, one patient refused further participation after study treatment was completed, and one patient withdrew consent because of unacceptable toxicity. PT, pertuzumab and trastuzumab.
Treatment Safety and Efficacy
AEs were as expected for this regimen (Appendix Table A1, online only). Grade 3 AEs included diarrhea (8%), anaphylaxis (1%), and left ventricular systolic dysfunction (1%).
pCR was observed in 22% of patients after four cycles of PT alone (18 of 83; 95% CI, 13 to 32). The assumption that those who withdrew consent did not obtain pCR led to a conservative estimate of 20% for pCR (18 of 88; 95% CI, 13 to 30). Of the patients who had histologically confirmed residual disease after 12 weeks of PT or clinical progression and received additional neoadjuvant nonstudy therapy, 32% (seven of 22) had pCR at the time of surgery. Seven (8%) experienced clinical progression on study therapy after two to four cycles (median, three cycles) of PT, and the study did not meet the early stopping rule because the proportion of clinical progression did not exceed 10%.
Baseline and Change in Biomarkers and Correlation With Response to Therapy
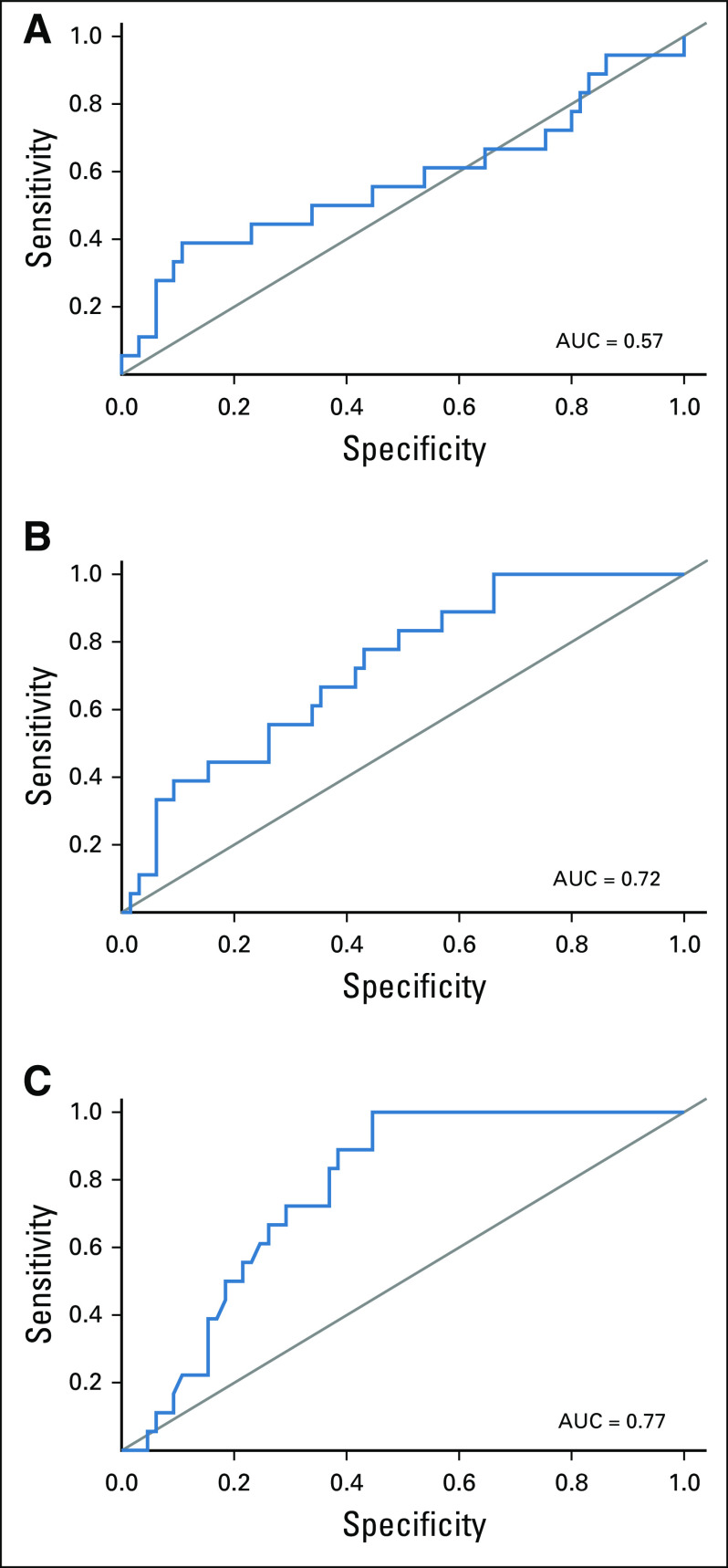
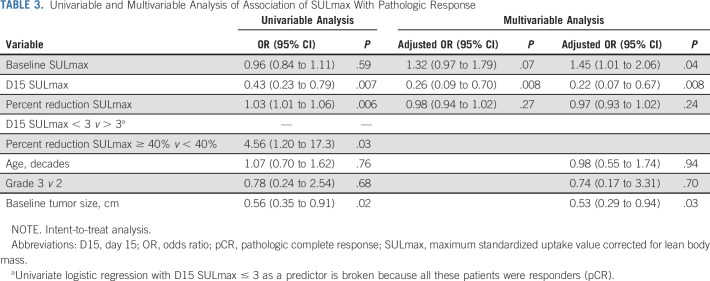
All 88 enrolled patients underwent baseline and C1D15 FDG-PET-CT. The same scanner at each site was used for baseline and C1D15 scan in 90% of cases, with representative images provided (Appendix Fig A2, online only). The mean injected FDG dose was 10.92 ± 2.3 mCi FDG. The mean radiotracer uptake time was 64 ± 11.5 minutes. Four patients subsequently withdrew consent, and one patient had incomplete data, preserving 83 evaluable cases for the ITT analysis. Summary statistics for baseline, C1D15, and percent change in SULmax are provided in Appendix Table A2 (online only). Baseline SULmax was not significantly associated with pCR (Table 2). ROC analysis of baseline SULmax yielded an AUC value of 0.57 (80% CI, 0.46 to 0.69) for discriminating patients with and without pCR (Fig 2). Univariable logistic regression suggested that baseline SULmax is negatively associated with the odds of obtaining pCR, although the association did not reach statistical significance (Table 3).
TABLE 2.
Baseline, D15, and Percent Change in SULmax Between pCR and No pCR
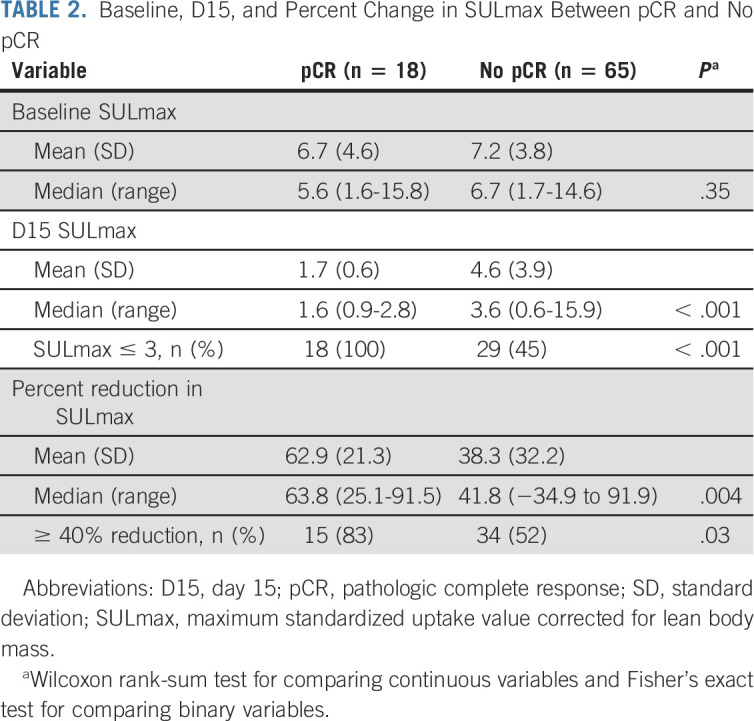
FIG 2.
Receiver operating characteristic curves for (A) baseline and (B) percent of change in SULmax and (C) 15 days after PT initiation (C1D15). AUC, area under the curve; PT, pertuzumab and trastuzumab; SULmax, maximum standardized uptake value corrected for lean body mass.
TABLE 3.
Univariable and Multivariable Analysis of Association of SULmax With Pathologic Response
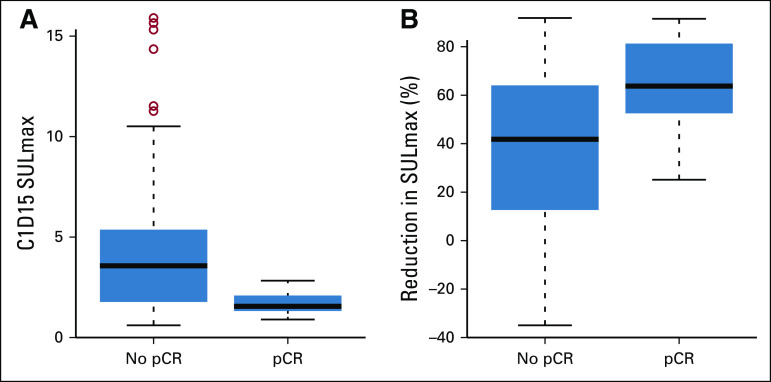
We observed a significant difference in median percent reduction in SULmax by C1D15 between patients who obtained pCR and those who did not (63.8% v 41.8%; P = .004; Table 2; Fig 3), and the univariable logistic regression estimated an odds ratio (OR) of 1.03 (95% CI, 1.01 to 1.06) associated with each additional 1% reduction in SULmax (Table 3). Similar results were observed in multivariable logistic regression that also adjusted for age, grade, and tumor size (Appendix Table A3, online only). The ROC analysis, with percent reduction in SULmax as the predictor, yielded an AUC of 0.72 (80% CI, 0.64 to 0.80; P = .12) in the evaluable patients, thus not rejecting the null hypothesis for the primary end point (Fig 2). The exploratory cutoff of ≥ 40% versus < 40% reduction in SULmax yielded a sensitivity of 83% and a specificity of 48% for identifying patients who obtained pCR. Compared with other cut points examined, including the cutoff value of 52.5% reduction determined on the basis of Youden's index,18 the threshold of 40% is considered clinically optimal for its high NPV (91%) for pCR (ie, declines in SUV < 40% predict lack of pCR; Appendix Table A4, online only).
FIG 3.
Box plots of (A) 15 days after PT initiation (C1D15), and (B) percent reduction in SULmax in patients with pCR versus no pCR. The horizontal line inside the box shows the median. The lower and upper hinges of the box represent the 25th and 75th percentiles, respectively. The circles represent actual values of D15 and percent reduction in SULmax. pCR, pathologic complete response; PT, pertuzumab and trastuzumab; SULmax, maximum standardized uptake value corrected for lean body mass.
Based on emerging knowledge regarding the role of FDG-PET-CT in assessing response to anticancer therapy and recognition that a minimum uptake of FDG at baseline is required to allow identification of a meaningful decline in tracer uptake from baseline, as is now incorporated into the PERCIST 1.0 criteria, an exploratory subset analysis was performed by excluding patients with low FDG avidity tumors (n = 7), defined as baseline tumor SULmax < 2. The subgroup ROC analysis (n = 76), with percent reduction in SULmax as the predictor, yielded an AUC of 0.78 (80% CI, 0.71 to 0.86; P = .01), rejecting the null hypothesis of AUC ≤ 0.65. The exploratory cutoff in this case of ≥ 50% versus < 50% reduction in SULmax yielded a sensitivity of 93% and a specificity of 53% for identifying patients obtaining pCR. Compared with other cut points examined, including the cutoff value of 52.5% reduction determined based on Youden's index,18 the threshold of 50% is considered clinically optimal for its high NPV (97%) for pCR (ie, declines in SUV < 50% predict lack of pCR; Appendix Table A5, online only).
Among the 83 evaluable patients, a significant difference was observed in median C1D15 SULmax between patients who obtained pCR and those who did not (1.6 v 3.6; P < .001; Table 2; Fig 3), and the univariable logistic regression estimated an OR of 0.43 (95% CI, 0.23 to 0.79) for a one-unit increase in C1D15 SULmax (Table 3). The association remained statistically significant after adjusting for age, grade, and tumor size (Appendix Table A3). The exploratory ROC analysis, with C1D15 SULmax as the predictor, yielded an AUC of 0.77 (80% CI, 0.70 to 0.83; P = .009; Fig 2). The cutoff of ≤ 3 versus > 3 in C1D15 SULmax yielded a high NPV for pCR over other cut points and maximized Youden's index. A significantly higher proportion of C1D15 SULmax ≤ 3 was observed in patients who obtained pCR versus those who did not (100% v 45%; P < .001). NPV and positive predictive value for pCR associated with this retrospectively determined threshold were 100% and 38%, respectively.
Multivariable logistic regression that adjusted for baseline SULmax and percent reduction in SULmax revealed that C1D15 SULmax remained significantly predictive of pCR (adjusted OR, 0.26; 95% CI, 0.09 to 0.70; Table 3). The inclusion of age, grade, and tumor size in the regression model did not change the predictive value of the PET parameters (Appendix Table A3). Tumor stage was not included in the analysis because no patients with stage III disease obtained pCR.
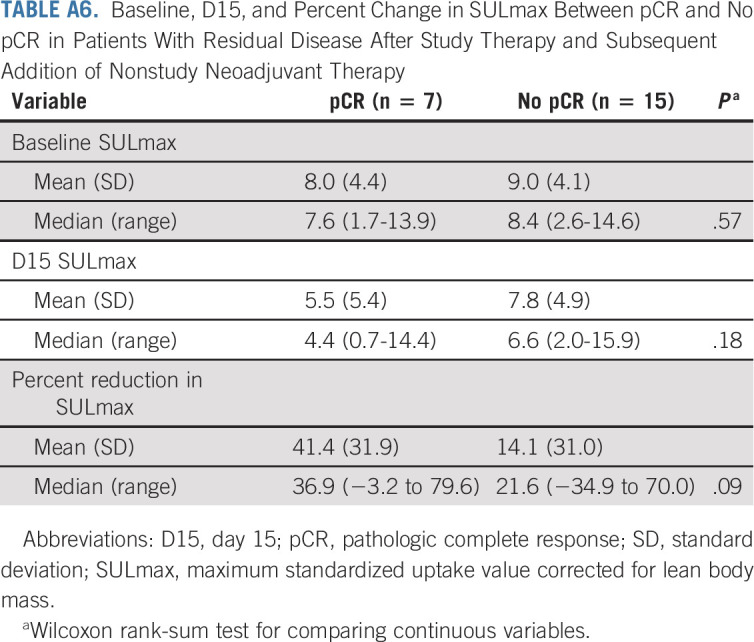
Among the 22 patients who received a subsequent addition of nonstudy neoadjuvant therapy (Fig 1), a predefined exploratory analysis was performed to correlate SULmax with pCR. Baseline, median percent reduction, and C1D15 SULmax were not significantly associated with pCR in this small patient population (Appendix Table A6, online only).
DISCUSSION
In the TBCRC026 trial, we investigated whether early changes in SULmax on FDG-PET-CT can predict pCR after HER2-directed therapy with PT alone (no chemotherapy) in ER-negative, HER2-positive early-stage breast cancer. In an ITT analysis, our study did not reject the null hypothesis related to the AUC of percent change in SULmax by C1D15. However, we demonstrated that a percent reduction in SULmax by C1D15 and C1D15 SULmax was significantly different between patients who obtained pCR after four cycles of PT and those who did not. ROC analysis revealed a higher proportion of SULmax reduction of ≥ 40% in those who obtained pCR with high sensitivity (83%) and NPV (91%). The exploratory analysis of C1D15 SULmax ≤ 3 may have an even greater sensitivity (100%) and NPV (100%) as a predictor of pCR. Because 56% of evaluable patients had C1D15 SULmax ≤ 3, both biomarkers have the potential to be useful early response assessment tools. Finally, an exploratory subset analysis supports the exclusion of patients with low FDG avidity tumors at baseline (SULmax < 2) from the primary analysis of future studies evaluating functional change in FDG uptake after the first cycle of therapy.
Our results suggest that optimized FDG-PET-CT quantitative imaging–based biomarkers may facilitate intensification and de-intensification of neoadjuvant therapy. Because of a high NPV, reduction in SULmax on FDG-PET-CT during the first cycle of PT may predict most optimally those patients who will not achieve pCR with PT alone and should be recommended alternative regimens in future clinical trials. One can consider an analogy to our most important predictive biomarkers in breast cancer, where ER- and HER2-negative phenotypes have a very high NPV for lack of benefit from targeted therapies.13,19
We previously reported that early change in SULmax on FDG-PET-CT by C1D15 after initiating neoadjuvant chemotherapy is successful in predicting pCR in patients with HER2-negative breast cancer.6 A limited number of studies have been performed in patients with HER2-positive breast cancer. In patients receiving trastuzumab emtansine for metastatic disease, a combination of results of pretreatment HER2–PET-CT with [89Zr]trastuzumab with those of early FDG-PET-CT metabolic response assessment (baseline and before the second cycle of trastuzumab emtansine) showed promise in predicting response.20 The NeoALLTO substudy identified that metabolic changes detected on FDG-PET-CT in early-stage breast tumors 2 weeks after commencing HER2-directed therapy predict for pCR rates that are two fold higher in metabolic responders compared with nonresponders by imaging criteria.8 The TBCRC026 trial, however, aimed to identify an optimal SULmax cut point that could help predict response to PT. This is in contrast to the NeoALLTO substudy that used a predefined cut point for response per European Organisation for Research and Treatment of Cancer imaging criteria at 2 weeks (> 15% reduction in SUV), which may not represent the most optimal cut point in the study population to predict response and may be below the reproducibility limits of sequential quantitative 18F-FDG-PET-CT studies.10
PT in combination with chemotherapy is now a standard of care in the high-risk early breast cancer setting yet can be associated with serious toxicities.21 Investigators have thus attempted to de-escalate therapy. For example, 12 weeks of adjuvant paclitaxel and 1 year of trastuzumab were associated with high survival rates among women with small, node-negative HER2-positive breast cancer.22 Chemotherapy-free approaches have also been investigated using dual HER2-directed therapy, concurrent with endocrine therapy for those with ER-positive cancers.5,23,24 Pertuzumab- or lapatinib-based approaches demonstrate similar pCR rates to the 22% rate that we observed with neoadjuvant PT alone in this ER-negative, HER2-positive subtype.
Studies in the neoadjuvant setting are ideal for investigation of promising predictors of sensitivity to HER2-directed therapy. In the PAMELA trial, investigators administered 18 weeks of neoadjuvant lapatinib and trastuzumab (and endocrine therapy if ER-positive) in patients with HER2-positive early breast cancer. Stromal tumor–infiltrating lymphocytes and tumor cellularity at C1D15 were independently associated with pCR.25 The HER2-enriched intrinsic subtype (defined by PAM50) was also identified as a potential predictor of pCR.23 In a study that investigated 12 weeks of lapatinib and trastuzumab, TBCRC006 investigators reported that activation of the phosphoinositide 3-kinase pathway is associated with resistance to therapy.26 Approaches that use a combination of imaging-/blood-/ and tissue-based biomarkers may provide the most optimal biomarker of response to HER2-directed therapy without chemotherapy.
Strengths of our study include its multicenter prospective design and inclusion of a homogenous group of patients with ER-negative, HER2-positive breast cancer. We chose the C1D15 time point for the second FDG-PET-CT scan to define as early as possible in the treatment course of those patients who might require an alternative approach. The exploratory single C1D15 time point appears promising as a predictive biomarker that if confirmed would indicate that baseline imaging may not be required. Limitations of our study include the identification of baseline primary tumor SULmax value below liver reference value (mean + 2 × standardized deviation, n = 4 patients), which may affect accurate measurement of percent change in SULmax.15 These patients were included in our original analysis as they were eligible per protocol, but may be excluded in future studies.
This approach to biomarker development that incorporates FDG-PET-CT is feasible across multiple sites, is less invasive than performing a tumor biopsy for biomarker evaluation, and has been shown to be useful in other tumor types.27,28 We identified percentage change and C1D15 cutoffs for SULmax that best predict resistance to HER2-directed therapy with PT and will design prospective studies in which we will determine the clinical utility of altering therapy based on early changes in SULmax. We will also investigate other imaging parameters including those described in the PET Response Criteria in Solid Tumors version 1.0.15
In conclusion, the results have the potential to provide a more individualized approach to neoadjuvant therapy in women with stage II or III, ER-negative and HER2-positive breast cancer. Such an approach could identify patients who may receive HER2-directed therapy alone and be spared chemotherapy as well as those who require an aggressive approach.
ACKNOWLEDGMENT
We thank Martin Lodge for imaging assistance during study initiation. We also thank Zhe Zhang, Susan Hilsenbeck, Stacie Jeter, and Bridget Walsh for valuable contribution during study design and conduct.
APPENDIX
Supplementary Methods
Corrective actions taken to ensure accuracy of data presented in the revised manuscript titled “Updated Results of TBCRC026: Phase II Trial Correlating Standardized Uptake Value With Pathological Complete Response to Pertuzumab and Trastuzumab in Breast Cancer” (10.1200/JCO.21.00280).
As stated in the retraction notice,12 errors in pathologic complete response (pCR) data were identified in the original publication of the TBCRC026 study.11 These errors related to data entry in the study master database, predominantly because of misclassification of ypT1a and microinvasive disease as pCR. A second independent pathologist was asked to independently review all pathology reports, and ultimately, 10 cases required reclassification from pCR to no pCR.
Since uncovering these findings in late May 2020, our study team has taken the following actions to rectify this issue:
Reports for all pathology cases were independently reviewed by two breast cancer pathologists. The second pathologist had not been previously involved with the trial.
The primary and secondary end point data were reanalyzed by the study statistician.
The revised data and proposed revisions to the manuscript were independently reviewed by the lead statistician for the Translational Breast Cancer Research Consortium.
The revised data were also assessed by the group statistician for a proposed imaging study that will independently validate the findings from TBCRC026 published in Journal of Clinical Oncology (JCO).
The revised analysis and proposed corrections in the published JCO manuscript were presented to the TBCRC Executive Committee in preparation of our submission of these materials to JCO.
As a result of this experience, the TBCRC leadership is reviewing procedures related to the analyses of primary objectives of its trials, to provide assurance that clinical trial investigators approve of all data related to trial conduct and reporting.
Reanalysis of the data has shown that we are now unable to reject the null hypothesis as outlined in the predefined analysis plan for the primary objective. Although the primary objective did not meet the predefined statistical boundaries, the data continue to show that the absence of early change in maximum standardized uptake value corrected for lean body mass is associated with a lower likelihood of achieving pathologic complete response after four cycles of pertuzumab/trastuzumab in early-stage human epidermal growth factor receptor 2–positive breast cancer, and the observed negative predictive value assessed as part of the primary objective remains essentially unchanged.
We believe that the revised primary results of this study (10.1200/JCO.21.00280) remain critical for investigators initiating clinical trials with similar treatment or end points. Finally, an exploratory subset analysis included in the revised manuscript provides important supportive data for the PERCIST 1.0 criteria of excluding low baseline fluorodeoxyglucose avidity tumors from the primary analysis of future studies.
FIG A1.
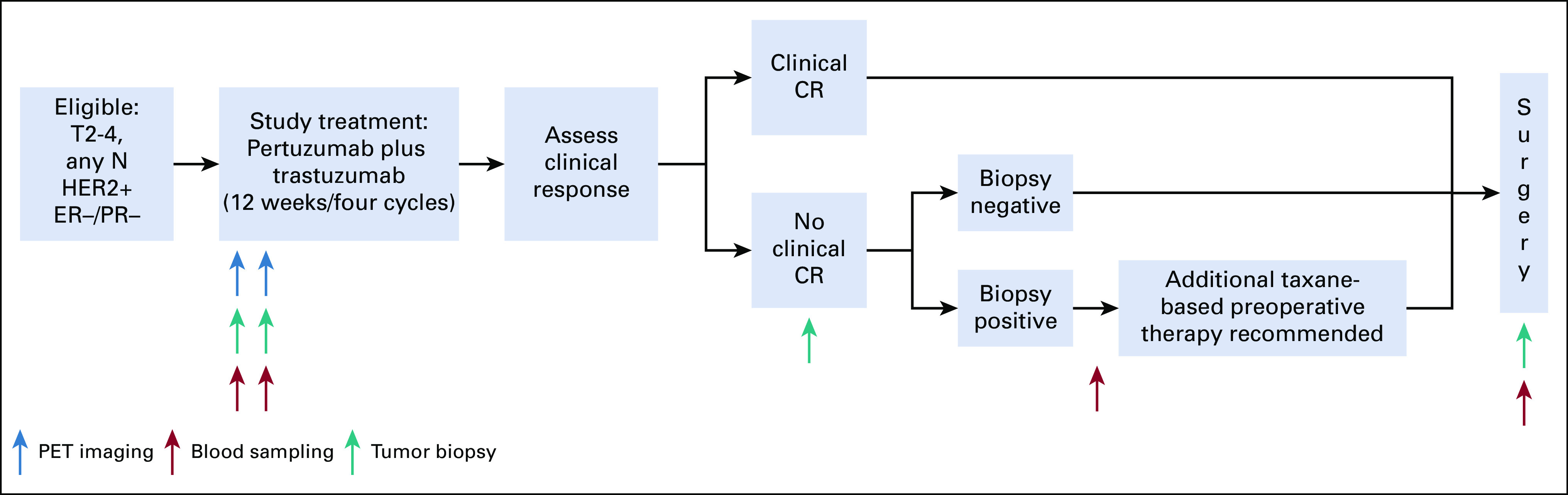
TBCRC026 study schema. CR, complete response; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PET, positron emission tomography; PR, progesterone receptor.
FIG A2.
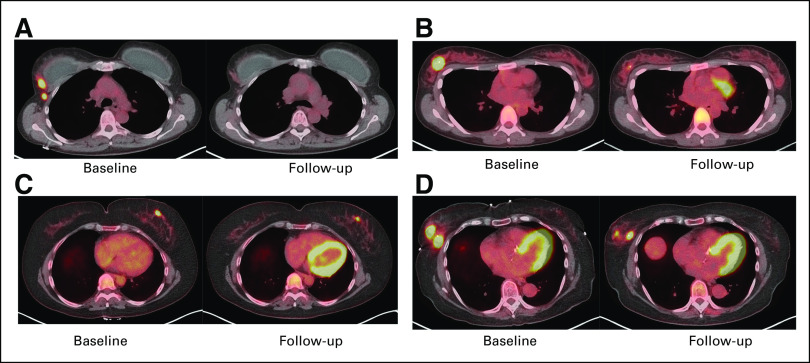
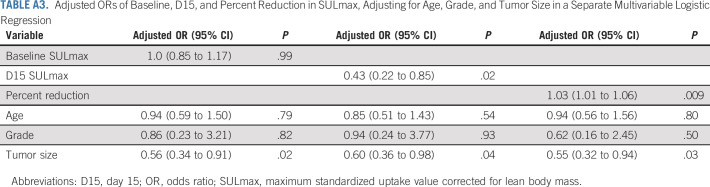
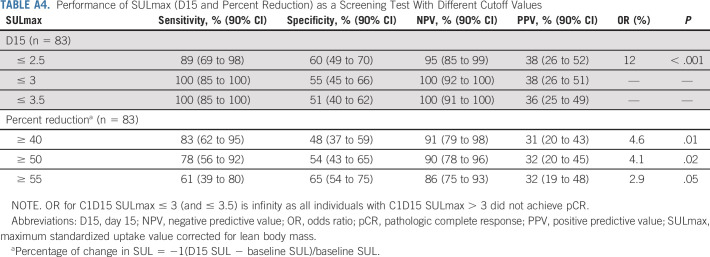
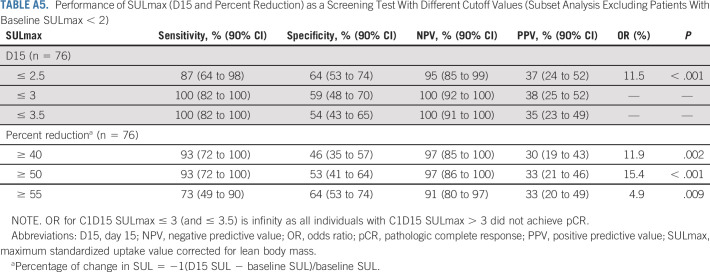
Sample 18F-FDG PET images (baseline and D15) in patients (A and B) with and (C and D) without pCR. 18F-FDG, 18F-labeled fluorodeoxyglucose; pCR, pathologic complete response; PET, positron emission tomography.
TABLE A1.
Treatment-Related Adverse Events Observed in Five or More Patients
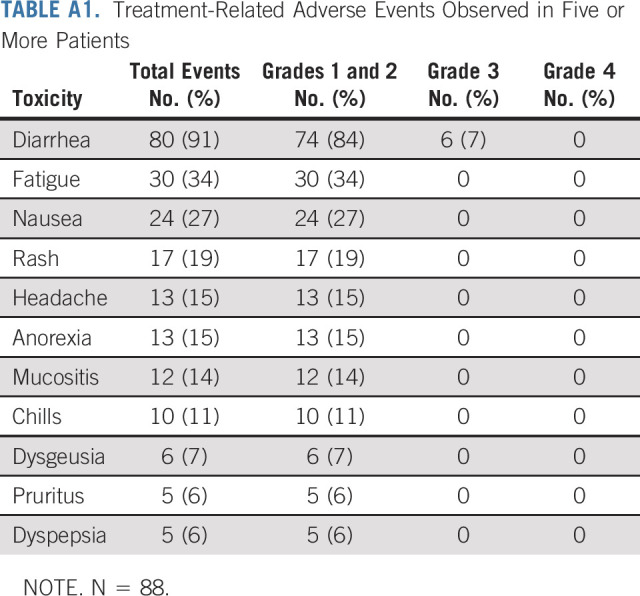
TABLE A2.
SULmax Summary Statistics
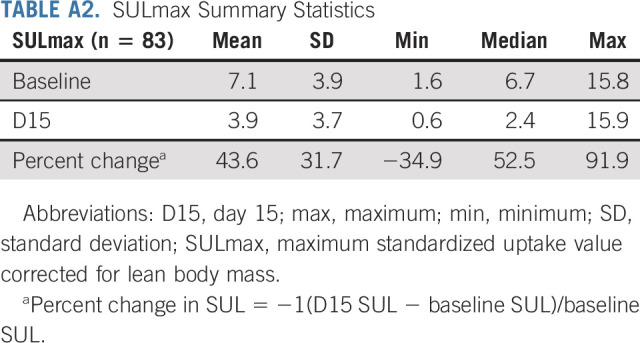
TABLE A3.
Adjusted ORs of Baseline, D15, and Percent Reduction in SULmax, Adjusting for Age, Grade, and Tumor Size in a Separate Multivariable Logistic Regression
TABLE A4.
Performance of SULmax (D15 and Percent Reduction) as a Screening Test With Different Cutoff Values
TABLE A5.
Performance of SULmax (D15 and Percent Reduction) as a Screening Test With Different Cutoff Values (Subset Analysis Excluding Patients With Baseline SULmax < 2)
TABLE A6.
Baseline, D15, and Percent Change in SULmax Between pCR and No pCR in Patients With Residual Disease After Study Therapy and Subsequent Addition of Nonstudy Neoadjuvant Therapy
Roisin M. Connolly
Research Funding: Genentech/Roche, Puma Biotechnology, Novartis, Merck, Macrogenics
Other Relationship: Pfizer
Lilja Solnes
Consulting or Advisory Role: Progenics
Research Funding: AAA/Novartis, Progenics
Vandana Abramson
Employment: HCA Healthcare
Consulting or Advisory Role: Eisai, Daiichi Sankyo, Abbvie
Research Funding: Genentech/Roche, Lilly
Lisa A. Carey
Research Funding: Innocrin Pharma, Syndax, Immunomedics, Novartis, NanoString Technologies, Abbvie, Seattle Genetics
Patents, Royalties, Other Intellectual Property: Royalty-sharing agreement, investorship interest in licensed IP to startup company, Falcon Therapeutics, that is designing neural stem cell-based therapy for glioblastoma multiforme
Uncompensated Relationships: Sanofi, Novartis, G1 Therapeutics, Genentech/Roche, GlaxoSmithKline, Exact Sciences, AstraZeneca/Daiichi Sankyo, Aptitude Health
Open Payments Link: https://openpaymentsdata.cms.gov/physician/179671
Minetta C. Liu
Research Funding: Eisai, Seattle Genetics, Novartis, Roche/Genentech, GRAIL, Merck, Tesaro, Menarini Silicon Biosystems, Genomic Health
Travel, Accommodations, Expenses: Grail, Merck, Menarini Silicon Biosystems, Pfizer, Genomic Health, AstraZeneca, Ionis Pharmaceuticals
Mothaffar Rimawi
Consulting or Advisory Role: Macrogenics, Daiichi Sankyo, Seattle Genetics, Genentech
Research Funding: Pfizer
Jennifer Specht
Honoraria: Nektar, Genomic Health, Daiichi Sankyo
Consulting or Advisory Role: Genomic Health, Nektar, Daiichi Sankyo
Research Funding: Pfizer, Nektar, Merck, Myriad Pharmaceuticals, Cascadian Therapeutics, Pfizer, Minerva Biotechnologies, Seattle Genetics, Novartis, Chalasani, Xencor, Johns Hopkins University, Genentech, Dana-Farber Cancer Institute, NCI
Travel, Accommodations, Expenses: Nektar
Anna Maria Storniolo
Employment: Sophren Therapeutics, Q32Bio Inc
Leadership: Sophren Therapeutics, Q32Bio Inc
Stock and Other Ownership Interests: Gilead Sciences, Moderna Therapeutics, Merck, Lilly, Gilead, Pfizer, Meridian Bioscience Inc
Consulting or Advisory Role: Q32bio, Tri-I TDI, Deerfield Management, Atlas Venture, Boston Pharmaceuticals
Research Funding: QUE Oncology, Pfizer
Vicente Valero
Honoraria: Genentech/Roche, Merck, Novartis
Consulting or Advisory Role: Genentech/Roche, Novartis, Merck
Travel, Accommodations, Expenses: Genentech/Roche
Christos Vaklavas
Employment: Flatiron Health
Honoraria: Guidepoint Global
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Genentech, Roche, Pfizer, Incyte, Pharmacyclics, Novartis, TRACON Pharma, Innocrin Pharma, Zymeworks, H3 Biomedicine
Other Relationship: Puma Biotechnology, Takeda, Daiichi Sankyo
Uncompensated Relationships: Genentech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1306968
Ian E. Krop
Employment: AMAG Pharmaceuticals, Freeline Therapeutics
Leadership: AMAG Pharmaceuticals, Freeline Therapeutics
Stock and Other Ownership Interests: AMAG Pharmaceuticals, Freeline Therapeutics, Vertex
Honoraria: Genentech/Roche, AstraZeneca, Celltrion
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Daiichi Sankyo, Macrogenics, Taiho Pharmaceutical, Context Therapeutics, Novartis, Merck, Ionis Pharmaceuticals, Bristol Myers Squibb, AstraZeneca
Research Funding: Genentech, Pfizer
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks
Research Funding: Genentech, AstraZeneca
Other Relationship: InfiniteMD
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceuticals
Research Funding: Biomarin, Celldex
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Ashley Cimino-Mathews
Employment: Vivante Health
Honoraria: Bristol Myers Squibb, Roche
Research Funding: Bristol Myers Squibb
Ben H. Park
Leadership: Loxo
Stock and Other Ownership Interests: Loxo, Celcuity
Honoraria: AstraZeneca
Consulting or Advisory Role: Horizon Discovery, Foundation Medicine, Loxo, Casdin Capital, H3 Biomedicine, Jackson Laboratory for Genomic Medicine, Lilly, Celcuity, Sermonix Pharmaceuticals, Pathovax
Research Funding: Abbvie, Pfizer, GE Healthcare, Lilly
Patents, Royalties, Other Intellectual Property: Royalties paid through inventions at Johns Hopkins University by Horizon Discovery Ltd
Travel, Accommodations, Expenses: Lilly, Loxo
Uncompensated Relationships: Tempus
Richard L. Wahl
Stock and Other Ownership Interests: Progenics
Honoraria: Siemens Healthineers, Bristol Myers Squibb, Jubilant DraxImage, Actinium Medical Advisory Board
Consulting or Advisory Role: Clarity Pharmaceuticals
Research Funding: Whiterabbit AI, Siemens Healthineers, Bristol Myers Squibb, Actinium Pharmaceuticals
Travel, Accommodations, Expenses: Siemens Healthineers, Actinium Pharmaceuticals
Other Relationship: Society of Nuclear Medicine
Vered Stearns
Research Funding: Abbvie, Pfizer, Novartis, Puma Biotechnology, Biocept
Other Relationship: Immunomedics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5 2018 (abstr 511, poster discussion).
SUPPORT
TBCRC and its foundation partners (The AVON Foundation, The Breast Cancer Research Foundation, and Susan G. Komen for the Cure), SKCCC Core Grant (P30-CA006973), NCI Quantitative Imaging Network (QIN) contract (5U01CA140204), and Genentech Inc including supply of pertuzumab and trastuzumab. Grant funding was obtained from the American Society of Clinical Oncology (ASCO) Conquer Cancer Foundation Career Development Award (2013) and the AVON Center of Excellence.
DATA SHARING STATEMENT
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.00280.
AUTHOR CONTRIBUTIONS
Conception and design: Roisin M. Connolly, Anna Maria Storniolo, Ian E. Krop, Eric P. Winer, Antonio C. Wolff, Ben H. Park, Richard L. Wahl, Vered Stearns
Administrative support: Ashley Carpenter, Richard L. Wahl, Vered Stearns
Financial support: Vered Stearns
Provision of study materials or patients: Minetta C. Liu, Mothaffar Rimawi, Jennifer Specht, Christos Vaklavas, Eric P. Winer, Melissa Camp, Vered Stearns
Collection and assembly of data: Roisin M. Connolly, Lilja Solnes, Ashley Carpenter, Katy Gaffney, Vandana Abramson, Minetta C. Liu, Mothaffar Rimawi, Jennifer Specht, Vicente Valero, Anna Maria Storniolo, Christos Vaklavas, Melissa Camp, Robert S. Miller, Ashley Cimino-Mathews, Richard L. Wahl
Data analysis and interpretation: Roisin M. Connolly, Jeffrey P. Leal, Lilja Solnes, Chiung-Yu Huang, Lisa A. Carey, Minetta C. Liu, Mothaffar Rimawi, Jennifer Specht, Vicente Valero, Anna Maria Storniolo, Antonio C. Wolff, Ashley Cimino-Mathews, Richard L. Wahl, Vered Stearns
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Updated Results of TBCRC026: Phase II Trial Correlating Standardized Uptake Value With Pathological Complete Response to Pertuzumab and Trastuzumab in Breast Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Roisin M. Connolly
Research Funding: Genentech/Roche, Puma Biotechnology, Novartis, Merck, Macrogenics
Other Relationship: Pfizer
Lilja Solnes
Consulting or Advisory Role: Progenics
Research Funding: AAA/Novartis, Progenics
Vandana Abramson
Employment: HCA Healthcare
Consulting or Advisory Role: Eisai, Daiichi Sankyo, Abbvie
Research Funding: Genentech/Roche, Lilly
Lisa A. Carey
Research Funding: Innocrin Pharma, Syndax, Immunomedics, Novartis, NanoString Technologies, Abbvie, Seattle Genetics
Patents, Royalties, Other Intellectual Property: Royalty-sharing agreement, investorship interest in licensed IP to startup company, Falcon Therapeutics, that is designing neural stem cell-based therapy for glioblastoma multiforme
Uncompensated Relationships: Sanofi, Novartis, G1 Therapeutics, Genentech/Roche, GlaxoSmithKline, Exact Sciences, AstraZeneca/Daiichi Sankyo, Aptitude Health
Open Payments Link: https://openpaymentsdata.cms.gov/physician/179671
Minetta C. Liu
Research Funding: Eisai, Seattle Genetics, Novartis, Roche/Genentech, GRAIL, Merck, Tesaro, Menarini Silicon Biosystems, Genomic Health
Travel, Accommodations, Expenses: Grail, Merck, Menarini Silicon Biosystems, Pfizer, Genomic Health, AstraZeneca, Ionis Pharmaceuticals
Mothaffar Rimawi
Consulting or Advisory Role: Macrogenics, Daiichi Sankyo, Seattle Genetics, Genentech
Research Funding: Pfizer
Jennifer Specht
Honoraria: Nektar, Genomic Health, Daiichi Sankyo
Consulting or Advisory Role: Genomic Health, Nektar, Daiichi Sankyo
Research Funding: Pfizer, Nektar, Merck, Myriad Pharmaceuticals, Cascadian Therapeutics, Pfizer, Minerva Biotechnologies, Seattle Genetics, Novartis, Chalasani, Xencor, Johns Hopkins University, Genentech, Dana-Farber Cancer Institute, NCI
Travel, Accommodations, Expenses: Nektar
Anna Maria Storniolo
Employment: Sophren Therapeutics, Q32Bio Inc
Leadership: Sophren Therapeutics, Q32Bio Inc
Stock and Other Ownership Interests: Gilead Sciences, Moderna Therapeutics, Merck, Lilly, Gilead, Pfizer, Meridian Bioscience Inc
Consulting or Advisory Role: Q32bio, Tri-I TDI, Deerfield Management, Atlas Venture, Boston Pharmaceuticals
Research Funding: QUE Oncology, Pfizer
Vicente Valero
Honoraria: Genentech/Roche, Merck, Novartis
Consulting or Advisory Role: Genentech/Roche, Novartis, Merck
Travel, Accommodations, Expenses: Genentech/Roche
Christos Vaklavas
Employment: Flatiron Health
Honoraria: Guidepoint Global
Consulting or Advisory Role: Daiichi Sankyo
Research Funding: Genentech, Roche, Pfizer, Incyte, Pharmacyclics, Novartis, TRACON Pharma, Innocrin Pharma, Zymeworks, H3 Biomedicine
Other Relationship: Puma Biotechnology, Takeda, Daiichi Sankyo
Uncompensated Relationships: Genentech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1306968
Ian E. Krop
Employment: AMAG Pharmaceuticals, Freeline Therapeutics
Leadership: AMAG Pharmaceuticals, Freeline Therapeutics
Stock and Other Ownership Interests: AMAG Pharmaceuticals, Freeline Therapeutics, Vertex
Honoraria: Genentech/Roche, AstraZeneca, Celltrion
Consulting or Advisory Role: Genentech/Roche, Seattle Genetics, Daiichi Sankyo, Macrogenics, Taiho Pharmaceutical, Context Therapeutics, Novartis, Merck, Ionis Pharmaceuticals, Bristol Myers Squibb, AstraZeneca
Research Funding: Genentech, Pfizer
Eric P. Winer
Honoraria: Genentech/Roche, Genomic Health
Consulting or Advisory Role: Leap Therapeutics, Seattle Genetics, Jounce Therapeutics, GlaxoSmithKline, Carrick Therapeutics, Lilly, G1 Therapeutics, Syros Pharmaceuticals, Genentech/Roche, Gilead Sciences, Zymeworks
Research Funding: Genentech, AstraZeneca
Other Relationship: InfiniteMD
Antonio C. Wolff
Consulting or Advisory Role: Ionis Pharmaceuticals
Research Funding: Biomarin, Celldex
Patents, Royalties, Other Intellectual Property: Antonio Wolff has been named as inventor on one or more issued patents or pending patent applications relating to methylation in breast cancer, and has assigned his rights to JHU, and participates in a royalty sharing agreement with JHU
Open Payments Link: https://openpaymentsdata.cms.gov/physician/357301/summary
Ashley Cimino-Mathews
Employment: Vivante Health
Honoraria: Bristol Myers Squibb, Roche
Research Funding: Bristol Myers Squibb
Ben H. Park
Leadership: Loxo
Stock and Other Ownership Interests: Loxo, Celcuity
Honoraria: AstraZeneca
Consulting or Advisory Role: Horizon Discovery, Foundation Medicine, Loxo, Casdin Capital, H3 Biomedicine, Jackson Laboratory for Genomic Medicine, Lilly, Celcuity, Sermonix Pharmaceuticals, Pathovax
Research Funding: Abbvie, Pfizer, GE Healthcare, Lilly
Patents, Royalties, Other Intellectual Property: Royalties paid through inventions at Johns Hopkins University by Horizon Discovery Ltd
Travel, Accommodations, Expenses: Lilly, Loxo
Uncompensated Relationships: Tempus
Richard L. Wahl
Stock and Other Ownership Interests: Progenics
Honoraria: Siemens Healthineers, Bristol Myers Squibb, Jubilant DraxImage, Actinium Medical Advisory Board
Consulting or Advisory Role: Clarity Pharmaceuticals
Research Funding: Whiterabbit AI, Siemens Healthineers, Bristol Myers Squibb, Actinium Pharmaceuticals
Travel, Accommodations, Expenses: Siemens Healthineers, Actinium Pharmaceuticals
Other Relationship: Society of Nuclear Medicine
Vered Stearns
Research Funding: Abbvie, Pfizer, Novartis, Puma Biotechnology, Biocept
Other Relationship: Immunomedics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer N Engl J Med 366109–1192012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial Lancet Oncol 1325–322012 [DOI] [PubMed] [Google Scholar]
- 3.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol 242278–22842013 [DOI] [PubMed] [Google Scholar]
- 4.Prowell TM, Pazdur R.Pathological complete response and accelerated drug approval in early breast cancer N Engl J Med 3662438–24412012 [DOI] [PubMed] [Google Scholar]
- 5.Nitz UA, Gluz O, Christgen M, et al. De-escalation strategies in HER2-positive early breast cancer (EBC): Final analysis of the WSG-ADAPT HER2+/HR− phase II trial: Efficacy, safety, and predictive markers for 12 weeks of neoadjuvant dual blockade with trastuzumab and pertuzumab ± weekly paclitaxel Ann Oncol 282768–27722017 [DOI] [PubMed] [Google Scholar]
- 6.Connolly RM, Leal JP, Goetz MP, et al. TBCRC 008: Early change in 18F-FDG uptake on PET predicts response to preoperative systemic therapy in human epidermal growth factor receptor 2-negative primary operable breast cancer J Nucl Med 5631–372015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahl RL, Zasadny K, Helvie M, et al. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation J Clin Oncol 112101–21111993 [DOI] [PubMed] [Google Scholar]
- 8.Gebhart G, Gamez C, Holmes E, et al. 18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: Results from Neo-ALTTO J Nucl Med 541862–18682013 [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial Lancet 379633–6402012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92205–2162000 [DOI] [PubMed] [Google Scholar]
- 11.Connolly RM, Leal JP, Solnes L, et al. TBCRC026: Phase II trial correlating standardized uptake value with pathologic complete response to pertuzumab and trastuzumab in breast cancer J Clin Oncol 37714–7222019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Connolly RM, Leal JP, Solnes L, et al. TBCRC026: Phase II trial correlating standardized uptake value with pathologic complete response to pertuzumab and trastuzumab in breast cancer J Clin Oncol 37714–7222019[Retraction: J Clin Oncol39 23192021] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update J Clin Oncol 313997–40132013 [DOI] [PubMed] [Google Scholar]
- 14.Graham MM, Wahl RL, Hoffman JM, et al. Summary of the UPICT protocol for 18F-FDG PET/CT imaging in oncology clinical trials J Nucl Med 56955–9612015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors J Nucl Med 50122S–150S2009suppl 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong ER, DeLong DM, Clarke-Pearson DL.Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach Biometrics 44837–8451988 [PubMed] [Google Scholar]
- 17.R Version 3.4.2 Software Package. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org [Google Scholar]
- 18.Youden WJ.Index for rating diagnostic tests Cancer 332–351950 [DOI] [PubMed] [Google Scholar]
- 19.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer J Clin Oncol 282784–27952010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebhart G, Lamberts LE, Wimana Z, et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial Ann Oncol 27619–6242016 [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Swain SM.Pertuzumab for the treatment of breast cancer: A safety review Expert Opin Drug Saf 15853–8632016 [DOI] [PubMed] [Google Scholar]
- 22.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer N Engl J Med 372134–1412015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llombart-Cussac A, Cortes J, Pare L, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): An open-label, single-group, multicentre, phase 2 trial Lancet Oncol 18545–5542017 [DOI] [PubMed] [Google Scholar]
- 24.Rimawi MF, Mayer IA, Forero A, et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: TBCRC 006 J Clin Oncol 311726–17312013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nuciforo P, Pascual T, Cortes J, et al. A predictive model of pathologic response based on tumor cellularity and tumor-infiltrating lymphocytes (CelTIL) in HER2-positive breast cancer treated with chemo-free dual HER2 blockade Ann Oncol 29170–1772018 [DOI] [PubMed] [Google Scholar]
- 26.Rimawi MF, De Angelis C, Contreras A, et al. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer Breast Cancer Res Treat 167731–7402018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial Lancet 3791791–17992012 [DOI] [PubMed] [Google Scholar]
- 28.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: The MUNICON phase II trial Lancet Oncol 8797–8052007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A data sharing statement provided by the authors is available with this article at DOI https://doi.org/10.1200/JCO.21.00280.