Abstract
PURPOSE
Hematopoietic cell transplantation (HCT) is curative for hematologic disorders, but outcomes are historically inferior when using HLA-mismatched donors. Despite unrelated donor registries listing > 38 million volunteers, 25%-80% of US patients lack an HLA-matched unrelated donor, with significant disparity across ethnic groups. We hypothesized that HCT with a mismatched unrelated donor (MMUD) using post-transplant cyclophosphamide (PTCy), a novel strategy successful in overcoming genetic disparity using mismatched related donors, would be feasible and increase access to HCT.
PATIENTS AND METHODS
We performed a prospective phase II study of MMUD bone marrow HCT with PTCy for patients with hematologic malignancies. The primary end point was 1-year overall survival (OS), hypothesized to be 65% or better. 80 patients enrolled at 11 US transplant centers (December 2016-March 2019). Following myeloablative or reduced-intensity conditioning–based HCT, patients received PTCy on days +3, +4, with sirolimus and mycophenolate mofetil starting on day +5. We compared outcomes to Center for International Blood and Marrow Transplant Research contemporary controls receiving PTCy.
RESULTS
Notably, 48% of patients enrolled were ethnic minorities. 39% of pairs were matched for 4-6 out of 8 HLA alleles. The primary end point was met, with 1-year OS of 76% (90% CI, 67.3 to 83.3) in the entire cohort, and 72% and 79% in the myeloablative and reduced-intensity conditioning strata, respectively. Secondary end points related to engraftment and graft-versus-host-disease were reached. Multivariate analysis comparing the study group with other mismatched HCT controls found no significant differences in OS.
CONCLUSION
Our prospective study demonstrates the feasibility and effectiveness of HCT with an MMUD in the setting of PTCy. Remarkably, nearly half of the study participants belonged to an ethnic minority population, suggesting this approach may significantly expand access to HCT.
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) is a well-established potentially curative therapy for hematologic diseases. The best outcomes are seen in the setting of a well-matched donor, historically an HLA-matched sibling, although more recently, similar outcomes are achieved using a graft from an HLA-matched (at HLA-A, -B, -C, -DRB1, or 8 out of 8 alleles) unrelated donor (URD).1,2 HLA-matched sibling donor availability ranges from 13% to 51%,3 and although international URD registries list > 38 million volunteer donors, a substantial proportion of patients still lack a matched URD (MUD), and patients from racial or ethnic minorities are disproportionately disadvantaged. The National Marrow Donor Program/Be The Match (NMDP) performed an analysis with > 10.5 million URD and found the probability of identifying an MUD for a Caucasian patient of 75% compared to 19% for an African-American patient.4
Although HCT with a mismatched donor (MMUD) is possible, survival for these patients is historically inferior, with increased graft-versus-host disease (GVHD) and graft failure when standard calcineurin inhibitor-based GVHD prophylaxis is used.5,6 Addition of T-cell–depleting agents7,8 or ex vivo depletion of T cells effectively reduces GVHD but increases the risks of other adverse outcomes such as infection, graft failure, and relapse, with the net effect of lowering survival.
A novel strategy for GVHD prevention using post-HCT high-dose cyclophosphamide (PTCy) is based on the immunobiologic rationale that recently activated, alloreactive effector T cells (responsible for GVHD) are selectively sensitive to the toxic effects of this drug and that regulatory T-cell function is preserved, avoiding more widespread immunosuppressive effects.9-11 PTCy effectively overcomes the barriers associated with multiple HLA-mismatches in the mismatched related (haploidentical) donor setting, with acceptable rates of engraftment, GVHD, and survival demonstrated in several single-center and multicenter clinical trials.12,13 Single-center data expanding this approach to the use of MMUD showed safety and feasibility.14
The goals of our phase II prospective clinical trial were therefore twofold: first, to improve outcomes of MMUD HCT using PTCy, and second, to increase access to HCT for underserved patients, especially racial or ethnic minorities, lacking an HLA-matched donor.
PATIENTS AND METHODS
Study Design and Eligibility
This study was sponsored by the NMDP, opened at 11 centers in the United States, and was approved by the NMDP central institutional review board (n = 2) or the transplant center (TC) institutional review board (n = 9) (ClinicalTrials.gov identifier: NCT02793544). All patients (cases and contemporary controls) provided written informed consent.
Eligible patients were 15-71 years old; had a diagnosis of acute or chronic leukemia, myelodysplastic syndrome, or lymphoma; were eligible for a standard-of-care first allogeneic HCT using bone marrow (BM) as the stem cell source; had a Karnofsky or Lansky performance score (KPS or LPS) of ≥ 60%; and adequate pulmonary, renal, cardiac, and liver function. Patients were excluded if they had a suitable HLA-identical sibling or MUD available. Patients with HIV were permitted on study if additional eligibility requirements were met (Protocol, online only).
Study Treatment
All patients underwent standard pre-transplant evaluations and staging procedures. Donor searches were performed by NMDP per TC practice. The study had two nonrandomized conditioning intensity strata, either myeloablative (MAC) or reduced-intensity conditioning (RIC), with the strata selected at the TC's discretion. TCs selected among three MAC regimens: cyclophosphamide and total body irradiation (TBI); busulfan and cyclophosphamide; or fludarabine and busulfan. One RIC regimen was used: fludarabine, cyclophosphamide, and low-dose TBI. Thereafter, patients received a fresh BM graft on day 0, PTCy on days +3, +4, and sirolimus and mycophenolate mofetil starting on day +5. Supportive care was per TC policy. Relapse-prevention strategies were allowed at center discretion, although donor leukocyte infusions were not permitted before day 100.
Evaluation and Toxicity Assessments
The schedule of patient assessments is shown in the Data Supplement (online only). Clinician assessments of toxicity (National Cancer Institute's Common Terminology Criteria for Adverse Events version 4.03) were performed, and all grade 5 and unexpected grade 3 and 4 toxicities were reported. Monitoring of two key safety end points (overall mortality and grade 2-4 acute GVHD [aGVHD] by day +100) was conducted weekly, with protocol-defined triggers for study pause and data safety monitoring board review. No stopping rules were triggered. GVHD was graded and treated per institutional guidelines using standard criteria.15
Study End Point and Statistical Analysis
The primary end point of this study was 1-year overall survival (OS). Sample size was based on estimation of the 1-year OS using 90% CIs within each of two strata; a sample size of 40 patients in each stratum was expected to have a 90% CI width of 27% when the 1-year OS was 65%. Correspondingly, when the true OS percentage is 65%, there is 80% power to rule out an OS percentage of ≤45% at α = .10 (two-sided). Hypotheses for key secondary end points included that engraftment would be > 90% and that the incidence of grade 3-4 aGVHD would be < 15% at 100 days. Primary graft failure (PGF) was defined as lack of donor-derived neutrophil engraftment (< 5% donor chimerism) by day +56.
OS, progression-free survival, grade 3-4 aGVHD-free survival, and GVHD-free, relapse-free survival (GRFS) were estimated using the Kaplan-Meier method.16 Cumulative incidence of nonrelapse mortality (NRM), relapse, grade 2-4 aGVHD, grade 3-4 aGVHD, chronic GVHD (cGVHD), and hematologic recovery were calculated using the Aalen-Johansen estimator to account for competing risks17of relapse for NRM and death for all other end points; 90% CI are reported for all outcomes.
We performed an unplanned analysis of comparator data, which was available from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry. All patients in the United States whose data were reported to CIBMTR and underwent a first allogeneic HCT between December 2016 and March 2019 using a MMUD with a peripheral blood stem cell (PBSC) graft or a mismatched related donor with a PBSC or a BM graft, received PTCy as GVHD prophylaxis, and otherwise met eligibility criteria for the phase II clinical trial were included. All analyses used standard stepwise Cox regression modeling,18 with results expressed as hazard ratios (HR) with 95% CI. All variables studied met the assumptions for proportionality. All P values are two-sided; P values < .05 were considered to indicate statistical significance for the comparator analysis. Analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
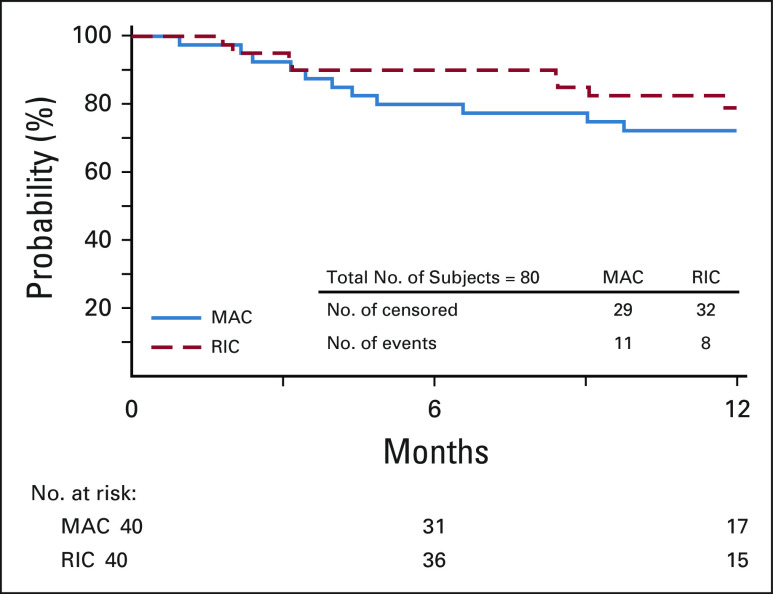
Eighty patients (including four HIV-positive patients) were enrolled between December 2016 and March 2019. Patient, donor, and transplant characteristics, divided by strata, are shown in Table 1. Most of the transplants on the MAC strata were performed for leukemia or myelodysplastic syndrome (n = 39), whereas a significant number (n = 16) received an HCT for lymphoma or Hodgkin disease on the RIC strata. The median age of patients differed by strata (MAC = 49; RIC = 60). A large proportion of patients had high-risk features before HCT, including 54% with an HCT-CI score of ≥ 3 (MAC = 60%; RIC = 48%) and 34% with a KPS < 90 (MAC = 35%; RIC = 33%). Of note, 48% of patients were from racial or ethnic minority groups (MAC = 58%; RIC = 38%). The average donor age was 29 years (range, 18-56 years), and 39% of patients received a graft that was mismatched for > 1 HLA allele. Total nucleated cell and CD34+ counts infused were 2.8 × 108 (range, 0.76-520.8) and 2.66 × 106 (0.39-6.23) per kg of recipient body weight, respectively.
TABLE 1.
Baseline Characteristics for Clinical Trial Patients by Conditioning Intensity
Study End Points and Toxicity
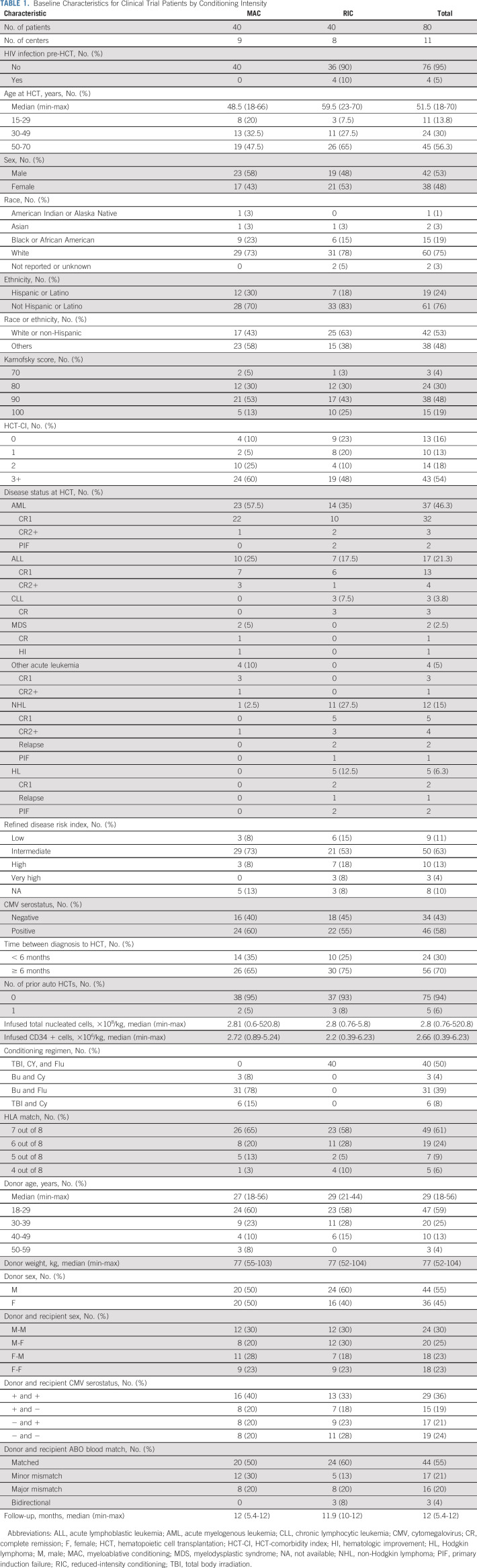
The study achieved its primary end point with 1-year OS of 76% (90% CI, 67.3 to 83.3) in the entire cohort; survival was 72% (90% CI, 59.9 to 83.1) and 79% (90% CI, 66.9 to 88.8) in the MAC and RIC strata, respectively (Fig 1). OS did not differ by HLA match grade; in 7 out of 8 matched pairs, this was 75% (90% CI, 63.4 to 84.3) and in 4-6 out of 8, it was 77% (90% CI, 64.1 to 88.4).
FIG 1.
OS, stratified by conditioning intensity. MAC, myeloablative conditioning; OS, overall survival; RIC, reduced-intensity conditioning.
Engraftment.
There were no cases of PGF in the MAC strata, whereas the incidence of PGF was 7.5% (< 5% donor chimerism in whole blood) in the RIC strata.
GVHD.
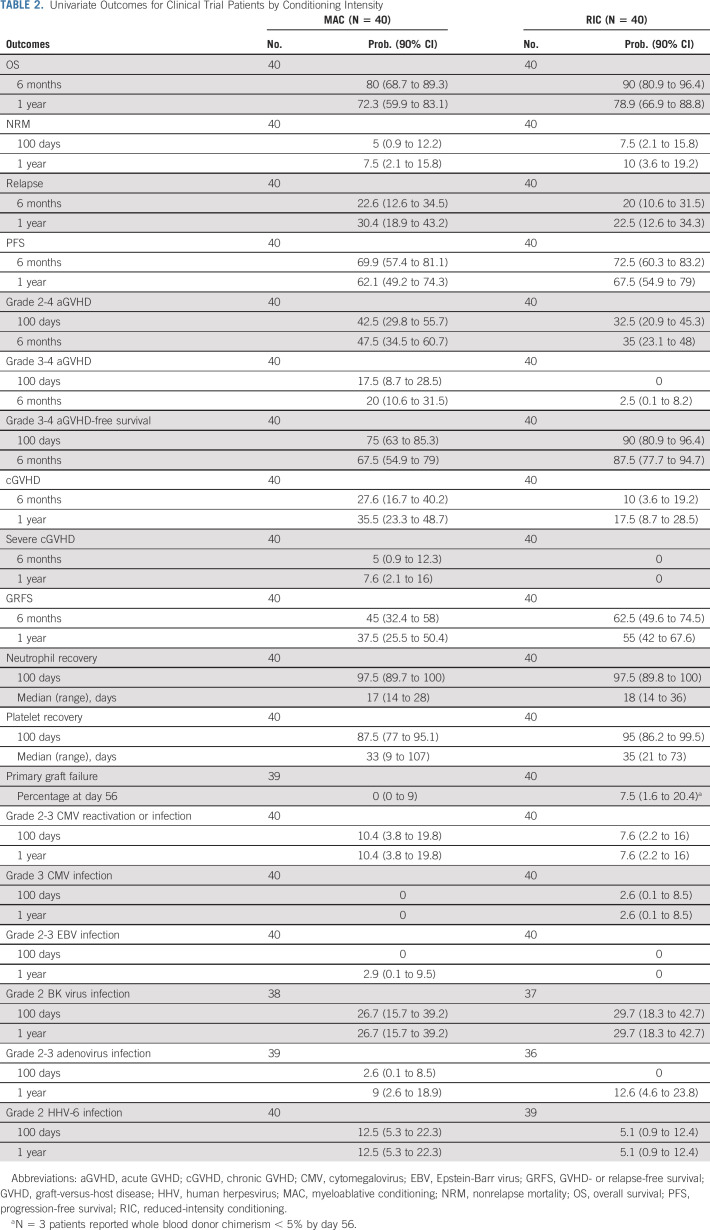
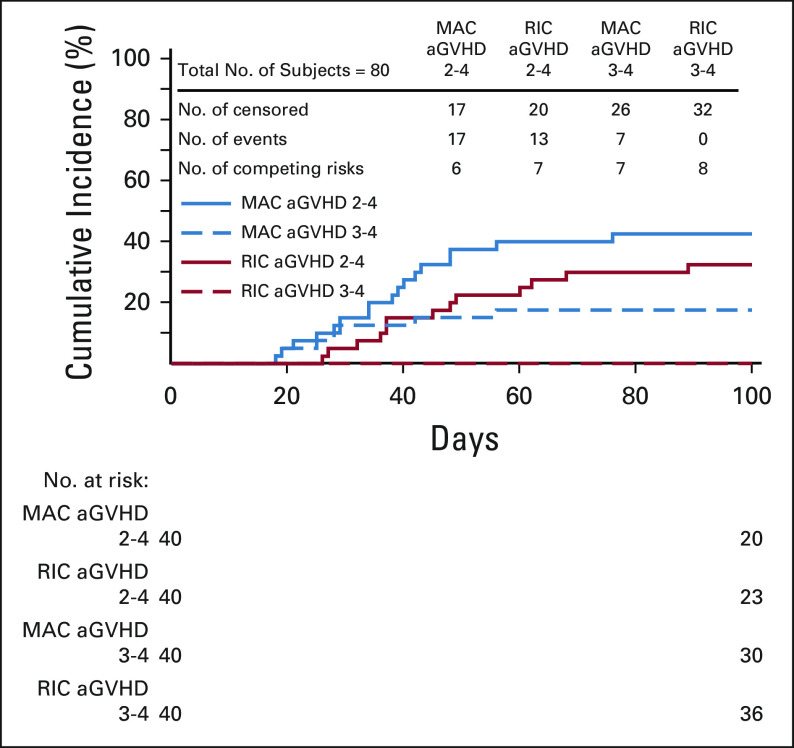
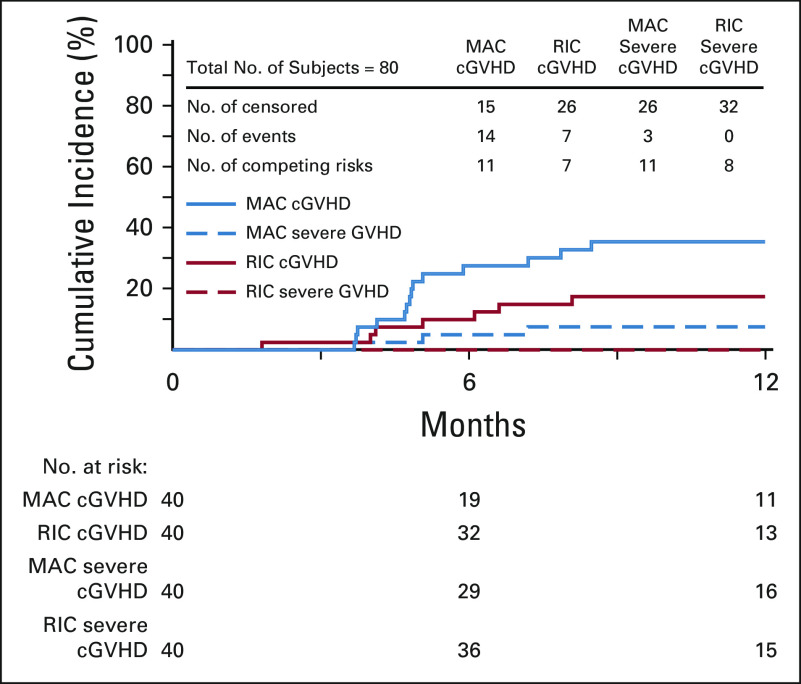
Patients receiving MAC had grade 2-4 and 3-4 aGVHD rates at day +100 of 43% and 18%, respectively, with cGVHD at 1 year of 36%. Patients receiving RIC had grade 2-4 and 3-4 aGVHD rates at day +100 of 33% and 0%, respectively, with cGVHD at 1 year of 18% (Table 2, Figs 2 and 3).
TABLE 2.
Univariate Outcomes for Clinical Trial Patients by Conditioning Intensity
FIG 2.
aGVHD by strata. aGVHD, acute graft-versus-host disease; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
FIG 3.
cGVHD by strata. cGVHD, chronic graft-versus-host disease; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
NRM and relapse.
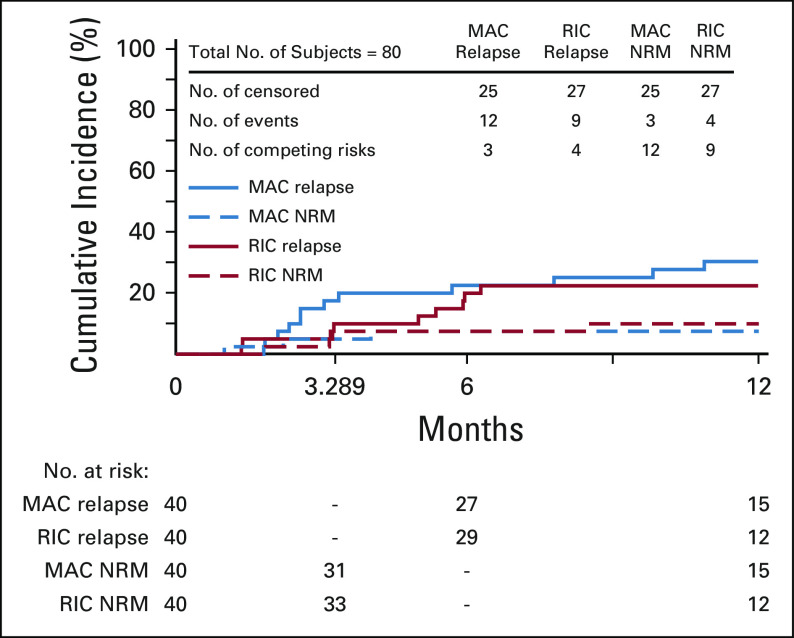
In MAC, NRM and relapse at 1 year were 8% and 30%, respectively, whereas in RIC, these were 10% and 23%, respectively (Table 2, Fig 4).
FIG 4.
Relapse or NRM by strata. MAC, myeloablative conditioning; NRM, nonrelapse mortality; RIC, reduced-intensity conditioning.
Toxicity.
Cumulative rates of clinically meaningful viral infection (ie, those with end-organ damage or requiring treatment) were ≤ 11% at 1 year (Table 2), except for BK virus (27% in MAC and 30% in RIC), adenovirus (13% in RIC) and human herpesvirus 6 (HHV6) (13% in MAC). The most common cause of death was disease relapse. Three patients died of infections (none of which were viral), and all other deaths were related to organ toxicity (Appendix Table A1, online only). Of four patients with HIV, three were transplanted with lymphoma, one of whom is alive and two of whom died because of lymphoma relapse or progression; the fourth patient, transplanted for acute leukemia, died of a fungal infection. Eleven serious unexpected events were reported in four patients; the data safety monitoring board determined that eight were possibly related, one unlikely to be related, and two unrelated to the trial procedures.
Although not protocol-specified end points, two novel composite HCT end points were assessed. GRFS (events including grade 3-4 aGVHD, cGVHD requiring systemic treatment, relapse, and death) at 1 year was 38% and 55% in the MAC and RIC strata, respectively. Grade 3-4 aGVHD-free survival at 6 months was 68% and 88% in the MAC and RIC strata, respectively.
Comparison to Contemporaneous Real-World Data Collected by the CIBMTR Registry
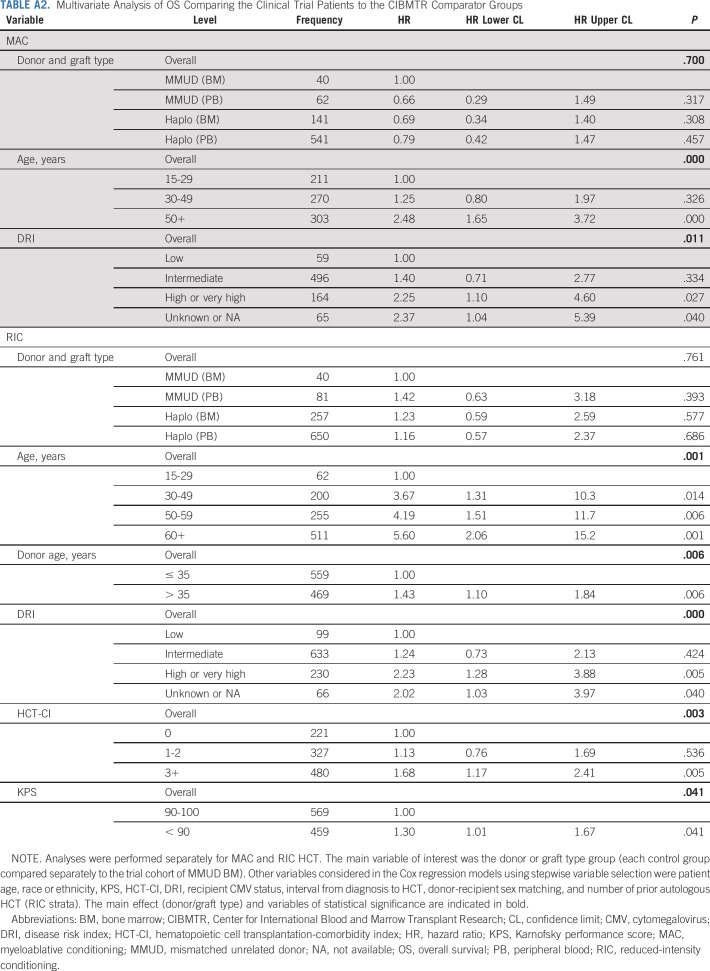
Three cohorts of contemporary registry controls were studied: MMUD receiving PBSC grafts (n = 143), mismatched related donor recipients receiving BM grafts (n = 398), or PBSC grafts (n = 1,191). In multivariate analysis, OS at 1 year was not significantly different between the four groups (P = .761) (Appendix Table A2, online only). Use of older donors (> 35 years old) was associated with higher mortality in the RIC setting (HR, 1.43; CI, 1.10 to 1.84; P = .006) (Appendix Table A2). Data on additional characteristics and end points are in the Data Supplement.
DISCUSSION
The results from this prospective phase II multicenter clinical trial confirm the feasibility of HCT from an MMUD using PTCy, with 1-year OS of > 70%. If confirmed in larger studies, this greatly extends the donor options for patients in need of HCT, particularly those from ethnically diverse backgrounds.
The results in our RIC strata are comparable to those reported in the single-center trial,14 with a 33% incidence of grade 2-4 aGVHD (no grade 3 or 4) and 18% cGVHD (no severe cGVHD). Patients on the MAC strata experienced higher rates of aGVHD compared with our RIC strata, however without GVHD-related mortality. MAC has been associated with a higher risk of GVHD compared to RIC.19 In addition, baseline characteristics differed somewhat between the MAC and RIC strata. Patients receiving MAC were more likely to be from racial or ethnic minorities and had more pre-HCT comorbidities, both factors associated with higher risks of GVHD.20,21 Strategies (untested in the MMUD setting) to reduce GVHD may be the addition of antithymocyte globulin22 or altering the timing of immunosuppressive drugs.23
We added sirolimus and mycophenolate mofetil to PTCy for GVHD prophylaxis, as in Kasamon et al.14 Sirolimus, a mechanistic target of rapamycin inhibitor, was selected in preference to the more commonly used calcineurin inhibitor (CNI), tacrolimus, because of synergism with PTCy seen in preclinical studies24; however, recent data in the mismatched related setting suggest similar outcomes with either sirolimus or tacrolimus.25
Abatacept, a selective inhibitor of T-cell costimulation, has also shown promise in minimally MMUD (7 out of 8 matched) HCTs, although studying this agent in the setting of multiple HLA mismatches is required. A phase II study in 43 patients receiving MMUD grafts and a CNI plus methotrexate showed grade 3-4 aGVHD at day +100 of 2.3% (patients received RIC [N = 10] or MAC [N = 33]).26 Seven additional patients transplanted for sickle cell disease with an MMUD, in a phase I multicenter study, received abatacept and CNI plus methotrexate. Grade 3-4 aGVHD at day +100 was 7%. Strategies to facilitate the use of MMUDs (either related27 or unrelated) in this disease are particularly relevant as some family members are excluded because of being affected by the disease, as well as paucity of MUD.28
As in early studies testing PTCy in the mismatched related setting, we used BM exclusively because of the higher incidence of GVHD associated with the use of PBSC.29 More recently, several investigators have switched the graft source from BM to PBSC in the PTCy setting30 because of concerns for higher disease relapse rates in aggressive disease,31,32 an increased incidence of graft failure,29 and the lower rates of GVHD in general in the PTCy setting33 (compared with standard GVHD prophylaxis).34 Despite the increase in cGVHD, PBSC has become the graft source of choice in most HCT settings,35 because of the concerns listed above, as well as the more predictable quality and ease of access of PBSC. Some studies using PTCy now report that the use of PBSC is associated with similar rates of aGVHD, and other outcomes, compared with BM,36 and this will be prospectively studied in our upcoming clinical trial.
Initial results in the mismatched related PTCy setting showed a higher-than-expected incidence of disease relapse,13 although this was not consistently shown in later studies.34 Relapse rates remain an issue to be addressed. In our study, those receiving MAC had the highest risk of disease relapse, perhaps reflecting the risk of the underlying disease (more patients with leukemia were transplanted with MAC). This may be mitigated by the use of PBSC, particularly for patients with high-risk disease.
Few studies have systematically collected and reported data on viral infections, making the data collected in our study difficult to interpret in the context of similar transplant approaches. The incidence of significant life-threatening viral infections (eg, cytomegalovirus and Epstein-Barr virus) was low, and no deaths in study participants were attributed to a viral infection. Virtual absence of post-transplant lymphoproliferative disease with PTCy has been previously noted.37 Rates of BK infection were relatively high, which warrants further investigation.
An important finding in this study is that 48% of the patients enrolled were from racial or ethnic minorities. Such patients have long faced poorer outcomes because of inequalities in access to HCT,38,39 greater likelihood of being transplanted with a mismatched graft,38 and low enrollment into clinical trials.40,41 Registry modeling has shown that simply increasing the number of minority volunteer donors cannot completely close the access gap (M. Maiers, personal communication, October 2020); thus, strategies, such as the use of PTCy, to improve outcomes when using MMUDs are urgently needed. NMDP data show that when an 8 out of 8 MUD is not available, a 7 out of 8 MMUD can be identified for 53% of African-American patients, and expanding the match grade to < 7 out of 8 would result in an identified donor for every patient (M. Maiers, personal communication, October 2020). Thirty-nine percent of patients in our study received cells from a donor matched for < 7 out of 8 HLA alleles, and no significant difference based on the degree of disparity was found. Although the numbers in our study are too small to be definitive, this warrants further study. Indeed, there are data in the mismatched related PTCy setting suggesting that greater degrees of HLA disparity may be associated with better outcomes.42
A recent article in this Journal,43 publishing the ASCO Recommendations for Promoting Health Equity, includes several recommendations to facilitate equitable access to research. In her editorial, Dr Pierce, ASCO President, states “We should not rest until the opportunity to participate in clinical trials is available to—and represents—all patients with cancer, not just the 5% who enroll today.”44(p3361) Our study included a remarkable number of patients from racial or ethnic minorities, which compares favorably to clinical trials in hematologic diseases and HCT and indicates the willingness of people from ethnic minorities to participate in research that address issues of importance to them.45 A recent review of randomized controlled trials in patients with multiple myeloma, a disease which disproportionately affects black people,40 found enrollment of 13%-21% of patients representing racial minorities. The Blood and Marrow Transplantation Clinical Trial Network, a National Institutes of Health–funded clinical trial network addressing important questions in HCT, reports between 8% and 37% racial or ethnic minority patients enrolled on its studies.46
This study clearly shows that MMUD transplants are feasible, safe, and effective, with results similar to the results of mismatched related HCT (although the comparison to the CIBMTR data should be treated with caution as this analysis was unplanned and does not represent prospectively randomly assigned patients). The small numbers preclude assessment of many important questions that remain to be answered in future studies, including random assignment between donor types addressing the impact of stem cell source, disease, and conditioning regimen, as well as factors that may favor use of one donor type over the other. Important factors favoring mismatched related donors may include speed of graft provision and cost. Factors favoring MMUD may include the ability to select a younger donor,47 or a donor better matched with respect to virologic serostatus and ABO blood type.34 Selection of younger donors (whether mismatched related or unrelated) was associated with significantly better OS, as well as several other end points, in our comparator analysis. Donor-specific antibodies, a significant issue in the mismatched related setting (associated with increased graft failure),48 can be completely avoided in the MMUD setting. Finally, selection of donors with rare genetic polymorphisms can be prioritized. We included HIV-positive patients in our study and prioritized selection of CCR5-Δ32 homozygous donors, as CCR5-Δ32 homozygous donors have been associated with cure of HIV following HCT.49 A suitable donor (ie, one who was CCR5-Δ32 homozygous) could be identified for all four patients, comparing favorably to the probability of 30% or less in the MUD setting.50
In conclusion, this phase II prospective clinical trial using MMUDs in the setting of PTCy showed excellent survival, acceptable rates of GVHD, and enrolled high numbers of patients from racial or ethnic minority groups. Limitations related to the use of BM, sirolimus, and the best RIC option are the focus of our upcoming study, and future research must address strategies to incorporate predictors for GVHD51 and to mitigate relapse.
Appendix
TABLE A1.
Primary Cause of Death by 1 Year for Clinical Trial Patients
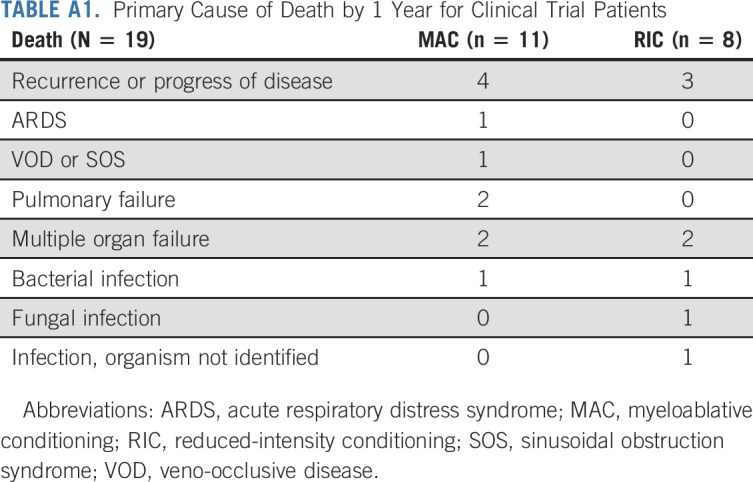
TABLE A2.
Multivariate Analysis of OS Comparing the Clinical Trial Patients to the CIBMTR Comparator Groups
Bronwen E. Shaw
Honoraria: Therakos
Consulting or Advisory Role: Orca Bio
Brent R. Logan
Consulting or Advisory Role: Daiichi Sankyo, Enlivex Therapeutics Ltd, Gamida Cell
Farhad Khimani
Research Funding: Bristol Myers Squibb
Brian C. Shaffer
Consulting or Advisory Role: Hansa Biopharma
Research Funding: Miltenyi Biotec
Nirav N. Shah
Stock and Other Ownership Interests: Exelixis, Geron
Honoraria: Miltenyi Biotec, Loxo/Lilly
Consulting or Advisory Role: Kite, a Gilead company, Celgene, Verastem, Loxo/Lilly, Legend Biotech, TG Therapeutics, Epizyme
Research Funding: Miltenyi Biotec
Travel, Accommodations, Expenses: Miltenyi Biotec
Alisha Mussetter
Research Funding: Magenta Therapeutics Inc
Xiao-Ying Tang
Research Funding: Jazz Pharmaceuticals, OncoImmune, Gamida Cell, Merck, Kyowa Kirin International, Bristol Myers Squibb
John M. McCarty
Honoraria: Kite, a Gilead company, Anthem Wellpoint
Research Funding: Celgene/Bristol Myers Squibb, Celgene, FATE Therapeutics, Seattle Genetics
Nosha Farhadfar
Consulting or Advisory Role: Incyte
Research Funding: CSL Behring
Nancy M. Hardy
Consulting or Advisory Role: Kite, a Gilead company, Gilead Sciences, American Gene Technologies
Research Funding: Incyte, Takeda
Travel, Accommodations, Expenses: Kite/Gilead
Uncompensated Relationships: GPB
Claudio Anasetti
Stock and Other Ownership Interests: Ionis Pharmaceuticals
Honoraria: Gilead Sciences
Consulting or Advisory Role: Gilead Sciences
Miguel-Angel Perales
Stock and Other Ownership Interests: NexImmune
Consulting or Advisory Role: Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed, Medigene, Takeda, Nektar, Abbvie, Cidara Therapeutics, Celgene, Kite/Gilead, Bristol Myers Squibb, Omeros
Research Funding: Incyte, Miltenyi Biotec, Novartis
Krishna V. Komanduri
Honoraria: Takeda, Kadmon, Kite/Gilead, Kiadis Pharma, Novartis, Incyte, Autolus
Consulting or Advisory Role: Kiadis Pharma, Kite/Gilead, Novartis, Takeda, Incyte, Autolus, Kadmon, Genentech/Roche, Iovance Biotherapeutics, Gamida Cell
Expert Testimony: Kite/Gilead
Leo Luznik
Consulting or Advisory Role: Gilead Sciences, Talaris Therapeutics, Precision BioSciences, Rubius Therapeutics, WindMIL Therapeutics
Research Funding: Genentech, Amgen
Patents, Royalties, Other Intellectual Property: Patent holder WindMIL Therapeutics
Uncompensated Relationships: WindMIL Therapeutics
Maxim Norkin
Research Funding: Celgene
Joseph A. Pidala
Consulting or Advisory Role: Syndax, CTI BioPharma Corp, Amgen, Regeneron
Steven M. Devine
Honoraria: Kiadis Pharma
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: Orca Bio, Kiadis Pharma
Travel, Accommodations, Expenses: Orca Bio
Mary M. Horowitz
Consulting or Advisory Role: Magenta Therapeutics, Janssen Research & Development, Medac
Research Funding: Biovitrum, Jazz Pharmaceuticals, Magenta Therapeutics, Novartis, Kite/Gilead, Actinium Pharmaceuticals, Amgen, Amneal Pharmaceuticals, Anthem, Bluebird Bio, Bristol Myers Squibb, Chimerix, CSL Behring, Cyto-Sen Therapeutics, Daiichi Sankyo, Gamida Cell, GlaxoSmithKline, Mesoblast, Miltenyi Biotec, Neovii, Oncoimmune, Pfizer, Pharmacyclics, Regeneron, Sanofi, Seattle Genetics, Shire
Javier Bolaños-Meade
Other Relationship: Incyte
No other potential conflicts of interest were reported.
See accompanying editorial on page 1951
DISCLAIMER
The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration or any other agency of the US Government.
PRIOR PRESENTATION
Presented in part at the Transplantation and Cellular Therapy Meetings, Orlando, FL, February 19-23, 2020; the American Society of Hematology Meetings, San Diego, CA, December 5, 2020; and the National Marrow Donor Program ONE Forum, Minneapolis, MN, November 6, 2020.
SUPPORT
Supported by The National Marrow Donor Program/Be The Match, the Be The Match Foundation, and the Center for International Blood and Marrow Transplant Research (CIBMTR), which is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute, the National Heart, Lung and Blood Institute, and the National Institute of Allergy and Infectious Diseases; HHSH250201700006C and HHSH250201700007C from the Health Resources and Services Administration; and N00014-18-1-2850, N00014-18-1-2888, and N00014-20-1-2705 from the Office of Naval Research. The CIBMTR is also supported by the following commercial entities: AbbVie; Actinium Pharmaceuticals Inc; Adaptive Biotechnologies; Adienne SA; Allovir Inc; Amgen Inc; Angiocrine Bioscience; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics Inc; Bluebird Bio Inc; Bristol Myers Squibb Co; Celgene Corp; CSL Behring; CytoSen Therapeutics Inc; Daiichi Sankyo Co, Ltd; Gamida-Cell, Ltd; Genentech Inc; HistoGenetics Inc; Incyte Corporation; Janssen Biotech Inc; Jazz Pharmaceuticals Inc; Johnson & Johnson; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt LLC; Merck & Company Inc; Merck Sharp & Dohme Corp; Millennium, the Takeda Oncology Co; Miltenyi Biotec Inc; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune Inc; Orca Biosystems Inc; Pfizer Inc; Pharmacyclics LLC; Sanofi Genzyme; Shire; Sobi Inc; Stemcyte; Takeda Pharma; Terumo BCT; Viracor Eurofins; Vor Bio Pharma; Xenikos BV.
CLINICAL TRIAL INFORMATION
B.E.S. and A.M.J-J. contributed equally as first authors.
DATA SHARING STATEMENT
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
AUTHOR CONTRIBUTIONS
Conception and design: Bronwen E. Shaw, Antonio Martin Jimenez-Jimenez, Linda J. Burns, Brent R. Logan, Farhad Khimani, Brian C. Shaffer, John M. McCarty, Hannah Choe, Richard F. Ambinder, Claudio Anasetti, Miguel-Angel Perales, Alan Howard, Krishna V. Komanduri, Maxim Norkin, Joseph A. Pidala, Voravit Ratanatharathorn, Dennis L. Confer, Mary M. Horowitz, Javier Bolaños-Meade
Administrative support: Dennis L. Confer
Provision of study materials or patients: Antonio Martin Jimenez-Jimenez, Brian C. Shaffer, Brian C. Shaffer, Asif Alavi, Katarzyna Jamieson, Miguel-Angel Perales, Alan Howard, Leo Luznik, Javier Bolaños-Meade
Collection and assembly of data: Bronwen E. Shaw, Antonio Martin Jimenez-Jimenez, Farhad Khimani, Brian C. Shaffer, Alisha Mussetter, Xiao-Ying Tang, John M. McCarty, Katarzyna Jamieson, Hannah Choe, Richard F. Ambinder, Maxim Norkin, Dennis L. Confer, Steven M. Devine, Javier Bolaños-Meade
Data analysis and interpretation: Bronwen E. Shaw, Antonio Martin Jimenez-Jimenez, Linda J. Burns, Brent R. Logan, Brian C. Shaffer, Nirav N. Shah, Alisha Mussetter, Xiao-Ying Tang, John M. McCarty, Asif Alavi, Nosha Farhadfar, Nancy M. Hardy, Hannah Choe, Richard F. Ambinder, Claudio Anasetti, Miguel-Angel Perales, Stephen R. Spellman, Alan Howard, Krishna V. Komanduri, Leo Luznik, Maxim Norkin, Joseph A. Pidala, Steven M. Devine, Mary M. Horowitz, Javier Bolaños-Meade
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
National Marrow Donor Program–Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Bronwen E. Shaw
Honoraria: Therakos
Consulting or Advisory Role: Orca Bio
Brent R. Logan
Consulting or Advisory Role: Daiichi Sankyo, Enlivex Therapeutics Ltd, Gamida Cell
Farhad Khimani
Research Funding: Bristol Myers Squibb
Brian C. Shaffer
Consulting or Advisory Role: Hansa Biopharma
Research Funding: Miltenyi Biotec
Nirav N. Shah
Stock and Other Ownership Interests: Exelixis, Geron
Honoraria: Miltenyi Biotec, Loxo/Lilly
Consulting or Advisory Role: Kite, a Gilead company, Celgene, Verastem, Loxo/Lilly, Legend Biotech, TG Therapeutics, Epizyme
Research Funding: Miltenyi Biotec
Travel, Accommodations, Expenses: Miltenyi Biotec
Alisha Mussetter
Research Funding: Magenta Therapeutics Inc
Xiao-Ying Tang
Research Funding: Jazz Pharmaceuticals, OncoImmune, Gamida Cell, Merck, Kyowa Kirin International, Bristol Myers Squibb
John M. McCarty
Honoraria: Kite, a Gilead company, Anthem Wellpoint
Research Funding: Celgene/Bristol Myers Squibb, Celgene, FATE Therapeutics, Seattle Genetics
Nosha Farhadfar
Consulting or Advisory Role: Incyte
Research Funding: CSL Behring
Nancy M. Hardy
Consulting or Advisory Role: Kite, a Gilead company, Gilead Sciences, American Gene Technologies
Research Funding: Incyte, Takeda
Travel, Accommodations, Expenses: Kite/Gilead
Uncompensated Relationships: GPB
Claudio Anasetti
Stock and Other Ownership Interests: Ionis Pharmaceuticals
Honoraria: Gilead Sciences
Consulting or Advisory Role: Gilead Sciences
Miguel-Angel Perales
Stock and Other Ownership Interests: NexImmune
Consulting or Advisory Role: Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed, Medigene, Takeda, Nektar, Abbvie, Cidara Therapeutics, Celgene, Kite/Gilead, Bristol Myers Squibb, Omeros
Research Funding: Incyte, Miltenyi Biotec, Novartis
Krishna V. Komanduri
Honoraria: Takeda, Kadmon, Kite/Gilead, Kiadis Pharma, Novartis, Incyte, Autolus
Consulting or Advisory Role: Kiadis Pharma, Kite/Gilead, Novartis, Takeda, Incyte, Autolus, Kadmon, Genentech/Roche, Iovance Biotherapeutics, Gamida Cell
Expert Testimony: Kite/Gilead
Leo Luznik
Consulting or Advisory Role: Gilead Sciences, Talaris Therapeutics, Precision BioSciences, Rubius Therapeutics, WindMIL Therapeutics
Research Funding: Genentech, Amgen
Patents, Royalties, Other Intellectual Property: Patent holder WindMIL Therapeutics
Uncompensated Relationships: WindMIL Therapeutics
Maxim Norkin
Research Funding: Celgene
Joseph A. Pidala
Consulting or Advisory Role: Syndax, CTI BioPharma Corp, Amgen, Regeneron
Steven M. Devine
Honoraria: Kiadis Pharma
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: Orca Bio, Kiadis Pharma
Travel, Accommodations, Expenses: Orca Bio
Mary M. Horowitz
Consulting or Advisory Role: Magenta Therapeutics, Janssen Research & Development, Medac
Research Funding: Biovitrum, Jazz Pharmaceuticals, Magenta Therapeutics, Novartis, Kite/Gilead, Actinium Pharmaceuticals, Amgen, Amneal Pharmaceuticals, Anthem, Bluebird Bio, Bristol Myers Squibb, Chimerix, CSL Behring, Cyto-Sen Therapeutics, Daiichi Sankyo, Gamida Cell, GlaxoSmithKline, Mesoblast, Miltenyi Biotec, Neovii, Oncoimmune, Pfizer, Pharmacyclics, Regeneron, Sanofi, Seattle Genetics, Shire
Javier Bolaños-Meade
Other Relationship: Incyte
No other potential conflicts of interest were reported.
REFERENCES
- 1.Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors—The ALL-SCT-BFM-2003 trial J Clin Oncol 331265–12742015 [DOI] [PubMed] [Google Scholar]
- 2.Saber W, Opie S, Rizzo JD, et al. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia Blood 1193908–39162012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besse K, Maiers M, Confer D, et al. On modeling human leukocyte antigen-identical sibling match probability for allogeneic hematopoietic cell transplantation: Estimating the need for an unrelated donor source Biol Blood Marrow Transplant 22410–4172016 [DOI] [PubMed] [Google Scholar]
- 4.Gragert L, Eapen M, Williams EP, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry N Engl J Med 371339–3482014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: Guidelines from the NMDP/CIBMTR Blood 134924–9342019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation Blood 1242596–26062014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali R, Ramdial J, Algaze S, et al. The Role of anti-thymocyte globulin or alemtuzumab-based serotherapy in the prophylaxis and management of graft-versus-host disease. Biomedicines. 2017;5:67. doi: 10.3390/biomedicines5040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies Blood 1176963–69702011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayumi H, Umesue M, Nomoto K.Cyclophosphamide-induced immunological tolerance: An overview Immunobiology 195129–1391996 [DOI] [PubMed] [Google Scholar]
- 10.Kanakry CG, Ganguly S, Zahurak M, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013;5:211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wachsmuth LP, Patterson MT, Eckhaus MA, et al. Post-transplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression J Clin Invest 1292357–23732019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase II trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts Blood 118282–2882011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide Biol Blood Marrow Transplant 14641–6502008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasamon YL, Ambinder RF, Fuchs EJ, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide Blood Adv 1288–2922017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment Bone Marrow Transplant 531401–14152018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P.Nonparametric estimation from incomplete observations J Am Stat Assoc 53457–4801958 [Google Scholar]
- 17.Aalen OO, Johansen S.An empirical transition matrix for non-homogeneous Markov chain based on censored observations Scand J Statist 5141–1501978 [Google Scholar]
- 18.Cox D.Regression models and life-tables J R Statist Soc Ser B (Methodological) 34187–2201972 [Google Scholar]
- 19.Scott BL, Pasquini MC, Logan BR, et al. Myeloablative versus reduced-intensity hematopoietic cell transplantation for acute myeloid leukemia and myelodysplastic syndromes J Clin Oncol 351154–11612017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorror ML, Martin PJ, Storb RF, et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality Blood 124287–2952014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton BK, Rybicki L, Sekeres M, et al. Racial differences in allogeneic hematopoietic cell transplantation outcomes among African Americans and Whites Bone Marrow Transplant 50834–8392015 [DOI] [PubMed] [Google Scholar]
- 22.Salas MQ, Law AD, Lam W, et al. Safety and efficacy of haploidentical peripheral blood stem cell transplantation for myeloid malignancies using post-transplantation cyclophosphamide and anti-thymocyte globulin as graft-versus-host disease prophylaxis Clin Hematol Int 1105–1132019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruggeri A, Labopin M, Battipaglia G, et al. Timing of post-transplantation cyclophosphamide administration in haploidentical transplantation: A comparative study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation Biol Blood Marrow Transplant 261915–19222020 [DOI] [PubMed] [Google Scholar]
- 24.Fitzhugh CD, Weitzel RP, Hseih MM, et al. Sirolimus and post transplant Cy synergistically maintain mixed chimerism in a mismatched murine model Bone Marrow Transplant 481335–13412013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmariah H, Kasamon YL, Zahurak M, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non-first-degree related donors Biol Blood Marrow Transplant 241099–11022018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watkins B, Qayed M, Bratrude B, et al. T cell costimulation blockade with abatacept nearly eliminates early severe acute graft versus host disease after HLA-mismatched (7/8 HLA matched) unrelated donor transplant, with a favorable impact on disease-free and overall survival. Blood. 2017;130:212. [Google Scholar]
- 27.Bolaños-Meade J, Fuchs EJ, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease Blood 1204285–42912012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ngwube A, Shah N, Godder K, et al. Abatacept is effective as GVHD prophylaxis in unrelated donor stem cell transplantation for children with severe sickle cell disease Blood Adv 43894–38992020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors N Engl J Med 3671487–14962012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Malki MM, Tsai NC, Palmer J, et al. A phase II trial of post-transplant cyclophosphamide as graft-versus-host disease prophylaxis in HLA-mismatched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2020;26:S188. suppl. [Google Scholar]
- 31.Bensinger WI.Allogeneic transplantation: Peripheral blood vs. bone marrow Curr Opin Oncol 24191–1962012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide J Clin Oncol 353002–30092017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battipaglia G, Labopin M, Kröger N, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation Blood 134892–8992019 [DOI] [PubMed] [Google Scholar]
- 34.Shaw BE.Related haploidentical donors are a better choice than matched unrelated donors: Counterpoint Blood Adv 1401–4062017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States Biol Blood Marrow Transplant 26e177–e1822020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson TM, O'Donnell PV, Fuchs EJ, et al. Haploidentical bone marrow and stem cell transplantation: Experience with post-transplantation cyclophosphamide Semin Hematol 5390–972016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanakry JA, Kasamon YL, Bolaños-Meade J, et al. Absence of post-transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis Biol Blood Marrow Transplant 191514–15172013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majhail NS, Nayyar S, Santibanez ME, et al. Racial disparities in hematopoietic cell transplantation in the United States Bone Marrow Transplant 471385–13902012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pidala J, Kim J, Schell M, et al. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and -DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization Bone Marrow Transplant 48346–3502013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohyuddin GR, Koehn K, Costa L, et al. Enrolment of racial minorities across 15 years of multiple myeloma randomised trials; calling on researchers to become agents of change Lancet Haematol 7e704–e7062020 [DOI] [PubMed] [Google Scholar]
- 41.Williams S, Ayers AA, Hildebrandt MT, et al. Strategies for overcoming disparities for patients with hematologic malignancies and for improving enrollment on clinical trials Oncology (Williston Park) 34216–2232020 [PubMed] [Google Scholar]
- 42.Zou J, Ciurea SO, Kongtim P, et al. Molecular disparity in human leukocyte antigens is associated with outcomes in haploidentical stem cell transplantation Blood Adv 43474–34852020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel MI, Lopez AM, Blackstock W, et al. Cancer disparities and health equity: A policy statement from the American Society of Clinical Oncology J Clin Oncol 383439–34482020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierce LJ.A time to dig deeper and take meaningful action J Clin Oncol 383361–33622020 [DOI] [PubMed] [Google Scholar]
- 45.Unger JM, Hershman DL, Till C, et al. “When offered to participate”: A systematic review and meta-analysis of patient agreement to participate in cancer clinical trials J Natl Cancer Inst 113244–2572021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.BMT CTN 2020 progress report. https://web.emmes.com/study/bmt2/public/Progress%20report/2020%20BMT%20CTN%20Progress%20Report%20Public%20Version.pdf
- 47.Shaw BE, Logan BR, Spellman SR, et al. Development of an unrelated donor selection score predictive of survival after HCT: Donor age matters most Biol Blood Marrow Transplant 241049–10562018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation Transplantation 881019–10242009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou S, Glynn S, Kuritzkes D, et al. Hematopoietic cell transplantation and HIV cure: Where we are and what next? Blood 1223111–31152013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutter G, Schneider T, Thiel E. Transplantation of selected or transgenic blood stem cells—a future treatment for HIV/AIDS? J Int AIDS Soc. 2009;12:10. doi: 10.1186/1758-2652-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartwell MJ, Özbek U, Holler E, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2017;2:e89798. doi: 10.1172/jci.insight.89798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accord with the National Institutes of Health Data Sharing Policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.