Abstract
PURPOSE
To determine the incidence of serious chronic health conditions among survivors of pediatric Hodgkin lymphoma (HL), compare by era of therapy and by selected cancer therapies, and provide estimates of risks associated with contemporary therapy.
METHODS
Assessing 2,996 5-year HL survivors in the Childhood Cancer Survivor Study diagnosed from 1970 to 1999, we examined the cumulative incidence of severe to fatal chronic conditions (grades 3-5) using self-report conditions, medically confirmed subsequent malignant neoplasms, and cause of death based on the National Death Index. We used multivariable regression models to estimate hazard ratios (HRs) per decade and by key treatment exposures.
RESULTS
HL survivors were of a mean age of 35.6 years (range, 12-58 years). The cumulative incidence of any grade 3-5 condition by 35 years of age was 31.4% (95% CI, 29.2 to 33.5). Females were twice as likely (HR, 2.1; 95% CI, 1.8 to 2.4) to have a grade 3-5 condition compared with males. From the 1970s to the 1990s, there was a 20% reduction (HR, 0.8; 95% CI, 0.7 to 0.9) in decade-specific risk of a grade 3-5 condition (P trend = .002). In survivors who had a recurrence and/or hematopoietic cell transplant, the risk of a grade 3-5 condition was substantially elevated, similar to that of survivors treated with high-dose, extended-field radiotherapy (HR, 1.2; 95% CI, 0.9 to 1.5). Compared with survivors treated with chest radiotherapy ≥ 35 Gy in combination with an anthracycline or alkylator, a contemporary regimen for low-intermediate risk HL was estimated to lead to a 40% reduction in risk of a grade 3-5 condition (HR, 0.6; 95% CI, 0.4 to 0.8).
CONCLUSION
This study demonstrates that risk-adapted therapy for pediatric HL has resulted in a significant reduction in serious long-term outcomes.
INTRODUCTION
Over the past 5 decades, pediatric Hodgkin lymphoma (HL) has served as an oncologic model for the maximize cure-minimize cost paradigm. Because of its radiosensitivity, extended-field radiotherapy used in the 1970s produced favorable survival rates in patients with HL. Data from the SEER Program reported an 80.9% 5-year survival rate for children and adolescents in the United States, 0-19 years of age, and diagnosed with HL (all stages) from 1975 through 1977.1 Indeed, although 5-year survival rates now exceed 95%,1 much of the change in therapy in recent years has focused on minimizing risk of long-term and late effects.2–5 Several recent reviews have detailed these changes in therapy.6,7 Briefly, the use of high-dose, wide-field radiotherapy has decreased, concurrent with the introduction and refinement of multimodal therapy.
CONTEXT
Key Objective
Determine whether the risk of serious outcomes have been reduced with changes in Hodgkin lymphoma therapy.
Knowledge Generated
Risk-adapted therapy for pediatric Hodgkin lymphoma appears to have resulted in a lower likelihood of serious late effects. Importantly, females have about twice the likelihood of having a serious chronic condition as males.
Relevance
Findings from this study can help guide upfront therapy and monitoring for late effects, as well as better understand some of the potential long-term tradeoffs of various cancer therapies.
When compared to other cancer groups or noncancer populations, HL survivors, depending on their treatment exposures, have substantially elevated risks for late mortality,8–12 serious morbidity,11,13–16 subsequent malignant neoplasms (SMNs)17–26 cardiovascular disease,27–30 stroke,31,32 pulmonary disease,11 infertility and gonadal dysfunction,33–35 diabetes, and other endocrinopathies.11,36,37 The concept of risk-adapted therapy was introduced in the treatment of HL in the 1990s, with a goal of avoiding unnecessary therapy for individuals with a lower risk of disease progression or recurrence while providing more intense upfront therapy for individuals with high-risk disease (Data Supplement, online only).6,38,39 Although some studies suggest that risk-adapted therapy will be associated with less long-term morbidity, this remains understudied.
In this study that used the Childhood Cancer Survivor Study (CCSS) cohort of HL survivors diagnosed from 1970 to 1999, we aimed to determine the incidence of serious chronic health conditions and compare them by era of therapy and by major treatment groupings. We hypothesized that the risk of a serious chronic condition, including fatal conditions, will be significantly lower for: (1) survivors diagnosed from 1990 to 1999 compared with those diagnosed from 1970 to 1979; (2) survivors treated with hybrid multimodality therapy, such as cyclophosphamide, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, and vinblastine (COPP-ABV) or doxorubicin, bleomycin, vincristine, etoposide, prednisone, and cyclophosphamide (ABVE-PC) with involved-field radiotherapy (IFRT; radiotherapy that targets the tumor and adjacent lymph nodes) compared with those treated with only mantle radiotherapy (that targets most supradiaphragmatic lymph nodes); and (3) survivors treated with multiagent chemotherapy without radiation compared with all other groups. Furthermore, we aimed to estimate the risks of serious chronic health conditions with contemporary HL therapy, based upon exposures from two recent Children's Oncology Group (COG) protocols—AHOD003140 and AHOD0431.41
METHODS
Study Design and Participants
The CCSS is a multi-institutional retrospective cohort study with longitudinal follow-up of survivors of common childhood cancers diagnosed before 21 years of age at one of 31 institutions in the United States and Canada. Survivors were eligible if they were diagnosed with cancer between January 1, 1970, and December 31, 1999, and had survived at least 5 years. For this analysis, we included all HL survivors who completed the baseline questionnaire (Data Supplement). The design and methods of the study have been described previously.42,43 The institutional review boards of participating institutions approved the CCSS protocol, and participants provided informed consent.
Procedures
Information on cancer treatments received within 5 years of initial diagnosis was abstracted from medical records, including chemotherapy cumulative doses and body region-specific radiation dose and field.43 Anthracycline doses were standardized as doxorubicin-equivalent dose,44,45 and alkylating agents were summarized as cyclophosphamide-equivalent dose.46 Information on salvage therapy was established by reports of recurrence and bone marrow transplant from medical records, from self-reports independently verified by additional questionnaires, or review of pathology reports. Treatment decade was categorized as 1970-1979, 1980-1989, or 1990-1999 according to date of cancer diagnosis.
Outcomes
Participants reported age at first occurrence of health outcomes via a series of multi-item, organ system-based questions on the baseline questionnaire, and subsequently on up to four follow-up questionnaires.47 Subsequent neoplasms reported by questionnaire or identified by National Death Index (NDI) searches were confirmed by review of pathology reports and/or medical records. Fatal conditions were ascertained by cause of death information from an NDI search through December 31, 2013. Using a well-established algorithm that incorporates self-reported chronic conditions, medical record confirmed subsequent cancers, and cause of death where applicable, a multi-disciplinary team reviewed and adjudicated all conditions graded and scored according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (v4.03) as previously described.16,48 For this study, we only included severe or disabling (grade 3), life-threatening (grade 4), or fatal (grade 5) chronic health conditions (Data Supplement). Because questionnaires captured age at first occurrence for each condition, multiple occurrences of the same condition were not ascertained, with the exception of subsequent neoplasms. We focused on three common groups of morbidity observed in HL survivors: cardiopulmonary disease, SMNs, and endocrinopathies. For cardiopulmonary disease, we combined cardiovascular and pulmonary outcomes.
Estimating Risk Based Upon Contemporary Therapy
To estimate risk of chronic conditions with contemporary therapy, we created a contemporary-regimen prototype cohort based upon recent COG protocols for low-risk (AHOD0431)41 and intermediate-risk (AHOD0031)40 HL. For this approach, we evaluated all CCSS HL survivors treated with a cumulative doxorubicin dose of 1-249 mg/m2, a cumulative cyclophosphamide dose between 2,000 and 3,900 mg/m2, and any dose of prednisone or vincristine (Data Supplement). Both AHOD0431 and AHOD0031 randomly assigned patients to receive or not receive 21 Gy IFRT. Because the number of HL survivors in the CCSS cohort who did not receive any IFRT was relatively small, for our prototype low-intermediate risk regimen, we grouped those who were treated with ≤ 26.0 Gy IFRT (n = 156) and those with no IFRT (n = 73).
Statistical Analysis
Outcomes assessed included any grade 3-5 condition and conditions specific to organ systems (cardiopulmonary disease, SMN, or endocrinopathy).The cumulative incidence of each type of chronic condition was estimated as a function of age, based on time to first condition, with death from other than the chronic condition type of interest as a competing risk and censoring at the age at last contact for those not experiencing any of the above. Left truncation accounted for staggered age of entry into the cohort at 5 years post diagnosis, and since very few Hodgkin survivors were diagnosed before 10 years of age, curves start at 15 years of age.49 The primary comparisons between cumulative incidence curves for various covariates were based on Wald tests comparing estimates at 30, 35, or 40 years of age, depending on which timepoint allowed sufficient follow-up times for all subgroups.
Cox proportional hazards models estimated hazard ratios (HRs) and 95% CIs for first occurrence of a grade 3-5 condition, using age as the time scale with survivors entering the analysis at 5 years post diagnosis. Models were adjusted for sex and age at diagnosis (0-9, 10-14, 15-20), and censored at 35 or 40 years of age, as appropriate. Race was examined but did not contribute to the models. One set of multivariable models evaluated the impact of treatments with recurrence or autologous or allogeneic hematopoietic cell transplant (HCT) included as time-dependent covariates based on earliest age. Another set of models examined specific treatment combinations to represent changes in protocol over era. Some chronic condition events were identified only by death certificate information, and thus, for these individuals, follow-up was extended to their death date, which is always after their last survey date. To examine the potential bias incurred by this assumption, we also carried out sensitivity analyses examining the impact of censoring these individuals at their last survey and since no marked differences were noted, results presented here use events ascertained from the death certificate. SAS version 9.4 was used for all analyses.
RESULTS
The cohort included 2,996 5-year HL survivors, of which 1,097 (36.6%) were diagnosed from 1970 to 1979, 1,057 (35.3%) from 1980 to 1989, and 842 (28.1%) from 1990 to 1999 (Table 1). Almost half (47.4%) were female, and 17.4% were racial or ethnic minorities. Nearly half (48.0%) were diagnosed between 15 and 20 years of age. Of the 2,996 5-year survivors enrolled in CCSS, 9.9% (296) had either progressive disease or relapse and subsequently had salvage therapy and/or an autologous or allogeneic HCT before entry into CCSS (ie, before 5 years post cancer diagnosis). Twenty-two percent were deceased by the time of the NDI search in 2013 (deaths at mean [standard deviation] age 37.7 [10.8] years) and age at last follow-up at mean (standard deviation) ages of 35.8 (8.1) years among those still alive (Data Supplement; Table 1).
TABLE 1.
Sociodemographics and Treatment Characteristics of 5-Year Survivors of Hodgkin Lymphoma Diagnosed Between 1970 and 1999, by Era
Treatment exposures evolved over the 3-decade period (Fig 1; Table 1). In the latter decades, there was an elimination or reduction of dose and volume of radiation. Concurrently, there was an increased use of intermediate-dose alkylators (ie, cumulative cyclophosphamide-equivalent doses of 5,000-9,999 mg/m2) and intermediate-dose anthracyclines (ie, cumulative doxorubicin-equivalent doses of 100-299 mg/m2).
FIG 1.
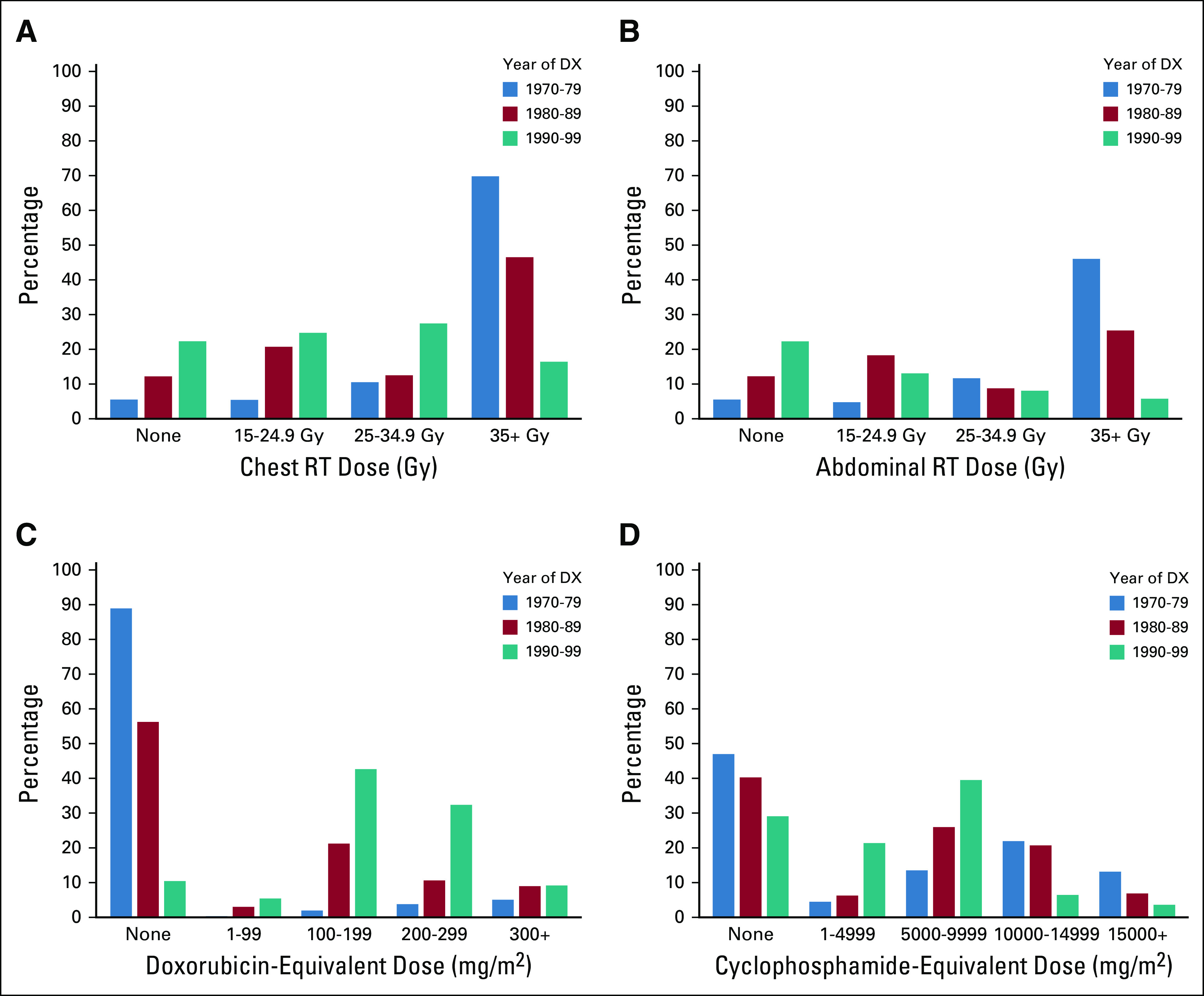
Treatment exposures by era: (A) chest radiotherapy, (B) abdominal radiotherapy, (C) anthracyclines, and (D) alkylating agents. DX, diagnosis; RT, radiotherapy.
By 35 years of age, the cumulative incidence of at least one grade 3-5 chronic condition for the cohort was 31.4% (95% CI, 29.2 to 33.5) (Fig 2). The cumulative incidence by 35 years of age of a grade 3-5 endocrinopathy, cardiopulmonary disease, or SMN were similar: 13.3% (95% CI, 11.7 to 15.1), 10.1% (14.5 to 17.9), and 9.4% (8.1 to 10.8), respectively. Females were twice as likely (HR, 2.1; 95% CI, 1.8 to 2.4) to have a grade 3-5 condition. The cumulative incidence of a grade 3-5 condition by 35 years of age was females 41.3% (95% CI, 37.6 to 44.8) and males 23.1% (95% CI, 20.5 to 25.8). This difference was driven by both SMNs and endocrinopathies (Data Supplement). From the 1970s to 1990s, the decade-specific risk of a grade 3-5 condition declined by 20% (HR, 0.8; 95% CI, 0.7 to 0.9; P trend = .002). This reduction was most noticeable for SMNs, with the cumulative incidence at 35 years of age decreasing from 10.9% (95% CI, 8.9 to 13.1) in the 1970s to 5.7% (95% CI, 3.6 to 8.3) in the 1990s.
FIG 2.

Cumulative incidence of grade 3-5 conditions: (A) overall and by chronic condition, (B) sex, and (C) era of treatment. Endo, endocrinopathy; SMN, subsequent malignant neoplasm.
High-dose (≥ 35 Gy) radiation fields confined to the chest and neck (ie, mantle field), compared with high-dose, extended-field radiotherapy (ie, total lymphoid irradiation), demonstrated a 50% decrease in SMN risk (HR, 0.5; 95% CI, 0.3 to 0.8), but without significant change in other grade 3-5 conditions (Fig 3; Data Supplement). Addition of an alkylator (eg, mechlorethamine, vincristine, procarbazine, prednisone [MOPP] or COPP regimens) or anthracycline (eg, doxorubicin, bleomycin, vinblastine, dacarbazine) to high-dose chest radiation did not statistically increase the risk of grade 3-5 conditions. However, with reduction of radiation doses to < 35 Gy (ie, 15-34 Gy) combined with a hybrid regimen, including both an anthracycline and alkylator (eg, COPP-ABV, ABVE-PC), the risk of grade 3-5 conditions declined by 30% (HR, 0.7; 95% CI, 0.5 to 0.9) compared with survivors treated with high-dose extended-field radiotherapy without chemotherapy (Data Supplement). The most dramatic decrease in risk was observed in survivors treated with a hybrid chemotherapeutic regimen including an anthracycline and an alkylator and no chest radiotherapy (HR, 0.3; 95% CI, 0.2 to 0.4). In survivors who had salvage therapy and/or HCT for a recurrence, the risk of a grade 3-5 condition was 4.7-fold higher (95% CI, 2.8 to 7.8) compared with that of survivors treated with hybrid chemotherapeutic regimen alone (Data Supplement). Moreover, the risk of a grade 3-5 condition in survivors who had a recurrence and/or HCT was similar to that observed for survivors treated with high-dose extended-field radiotherapy (HR, 1.2; 95% CI, 0.9 to 1.5).
FIG 3.
Cumulative incidence of grade 3-5 conditions (any grade 3-5 condition [Any], SMN, CPD, or Endo), by select treatment groups (extended-field radiotherapy [Extended]; Treatment for relapse with or without an autologous or allogeneic hematopoietic cell transplant [Salvage]; chest radiotherapy ≥ 35 Gy without any chemotherapy [chest RT ≥ 35 Gy, no chemo]; chest radiotherapy ≥ 35 Gy with chemotherapy [chest RT ≥ 35 Gy plus chemo]; chest radiotherapy < 35 Gy with chemotherapy [chest RT < 35 Gy plus chemo]; and chemotherapy without chest radiotherapy [chemo without chest RT]). CPD, cardiopulmonary disease; Endo, endocrinopathy; RT, radiotherapy; SMN, subsequent malignant neoplasm.
In multivariable treatment models adjusted for era, sex, and age at diagnosis, there was an elevated risk of cardiopulmonary disease associated with regimens that included an anthracycline (Data Supplement). There were statistically significant reductions for the 1990-1999 treatment era in risk for any chronic condition, SMN, and cardiopulmonary conditions, but not for endocrine outcomes. Inclusion of high-dose alkylating agent chemotherapy (cyclophosphamide-equivalent dose ≥ 10 g/m2) was associated with an increased risk of a grade 3-5 condition, compared with those not treated with an alkylator (HR, 1.3, 95% CI, 1.1 to 1.7). Reduction in mantle field radiation dose to below 35 Gy was associated with a decrease in risk compared with higher doses (HR, 2.3, 95% CI, 1.5 to 3.5 for < 35 Gy and HR, 3.0, 95% CI, 2.0 to 4.6 for ≥ 35 Gy; both v no chest RT; P = .04 for HRs 2.3 v 3.0; Data Supplement).
We estimated the cumulative incidence of grade 3-5 conditions by 30 years of age for the prototype contemporary-regimen for low-intermediate risk HL (Table 2). Because of the smaller number of survivors in this group, estimates beyond 30 years of age were unstable. At 30 years of age, the cumulative incidence of any condition was 14.5% (95% CI, 8.6 to 21.9). Compared to survivors treated with chest RT ≥ 35 Gy, with a cumulative incidence of 25.1% (95% CI, 21.2 to 29.2), the prototype contemporary-regimen was associated with a 40% reduction in risk of a grade 3-5 condition (HR, 0.6; 95% CI, 0.4 to 0.9, not shown in table).
TABLE 2.
Cumulative Incidence (95% CI) by 30 Years of Age for Having At Least One Grade 3-5 Chronic Health Condition (Severe, Disabling, Life-Threatening, or Fatal) by Select Treatment Group
DISCUSSION
Contemporary treatment strategies for HL have served as a paradigm for optimizing cancer therapy based on clinical risk-stratification while minimizing treatment-related morbidity. In this large and diverse cohort of almost 3,000 HL survivors treated in North America over three decades, we demonstrate that risk-adapted therapy has significantly reduced serious long-term outcomes. Furthermore, we have documented that the lowest rates of late effects, including SMNs and cardiopulmonary disease, were achieved when radiation was not included in the treatment plan. In addition, our data suggest that contemporary therapy for low- and intermediate-risk HL may further reduce long-term risk. Finally, our results highlight the disparity in outcomes by sex; females experienced a two-fold higher risk of serious chronic conditions in comparison with males, which was consistent across decades and largely the result of radiation-associated SMNs and endocrinopathies.
Most recent studies of HL survivors have focused on a single outcome category, such as SMNs or cardiovascular disease. Although Bhakta et al50 reported cumulative morbidity burden among pediatric HL survivors, our study uniquely provides an in-depth evaluation of multisystem morbidity with a composite view of outcomes demonstrating the tradeoffs between initial and salvage therapies. By 40 years of age, the cumulative incidence of a serious chronic condition was 44%, with a fairly similar distribution between cardiopulmonary disease, endocrinopathies, and SMNs. Importantly, we observed a 20% reduction in risk of a serious chronic condition with each decade interval from 1970s to 1990s. The most notable reduction in risk was observed if no chest radiation was needed to achieve a cure; there was a 70% reduction in the risk of a serious chronic condition among survivors treated without any chest radiation compared with those treated with extended-field radiation. Although we did not have an adequate number of survivors to estimate risks associated with contemporary COG chemotherapy alone regimens, we anticipate that this risk will be lower compared with that of survivors treated with chest radiation plus a hybrid chemotherapy regimen. Importantly, when planning therapy, reduction in toxicity risk must be balanced with risk of relapse and the substantial increase in risk for serious chronic conditions associated with salvage therapy. Among survivors with progressive disease or recurrence who were treated with salvage therapy, we observed a higher but not statistically significant risk for serious chronic conditions than among those treated with high-dose, extended-field radiation used in the 1970s.
When evaluating the risk of different chemotherapeutic regimens, we observed an increased risk of cardiopulmonary disease with anthracycline-based protocols compared with those treated with a MOPP or COPP protocol. However, with these latter regimens, we observed an increased risk of an endocrinopathy. Maintaining the doxorubicin-equivalent dose below 300 mg/m2 and the cyclophosphamide-equivalent dose below 4,000 mg/m2 substantially reduced long-term morbidity. Our estimates of contemporary low-intermediate risk protocols suggest that the long-term risk will be further reduced. The long-term benefits of contemporary radiation delivery, such as intensity-modulated radiation therapy, involved nodal radiation therapy, or proton beam radiotherapy, could not be determined in this study as these approaches became standard practice only in the past decade.
It is well known that females have a higher risk of second cancers following chest radiation, primarily driven by breast cancer.22,51–53 In this study, we noted that females had a two-fold increased risk compared with males, with risk extending across decades for SMNs and endocrinopathies. Importantly, females also had a cardiopulmonary risk that was similar to that of males, regardless of attained age. It is imperative that clinicians are cognizant of this risk and avoid attributing new symptoms, such as chest pain, to anxiety, musculoskeletal pain, or GI symptoms.
When interpreting the results of this study, it is important to understand its strengths and limitations. The cohort is the most ethnically and geographically diverse population of HL survivors assembled worldwide with detailed treatment information. Notably, CCSS HL survivors are similar in terms of age at diagnosis, sex, race, and time since diagnosis to those reported in SEER, indicating CCSS is representative of the larger US population of childhood HL survivors.54 Regardless, it remains possible that the health profiles of 5-year childhood cancer survivors who participate in CCSS from more recent eras may differ from those in earlier eras because of potential participation bias at cohort entry. Without knowledge about the health of nonparticipants, it is not possible to assess this. SMNs, a key contributor to long-term morbidity, were confirmed by pathology reports or when unavailable by medical records. Although nonfatal chronic conditions were self-reported, we used an extensively applied and conservative algorithm focusing only on more serious outcomes.16,48 Importantly, the prevalence and incidence of chronic conditions that we previously reported for the entire CCSS cohort has been confirmed by studies using medical evaluations or population-based registries with the notable difference that estimates based upon self-report underestimate what is identified by medical evaluations.15,48,55–58 As in any cohort study with longitudinal follow-up, rates of participation are important. Within CCSS, there is some evidence that subjects with longitudinal data for chronic condition outcomes may be somewhat more likely to be ill than those who do not have longitudinal reporting, and thus there is potential for overestimating rates of adverse health outcomes. Next, it is important to recognize that this analysis does not provide a full picture of the tradeoffs between therapeutic regimens, as our cohort only includes five-year survivors. Moreover, when balancing the risk-benefit of different regimens, it is essential to remember that patients who relapse and subsequently require salvage therapy have a substantially elevated risk of serious late effects. Another limitation is that the general population trends in the incidence rates of cancer and cardiovascular disease have not been taken into account. A substantial decline in rates of cancer or cardiovascular disease over the calendar time in the period under study could also contribute to the observed trends. Finally, we are limited in what we can report regarding current therapy, including novel radiotherapy approaches (eg, proton radiotherapy or involved node radiotherapy) or targeted agents (eg, brentuximab vedotin or PD-1 inhibitors) and response-adapted regimens; however, that is the very essence of survivorship research, as it generally takes 15 or more years following diagnosis to establish robust estimates of the long-term complications of therapy.
In conclusion, this study documents and quantifies the clinically significant reductions in the risk of long-term serious health problems with risk-adapted therapy for pediatric HL.
PRIOR PRESENTATION
Presented as an Oral Presentation at the 2018 ASCO Annual Meeting, Chicago, IL, June 1-5, 2018.
SUPPORT
Supported by grants from the National Cancer Institute (U24CA55727, PI: G.T. Armstrong; K05CA160724, PI: K.C. Oeffinger; K07CA134935, PI: T.O. Henderson) and the Meg Berté Owen Foundation. Support to St Jude Children's Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
AUTHOR CONTRIBUTIONS
Conception and design: Kevin C. Oeffinger, Melissa M. Hudson, Wendy M. Leisenring, Suzanne L. Wolden, Gregory T. Armstrong, Leslie L. Robison
Financial support: Gregory T. Armstrong, Leslie L. Robison
Administrative support: Gregory T. Armstrong, Leslie L. Robison
Provision of study materials or patients: Melissa M. Hudson, Suzanne L. Wolden, Louis S. Constine, Lisa R. Diller, Gregory T. Armstrong, Leslie L. Robison
Collection and assembly of data: Kayla L. Stratton, Melissa M. Hudson, Wendy M. Leisenring, Rebecca M. Howell, Susan A. Smith, Gregory T. Armstrong, Leslie L. Robison
Data analysis and interpretation: Kevin C. Oeffinger, Kayla L. Stratton, Melissa M. Hudson, Wendy M. Leisenring, Tara O. Henderson, Suzanne L. Wolden, Louis S. Constine, Lisa R. Diller, Charles A. Sklar, Paul C. Nathan, Sharon M. Castellino, Dana Barnea, Susan A. Smith, Raymond J. Hutchinson, Gregory T. Armstrong, Leslie L. Robison
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Risk-Adapted Therapy for Pediatric Hodgkin Lymphoma on Risk of Long-Term Morbidity: A Report From the Childhood Cancer Survivor Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975 2017. https://seer.cancer.gov/csr/1975_2017/
- 2.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma JAMA 3072609–26162012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jhawar SR, Rivera-Nunez Z, Drachtman R, et al. Association of combined modality therapy vs chemotherapy alone with overall survival in early-stage pediatric Hodgkin lymphoma JAMA Oncol 5689–6952019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marks LJ, Pei Q, Bush R, et al. Outcomes in intermediate-risk pediatric lymphocyte-predominant Hodgkin lymphoma: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2018;65:e27375. doi: 10.1002/pbc.27375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flerlage JE, Metzger ML, Bhakta N.The management of Hodgkin lymphoma in adolescents and young adults: Burden of disease or burden of choice? Blood 132376–3842018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies Pediatr Blood Cancer 58334–3432012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgson DC, Hudson MM, Constine LS.Pediatric Hodgkin lymphoma: Maximizing efficacy and minimizing toxicity Semin Radiat Oncol 17230–2422007 [DOI] [PubMed] [Google Scholar]
- 8.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study J Clin Oncol 193163–31722001 [DOI] [PubMed] [Google Scholar]
- 9.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries J Clin Oncol 193173–31812001 [DOI] [PubMed] [Google Scholar]
- 10.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer JAMA 304172–1792010 [DOI] [PubMed] [Google Scholar]
- 11.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study Blood 1171806–18162011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study J Natl Cancer Inst 1001368–13792008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study J Clin Oncol 321218–12272014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings J Clin Oncol 272339–23552009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer JAMA 2972705–27152007 [DOI] [PubMed] [Google Scholar]
- 16.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer N Engl J Med 3551572–15822006 [DOI] [PubMed] [Google Scholar]
- 17.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study J Natl Cancer Inst 1021083–10952010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma J Clin Oncol 251489–14972007 [DOI] [PubMed] [Google Scholar]
- 19.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence J Clin Oncol 182435–24432000 [DOI] [PubMed] [Google Scholar]
- 20.Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer JAMA 3052311–23192011 [DOI] [PubMed] [Google Scholar]
- 21.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease J Natl Cancer Inst 94182–1922002 [DOI] [PubMed] [Google Scholar]
- 22.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer J Clin Oncol 322217–22232014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma Blood 124319–327quiz 4662014 [DOI] [PubMed] [Google Scholar]
- 24.Henderson TO, Oeffinger KC, Whitton J, et al. Secondary gastrointestinal cancer in childhood cancer survivors: A cohort study Ann Intern Med 156757–7662012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morton LM, Dores GM, Curtis RE, et al. Stomach cancer risk after treatment for Hodgkin lymphoma J Clin Oncol 313369–33772013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniels LA, Krol AD, Schaapveld M, et al. Long-term risk of secondary skin cancers after radiation therapy for Hodgkin's lymphoma Radiother Oncol 109140–1452013 [DOI] [PubMed] [Google Scholar]
- 27.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma Blood 1091878–18862007 [DOI] [PubMed] [Google Scholar]
- 28.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer J Clin Oncol 313673–36802013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin's lymphoma: An analysis from the St Jude Lifetime Cohort Study Lancet Oncol 171325–13342016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Bruin ML, Dorresteijn LD, van't Veer MB, et al. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma J Natl Cancer Inst 101928–9372009 [DOI] [PubMed] [Google Scholar]
- 32.Bowers DC, McNeil DE, Liu Y, et al. Stroke as a late treatment effect of Hodgkin's disease: A report from the Childhood Cancer Survivor Study J Clin Oncol 236508–65152005 [DOI] [PubMed] [Google Scholar]
- 33.Sklar CA, Mertens AC, Mitby P, et al. Premature menopause in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study J Natl Cancer Inst 98890–8962006 [DOI] [PubMed] [Google Scholar]
- 34.Green DM, Kawashima T, Stovall M, et al. Fertility of male survivors of childhood cancer: A report from the Childhood Cancer Survivor Study J Clin Oncol 28332–3392010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study J Clin Oncol 272677–26852009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: A report for the Childhood Cancer Survivor Study Arch Intern Med 1691381–13882009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Nimwegen FA, Schaapveld M, Janus CP, et al. Risk of diabetes mellitus in long-term survivors of Hodgkin lymphoma J Clin Oncol 323257–32632014 [DOI] [PubMed] [Google Scholar]
- 38.Nachman JB, Sposto R, Herzog P, et al. Randomized comparison of low-dose involved-field radiotherapy and no radiotherapy for children with Hodgkin's disease who achieve a complete response to chemotherapy J Clin Oncol 203765–37712002 [DOI] [PubMed] [Google Scholar]
- 39.Hudson MM.Pediatric Hodgkin's therapy: Time for a paradigm shift J Clin Oncol 203755–37572002 [DOI] [PubMed] [Google Scholar]
- 40.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: A report from the Children's Oncology Group Study AHOD0031 J Clin Oncol 323651–36582014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller FG, Castellino SM, Chen L, et al. Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: A report from the Children's Oncology Group Cancer 1243210–32192018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study J Clin Oncol 272319–23272009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research J Clin Oncol 272308–23182009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feijen EA, Leisenring WM, Stratton KL, et al. Equivalence ratio for daunorubicin to doxorubicin in relation to late heart failure in survivors of childhood cancer J Clin Oncol 333774–37802015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feijen EAM, Leisenring WM, Stratton KL, et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity JAMA Oncol 5864–8712019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study Pediatr Blood Cancer 6153–672014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St. Jude Children's Research Hospital: The Childhood Cancer Survivor Study. https://ccss.stjude.org/tools-and-documents/questionnaires.html
- 48.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970-99: A report from the Childhood Cancer Survivor Study cohort Lancet Oncol 191590–16012018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geskus RB.Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring Biometrics 6739–492011 [DOI] [PubMed] [Google Scholar]
- 50.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet 3902569–25822017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease N Engl J Med 334745–7511996 [DOI] [PubMed] [Google Scholar]
- 52.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group J Clin Oncol 214386–43942003 [DOI] [PubMed] [Google Scholar]
- 53.Moskowitz CS, Chou JF, Neglia JP, et al. Mortality after breast cancer among survivors of childhood cancer: A report from the Childhood Cancer Survivor Study J Clin Oncol 372120–21302019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: Prevalence and burden of morbidity Cancer Epidemiol Biomarkers Prev 24653–6632015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oeffinger KC, Robison LL.Childhood cancer survivors, late effects, and a new model for understanding survivorship JAMA 2972762–27642007 [DOI] [PubMed] [Google Scholar]
- 56.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer JAMA 3092371–23812013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Fine Licht S, Winther JF, Gudmundsdottir T, et al. Hospital contacts for endocrine disorders in Adult Life After Childhood Cancer in Scandinavia (ALiCCS): A population-based cohort study Lancet 3831981–19892014 [DOI] [PubMed] [Google Scholar]
- 58.Oeffinger KC, Sklar CA.Childhood cancer, endocrine disorders, and cohort studies Lancet 3831950–19522014 [DOI] [PMC free article] [PubMed] [Google Scholar]





