INTRODUCTION
Increasing insights into the hallmarks of cancer have reshaped the treatment landscape for many solid tumors and have led to the availability of novel and highly effective targeted agents. It is essential that these agents are evaluated across the spectrum of patients with cancer such that as many as possible can benefit. This is particularly relevant to the care of older adults. Almost 60% of patients are ≥ 70 years of age at the time of diagnosis of cancer, yet older adults remain under-represented in clinical trials.1 Older patients are also a highly heterogeneous population, and the older patients who are recruited to clinical trials may not be representative of the general population of older patients with cancer. This problem is compounded by failure to include patient-reported outcomes and adequately describe comorbidities, functional status, and frailty in many final study reports. These factors might lead clinicians to overestimate or underestimate both the efficacy and toxicity of novel targeted agents in the older age group.2
CONTEXT
Key Objective
Are targeted agents safe and effective systemic treatment options for older adults with solid tumors?
Knowledge Generated
Older individuals represent a significant proportion of patients with cancer. However, evidence regarding the efficacy and safety of most monoclonal antibodies, antibody-drug conjugates, and small molecules are very limited in the older population. These evidence gaps may lead to incorrect assumptions regarding safety, side effects, and efficacy. This may mean that potentially beneficial treatments are withheld owing to concerns regarding lack of supportive evidence. Conversely, older patients may be exposed to undue risk of side effects owing to underestimates of toxicity.
Relevance
This review highlights data gaps. It is critical for the oncology community that investigators and regulatory authorities are aware of these issues, such that they can be addressed for the benefit of older patients with cancer.
This review aims to explore the efficacy and safety of three common groups of targeted agents: monoclonal antibodies (MoAb), antibody-drug conjugates (ADC), and small molecules in the management of common solid tumors in older adults. We prioritized registration or phase III and age-specific trial data, where available, to provide a broad overview of the current state of evidence and to identify gaps in knowledge.
MoAb
Several MoAbs designed to bind specific receptors and additional epitopes have demonstrated efficacy and safety in the management of a number of malignancies in both the curative and palliative setting. However, they have very different safety profiles and data in older adults are still limited, particularly for the most recently developed agents (Data Supplement, online only).
Antihuman Epidermal Growth Factor Receptor 2 MoAb
MoAb targeting the human epidermal growth factor receptor 2 (HER2) are standard of care for the management of both early and advanced HER2-positive breast cancer (BC).
Trastuzumab is a MoAb targeting the extracellular domain of HER2, improves survival and risk of recurrence compared with chemotherapy alone, and is well tolerated in older patients.3 The existing data suggest that older patients derive similar benefit from adjuvant trastuzumab as younger patients, albeit with a 5% rate of cardiotoxicity. However, trastuzumab-induced cardiotoxicity is usually reversible, and most older patients are able to complete one year of trastuzumab.4 Irrespective, shorter courses of adjuvant trastuzumab may also be considered to decrease cardiac risk without necessarily compromising outcomes.5
Pertuzumab is a humanized monoclonal antibody that binds to the extracellular component of HER2, preventing it from dimerizing to other HER receptors. Few older patients were recruited to the pivotal phase III studies where pertuzumab was added to trastuzumab and chemotherapy in the adjuvant and metastatic settings; 13% and 16% patients were ≥ 65 years of age, respectively.6,7 Nonetheless, pertuzumab adds limited additional toxicity and can safely be combined with chemotherapy or trastuzumab in fit older patients. The importance of combining chemotherapy with pertuzumab and trastuzumab in the palliative setting was demonstrated by the European Organisation for Research and Treatment of Cancer 75111-10114 trial in women ≥ 70 years or ≥ 60 years of age with vulnerabilities.8 This study showed superior median progression-free survival (PFS) when metronomic cyclophosphamide was added to pertuzumab or trastuzumab compared with dual antibodies alone.
Antiepidermal Growth Factor Receptor MoAb
Antiepidermal growth factor receptor (EGFR) therapies inhibit the EGFR pathway, which regulates growth, survival, and proliferation in some cancers. EGFR inhibition is used frequently in lung, colorectal, and head or neck cancers. Monoclonal antibodies bind to the extracellular domain of EGFR preventing ligand binding.
Use of MoAb targeting EGFR, such as cetuximab and panitumumab, is common in colorectal cancers that are wild-type for KRAS and NRAS mutations. These agents are most often used in addition to systemic chemotherapy, but also have activity as single agent. Although no large phase III studies have specifically examined the use of MoAb in older adults, post hoc subgroup analyses of several large phase III studies have shown similar efficacy between young and old patients.9 In a German cohort study of more than 300 older adults with colorectal cancer, treatment with cetuximab combined with chemotherapy had similar objective response rate (ORR), median PFS and overall survival (OS), and incidence of rash between older and younger patients.10 Some small studies have also explored the use of anti-EGFR monotherapy in frail populations deemed unfit for chemotherapy (based on performance status [PS] or physician's judgment) with modest efficacy results in responses, median PFS and OS, but good tolerability including rash in 15.2%-16.7% of patients.11,12 The most often encountered toxicity is an acneiform skin rash that occurs in majority of patients (65%-90%) with around 20% having a more severe form; however, there does not appear to be any difference in the occurrence of skin toxicities by age.13 As skin rash is a common toxicity for all agents targeting the EGFR pathway, it can be useful to pre-emptively treat with moisturizers, doxycycline, and/or topical hydrocortisone to reduce the severity and occurrence of the rash.14
Antiangiogenesis
Angiogenesis via growth factors such as vascular endothelial growth factor (VEGF) supports the growth of many solid tumors.15 Therefore, antiangiogenic agents deprive tumors of the excessive vessels needed for growth. Several VEGF targeting MoAb are used in the clinic, including bevacizumab, aflibercept, and ramucirumab. Of the agents that directly target VEGF, bevacizumab was the first to be approved by the US Food and Drug Administration and thus has the most accumulated efficacy and safety data as well as most data in older adult populations.16 Anti-VEGF agents share common class-related side effects, including hypertension, thromboembolic events (such as myocardial infarction and cerebrovascular events), and wound-healing complications.16 Given concomitant comorbid conditions are often present in older adults with cancer, these agents are often used with caution in those with pre-existing refractory hypertension or a recent history of myocardial infarction or stroke.
A recent meta-analysis of 1,652 older patients with metastatic colorectal cancer from 10 studies examined the clinical benefit of the addition of bevacizumab with fluorouracil and found statistically significant benefits in OS and PFS with the addition of bevacizumab, whereas there was no significant benefit found with the addition of either oxaliplatin or irinotecan.17 More specifically, two prospective studies have demonstrated the additional benefit of bevacizumab therapy in older adults with cancer. The Avastin in the Elderly With Xeloda (AVEX) study randomly assigned 280 patients 70 years of age and older deemed ineligible for doublet chemotherapy (as per investigator's judgment). Participants were randomly assigned to capecitabine twice daily with or without the addition of bevacizumab. PFS was significantly longer in the bevacizumab arm, and the combination was overall deemed well tolerated.18 Similarly, the randomized phase II study PRODIGE 20 evaluated the benefit of bevacizumab in combination with chemotherapy (fluorouracil monotherapy or physician choice) in adults 75 years of age or older. PFS was improved in favor of the bevacizumab arm with no discernible worsening in health-related quality of life (HRQoL) metrics.19 Furthermore, the investigators found that baseline impairment in instrumental activities of daily living was the strongest predictor of both efficacy and tolerability in this population.20
ADC
ADCs allow for more targeted and efficient drug delivery, which may be particularly valuable to spare older patients the additional toxicities associated with the use of cytotoxics.
Trastuzumab emtansine (T-DM1) comprises trastuzumab attached to a microtubule inhibitor payload DM1 through a noncleavable linker drug. In the KATHERINE study, adjuvant T-DM1 approximately halved the risk of recurrence for patients with residual HER2-positive BC at surgery after neoadjuvant therapy, compared with trastuzumab alone.21 Nonetheless, < 10% of women enrolled in this study were ≥ 65 years old, and T-DM1 was associated with higher rates of thrombocytopenia, peripheral neuropathy, fatigue, and treatment discontinuations. Therefore, adjuvant T-DM1 may be considered for fit, older patients along with careful monitoring of toxicities. TDM-1 has been extensively studied in the advanced disease setting, where it has been found to be superior to lapatinib and capecitabine and the treatment of physicians' choice.8,22 Although older patients were under-represented in its registration studies, a higher incidence of grade ≥ 3 adverse events and discontinuations on T-DM1 in older versus younger patients was reported in a phase 3b safety study.23 Conversely, its cardiotoxicity profile appears favorable compared with trastuzumab (1%-2.7% in the registration and in the safety trials).
There are no specific data regarding newer ADCs in older patients (Data Supplement). However, the rates of pneumonitis, diarrhea, and neutropenia observed with novel agents such as trastuzumab deruxtecan or sacituzumab govitecan require careful monitoring, especially in older patients.
SMALL MOLECULES
Small molecules are effective inhibitors of a broad range of intracellular and extracellular proteins and are established standards of care for many malignancies. However, they have a heterogeneous safety profile that is not universally defined in older adults (Data Supplement).
HER2 Inhibitors
Lapatinib is an oral reversible dual tyrosine kinase inhibitor (TKI) targeting the tyrosine-kinase domains of both HER1 and HER2. It can be used in combination with other drugs to overcome de novo or acquired resistance to first-line anti-HER2 agents for advanced HER2-positive BC. The combination of lapatinib and capecitabine was evaluated in patients ≥ 65 years of age in two retrospective analyses that showed that the combination was effective and tolerable.24,25 More recently, lapatinib plus trastuzumab was assessed in 40 patients with advanced BC and a median age of 72 years and showed activity, although toxicity management remains a concern.26 Although cardiotoxicity is rare and not influenced by age,27 a pooled analysis of nine studies including 13% of older women demonstrated higher rates of grade 3 diarrhea (33% v 19%) of longer duration in older versus younger women.28 Given the overlapping toxicities with capecitabine, the combination of lapatinib plus endocrine therapy is attractive in older patients with hormone receptor-positive, HER2-positive BC. The EGF30008 trial population included 44% of older patients and demonstrated that lapatinib plus letrozole was a tolerable regimen29 and more effective than letrozole alone.
Neratinib is an oral irreversible pan-HER (HER1, 2, and 4) TKI. Although data specific to older individuals are lacking, neratinib was evaluated in combination with various chemotherapy agents (vinorelbine, capecitabine, and paclitaxel) in phase II-III trials enrolling only 5%-18% of patients ≥ 65 years of age and documenting high rates of clinically significant diarrhea and worse HRQoL.30-33 The NALA study recruited more than 20% of individuals ≥ 65 years of age and showed grade ≥ 3 diarrhea in 24% of patients,34 which may be a concern for the older age group in view of the risk of dehydration. Similarly, in the adjuvant setting, the ExteNET trial documented grade ≥ 3 diarrhea in 40% of patients in the overall population (including only 12% of older individuals) and higher rates of discontinuation above the age of 65 years (45%).35,36
New-generation TKIs have greater affinity for the kinase domain and/or a broader spectrum of targets. Tucatinib selectively inhibits HER2 but not HER1. The HER2CLIMB study recruited 18.9% of patients ≥ 65 years of age and demonstrated improved PFS and OS in those randomly assigned to tucatinib with capecitabine and trastuzumab compared with capecitabine or trastuzumab alone.37 Grade ≥ 3 diarrhea occurred in 12.9% of patients receiving the combination.
EGFR TKIs
EGFR TKIs are the first-line treatment for EGFR-mutated non–small-cell lung cancer (NSCLC). First- and second-generation EGFR TKIs such as gefitinib, erlotinib, and afatinib resulted in higher ORR (56%-83% v 23%-47%), prolonged PFS, and better HRQoL compared with chemotherapy.38 In all these phase III trials, median age was approximately 60 years, but a meta-analysis demonstrated that the PFS improvement did not differ by age or Eastern Cooperative Oncology Group (ECOG) PS.39 Four prospective studies40-43 and four retrospective studies44-47 in older patients demonstrated comparable results to these phase III trials and one study also showed a significant HRQoL improvement. Although toxicity is comparable for all EGFR TKIs, afatinib is associated with higher frequency and higher grades. In comparison with gefitinib or erlotinib, osimertinib, a third-generation EGFR TKI, showed prolonged PFS and OS as well as a more favorable toxicity profile and better HRQoL.48 Subgroup analysis demonstrated similar PFS and OS benefit for patients younger and older than 65 years. The favorable toxicity profile was also observed in second-line trials specific in older patients, although prolonged QT (2.8%), left ventricular ejection fraction decrease (2.8%), and pneumonitis (11%) should be closely monitored.49,50
Anaplastic Lymphoma Kinase Inhibitors
Anaplastic lymphoma kinase (ALK) TKIs are the first-line treatment for NSCLC with ALK rearrangements. Patients with this type of NSCLC tend to be younger with a median age in phase III trials ranging from 51 to 61 years.51-54 As a consequence, there are limited data with ALK TKIs in older patients. The first generation ALK TKI crizotinib demonstrated an improved ORR and PFS when compared with first-line chemotherapy.51 The PFS benefit was comparable in younger (< 65 years) and older (≥ 65 years) patients. More recently, alectinib, brigatinib and lorlatinib showed longer PFS when compared with crizotinib, again with similar benefits in patients < 65 years and ≥ 65 years.52-54 The toxicity profile differs for the different ALK TKIs and may guide the preferred treatment of choice. Importantly, lorlatinib has been associated with peripheral neuropathy as well as cognitive effects such as memory impairment, disturbance in attention, confusion, amnesia, and delirium, which may be of importance in older patients.54 In a prospective phase II study in patients with ALK rearranged NSCLC and poor ECOG PS, median age was 72 years (range, 35-84 years) and treatment with an ALK TKI resulted in ORR 72.2%, a median PFS of 10.1 months, as well as an improvement of ECOG PS in 83.3%.55 In a retrospective analysis of patients treated outside of clinical trials with crizotinib, ceritinib, or alectinib, age did not affect PFS, but older patients (≥ 65 years) were more likely to develop toxicity, especially with regards to diarrhea, nausea, creatinine elevation, and fluid retention.56 In this study, alectinib was the only drug that was not associated with high-grade toxicity or drug discontinuation because of toxicity.
Mesenchymal-Epithelial Transition Factor Inhibitors
In patients with mesenchymal-epithelial transition factor (MET) exon 14 skipping mutated NSCLC, the MET TKIs tepotinib and capmatinib demonstrated promising response rates (33.3%-72%) and PFS in first and further line.57,58 Patients with such a mutation are in general older with median age in both studies ranging from 71 to 74 years. ORR in older patients was equally promising in both studies. Peripheral edema is one of the most frequent toxicities of both MET TKIs and may affect mobility and functional status in older patients.
BRAF/MEK Inhibitors
BRAF/MEK inhibitors in combination have been the mainstay of treatment in melanoma patients with BRAF mutations both in the adjuvant and metastatic settings. The role of dabrafenib and trametinib has been investigated in the adjuvant setting in patients with stage III malignant melanoma with BRAF v600 mutations. In this study, 158/712 (22.2%) of the patients were ≥ 65 years and these patients experienced a similar improvement in relapse-free survival with BRAF/MEK inhibition.59
In metastatic melanoma, the BRAF/MEK combination therapy has been shown to be superior to single-agent inhibitors in several phase III trials.60-62 In these pivotal studies, between 24% and 28% of patients recruited were 65 years of age and older and were found to have similar benefits with BRAF/MEK inhibitors as the younger patients. Unfortunately, none of these trials had analyzed the occurrence of adverse events based on age.
In general, there were no phase III randomized controlled trials involving BRAF/MEK inhibitors that only recruited older adults. However, most recent studies have subgroup analysis specific to older adults that provide sufficient evidence on efficacy to support the use of these drugs in these patients.
Multitarget Tyrosine Kinase Inhibitors
Multitargeted TKIs (mTKIs) are the mainstay of treatment for many different tumors in the adjuvant and palliative setting. In older adults with metastatic renal cell carcinoma (mRCC), a few mTKIs have shown efficacy in first and subsequent lines of treatment based on a systematic review.63 Sorafenib was the first mTKI approved in mRCC. A subgroup analysis of a phase III trial found similar ORR and PFS in older and younger patients.64 In terms of safety, older patients on sorafenib were more likely to report GI toxicity and fatigue when compared with their younger counterparts.64 Subsequently, a series of other mTKIs, such as sunitinib, pazopanib, axitinib, cabozantinib, and lenvatinib, have been approved for mRCC, with all subgroup analyses of the pivotal phase III studies showing that older adults derive similar survival benefits as younger adults. A pooled analysis of six trials showed higher rates of toxicities in older adults compared with their younger counterparts despite comparable median PFS and OS outcomes.65 Higher rates of toxicity with increasing age are also confirmed in a recent retrospective analysis of patients treated with standard first-line treatment options including TKIs.66
In advanced hepatocellular carcinoma, a number of mTKIs (sorafenib, cabozantinib, regorafenib, and lenvatinib) have been approved based on phase III randomized controlled trials.67-70 Most of the studies (except for sorafenib) have shown subgroup analyses for efficacy, favoring their use in patients ≥ 65 years. In GI stromal tumors, sunitinib, imatinib, and regorafenib had similar efficacy in older adults recruited in the phase III studies.71-73 In the adjuvant GI stromal tumor setting, imatinib was also shown to be beneficial for older adults in terms of recurrence-free survival.74 Regorafenib had benefited patients ≥ 65 years of age with metastatic colorectal cancer based on subgroup analysis in the pivotal phase III study.75 Cabozantinib and lenvatinib have been shown to be effective in prolonging survival in adults ≥ 65 years of age with metastatic thyroid cancers.76,77
Cyclin-Dependent Kinase4/6 Inhibitors
Circumventing programs regulating cell proliferation governed by cyclin-dependent kinase (CDK) is a hallmark of cancer. CDK 4/6 inhibitors have recently transformed the treatment landscape of BC.78
Their efficacy does not change based on age. In a pooled analysis of the PALOMA studies of palbociclib, older patients represented 41.3% and 24.8% of those treated with an aromatase inhibitor (AI) and with fulvestrant, respectively.79 The PFS benefit was maintained in older patients, as were HRQoL outcomes, although myelosuppression was more frequent above the age of 75 years. A subgroup analysis of the MONALEESA-2 study of ribociclib (with 44.1% of patients ≥ 65 years of age) confirmed similar PFS benefits regardless of age and higher rates of grade 1-2 anemia and fatigue in the older cohort.80 Similarly, a pooled analysis of the MONARCH 2 and 3 trials of abemaciclib included 40.2% of individuals ≥ 65 years of age81: the study showed consistent PFS benefit across age groups along with higher rates of clinically relevant grade 2-3 diarrhea, grade 2-3 nausea, and any grade fatigue in older versus younger patients. A pooled analysis of three randomized controlled studies of first-line CDK4/6 inhibitors plus an AI documented a similar PFS benefit regardless of age but also higher rates of toxicity and dose modifications above the age of 75 years.82
CDK4/6 inhibitors are a suitable option for older patients.83 However, more research is warranted to elucidate their efficacy, safety, and impact on HRQoL in less selected populations and based on fitness. In selected cases, pursuing endocrine therapy alone might still be appropriate. The APPALACHES study (ClinicalTrials.gov identifier: NCT03609047) is investigating the role of palbociclib as an alternative to chemotherapy for older patients with early BC in the adjuvant setting.
Mammalian Target of Rapamycin Inhibitors
Everolimus is an inhibitor of mammalian target of rapamycin and is standard of care in combination with exemestane for patients with advanced ER-positive, HER2-negative BC progressing on AIs based on the BOLERO2 study findings.84 A separate study analysis documented similar efficacy of everolimus in older (> 70 years) versus younger individuals.85 However, higher rates of toxicity were seen in the older age group, including decreased appetite, dyspnea, anemia, fatigue, increased creatinine, and urinary tract infections. Grade ≥ 3 toxicities and discontinuations were also more frequent in those ≥ 70 years of age and importantly higher rates of treatment-related deaths were observed in older patients receiving everolimus versus those on placebo (7.7% v 0.0%) with no differences in the younger age group.
A pooled analysis confirmed higher rates of treatment discontinuations in patients ≥ 70 years of age on everolimus or exemestane.86 Finally, 26% of patients enrolled in the expanded-access BALLET trial were ≥ 70 years of age: similarly, the study reported higher rates of treatment discontinuations (23.8% v 13.0%) and dose reductions or interruptions (60.5% v 54.2%) in this age group.87 Therefore, everolimus should be used with caution in older adults in the context of its toxicity profile.
Phosphoinositide 3-Kinase Inhibitors
Alpelisib is an alpha isoform-specific phosphoinositide 3-kinase inhibitor approved in combination with fulvestrant for the treatment of ER-positive, HER2-negative advanced BC based on the SOLAR-1 study results.88 The median age of patients recruited was 63 years (range, 25-92 years). The trial did not report age-specific data. However, the higher prevalence of diabetes and renal disease in older patients requires careful consideration in the context of the higher rates of hyperglycemia (36.6% v 0.7%), diarrhea (57.7% v 6.7%), and discontinuations because of adverse events (25.0% v 4.2%) with the combination versus fulvestrant or placebo.
Poly ADP-Ribose Polymerase Inhibitors
Poly ADP-ribose polymerase (PARP) inhibitors have become standard of care for selected patients with ovarian cancer (OC) or BC.
A phase II trial of maintenance olaparib versus placebo in patients with advanced OC and a breast cancer type 1 susceptibility protein (BRCA1) or breast cancer type 2 susceptibility protein (BRCA2) mutation in remission following platinum-based chemotherapy did not show PFS benefit in those ≥ 65 years of age, although this might be because of the small proportion of older individuals enrolled.89,90 A pooled analysis of 78 older individuals enrolled in eight phase I-II trials of olaparib documented no differences in its safety in those older versus younger than 65 years.91 However, this analysis included a very selected and fit population with only a minority of patients ≥ 75 years of age. An observational study of olaparib maintenance in 20 women ≥ 65 years of age treated in Denmark showed grade ≥ 3 toxicities in 30% of patients with a median PFS of 6 months.92
The phase III NOVA trial of maintenance niraparib versus placebo for platinum-sensitive advanced OC enrolled more than one third of patients ≥ 65 years of age and confirmed PFS benefit also in the older age group.93 The study documented no impact on HRQoL. A subgroup analysis of patients ≥ 70 years of age confirmed PFS benefit regardless of BRCA status and similar safety compared with their younger counterparts.94
Maintenance rucaparib was compared with placebo in patients with advanced OC in remission after ≥ 2 lines of platinum-based chemotherapy in the phase III ARIEL study.95 Only 38% of enrolled patients were ≥ 65 years of age. A PFS benefit was documented in those 65-74 years of age.96
The registration trials of olaparib and talazoparib recruited a young BC population (median age, 45 and 44 years, respectively) and did not report age-specific analyses.97,98 Nevertheless, both studies demonstrated consistent HRQoL benefit in patients receiving PARP inhibitors versus treatment of physician's choice.99,100 Although the risk of nausea or vomiting and drug interactions should be carefully considered, this aspect may make PARP inhibitors an attractive option (compared with chemotherapy) for the management of BC in older BRCA carriers.
SUMMARY
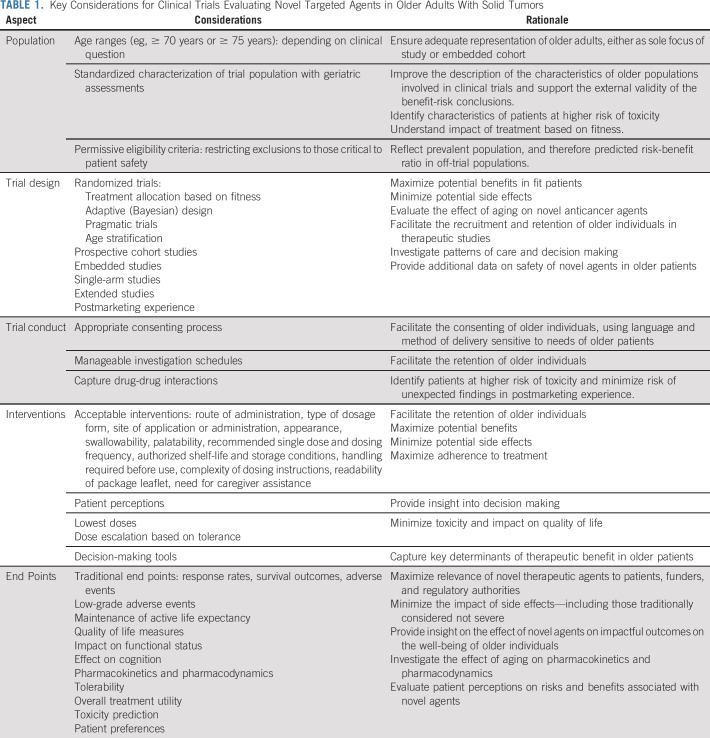
Over the past decade, significant strides have been made with the incorporation of novel targeted therapies into everyday clinical practice. A central tenet of evidence-based medicine is that an intervention should be ○proven in the population where it is to be applied. Despite the wealth of clinical trial data, there is persistent imbalance between the clinical trials of novel agents that recruited younger patient populations compared with the prevalent cancer populations. Where evidence exists, it is suggestive that fit older patients enrolled in clinical trials derive just as much benefit from targeted therapies as younger patients (as biologically one might expect). However, relatively little is known about efficacy in older patients who are frail and with comorbidities. Moreover, the impact of toxicities in all older patients is poorly described. This is an important evidence gap as it may mean either exposure to older patients to risk of excessive toxicity, but also (perhaps unwarranted) withholding of advantageous therapies because of lack of data. This is recognized by regulatory authorities in Europe101 and the United States.102 In Table 1, we propose a suggested framework for the evaluation of novel targeted agents in older patients. It is incumbent upon the cancer community to address these gaps in knowledge to expand the evidence base such that older patients have the opportunity to benefit from the targeted approach of modern cancer therapy.
TABLE 1.
Key Considerations for Clinical Trials Evaluating Novel Targeted Agents in Older Adults With Solid Tumors
ACKNOWLEDGMENT
N.M.L.B. and A.R. would like to acknowledge the support of the Cridlan Ross Smith Charitable Trust and the NIHR Biomedical Research Center at The Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London.
SUPPORT
G.W. is supported in part by the National Cancer Institute of the National Institutes of Health (K08CA234225).
AUTHOR CONTRIBUTIONS
Conception and design: Nicolò Matteo Luca Battisti, Lore Decoster, Grant R. Williams, Alistair Ring
Collection and assembly of data: Nicolò Matteo Luca Battisti, Grant R. Williams, Ravindran Kanesvaran, Hans Wildiers, Alistair Ring
Data analysis and interpretation: Nicolò Matteo Luca Battisti, Lore Decoster, Grant R. Williams, Ravindran Kanesvaran, Hans Wildiers, Alistair Ring
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Targeted Therapies in Older Adults With Solid Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Scher KS, Hurria A.Under-representation of older adults in cancer registration trials: Known problem, little progress J Clin Oncol 302036–20382012 [DOI] [PubMed] [Google Scholar]
- 2.Wildiers H, de Glas NA.Anticancer drugs are not well tolerated in all older patients with cancer Lancet Healthy Longevity 1e43–e72020 [DOI] [PubMed] [Google Scholar]
- 3.Brollo J, Curigliano G, Disalvatore D, et al. Adjuvant trastuzumab in elderly with HER-2 positive breast cancer: A systematic review of randomized controlled trials Cancer Treat Rev 3944–502013 [DOI] [PubMed] [Google Scholar]
- 4.Vaz-Luis I, Keating NL, Lin NU, et al. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: A population-based study J Clin Oncol 32927–9342014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial Lancet 3932599–26122019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer N Engl J Med 366109–1192012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer N Engl J Med 377122–1312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wildiers H, Tryfonidis K, Dal Lago L, et al. Pertuzumab and trastuzumab with or without metronomic chemotherapy for older patients with HER2-positive metastatic breast cancer (EORTC 75111-10114): An open-label, randomised, phase 2 trial from the Elderly Task Force/Breast Cancer Group Lancet Oncol 19323–3362018 [DOI] [PubMed] [Google Scholar]
- 9.Gilabert M, Ries P, Chanez B, et al. Place of anti-EGFR therapy in older patients with metastatic colorectal cancer in 2020 J Geriatr Oncol 111229–12362020 [DOI] [PubMed] [Google Scholar]
- 10.Jehn CF, Böning L, Kröning H, et al. Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer Br J Cancer 106274–2782012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sastre J, Massuti B, Pulido G, et al. First-line single-agent panitumumab in frail elderly patients with wild-type KRAS metastatic colorectal cancer and poor prognostic factors: A phase II study of the Spanish Cooperative Group for the Treatment of Digestive Tumours Eur J Cancer 511371–13802015 [DOI] [PubMed] [Google Scholar]
- 12.Terazawa T, Kato T, Goto M, et al. Phase II study of panitumumab monotherapy in chemotherapy-naive frail or elderly patients with unresectable RAS wild-type colorectal cancer: OGSG 1602 Oncologist 2617–e472021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabbrocini G, Panariello L, Caro G, et al. Acneiform rash induced by EGFR inhibitors: Review of the literature and new insights Skin Appendage Disord 131–372015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacouture ME, Anadkat MJ, Bensadoun RJ, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities Support Care Cancer 191079–10952011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellou S, Pentheroudakis G, Murphy C, et al. Anti-angiogenesis in cancer therapy: Hercules and hydra Cancer Lett 338219–2282013 [DOI] [PubMed] [Google Scholar]
- 16.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. 2012;2:a006577. doi: 10.1101/cshperspect.a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landre T, Maillard E, Taleb C, et al. Impact of the addition of bevacizumab, oxaliplatin, or irinotecan to fluoropyrimidin in the first-line treatment of metastatic colorectal cancer in elderly patients Int J Colorectal Dis 331125–11302018 [DOI] [PubMed] [Google Scholar]
- 18.Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial Lancet Oncol 141077–10852013 [DOI] [PubMed] [Google Scholar]
- 19.Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: A randomized phase II trial-PRODIGE 20 study results. Ann Oncol. 2018;29:2270. doi: 10.1093/annonc/mdx808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aparicio T, Bouche O, Francois E, et al. Geriatric analysis from PRODIGE 20 randomized phase II trial evaluating bevacizumab + chemotherapy versus chemotherapy alone in older patients with untreated metastatic colorectal cancer Eur J Cancer 9716–242018 [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer N Engl J Med 380617–6282019 [DOI] [PubMed] [Google Scholar]
- 22.Krop IE, Kim S-B, Martin AG, et al. Trastuzumab emtansine versus treatment of physician's choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): Final overall survival results from a randomised open-label phase 3 trial Lancet Oncol 18743–7542017 [DOI] [PubMed] [Google Scholar]
- 23.Montemurro F, Ellis P, Anton A, et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: Primary results from the KAMILLA study cohort 1 Eur J Cancer 10992–1022019 [DOI] [PubMed] [Google Scholar]
- 24.Cetin B, Benekli M, Dane F, et al. Lapatinib plus capecitabine for HER2-positive advanced-stage breast cancer in elderly women: Review of the anatolian society of medical oncology (ASMO) experience Breast Care (Basel) 867–702013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crivellari D, Spazzapan S, Lombardi D, et al. Lapatinib-based therapy in heavily pretreated HER2-positive metastatic breast cancer: A single institution experience Tumori 9833–382012 [DOI] [PubMed] [Google Scholar]
- 26.O'Connor T, Soto-Perez-de-Celis E, Blanchard S, et al. Abstract P5-21-08: Tolerability of the combination of lapatinib and trastuzumab in older patients with HER2 positive metastatic breast cancer. Cancer Res. 2018;78(4 suppl):P5-21-08-P5-21-08. [Google Scholar]
- 27.Choi HD, Chang MJ.Cardiac toxicities of lapatinib in patients with breast cancer and other HER2-positive cancers: A meta-analysis Breast Cancer Res Treat 166927–9362017 [DOI] [PubMed] [Google Scholar]
- 28.Crown JP, Burris HA, III, Boyle F, et al. Pooled analysis of diarrhea events in patients with cancer treated with lapatinib Breast Cancer Res Treat 112317–3252008 [DOI] [PubMed] [Google Scholar]
- 29.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer J Clin Oncol 275538–55462009 [DOI] [PubMed] [Google Scholar]
- 30.Awada A, Colomer R, Inoue K, et al. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: The NEfERT-T randomized clinical trial JAMA Oncol 21557–15642016 [DOI] [PubMed] [Google Scholar]
- 31.Awada A, Dirix L, Manso Sanchez L, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy Ann Oncol 24109–1162013 [DOI] [PubMed] [Google Scholar]
- 32.Chow LW, Xu B, Gupta S, et al. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer Br J Cancer 1081985–19932013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saura C, Garcia-Saenz JA, Xu B, et al. Safety and efficacy of neratinib in combination with capecitabine in patients with metastatic human epidermal growth factor receptor 2-positive breast cancer J Clin Oncol 323626–36332014 [DOI] [PubMed] [Google Scholar]
- 34.Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Phase III NALA trial J Clin Oncol 383138–31492020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial Lancet Oncol 17367–3772016 [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial Lancet Oncol 181688–17002017 [DOI] [PubMed] [Google Scholar]
- 37.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer N Engl J Med 382597–6092020 [DOI] [PubMed] [Google Scholar]
- 38.Hsu WH, Yang JC, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC Ann Oncol 29i3–i92018suppl 1 [DOI] [PubMed] [Google Scholar]
- 39.Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: A meta-analysis J Clin Oncol 331958–19652015 [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K, Saito H, Hasegawa Y, et al. First-line gefitinib therapy for elderly patients with non-small cell lung cancer harboring EGFR mutation: Central Japan Lung Study Group 0901 Cancer Chemother Pharmacol 74721–7272014 [DOI] [PubMed] [Google Scholar]
- 41.Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study J Thorac Oncol 71417–14222012 [DOI] [PubMed] [Google Scholar]
- 42.Inoue Y, Inui N, Asada K, et al. Phase II study of erlotinib in elderly patients with non-small cell lung cancer harboring epidermal growth factor receptor mutations Cancer Chemother Pharmacol 76155–1612015 [DOI] [PubMed] [Google Scholar]
- 43.Imai H, Kaira K, Suzuki K, et al. A phase II study of afatinib treatment for elderly patients with previously untreated advanced non-small-cell lung cancer harboring EGFR mutations Lung Cancer 12641–472018 [DOI] [PubMed] [Google Scholar]
- 44.Tateishi K, Ichiyama T, Hirai K, et al. Clinical outcomes in elderly patients administered gefitinib as first-line treatment in epidermal growth factor receptor-mutated non-small-cell lung cancer: Retrospective analysis in a Nagano Lung Cancer Research Group Study. Med Oncol. 2013;30:450. doi: 10.1007/s12032-012-0450-2. [DOI] [PubMed] [Google Scholar]
- 45.Uruga H, Kishi K, Fujii T, et al. Efficacy of gefitinib for elderly patients with advanced non-small cell lung cancer harboring epidermal growth factor receptor gene mutations: A retrospective analysis Intern Med 49103–1072010 [DOI] [PubMed] [Google Scholar]
- 46.Corre R, Gervais R, Guisier F, et al. Octogenarians with EGFR-mutated non-small cell lung cancer treated by tyrosine-kinase inhibitor: A multicentric real-world study assessing tolerance and efficacy (OCTOMUT study) Oncotarget 98253–82622018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuwako T, Imai H, Masuda T, et al. First-line gefitinib treatment in elderly patients (aged ≥75 years) with non-small cell lung cancer harboring EGFR mutations Cancer Chemother Pharmacol 76761–7692015 [DOI] [PubMed] [Google Scholar]
- 48.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer N Engl J Med 378113–1252018 [DOI] [PubMed] [Google Scholar]
- 49.Nakao A, Hiranuma O, Uchino J, et al. Final results from a phase II trial of osimertinib for elderly patients with epidermal growth factor receptor t790m-positive non-small cell lung cancer that progressed during previous treatment. J Clin Med. 2020;9:1762. doi: 10.3390/jcm9061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuta H, Uemura T, Yoshida T, et al. Efficacy and safety data of osimertinib in elderly patients with NSCLC who harbor the EGFR T790M mutation after failure of initial EGFR-TKI treatment Anticancer Res 385231–52372018 [DOI] [PubMed] [Google Scholar]
- 51.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer N Engl J Med 3712167–21772014 [DOI] [PubMed] [Google Scholar]
- 52.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer N Engl J Med 377829–8382017 [DOI] [PubMed] [Google Scholar]
- 53.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer N Engl J Med 3792027–20392018 [DOI] [PubMed] [Google Scholar]
- 54.Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer N Engl J Med 3832018–20292020 [DOI] [PubMed] [Google Scholar]
- 55.Iwama E, Goto Y, Murakami H, et al. Survival analysis for patients with ALK rearrangement-positive non-small cell lung cancer and a poor performance status treated with alectinib: Updated results of lung oncology group in kyushu 1401 Oncologist 25306–e6182020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedas A, Peled N, Maimon Rabinovich N, et al. Efficacy and safety of ALK tyrosine kinase inhibitors in elderly patients with advanced ALK-positive non-small cell lung cancer: Findings from the real-life cohort Oncol Res Treat 42275–2822019 [DOI] [PubMed] [Google Scholar]
- 57.Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations N Engl J Med 383931–9432020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer N Engl J Med 383944–9572020 [DOI] [PubMed] [Google Scholar]
- 59.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma N Engl J Med 3771813–18232017 [DOI] [PubMed] [Google Scholar]
- 60.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib N Engl J Med 37230–392015 [DOI] [PubMed] [Google Scholar]
- 61.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial Lancet 386444–4512015 [DOI] [PubMed] [Google Scholar]
- 62.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma N Engl J Med 3711867–18762014 [DOI] [PubMed] [Google Scholar]
- 63.Kanesvaran R, Le Saux O, Motzer R, et al. Elderly patients with metastatic renal cell carcinoma: Position paper from the international society of geriatric oncology Lancet Oncol 19e317–e262018 [DOI] [PubMed] [Google Scholar]
- 64.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma N Engl J Med 356125–1342007 [DOI] [PubMed] [Google Scholar]
- 65.Hutson TE, Bukowski RM, Rini BI, et al. Efficacy and safety of sunitinib in elderly patients with metastatic renal cell carcinoma Br J Cancer 1101125–11322014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermansen CK, Donskov F.Outcomes based on age in patients with metastatic renal cell carcinoma treated with first line targeted therapy or checkpoint immunotherapy: Older patients more prone to toxicity J Geriatr Oncol 12827–8332021 [DOI] [PubMed] [Google Scholar]
- 67.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma N Engl J Med 359378–3902008 [DOI] [PubMed] [Google Scholar]
- 68.Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma N Engl J Med 37954–632018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial Lancet 3911163–11732018 [DOI] [PubMed] [Google Scholar]
- 70.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial Lancet 38956–662017 [DOI] [PubMed] [Google Scholar]
- 71.Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial Lancet 381295–3022013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial Lancet 3681329–13382006 [DOI] [PubMed] [Google Scholar]
- 73.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033 J Clin Oncol 26626–6322008 [DOI] [PubMed] [Google Scholar]
- 74.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial JAMA 3071265–12722012 [DOI] [PubMed] [Google Scholar]
- 75.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial Lancet 381303–3122013 [DOI] [PubMed] [Google Scholar]
- 76.Schlumberger M, Elisei R, Muller S, et al. Overall survival analysis of EXAM, a phase III trial of cabozantinib in patients with radiographically progressive medullary thyroid carcinoma Ann Oncol 282813–28192017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer N Engl J Med 372621–6302015 [DOI] [PubMed] [Google Scholar]
- 78.Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: Past, present, and future Lancet 395817–8272020 [DOI] [PubMed] [Google Scholar]
- 79.Rugo HS, Turner NC, Finn RS, et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: A pooled analysis of randomised PALOMA clinical studies Eur J Cancer 101123–1332018 [DOI] [PubMed] [Google Scholar]
- 80.Sonke GS, Hart LL, Campone M, et al. Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial Breast Cancer Res Treat 167659–6692018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goetz MP, Okera M, Wildiers H, editors. Safety and Efficacy of Abemaciclib Plus Endocrine Therapy in Elderly Patients With HR+, HER2- Advanced Breast Cancer: An Age-Specific Subgroup Analysis of MONARCH2 and 3 Trials (abstract P1-19-10). San Antonio Breast Cancer Symposium. San Antonio, TX: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Howie LJ, Singh H, Bloomquist E, et al. Outcomes of older women with hormone receptor-positive, human epidermal growth factor receptor-negative metastatic breast cancer treated with a CDK4/6 inhibitor and an aromatase inhibitor: An FDA pooled analysis J Clin Oncol 373475–34832019 [DOI] [PubMed] [Google Scholar]
- 83.Battisti NML, De Glas N, Sedrak MS, et al. Use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in older patients with ER-positive HER2-negative breast cancer: Young International Society of Geriatric Oncology review paper. Ther Adv Med Oncol. 2018;10:1758835918809610. doi: 10.1177/1758835918809610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piccart M, Hortobagyi GN, Campone M, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2† Ann Oncol 252357–23622014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pritchard KI, Burris HA, III, Ito Y, et al. Safety and efficacy of everolimus with exemestane vs. exemestane alone in elderly patients with HER2-negative, hormone receptor-positive breast cancer in BOLERO-2 Clin Breast Cancer 13421–432.e82013 [DOI] [PubMed] [Google Scholar]
- 86.Freedman RA, Tolaney SM.Efficacy and safety in older patient subsets in studies of endocrine monotherapy versus combination therapy in patients with HR+/HER2- advanced breast cancer: A review Breast Cancer Res Treat 167607–6142018 [DOI] [PubMed] [Google Scholar]
- 87.Jerusalem G, Mariani G, Ciruelos EM, et al. Safety of everolimus plus exemestane in patients with hormone-receptor-positive, HER2-negative locally advanced or metastatic breast cancer progressing on prior non-steroidal aromatase inhibitors: Primary results of a phase IIIb, open-label, single-arm, expanded-access multicenter trial (BALLET) Ann Oncol 271719–17252016 [DOI] [PubMed] [Google Scholar]
- 88.André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer N Engl J Med 3801929–19402019 [DOI] [PubMed] [Google Scholar]
- 89.Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer N Engl J Med 3661382–13922012 [DOI] [PubMed] [Google Scholar]
- 90.Ledermann JA, Harter P, Gourley C, et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial Lancet Oncol 171579–15892016 [DOI] [PubMed] [Google Scholar]
- 91.Dockery LE, Tew WP, Ding K, et al. Tolerance and toxicity of the PARP inhibitor olaparib in older women with epithelial ovarian cancer Gynecol Oncol 147509–5132017 [DOI] [PubMed] [Google Scholar]
- 92.Liposits G, Wulff CN, Otland A, et al. Olaparib treatment in older patients with ovarian cancer: Need for “real-world” data beyond clinical trials. Ecancermedicalscience. 2020;14:1104. doi: 10.3332/ecancer.2020.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer N Engl J Med 3752154–21642016 [DOI] [PubMed] [Google Scholar]
- 94.Fabbro M, Moore KN, Dørum A, et al. Efficacy and safety of niraparib as maintenance treatment in older patients (≥ 70 years) with recurrent ovarian cancer: Results from the ENGOT-OV16/NOVA trial Gynecol Oncol 152560–5672019 [DOI] [PubMed] [Google Scholar]
- 95.Coleman RL, Oza AM, Lorusso D, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial Lancet 3901949–19612017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ledermann JA, Oza AM, Lorusso D, et al. The effect of age on efficacy and safety outcomes with rucaparib: A post hoc exploratory analysis of ARIEL3, a phase III, randomized, placebo-controlled maintenance study in patients with recurrent ovarian carcinoma Gynecol Oncol 1544–52019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N Engl J Med 377523–5332017 [DOI] [PubMed] [Google Scholar]
- 98.Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation N Engl J Med 379753–7632018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ettl J, Quek RGW, Lee KH, et al. Quality of life with talazoparib versus physician's choice of chemotherapy in patients with advanced breast cancer and germline BRCA1/2 mutation: Patient-reported outcomes from the EMBRACA phase III trial Ann Oncol 291939–19472018 [DOI] [PubMed] [Google Scholar]
- 100.Robson M, Ruddy KJ, Im SA, et al. Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial Eur J Cancer 12020–302019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. https://www.ema.europa.eu/en/human-regulatory/research-development/medicines-older-people
- 102. https://www.fda.gov/media/135804/download