INTRODUCTION
Cancer is a disease of older adults. The median age at cancer diagnosis is 66 years and median age at cancer-related death is 72 years.1 Older adults represent one of the fastest growing populations of patients with cancer.2 The development of immunotherapy, particularly immune checkpoint inhibitors (ICIs), has revolutionized modern cancer care for patients of all ages including older adults. Inhibitors of cytotoxic T-lymphocyte-associated antigen 4 and programmed death receptor-1 (PD-1) and its ligand (PD-L1) are associated with improved overall survival (OS) for many tumor types3 with durable responses for a subset of patients.4 On average, ICIs have a favorable toxicity profile compared with cytotoxic chemotherapy,5 although with rare but serious immune-related adverse events (irAEs). Since the 2011 US Food and Drug Administration approval of the cytotoxic T-lymphocyte-associated antigen-4 inhibitor ipilimumab for melanoma,6 the number of approved ICIs has continued to increase with new indications for various tumor types and even tumor-agnostic settings (eg, PD-1 inhibitor pembrolizumab for microsatellite instability-high solid tumors7).
CONTEXT
Key Objective
What is known about immune checkpoint inhibitor (ICI) clinical efficacy and toxicity among older adults with cancer, and how does toxicity management differ in this vulnerable population?
Knowledge Generated
Among fit older adults included in clinical trials, ICI efficacy and toxicity are comparable with younger adults, although efforts to study ICI use among frail older adults cared for in everyday practice are ongoing. Toxicity management among older adults must consider comorbidities and ideally include primary care teams and caregivers.
Relevance
The data reviewed here can help clinicians assess the benefits and harms of immunotherapy using an individualized approach aimed at improving goal-concordant care and patient outcomes among older adults with cancer.
Although these immunotherapy advances are promising for many patients with cancer, they also introduce new challenges for the care of older adults with cancer. Older adults—especially frail older adults—remain underrepresented in cancer clinical trials.8 Given ICIs' more favorable toxicity profile, some frail older adults with cancer who may not have previously been offered cancer-directed therapy might now be evaluated for treatment with ICIs. Additionally, aging is associated with immune system changes that may affect immunotherapy outcomes in older adults. Therefore, it is important to review available data on ICI efficacy and toxicity in older adults with cancer and understand the limitations of the current evidence.
In this review, we discuss immunologic aging, ICI clinical efficacy among older adults focusing on four major tumor types (non–small-cell lung cancer [NSCLC], melanoma, renal cell carcinoma [RCC], and urothelial carcinoma), immunotherapy toxicity and management of toxicity in older adults, and future directions for geriatric oncology research in this rapidly growing space.
IMMUNOLOGIC AGING AND POTENTIAL IMPACTS ON IMMUNOTHERAPY
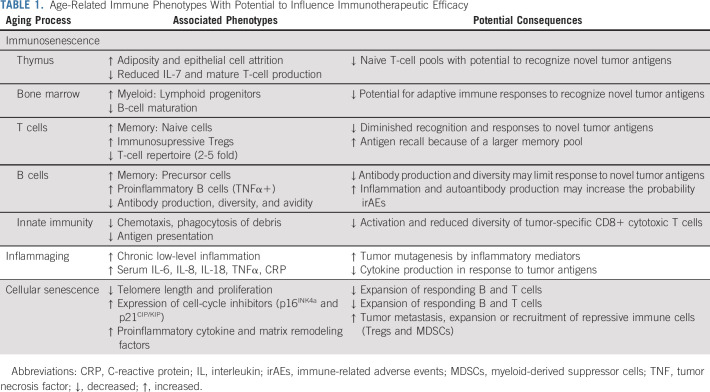
The immune system is gradually remodeled as a consequence of normal, physiologic aging. This process, known as immunosenescence, is characterized by: (1) changes in the microenvironment of lymphoid organs such as the bone marrow and thymus, (2) shifts in the relative abundance of immune cell subsets, and (3) alterations in the makeup of circulating cytokines, which control immune homeostasis9 (Table 1). Whether immunosenescence is a process that occurs on its own or in response to other age-related physiologic declines throughout the body is unclear. Regardless, intrinsic differences in the immune systems of older adults may influence the efficacy and/or toxicity of cancer immunotherapies.
TABLE 1.
Age-Related Immune Phenotypes With Potential to Influence Immunotherapeutic Efficacy
All three hallmarks of immunosenescence remodel the adaptive immune system. Aging of the bone marrow microenvironment reinforces the skewed differentiation of hematopoietic stem cells into myeloid over lymphoid progenitors, resulting in the production of fewer immature B and T cells.10,11 Changes in the post-pubescent microenvironment of the thymus, bone marrow, and lymph nodes further compromise the maturation of these immature immune cells.9,12 Although peripheral signals initially maintain the circulating pool of antigen-inexperienced (naive) T cells in adults, encounters with environmental and self-antigens cause an increasing number of naive T cells to differentiate into effector and effector memory cells.9 One consequence of this expanding memory pool is a reduction of immunologic space, which causes a two- to five-fold decrease in the T-cell repertoire and may limit the expansion of additional T-cell clones.13 Beyond these events, the age-related remodeling germinal center constituents promote the expansion of proinflammatory B cells and limit the production of high-affinity antibodies.14
Age-related shifts in circulating cytokines and chemokines, known as inflammaging, can also affect adaptive immunity. Inflammaging is the persistent, low-level activation of inflammatory responses in the absence of infection and is associated with morbidity and mortality among older adults.15 Several aging phenotypes contribute to inflammaging. One is the reduced capacity of innate immune cells to traffic to areas of tissue damage and eliminate inflammatory debris.16 Others include increasing interactions between the immune system and microbiota because of growing intestinal permeability, heightened activation of the coagulation and complement systems, and cellular senescence.15
Cellular senescence differs from immunosenescence. It is an age-related process that occurs in individual cells, rather than a combination of physiologic changes. Cellular senescence is an irreversible cell cycle arrest characterized by telomere attrition, epigenetic and metabolic rewiring, secretion of proinflammatory and matrix remodeling factors, and the persistent expression of cell-cycle inhibitors (eg, p16INK4a and p21CIP/KIP 17). Senescent cells accumulate with age and studies in animal models suggest that the elimination of these cells can stave off age-related disease.17 Although cellular and immunosenescence often go hand-in-hand, emerging data suggest that these are separate consequences of physiologic aging.18
How immunosenescence, inflammaging, and cellular senescence influence responses to immunotherapy is unclear. Although an expanded pool of effector memory cells might allow for more rapid and robust antigen responses, higher levels of self-reactive T cells and inflammaging may increase the propensity for irAEs. Few studies have examined how the immune microenvironments targeted by ICIs age or the relationship between immune aging and T-cell exhaustion.18 Therefore, it remains difficult to predict how ICIs might function in older adults with cancer. Evidence that the immune system may age in response to the patient's cancer or treatment also suggests that individuals of the same chronologic age can have very different immunologic set points before beginning therapy.19 Exploring these questions is the ongoing work of geriatric oncologists and aging scientists.
CLINICAL EFFICACY OF IMMUNOTHERAPY AMONG OLDER ADULTS
Non–Small-Cell Lung Cancer
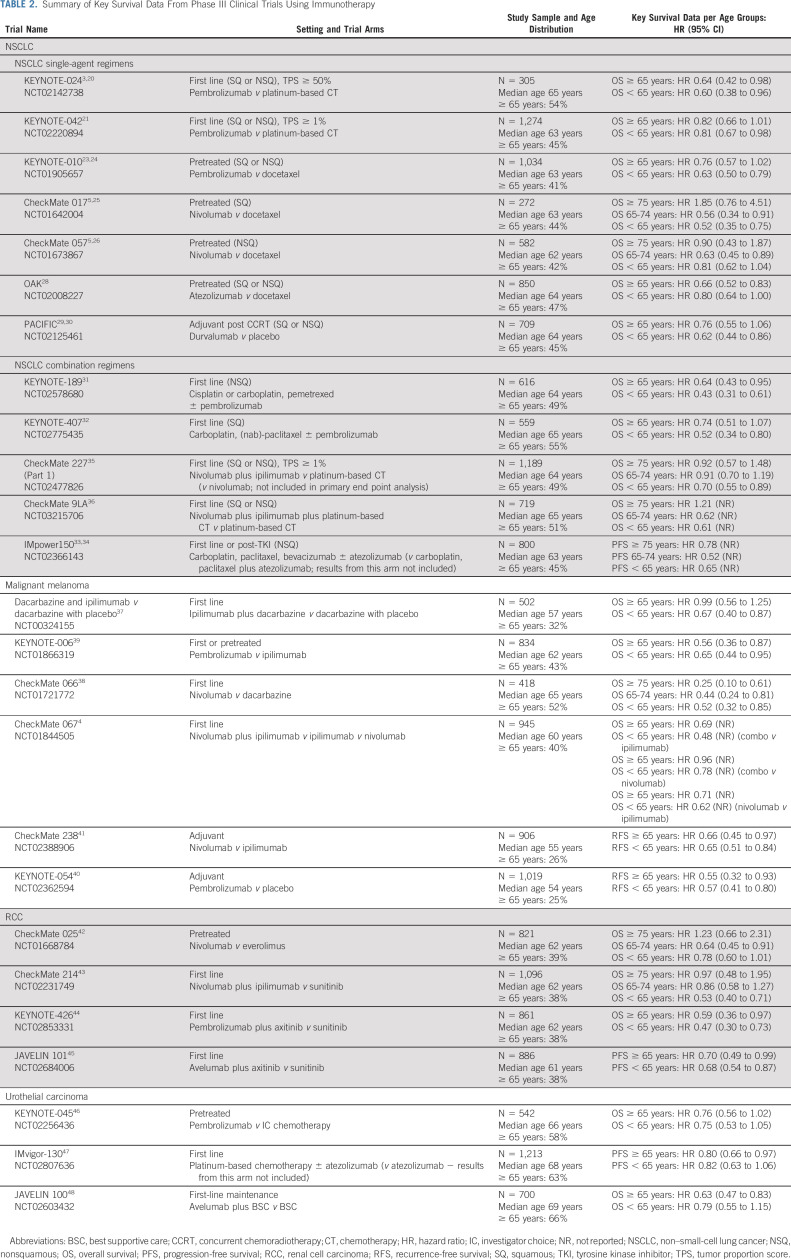
Older adults with NSCLC represent the largest subset of older patients treated with ICIs. Yet, the proportion of older adults (≥ 65 years) in the pivotal phase III clinical trials accounted for only 41%-55% of participants (Table 2).
TABLE 2.
Summary of Key Survival Data From Phase III Clinical Trials Using Immunotherapy
Single-Agent ICI in NSCLC
For untreated advanced or metastatic NSCLC, the phase III KEYNOTE-024 confirmed superior OS with pembrolizumab versus platinum-based chemotherapy in high PD-L1 expressers (≥ 50%).3,20 KEYNOTE-042 included patients with PD-L1 expression ≥ 1% and confirmed an OS benefit, yet in smaller magnitude compared with high-expressers.21 No benefit was observed in the prespecified exploratory analysis in the PD-L1 1%-49% subgroup. In PD-L1 high-expressers, the OS improvement was similar across age groups (Table 2) with a pooled analysis confirming this for older adults (hazard ratio [HR], 0.40; 95% CI, 0.25 to 0.64).22
In the setting of pretreated patients with advanced NSCLC, multiple phase III trials demonstrated the OS superiority of pembrolizumab, nivolumab, and atezolizumab versus docetaxel (Table 2). In KEYNOTE-010, the improvement with pembrolizumab (PD-L1 ≥ 1%) was less clear in older patients.23,24 In CheckMate 017 and CheckMate 057, nivolumab benefited older adults similar to younger adults, particularly within the nonsquamous histology subtype.5,25,26 Data on patients ≥ 75 years of age were reported, but the small number of older patients (n = 72, 8% of patients) limited conclusions. The phase IIIB/IV CheckMate 15327 trial reported that older patients (≥ 70 years), who accounted for 39% of patients, had a similar OS to younger patients. Last, the OAK trial with atezolizumab enrolled a higher proportion of older patients (47%), who had a greater magnitude of OS improvement.28
In unresectable stage III NSCLC, the use of durvalumab after concurrent chemoradiotherapy significantly improved OS versus placebo in the PACIFIC trial.29,30 However, this benefit was less clear for older patients (45% of patients) in the intent-to-treat analysis (Table 2).
Combination ICI Regimens in NSCLC
In the setting of untreated advanced or metastatic disease, the phase III trials KEYNOTE-189 and KEYNOTE-407 demonstrated OS superiority of pembrolizumab in combination with chemotherapy.31,32 These trials enrolled a considerable number of older adults (≥ 65 years; KEYNOTE-189: n = 304, 49%; KEYNOTE-407: n = 305, 55%), but older adults had a smaller magnitude of benefit, particularly in the squamous subtype (Table 2). The phase III IMpower150 trial confirmed the superiority of adding atezolizumab to chemotherapy and bevacizumab.33,34 The improvement in older patients was comparable to younger patients, but the subgroup ≥ 75 years was small (n = 78; 10%) and inconclusive.
More recently, combinations of different ICIs with or without chemotherapy have also been investigated (Table 2). CheckMate 227 confirmed the superiority of nivolumab plus ipilimumab versus standard platinum-based chemotherapy.35 However, this benefit was not clear in the older subgroups. CheckMate 9LA reported on the combination of nivolumab plus ipilimumab and platinum-based chemotherapy with a similar improvement in OS across age groups, yet with limited data on those ≥ 75 years of age (n = 70, 10%).36
Malignant Melanoma
Patients diagnosed with melanoma are generally younger, which is reflected in the proportion of older patients (≥ 65 years) enrolled in the pivotal trials (25%-52%; Table 2). ICIs have become the backbone of melanoma treatment. In the phase III trial of ipilimumab versus dacarbazine, too few older adults were enrolled to confirm the OS benefit in this subgroup.37 Nivolumab and pembrolizumab confirmed their superiority in the phase III trials CheckMate 066 and KEYNOTE-006, respectively.38,39 These trials were more inclusive of older adults and confirmed an OS advantage (Table 2). The landmark phase III trial CheckMate 067 investigated a combination of nivolumab and ipilimumab versus ipilimumab versus nivolumab.4 Both nivolumab arms were superior to ipilimumab alone. Although this study was not powered to compare the nivolumab-containing arms, it provided data suggesting superiority of the combination ICI arm. These OS improvements were clear across all age groups, but the magnitude of benefit was smaller among older adults (40% of patients, Table 2).
In the adjuvant setting for resectable melanoma, the phase III trials KEYNOTE-054 and CheckMate 238 demonstrated improvements in recurrence-free survival with pembrolizumab and nivolumab, respectively. Although these trials recruited the smallest proportion of older adults (25%-26%), the survival improvement was similar across age groups.40,41
Renal Cell Carcinoma
The proportion of older adults (≥ 65 years) enrolled in the pivotal phase III ICI trials for RCC has been steady at approximately 38% but with very small numbers ≥ 75 years of age, which precludes any conclusions in the oldest age group (Table 2). The role of ICI in RCC is currently limited to the advanced or metastatic setting where an increasing number of drugs have become available and treatment decisions are often made based on drug access, toxicity profile, and prognostic risk of patients. In pretreated patients, the phase III trial CheckMate 025 demonstrated the superiority of nivolumab over everolimus, regardless of age.42 In the first-line setting, the landmark phase III trial CheckMate 214 confirmed the OS superiority of nivolumab and ipilimumab versus sunitinib, particularly in RCC of intermediate or poor prognostic risk.43 However, the improvement with this ICI combination was not clear for older adults. The phase III trials KEYNOTE-426 and JAVELIN-101 investigated pembrolizumab and avelumab, respectively, combined with axitinib versus sunitinib, confirming the ICI arm's superiority regardless of age.44,45
Urothelial Carcinoma
The pivotal phase III trials of ICIs for advanced or metastatic urothelial carcinoma enrolled the highest proportion of older adults (≥ 65 years), accounting for up to 66% of participants (Table 2). KEYNOTE-045 confirmed the superiority of pembrolizumab versus investigator choice of chemotherapy after platinum-based chemotherapy.46 IMvigor-130 confirmed the superiority of combining atezolizumab to platinum-based chemotherapy upfront.47 Last, JAVELIN-100 confirmed the role of avelumab as a maintenance treatment after platinum-based chemotherapy.48 All these treatments have proven to be at least as effective in older adults as younger adults.
Real-World ICI Effectiveness in Older Adults
Although a large meta-analysis of PD-L1 inhibitor clinical trials found comparable efficacy among older and younger adults,49 an important limitation of these landmark ICI trials is the exclusion of vulnerable or frail older adults. The majority of these trials excluded patients with organ dysfunction or an Eastern Cooperative Oncology Group (ECOG) performance status ≥ 2, which limits the generalizability to more frail older adults routinely cared for in clinical practice. A few small retrospective studies have examined ICI effectiveness in older adults outside of clinical trials and found no difference in OS by age.50,51 However, one study of patients with NSCLC who were treated with PD-L1 inhibitors found that adults ≥ 80 years of age had a higher hazard for death compared with younger adults < 60 years of age (HR, 2.74; 95% CI, 1.43 to 5.25; P = .002).52
IMMUNOTHERAPY TOXICITY IN OLDER ADULTS
Although immunotherapy enhances the immune system to treat cancer, it can also cause the immune system to damage normal, healthy tissue and cause irAEs. Targeting the immune system has been a major advancement in the treatment of many cancers; yet, it is accompanied by any-grade irAEs in 30%-65% and high-grade irAEs in 5%-10% of treated patients.53,54 These autoimmune phenomena can occur anywhere in the body causing inflammation of any organ including but not limited to the liver (hepatitis), kidneys (nephritis), brain (encephalitis), colon (colitis), or lungs (pneumonitis; Fig 1). The most frequently affected organs are the endocrine system, the skin, the colon, the lung, and the liver. More rarely, the neurologic system and the kidneys can be affected.55
FIG 1.
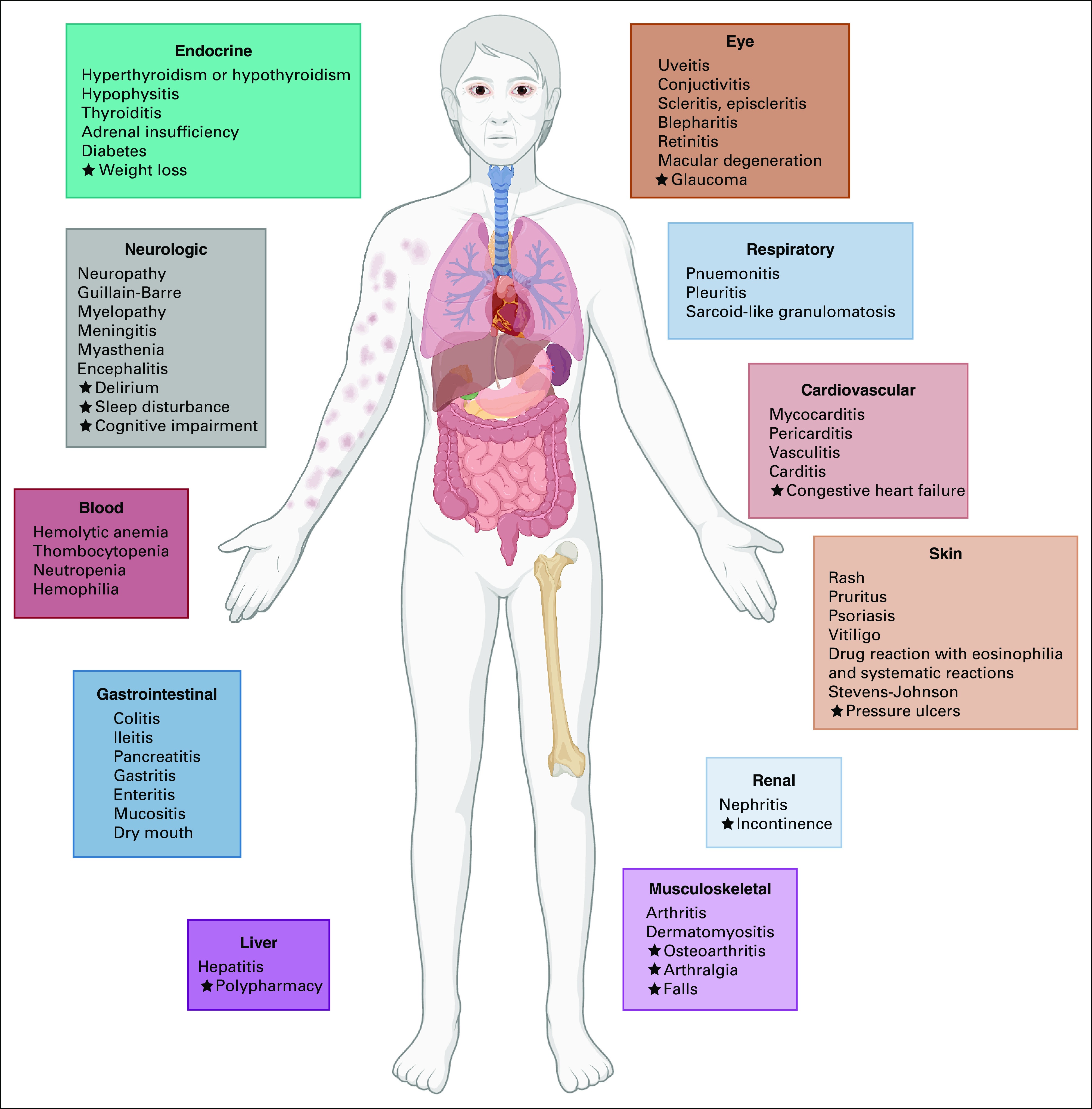
Immune-related adverse events and geriatric syndromes to consider among older adults with cancer. Star indicates additional comorbidities and geriatric syndromes to consider.
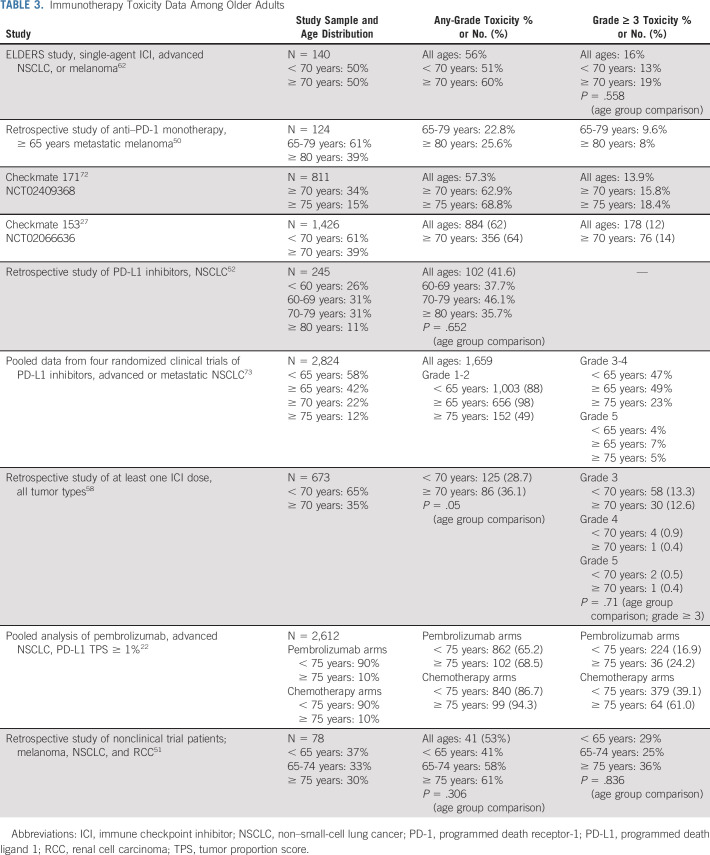
Overall, few studies have focused on irAEs specifically among older adults (Table 3). Among older adults, irAEs occur with a similar incidence and severity as younger adults. However, age-based comparisons are not always reported in the initial or post hoc 5-year clinical trial results.56 A meta-analysis of clinical trials demonstrated that treatment-related adverse events occurred in 66% of patients receiving PD-1 and PD-L1 inhibitors, with grade ≥ 3 adverse events occurring in 14% of patients, the majority of which were irAEs.57 Data are lacking on irAE severity or type based on age. The majority of older adults are treated in the community oncology setting, further limiting generalizability of clinical trial data to older adults.
TABLE 3.
Immunotherapy Toxicity Data Among Older Adults
The largest prospective clinical trial with a preplanned older adult subgroup analysis is the Checkmate 153.27 Among 1,426 patients treated with nivolumab, 556 (39%) were ≥ 70 years of age. This study highlighted the safety and tolerability of single-agent nivolumab. A few retrospective single-center institution studies demonstrated no difference in incidence or severity of irAEs among older versus young adults treated with ICIs (Table 3).51,58 One study demonstrated no improvement in OS (HR, 0.86; 95% CI, 0.55 to 1.33, P = .49) among older adults (≥ 70 years) treated with ICIs for advanced cancer and who experienced a high-grade irAE (grade ≥ 3) compared with those who did not experience a high-grade irAE.58 By contrast, among younger adults < 70 years of age who experienced high-grade irAEs, OS was significantly longer (HR, 0.33; 95% CI, 0.21 to 0.52, P < .001) compared with those who did not experience a high-grade irAE.58
Furthermore, there is limited information about risk factors for the development of irAE toxicity, specifically among older adults. A meta-analysis did find that older age is a risk factor for fatal ICI toxicities.59 Older adults are more vulnerable because of comorbidities and other geriatric syndromes, particularly frailty, polypharmacy (≥ 6 medications), cognitive impairment, and falls. In addition, age-related organ decline may be because of normal physiologic aging, a geriatric syndrome, or a co-occurring irAE (Fig 1). A few studies have linked geriatric syndromes or clinical risk factors to ICI toxicity outcomes in older adults. Patients in a small study (N = 28) with impairment in instrumental activities of daily living received fewer cycles of ICIs.60 Another study of 75 older adults with NSCLC receiving ICIs had a higher risk of death if they had an ECOG PS ≥ 2 but there were no significant associations with age, sex, comorbidity, or line of treatment.61
The recently published ELDERS study is one of the first and largest prospective observational cohort studies designed specifically to address the safety of ICIs among older adults.62 Patients with advanced NSCLC or malignant melanoma starting single-agent ICI (N = 140) were enrolled into two age-based cohorts (≥ 70 years and < 70 years). Half of the participants (n = 70) were in the older cohort. Frailty and geriatric assessments were prospectively implemented in the study design to characterize older adults beyond chronologic age. Half of the older patients enrolled were considered vulnerable or frail based on Geriatric-8 (G8) screening test (≤ 14 points). This superiority study was negative for its primary end point with no significant difference in the incidence of grade 3-5 irAEs between older and younger adults (18.6% v 12.9%; odds ratio, 1.55; 95% CI, 0.61 to 3.89; P = .35). Frail older adults with a positive G8 screening test had a higher risk of hospital admissions (P = .03) and death (P = .01), but not a higher incidence of irAEs.62
MANAGEMENT OF IMMUNOTHERAPY TOXICITY
The management of irAEs for older adults in general is similar to that recommended for the general adult population. A few key principles—namely to prevent, anticipate, detect, treat, and monitor—apply to both older and younger adults.63 Older adults may have poorer functional reserve, multiple comorbidities, and poorer social support compared with their younger counterparts, which may increase their risk of poor outcomes from irAEs.64 Guidelines are available to provide guidance on how to treat irAEs.65,66 However, none of these guidelines specifically address the issue of treatment of irAEs among older adults because of a paucity of data.
One way to potentially reduce the risk of developing severe irAEs in older adults with cancer is to incorporate a geriatric assessment. A geriatric assessment may identify impairments or vulnerabilities with functional status, mood, cognition, polypharmacy, comorbidities, and social support, which can then be optimized before initiation of and during ICI therapy.67 A thorough history, physical examination, and laboratory investigations are important before ICI initiation with close surveillance during treatment to prevent and detect potential irAEs early among older adults.63 Timely intervention of low-grade irAEs may help prevent older adults from developing severe irAEs, who have a higher risk of causing morbidity and mortality compared with younger adults.58 A positive G8 screen or older age has not been associated with higher risk of severe irAEs, but they have been associated with hospital admissions and risk of death.58,62 These data suggest that recovery of baseline function after development of severe irAEs may be less likely among frail, older adults.
Education of older adults and caregivers about the common and subtle symptoms of irAEs is important to empower them to detect and recognize these symptoms and contact their care team early in the toxicity timeline.68 Education of clinicians including primary care teams is equally important in order for them to recognize these symptoms as well, evaluate patients accordingly, and collaborate with oncologists to initiate the appropriate treatment when indicated (i.e., corticosteroids). irAE management is typically based on toxicity grade. ASCO has published clinical practice guidelines for the management of irAEs based on a systematic review of 204 publications.65 However, as older adults are poorly represented in clinical trials, there are limited data regarding how best to treat toxicities in this unique and heterogeneous group of patients.
Ideally, any treatment planned for irAEs for older adults is tailored within the context of their preexisting comorbidities. For example, corticosteroids form the current mainstay of irAE treatment, especially for patients with grade ≥ 2 irAEs.65 However, older adults are more likely to have chronic illnesses such as diabetes or osteoporosis, which can worsen with steroid therapy. In patients with diabetes, older adults may require closer glucose monitoring and may need to escalate their diabetic medication regimen, at least temporarily until corticosteroids can be tapered. Older adults are also more likely to develop delirium from corticosteroids and need to be counseled in advance, along with their caregivers, for signs of altered mental status.69 Patients who experience refractory irAEs may need a longer duration of corticosteroids or other immunosuppressive therapies. Older adults on long-term immunosuppressive therapies will be at higher risk of opportunistic infections and may need to be treated with antibiotic prophylaxis.70
Older adults with grade 1-2 irAEs can typically be managed in the outpatient setting. However, they may require closer monitoring with more frequent follow-up care to evaluate for worsening symptoms, which may require inpatient evaluation and management. Some monitoring of low-grade irAEs such as bowel movements for colitis or oxygen saturation levels for pneumonitis can be done at home but may require the assistance of caregivers or home nursing.68
In conclusion, immunotherapy has changed the treatment paradigm for many older adults with cancer, allowing for more tolerable treatment options with the potential for durable responses. Overall, based on the available evidence, ICI clinical efficacy and toxicity among fit older adults included in clinical trials is comparable to younger adults and studies of ICI therapy in an aging immune system are ongoing.
Efforts to study ICI effectiveness and toxicity among frail older adults in everyday clinical practice are important to expand the evidence base to patients who were excluded from the landmark immunotherapy trials (eg, ECOG performance status ≥ 2 and organ dysfunction), yet are routinely treated in oncology clinics. Upcoming data from immunotherapy trials designed specifically for older adults such as Alliance A171901 (ClinicalTrials.gov identifier: NCT04533451) are highly anticipated to improve our understanding of ICIs in older adults. Alliance A171901 is an ongoing phase II trial that examines adverse events, OS, and quality of life among adults age ≥ 70 with advanced or metastatic lung adenocarcinoma treated with first-line pembrolizumab with or without chemotherapy based on oncologist's or patient's choice. Unlike most clinical trials, Alliance A171901 does not include a performance status eligibility criterion. Therefore, older adults who are deemed fit enough for pembrolizumab by their oncologist (but may not have an ECOG performance status of 0-1) can be enrolled, which will result in a more generalizable older patient sample. This more inclusive clinical trial design can be applied to study many cancer treatment regimens for any tumor type where data on toxicity and tolerance in older adults would improve clinical care.
In addition, active areas of geriatric oncology research include understanding who is at highest risk of ICI toxicity and expanding the definition of cancer treatment toxicity to include functional status and quality of life. The Cancer and Aging Research Group chemotherapy toxicity calculator predicts grade ≥ 3 AEs among older adults receiving chemotherapy,71 but this predictive model was developed before the introduction of immunotherapy into clinical practice. We need predictive models for immunotherapy and chemoimmunotherapy toxicity among older adults including both traditional AE outcomes (eg, grade ≥ 3 AEs) and geriatric outcomes important to older adults (eg, functional status and ability to live independently). Furthermore, we need predictive models to understand risk factors for poor recovery of function after experiencing severe AEs from ICIs. Prospective cohort studies of functional status and quality of life among older adults receiving immunotherapy are ongoing. Together, these efforts to individualize assessments of benefits and harms of immunotherapy for older adults will help guide treatment decision making to improve goal-concordant care and patient outcomes.
Carolyn J. Presley
Consulting or Advisory Role: PotentiaMetrics, Onc Live
Fabio Gomes
Honoraria: AstraZeneca
Christin E. Burd
Stock and Other Ownership Interests: Abbvie, Luminex, GE Healthcare, Gilead Sciences
Ravindran Kanesvaran
Honoraria: Astellas Pharma, Novartis, Janssen, MSD Oncology, Bristol-Myers Squibb
Consulting or Advisory Role: Pfizer, Astellas Pharma, Novartis, MSD Oncology, Janssen Oncology
Research Funding: Sanofi, Janssen
Travel, Accommodations, Expenses: Astellas Pharma, MSD Oncology, Bristol-Myers Squibb
Melisa L. Wong
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
No other potential conflicts of interest were reported.
SUPPORT
C.J.P. is supported by the National Institute on Aging (R03AG064374) and the Ohio State University Comprehensive Cancer Center. C.E.B. is supported by the National Institute on Aging (R01AG059711). M.L.W. is supported by the National Institute on Aging (R03AG056439, K76AG064431) and the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center. The Cancer and Aging Research Group also supported this review (R33AG059206).
AUTHOR CONTRIBUTIONS
Conception and design: Carolyn J. Presley, Melisa L. Wong
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Immunotherapy in Older Adults With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Carolyn J. Presley
Consulting or Advisory Role: PotentiaMetrics, Onc Live
Fabio Gomes
Honoraria: AstraZeneca
Christin E. Burd
Stock and Other Ownership Interests: Abbvie, Luminex, GE Healthcare, Gilead Sciences
Ravindran Kanesvaran
Honoraria: Astellas Pharma, Novartis, Janssen, MSD Oncology, Bristol-Myers Squibb
Consulting or Advisory Role: Pfizer, Astellas Pharma, Novartis, MSD Oncology, Janssen Oncology
Research Funding: Sanofi, Janssen
Travel, Accommodations, Expenses: Astellas Pharma, MSD Oncology, Bristol-Myers Squibb
Melisa L. Wong
Employment: Roche/Genentech
Stock and Other Ownership Interests: Roche/Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017. Bethesda, MD: National Cancer Institute; [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: Burdens upon an aging, changing nation J Clin Oncol 272758–27652009 [DOI] [PubMed] [Google Scholar]
- 3.Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) 50% Ann Oncol 31S1181–S11822020 [Google Scholar]
- 4.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma N Engl J Med 3771345–13562017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gettinger S, Borghaei H, Brahmer J, et al. Five-year outcomes from the randomized, phase 3 trials CheckMate 017/057: Nivolumab vs docetaxel in previously treated NSCLC J Thorac Oncol 14S244–S2452019 [Google Scholar]
- 6.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma N Engl J Med 363711–7232010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 8.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions CA Cancer J Clin 7178–922020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolich-Zugich J.The twilight of immunity: Emerging concepts in aging of the immune system Nat Immunol 1910–192018 [DOI] [PubMed] [Google Scholar]
- 10.Denkinger MD, Leins H, Schirmbeck R, et al. HSC aging and senescent immune remodeling Trends Immunol 36815–8242015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas-Madrid D, Muniategui A, Sanchez-Caballero I, et al. Improving miRNA-mRNA interaction predictions. BMC Genomics. 2014;15(suppl 10):S2. doi: 10.1186/1471-2164-15-S10-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasca D, Diaz A, Romero M, et al. B cell immunosenescence Annu Rev Cell Dev Biol 36551–5742020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi Q, Liu Y, Cheng Y, et al. Diversity and clonal selection in the human T-cell repertoire Proc Natl Acad Sci USA 11113139–131442014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kogut I, Scholz JL, Cancro MP, et al. B cell maintenance and function in aging Semin Immunol 24342–3492012 [DOI] [PubMed] [Google Scholar]
- 15.Franceschi C, Campisi J.Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases J Gerontol A Biol Sci Med Sci 69S4–S92014suppl 1 [DOI] [PubMed] [Google Scholar]
- 16.Solana R, Tarazona R, Gayoso I, et al. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans Semin Immunol 24331–3412012 [DOI] [PubMed] [Google Scholar]
- 17.Di Micco R, Krizhanovsky V, Baker D, et al. Cellular senescence in ageing: From mechanisms to therapeutic opportunities Nat Rev Mol Cell Biol 2275–952020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pawelec G, Bronikowski A, Cunnane SC, et al. The conundrum of human immune system “senescence”. Mech Ageing Dev. 2020;192:111357. doi: 10.1016/j.mad.2020.111357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaPak KM, Burd CE.The molecular balancing act of p16(INK4a) in cancer and aging Mol Cancer Res 12167–1832014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer N Engl J Med 3751823–18332016 [DOI] [PubMed] [Google Scholar]
- 21.Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial Lancet 3931819–18302019 [DOI] [PubMed] [Google Scholar]
- 22.Nosaki K, Saka H, Hosomi Y, et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1-positive advanced non-small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies Lung Cancer 135188–1952019 [DOI] [PubMed] [Google Scholar]
- 23.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial Lancet 3871540–15502016 [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Garon EB, Kim DW, et al. Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro Ann Oncol 29X42–X432018 [Google Scholar]
- 25.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer N Engl J Med 373123–1352015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer N Engl J Med 3731627–16392015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spigel DR, McCleod M, Jotte RM, et al. Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older or with poor performance status (CheckMate 153) J Thorac Oncol 141628–16392019 [DOI] [PubMed] [Google Scholar]
- 28.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial Lancet 389255–2652017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer N Engl J Med 3771919–19292017 [DOI] [PubMed] [Google Scholar]
- 30.Faivre-Finn C, Vicente D, Kurata T, et al. Durvalumab after chemoradiotherapy in stage III NSCLC: 4-year survival update from the phase III PACIFIC trial Ann Oncol 31S1178–S11792020 [Google Scholar]
- 31.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer N Engl J Med 3782078–20922018 [DOI] [PubMed] [Google Scholar]
- 32.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer N Engl J Med 3792040–20512018 [DOI] [PubMed] [Google Scholar]
- 33.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC N Engl J Med 3782288–23012018 [DOI] [PubMed] [Google Scholar]
- 34.Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol. 2018;36 suppl; abstr 9002. [Google Scholar]
- 35.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer N Engl J Med 3812020–20312019 [DOI] [PubMed] [Google Scholar]
- 36.Reck M, Ciuleanu T-E, Dols MC, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38 suppl; abstr 9501. [Google Scholar]
- 37.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma N Engl J Med 3642517–25262011 [DOI] [PubMed] [Google Scholar]
- 38.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation N Engl J Med 372320–3302014 [DOI] [PubMed] [Google Scholar]
- 39.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma N Engl J Med 3722521–25322015 [DOI] [PubMed] [Google Scholar]
- 40.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma N Engl J Med 3781789–18012018 [DOI] [PubMed] [Google Scholar]
- 41.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma N Engl J Med 3771824–18352017 [DOI] [PubMed] [Google Scholar]
- 42.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma N Engl J Med 3731803–18132015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma N Engl J Med 3781277–12902018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma N Engl J Med 3801116–11272019 [DOI] [PubMed] [Google Scholar]
- 45.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma N Engl J Med 3801103–11152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma N Engl J Med 3761015–10262017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galsky MD, Arija JÁA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial Lancet 3951547–15572020 [DOI] [PubMed] [Google Scholar]
- 48.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma N Engl J Med 3831218–12302020 [DOI] [PubMed] [Google Scholar]
- 49.Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: A meta-analysis. J Immunother Cancer. 2018;6:26. doi: 10.1186/s40425-018-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Betzalel G, Steinberg-Silman Y, Stoff R, et al. Immunotherapy comes of age in octagenarian and nonagenarian metastatic melanoma patients Eur J Cancer 10861–682019 [DOI] [PubMed] [Google Scholar]
- 51.Sattar J, Kartolo A, Hopman WM, et al. The efficacy and toxicity of immune checkpoint inhibitors in a real-world older patient population J Geriatr Oncol 10411–4142019 [DOI] [PubMed] [Google Scholar]
- 52.Lichtenstein MRL, Nipp RD, Muzikansky A, et al. Impact of age on outcomes with immunotherapy in patients with non-small cell lung cancer J Thorac Oncol 14547–5522019 [DOI] [PubMed] [Google Scholar]
- 53.Baxi S, Yang A, Gennarelli RL, et al. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: Systematic review and meta-analysis. BMJ. 2018;360:k793. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helissey C, Vicier C, Champiat S.The development of immunotherapy in older adults: New treatments, new toxicities? J Geriatr Oncol 7325–3332016 [DOI] [PubMed] [Google Scholar]
- 55.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma N Engl J Med 37323–342015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robert C, Ribas A, Schachter J, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study Lancet Oncol 201239–12512019 [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: A systematic review and meta-analysis JAMA Oncol 51008–10192019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johns A, Wei L, Grogan M, et al. Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer J Geriatr Oncol 12813–8192021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis JAMA Oncol 41721–17282018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welaya K, Loh KP, Messing S, et al. Geriatric assessment and treatment outcomes in older adults with cancer receiving immune checkpoint inhibitors J Geriatr Oncol 11523–5282020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muchnik E, Loh KP, Strawderman M, et al. Immune checkpoint inhibitors in real-world treatment of older adults with non–small cell lung cancer J Am Geriatr Soc 67905–9122019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes F, Lorigan P, Woolley S, et al. A prospective cohort study on the safety of checkpoint inhibitors in older cancer patients: The ELDERS study. ESMO Open. 2021;6:100042. doi: 10.1016/j.esmoop.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper Ann Oncol 27559–5742016 [DOI] [PubMed] [Google Scholar]
- 64.van Holstein Y, Kapiteijn E, Bastiaannet E, et al. Efficacy and adverse events of immunotherapy with checkpoint inhibitors in older patients with cancer Drugs Aging 36927–9382019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline J Clin Oncol 361714–17682018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up Ann Oncol 28iv119–iv1422017 [DOI] [PubMed] [Google Scholar]
- 67.Kanesvaran R, Cordoba R, Maggiore R.Immunotherapy in older adults with advanced cancers: Implications for clinical decision-making and future research Am Soc Clin Oncol Ed Book 38400–4142018 [DOI] [PubMed] [Google Scholar]
- 68.Bhandari S, Gill AS, Perez CA, et al. Management of immunotherapy toxicities in older adults Semin Oncol 45226–2312018 [DOI] [PubMed] [Google Scholar]
- 69.Manzo C, Serra-Mestres J, Castagna A, et al. Behavioral, psychiatric, and cognitive adverse events in older persons treated with glucocorticoids. Medicines (Basel) 2018;5:82. doi: 10.3390/medicines5030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kyi C, Hellmann MD, Wolchok JD, et al. Opportunistic infections in patients treated with immunotherapy for cancer. J Immunother Cancer. 2014;2:19. doi: 10.1186/2051-1426-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study J Clin Oncol 293457–34652011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations Eur J Cancer 127160–1722020 [DOI] [PubMed] [Google Scholar]
- 73.Marur S, Singh H, Mishra-Kalyani P, et al. FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies Semin Oncol 45220–2252018 [DOI] [PubMed] [Google Scholar]