Abstract
PURPOSE
Severe (grade 3-4) acute graft-versus-host disease (AGVHD) is a major cause of death after unrelated-donor (URD) hematopoietic cell transplant (HCT), resulting in particularly high mortality after HLA-mismatched transplantation. There are no approved agents for AGVHD prevention, underscoring the critical unmet need for novel therapeutics. ABA2 was a phase II trial to rigorously assess safety, efficacy, and immunologic effects of adding T-cell costimulation blockade with abatacept to calcineurin inhibitor (CNI)/methotrexate (MTX)-based GVHD prophylaxis, to test whether abatacept could decrease AGVHD.
METHODS
ABA2 enrolled adults and children with hematologic malignancies under two strata: a randomized, double-blind, placebo-controlled stratum (8/8-HLA-matched URD), comparing CNI/MTX plus abatacept with CNI/MTX plus placebo, and a single-arm stratum (7/8-HLA-mismatched URD) comparing CNI/MTX plus abatacept versus CNI/MTX CIBMTR controls. The primary end point was day +100 grade 3-4 AGVHD, with day +180 severe-AGVHD-free-survival (SGFS) a key secondary end point. Sample sizes were calculated using a higher type-1 error (0.2) as recommended for phase II trials, and were based on predicting that abatacept would reduce grade 3-4 AGVHD from 20% to 10% (8/8s) and 30% to 10% (7/8s). ABA2 enrolled 142 recipients (8/8s, median follow-up = 716 days) and 43 recipients (7/8s, median follow-up = 708 days).
RESULTS
In 8/8s, grade 3-4 AGVHD was 6.8% (abatacept) versus 14.8% (placebo) (P = .13, hazard ratio = 0.45). SGFS was 93.2% (CNI/MTX plus abatacept) versus 82% (CNI/MTX plus placebo, P = .05). In the smaller 7/8 cohort, grade 3-4 AGVHD was 2.3% (CNI/MTX plus abatacept, intention-to-treat population), which compared favorably with a nonrandomized matched cohort of CNI/MTX (30.2%, P < .001), and the SGFS was better (97.7% v 58.7%, P < .001). Immunologic analysis revealed control of T-cell activation in abatacept-treated patients.
CONCLUSION
Adding abatacept to URD HCT was safe, reduced AGVHD, and improved SGFS. These results suggest that abatacept may substantially improve AGVHD-related transplant outcomes, with a particularly beneficial impact on HLA-mismatched HCT.
INTRODUCTION
Allogeneic hematopoietic cell transplant (HCT) is an effective treatment for aggressive hematologic malignancies, often representing the only option for cure. For patients lacking HLA-matched related donors, unrelated donors (URDs) are often used. The major disadvantage of URD HCT is an increased risk for nonrelapse mortality (NRM) mediated by severe acute graft-versus-host disease (AGVHD), chronic GVHD (CGVHD), and infection.1-7 The use of an HLA-mismatched URD accentuates these risks, with exceedingly high rates of severe AGVHD (up to 37%3) driving substantial NRM (up to 45%3). Notably, although the majority of patients in ethnic minorities will not have an HLA-matched URD, most of these patients will have 7/8 URD donors available,8 underscoring the importance of improving the safety of these high-risk transplants.
CONTEXT
Key Objective
The ABA2 trial addressed a critical question in unrelated-donor (URD) hematopoietic stem cell transplant (HCT): Can we significantly reduce acute graft-versus-host disease (AGVHD) after URD HCT by adding T-cell costimulation blockade with abatacept?
Knowledge Generated
ABA2 demonstrated that when a short course of abatacept was added to standard AGVHD prevention, rates of AGVHD significantly decreased in HLA-matched and HLA-mismatched URD HCT, with particularly striking results in HLA-mismatched HCT. ABA2 also demonstrated that this decrease in AGVHD was not accompanied by an increase in the risk of relapse or infectious complications, two major safety indicators for GVHD studies.
Relevance
ABA2 is the first multicenter trial of targeted T-cell costimulation blockade for GVHD prevention. The results from this trial suggest that the addition of abatacept for AGVHD prevention will be clinical practice-changing for URD HCT.
Preclinical data demonstrate that the costimulation blockade agent, cytotoxic T-cell lymphocyte-4-immunoglobulin (abatacept) can prevent GVHD.9-16 These results provided the rationale for the first-in-disease trial of abatacept for GVHD prevention (ClinicalTrials.gov identifier: NCT01012492)17 that established the feasibility and general safety of the approach. This phase II trial of abatacept for severe AGVHD prevention (ABA2, ClinicalTrials.gov identifier: NCT01743131) was designed to test the hypothesis that abatacept could lower the risk of severe AGVHD for patients receiving either 7/8 or 8/8 URD HCT, thereby improving transplant outcomes.
METHODS
Patients and Treatment
ABA2 is a phase II study of abatacept plus standard calcineurin inhibition (CNI; either cyclosporine or tacrolimus) and methotrexate (MTX; 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11). CNI was continued through day +100, and weaned between days 100-180 post-transplant as tolerated. For patients receiving abatacept, four doses were delivered, 10 mg/kg/dose, on days −1, +5, +14, and +28. Pretransplant conditioning used one of four myeloablative regimens: busulfan/fludarabine, busulfan/cyclophosphamide, total body irradiation/cyclophosphamide, and fludarabine/melphalan (details in the ABA2 Protocol, online only).
Random Assignment and Masking
For patients receiving 8/8 HLA (HLA A, B, C, and DR)–matched HCT, a randomized double-blind placebo-controlled design was used, with patients randomly assigned 1:1 to abatacept or placebo, using nonadaptive random assignment with a block size of 8, stratified by patient age (≤ 21 v > 21 years). For patients receiving 7/8 HLA-matched grafts, a single-arm open-label design was used, with comparison to a prespecified control cohort from the Center for International Blood and Marrow Transplant Research (CIBMTR). The process for identifying CIBMTR controls was masked: the statistician had no access to outcome data. It is important to note that the 7/8 stratum was also initially designed as a randomized, double-blind placebo-controlled cohort. However, the 7/8 stratum was experiencing very slow recruitment (Fig 1) and participating centers were queried to determine barriers to enrollment. This revealed a major reluctance by treating physicians to randomly assign 7/8 transplant recipients to placebo, given the attendant high risks of severe AGVHD and NRM with standard CNI/MTX prophylaxis.3,7 The trial was therefore amended such that all patients receiving 7/8 URD HCT were assigned to CNI/MTX plus abatacept as an open-label single-arm stratum. The 7/8 stratum enrolled 41 patients in the subsequent 23 months (an approximately 10-fold increased rate of enrollment).
FIG 1.
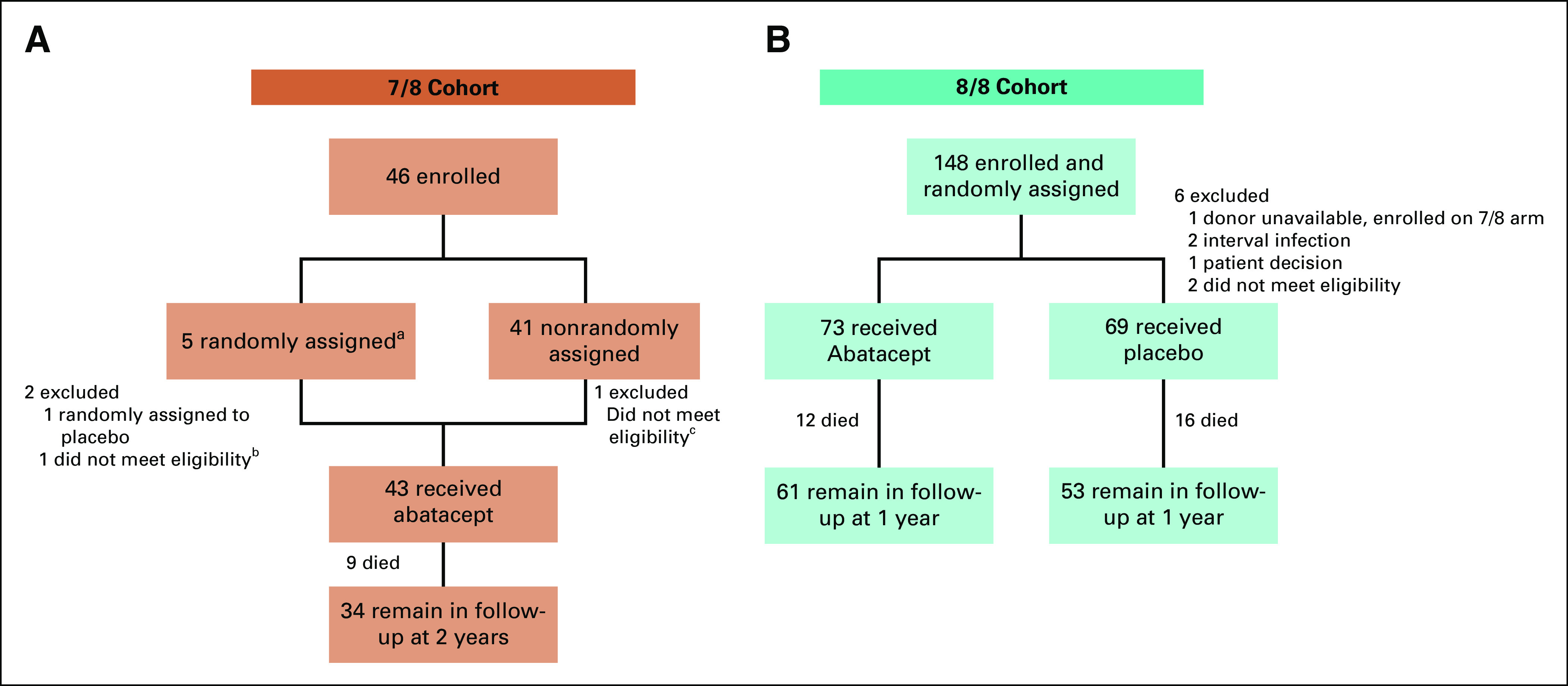
CONSORT diagrams. (A) 7/8 stratum. (B) 8/8 stratum. aPatients were randomly assigned between March 1, 2013, and December 9, 2014. Following a protocol amendment, subjects in the 7/8 cohort were nonrandomly assigned to receive abatacept. The remaining 7/8 patients were randomly assigned between December 19, 2014, and November 30, 2016. bPatient determined to be ineligible because of disease progression prior to day 1. cPatient excluded because of confirmation of Li-Fraumeni Syndrome prior to day 1.
End Points
The primary end point was the cumulative incidence of severe (grade 3-4) AGVHD at day +100. Day +180 severe-AGVHD-free-survival (SGFS) was a key secondary end point. Other end points included: (1) grade 3-4 AGVHD at day +180; (2) grade 2-4 AGVHD at days +100 and +180; (3) CGVHD at 1 year; (4) NRM; (5) relapse; (6) relapse-free survival (RFS); (7) overall survival (OS); (8) Cytomegalovirus reactivation and disease; (9) Epstein-Barr virus reactivation and post-transplant lymphoproliferative disease; (10) hematologic recovery; and (11) donor engraftment. During post-hoc analysis, the cumulative incidence of steroid-refractory AGVHD (defined by lack of response at day +28 or the need for second-line therapy) was compared in 8/8s (the necessary treatment data were not available for the CIBMTR 7/8 controls).
Statistical Analysis
The statistical analysis for ABA2 was stipulated by the Protocol and the Statistical Analysis Plan. This analysis was performed in the modified intent-to treat (ITT) population, which included all patients who received at least one dose of study medication. A screening phase-II design18 (Bayesian type I error probability < 0.2 and Bayesian power > 0.8) was used for sample size calculations.18 In the analyses of study end points, the primary end point was analyzed first, and secondary or exploratory end points were only evaluated if the primary end point met the prespecified statistical threshold. The decision rule was to reject the null hypothesis if P8/8 < .2 for the 8/8 HLA-matched URD and P7/8 < .2 for the 7/8 HLA-mismatched URD. For the 8/8 stratum, a sample size of 70 per arm was calculated to achieve 80% Bayesian power to detect a reduction in severe AGVHD from 20% to 10% (Bayesian type I probability < 0.2). For the 7/8 stratum, a sample size of 40 patients was calculated to achieve 80% Bayesian power to detect a reduction in severe AGVHD from 30% to 10% (Bayesian type I probability < 0.2). Additional statistical and methodological details are provided in the Data Supplement (online only).
RESULTS
Patients
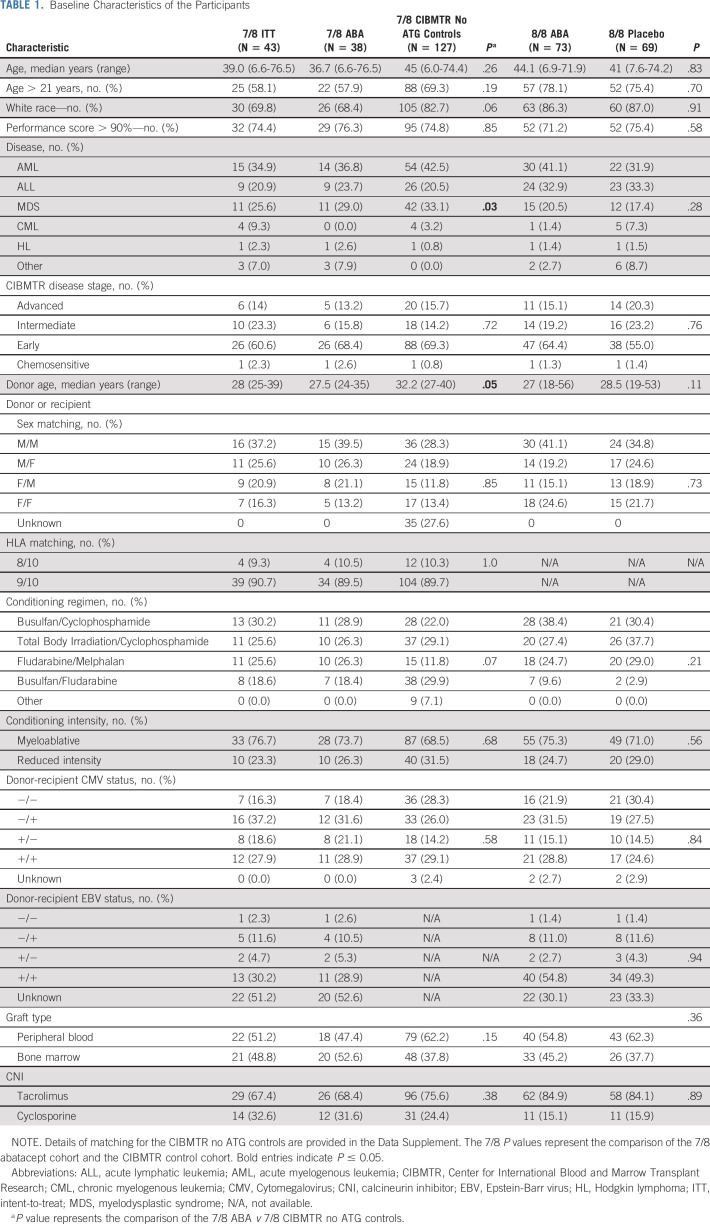
Figure 1 depicts the CONSORT diagram for the ABA2 study. Baseline characteristics for the 7/8 and 8/8 cohorts are shown in Table 1. For 8/8s, the abatacept and placebo groups were balanced with respect to all parameters tested. The 7/8 cohort was well matched with CIBMTR CNI/MTX controls for all variables except overall disease type, with the apparent mismatch in overall disease type resulting from a nonuniform case:control matching ratio for the abatacept cohort (ie, some patients receiving abatacept were matched 1:2 v 1:3 or 1:4). Although this covariate imbalance is present overall, analysis of time-dependent outcomes accounting for the matched-pair design eliminated the effect of this imbalance on parameter estimation.
TABLE 1.
Baseline Characteristics of the Participants
Efficacy Outcomes
Acute GVHD.
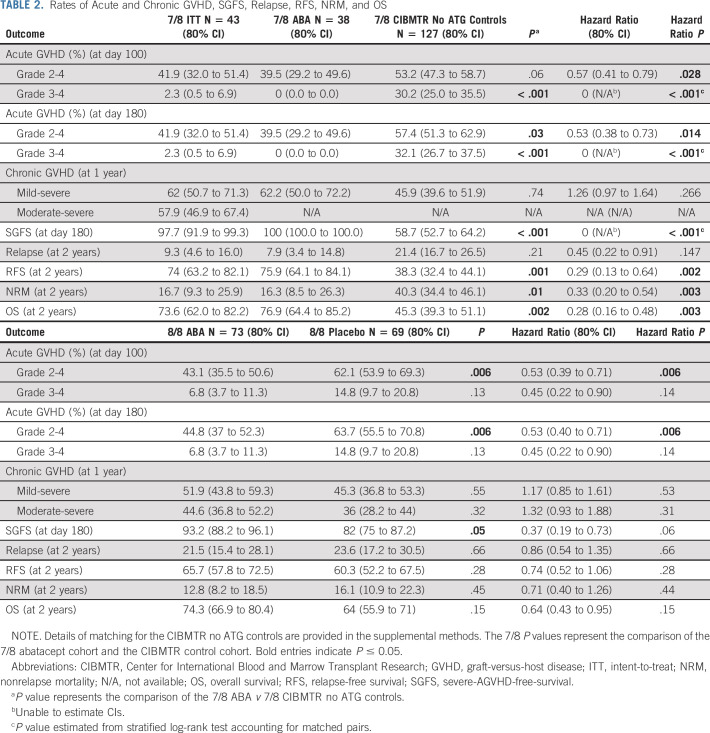
ABA2 demonstrated improved AGVHD in 8/8s receiving abatacept when compared with the randomly assigned placebo group based on the prespecified phase II type I error (0.2). 7/8s also demonstrated a sizable AGVHD benefit when compared with a nonrandomly assigned control group and historical references in the literature.3-5,7 The primary end point of ABA2 was the cumulative incidence of severe AGVHD at day +100. As shown in Table 2 and Figure 2A, in 7/8s, patients receiving abatacept demonstrated a substantial decrease in the day +100 CI of severe (grades 3-4) AGVHD, with 2.3% (ITT population, 0% in the CIBMTR CNI/MTX comparator cohort) severe AGVHD in patients receiving CNI/MTX plus abatacept versus 30.2% in CNI/MTX CIBMTR controls (P < .001, hazard ratio [HR], 0.0 for the ABA2 v CIBMTR CNI/MTX comparison), greatly exceeding the expectations of the trial's statistical design. The severe AGVHD outcomes for the CIBMTR controls mirrored those documented in multiple previous studies of 7/8 URD HCT,3,7 supporting the comparability of this control cohort with the 7/8 URD HCT population as a whole. In post-hoc analysis, we also compared recipients of 7/8 HCT who received CNI/MTX plus abatacept to CIBMTR controls receiving CNI/MTX plus anti-thymocyte globulin (ATG), and observed similarly superior outcomes in the abatacept group (Data Supplement). The randomized double-blind 8/8 stratum also demonstrated a decrease in severe AGVHD, from 14.8% in placebo to 6.8% in patients receiving abatacept (P = .13, HR, 0.45; 80% CI, 0.22 to 0.9, Table 2, Fig 2B). Because the sample size for ABA2 was designed with an alpha error of 0.2, the 8/8 stratum did reach the protocol-specified statistical end point (despite P > .05). Other AGVHD end-points were also evaluated as exploratory analyses, which all demonstrated significant improvement compared with controls (Figs 2C and 2D, Data Supplement).
TABLE 2.
Rates of Acute and Chronic GVHD, SGFS, Relapse, RFS, NRM, and OS
FIG 2.
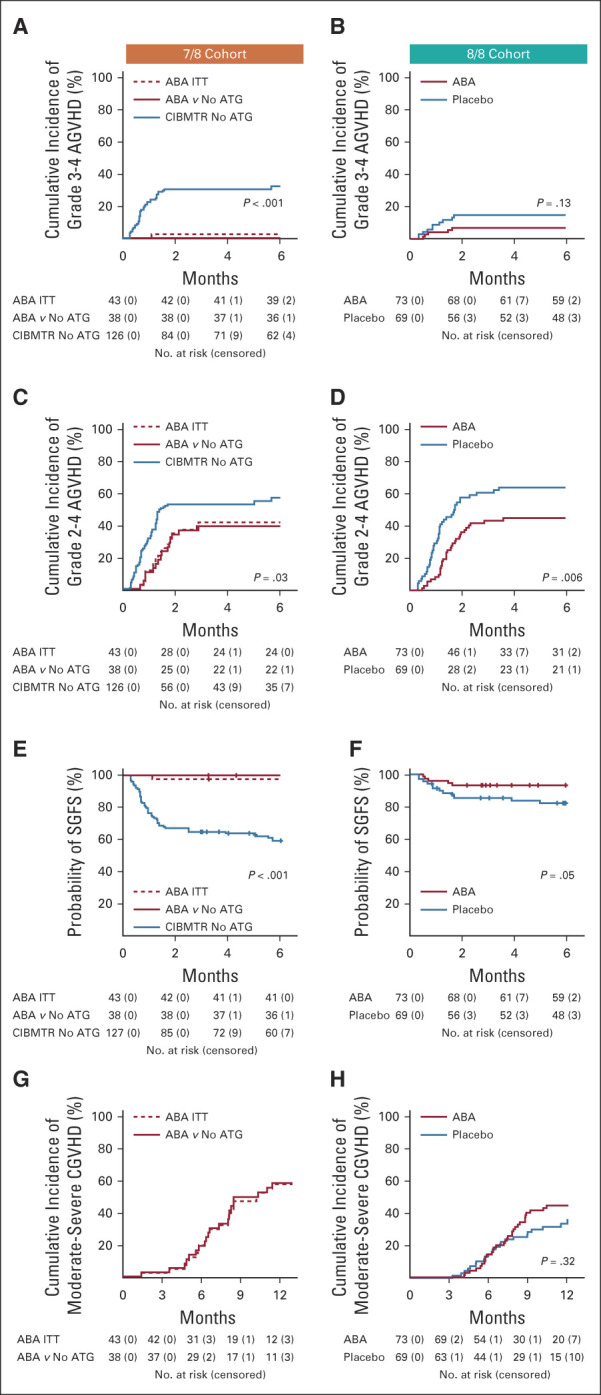
Key GVHD outcomes. (A) 7/8 Cohort, cumulative incidence of grade 3-4 AGVHD. Number of patients at risk and censored are listed below the graph. (B) 8/8 Cohort, cumulative incidence of grade 3-4 AGVHD. Number of patients at risk and censored are listed below the graph. (C) 7/8 Cohort, cumulative incidence of grade 2-4 AGVHD. Number of patients at risk and censored are listed below the graph. (D) 8/8 Cohort, cumulative incidence of grade 3-4 AGVHD. Number of patients at risk and censored are listed below the graph. (E) 7/8 Cohort: cumulative incidence of severe AGVHD-free-survival (SGFS). Number of patients at risk and censored are listed below the graph. (F) 8/8 Cohort: cumulative incidence of SGFS. Number of patients at risk and censored are listed below the graph. (G) 7/8 Cohort: cumulative incidence of moderate-severe CGVHD. Number of patients at risk and censored are listed below the graph. (H) 8/8 Cohort: Cumulative incidence of moderate-severe CGVHD. Number of patients at risk and censored are listed below the graph. 7/8 Cohort: Blue: CIBMTR control cohort. Solid red: 7/8 abatacept cohort, matched to the CIBMTR controls. Dashed red: 7/8 intention-to-treat (ITT) cohort. P values represent comparison of 7/8 abatacept cohort and CIBMTR control cohort. 8/8 Cohort: Blue: placebo. Red: abatacept. ABA, abatacept; AGVHD, acute graft-versus-host disease; ATG, anti-thymocyte globulin; CGVHD, chronic GVHD; CIBMTR, Center for International Blood and Marrow Transplant Research.
Day 180 SGFS.
Because abatacept is a novel agent for AGVHD prophylaxis, in addition to measuring efficacy, we also measured early treatment failure using the composite SGFS end point, which includes both severe AGVHD and death within 180 days as events. A similar end point (SGFS through 100 days) was described in a study of ATG for GVHD prevention by Finke et al19 in 2009, with extension to 180 days in ABA2 based on FDA recommendations. As shown in Figures 2F and 2G, Table 2, and the Data Supplement, in both the 7/8 and 8/8 cohorts, day +180 SGFS outcomes for patients receiving abatacept were superior to those receiving standard prophylaxis. In the 7/8 cohort, SGFS was 97.7% (abatacept ITT) versus 58.7% (CNI/MTX) (P < .001, HR, 0.00). In the 8/8 cohort, SGFS was 93.2% (abatacept) versus 82.0% (placebo, P = .05, HR, 0.37; 80% CI, 0.19 to 0.73).
Chronic GVHD.
The four-dose abatacept prophylaxis regimen did not improve CGVHD. Thus, for the 7/8 and 8/8 strata, patients receiving abatacept demonstrated a 1-year mild-severe CGVHD cumulative incidence of 62.0% and 51.9%, respectively, compared with 45.9% and 45.3% for CIBMTR and placebo controls (P = .74; HR, 1.26; 80% CI, 0.97 to 1.64; and P = .55, HR, 1.17; 80% CI, 0.85 to 1.61, respectively). Although the rate of moderate-severe CGVHD was not available for CIBMTR controls, this comparison was made for the 8/8 stratum, demonstrating 44.6% and 36% for patients receiving abatacept and placebo controls, respectively (P = .32, HR, 1.32; 80% CI, 0.93 to 1.88, Fig 2I). The 1-year moderate-severe CGVHD cumulative incidence for the 7/8 patients receiving abatacept was 57.9% (Fig 2H).
Hematologic reconstitution, donor engraftment, viral reactivation, and disease.
As shown in the Data Supplement, both the 7/8 and 8/8 strata demonstrated successful neutrophil and platelet reconstitution, donor engraftment, and leukocyte reconstitution post-transplant.
In addition, there was no statistically significant difference in Cytomegalovirus or Epstein-Barr virus viral reactivation or end-organ disease in 8/8 patients (Data Supplement). 7/8 patients could not be directly compared for this end point, as viral reactivation data were not collected by the CIBMTR. Additional details for these end points are found in the Data Supplement.
Relapse.
The addition of abatacept did not increase relapse (7/8s HR, 0.45; 80% CI, 0.22 to 0.91, P = .21 and 8/8s HR, 0.86; 80% CI, 0.54 to 1.35, P = .66, Table 2). The 2-year relapse point estimate was 9.3% (4.6%-16.0%) in the 7/8s ITT, compared with 21.4% (16.7%-26.5%) in the CNI/MTX CIBMTR controls. In the 8/8 stratum, 2-year relapse was 21.5% 15.4%-28.1%) for abatacept versus 23.6% (17.2%-30.5%) for placebo.
Survival end points.
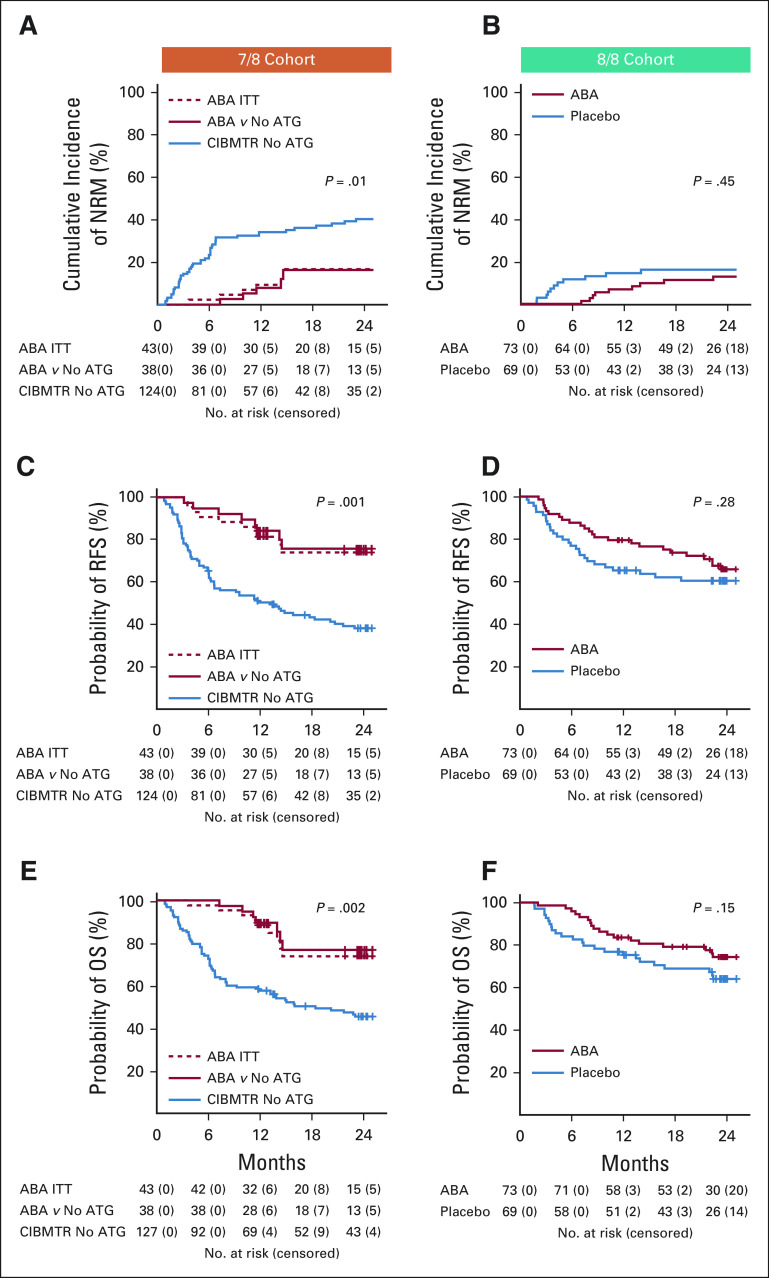
In addition to day +180 SGFS, other survival indicators were also tracked in ABA2, including NRM, RFS, and OS (Table 2, Figs 3A-3F and the Data Supplement, causes of death shown in the Data Supplement). In 7/8s, abatacept significantly improved each of these outcomes (Figs 3A, 3C, and 3E), with survival advantages stable through 2 years for the ABA2 versus CNI/MTX CIBMTR control comparisons (Table 2). To further interrogate the impact of abatacept in the nonrandomized 7/8 stratum, a post-hoc multivariable regression was also performed. This analysis supported the salutary effect of abatacept on key study outcomes (Data Supplement). In the 8/8 stratum, no statistically significant benefit was observed for NRM or OS with a median follow-up of alive patients of 716 days (Table 2 and Figs 3B and 3F). These results are consistent with the major impact of abatacept being on AGVHD rather than on CGVHD and underscore the fact that reducing NRM after HLA-mismatched (7/8) URD HCT is more closely linked with control of AGVHD than after 8/8 URD HCT.
FIG 3.
Key transplant outcomes. (A) 7/8 Cohort, cumulative incidence of nonrelapse mortality (NRM). Number of patients at risk and censored are listed below the graph. (B) 8/8 Cohort, cumulative incidence of NRM. Number of patients at risk and censored are listed below the graph. (C) 7/8 Cohort, cumulative incidence of relapse-free survival (RFS). Number of patients at risk and censored are listed below the graph. (D) 8/8 Cohort, cumulative incidence of RFS. Number of patients at risk and censored are listed below the graph. (E) 7/8 Cohort, cumulative incidence of. Number of patients at risk and censored are listed below the graph. (F) 8/8 Cohort, cumulative incidence of OS. Number of patients at risk and censored are listed below the graph. 7/8 Cohort: Blue: CIBMTR control cohort. Solid red: 7/8 abatacept cohort, matched to the CIBMTR controls. Dashed red: 7/8 intention-to-treat (ITT) cohort. P values represent comparison of 7/8 abatacept cohort and CIBMTR control cohort. 8/8 Cohort: Blue: Placebo. Red: Abatacept. ABA, abatacept; ATG, anti-thymocyte globulin; CIBMTR, Center for International Blood and Marrow Transplant Research; OS, overall survival.
Flow cytometric and transcriptomic analyses reveal control of CD4+ T-cell activation and effector differentiation with abatacept.
Because CD4+ T cells have been consistently documented to be the main therapeutic target of abatacept,17 we interrogated this cell population in patients who received abatacept compared with placebo. Flow cytometric analysis revealed that patients receiving abatacept demonstrated a significant preservation of the relative proportions of naive CD4 T cells during hematologic reconstitution (Figs 4A-4C, Data Supplement). These shifts were not impacted by the degree of HLA matching and were further accentuated in patients receiving abatacept who were protected from grades 3-4 AGVHD (Fig 4C). To further interrogate the immunologic interaction between exposure to abatacept and protection from severe AGVHD, RNASeq was performed on CD4+ T cells isolated on day +21 to +28 from ABA2 patients (both 7/8 and 8/8 cohorts), as well as a cohort of 12 healthy controls (Fig 4D). Weighted Gene Correlation Network Analysis20 identified self-assembling gene modules (SAMs, Fig 4E) that were correlated with clinical variables, including prophylaxis regimen and AGVHD (Data Supplement). The most pertinent SAM encapsulates a 476-gene module (Fig 4E, turquoise SAM) that strongly correlated with patients receiving CNI/MTX prophylaxis and away from patients receiving abatacept (Fig 4F). Even more noteworthy, embedded within the turquoise module was a 93-gene subset that not only correlated strongly with CNI/MTX prophylaxis, but also demonstrated increasing expression with increasing grade of AGVHD (Fig 4G, Data Supplement), thus identifying the first human T-cell transcriptional network associated with AGVHD severity.
FIG 4.
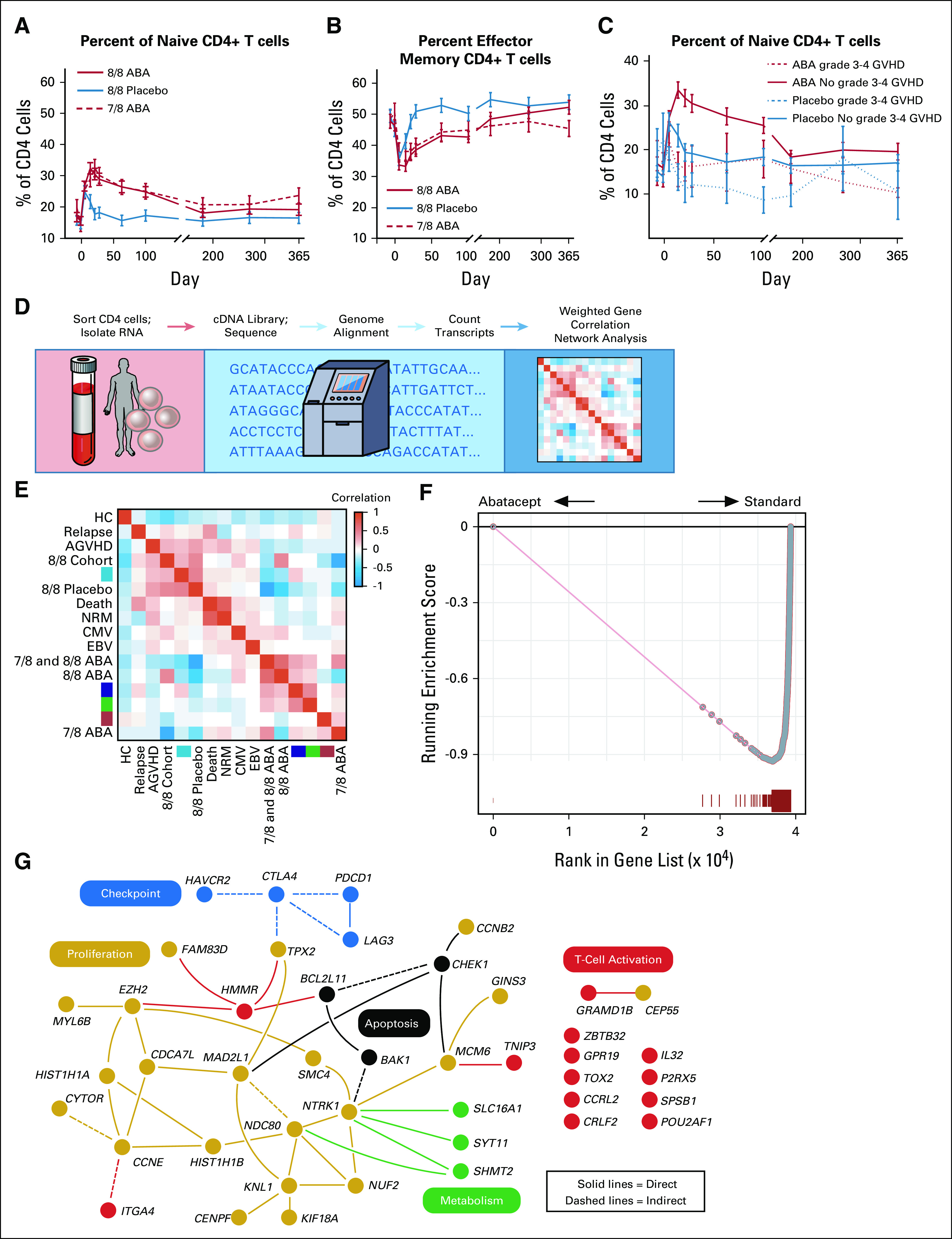
Post-transplant immunologic analysis. (A) Percent of CD4+ naive T cells (compared with total CD4+ T cells). ANOVA P value = .00004; 8/8 ABA versus 7/8 ABA P = .99; 8/8 ABA versus 8/8 placebo P = .0001; 7/8 ABA versus 8/8 placebo P = .0016. (B) Percent of CD4+ effector-memory T cells (compared with total CD4+ T cells). ANOVA P value = .0052; 8/8 ABA versus 7/8 ABA P = .52; 8/8 ABA versus 8/8 Placebo P = .0034; 7/8 ABA versus 8/8 placebo P = .18. (C) Percent of CD4+ naive T cells (compared with total CD4+ T cells), dichotomized by grade 3-4 AGVHD. ABA patients with no grade 3-4 GVHD versus ABA patients with grade 3-4 GVHD P = .024. In A-C, data are shown as the percentage ± SEM. In A-B, dashed red = 7/8 ABA intent-to treat; solid red = 8/8 ABA; solid blue = 8/8 placebo; in C, dashed red = 8/8 ABA with grade 3-4 AGVHD; solid red = 8/8 ABA without grade 3-4 AGVHD; dashed blue: 8/8 placebo with grade 3-4 AGVHD; solid blue = 8/8 placebo without grade 3-4 AGVHD. (D) Transcriptomic analysis pipeline. (E) Weighted gene coexpression network analysis heatmap. Heatmap demonstrates the correlation (using a blue-red false color scale) of weighted gene coexpression network analysis gene modules (colored turquoise, blue, green, and red) in transcriptomic samples grouped by clinical metadata. Row or column order is determined by hierarchical clustering of the module eigengene with clinical metadata for each sample. (F) Gene set enrichment analysis of the turquoise WGCNA module in a ranked list of differentially expressed genes between patients receiving abatacept (left) and patients receiving standard prophylaxis (right). The gene list was generated with a cutoff of FDR < 0.001 by gene-set permutation testing. (G) Ingenuity pathway analysis of the 93-gene subset of the turquoise module. Yellow: proliferation genes; black: apoptosis genes; blue: checkpoint genes; green: metabolism genes; red: T-cell activation genes. Solid lines: direct interactions; dashed lines: indirect interactions. ABA, abatacept; AGVHD, acute graft-versus-host disease; CMV, Cytomegalovirus; EBV, Epstein-Barr virus; FDR, False Discovery Rate; HC, hematopoietic cell; NRM, nonrelapse mortality.
DISCUSSION
There is a critical unmet need in HCT for safe and effective transplant strategies for patients lacking an HLA-matched sibling donor. This is especially true for patients who also lack an 8/8 HLA-matched URD, a population that is highly skewed toward those in specific ethnic groups (including Black, Hispanic, Asian Pacific Islanders, amongst others).8 Although both cord blood and haploidentical transplants are reasonable alternatives for these patients, challenges with these graft sources remain, including slow immune recovery and infections with both graft sources.21-26 The ABA2 study was designed to test whether targeted in vivo CD80/86 costimulation blockade with abatacept could improve outcomes for both 7/8 and 8/8 URD HCT.
ABA2 used a dual-strata trial design to account for the different a priori risks of severe AGVHD and AGVHD-associated mortality for patients receiving transplants from 7/8- versus 8/8-HLA matched donors. The inclusion of the 7/8 cohort in ABA2, even without random assignment, was important: these high-risk patients are often either excluded from randomized, placebo-controlled trials, or nominally included, but with acknowledgment that few high-risk patients will be enrolled. This contributes to the lack of representation of these high-risk patients (often including large proportions of ethnic minorities) in clinical trial data, and to the lack of thorough evaluation of new agents in these transplant patients, who could arguably derive the greatest benefit from novel therapies. However, despite the relevance of the 7/8 cohort, several limitations are noted in the single-arm, historical control design. These include inherent differences in characteristics between the donors and recipients on the 7/8 ABA2 versus control arms, which may be clinically meaningful, despite the lack of statistically significant differences calculated. They also include the difference in transplant time period between the 7/8 ABA2 cohort and the CIBMTR controls, which, although necessary to obtain adequate control numbers, could introduce unmeasured effects on outcomes. An additional limitation, which is gaining more importance with evolving transplant practices, was the inability to include post-transplant cyclophosphamide (PT-Cy) in the CIBMTR control cohort because of inadequate number of these controls in the CIBMTR database. Given the potential promise of both abatacept-based and PT-Cy-based27 GVHD prevention strategies, future prospective trials directly comparing these approaches may be warranted.
The ABA2 study demonstrated that the addition of four peritransplant doses of abatacept improved AGVHD outcomes, with strikingly positive results in 7/8 URD HCT. Thus, for 7/8 patients, the abatacept cohort demonstrated substantial improvements in grade 3-4 AGVHD, grade 2-4 AGVHD, and day +180 SGFS compared with CNI/MTX controls. Given that the 7/8 stratum was single arm, it was important to evaluate how representative the outcomes from both the enrolled patient cohort and the CIBMTR controls were compared with the general 7/8 URD transplant population. With respect to the CIBMTR controls, their outcomes closely mirrored those previously reported for 7/8 URD HCT,3,7 supporting the conclusion that improved results with abatacept were not because of spuriously poor outcomes in the controls. With respect to the enrolled 7/8 patient cohort, the positive outcomes in ABA2 patients were unlikely to be because of ABA2 patients being a highly selected population with intrinsically lower risk of GVHD. Although abatacept improved acute GVHD in ABA2 patients, it did not affect chronic GVHD. This observation strengthens the inference that the positive AGVHD outcomes were because of the efficacy of the four-dose abatacept regimen in preventing AGVHD, rather than to an intrinsically lower risk of all-cause GVHD in the enrolled patients. Furthermore, multivariable analysis of several key outcomes in the 7/8 cohort (Data Supplement) supported the significant improvement that abatacept made in this cohort.
The substantial improvement in outcomes for 7/8 patients generated an additional, unexpected observation: although not a controlled analysis, the NRM, RFS, and OS statistics from the 7/8 abatacept cohort compared favorably with the 8/8 cohort receiving CNI/MTX plus placebo (Table 2). This observation is consistent with the biology of CD28:CD80/86-directed costimulation blockade, which inhibits signaling events downstream from initial T cell receptor–HLA interactions.28 These observations suggest that a regimen containing CNI/MTX plus abatacept could potentially ‘level the playing field’ for those patients (often ethnic minorities) for whom the only URD option is HLA-mismatched. Moreover, the survival results for the 7/8 ABA2 patients compare favorably with a retrospective study of PT-Cy versus ATG in HLA-mismatched URD transplants, recently published by the European Bone Marrow Transplant Consortium,29 further suggesting the importance abatacept as part of the armamentarium of GVHD prophylaxis agents available clinically.
The survival outcomes in ABA2 were driven by the combined decrease in AGVHD and the favorable safety profile, with the placebo-controlled analysis of safety end points in the 8/8 stratum adding rigor to this conclusion. Most notably, abatacept did not appear to increase the risk of disease relapse in either 7/8 or 8/8 HCT, a key safety outcome when adding an adjunctive immunomodulating agent to transplants for hematologic malignancies. It is important to note that long-term analysis of this end point is still ongoing, with follow-up of all ABA2 patients planned through 5 years post-transplant. As noted above, one important exception to the improvement in outcomes with abatacept was in the area of CGVHD, with abatacept not improving CGVHD outcomes in either of the 7/8 and 8/8 cohorts compared with controls. This outcome may be expected, given the short course of abatacept that was administered in this trial (four doses, with the final dose on day +28, and lack of drug exposure expected by day +10017). The improvement in survival statistics despite equivalent rates of CGVHD in the 7/8 stratum underscores the impact that severe AGVHD makes on survival after URD transplant, especially after HLA-mismatched HCT. Recent smaller studies have suggested that abatacept may have activity in treating CGVHD, and that increasing the number abatacept doses could prevent both CGVHD and AGVHD.30,31 Thus, there is rationale for rigorously testing an extended dosing schedule to determine whether longer exposure could improve chronic as well as acute GVHD outcomes.
Flow cytometric analysis provided compelling evidence for substantial restraint of effector-memory CD4+ T-cell expansion with abatacept, and for the similarity of abatacept's immunologic effect on CD4+ T-cell reconstitution in both the 7/8 and 8/8 cohorts. This similarity in the immunologic impact of abatacept may have led to the downstream clinical results of ABA2, with comparable outcomes in the 7/8 and 8/8 cohorts. Transcriptomic analysis of CD4+ T cells from ABA2 patients enabled a fundamental advance in our understanding of the immune networks, rather than selected genes,32-34 driving AGVHD. Abatacept not only downregulated the expression of genes in these networks compared with CNI/MTX, but also decoupled their expression from AGVHD severity. These results suggest a profound reprograming of T-cell activation with abatacept that is correlated with control of AGVHD.
In summary, the ABA2 trial demonstrated the safety and efficacy of in vivo costimulation blockade with abatacept in preventing both moderate-severe and severe AGVHD after 7/8 URD HCT, with a significant impact on moderate-severe AGVHD and steroid-refractory AGVHD in 8/8 patients. The substantial improvement in survival indicators in the 7/8 cohort suggest that the addition of abatacept could be clinical practice-changing for these otherwise high-risk transplants.
ACKNOWLEDGMENT
We gratefully acknowledge the patients who participated in this study and their families. We thank the clinical research staff and caregivers at all participating sites. Steve Moskowitz of Advanced Medical Graphics performed medical illustration. Carly Ziegler provided assistance with Ingenuity Pathway Analysis for the CD4+ transcriptomic visualization. Dr Daniel Promislow provided assistance with the linear regression analysis of the transcriptomic data.
Benjamin Watkins
Consulting or Advisory Role: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent pending for “Method to prevent relapse after transplant”
Muna Qayed
Consulting or Advisory Role: Novartis, Mesoblast
Travel, Accommodations, Expenses: Novartis
Steve Bosinger
Research Funding: GlaxoSmithKline
Victor Tkachev
Travel, Accommodations, Expenses: Regeneron
Ted A. Gooley
Consulting or Advisory Role: Kiadis Pharma, Pharmacyclics, REGiMMUNE
Marcelo C. Pasquini
Consulting or Advisory Role: Pfizer, Medigene, Celgene, Amgen
Research Funding: Kite/Gilead, Novartis, Celgene, Bristol Myers Squibb
Roger Giller
Research Funding: Bristol-Myers Squibb, Jazz Pharmaceuticals, Atara
Maxim Norkin
Research Funding: Celgene
Nosha Farhadfar
Consulting or Advisory Role: Incyte
Research Funding: CSL Behring
Michael A Pulsipher
Honoraria: Amgen, Bellicum Pharmaceuticals, Miltenyi Biotec
Consulting or Advisory Role: Novartis, CSL Behring, Jasper Therapeutics, Novartis
Speakers' Bureau: Novartis
Research Funding: Adaptive Biotechnologies, Miltenyi Biotec
Travel, Accommodations, Expenses: Medac, Bellicum Pharmaceuticals, Miltenyi Biotec
Shalini Shenoy
Honoraria: Novartis, Jazz
Consulting or Advisory Role: as listed previously, California Institute of Regenerative Medicine
Travel, Accommodations, Expenses: as listed above
Aleksandra Petrovic
Consulting or Advisory Role: Orchard, CSL Behring
Kirk R. Schultz
Honoraria: Shire
Consulting or Advisory Role: MesoScale Discovery, Juno Therapeutics
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Gregory A. Yanik
Research Funding: Jazz Pharmaceuticals
Edmund K. Waller
Employment: Cambium Medical Technologies
Leadership: Cambium Medical Technologies, Cambium Oncology, Cambium Oncology
Stock and Other Ownership Interests: Cambium Medicl Technologies, Cambium Oncology, Cerus, Chimerix
Honoraria: Novartis, Partners, Verastem, Kite Pharma, Pharmacyclics, Karyopharma
Consulting or Advisory Role: Novartis, Verastem, Pharmacyclics, Karyopharm Therapeutics, Partners Healthcare, Kite Pharma
Research Funding: Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare, Partners Healthcare
Patents, Royalties, Other Intellectual Property: Received Royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies
Travel, Accommodations, Expenses: Pharmacyclics
John E. Levine
Consulting or Advisory Role: Incyte, Novartis, Talaris, Bluebird Bio, Mesoblast
Research Funding: Incyte, Kamada, Biogen
Patents, Royalties, Other Intellectual Property: GVHD biomarkers patent licensed to Viracor
James L. Ferrara
Honoraria: Incyte, Mallinckrodt, Equillium, Kamda
Consulting or Advisory Role: Omeros
Research Funding: Kamada, Incyte
Patents, Royalties, Other Intellectual Property: Royalties from an IP license
Travel, Accommodations, Expenses: Incyte
Bruce R. Blazar
Stock and Other Ownership Interests: Five Prime Therapeutics, BlueRock Therapeutics, Tmunity Therapeutics, Inc, Magenta Therapeutics
Honoraria: Kadmon, REGiMMUNE, Dr Reddy's Laboratories, Incyte, GT Biopharma, bioverativ
Consulting or Advisory Role: Kadmon, BlueRock Therapeutics, Magenta Therapeutics
Research Funding: Tmunity Therapeutics, Inc, Fate Therapeutics, BlueRock Therapeutics, Alpine Immune Sciences, RXI Pharmaceuticals, Abbvie
Travel, Accommodations, Expenses: Incyte, Magenta Therapeutics, Dr Reddy's Laboratories, bioverativ, Intellia Therapeutics, Rheos
Amelia Langston
Research Funding: Chimerix, Astellas Pharma, Incyte, Takeda, Jazz Pharmaceuticals, Kadmon, Novartis
Leslie S. Kean
Honoraria: Gilead Sciences
Consulting or Advisory Role: HiFiBio, Forty Seven, EMD Serono
Research Funding: Magenta Therapeutics, Bluebird Bio, Kyocera, Regeneron, Gilead Sciences, BEAM Therapeutics, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Licensing Fees for ABA2 clinical trial data
No other potential conflicts of interest were reported.
SUPPORT
Supported by: National Institutes of Health, FDA Orphan Drug Grant (NIH R01-FD004099), National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL095791), Bristol-Myers Squibb, CURE Childhood Cancer (L.S.K.); National Heart, Lung, and Blood Institute (K23 HL136900); National Institute of Child Health and Human Development (K12 HD 72245-4) (B.W.); American Cancer Society (129701-MRSG-16-153-01-CCE) (S.F.); and National Center for Advancing Translational Sciences (KL2TR000455) (M.Q.).
B.W. and M.Q. contributed equally to this work. A.L., J.T.H., and L.S.K. are cosenior authors. A.G.T. is posthumous.
DATA SHARING STATEMENT
Data collected for this study will be made available with publication to anyone who wishes to access the data for any purpose. This will include the protocol with all amendments, informed consent forms, complete de-identified patient data sets, and analytic or statistical code. Data will be available indefinitely at (http://www.keanlab.com).
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin Watkins, Muna Qayed, Marcelo C. Pasquini, Andre Rogatko, Mourad Tighiouart, Edmund K. Waller, Amelia Langston, John T. Horan, Leslie S. Kean
Provision of study materials or patients: Muna Qayed, James Rhodes, Sung W. Choi, Jeffery Davis, Christine Duncan, Roger Giller, Michael Grimley, Andrew C. Harris, David Jacobsohn, Nahal Lalefar, Maxim Norkin, Nosha Farhadfar, Michael A. Pulsipher, Shalini Shenoy, Aleksandra Petrovic, Kirk R. Schultz, Amelia Langston, John T. Horan, Leslie S. Kean
Collection and assembly of data: Benjamin Watkins, Muna Qayed, Brandi Bratrude, Kayla Betz, Yvonne Suessmuth, Alison Yu, Shauna Sinclair, Scott Furlan, Steve Bosinger, James Rhodes, Audrey Grizzle Tumlin, Alexandria Narayan, Kayla Cribbin, Marcelo C. Pasquini, Kyle Hebert, Urvi Kapoor, Catherine Bresee, Sung W. Choi, Jeffrey Davis, Christine Duncan, Roger Giller, Michael Grimley, David Jacobsohn, Nahal Lalefar, Maxim Norkin, Nosha Farhadfar, Michael A. Pulsipher, Shalini Shenoy, Aleksandra Petrovic, Kirk R. Schultz, John E. Levine, Amelia Langston, John T. Horan, Leslie S. Kean
Data analysis and interpretation: Benjamin Watkins, Muna Qayed, Kayla Betz, Yvonne Suessmuth, Scott Furlan, Victor Tkachev, Scott Gillespie, Ted A. Gooley, Marcelo C. Pasquini, Kyle Hebert, Urvi Kapoor, Andre Rogatko, Mourad Tighiouart, Sungjin Kim, Roger Giller, Michael Grimley, Andrew C. Harris, David Jacobsohn, Maxim Norkin, Michael A. Pulsipher, Shalini Shenoy, Gregory A. Yanik, Edmund K. Waller, John E. Levine, James L. Ferrara, Bruce R. Blazar, John T. Horan, Leslie S. Kean
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Trial of Costimulation Blockade With Abatacept for Prevention of Acute GVHD
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benjamin Watkins
Consulting or Advisory Role: Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent pending for “Method to prevent relapse after transplant”
Muna Qayed
Consulting or Advisory Role: Novartis, Mesoblast
Travel, Accommodations, Expenses: Novartis
Steve Bosinger
Research Funding: GlaxoSmithKline
Victor Tkachev
Travel, Accommodations, Expenses: Regeneron
Ted A. Gooley
Consulting or Advisory Role: Kiadis Pharma, Pharmacyclics, REGiMMUNE
Marcelo C. Pasquini
Consulting or Advisory Role: Pfizer, Medigene, Celgene, Amgen
Research Funding: Kite/Gilead, Novartis, Celgene, Bristol Myers Squibb
Roger Giller
Research Funding: Bristol-Myers Squibb, Jazz Pharmaceuticals, Atara
Maxim Norkin
Research Funding: Celgene
Nosha Farhadfar
Consulting or Advisory Role: Incyte
Research Funding: CSL Behring
Michael A Pulsipher
Honoraria: Amgen, Bellicum Pharmaceuticals, Miltenyi Biotec
Consulting or Advisory Role: Novartis, CSL Behring, Jasper Therapeutics, Novartis
Speakers' Bureau: Novartis
Research Funding: Adaptive Biotechnologies, Miltenyi Biotec
Travel, Accommodations, Expenses: Medac, Bellicum Pharmaceuticals, Miltenyi Biotec
Shalini Shenoy
Honoraria: Novartis, Jazz
Consulting or Advisory Role: as listed previously, California Institute of Regenerative Medicine
Travel, Accommodations, Expenses: as listed above
Aleksandra Petrovic
Consulting or Advisory Role: Orchard, CSL Behring
Kirk R. Schultz
Honoraria: Shire
Consulting or Advisory Role: MesoScale Discovery, Juno Therapeutics
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Gregory A. Yanik
Research Funding: Jazz Pharmaceuticals
Edmund K. Waller
Employment: Cambium Medical Technologies
Leadership: Cambium Medical Technologies, Cambium Oncology, Cambium Oncology
Stock and Other Ownership Interests: Cambium Medicl Technologies, Cambium Oncology, Cerus, Chimerix
Honoraria: Novartis, Partners, Verastem, Kite Pharma, Pharmacyclics, Karyopharma
Consulting or Advisory Role: Novartis, Verastem, Pharmacyclics, Karyopharm Therapeutics, Partners Healthcare, Kite Pharma
Research Funding: Novartis, Amgen, Juno Therapeutics, Verastem, Partners Healthcare, Partners Healthcare
Patents, Royalties, Other Intellectual Property: Received Royalties from patent on preparing platelet lysate that has been licensed to Cambium Medical Technologies
Travel, Accommodations, Expenses: Pharmacyclics
John E. Levine
Consulting or Advisory Role: Incyte, Novartis, Talaris, Bluebird Bio, Mesoblast
Research Funding: Incyte, Kamada, Biogen
Patents, Royalties, Other Intellectual Property: GVHD biomarkers patent licensed to Viracor
James L. Ferrara
Honoraria: Incyte, Mallinckrodt, Equillium, Kamda
Consulting or Advisory Role: Omeros
Research Funding: Kamada, Incyte
Patents, Royalties, Other Intellectual Property: Royalties from an IP license
Travel, Accommodations, Expenses: Incyte
Bruce R. Blazar
Stock and Other Ownership Interests: Five Prime Therapeutics, BlueRock Therapeutics, Tmunity Therapeutics, Inc, Magenta Therapeutics
Honoraria: Kadmon, REGiMMUNE, Dr Reddy's Laboratories, Incyte, GT Biopharma, bioverativ
Consulting or Advisory Role: Kadmon, BlueRock Therapeutics, Magenta Therapeutics
Research Funding: Tmunity Therapeutics, Inc, Fate Therapeutics, BlueRock Therapeutics, Alpine Immune Sciences, RXI Pharmaceuticals, Abbvie
Travel, Accommodations, Expenses: Incyte, Magenta Therapeutics, Dr Reddy's Laboratories, bioverativ, Intellia Therapeutics, Rheos
Amelia Langston
Research Funding: Chimerix, Astellas Pharma, Incyte, Takeda, Jazz Pharmaceuticals, Kadmon, Novartis
Leslie S. Kean
Honoraria: Gilead Sciences
Consulting or Advisory Role: HiFiBio, Forty Seven, EMD Serono
Research Funding: Magenta Therapeutics, Bluebird Bio, Kyocera, Regeneron, Gilead Sciences, BEAM Therapeutics, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: Licensing Fees for ABA2 clinical trial data
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bunin N, Carston M, Wall D, et al. Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission Blood 993151–31572002 [DOI] [PubMed] [Google Scholar]
- 2.Bunin NJ, Davies SM, Aplenc R, et al. Unrelated donor bone marrow transplantation for children with acute myeloid leukemia beyond first remission or refractory to chemotherapy J Clin Oncol 264326–43322008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation Blood 1104576–45832007 [DOI] [PubMed] [Google Scholar]
- 4.Shaw PJ, Kan F, Woo Ahn K, et al. Outcomes of pediatric bone marrow transplantation for leukemia and myelodysplasia using matched sibling, mismatched related, or matched unrelated donors Blood 1164007–40152010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolfrey A, Klein JP, Haagenson M, et al. HLA-C antigen mismatches are associated with worse outcomes in unrelated donor peripheral blood stem cell transplantation Biol Blood Marrow Transplant 17885–8922011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochs L, Shu XO, Miller J, et al. Late infections after allogeneic bone marrow transplantations: Comparison of incidence in related and unrelated donor transplant recipients Blood 863979–39861995 [PubMed] [Google Scholar]
- 7.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation Blood 119296–3072012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry N Engl J Med 371339–3482014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Fukuda T, Thakar MS, et al. Immunomodulatory effects induced by cytotoxic T lymphocyte antigen 4 immunoglobulin with donor peripheral blood mononuclear cell infusion in canine major histocompatibility complex-haplo-identical non-myeloablative hematopoietic cell transplantation Cytotherapy 131269–12802011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves SS, Stone D, Loretz C, et al. Establishment of long-term tolerance to SRBC in dogs by recombinant canine CTLA4-Ig Transplantation 88317–3222009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts N Engl J Med 3401704–17141999 [DOI] [PubMed] [Google Scholar]
- 12.Miller WP, Srinivasan S, Panoskaltsis-Mortari A, et al. GvHD after haploidentical transplant: A novel, MHC-defined rhesus macaque model identifies CD28-negative CD8+ T cells as a reservoir of breakthrough T cell proliferation during costimulation blockade and sirolimus-based immunosuppression Blood 1165403–54182010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohata J, Sakurai J, Saito K, et al. Differential graft-versus-leukaemia effect by CD28 and CD40 co-stimulatory blockade after graft-versus-host disease prophylaxis Clin Exp Immunol 12961–682002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamada K, Tamura H, Flies D, et al. Blockade of LIGHT/LTbeta and CD40 signaling induces allospecific T cell anergy, preventing graft-versus-host disease J Clin Invest 109549–5572002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor PA, Friedman TM, Korngold R, et al. Tolerance induction of alloreactive T cells via ex vivo blockade of the CD40:CD40L costimulatory pathway results in the generation of a potent immune regulatory cell Blood 994601–46092002 [DOI] [PubMed] [Google Scholar]
- 16.Via CS, Rus V, Nguyen P, et al. Differential effect of CTLA4Ig on murine graft-versus-host disease (GVHD) development: CTLA4Ig prevents both acute and chronic GVHD development but reverses only chronic GVHD J Immunol 1574258–42671996 [PubMed] [Google Scholar]
- 17.Koura DT, Horan JT, Langston AA, et al. In vivo T cell costimulation blockade with abatacept for acute graft-versus-host disease prevention: A first-in-disease trial Biol Blood Marrow Transplant 191638–16492013 [DOI] [PubMed] [Google Scholar]
- 18.Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials J Clin Oncol 237199–72062005 [DOI] [PubMed] [Google Scholar]
- 19.Finke J, Bethge WA, Schmoor C, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial Lancet Oncol 10855–8642009 [DOI] [PubMed] [Google Scholar]
- 20.Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts Blood 118282–2882011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors N Engl J Med 3441815–18222001 [DOI] [PubMed] [Google Scholar]
- 23.Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts Biol Blood Marrow Transplant 201573–15792014 [DOI] [PubMed] [Google Scholar]
- 24.Sauter C, Abboud M, Jia X, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin Biol Blood Marrow Transplant 171460–14712011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szabolcs P, Niedzwiecki D.Immune reconstitution after unrelated cord blood transplantation Cytotherapy 9111–1222007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia Blood 1261033–10402015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battipaglia G, Labopin M, Kroger N, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation Blood 134892–8992019 [DOI] [PubMed] [Google Scholar]
- 28.Bluestone JA, St Clair EW, Turka LA.CTLA4Ig: Bridging the basic immunology with clinical application Immunity 24233–2382006 [DOI] [PubMed] [Google Scholar]
- 29.Battipaglia G, Labopin M, Kroger N, et al. Post-transplant cyclophosphamide versus antithymocyte globulin in HLA-mismatched unrelated donors transplantation Blood 134892–8992019 [DOI] [PubMed] [Google Scholar]
- 30.Nahas MR, Soiffer RJ, Kim HT, et al. Phase 1 clinical trial evaluating abatacept in patients with steroid-refractory chronic graft-versus-host disease Blood 1312836–28452018 [DOI] [PubMed] [Google Scholar]
- 31.Jaiswal SR, Bhakuni P, Zaman S, et al. T cell costimulation blockade promotes transplantation tolerance in combination with sirolimus and post-transplantation cyclophosphamide for haploidentical transplantation in children with severe aplastic anemia Transpl Immunol 43-4454–592017 [DOI] [PubMed] [Google Scholar]
- 32.Khandelwal P, Chaturvedi V, Owsley E, et al. CD38(bright)CD8(+) T cells associated with the development of acute GVHD are activated, proliferating, and cytotoxic trafficking cells Biol Blood Marrow Transplant 261–62020 [DOI] [PubMed] [Google Scholar]
- 33.Paczesny S.Biomarkers for posttransplantation outcomes Blood 1312193–22042018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paczesny S, Braun TM, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]