INTRODUCTION
Significant progress has been made toward improving the care of older adults with cancer. As described elsewhere in this Special Series, the evidence base in geriatric oncology has grown substantially since the early calls for prioritizing this growing population. Nonetheless, oncologists still face uncertainty when making management decisions for older adults. This uncertainty arises from the persistent gap between healthier patients who are enrolled in clinical trials and frailer older patients who are typically treated in community practices.1,2 Adding to this complexity is the involvement in decision making of caregivers and other key persons, with varying influences on the decision structure and choice process.3
CONTEXT
Key Objective
This conceptual review on medical decision making in older adults with cancer synthesizes the evidence, identifies key knowledge gaps, and provides a decision framework that incorporates current best practices.
Knowledge Generated
Although the evidence base in geriatric oncology is expanding, oncologists must continue to make treatment decisions under considerable uncertainty. Our decision framework helps optimize decision making on the basis of three principles: (1) determining age-related vulnerability using the geriatric assessment, (2) considering the overall benefits and harms of cancer treatments for older adults in light of this vulnerability, and (3) incorporating patient values and preferences.
Relevance
Using this framework matches treatment intensity with age-related vulnerability and aligns expected outcomes with preferences, thus minimizing undertreatment and overtreatment in older adults with cancer. This framework can be adapted to all cancer types and treatment modalities and will strengthen as the evidence in geriatric oncology continues to grow.
Our purpose in this review is to assist oncologists in navigating this complexity by summarizing what is known regarding shared decision making in older adults with cancer. Our goals are to synthesize the evidence, identify knowledge gaps, and provide a decision framework that incorporates current best practices. We will focus on decision making for older adults with cancer diagnoses that require a treatment decision. Decision making related to cancer screening in older adults is an important, well-studied, but separate topic covered elsewhere.4 Our framework can be adapted to any cancer type or treatment modality, and we emphasize the role of the geriatric assessment (GA) in guiding the decision-making process. Throughout, we will illustrate our framework using two case examples that reflect notable uncertainty (Table 1).
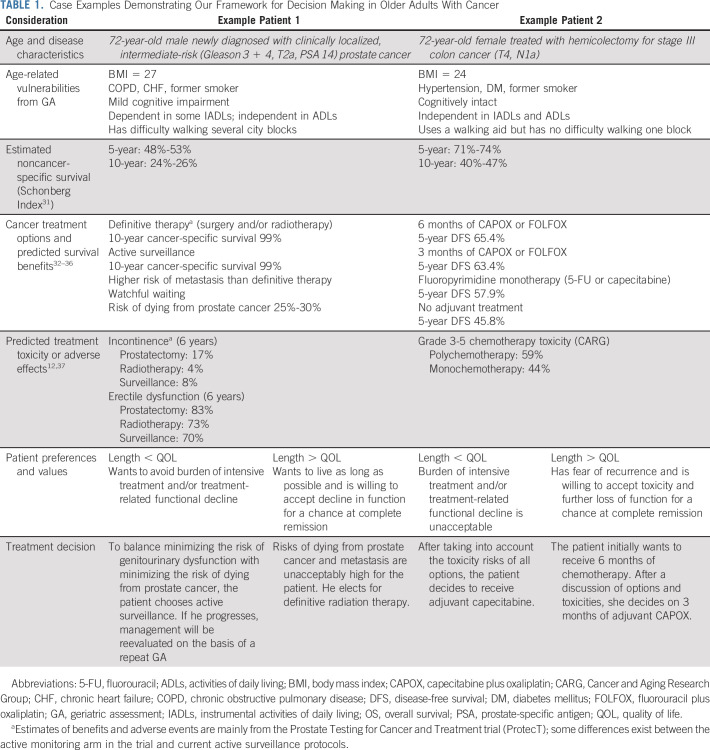
TABLE 1.
Case Examples Demonstrating Our Framework for Decision Making in Older Adults With Cancer
It is important to distinguish our approach from other perspectives.5 Our focus is on clinical decision making for individual patients, not decision analysis for populations. These individualized decisions follow the principles of bioethics in respecting patient autonomy, practicing nonmaleficence and beneficence, and seeking justice.6 Decisions regarding populations rely on principles of resource allocation, cost-effectiveness, and policy making7—also separate topics that will not be discussed here. Our aim is to review the elements of sound, shared decision making in older adults with cancer considering cancer treatment to maximize benefits and minimize harms for each individual patient. In following this process for each older adult, we believe that allocation of cancer treatments within a population will more often be directed to those most likely to benefit.
FOUNDATION AND FRAMEWORK FOR DECISION MAKING IN OLDER ADULTS WITH CANCER
Our framework for clinical decision making in older adults with cancer is built on a foundation of evidence-based medicine (EBM). The core tenets of EBM best guide clinicians in translating empirical evidence into practice. These tenets comprise the need to consider the totality of evidence for a given question, the need to appraise the quality of the evidence, and the need to incorporate a patient's values and preferences—especially when the evidence is limited and uncertainty is highest.8 A fundamental step in improving decision making in older adults with cancer is continuing to build the underlying evidence base, especially by including high-quality randomized controlled trials. Following years of exclusion of older adults from clinical trials, the National Institutes of Health’s Inclusion Across the Lifespan policy now requires that funded clinical studies represent more of the older cancer population who are most likely to be candidates for therapies.9 Complementing the expansion of trial eligibility is the need for rigorous observational studies investigating treatment effects in frailer patients who are excluded from trials but often receive novel therapies in practice.10,11
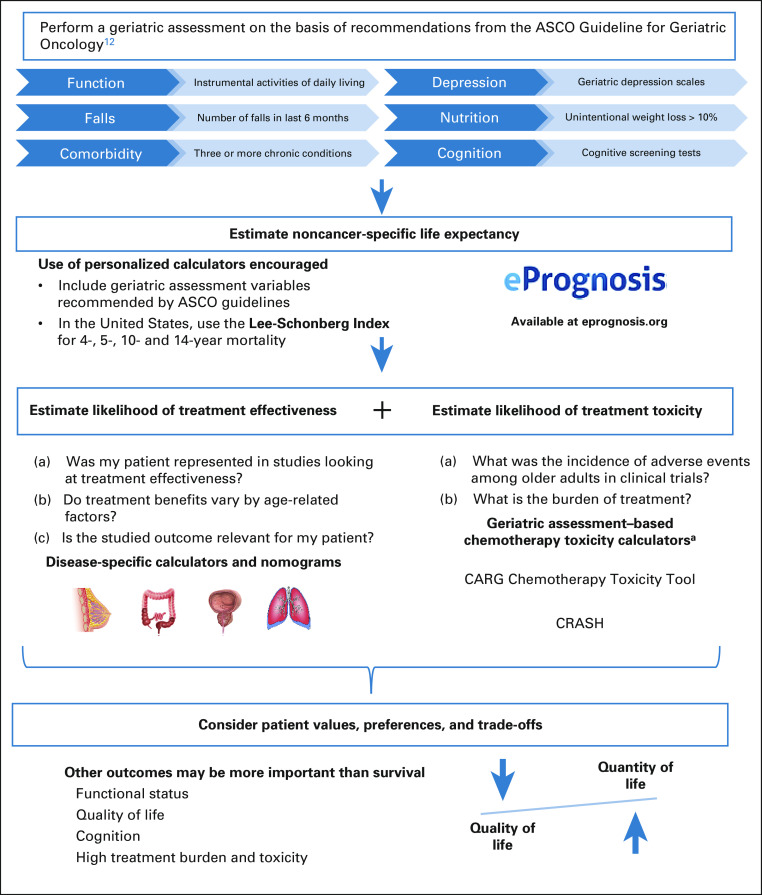
Meanwhile, oncologists must continue to make decisions in older adults with cancer on the basis of the available evidence. To optimize clinical decision making, we propose a framework (Fig 1) that synthesizes the available evidence and synergizes guidelines and recommendations espoused by ASCO,12 the National Comprehensive Cancer Network,13 the International Society of Geriatric Oncology,14 and the American Geriatrics Society.15 Our framework is based on three principles: (1) determining age-related vulnerability using the GA, (2) considering the benefits and harms of cancer treatments in light of this vulnerability, and (3) considering patient values, preferences, and trade-offs (balancing desirable but conflicting outcomes, eg, prolonging survival while minimizing treatment burden and toxicity).
FIG 1.
Framework for decision making in older adults with cancer. aCurrent toxicity calculators exist for chemotherapy only. For surgical risks, consider the ACS NSQIP Surgical Risk Calculator, which was recently updated to include outcomes for older adults.41 ACS, American College of Surgeons; CARG, Cancer and Aging Research Group; CRASH, Chemotherapy Risk Assessment Scale for High-Age Patients; NSQIP, National Surgical Quality Improvement Program.
The GA forms the core of this framework because it best identifies older adults who are resilient enough to tolerate intensive cancer treatments for the best chances of controlling their cancer. In addition, the GA can identify older adults who are frail and vulnerable and therefore at high risk of treatment toxicity.16,17 Finally, the GA can be used to estimate life expectancy. We highlight below how the GA is essential for all three principles of shared decision making in older adults with cancer.
CONSIDERING BENEFITS OF CANCER TREATMENT IN OLDER ADULTS
To determine whether a cancer treatment is beneficial in an older patient, one must first estimate whether the patient's cancer will cause symptoms in their remaining lifetime. This determination involves evaluating the aggressiveness of the patient's malignancy and estimating their noncancer-specific life expectancy. Estimation of noncancer-specific life expectancy helps frame the probability of dying from another condition before one's cancer causes significant symptoms. For example, early-stage prostate cancer, although indolent, is still likely to affect a fit 55-year-old male with minimal comorbidities because of his long remaining life expectancy. It is less likely to affect a vulnerable 72-year-old with multiple comorbidities whose 5- and 10-year mortality risks are already high (Table 1).
To estimate noncancer-specific life expectancy, clinicians can use prognostic calculators such as those found on the ePrognosis website.18 These calculators are derived from prognostic models that have been developed and validated in thousands of patients for use in a variety of settings—from older adults living in the community to those nearing end of life.19,20 These calculators rely on variables from the GA, such as functional status, comorbidities, and cognition (of note, these models were derived from US populations).
If a cancer is likely to affect a patient during their remaining lifetime, then evidence must be appraised for beneficial treatments. For older adults, this appraisal includes the following: (1) Was my patient represented in the study of effectiveness in terms of age, comorbidities, and health status (generalizability)? (2) Does the treatment benefit vary by any one of these age-related factors (heterogeneity of treatment effect21)? (3) Is the studied outcome important to the older patient in front of me? The generalizability of trial results is often limited in older adults treated in practice. Assessment of heterogeneity in treatment effects is also limited since even those older adults included in trials have less chronic conditions and better health status than most older adults treated in practice.1–3,22 Nonetheless, one can reasonably assume that, in terms of controlling disease, the effectiveness of cancer therapies is similar by age.23
The third question, regarding whether the outcome investigated as a treatment benefit is relevant to the particular older adult, is often difficult to answer. Most outcomes studied in trials are survival, survival surrogates (eg, progression-free survival), or other disease-specific outcomes (eg, biomarkers and tumor response and/or regression).24,25 Yet how tumor shrinkage or biomarker reduction translates to improvements in function, quality, or even quantity of life is often unclear.26–29 This lack of relevant outcomes valued by older adults has led to efforts to encourage not only the inclusion of older patients in trials but also the incorporation of patient-centered outcomes such as function and quality of life (QOL).11,30 These efforts will generate better evidence to inform not only whether an older patient is likely to experience a benefit from cancer treatment in their remaining lifetime, but also whether that benefit is meaningful. Without this evidence, oncologists must rely on their experience and judgment but should clearly communicate this uncertainty (ie, lack of data). Receiving input and comanagement from different specialties—especially multidisciplinary teams involving geriatricians, primary care physicians, palliative care experts, and/or allied health professionals—can improve patient-centered outcomes even when evidence is lacking.
CONSIDERING HARMS OF CANCER TREATMENT IN OLDER ADULTS
If a cancer treatment is found to provide patient-centered benefit, then the oncologist must weigh that benefit against the risks of toxicities, ie, the harms. These harms vary across treatment intensity (high-risk surgery v stereotactic radiation) and patient health status (increased toxicity in patients with many geriatric syndromes). For example, consider a 72-year-old fit female with stage III colon cancer deciding among various adjuvant treatment options after hemicolectomy (Table 1). Each option offers improvement in disease-free survival compared with no adjuvant therapy, but these benefits must be weighed against the risk of severe toxicity.
Moreover, the burden of treatment adherence must also be considered as time spent in infusion clinics or in the hospital after surgery is time spent away from home and family.38 Finally, the financial toxicity of cancer treatments is especially relevant in older adults.39,40 The exorbitant and escalating costs of cancer treatments may significantly and unduly affect QOL for many patients.
To estimate the harms from a given cancer treatment in an older adult, clinicians should apply the same three appraisal questions as when estimating treatment benefit: (1) generalizability, (2) heterogeneity of treatment effect, and (3) relevance of outcomes measured. Even if there is little difference in the likelihood of cancer control by a given treatment between older and younger adults, harms may vary significantly by age group.
Consider the following examples. For patients with early-stage breast cancer, surgical resection is considered first-line therapy on the basis of consensus guidelines and trial data.42–44 However, Tang et al45 reported that frail older nursing home residents who underwent breast cancer surgery experienced 30%-40% 1-year mortality; among survivors, 56-60% experienced functional decline. For patients with primary CNS lymphoma, high-dose methotrexate-based chemotherapy is considered first-line therapy on the basis of trial outcomes,46 but patients treated in real-world settings are significantly older, have worse performance status, and experience worse outcomes47; Houillier et al48 reported that a subgroup of 239 adults with a median age of 73 years had 25% mortality within 6 months that was largely attributed to complications of comorbidities and treatment toxicity. For acute myeloid leukemia (AML), intensive multiagent chemotherapy regimens have historically been considered first-line on the basis of trials in younger, fitter patients,49 but older patients treated in practice with intensive regimens have significantly worse outcomes; Kantarjian et al50 reported that patients age ≥ 70 years demonstrated 35% mortality at 8 weeks despite reasonable cancer response rates. The last example reinforces the need for caution when generalizing treatment benefits founded on cancer-specific response criteria in a fitter population to frailer adults treated in practice. Cancer response, progression-free survival, and cancer-specific survival may not always correlate with overall survival; the latter is also influenced by treatment toxicity, comorbidities, and other age-related factors.24–26,51
Results of the last study50 also reinforce the need to better estimate the likelihood of treatment toxicity in older adults, identifying those at increased risk of harm. The GA performs better at discriminating this risk than traditional performance status measures used in oncology (Eastern Cooperative Oncology Group and Karnofsky).17 Considering chemotherapy toxicity, ASCO recommends the Cancer and Aging Research Group (CARG) and the Chemotherapy Risk Assessment Scale for High-Age Patients risk scores.12,52 More cancer-specific prediction models are being developed with the promise of more accurate and precise predictions of toxicity in comparison with the general CARG toxicity tools.53
After estimating toxicity risk through the identification of age-related vulnerabilities, oncologists can decide to (1) adjust treatment decisions (eg, up-front dose reduction for chemotherapy) and/or (2) prescribe appropriate interventions for modifiable GA deficits.54,55 Recent trials in older adults with cancer suggest that, through these two mechanisms, GA-guided care can mitigate toxicity and unplanned healthcare utilization without sacrificing treatment benefit.56–58 Further work is needed to better assess how novel cancer treatments affect relevant patient-centered outcomes in older adults such as function and QOL (appraisal question 3). As with treatment benefits, oncologists should be honest about the uncertainty regarding potential harms to patient-centered outcomes, and engagement with multidisciplinary teams can help minimize losses to function and QOL.
CONSIDERING VALUES, PREFERENCES, AND TRADE-OFFS IN OLDER ADULTS
Once benefits and harms are estimated, the oncologist must balance trade-offs in the context of an older adult's values and preferences. Treatment benefits, harms, and their relative importance may be perceived differently by older versus younger patients. Younger adults, with a long noncancer life expectancy, are more often willing to tolerate the risks and burdens of intensive therapies to preserve life expectancy.59 In contrast, older patients are less often willing to sacrifice QOL in exchange for quantity.60–62
To determine the relative importance of treatment benefits and harms, oncologists must elicit the patient's values and preferences regarding their health. Several validated tools can elicit preferences. The Patient Priorities Care website63 provides conversation guides for both clinicians and patients and caregivers.64 The Prepare for Your Care website65 provides handouts and interactive modules that help a patient list specific priorities (eg, participating in hobbies) and specific fears (eg, not being able to feed, bathe, or take care of one's self) and gauge whether quality and quantity of life are more or equally important compared with each other.66 Once elicited, values and preferences can help determine which treatment benefits are most desired, which harms are unacceptable, and whether a given treatment constitutes a net benefit or net harm. Comparing the time to benefits with the time to harms must also be considered within an older adult's remaining life expectancy.67,68
For example, to the vulnerable older male with prostate cancer, fear of metastasis in the future may drive an up-front desire for definitive treatment, accepting the immediate risks to genitourinary functioning within his remaining lifetime (Table 1). Conversely, if he values maintaining genitourinary functioning for as long as possible—accepting a higher risk of future metastasis or even prostate-related death—then active surveillance or watchful waiting likely provides the greatest net benefit. Similarly, to the fit older woman with stage III colon cancer following hemicolectomy, the desire to extend life without recurrence for as long as possible may drive more intensive adjuvant therapy (Table 1). On the other hand, if she values QOL more than quantity, then a less intensive option balances gains in disease-free survival with risk of severe toxicity that threatens her function and QOL.
The Patient Priorities Care63 and Prepare for Your Care65 websites are examples of decision aids that have shown usefulness in engaging patients with cancer in the decision-making process.69 Another example gaining popularity in oncology is the Best Case/Worst Case tool, which was originally developed for high-stakes decisions regarding surgery70 but has been increasingly used in scenarios involving other forms of cancer treatment. To illustrate, Kiely et al71 used data from 36 randomized trials of breast cancer to devise the best-case and worst-case scenarios for survival benefits in patients starting first-line chemotherapy. These scenarios may help patients visualize more concretely the best and worst outcomes to balance trade-offs in light of their preferences.
CAREGIVERS AND CULTURE
A comprehensive understanding of aging and decision making must go beyond individual patient characteristics and consider contextual factors. Real-life decision making is embedded in a social context, in which other individuals, especially family members, caregivers, and members of the healthcare team, provide input on behalf of patients.72 Conversely, family members may attempt to shield their older relatives from pain by withholding information about prognosis and treatment. The influence of family members on decision making is higher among older individuals and especially among those from underrepresented racial and ethnic groups.73
Unfortunately, few studies of shared decision making in cancer treatment have focused on patients from underrepresented populations. A systematic review found that most studies exploring shared decision making included mainly non-Hispanic White participants.74 Models developed from such studies may not be appropriate for generalizing to patients from underrepresented populations, who in some instances have been shown to be more likely to make decisions with input from family members.73 Additionally, patients from racial and ethnic groups who have experienced discrimination within the healthcare system may be more likely to hold negative beliefs and mistrust.75 However, it is important to note that although decisional control and communication preferences may be influenced by race or language, stereotyping patients on the basis of their cultural or ethnic backgrounds should never be done. Instead, physicians should actively engage the patient and their caregivers in the decision-making process,76 develop and improve their communication skills,77 and build trust.
A helpful way to frame the multiple contextual factors that influence those involved in the decision-making process (patients, caregivers, community members, and clinicians) is that of the decision-making unit. This concept, initially developed for policy decision making, attempts to categorize the way in which groups of individuals work together in times of crisis to reach a common decision.78 In general, three types of decision-making units are recognized: those with a predominant leader, where a single individual has the power to make decisions alone if necessary; those acting as a single group, where all members collectively select a course of action in consultation with each other; and coalitions, in which all members act as separate individuals and provide input, with no single member having the power to decide for the others.79 Understanding the type of decision-making dynamics of older patients with cancer and identifying the prevailing values (egalitarianism, individualism, and fatalism) within the decision-making unit can be useful for guiding the shared decision-making process to reach an outcome that is satisfactory to all.
PSYCHOLOGY, COGNITIVE BIASES, AND INFORMED CONSENT
Oncologists should be aware of the various biases by which both they and their older patients can be influenced during the decision-making process (Table 2). At the heart of these biases is a failure to balance type 1 thinking, or intuitive and fast reasoning, with type 2 thinking, or deliberative and slow thinking.80 Too much of the former can occur when too little time is dedicated to the decision process, as in acute scenarios where time-sensitive decisions must be made. In such scenarios, emotions can cloud sound reasoning and judgment (ie, affect heuristic).81 Patient anxiety or depression can override EBM choices about treatment options in cancer.82 Anxiety can influence oncologists as well. An oncologist's fear of a patient's cancer spreading or recurring after choosing to withhold treatment may encourage more intensive treatment up front—even if limited evidence supports it.83 Conversely, erring on the side of pure, calculated reasoning can remove the humanistic component of decision making. Although there is debate about normative versus accurate decisions that are either too emotionally laden or too bare, experts in decision making generally agree that a healthy mix of both type 1 and type 2 thinking helps arrive at the best decisions.80
TABLE 2.
Selected Biases to Avoid in Decision Making Involving Older Adults With Cancer
A special bias affecting decision making in older adults is ageism: negative attitudes and stereotypes associated with aging.84 Ageism among clinicians may lead to foregoing beneficial therapies, poor access to therapeutic options, poor communication about competing outcomes, and a paternalistic approach to decision making (see Minimizing Undertreatment and Overtreatment, below).85 Furthermore, older adults may hold biases or assumptions about themselves (self-ageism), which lead to a lower likelihood to seek treatment for unmet medical needs.84 Ageism may also occur among caregivers, leading to an inappropriate reduction in patient autonomy.86 Decisions should never be made on the basis of chronologic age alone.
To facilitate informed consent in decision making, it is important to promote disease and treatment understanding (eg, treatment options, outcomes, and prognosis) with older patients and their caregivers. Assessment of prognostic understanding may occur in the forms of treatment goal or intent, illness perception, curability, and survival.87–90 Studies have demonstrated that 50%-73% of older adults with advanced cancer have poor prognostic understanding or that their prognostic understanding differs from that of their oncologist.87,89,91 In addition, 48%-52% of caregivers of older adults with advanced cancer have differing prognostic understanding from that of the patients and their oncologists.88,89 Poor patient prognostic understanding is associated with receipt of more aggressive care such as chemotherapy at the end of life.92 In addition, differing prognostic understanding between patients and their caregivers may be associated with lower utilization of hospice services.93 To date, few interventions, other than palliative care, have been shown to improve disease understanding.94
Inherent in the above discussion is the need to confirm decision-making capacity in an older patient, which requires (1) understanding, (2) expressing a choice, (3) appreciation, and (4) reasoning.95 This determination is made clinically (v legally in the courts, ie, competency) and concerns specific treatment decisions (v decision-making ability as a whole). Determination of capacity is especially important in the older population, given the increased prevalence of cognitive impairment. However, although the presence of cognitive impairment increases the chances of being impaired in one or more of the four abilities mentioned above, cognitive impairment by itself is insufficient to deem a patient incapacitated. If an older patient is deemed to lack capacity for a particular treatment decision, then their healthcare proxy should be involved in making the final decision as a surrogate representing the patient's values. If no proxy is available, then institutional and state policies regarding identification of a surrogate decision maker should be followed.99
There is also debate regarding the degree to which a clinician should be involved in assisting the patient with making the final decision. Consider in older adults with AML the choice between intensive induction chemotherapy versus lower-intensity outpatient treatments. Practice patterns vary widely, from physicians recommending a decision (with or without accounting for patient preferences) to patients making the final decision by themselves.100 Only limited evidence is available on what approach is best, but the extremes should be avoided: a fully clinician-determined decision without considering patient preference is paternalistic, and patients demanding treatments that are of no benefit is futility.6 It is a patient's right, however, to request that the physician make a recommendation for them, and in such circumstances the physician should do so on the basis of the patient's values. Some have even argued that stronger clinician-driven recommendations are the preferred approach when uncertainty regarding harms and benefits of treatment is highest, as it is unreasonable to believe the patient can navigate the complexity of knowing and interpreting all available evidence.101
MINIMIZING UNDERTREATMENT AND OVERTREATMENT
In an older patient with cancer, biased and uninformed decision making too often leads to one of the two adverse outcomes: undertreatment or overtreatment.102 One of ASCO's research priorities in 2021 is to investigate the use of personalized care guided by the GA to minimize undertreatment and overtreatment. Unfortunately, bias and imprecision exist in the very use of these terms that could paradoxically lead to overtreatment when trying to avoid undertreatment, and vice versa.102 Undertreatment of an older adult is often used to denote the prescription of less than a recommended or guideline therapy, not accounting for the underrepresentation of older adults in the evidence underlying guidelines and relevant GA measures. Overtreatment is variably used to denote either the prescription of an intensive treatment in a frail older adult or an intensive treatment of a cancer not expected to affect an older adult in their remaining lifetime. Only half of all articles advocated for use of the GA to aid in risk stratification for treatment intensity, and only a quarter advocated for its use to identify age-related vulnerabilities for supportive care alongside whatever cancer treatment was chosen.
Sound, shared decision making is essential to minimizing under- and overtreatment, but bias and imprecision in the use of these terms may affect the decision-making process. Our review found an overemphasis on disease-specific survival measures that neglects other risk factors and outcomes important in older adults, and an underemphasis on patient preferences and QOL that neglects their indispensability in determining the very goals of treatment by which under- and overtreatment should be judged. To help overcome this bias and imprecision, we propose new definitions of undertreatment and overtreatment (Table 3). These definitions shift disease-centric criteria to patient-centered criteria, with a broader focus on not only survival but also function and QOL. Incorporating these elements in the decision-making process will best minimize undertreatment and overtreatment in older adults with cancer.
TABLE 3.
Newly Proposed Definitions of Undertreatment and Overtreatment in Older Adults With Cancer101
In conclusion, given the complexity of cancer in older patients, the uncertainty in the face of incomplete evidence, and the potential for bias leading to under- or overtreatment, best practice for decision making in these patients requires a principled approach. We believe that use of our framework (Fig 1) will optimize decisions, best matching treatment intensity with age-related vulnerability and aligning expected outcomes with patient preferences. We stress the importance of focus on a process, informed by the GA, that actively engages the patient and their caregiver and allows for emotions, intuition (type 1 reasoning), and deliberative reasoning (type 2 reasoning). Strong evidence suggests that regardless of its impact on survival and function, such a decision-making process improves communication and satisfaction with care103—outcomes important in and of themselves. As the evidence accumulates regarding the benefits and harms of cancer treatment in older adults, it can be applied using this framework and evaluated across different scenarios to optimize decision making.
ACKNOWLEDGMENT
We wish to acknowledge Dr Susan Rosenthal for her editorial assistance.
Kah Poh Loh
Consulting or Advisory Role: Pfizer, Seattle Genetics
Enrique Soto-Perez-de-Celis
Research Funding: Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Harvard Translational Research in Aging Training Program (National Institute on Aging of the National Institutes of Health: T32AG023480) (C.D.); the Wilmot Research Fellowship and National Cancer Institute (K99 CA237744) (K.P.L.); the Conquer Cancer (The ASCO Foundation) Career Development Award and the National System of Researchers of Mexico (E.S.); Geriatric Oncology Research Infrastructure to Improve Clinical Care (NIA R33AG059206) and Mentoring the Next Generation of Geriatric Oncology Researchers in Patient-Oriented Research to Improve the Care of Older Adults With Cancer (NIA K24AG055693) (W.D.).
AUTHOR CONTRIBUTIONS
Conception and design: Clark DuMontier, Kah Poh Loh, Enrique Soto-Perez-de-Celis, William Dale
Administrative support: Clark DuMontier, William Dale
Financial support: Clark DuMontier, William Dale
Provision of study materials or patients: Clark DuMontier, Kah Poh Loh, Enrique Soto-Perez-de-Celis
Collection and assembly of data: Clark DuMontier, Kah Poh Loh, Enrique Soto-Perez-de-Celis
Data analysis and interpretation: Clark DuMontier, Kah Poh Loh, Enrique Soto-Perez-de-Celis, William Dale
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Decision Making in Older Adults With Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kah Poh Loh
Consulting or Advisory Role: Pfizer, Seattle Genetics
Enrique Soto-Perez-de-Celis
Research Funding: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: A systematic review of barriers and interventions CA Cancer J Clin 7178–922021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated with age disparities among cancer clinical trial participants JAMA Oncol 51769–17732019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadambi S, Loh KP, Dunne R, et al. Older adults with cancer and their caregivers—Current landscape and future directions for clinical care Nat Rev Clin Oncol 17742–7552020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter LC, Covinsky KE.Cancer screening in elderly patients: A framework for individualized decision making JAMA 2852750–27562001 [DOI] [PubMed] [Google Scholar]
- 5.Djulbegovic B, Elqayam S, Dale W.Rational decision making in medicine: Implications for overuse and underuse J Eval Clin Pract 24655–6652018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauchamp TL, Childress JF. Principles of Biomedical Ethics. ed 7. New York, NY: Oxford University Press; 2013. [Google Scholar]
- 7.Persad G, Wertheimer A, Emanuel EJ.Principles for allocation of scarce medical interventions Lancet 373423–4312009 [DOI] [PubMed] [Google Scholar]
- 8.Djulbegovic B, Guyatt GH.Progress in evidence-based medicine: A quarter century on Lancet 390415–4232017 [DOI] [PubMed] [Google Scholar]
- 9.Vaughan CP, Dale W, Allore HG, et al. AGS report on engagement related to the NIH inclusion across the lifespan policy J Am Geriatr Soc 67211–2172019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platt R, Brown JS, Robb M, et al. The FDA Sentinel Initiative—An evolving national resource N Engl J Med 3792091–20932018 [DOI] [PubMed] [Google Scholar]
- 11.Levit LA, Singh H, Klepin HD, et al. Expanding the evidence base in geriatric oncology: Action items from an FDA-ASCO workshop J Natl Cancer Inst 1101163–11702018 [DOI] [PubMed] [Google Scholar]
- 12.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology J Clin Oncol 362326–23472018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network . Older Adult Oncology (version 11.2020) https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf [Google Scholar]
- 14.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer J Clin Oncol 322595–26032014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd C, Smith CD, Masoudi FA, et al. Decision making for older adults with multiple chronic conditions: Executive summary for the American Geriatrics Society Guiding Principles on the Xare of Older Adults With Multimorbidity J Am Geriatr Soc 67665–6732019 [DOI] [PubMed] [Google Scholar]
- 16.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study J Clin Oncol 293457–34652011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer J Clin Oncol 342366–23712016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ePrognosis . Calculators. https://eprognosis.ucsf.edu/calculators/#/ [Google Scholar]
- 19.Lee SJ, Lindquist K, Segal MR, et al. Development and validation of a prognostic index for 4-year mortality in older adults JAMA 295801–8082006 [DOI] [PubMed] [Google Scholar]
- 20.Schonberg MA, Davis RB, McCarthy EP, et al. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey J Gen Intern Med 241115–11222009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kent DM, Paulus JK, van Klaveren D, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement Ann Intern Med 17235–452020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passaro A, Spitaleri G, Gyawali B, et al. Immunotherapy in non-small-cell lung cancer patients with performance status 2: Clinical decision making with scant evidence J Clin Oncol 371863–18672019 [DOI] [PubMed] [Google Scholar]
- 23.Helissey C, Vicier C, Champiat S.The development of immunotherapy in older adults: New treatments, new toxicities? J Geriatr Oncol 7325–3332016 [DOI] [PubMed] [Google Scholar]
- 24.Mailankody S, Prasad V.Overall survival in cancer drug trials as a new surrogate end point for overall survival in the real world JAMA Oncol 3889–8902017 [DOI] [PubMed] [Google Scholar]
- 25.Mailankody S, Prasad V.Overall survival vs disease-specific survival-reply JAMA Oncol 4586–5872018 [DOI] [PubMed] [Google Scholar]
- 26.Davis S, Tappenden P, Cantrell A. NICE Decision Support Unit Methods Development, A Review of Studies Examining the Relationship between Progression-Free Survival and Overall Survival in Advanced or Metastatic Cancer. London, United Kingdom: National Institute for Health and Care Excellence (NICE); 2012. [PubMed] [Google Scholar]
- 27.Gyawali B, Hey SP, Kesselheim AS.Assessment of the clinical benefit of cancer drugs receiving accelerated approval JAMA Intern Med 179906–9132019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gyawali B, Niraula S. Cancer treatment in the last 6 months of life: When inaction can outperform action. Ecancermedicalscience. 2018;12:826. doi: 10.3332/ecancer.2018.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang TJ, Gyawali B.Association between progression-free survival and patients' quality of life in cancer clinical trials Int J Cancer 1441746–17512019 [DOI] [PubMed] [Google Scholar]
- 30.Rocque GB, Williams GR.Bridging the data-free zone: Decision making for older adults with cancer J Clin Oncol 373469–34712019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ePrognosis . Lee Shonberg Index. https://eprognosis.ucsf.edu/leeshonberg.php [Google Scholar]
- 32.Sobrero AF, Puccini A, Shi Q, et al. A new prognostic and predictive tool to enhance shared decision making in stage III colon cancer. J Clin Oncol. 2020;38 doi: 10.1016/j.ejca.2020.07.031. suppl; abstr 4010. [DOI] [PubMed] [Google Scholar]
- 33.Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer N Engl J Med 3751415–14242016 [DOI] [PubMed] [Google Scholar]
- 34.Bekelman JE, Rumble RB, Chen RC, et al. Clinically localized prostate cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline J Clin Oncol 363251–32582018 [DOI] [PubMed] [Google Scholar]
- 35.Roswell Park. Calculator for Estimating Overall Life Expectancy and Lifetime Risk for Prostate Cancer Death in Newly Diagnosed Men Managed Without Definitive Local Therapy. https://www.roswellpark.org/apps/prostate_cancer_estimator/
- 36.Kim HL, Puymon MR, Qin M, et al. A method for using life tables to estimate lifetime risk for prostate cancer death J Natl Compr Canc Netw 8148–1542010 [DOI] [PubMed] [Google Scholar]
- 37.Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer N Engl J Med 3751425–14372016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chesney TR, Haas B, Coburn NG, et al. Patient-centered time-at-home outcomes in older adults after surgical cancer treatment. JAMA Surg. 2020;155:e203754. doi: 10.1001/jamasurg.2020.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arastu A, Patel A, Mohile SG, et al. Assessment of financial toxicity among older adults with advanced cancer. JAMA Netw Open. 2020;3:e2025810. doi: 10.1001/jamanetworkopen.2020.25810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giri S, Clark D, Al-Obaidi M, et al. Financial distress among older adults with cancer. JCO Oncol Pract. 10.1200/OP.20.00601 epub ahead of print on October 30, 2020. [DOI] [PMC free article] [PubMed]
- 41.Hornor MA, Ma M, Zhou L, et al. Enhancing the American College of Surgeons NSQIP surgical risk calculator to predict geriatric outcomes J Am Coll Surg 23088–100E12020 [DOI] [PubMed] [Google Scholar]
- 42.van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study Lancet Oncol 171158–11702016 [DOI] [PubMed] [Google Scholar]
- 43.Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer JAMA Surg 149267–2742014 [DOI] [PubMed] [Google Scholar]
- 44.National Comprehensive Cancer Network . Breast Cancer (version 1.2021) https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang V, Zhao S, Boscardin J, et al. Functional status and survival after breast cancer surgery in nursing home residents JAMA Surg 1531090–10962018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network . Central Nervous System Cancers (version 3) 2020. [Google Scholar]
- 47.Zeremski V, Koehler M, Fischer T, et al. Characteristics and outcome of patients with primary CNS lymphoma in a “real-life” setting compared to a clinical trial Ann Hematol 95793–7992016 [DOI] [PubMed] [Google Scholar]
- 48.Houillier C, Soussain C, Ghesquieres H, et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study Neurology 94e1027–e10392020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Comprehensive Cancer Network . Acute Myeloid Leukemia (version 2.2021) https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf [Google Scholar]
- 50.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia Blood 1164422–44292010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haslam A, Hey SP, Gill J, et al. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology Eur J Cancer 106196–2112019 [DOI] [PubMed] [Google Scholar]
- 52.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score Cancer 1183377–33862012 [DOI] [PubMed] [Google Scholar]
- 53.Magnuson A, Sedrak MS, Gross CP, et al. Development and validation of a risk tool for predicting severe toxicity in older adults receiving chemotherapy for early-stage breast cancer J Clin Oncol 39608–6182021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamaker ME, Te Molder M, Thielen N, et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients—A systematic review J Geriatr Oncol 9430–4402018 [DOI] [PubMed] [Google Scholar]
- 55.Mohile SG, Magnuson A, Pandya C, et al. Community oncologists' decision-making for treatment of older patients with cancer J Natl Compr Canc Netw 16301–3092018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohile SG, Mohamed MR, Culakova E, et al. A geriatric assessment (GA) intervention to reduce treatment toxicity in older patients with advanced cancer: A University of Rochester Cancer Center NCI Community Oncology Research Program cluster randomized clinical trial (CRCT) J Clin Oncol. 2020;38 suppl; abstr 12009. [Google Scholar]
- 57.Li D, Sun CL, Kim H, et al. Geriatric assessment-driven intervention (GAIN) on chemotherapy toxicity in older adults with cancer: A randomized controlled trial. J Clin Oncol. 2020;38 doi: 10.1001/jamaoncol.2021.4158. suppl; abstr 12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soo W-K, King M, Pope A, et al. Integrated geriatric assessment and treatment (INTEGERATE) in older people with cancer planned for systemic anticancer therapy. J Clin Oncol. 2020;38 doi: 10.1016/S2666-7568(22)00169-6. suppl; abstr 12011. [DOI] [PubMed] [Google Scholar]
- 59.Shrestha A, Martin C, Burton M, et al. Quality of life versus length of life considerations in cancer patients: A systematic literature review Psychooncology 281367–13802019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loh KP, Mohile SG, Epstein RM, et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults Cancer 1252506–25132019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried TR, Bradley EH, Towle VR, et al. Understanding the treatment preferences of seriously ill patients N Engl J Med 3461061–10662002 [DOI] [PubMed] [Google Scholar]
- 62.Fried TR, Tinetti ME, Iannone L, et al. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions Arch Intern Med 1711854–18562011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patient Priorities Care . https://patientprioritiescare.org/ [Google Scholar]
- 64.Tinetti ME, Naik AD, Dindo L, et al. Association of patient priorities-aligned decision-making with patient outcomes and ambulatory health care burden among older adults with multiple chronic conditions: A nonrandomized clinical trial JAMA Intern Med 1791688–16972019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.PREPARE: Prepare for Your Care . https://prepareforyourcare.org/welcome [Google Scholar]
- 66.Sudore RL, Boscardin J, Feuz MA, et al. Effect of the PREPARE website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: A randomized clinical trial JAMA Intern Med 1771102–11092017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holmes HM, Hayley DC, Alexander GC, et al. Reconsidering medication appropriateness for patients late in life Arch Intern Med 166605–6092006 [DOI] [PubMed] [Google Scholar]
- 68.Holmes HM, Min LC, Yee M, et al. Rationalizing prescribing for older patients with multimorbidity: Considering time to benefit Drugs Aging 30655–6662013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAlpine K, Lewis KB, Trevena LJ, et al. What is the effectiveness of patient decision aids for cancer-related decisions? A systematic review subanalysis JCO Clin Cancer Inform 21–132018 [DOI] [PubMed] [Google Scholar]
- 70.Kruser JM, Taylor LJ, Campbell TC, et al. “Best case/worst case”: Training surgeons to use a novel communication tool for high-risk acute surgical problems J Pain Symptom Manage 53711–719.e52017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kiely BE, Soon YY, Tattersall MH, et al. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials J Clin Oncol29:456-63, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Löckenhoff CE.Aging and decision-making: A conceptual framework for future research—A mini-review Gerontology 64140–1482018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hobbs GS, Landrum MB, Arora NK, et al. The role of families in decisions regarding cancer treatments Cancer 1211079–10872015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mead EL, Doorenbos AZ, Javid SH, et al. Shared decision-making for cancer care among racial and ethnic minorities: A systematic review Am J Public Health 103e15–e292013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf A, Alpert N, Tran BV, et al. Persistence of racial disparities in early-stage lung cancer treatment J Thorac Cardiovasc Surg 1571670–1679.e42019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo T, Spolverato G, Johnston F, et al. Factors that determine cancer treatment choice among minority groups J Oncol Pract 11259–2612015 [DOI] [PubMed] [Google Scholar]
- 77.Hawley ST, Morris AM.Cultural challenges to engaging patients in shared decision making Patient Educ Couns 10018–242017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hermann MG.How decision units shape foreign policy: A theoretical framework Int Stud Rev 347–812001 [Google Scholar]
- 79.Bernhardsdóttir ÁE.Crisis-Related Decision-Making and the Influence of Culture on the Behavior of Decision Makers Cross-Cultural Behavior in Crisis Preparedness and Response ed 1Springer, pp XVII, 198 [Google Scholar]
- 80.Kahneman D. Thinking, Fast and Slow. ed 1. New York, NY: Farrar, Straus and Giroux; 2011. [Google Scholar]
- 81.Mazzocco K, Masiero M, Carriero MC, et al. The role of emotions in cancer patients' decision-making. Ecancermedicalscience. 2019;13:914. doi: 10.3332/ecancer.2019.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dale W, Hemmerich J, Bylow K, et al. Patient anxiety about prostate cancer independently predicts early initiation of androgen deprivation therapy for biochemical cancer recurrence in older men: A prospective cohort study J Clin Oncol 271557–15632009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobson JO.The oncologist's wager J Clin Oncol 372947–29512019 [DOI] [PubMed] [Google Scholar]
- 84.Ayalon L, Tesch-Römer C.Contemporary Perspectives on Ageism, International Perspectives on Aging Springer; 2018, pp XXX, 564 [Google Scholar]
- 85.Lawler M, Selby P, Aapro MS, et al. Ageism in cancer care. BMJ. 2014;348:g1614. doi: 10.1136/bmj.g1614. [DOI] [PubMed] [Google Scholar]
- 86.Rostoft S, van den Bos F, Pedersen R, et al. Shared decision-making in older patients with cancer—What does the patient want? J Geriatr Oncol 12339–3422021 [DOI] [PubMed] [Google Scholar]
- 87.Thompson LL, Temel B, Fuh CX, et al. Perceptions of medical status and treatment goal among older adults with advanced cancer J Geriatr Oncol 11937–9432020 [DOI] [PubMed] [Google Scholar]
- 88.Loh KP, Soto Pérez de Celis E, Duberstein PR, et al. Patient and caregiver agreement on prognosis estimates for older adults with advanced cancer Cancer 127149–1592020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loh KP, Mohile SG, Lund JL, et al. Beliefs about advanced cancer curability in older patients, their caregivers, and oncologists Oncologist 24e292–e3022019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burns CM, Dixon T, Broom D, et al. Family caregiver knowledge of treatment intent in a longitudinal study of patients with advanced cancer Support Care Cancer 11629–6372003 [DOI] [PubMed] [Google Scholar]
- 91.El-Jawahri A, Nelson-Lowe M, VanDusen H, et al. Patient-clinician discordance in perceptions of treatment risks and benefits in older patients with acute myeloid leukemia Oncologist 24247–2542019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mack JW, Walling A, Dy S, et al. Patient beliefs that chemotherapy may be curative and care received at the end of life among patients with metastatic lung and colorectal cancer Cancer 1211891–18972015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Trevino KM, Prigerson HG, Shen MJ, et al. Association between advanced cancer patient-caregiver agreement regarding prognosis and hospice enrollment Cancer 1253259–32652019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non-small-cell lung cancer: Results of a randomized study of early palliative care J Clin Oncol 292319–23262011 [DOI] [PubMed] [Google Scholar]
- 95.Moye J, Marson DC.Assessment of decision-making capacity in older adults: An emerging area of practice and research J Gerontol B Psychol Sci Soc Sci 62P3–P112007 [DOI] [PubMed] [Google Scholar]
- 96.Savadori L, Caovilla J, Zaniboni S, et al. The affect heuristic in occupational safety Med Lav 106239–2492015 [PubMed] [Google Scholar]
- 97.Tversky A, Kahneman D.Judgment under uncertainty: Heuristics and biases Science 1851124–11311974 [DOI] [PubMed] [Google Scholar]
- 98.Tversky A, Kahneman D.The framing of decisions and the psychology of choice Science 211453–4581981 [DOI] [PubMed] [Google Scholar]
- 99.DeMartino ES, Dudzinski DM, Doyle CK, et al. Who decides when a patient can't? Statutes on alternate decision makers N Engl J Med 3761478–14822017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Loh KP, Abdallah M, Kadambi S, et al. Treatment decision-making in acute myeloid leukemia: A qualitative study of older adults and community oncologists Leuk Lymphoma 62387–3982021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fried TR.Shared decision making—Finding the sweet spot N Engl J Med 374104–1062016 [DOI] [PubMed] [Google Scholar]
- 102.DuMontier C, Loh KP, Bain PA, et al. Defining undertreatment and overtreatment in older adults with cancer: A scoping literature review J Clin Oncol 382558–25692020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohile SG, Epstein RM, Hurria A, et al. Communication with older patients with cancer using geriatric assessment: A cluster-randomized clinical trial from the National Cancer Institute Community Oncology Research Program JAMA Oncol 6196–2042019 [DOI] [PMC free article] [PubMed] [Google Scholar]