Abstract
PURPOSE
Brentuximab vedotin, an effective anti-CD30 antibody-drug conjugate approved for use in adults with classical Hodgkin lymphoma (HL), was introduced in this frontline trial to reduce prescribed radiation in children and adolescents with classical HL.
METHODS
Open-label, single-arm, multicenter trial for patients (age ≤ 18 years) with stage IIB, IIIB, or IV classical HL was conducted. Brentuximab vedotin replaced each vincristine in the OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) regimen according to GPOH-HD2002 treatment group 3 (TG3); two cycles of AEPA and four cycles of CAPDac. Residual node radiotherapy (25.5 Gy) was given at the end of all chemotherapy only to nodal sites that did not achieve a complete response (CR) at the early response assessment (ERA) after two cycles of therapy. Primary objectives were to evaluate the safety and efficacy (complete remission at ERA) of this combination and the 3-year event-free (EFS) and overall survival (OS). The trials are registered at ClinicalTrials.gov (identifier: NCT01920932).
RESULTS
Of the 77 patients enrolled in the study, 27 (35%) achieved complete remission at ERA and were spared radiation. Patients who were irradiated received radiation to individual residual nodal tissue. At a median follow-up of 3.4 years, the 3-year EFS was 97.4% (SE 2.3%) and the OS was 98.7% (SE 1.6%). One irradiated patient experienced disease progression at the end of therapy and now remains disease free more than 6 years following salvage therapy, and one unexpected death occurred. Only 4% of patients experienced grade 3 neuropathy.
CONCLUSION
The integration of brentuximab vedotin in the frontline treatment of pediatric high-risk HL is highly tolerable, facilitated significant reduction in radiation exposure, and yielded excellent outcomes.
INTRODUCTION
Pediatric patients with Hodgkin lymphoma (HL) have a long-term event-free survival (EFS) approximating 90% and an overall survival (OS) of 98%-99% with combined modality therapy.1,2 Even for patients with high-risk disease, the 5-year EFS ranges between 85% and 94%1,3-5 following risk-adapted therapy using consolidative involved field radiation therapy (IFRT). Although both chemotherapy and radiotherapy harbor risks of late effects, the focus in most contemporary pediatric trials has been to reduce radiation through risk and response-adapted therapy while maintaining excellent cure rates.4,6 For the past 2 decades, our multi-institutional consortium has attempted to build upon well-established combination chemotherapy while reducing radiation for those inadequately responding to chemotherapy with the goal of limiting late toxicities.7 In this trial, to maintain excellent outcomes without intensifying chemotherapy and its associated toxicities, we substituted brentuximab vedotin (Bv) for vincristine in the OEPA/COPDac (vincristine, etoposide, prednisone, and doxorubicin/cyclophosphamide, vincristine, prednisone, and dacarbazine) regimen followed by response-based residual node radiation (RNRT), building on the GPOH-HD2002 experience that achieved a 5-year EFS of 86.9% (SE 2.3%) in which all participants with high-risk HL received IFRT (19.8 Gy). Here, we report the results of HLHR13, the first frontline trial for pediatric patients with high-risk classical HL to incorporate this targeted therapy.
CONTEXT
Key Objective
To prospectively test the safety and efficacy of introducing targeted therapy (brentuximab vedotin [A]) into a well-established frontline treatment regimen (OEPA/COPDac) for pediatric patients with high-risk (stages IIB, IIIB, and IV) classical Hodgkin lymphoma.
Knowledge Generated
AEPA/CAPDac was well-tolerated and allowed for omission of radiotherapy in 35% of patients treated; the residual node radiation volumes in patients requiring radiation were very small compared with historical controls sparing healthy surrounding tissue. Only one patient relapsed (out of radiation field) and was successfully salvaged.
Relevance
Introduction of targeted therapy frontline in a response-adapted strategy yields excellent results and allows for tailoring of radiation therapy in patients with an inadequate early response. These results warrant further prospective testing in an effort to reduce long-term effects of therapy.
METHODS
Study Design and Participants
This study was a multicenter, investigator-initiated, single-arm trial sponsored by Seattle Genetics (IND [#118603], ClinicalTrials.gov identifier: NCT01920932), which enrolled patients at St Jude Children's Research Hospital (SJCRH), Stanford University Medical Center, Dana-Farber Cancer Institute, Maine Children's Cancer Program, Children's Hospital of Illinois in Peoria, and Massachusetts General Hospital. Eligibility criteria included age 18 years and younger with previously untreated Ann Arbor8 stages IIB, IIIB, or IV and classical (CD30+) HL (full eligibility criteria are provided in the Data Supplement, online only). The trial was approved by the institutional review board of each participating center and monitored by the SJCRH Data, Safety, and Monitoring Board. Informed consent was obtained as per institutional guidelines.
Procedures
Patients underwent protocol-required evaluations before the initiation of chemotherapy. Staging assessments included contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) of the neck/chest/abdomen and pelvis and [18F]fluoro-2-deoxyglucose positron emission tomography (PET/CT) scans.
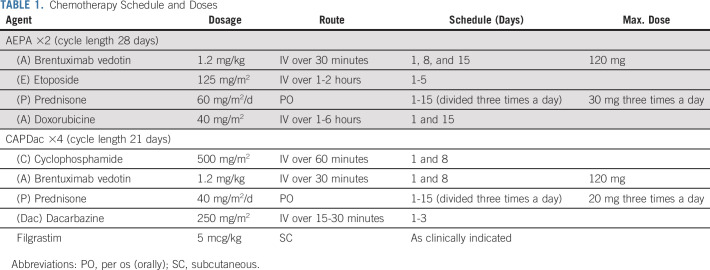
Chemotherapy consisted of two cycles of AEPA—Bv, etoposide, prednisone, and doxorubicin—followed by four cycles of CAPDac—cyclophosphamide, Bv, prednisone, and dacarbazine (Table 1). Filgrastim was recommended only for prolonged neutropenia to avoid chemotherapy delays.
TABLE 1.
Chemotherapy Schedule and Doses
After AEPA cycles, patients underwent an early response assessment (ERA) with PET/CT and contrast-enhanced CT scan or MRI. Conformal type RNRT was delivered to individual lymph nodes without complete response (CR, defined by PET Deauville 1-3 and ≥ 75% reduction in product of perpendicular diameters) at ERA compared with initial staging (full response criteria are given in the Data Supplement). Lymph nodes achieving CR at ERA were not irradiated, whereas those not achieving CR were targeted on radiation simulation studies after completion of chemotherapy, allowing further reduction in the targeted volume. A 1 cm margin was added to the target volumes. In the case of a residual nodal conglomerate, the whole conglomerate was targeted. Noncontiguous treatment volumes were encouraged to further reduce normal tissue radiation exposure. The prescribed dose was 25.5 Gy in 17 fractions of 1.5 Gy, 2-4 weeks after completion of chemotherapy. Photon-based intensity-modulated radiation therapy, 3D conformal approaches (in Gy), and proton beam radiation (Gy for relative biological effect) were allowed.
Follow-up evaluations occurred at scheduled intervals. CT scans of previously involved sites were performed at 1 and 2 years off therapy. Relapse was confirmed by biopsy.
Outcomes
Primary objectives were to evaluate efficacy (percent individuals in complete remission at ERA) and to compare EFS and OS with an historical control group treated with the Stanford V (prednisone, nitrogen mustard, doxorubicin, vincristine, etoposide, bleomycin, and vinblastine) regimen used in a previous consortium protocol (HOD99).9 An event was defined as relapse, disease progression, second malignancy (excluding basal cell carcinoma), or death from time of study enrollment to first event or censored at last follow-up if a patient had no event.
Secondary objectives were to evaluate the safety of Bv in the AEPA/CAPDac regimen in children with high-risk classical HL by describing acute hematologic, neuropathic, and infectious toxicities, according to the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.10
Exploratory objectives were to describe the means of Pain Quality Assessment Scale (PQAS—description in the Data Supplement) and subscales and to compare the ability of proton-based radiation to spare adjacent normal tissues compared with photon-based radiation. Using submitted radiation treatment plans (CT planning study, target volumes, and radiation dose distributions), photon and proton treatment plans were compared with HOD99 (which included treatment of classic involved fields based on initial extent of disease) where dose of radiation delivered was either 15 Gy or 25.5 Gy depending on whether a CR or < CR was obtained at 8-week response evaluation. Thirty-one patients had radiation treatment plans available for comparison. The volume of tissue that received 95% of the prescribed 25.5 Gy dose and the volume of tissue that received 5 Gy, 10 Gy and 15 Gy were compared by treatment study (current trial v HOD99) and modality (proton- or photon-based radiation). The integral dose to the patient (or total dose, calculated from the submitted radiation treatment plans) and the mean doses to the heart, breast (in female patients), and thyroid were compared. The volume of nodal tissue requiring radiation was compared by modality to see if there was a bias in the use of proton beam radiation based on tumor volume.
Statistical Analysis
The proportion of patients in CR at week 8 (ERA) of Stanford V chemotherapy on HOD99 was 17% (24 of 141). For the first primary efficacy objective testing of the early CR rate, a 1-sample exact binomial design led to 32 patients to detect a 20% increase in CR rate from 17% to 37% with 80% power and one-sided 5% type I error. The study was subsequently amended to enroll a total of 77 patients to assess improvement in EFS distribution against patient level data from HOD99 using a historical control design.11 The design assumed a Weibull model for the historical data, which included 141 subjects and 27 EFS events. Using a log-rank test with 10% type 1 error and 80% power, 77 patients were adequate to detect a hazard ratio of 0.4 with an assumed accrual duration of 5-6 years and a follow-up time of 2 years. The comparisons of radiation doses between the modalities were assessed by Student's t-test. All analyses were conducted with the use of SAS software, version 9.4 (SAS Institute).
Baseline characteristics and toxicities were summarized with descriptive statistics (Data Supplement). The CR rate based on the first 32 patients and its 90% and 95% exact CIs were estimated. The CR of all 77 patients was compared with the historical control with Fisher's exact test. The EFS and OS were estimated with Kaplan-Meier estimator. The comparisons of EFS and OS with the historical control were assessed by log-rank test. For the safety analysis, summary statistics are provided for all toxicities. The longitudinal means of PQAS subscales are estimated and visualized.
RESULTS
Patient Characteristics
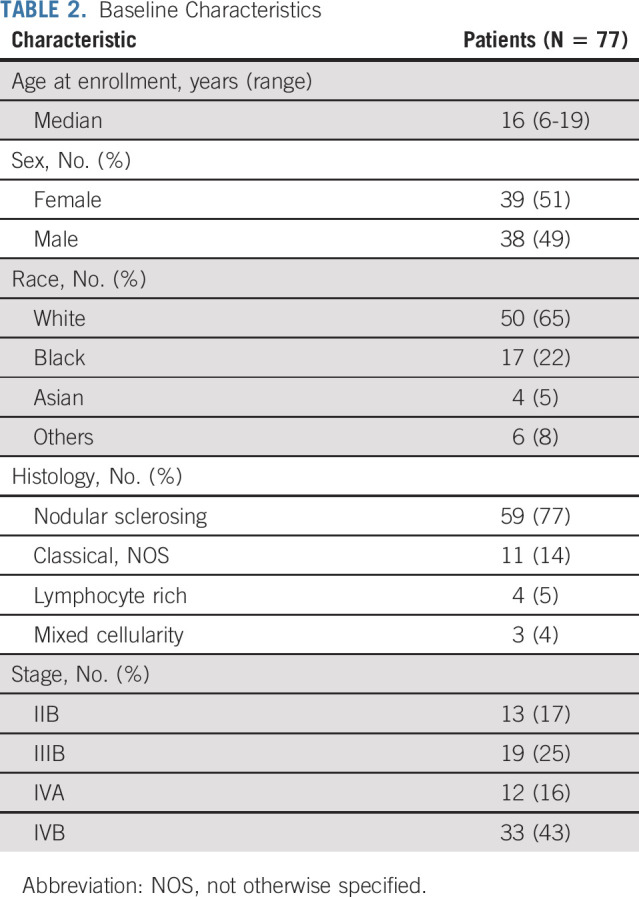
The trial enrolled 77 eligible patients from August 8, 2013, to July 9, 2018 (Table 2). The median (range) age at diagnosis was 16 (6-19) years; 51% were female, 65% White, and 22% Black. The most common histology was nodular sclerosing (77%), and most patients had stage IV (59%) disease.
TABLE 2.
Baseline Characteristics
Response to Therapy
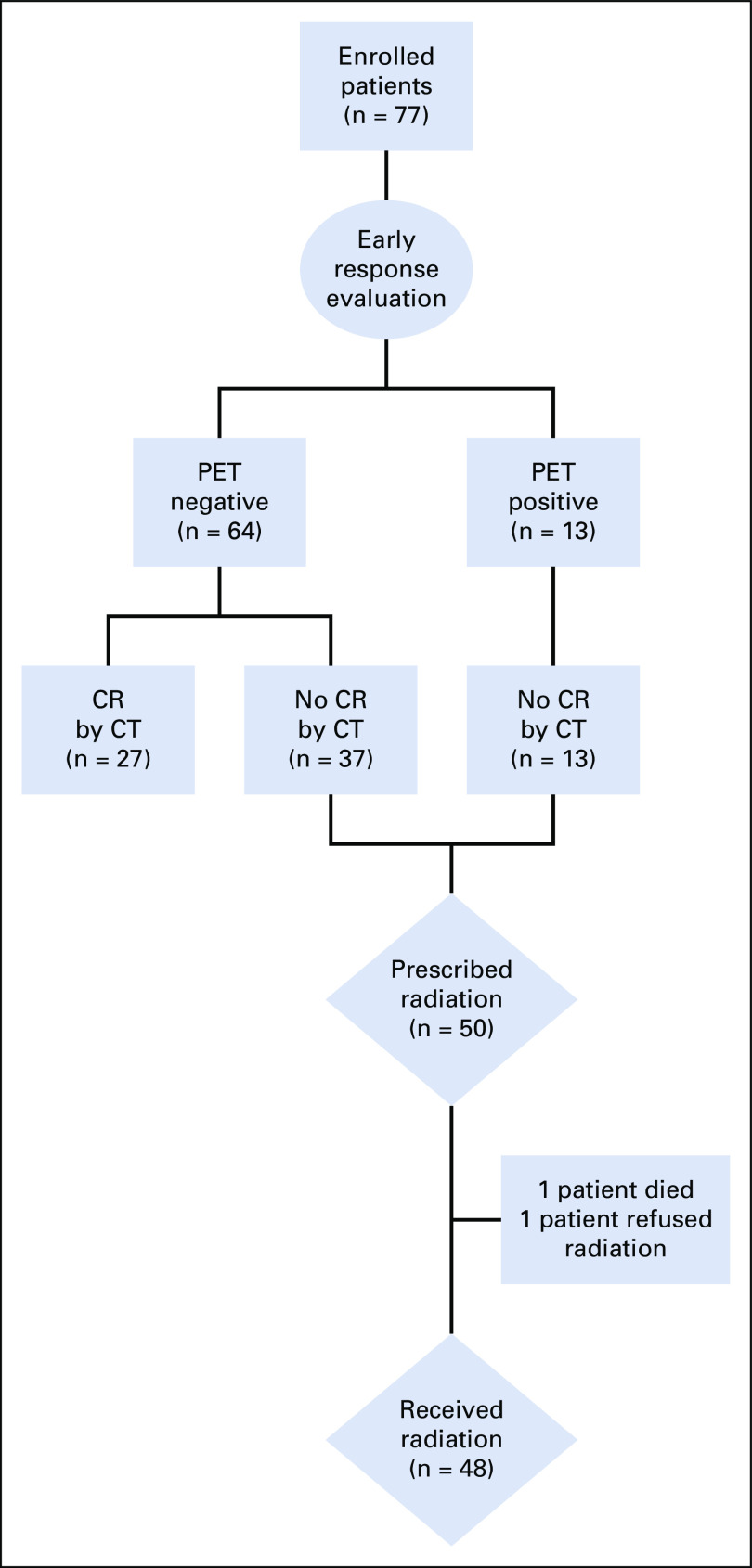
Patient demographics did not differ significantly between HLHR13 and the historical control (Data Supplement) by age (P = .248), sex (P = .778), race (P = .397), or histology (P = .194). The historical control group enrolled more patients with stage IIB (31% v 17%), whereas HLHR13 enrolled more patients with stage IIIB (25% v 14%) and IVB (43% v 37%) (P = .046). Compared with 17% of patients in the historical control that achieved a complete remission at ERA, 27 of 77 patients (35.1%; 95% CI, 24.5 to 46.8; P = .004) achieved CR after two cycles of AEPA and finished therapy without radiation (Fig 1). Of 50 patients prescribed radiation, 37 (74%) achieved a metabolic CR at all sites in the absence of an anatomic CR to at least one site and 13 (36%) achieved neither anatomic nor metabolic CR at all sites. Radiotherapy was not delivered in two patients as indicated because of death and refusal.
FIG 1.
Diagram with response evaluation and radiotherapy allocation. CR, complete response; CT, computed tomography; PET, positron emission tomography.
Outcome
EFS and OS.
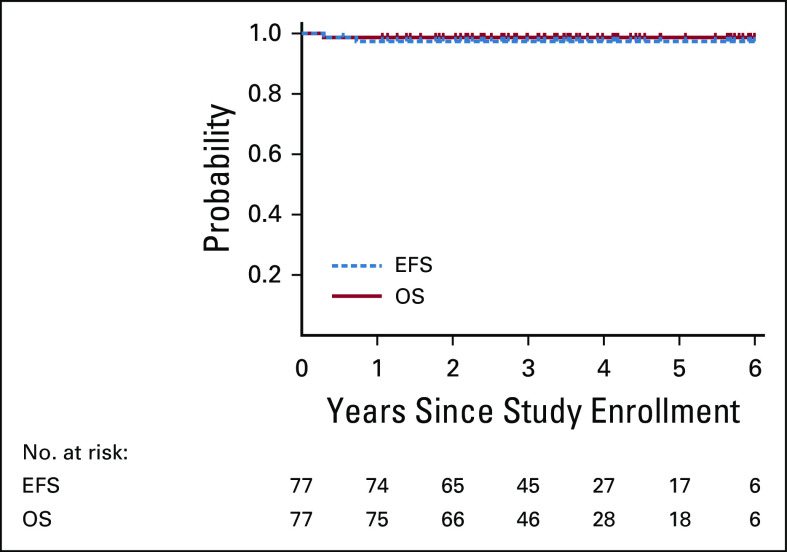
With a median follow-up time of 3.4 years, the 3-year EFS and OS for HLHR13 were 97.4% (SE 2.3%) and 98.7% (SE 1.6%), respectively (Fig 2), compared with 80.8% (SE 3.3%; P = .0008) and 96.5% (SE 1.6%; P = .311), respectively, in the HOD99 trial (Data Supplement). There were no events in the nonirradiated patients (25 of 77 patients). There was one biopsy-confirmed treatment failure among irradiated patients, a 16-year-old female with stage IIIB and bulky mediastinal disease who relapsed within 3 months from end of therapy. This patient received salvage therapy and remains disease free for ≥ 6 years. An otherwise healthy 11-year-old male with stage IVB disease who had not achieved CR at ERA died unexpectedly from fatal ventricular tachycardia during cycle 4 of chemotherapy. Autopsy revealed noninfectious pancarditis of undetermined etiology.
FIG 2.
Three-year EFS 97.4% (SE 2.3%) and OS 98.7% (SE 1.6%). EFS, event-free survival; OS, overall survival.
Safety.
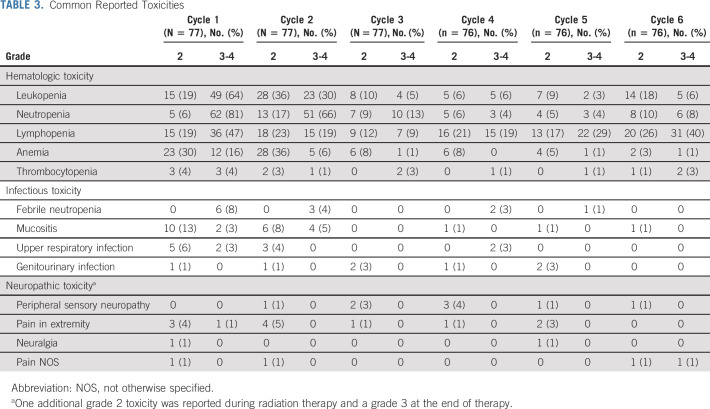
Therapy was well-tolerated and mostly limited to low-grade nausea, vomiting, and constipation (Table 3). The most common adverse events were hematologic, particularly during the first two cycles. Two patients (2.6%) required platelet transfusions; both patients received one transfusion during the first cycle, and one patient also during cycle 6 and radiation. Thirteen (17%) received packed red blood cell transfusions; 19 (25%) received filgrastim during the first cycle (n = 8), second cycle (n = 7), or both (n = 3) cycles. The median (range) duration of filgrastim administration was 2.8 (1-8) days. Eight patients (10%) were hospitalized 12 times for febrile neutropenia and nine during the first two cycles at a median (range) duration of hospitalization of 2 (2-21) days.
TABLE 3.
Common Reported Toxicities
There were seven (9%) cases of symptomatic osteonecrosis confirmed by imaging; three patients required core decompression, one of whom required total hip arthroplasty. The weight gain > 20% over baseline occurred in 45% of patients.
Neuropathic and non-neuropathic pain was rare, with only three (4%) grade 3 events. Of 26 adverse events related to neuropathy, 12 occurred during the first two chemotherapy cycles. The mean PQAS domain scores available for 64 patients across time did not exceed 2 on a 0-10 scale (Data Supplement). Surface pain sensations (itchy, cold, numb, sensitive, and tingling)12 were reported most often at cycle 1, day 1 (mean 1.54 [SD 1.66]) and declined with subsequent cycles. Deep pain sensations (aching, heavy, dull, cramping, and throbbing) were reported most frequently across all other cycles and after completion of therapy.
Radiation.
The single treatment failure occurred at a site of initial involvement but outside of the radiation field.
Volumetric radiation treatment plans were available for 47 of 48 patients (one patient received radiation at a nonstudy site). Patients treated with RNRT had significantly lower integral radiation dose compared with patients treated on HOD99 with IFRT (78.1 J v 249.6 J, P < .001). These significant differences were maintained when tissue exposure was assessed based on volume of tissue receiving 5 Gy (4.66 L v 9.12 L, P < .001), 10 Gy (2.92 L v 7.94 L, P < .001), and 15 Gy (1.86 L v 6.99 L, P < .001) and volume of tissue receiving 24.2 Gy, which was 95% of the prescribed dose (0.80 L v 4.72 L, P < .001). Doses to specific organs were also compared as the reduced RNRT paradigm in the current trial allowed for improved normal tissue protection compared with traditional involved fields used in HOD99. The mean heart dose was reduced to 5.29 Gy from 16.9 Gy (P < .001), and the mean thyroid dose was reduced to 4.46 Gy from 25.9 Gy (P < .001). In addition, there was a significant reduction in breast exposure in the female population, where the RNRT paradigm limited exposure to a mean breast dose of 3.21 Gy compared with 6.85 Gy in HOD99 (P = .004).
In an exploratory analysis, we compared normal tissue exposure in patients who received residual nodal radiation with proton beam (n = 15; 31%) with those treated with photon-based techniques. Patients treated with proton beam received significantly lower integral radiation dose compared with photons (Data Supplement: 36.7 J v 97.5 J, P = .002). This reduction in tissue exposure by proton beam was also found for the volume of tissue receiving 5 Gy (P = .002), 10 Gy (P = .013), and 15 Gy (P = .04). When higher radiation doses were assessed (volume of tissue receiving 24.2 Gy or 95% of the prescribed dose), there was a nonsignificant difference by modality (P = .05). To assess whether patients with larger residual tumor volumes might have been preferentially managed with one modality, the volume of gross residual disease requiring radiation therapy was compared by modality and was not statistically different (proton 93.5 mL v photon 155 mL, P = .28). Mean breast dose in female patients was significantly reduced with proton beam approach (0.5 Gy mean dose compared with 4.4 Gy, P = .004). There was a trend toward lower mean heart dose among patients treated with protons versus those treated with photons (4.0 Gy v 6.0 Gy, respectively, P = .12). The mean thyroid exposure was reduced from 5.9 Gy to 1.7 Gy with proton-based treatment (P = .05).
DISCUSSION
This is the first frontline trial for pediatric classical HL to incorporate Bv into a well-established chemotherapy regimen. The addition of brentuximab vedotin resulted in superior EFS and OS for patients with high-risk (stages IIB, IIIB, and IV) disease compared with previously published pediatric trials. Our 3-year EFS of 97.4% (SE 2.3%) of AEPA/CAPDac also compares favorably with outcomes on the GPOH-HD2002 that reported a 5-year EFS of 86.9% (SE 2.3%) and prescribed IFRT (19.8-35 Gy) to all sites in all high-risk (TG3- IIEB, IIIEA/B, IIIB, and IV) patients.1 Although the EuroNet-C1 trial is not yet published, a late interim analysis presented in 2016 reported a 4-year EFS of 86.8% for the TG3 group, of whom 67% received IFRT.13 The results of our study also demonstrate superiority to the ECHELON-1 study, which randomly assigned adult patients with stages III or IV disease to receive either 6 cycles of ABVD or A + AVD, substituting the bleomycin with Bv. In that study, the 2-year modified progression-free survival (time to progression, death, or non-CR requiring subsequent anticancer therapy) was 82.1% (95% CI, 73.7 to 80.4)14 with a cumulative anthracycline dose of 300 mg/m2 compared with 160 mg/m2 on our trial.15
Our study demonstrates that AEPA/CAPDac is well-tolerated, although the single fatality observed is concerning and warrants postmarketing toxicity monitoring of Bv. The episode of pancarditis was unexpected based on the patient's clinical history and the currently known toxicity profile of OEPA/COPDac and Bv. Hematotoxicity occurred at similar frequencies to that of OEPA/COPDac; however, grade 3 constipation, reported in 7.3% of patients receiving OEPA, was not observed in HLHR13.1 The ECHELON-1 study reported peripheral neuropathy in 67% of adult patients receiving Bv (20% grade 2 and 11% grade 3 neuropathy) and 10% requiring discontinuation of Bv,14 possibly compounded by the concomitant administration of vinblastine and a higher prevalence of comorbidities that increase adults' vulnerability to neuropathy. Notably, we observed a very low incidence of neuropathy (4%) by both clinician and patient report, and no participants required Bv dose reduction or discontinuation.
The role of radiation for adults with advanced-stage HL is controversial.16,17 Pediatric trials, however, endorse a response-adapted combined modality approach for all risk groups in an attempt to balance disease control and toxicity.2 Response-adapted low-dose radiation has become a hallmark of pediatric HL trials, although historically applied in an all or nothing approach using involved fields, where the patient qualifies for no radiation to any site (or only bulky mediastinal masses) or radiation to all involved sites even if only one site did not achieve the desired early response to therapy.2 The GPOH-HD95 trial omitted radiation in patients achieving an anatomic remission after completion of chemotherapy, leading to inferior outcome for intermediate- and high-risk patients who did not receive IFRT.6 The Children's Oncology Group trials have focused on increasing dose intensity to achieve rapid early response and limiting radiation to sites of initial bulky disease or sites with slow early response.4,18 Although elimination of radiation has been a goal for many pediatric prospective trials, our previous and current studies sought to evaluate the efficacy and reduction in toxicity with a modified radiation target volume and delivery technique. In this trial, we implemented RNRT to lymphatics not meeting response criteria. Using this approach, efficacy was maintained as evident in excellent EFS, OS, and a pattern of treatment failure without marginal or distant nodal recurrences. While maintaining efficacy, this trial significantly reduced the volume of irradiated tissue 3-fold compared with our previous trial, eliminated mandatory radiation for bulky disease, and reduced normal tissue exposures. Radiation volume reduction is a significant advantage for patients who do not achieve a favorable response. To determine if further improvement in the therapeutic ratio is possible, we compared patients who received proton beam radiation or photon-based radiation. Both organ-specific doses and general tissue exposures were lower in patients treated with proton beam, which resulted in a near 10-fold further reduction in mean breast exposure. Although the ultimate benefit for these patients will be determined in the assessment of late toxicities and second malignancies, the reduction in radiation exposure suggests that this is possible.
In conclusion, AEPA-CAPDac was highly tolerable, facilitated reduction in radiation exposure, and yielded superior 3-year EFS and OS compared with previously published trials for pediatric patients with high-risk HL. The favorable safety and toxicity profile of Bv in combination with chemotherapy for high-risk pediatric patients supports its prospective evaluation in a randomized trial. Longer follow-up is required to establish if this approach reduces risk of late-occurring toxicities such as second malignant neoplasms in this cohort of minimally irradiated patients.
ACKNOWLEDGMENT
This report is dedicated to the memory of Larry Kun (1946-2018), a radiation oncologist who provided inspiration and expertise to the treatment of children with Hodgkin lymphoma. We thank all the investigators and trial teams for their participation in this trial, particularly Ms Mariesha Williams, clinical research associate, for clinical coordination and data management and Arzu Onar, PhD, for her review of the statistical section. Our special thanks to all of our patients and their families for their altruism and dedication to this trial.
Monika L. Metzger
Research Funding: Seattle Genetics
Michael P. Link
Consulting or Advisory Role: Incyte, ADC Therapeutics, Lilly, Steba Biotech, Mesoblast, GlaxoSmithKline, Syndax
Research Funding: Seattle Genetics, Janssen Oncology
Jamie Flerlage
Research Funding: Seattle Genetics
Matthew J. Ehrhardt
Honoraria: Optum
Torunn I. Yock
Consulting or Advisory Role: Huron Consulting Services, LLC
Research Funding: MIM Software
Sue C. Kaste
Stock and Other Ownership Interests: GE Healthcare
Barry Shulkin
Consulting or Advisory Role: Navidea
Melissa M. Hudson
Consulting or Advisory Role: Oncology Research Information Exchange Network, Princess Máxima Center
Matthew J. Krasin
Consulting or Advisory Role: Debiopharm Group
No other potential conflicts of interest were reported.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report. The corresponding authors had full access to all of the data, contributed to the manuscript and gave final approval before submission.
SUPPORT
Supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA021765.
CLINICAL TRIAL INFORMATION
M.M.H. and M.J.K. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Monika L. Metzger, Michael P. Link, Amy L. Billett, Jamie Flerlage, Alison M. Friedmann, Pedro de Alarcon, Eric Larsen, Sarah S. Donaldson, Melissa M. Hudson, Matthew J. Krasin
Financial support: Monika L. Metzger
Administrative support: Monika L. Metzger
Provision of study materials or patients: Monika L. Metzger, Matthew J. Ehrhardt, Nickhill Bhakta, Sandra Luna-Fineman, Sarah S. Donaldson, Matthew J. Krasin
Collection and assembly of data: Monika L. Metzger, Amy L. Billett, Jamie Flerlage, Belinda N. Mandrell, Matthew J. Ehrhardt, Nickhill Bhakta, Alison M. Friedmann, Pedro de Alarcon, Sandra Luna-Fineman, Eric Larsen, Sue C. Kaste, Susan M. Hiniker, Melissa M. Hudson, Matthew J. Krasin
Data analysis and interpretation: Monika L. Metzger, Michael P. Link, Amy L. Billett, John T. Lucas Jr, Belinda N. Mandrell, Matthew J. Ehrhardt, Torunn I. Yock, Pedro de Alarcon, Sue C. Kaste, Barry Shulkin, Zhaohua Lu, Chen Li, Susan M. Hiniker, Sarah S. Donaldson, Melissa M. Hudson, Matthew J. Krasin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Excellent Outcome for Pediatric Patients With High-Risk Hodgkin Lymphoma Treated With Brentuximab Vedotin and Risk-Adapted Residual Node Radiation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Monika L. Metzger
Research Funding: Seattle Genetics
Michael P. Link
Consulting or Advisory Role: Incyte, ADC Therapeutics, Lilly, Steba Biotech, Mesoblast, GlaxoSmithKline, Syndax
Research Funding: Seattle Genetics, Janssen Oncology
Jamie Flerlage
Research Funding: Seattle Genetics
Matthew J. Ehrhardt
Honoraria: Optum
Torunn I. Yock
Consulting or Advisory Role: Huron Consulting Services, LLC
Research Funding: MIM Software
Sue C. Kaste
Stock and Other Ownership Interests: GE Healthcare
Barry Shulkin
Consulting or Advisory Role: Navidea
Melissa M. Hudson
Consulting or Advisory Role: Oncology Research Information Exchange Network, Princess Máxima Center
Matthew J. Krasin
Consulting or Advisory Role: Debiopharm Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mauz-Korholz C, Hasenclever D, Dorffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin's lymphoma: The GPOH-HD-2002 study J Clin Oncol 283680–36862010 [DOI] [PubMed] [Google Scholar]
- 2.Mauz-Korholz C, Metzger ML, Kelly KM, et al. Pediatric Hodgkin lymphoma J Clin Oncol 332975–29852015 [DOI] [PubMed] [Google Scholar]
- 3.Kelly KM, Sposto R, Hutchinson R, et al. BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: A report from the Children's Oncology Group Blood 1172596–26032011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk Hodgkin lymphoma: A report from the Children's Oncology Group study AHOD0031 J Clin Oncol 323651–36582014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz CL, Constine LS, Villaluna D, et al. A risk-adapted, response-based approach using ABVE-PC for children and adolescents with intermediate- and high-risk Hodgkin lymphoma: The results of P9425 Blood 1142051–20592009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorffel W, Ruhl U, Luders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: Final results of the multinational trial GPOH-HD95 J Clin Oncol 311562–15682013 [DOI] [PubMed] [Google Scholar]
- 7.Metzger ML, Weinstein HJ, Hudson MM, et al. Association between radiotherapy vs no radiotherapy based on early response to VAMP chemotherapy and survival among children with favorable-risk Hodgkin lymphoma JAMA 3072609–26162012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification Cancer Res 311860–18611971 [PubMed] [Google Scholar]
- 9.Metzger ML, Billett A, Link MP.The impact of drug shortages on children with cancer: The example of mechlorethamine N Engl J Med 3672461–24632012 [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute, NIH, US Department of Health and Human Services . Common Terminology Criteria for Adverse Events, Version 4.0. 2009. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf [Google Scholar]
- 11.Wu J, Xiong X.Group sequential design for randomized phase III trials under the Weibull model J Biopharm Stat 251190–12052015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victor TW, Jensen MP, Gammaitoni AR, et al. The dimensions of pain quality: Factor analysis of the Pain Quality Assessment Scale Clin J Pain 24550–5552008 [DOI] [PubMed] [Google Scholar]
- 13.Landman-Parker J, Wallace H, Hasenclever D, et al. Late-interim analysis of the first European protocol EuroNet PHL-C1 in classical HL (2007-2013) https://www.hodgkinsymposium.org/archive/2016/recording/57 International Symposium on Hodgkin Lymphoma (ISH10), 2016.
- 14.Connors JM, Jurczak W, Straus DJ, et al. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma N Engl J Med 378331–3442018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf CM, Reiner B, Kuhn A, et al. Subclinical cardiac dysfunction in childhood cancer survivors on 10-years follow-up correlates with cumulative anthracycline dose and is best detected by cardiopulmonary exercise testing, circulating serum biomarker, speckle tracking echocardiography, and tissue Doppler imaging. Front Pediatr. 2020;8:123. doi: 10.3389/fped.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockney NA, Yang JC.Radiation therapy for advanced-stage Hodgkin lymphoma Adv Radiat Oncol 5809–8162020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallamini A, Rossi A, Patti C, et al. Consolidation radiotherapy could be safely omitted in advanced Hodgkin lymphoma with large nodal mass in complete metabolic response after ABVD: Final analysis of the randomized GITIL/FIL HD0607 trial J Clin Oncol 383905–39132020 [DOI] [PubMed] [Google Scholar]
- 18.Kelly KM, Cole PD, Pei Q, et al. Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): A report from the Children's Oncology Group Br J Haematol 18739–482019 [DOI] [PMC free article] [PubMed] [Google Scholar]