INTRODUCTION
Hematologic malignancies (HMs) represent a varied set of diseases ranging from indolent to aggressive that are increasingly common in the growing older adult population. Representation of older adults in clinical trials remains low, particularly among those over 75 years old. As treatment options expand, questions remain regarding fitness for therapies, sequencing of therapies, management for vulnerable or frail patients, and strategies to optimize functional independence and quality of life (QOL). This review provides updates on new therapies for common HMs with an emphasis on older adult–specific evidence and the evolving role of a geriatric assessment (GA) in informing therapy selection and management.
CONTEXT
Key Objective
How do advances in therapeutics and geriatric assessment (GA) strategies inform personalized management for older adults with hematologic malignancies?
Knowledge Generated
Novel therapeutics, targeted therapies, and maintenance strategies are improving outcomes for older adults including those who have functional impairments and competing comorbid conditions. GA and frailty measures are predictive of treatment tolerance and outcomes across various hematologic malignancies.
Relevance
Integration of GA measures into practice can inform shared treatment decisions and guide management of identified vulnerabilities to enhance therapeutic benefit and quality of life.
MULTIPLE MYELOMA
Multiple myeloma (MM) is a disease of aging (median age at diagnosis 69 years). MM is not considered more biologically aggressive with aging, but older age is associated with advanced-stage disease.1,2 The therapeutic landscape is increasingly complex with combinations of drug classes including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), alkylating agents, corticosteroids, monoclonal antibodies (mABs), targeted agents, and cellular therapy (autologous hematopoietic cell transplantation [autoHCT], allogeneic hematopoietic cell transplantation [HCT], and chimeric antigen receptor T-cell therapy [CAR-T]). In the past 5 years, the US Food and Drug Administration has approved seven new therapies for MM. This dynamic field complicates treatment decisions for older adults as it pertains to treatment selection, expected toxicities, and therapy sequencing. Importantly, undertreatment of older adults with MM (≥ 65) is evident; recent large registries report that 38%-49% of patients with MM do not receive antimyeloma therapy.3,4 With MM treatment, 5-year myeloma-specific survival is improving (ages 66-79) from 26% during 1973-1979, to 32% during 1980-1999, to 41% from 2000-2009 (P < .05).5 Survival among younger adults (< 50) approaches 68%, whereas survival is inferior and unchanged for octogenarians.5
For newly diagnosed MM (NDMM), treatment strategies are framed as transplant eligible versus ineligible. A growing body of literature, however, recognizes that health status, and therefore eligibility, fluctuates.6 MM guidelines recommend frontline autoHCT as the standard of care (SOC) for eligible patients.7,8 AutoHCT with high-dose melphalan remains the cornerstone of therapy for MM, although randomized controlled trials evaluating tolerance of transplant are limited by upper age restrictions, centered around age 65.9 Long-term data from the IFM-2009 trial reported improved progression-free survival (PFS) with lenalidomide, bortezomib, and dexamethasone (RVD) before and after autoHCT compared with RVD alone for patients ≤ 65 years, 47.3 months vs 35.0 months respectively (P < .001).10 After 8 years, the median overall survival (OS) was not reached with no difference in OS rate by treatment arms.
Nontransplant strategies for older adults with NDMM include doublets, triplets, and quadruplet induction regimens based on patient fitness, disease biology (eg, cytogenetics), and shared decision making. The FIRST trial evaluated lenalidomide-dexamethasone continuously (Rd) versus 18 cycles (Rd18) versus melphalan-prednisone-thalidomide; PFS was superior with Rd, and OS was significantly improved in both Rd treatment arms; older age was an adverse risk factor.11 Prospective observational data evaluating real-world treatment in the United States reported that RVD is the most common induction strategy,12 based on the SWOG S0777 study where RVD resulted in superior PFS and OS in comparison with Rd with acceptable toxicity profiles.13 Triplet therapy in the MAIA trial evaluated continuous daratumumab (D)-Rd versus Rd and demonstrated improved PFS and overall response rate (ORR) with D-Rd, with more leukopenia and pneumonia in daratumumab-exposed patients.14 Other triplet induction strategies such as carfilzomib, lenalidomide, and dexamethasone in transplant-ineligible patients have not improved PFS compared with RVD and had higher rates of grade 3-5 treatment-related cardiac, pulmonary, and renal toxicity (P ≤ .0001).15 Quadruplet therapy in the ALCYONE study yielded OS advantages of daratumumab, bortezomib, melphalan, and prednisone followed by daratumumab maintenance, in comparison with bortezomib, melphalan, and prednisone, with a 40% reduction in the risk of death.16 For transplant-eligible high-risk patients, daratumumab and RVD (D-RVD) is gaining traction given high ORR of D-RVD versus RVD (odds ratio = 8.75 [95% CI, 1.08 to 71.01], P = .016).17 Studies reporting D-carfilzomib, lenalidomide, and dexamethasone efficacy and tolerability peri-autoHCT are forthcoming.18
Treatment for Relapsed or Refractory MM (RR MM) is complex, combining novel therapies (PIs and IMiDs) with next-generation agents (mABs, targeted) or with the use of cellular therapy. Relapsed therapies are crafted based on several factors including disease biology; tempo of disease relapse; tolerance, toxicity, and type of prior therapy; underlying health status; and shared decision making.19 Studies evaluating symptom trajectories show improved symptom burden and health-related QOL post-diagnosis with therapy that worsens again at time of relapse. Exploring tolerability of next-generation therapies is imperative. In one example evaluating idecabtagene vicleucel, B-cell maturation antigen–directed CAR-T cell for triple-class RRMM (refractory to IMiDs, PI, and mABs), 35% of patients were ≥ 65 years and the response or duration rates and PFS were similar for older adults versus younger adults with QOL improvement nine months post-infusion.20
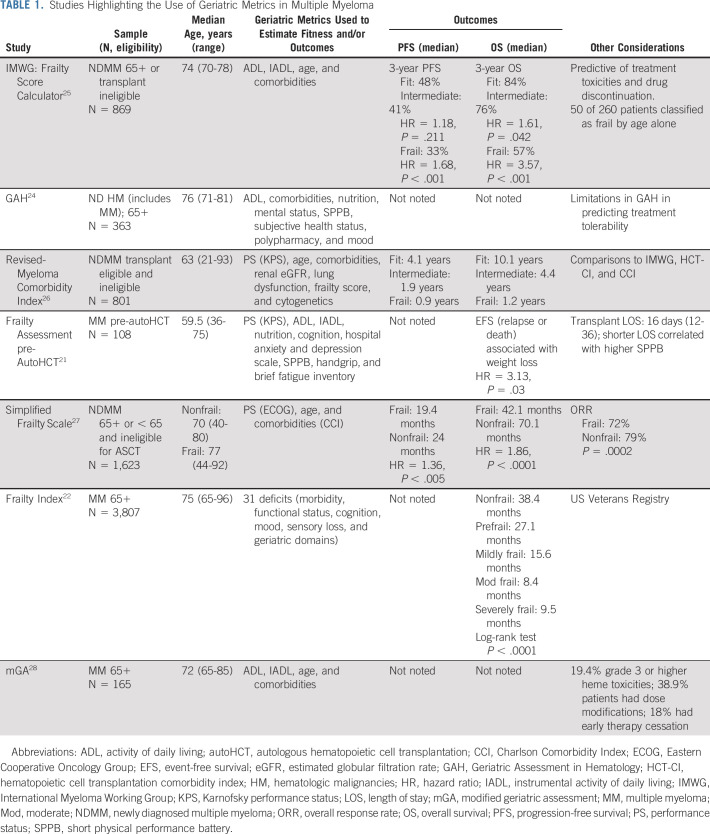
Objectively characterizing health status at each MM treatment decision point (diagnosis, transplant, and relapse) can right size therapy. GA metrics are well-characterized to identify vulnerability and have prognostic significance in MM. GA tools have been evaluated in NDMM before AutoHCT21 and in large registries.22 Several MM-specific geriatric tools are available to estimate treatment tolerance, each with limitations, but are better estimates of health status than age or comorbidities alone (Table 1). Well-established tools include the International Myeloma Working Group Frailty Score, Revised-Myeloma Comorbidity Index, and the Geriatric Assessment in Hematology scoring system.23-28 Robust characterization of health using GA, longitudinal functional assessment, and nonage-based clinical trials can improve morbidity and mortality for older adults with MM.
TABLE 1.
Studies Highlighting the Use of Geriatric Metrics in Multiple Myeloma
DIFFUSE LARGE B-CELL LYMPHOMA IN OLDER ADULTS
The most frequent non-Hodgkin lymphoma subtype among older adults is diffuse large B-cell lymphoma (DLBCL) (median age at diagnosis of 66 years). Management of older adults with DLBCL requires a multidisciplinary approach, where frailty, cognition, malnutrition, comorbidities, polypharmacy, social isolation, and depression are commonly seen. GA remains the gold standard to classify patients into frailty phenotypes. To simplify the GA, the Fondazione Italiana Linfomi consortium proposed the use of age, activities of daily living (ADLs), instrumental activities of daily living (IADLs), and the Cumulative Illness Rating Scale for Geriatrics to classify patients as fit, unfit, or frail.29,30 The International Society of Geriatric Oncology also published a position paper about the impact of prognosis, comorbidities, GA, and supportive care in selecting a best approach for older adults with DLBCL.31 Further considerations at initial assessment should include infection risk, growth factor support as a primary prophylaxis, the role of bone protection, and prephase therapy (eg, steroids and vincristine) in older patients with impaired performance status (PS) driven primarily by disease burden, which has been indirectly shown to reduce treatment-related mortality.32
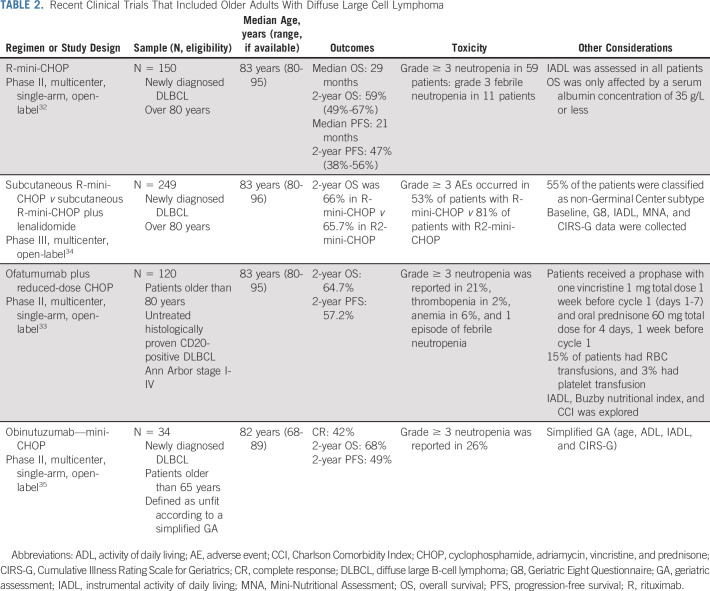
Trials that are not older adult–specific largely exclude octogenarians. The evidence base is predominantly limited to phase II trials and retrospective series (Table 2). The choice of dose intensity in anthracycline-fit older patients represents a trade-off between the risk of treatment-related toxicity and the risk of insufficient dosing resulting in inadequate efficacy. This is of relevance in this group of patients who have historically had few effective options at relapse or progression.
TABLE 2.
Recent Clinical Trials That Included Older Adults With Diffuse Large Cell Lymphoma
In 2011, Peyrade et al32 provided the first prospective phase II evidence to show that anthracycline-based immunochemotherapy provided curative potential in octogenarians with DLBCL. Over the last decade, the use of anthracycline-based immunochemotherapy has become more widespread. The regimen named mini rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone (adriamycin [25 mg/m2], cyclophosphamide [400 mg/m2], and vincristine [1 mg capped dose]) resulted in a 2-year OS of 59% with a survival plateau. Similar outcomes were seen when ofatumumab was investigated in place of rituximab and in the phase III SENIOR trial,33 where the 2-year PFS of R-miniCHOP arm (subcutaneous rituximab) was 67%.34 In a phase II multicenter trial to investigate miniCHOP plus obinutuzumab in older patients with DLBCL (≥ 65), prospectively defined as unfit according to a simplified comprehensive GA, the 2-year PFS and OS were not improved when compared with historical data obtained with R-miniCHOP in this group of patients.35 When analyzing intended dose intensity (IDI), IDI lower than 80% is associated with worse outcomes in patients 70-80 years old, whereas survival in those ≥ 80 years was similar independent of IDI.36 Similar results were reported in patients ≥ 80 years in the large Danish registry.37 R-mini-CHOP is a reasonable strategy and serves as the backbone of control and experimental arms for patients ≥ 80 years.
Several options exist for patients with a cardiac impairment who are not candidates for anthracycline-based treatment. Prospective phase II data support the use of gemcitabine-based regimens (R-GCVP and R-Gem-Ox).38 Sixty-two patients received R-CVP plus gemcitabine as an anthracycline substitute. The 2-year PFS was 49.8%, and the 2-year OS was 55.8%. Fifteen cardiac events were documented including three deaths. R-Gem-Ox-14 (rituximab, gemcitabine, and oxaliplatin) was recently assessed in 61 patients (median age 75 years).39 The 3-year PFS was 49% with survival equivalent in those older than 80 years. Taken together, these data suggest that gemcitabine-based chemoimmunotherapy provides durable DLBCL control in approximately 50% and is well-tolerated including in those with cardiac comorbidities. Another strategy is the use of liposomal formulation of adriamycin. Fifty patients with cardiac comorbidities have been evaluated using this novel formulation in the R-COMP regimen.40 The three-year PFS was 38%, and the 3-year OS was 50% in a similar population to those receiving R-GCVP. R-bendamustine has been studied in small trials, but outcomes are generally disappointing, with a median progression-free survival of 10 months.41
In patients classified as unfit for curative-intent treatment after a GA, QOL and symptom control would be the main goal of therapy. A large population-based series found that in patients > 85 years, OS was equivalent when CVP with or without R or CEOP with or without R was compared with rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone/R-CHOEP (RCHOP plus etoposide).37 In the Italian study, no benefit was seen in the poor prognosis group, where palliation would be the best strategy.29 Rituximab monotherapy, steroids, or no drug treatment may be entirely appropriate in the extremely frail or those wishing to avoid treatment-related adverse effects.
For RR DLBCL, recent advances have seen the advent of novel antibody-drug conjugates, anti-CD19 mABs, and anti-CD19–directed CAR-T therapy. Briefly, the conjugated anti-CD79b mAB polatuzumab vedotin has shown promising efficacy in combination with bendamustine-rituximab within a randomized phase II trial and is a licensed option in appropriately selected patients.42 The novel anti-CD19–directed mAB tafasitamab in combination with lenalidomide is very active, albeit in a low-risk cohort, not including primary refractory patients.43 Trials are actively investigating CAR-T, autoHCT, and immunochemotherapy in cohorts defined by fitness status.
MYELODYSPLASTIC SYNDROMES
Myelodysplastic syndromes (MDS) have one of the highest median ages at diagnosis, over 70 years. Accordingly, patients with these syndromes are often frail, and clinicians may fear doing more harm than good, especially in the context of lower-risk disease. As frailty and comorbidities frequently limit the goals of MDS treatment to improving function and/or QOL rather than cure, investigators have long recognized that assessing these measures is critical.
One of the advantages of the hypomethylating agent (HMA) azacitidine—the first drug approved for MDS in 2004—was that it was better tolerated than intensive chemotherapy or HCT. In the QOL analysis (n = 191; mean age 67.5),44 patients treated with azacitidine experienced less fatigue and dyspnea, and better physical functioning compared with those in the supportive care arm. Similar QOL benefits were eventually published for decitabine.45 Although neither analysis measured frailty per se, improved physical functioning and decreased symptoms likely translate to reduction in markers of frailty.
More recently, data regarding the specific impact of frailty in MDS have come to light through analyses of the MDS-CAN Canadian registry. The investigators evaluated frailty using the Rockwood Clinical Frailty Scale (N = 445; median age 71).46 Frailty enhanced Revised International Prognostic Scoring System prognostication, was independently associated with survival, and improved risk stratification more than simply factoring in comorbidity: 30% versus 5%.47 Independent contributions of frailty and comorbidity to outcomes were confirmed in a subsequent analysis of patients with MDS in Japan (N = 118; median age 73).48
The Canadian group developed a 42-item MDS-specific frailty index based on deficits in physical function, laboratory values, comorbidity, IADLs, QOL, and PS, which improves upon existing models of disease risk.49 They eventually parsed these down to 15 items,50 which are offered in an online calculator51 combining MDS risk score with metrics such as lactate dehydrogenase, 4-m gait speed, and ability to prepare meals.
Given the early publication of QOL benefits of HMAs, subsequent analyses revealing how frailty affects survival, and emerging data from the National Heart, Lung, and Blood Institute/National Cancer Institute MDS Natural History Study (N = 253; mean age 72), suggesting that frail patients have significantly worse QOL,52 studies of newer agents in MDS should rigorously incorporate one or both domains in their design and analyses. It is thus disheartening that the analyses used for approval of the two newest agents—luspatercept for anemia in lower-risk MDS with ring sideroblasts (MEDALIST; N = 229, median age 71)53 and oral cedazuridine/decitabine for intermediate- and higher-risk MDS (eg, ASTX727-01-B; N = 80, median age 69-72)54—do not specifically reference frailty or QOL data. For cedazuridine/decitabine, although oral medication is presumed to be easier to tolerate for patients with cancer, this may not be the case for the frail, for patients who have trouble adhering to prescribed regimens, or for whom regular course-correcting check-ins during infusion visits may be beneficial.55,56
Finally, given the success of venetoclax plus HMA therapy for newly diagnosed acute myeloid leukemia (AML) in older adults, including those with comorbidities precluding traditional induction, there has been intense interest in this combination for frail adults with MDS. In the AML trial that served as the basis for full venetoclax approval, VIALE-A,57 frailty was accounted for in the eligibility criteria, but not as an outcome. For the MDS trials of venetoclax combinations, there are few age- or frailty-related entry criteria, but some worry that dual treatments can make QOL or function worse.
These concerns may not be substantiated. For example, M15-531, a phase Ib, dose-escalation study of venetoclax plus azacitidine for higher-risk MDS (N = 78, median age 70)58 included the European Organization for Research and Treatment of Cancer QOL Questionnaire (QLQ-C30). In preliminary analyses, improvements in dyspnea, fatigue, and global QOL were observed and physical functioning was maintained throughout treatment. Moreover, an ongoing phase III study of the combination (NCT04401748) has as a secondary outcome time to deterioration of physical functioning measured by changes in the QLQC30's physical functioning domain. While not ideal, this is a reasonable proxy for increasing frailty and should help MDS clinicians characterize the impact that the combination is likely to have on function. Hopefully, future studies will also incorporate one of the many enhanced functional measures shown to be valid for this patient population.
ACUTE MYELOID LEUKEMIA
Most cases of AML are diagnosed among adults over age 65. Although outcomes are improving, age-related disparity persists with 5-year survival rates < 10% for those ≥ 65 years. Despite evidence of benefit from therapy,59 a large proportion of older adults receive no treatment for a new diagnosis of AML.60
Both disease- and patient-related factors contribute to poor outcomes. Disease-related factors (ie, unfavorable cytogenetic and molecular abnormalities, multidrug resistance phenotype, and secondary AML) contribute to poor response to conventional chemotherapy. Patient-specific factors (ie, comorbidities and functional limitations) contribute to poor treatment tolerance.61,62 Treatment tolerance and benefit are highly variable among older adults and inadequately predicted by age alone. PS is a useful proxy to predict toxicity risk; the interaction between older age and poor PS (ie, Eastern Cooperative Oncology Group PS ≥ 3) dramatically increases early mortality risk.63 By contrast, older adults with adequate PS represent a heterogeneous group for whom treatment toxicity and benefit are less predictable and additional assessments are required.
Evidence supports the benefit of antileukemic therapy for older adults59 (defined as age ≥ 60 years). Initial treatment considerations for nonacute promyelocytic leukemia AML fall into a framework of intensive therapy, less intensive therapy, or best supportive care (BSC). Randomized studies show a consistent survival benefit for antileukemic therapy versus BSC,59 suggesting that the BSC alone should be restricted to a shrinking minority of older adults (ie, those with pre-existing frailty, limited non-AML life expectancy, or who express clear preference to avoid therapy in favor of hospice care). When possible, older adults should receive care through or in coordination with specialized leukemia centers.64
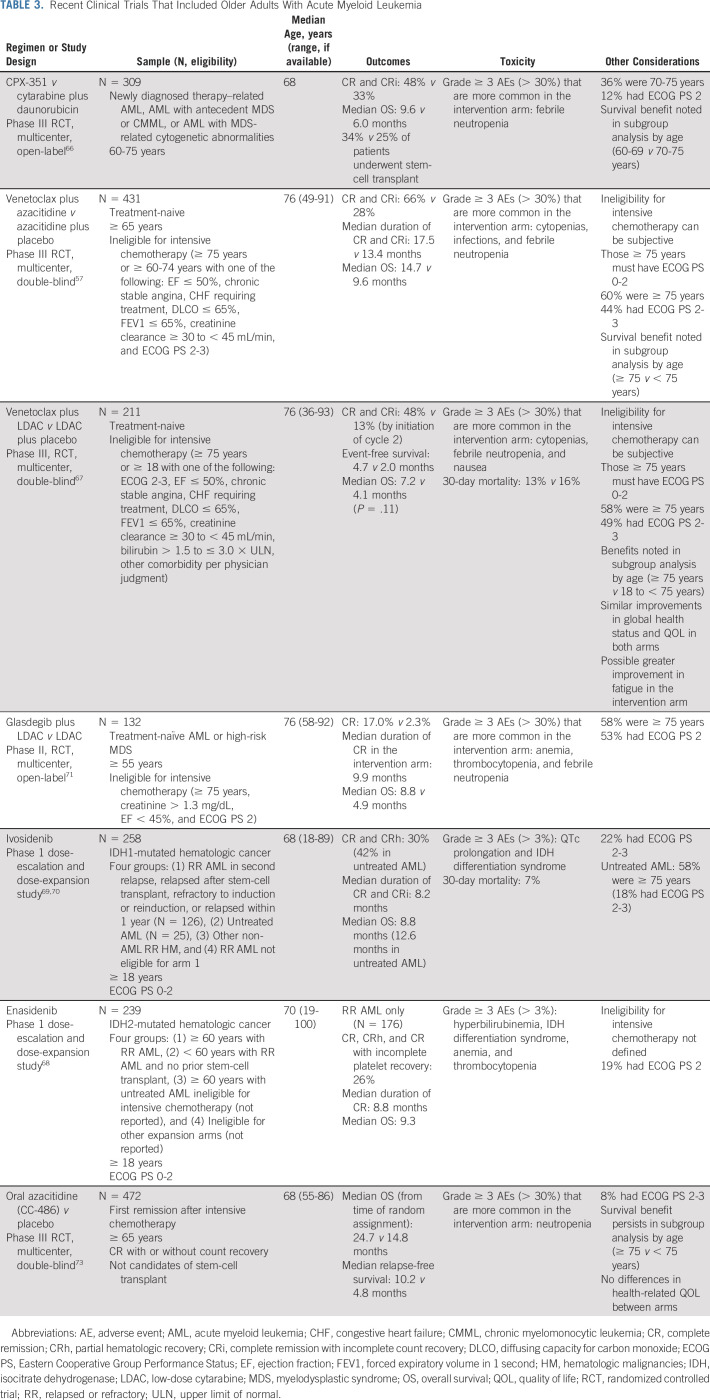
Therapeutic options have expanded significantly for older adults in recent years. Table 3 summarizes studies supporting new therapies. In general, intensive induction therapy, typically inclusive of anthracycline and cytarabine, is recommended for older adults with minimal comorbidity and good functional status (fit) in the setting of favorable- or intermediate-risk disease. Addition of the multitargeted kinase inhibitor midostaurin for FLT3-mutated AML enhances survival and should be considered for fit older adults despite lack of inclusion on the pivotal trial.65 The goal of treatment is to achieve remission, followed by postremission therapy to render long-term disease-free survivorship. An important advance is CPX-351, a dual-drug liposomal encapsulation of cytarabine and daunorubicin, which improved survival for older adults (age 60-75 years) with secondary AML (ie, therapy-related, antecedent MDS).66
TABLE 3.
Recent Clinical Trials That Included Older Adults With Acute Myeloid Leukemia
Many older adults may not be considered fit for intensive therapy or may prefer a less intensive approach. The addition of BCL-2 inhibitor venetoclax to HMAs or low-dose cytarabine has ushered in a new SOC option with improved remission rates and OS compared with single-agent therapy.57,67 Registration trials targeted an unfit population defined largely by comorbidities or age ≥ 75 years. Additional options for older adults use targeted therapies including isocitrate dehydrogenase inhibitors (enasidenib and ivosidenib) and the hedgehog inhibitor (glasdegib).68-71 The Beat-AML trial showed that a precision medicine approach to initial treatment for older adults is feasible and improves outcomes supporting the benefit of incorporating genomic data into early treatment decisions.72 Advances in postremission therapy, including HCT and use of oral azacitidine maintenance for those who are not transplant candidates, contribute to meaningful improvements in disease control and survival for older adults.73
Similar to other HM, defining fitness for therapies remains a challenge in AML.74 The decision is often based on provider judgment, chronologic age (≥ 75 years), and comorbid conditions. Algorithms exist to predict treatment response and mortality among older adults treated intensively although most rely on chronologic age as a surrogate for patient characteristics.75,76 Careful characterization of comorbidity burden (ie, HCT Comorbidity Index) adds predictive utility.77
GA is feasible and can further refine fitness for patients with AML.78 Dependence in ADLs and high comorbidity burden (HCT Comorbidity Index score > 3) predict shorter survival with less intensive therapy and can characterize individuals unfit for intensive therapy.61 Dependence in IADLs is associated with early discontinuation of HMA therapy.79 Among patients without low or modest comorbidity who are independent in ADLs, the use of objective physical performance testing (short physical performance battery) and cognition screening can further discriminate those who may be most resilient to intensive therapy.62 Practical screening tools include the 4-m walk test and the five-word recall.80,81
Inclusion of QOL information is critical for optimizing patient-centered care for AML. Observational studies show no clear difference in global QOL between intensive and less intensive treatment.82 Older adult survivors treated intensively experience improvements in QOL, largely driven by symptom improvement.78,83 Short-term declines in physical function, however, can be expected, which may affect candidacy for subsequent therapies.84 A recent analysis demonstrated that lower objectively measured function (short physical performance battery score) and depressive symptoms measured at postremission evaluation were independently associated with worse survival.85 These observations support the use of GA at key decision intervals to provide prognostic information and guide supportive care to improve fitness.
Finally, the approach to older adults with acute promyelocytic leukemia differs because of the high response and lower toxicity rates with modern therapies. Older adults regardless of fitness or age may benefit from treatment, the majority of whom can be treated with nonchemotherapy-based regimens.86
HCT AND CAR-T FOR OLDER ADULTS
HCT and CAR-T may favorably alter the natural history of high-risk HMs although treatment toxicities remain substantial. Advances in HCT have lifted traditional age limits; in 2018 transplant registry data, patients ≥ 60 years and ≥ 70 years represented 39% and 9% of allogeneic (allo) HCT, respectively, and 55% and 15% of autoHCT, respectively.87 Recent approvals of CAR-T included 23%-50% of patients ≥ 65 years in seminal studies.88-91
Autologous HCT
MM and B-cell non-Hodgkin lymphoma (NHL) constitute the major indications for autoHCT among older patients. The large randomized studies defining autoHCT as SOC for MM consolidation were tested in patients ≤ 65 years old or occasionally up to 70 years.92 Smaller randomized studies conducted 2 decades ago did not clearly establish a benefit of autografting for older patients with MM; however, meta-analysis of recent comparative studies suggests better survival applying autoHCT.93 The low transplant-related mortality (TRM) by day 100 of 1% following autoHCT for MM in those ≥ 70 years reinforces autoHCT as an option for older patients.94
Limited data exist on how autoHCT in DLBCL in response affects survival for older patients. TRM rates after autoHCT for NHL are higher than MM,95 warranting more careful appraisal of HCT candidacy in lymphoma.
Allogeneic HCT
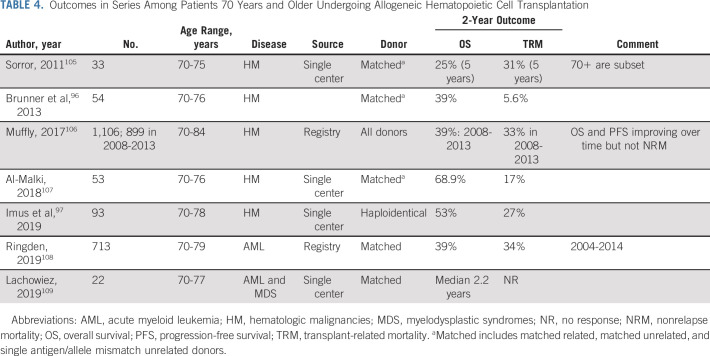
AlloHCT remains one of the most potent therapies against high-risk AML and MDS. Table 4 underscores the outcomes and danger, as registry data show around one-third of patients ≥ 70 years succumb to TRM. The studies also highlight lower TRM employing low-intensity regimens at the cost of higher relapse, particularly for AML.96,97
TABLE 4.
Outcomes in Series Among Patients 70 Years and Older Undergoing Allogeneic Hematopoietic Cell Transplantation
Observational comparative studies offer evidence, albeit of low quality, of a 10%-15% survival benefit of alloHCT for patients with AML ≥ 60 years old.98,99 In a prospective donor versus no donor design, Nakamura et al100 presented preliminary data of a 20% improved 3-year survival in patients with high-risk MDS of age 50-75 who were biologically assigned by donor match to alloHCT.
A GA in HCT may facilitate a broader concept of patient resilience and may vary based on the treatment approach; autoHCT with reduced dose melphalan at 140 mg/m2 may be safely performed with selected patient deficits, whereas alloHCT with an intermediate-intensity regimen necessitates greater resilience. Evidence supports6 pre-HCT GA uncovering deficits in a large proportion of auto/allo HCTs. Single-center studies have linked various pre-HCT functional measures with inferior outcome, primarily higher TRM after alloHCT and inferior PFS for autoHCT. One multicenter retrospective analysis applying the same panel of functional and cognitive tools found only cognitive impairment by a brief cognitive screen, not function, independently tracked with higher TRM among alloHCT patients ≥ 50 years.101 Investigators recently described a novel strategy to use GA-guided optimization to better select HCT candidates and further suggested fewer complications, less TRM, and better survival relative to historical controls.102
The era of cellular therapy has arrived with US Food and Drug Administration approval of three CAR-T products indicated for RR B-cell NHL in older adults; other approvals may emerge soon including CAR-T for MM. CAR-T therapy can produce deep and durable responses of around 40% in RR aggressive B-NHL; however, cytokine release syndrome and neurologic toxicities mandate careful consideration of candidacy and management after therapy.
Among those enrolled on the pivotal axicabtagene study (RR aggressive B-cell NHL), patients ≥ 65 years achieved similar ORR (92% older patients v 81% in patients < 65 years old) and reassuringly no difference in peak CAR-T expansion.103 Real-world data with axicabtagene likewise demonstrated higher CR at 72% for those patients ≥ 60 years old versus 55% in younger patients.104 The higher rates of grade 3 neurologic toxicity in the pivotal trial were not observed in the real-world data. Of interest, lisocabtagene maraleucel demonstrated particularly promising activity and safety (minimal grade 3+ neurotoxicity or no grade 3-5 cytokine release syndrome) in a study with 42% of patients with RR aggressive B-NHL ≥ 65 years of age.90
Risk stratification by GA or detailed health inventories have only been reported to date in a small number of older CAR-T recipients.110 Special attention to cardiovascular reserve and neurologic function is prudent based on the known toxicity profile. The feasibility and promising outcomes for adults in their seventh and eighth decade have been established for autoHCT, alloHCT, and CAR-T. Prospective studies among older adults to quantify risks and benefits are necessary. GA or other health tools to gauge patient resiliency may guide both candidacy if not strategies to mitigate toxicities.
In conclusion, therapies are expanding for older adults with HM. Personalized care requires careful consideration of disease- and patient-specific characteristics throughout the survivorship continuum. The use of GA can guide treatment selection and inform supportive care to optimize function and QOL. As the evidence supporting the use of GA measures in HMs increases, disease-specific guidelines should incorporate these data to inform evidence-based care.
Ashley E. Rosko
Consulting or Advisory Role: Association of Community Cancer Centers (ACCC), Medscape
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Raul Cordoba
Consulting or Advisory Role: Takeda, Kyowa-Kirin, Kite/Gilead, Incyte, Janssen, Celgene/Bristol-Myers Squibb
Speakers' Bureau: Abbvie, AstraZeneca, Roche
Research Funding: Pfizer
Travel, Accommodations, Expenses: Janssen, Abbvie, Roche
Andrew Artz
Employment: Radiology Partners
Stock and Other Ownership Interests: Radiology Partners
Consulting or Advisory Role: Biogen
Open Payments Link: https://openpaymentsdata.cms.gov/physician/331072
Kah Poh Loh
Consulting or Advisory Role: Pfizer, Seattle Genetics
Heidi D. Klepin
Consulting or Advisory Role: Genentech, Pfizer
Patents, Royalties, Other Intellectual Property: UpToDate contributor
Uncompensated Relationships: Genentech
No other potential conflicts of interest were reported.
SUPPORT
H.D.K. was supported by the National Institute on Aging (R33AG059206).
AUTHOR CONTRIBUTIONS
Conception and design: Ashley E. Rosko, Raul Cordoba, Gregory Abel, Andrew Artz, Heidi D. Klepin
Provision of study materials or patients: Kah Poh Loh
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Advances in Management for Older Adults With Hematologic Malignancies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ashley E. Rosko
Consulting or Advisory Role: Association of Community Cancer Centers (ACCC), Medscape
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Raul Cordoba
Consulting or Advisory Role: Takeda, Kyowa-Kirin, Kite/Gilead, Incyte, Janssen, Celgene/Bristol-Myers Squibb
Speakers' Bureau: Abbvie, AstraZeneca, Roche
Research Funding: Pfizer
Travel, Accommodations, Expenses: Janssen, Abbvie, Roche
Andrew Artz
Employment: Radiology Partners
Stock and Other Ownership Interests: Radiology Partners
Consulting or Advisory Role: Biogen
Open Payments Link: https://openpaymentsdata.cms.gov/physician/331072
Kah Poh Loh
Consulting or Advisory Role: Pfizer, Seattle Genetics
Heidi D. Klepin
Consulting or Advisory Role: Genentech, Pfizer
Patents, Royalties, Other Intellectual Property: UpToDate contributor
Uncompensated Relationships: Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Maura F, Bolli N, Angelopoulos N, et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat Commun. 2019;10:3835. doi: 10.1038/s41467-019-11680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avet-Loiseau H, Hulin C, Campion L, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: The Intergroupe Francophone du Myélome Experience J Clin Oncol 312806–28092013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakhri B, Fiala MA, Tuchman SA, et al. Undertreatment of older patients with newly diagnosed multiple myeloma in the era of novel therapies Clin Lymphoma Myeloma Leuk 18219–2242018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mian HS, Kouroukis CT, Pond GR, et al. Undertreatment of older patients with newly-diagnosed multiple myeloma: Real world evidence from a Canadian registry cohort. Blood. 2019;134:268. [Google Scholar]
- 5.Kristinsson SY, Anderson WF, Landgren O.Improved long-term survival in multiple myeloma up to the age of 80 years Leukemia 281346–13482014 [DOI] [PubMed] [Google Scholar]
- 6.Jayani R, Rosko A, Olin R, et al. Use of geriatric assessment in hematopoietic cell transplant J Geriatr Oncol 11225–2362020 [DOI] [PubMed] [Google Scholar]
- 7.Kumar SK, Callander NS, Hillengass J, et al. NCCN guidelines insights: Multiple myeloma, version 1.2020 J Natl Compr Canc Netw 171154–11652019 [DOI] [PubMed] [Google Scholar]
- 8.Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation Blood 1176063–60732011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma N Engl J Med 3761311–13202017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurore Perrot M, Lauwers-Cances V, Cazaubiel T, et al. Early versus late autologous stem cell transplant in newly diagnosed multiple myeloma: Long-term follow-up analysis of the IFM 2009 trial. Presented at 62nd ASH Annual Meeting, December, 2020, Virtual.
- 11.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma N Engl J Med 371906–9172014 [DOI] [PubMed] [Google Scholar]
- 12.Lee HC, Ailawadhi S, Gasparetto C, et al. Treatment patterns and outcomes in elderly patients with newly diagnosed multiple myeloma: Results from the Connect® MM Registry. Blood. 2019;134:3128. doi: 10.1038/s41408-021-00524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial Lancet 389519–5272017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma N Engl J Med 3802104–21152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar SK, Jacobus SJ, Cohen AD, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): A multicentre, open-label, phase 3, randomised, controlled trial Lancet Oncol 211317–13302020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial Lancet 395132–1412020 [DOI] [PubMed] [Google Scholar]
- 17.Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial Blood 136936–9452020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa LJ, Chhabra S, Godby KN, et al. Daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd) induction, autologous transplantation and post-transplant, response-adapted, measurable residual disease (MRD)-based Dara-KRd consolidation in patients with newly diagnosed multiple myeloma (NDMM) Blood. 2019;134:860. [Google Scholar]
- 19.Bazarbachi AH, Al Hamed R, Malard F, et al. Relapsed refractory multiple myeloma: A comprehensive overview Leukemia 332343–23572019 [DOI] [PubMed] [Google Scholar]
- 20.Weisel K, Einsele H, Goldschmidt H, et al. Quality of life in patients (pts) with relapsed and refractory multiple myeloma (RRMM) treated with the BCMA-DIRECTED CAR T cell therapy Idecabtagene vicleucel (IDE-CEL, bb2121): Results from the KarMMa trial Oncol Res Treat 4393–942020 [Google Scholar]
- 21.Rosko AE, Huang Y, Benson DM, et al. Use of a comprehensive frailty assessment to predict morbidity in patients with multiple myeloma undergoing transplant J Geriatr Oncol 10479–4852018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel BG, Luo S, Wildes TM, et al. Frailty in older adults with multiple myeloma: A study of US veterans JCO Clin Cancer Inform 4117–1272020 [DOI] [PubMed] [Google Scholar]
- 23.dela Rubia J, González BJ, Hernández Rivas JÁ, et al. GAH scale is a simple, comprehensive assessment tool in older patients with hematological malignancies that shows mortality prediction capacities. Clin Lymphoma Myeloma Leuk. 2015;15:e99. [Google Scholar]
- 24.Bonanad S, De la Rubia J, Gironella M, et al. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: The GAH scale J Geriatr Oncol 6353–3612015 [DOI] [PubMed] [Google Scholar]
- 25.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report Blood 1252068–20742015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelhardt M, Domm AS, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients Haematologica 102910–9212017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial Leukemia 34224–2332020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathwani N, Kurtin SE, Lipe B, et al. Integrating touchscreen-based geriatric assessment and frailty screening for adults with multiple myeloma to drive personalized treatment decisions JCO Oncol Pract 16e92–e992020 [DOI] [PubMed] [Google Scholar]
- 29.Tucci A, Martelli M, Rigacci L, et al. Comprehensive geriatric assessment is an essential tool to support treatment decisions in elderly patients with diffuse large B-cell lymphoma: A prospective multicenter evaluation in 173 patients by the Lymphoma Italian Foundation (FIL) Leuk Lymphoma 56921–9262015 [DOI] [PubMed] [Google Scholar]
- 30.Merli F, Luminari S, Tucci A, et al. Simplified geriatric assessment in older patients with diffuse large B-cell lymphoma: The prospective elderly project of the Fondazione Italiana Linfomi J Clin Oncol39:1214-1222, 2021 [DOI] [PubMed]
- 31.Morrison VA, Hamlin P, Soubeyran P, et al. Approach to therapy of diffuse large B-cell lymphoma in the elderly: The International Society of Geriatric Oncology (SIOG) expert position commentary Ann Oncol 261058–10682015 [DOI] [PubMed] [Google Scholar]
- 32.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: A multicentre, single-arm, phase 2 trial Lancet Oncol 12460–4682011 [DOI] [PubMed] [Google Scholar]
- 33.Peyrade F, Bologna S, Delwail V, et al. Combination of ofatumumab and reduced-dose CHOP for diffuse large B-cell lymphomas in patients aged 80 years or older: An open-label, multicentre, single-arm, phase 2 trial from the LYSA group Lancet Haematol 4e46–e552017 [DOI] [PubMed] [Google Scholar]
- 34.Oberic L, Peyrade F, Puyade M, et al. Subcutaneous rituximab-MiniCHOP compared with subcutaneous rituximab-MiniCHOP plus lenalidomide in diffuse large B-cell lymphoma for patients age 80 years or older J Clin Oncol39:1203-1213, 2021 [DOI] [PubMed]
- 35.Merli F, Cavallo F, Salvi F, et al. Obinutuzumab and miniCHOP for unfit patients with diffuse large B-cell lymphoma. A phase II study by Fondazione Italiana Linfomi J Geriatr Oncol 1137–402020 [DOI] [PubMed] [Google Scholar]
- 36.Eyre TA, Martinez-Calle N, Hildyard C, et al. Impact of intended and relative dose intensity of R-CHOP in a large, consecutive cohort of elderly diffuse large B-cell lymphoma patients treated with curative intent: No difference in cumulative incidence of relapse comparing patients by age J Intern Med 285681–6922019 [DOI] [PubMed] [Google Scholar]
- 37.Juul MB, Jensen PH, Engberg H, et al. Treatment strategies and outcomes in diffuse large B-cell lymphoma among 1011 patients aged 75 years or older: A Danish population-based cohort study Eur J Cancer 9986–962018 [DOI] [PubMed] [Google Scholar]
- 38.Fields PA, Townsend W, Webb A, et al. De novo treatment of diffuse large B-cell lymphoma with rituximab, cyclophosphamide, vincristine, gemcitabine, and prednisolone in patients with cardiac comorbidity: A United Kingdom National Cancer Research Institute trial J Clin Oncol 32282–2872014 [DOI] [PubMed] [Google Scholar]
- 39.Shen QD, Zhu HY, Wang L, et al. Gemcitabine-oxaliplatin plus rituximab (R-GemOx) as first-line treatment in elderly patients with diffuse large B-cell lymphoma: A single-arm, open-label, phase 2 trial Lancet Haematol 5e261–e2692018 [DOI] [PubMed] [Google Scholar]
- 40.Luminari S, Viel E, Ferreri AJM, et al. Nonpegylated liposomal doxorubicin combination regimen in patients with diffuse large B-cell lymphoma and cardiac comorbidity. Results of the HEART01 phase II trial conducted by the Fondazione Italiana Linfomi Hematol Oncol 3668–752018 [DOI] [PubMed] [Google Scholar]
- 41.Storti S, Spina M, Pesce EA, et al. Rituximab plus bendamustine as front-line treatment in frail elderly (>70 years) patients with diffuse large B-cell non-Hodgkin lymphoma: A phase II multicenter study of the Fondazione Italiana Linfomi Haematologica 1031345–13502018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma J Clin Oncol 38155–1652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study Lancet Oncol 21978–9882020 [DOI] [PubMed] [Google Scholar]
- 44.Kornblith AB, Herndon JE, II, Silverman LR, et al. Impact of azacytidine on the quality of life of patients with myelodysplastic syndrome treated in a randomized phase III trial: A Cancer and Leukemia Group B study J Clin Oncol 202441–24522002 [DOI] [PubMed] [Google Scholar]
- 45.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: Results of a phase III randomized study Cancer 1061794–18032006 [DOI] [PubMed] [Google Scholar]
- 46.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people CMAJ 173489–4952005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buckstein R, Wells RA, Zhu N, et al. Patient-related factors independently impact overall survival in patients with myelodysplastic syndromes: An MDS-CAN prospective study Br J Haematol 17488–1012016 [DOI] [PubMed] [Google Scholar]
- 48.Sakatoku K, Takeoka Y, Miura A, et al. Combination of frailty status and comorbidity score improves the stratification of survival in patients with myelodysplastic syndrome owing to good predictive capability for infection-related mortality Clin Lymphoma Myeloma Leuk 19799–8052019 [DOI] [PubMed] [Google Scholar]
- 49.Starkman R, Alibhai S, Wells RA, et al. An MDS-specific frailty index based on cumulative deficits adds independent prognostic information to clinical prognostic scoring Leukemia 341394–14062020 [DOI] [PubMed] [Google Scholar]
- 50.Wan BA, Nazha A, Starkman R, et al. Revised 15-item MDS-specific frailty scale maintains prognostic potential Leukemia 343434–34382020 [DOI] [PubMed] [Google Scholar]
- 51.QxMD: Myelodysplastic syndrome (MDS) specific frailty index. https://qxcalc.app.link/mdsfrailty
- 52.Abel G, Hebert D, Lee C, et al. Patient-reported outcomes and frailty among participants in the NHLBI MDS natural history study (abstract) Blood. 2020;136(suppl 1):1663. [Google Scholar]
- 53.Fenaux P, Platzbecker U, Mufti GJ, et al. Luspatercept in patients with lower-risk myelodysplastic syndromes N Engl J Med 382140–1512020 [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Manero G, Griffiths EA, Steensma DP, et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study Blood 136674–6832020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacobs JM, Ream ME, Pensak N, et al. Patient experiences with oral chemotherapy: Adherence, symptoms, and quality of life J Natl Compr Canc Netw 17221–2282019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xavier FD, Ferreira FSB, Abreu RM.Treatment of elderly patients with refractory/relapsed multiple myeloma: Oral drugs adherence and the COVID-19 outbreak Oncotarget 114371–43862020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia N Engl J Med 383617–6292020 [DOI] [PubMed] [Google Scholar]
- 58.Garcia J, Wei H, Borate U, et al. Safety, efficacy, and patient-reported outcomes of venetoclax in combination with azacitidine for the treatment of patients with higher-risk myelodysplastic syndrome: A phase 1b study (abstract) Blood. 2020;136(suppl 1):656. [Google Scholar]
- 59.Sekeres MA, Guyatt G, Abel G, et al. American Society of Hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults Blood Adv 43528–35492020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeidan AM, Podoltsev NA, Wang X, et al. Temporal patterns and predictors of receiving no active treatment among older patients with acute myeloid leukemia in the United States: A population-level analysis Cancer 1254241–42512019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deschler B, Ihorst G, Platzbecker U, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome Haematologica 98208–2162013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia Blood 1214287–42942013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia Blood 1073481–34852006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeidan AM, Podoltsev NA, Wang X, et al. Patterns of care and clinical outcomes with cytarabine-anthracycline induction chemotherapy for AML patients in the United States Blood Adv 41615–16232020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation N Engl J Med 377454–4642017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia J Clin Oncol 362684–26922018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei A, Strickland S, Hou J-Z, et al. Venetoclax with low-dose cytarabine induces rapid, deep, and durable responses in previously untreated older adults with AML ineligible for intensive chemotherapy. Blood. 2018;132:284. [Google Scholar]
- 68.Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia Blood 130722–7312017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roboz GJ, DiNardo CD, Stein EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia Blood 135463–4712020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML N Engl J Med 3782386–23982018 [DOI] [PubMed] [Google Scholar]
- 71.Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome Leukemia 33379–3892019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burd A, Levine RL, Ruppert AS, et al. Precision medicine treatment in acute myeloid leukemia using prospective genomic profiling: Feasibility and preliminary efficacy of the Beat AML Master Trial Nat Med 261852–18582020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei AH, Döhner H, Pocock C, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission N Engl J Med 3832526–25372020 [DOI] [PubMed] [Google Scholar]
- 74.Borlenghi E, Pagani C, Zappasodi P, et al. Validation of the “fitness criteria” for the treatment of older patients with acute myeloid leukemia: A multicenter study on a series of 699 patients by the Network Rete Ematologica Lombarda (REL) J Geriatr Oncol 12550–5562021 [DOI] [PubMed] [Google Scholar]
- 75.Rollig C, Thiede C, Gramatzki M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: Results of 909 patients entered into the prospective AML96 trial Blood 116971–9782010 [DOI] [PubMed] [Google Scholar]
- 76.Krug U, Rollig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: A web-based application for prediction of outcomes Lancet 3762000–20082010 [DOI] [PubMed] [Google Scholar]
- 77.Sorror ML, Storer BE, Nyland J, et al. Revised acute myeloid leukemia composite model using the 2017 European LeukemiaNet Risk Classification JAMA Oncol 51062–10642019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klepin HD, Ritchie E, Major-Elechi B, et al. Geriatric assessment among older adults receiving intensive therapy for acute myeloid leukemia: Report of CALGB 361006 (Alliance) J Geriatr Oncol 11107–1132020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Molga A, Wall M, Chhetri R, et al. Comprehensive geriatric assessment predicts azacitidine treatment duration and survival in older patients with myelodysplastic syndromes J Geriatr Oncol 11114–1202020 [DOI] [PubMed] [Google Scholar]
- 80.Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength and clinical outcomes in older patients with hematologic malignancies Blood 134374–3822019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hshieh TT, Jung WF, Grande LJ, et al. Prevalence of cognitive impairment and association with survival among older patients with hematologic cancers JAMA Oncol 4686–6932018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Jawahri A, Abel GA, Traeger L, et al. Quality of life and mood of older patients with acute myeloid leukemia (AML) receiving intensive and non-intensive chemotherapy Leukemia 332393–24022019 [DOI] [PubMed] [Google Scholar]
- 83.Timilshina N, Breunis H, Tomlinson GA, et al. Long-term recovery of quality of life and physical function over three years in adult survivors of acute myeloid leukemia after intensive chemotherapy Leukemia 3315–252019 [DOI] [PubMed] [Google Scholar]
- 84.Klepin HD, Tooze JA, Pardee TS, et al. Effect of intensive chemotherapy on physical, cognitive, and emotional health of older adults with acute myeloid leukemia J Am Geriatr Soc 641988–19952016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saad M, Loh KP, Tooze JA, et al. Geriatric assessment and survival among older adults receiving postremission therapy for acute myeloid leukemia Blood 1362715–27192020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klepin HD, Neuendorff NR, Larson RA, et al. Treatment of acute promyelocytic leukemia in older patients: Recommendations of an International Society of Geriatric Oncology (SIOG) task force J Geriatr Oncol 111199–12092020 [DOI] [PubMed] [Google Scholar]
- 87.D'Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States Biol Blood Marrow Transpl 26e177–e1822020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma N Engl J Med 3772531–25442017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma N Engl J Med 38045–562019 [DOI] [PubMed] [Google Scholar]
- 90.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study Lancet 396839–8522020 [DOI] [PubMed] [Google Scholar]
- 91.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma N Engl J Med 3821331–13422020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stadtmauer EA, Pasquini MC, Blackwell B, et al. Autologous transplantation, consolidation, and maintenance therapy in multiple myeloma: Results of the BMT CTN 0702 trial J Clin Oncol 37589–5972019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mian H, Mian OS, Rochwerg B, et al. Autologous stem cell transplant in older patients (age ≥65) with newly diagnosed multiple myeloma: A systematic review and meta-analysis J Geriatr Oncol 1193–992020 [DOI] [PubMed] [Google Scholar]
- 94.Munshi PN, Vesole D, Jurczyszyn A, et al. Age no bar: A CIBMTR analysis of elderly patients undergoing autologous hematopoietic cell transplantation for multiple myeloma Cancer 1265077–50872020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jantunen E, Canals C, Rambaldi A, et al. Autologous stem cell transplantation in elderly patients (≥ 60 years) with diffuse large B-cell lymphoma: An analysis based on data in the European Blood and Marrow Transplantation registry Haematologica 931837–18422008 [DOI] [PubMed] [Google Scholar]
- 96.Brunner AM, Kim HT, Coughlin E, et al. Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies Biol Blood Marrow Transpl 191374–13802013 [DOI] [PubMed] [Google Scholar]
- 97.Imus PH, Tsai HL, Luznik L, et al. Haploidentical transplantation using posttransplant cyclophosphamide as GVHD prophylaxis in patients over age 70 Blood Adv 32608–26162019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ustun C, Le-Rademacher J, Wang HL, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): An alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study Leukemia 332599–26092019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Versluis J, Hazenberg CL, Passweg JR, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: A time-dependent analysis Lancet Haematol 2e427–e4362015 [DOI] [PubMed] [Google Scholar]
- 100.Nakamura R, Saber W, Martens MJ, et al. A multi-center biologic assignment trial comparing reduced intensity allogeneic hematopoietic cell transplantation to hypomethylating therapy or best supportive care in patients aged 50-75 with advanced myelodysplastic syndrome: Blood and Marrow Transplant Clinical Trials Network Study 1102 Blood 136(suppl 1) 19–212020 [Google Scholar]
- 101.Olin RL, Fretham C, Pasquini MC, et al. Cognitive impairment is associated with inferior survival and increased non-relapse mortality in older allogeneic hematopoietic cell transplant (alloHCT) recipients: A multicenter retrospective study. Blood. 2019;134:4606. [Google Scholar]
- 102.Derman BA, Kordas K, Ridgeway J, et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults Blood Adv 33488–34982019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neelapu SS, Jacobson CA, Oluwole OO, et al. Outcomes of older patients in ZUMA-1, a pivotal study of axicabtagene ciloleucel in refractory large B-cell lymphoma Blood 1352106–21092020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: Results from the US Lymphoma CAR T Consortium J Clin Oncol 383119–31282020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sorror ML, Sandmaier BM, Storer BE, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies JAMA 3061874–18832011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muffly L, Pasquini MC, Martens M, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States Blood 1301156–11642017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Malki MM, Nathwani N, Yang D, et al. Melphalan-based reduced-intensity conditioning is associated with favorable disease control and acceptable toxicities in patients older than 70 with hematologic malignancies undergoing allogeneic hematopoietic stem cell transplantation Biol Blood Marrow Transplant 241828–18352018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ringden O, Boumendil A, Labopin M, et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients age > 69 years with acute myelogenous leukemia: On behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation Biol Blood Marrow Transplant 251975–19832019 [DOI] [PubMed] [Google Scholar]
- 109.Lachowiez C, Cook RJ, Hayes-Lattin B, et al. Allogeneic transplantation outcomes amongst a contemporary cohort of high-risk myelodysplastic syndrome and acute myeloid leukemia patients aged ≥70 years Hematol Oncol Stem Cell Ther 12105–1092019 [DOI] [PubMed] [Google Scholar]
- 110.Lin RJ, Lobaugh SM, Pennisi M, et al. Impact and safety of chimeric antigen receptor T-cell therapy in older, vulnerable patients with relapsed/refractory large B-cell lymphoma Haematologica 106255–2582021 [DOI] [PMC free article] [PubMed] [Google Scholar]