Abstract
Due to soil changes, high density planting, and the use of straw-returning methods, wheat common root rot (spot blotch), Fusarium crown rot (FCR), and sharp eyespot (sheath blight) have become severe threats to global wheat production. Only a few wheat genotypes show moderate resistance to these root and crown rot fungal diseases, and the genetic determinants of wheat resistance to these devastating diseases are poorly understood. This review summarizes recent results of genetic studies of wheat resistance to common root rot, Fusarium crown rot, and sharp eyespot. Wheat germplasm with relatively higher resistance are highlighted and genetic loci controlling the resistance to each disease are summarized.
Keywords: wheat, resistance, common rot root, spot blotch, Fusarium crown rot, sharp eyespot
Introduction
Long-term environmental changes have greatly affected crop diseases. For example, the higher temperatures associated with global warming may increase the severity of many plant diseases (Cohen and Leach, 2020). Bursts of wheat stem base rot diseases, including common root rot (spot blotch), Fusarium crown rot, and sharp eyespot, are highly correlated with crop rotation practices. The large-scale application of wheat-maize rotation in the North China wheat cultivation area has dramatically changed the organic carbon, fertilization state, and nitrogen balance of the soil (Zhao et al., 2006; Wang et al., 2015). The disease suppressive capacity of the soil microbiome is also highly dependent on crop rotational diversity (Peralta et al., 2018).
Pathogenic Profiles
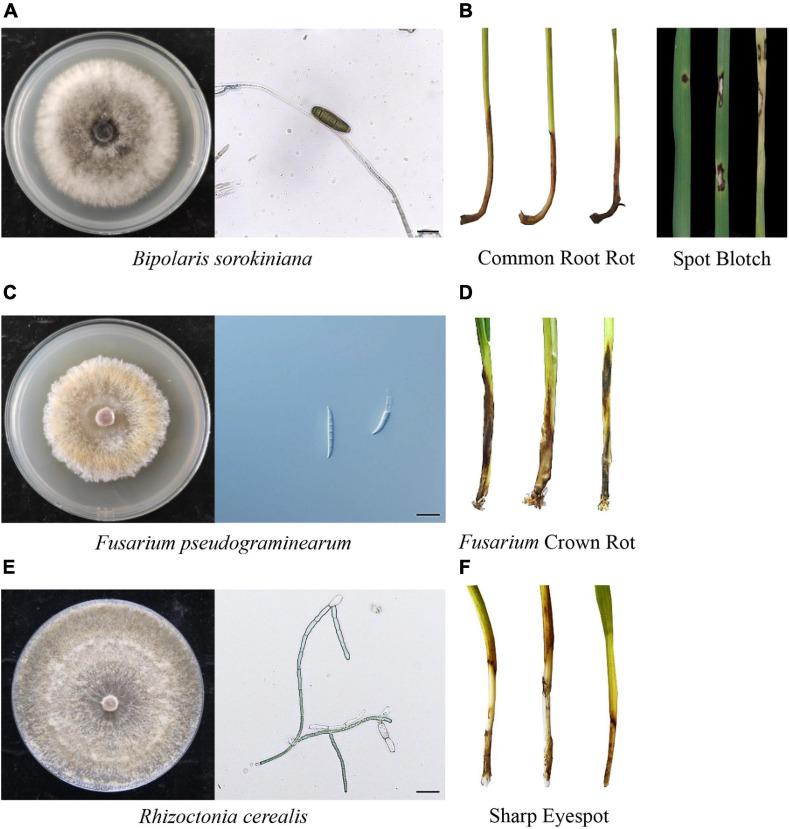
Wheat common root rot is caused by Bipolaris sorokiniana infection (Figure 1A, teleomorph Cochliobolus sativus) in the root and stem base of wheat plants. Severe infections of this fungal pathogen in the root and crown of seedlings may kill plants. B. sorokiniana can also induce phenotypes of leaf spot (spot blotch, Helminthosporium leaf blight, or foliar blight, Figure 1B), seedling wilt, head blight, and black point in Triticeae crops (Kumar et al., 2002). The average yield loss caused by B. sorokiniana ranges from 15 to 20%, but under favorable heat and drought conditions this disease can decrease wheat production by 70% and reduce seed quality (Sharma and Duveiller, 2007). This fungal pathogen accumulates several toxins to kill or weaken plant cells, including prehelminthosporol, helminthosporol, helminthosporic acid, sorokinianin, and bipolaroxin (Kumar et al., 2002; Gupta et al., 2018). However, the potential negative effects of B. sorokiniana-infected wheat grains (black point) on food safety have not been investigated in detail. B. sorokiniana has a very wide host range, as it can infect wheat, barley, maize, rice, and many other grass species (Gupta et al., 2018). Multiple-year Triticeae crop rotations of wheat and barley greatly promote the severity of common root rot caused by B. sorokiniana (Conner et al., 1996). Maize crops and returned straws may also be infected by this fungus, so common root rot and spot blotch have been more frequently observed in areas of wheat cultivation in North China where methods of large-scale wheat-maize rotation and straw returning have been applied. Wheat resistance to B. sorokiniana was largely associated with accumulation of reactive oxygen species (ROS) and transcriptional activation of pathogenesis-related protein (PR) genes (Kumar et al., 2001; Wang et al., 2018b).
FIGURE 1.
Pathogenic profiles of Bipolaris sorokiniana, Fusarium pseudograminearum, and Rhizoctonia cerealis. (A) B. sorokiniana was cultivated on potato dextrose agar (PDA) medium and spores were directly collected. (B) Common root rot and spot blotch caused by B. sorokiniana. Infected wheat plants were easily pulled out, the stem base and root system felt wet, and black and brown striped spots can be observed in both the stem base and lower leaves. (C) F. pseudograminearum cultivated on PDA medium. Spores of F. pseudograminearum can be induced on carboxymethyl cellulose sodium (CMC) medium. (D) Fusarium crown rot caused by F. pseudograminearum. The stem base of infected wheat plants became dry and fragile, and was easily broken apart. Additionally, dark and red brown rot can be observed in the stem base. (E) R. cerealis was cultivated on PDA medium. (F) Sharp eyespot caused by R. cerealis. The typical lesions on wheat stem are elliptical or exhibit an “eye” shape with sharply dark brown borders. Scale bar = 20 μm.
Fusarium crown rot (FCR) is caused by infection of Fusarium pseudograminearum (Figure 1C), or other Fusarium pathogens including F. culmorum, F. avenaceum, and F. graminearum. These fungal species infect the coleoptile, leaf sheath, and stem base of wheat seedlings, generating browning and decay phenotypes (Figure 1D). Fusarium pathogens are found globally in arid and semi-arid wheat planting areas (Kazan and Gardiner, 2018). FCR infection caused an estimated 35% yield loss of winter wheat in the Northwest Pacific region of the United States (Smiley et al., 2005). When FCR-infected plants are co-infected with Fusarium Head Blight (FHB), wheat seeds are likely to be contaminated by fungal toxins such as deoxynivalenol (DON) and nivalenol (NIV), which greatly threaten the health of human and livestock (Monds et al., 2005; Obanor and Chakraborty, 2014). Maize also can be infected with various Fusarium pathogens, and the fungi from infected plants can remain active in returned straw debris for as long as 5 years (Burgess et al., 2001). For these reasons, FCR is a growing threat to wheat cultivation in wheat-maize rotation regions in North China. Based on previous omics studies, wheat resistance to FCR was associated with transcriptional activations of transcription factor, cellular transport and detoxification genes, as well as protein accumulations in photosynthesis, secondary metabolite biosynthesis, phenylpropanoid biosynthesis, and glutathione metabolism (Powell et al., 2017; Qiao et al., 2021).
Wheat sharp eyespot (sheath blight) is caused by infection of Rhizoctonia cerealis (Figure 1E) in the root and stem base of wheat plants, generating disease symptoms of stem eyespot (Figure 1F), crown rot, seedling fatal damage, and head blight. Wheat sharp eyespot is a typical soil-borne fungal disease that is prevalent worldwide (Hamada et al., 2011). R. cerealis also has a broad host range, including many cereals. This fungal pathogen can survive in soil or on infected crop residues for a long time. Consequently, practices of wheat-maize rotation and straw-returning have greatly facilitated the burst of this disease in China during the last two decades (Ren et al., 2020). In 2005, approximately 8 million ha of wheat fields in China were infected with sharp eyespot, with an estimated yield loss of about 530,000 tons (McBeath and McBeath, 2010). Sharp eyespot also significantly decreases wheat grain quality (Lemańczyk and Kwaśna, 2013). Wheat resistance to sharp eyespot seemed to be dependent on a complex defense pathway including genes encoding nucleotide binding site-leucine rich repeat (NBS-LRR) protein, ethylene response factor (ERF) transcription factor, and AGC kinase (Zhu et al., 2014a, 2015, 2017).
These three diseases can have similar phenotypes, causing stem base rot and head blight, but there are differences as well. Common root rot caused by B. sorokiniana weakens infected wheat plants so they can be easily pulled out. Additionally the stem base and root system feel wet, and black and brown striped spots form on both the stem base and lower leaves (Figure 1B). For FCR caused by F. pseudograminearum, the stem base of the infected wheat plant becomes dry and fragile, and dark brown rot can be observed in the stem base (Figure 1D). For sharp eyespot caused by R. cerealis, lesions on the wheat stem are elliptical or have a “eye” shape, with sharply dark brown borders (Figure 1F).
Progress in Dissecting the Genetics of Wheat Resistance to Common Root Rot (Spot Blotch)
The use of wheat resistant cultivars remains the most efficient and economical way to control common root rot (spot blotch). However, there are currently insufficient germplasm resources with resistance to common root rot to meet the growing needs for global wheat breeding applications and there have been few studies to identify the genetic loci that control resistance to common root rot (Gupta et al., 2018). Early efforts focused on the introgression of common root rot resistant loci from Thinopyrum ponticum, a wheat relative (Li et al., 2004). Wheat breeding programs for common root rot resistance have had limited success because analysis of complex quantitative trait loci (QTL) is required (Joshi et al., 2004). Using bi-parental populations and linkage mapping, four genetic loci with major resistant effect were identified and designated as Sb genes. Sb1 was discovered in the bread wheat line “Saar,” was mapped to chromosome 7DS, and is associated with the wheat leaf rust resistance gene Lr34 (Lillemo et al., 2013). The Lr34/Yr18/Pm38 gene encodes a ATP-binding cassette (ABC) transporter that confers broad-spectrum resistance to multiple foliar fungal diseases, including leaf rust, stripe rust, and powdery mildew (Krattinger et al., 2009). Another minor QTL linked to Lr46 on chromosome 1BL was also identified from “Saar.” The Lr46 gene is associated with resistance to leaf rust in adult plants and is also associated with the stripe rust resistance gene Yr29 (William et al., 2003). The Sb2 gene was identified in bread wheat cultivar “Yangmai 6,” which significantly reduced the spot blotch disease severity on wheat leaves (Kumar et al., 2015). The Sb2 gene was mapped to chromosome 5BL between simple sequence repeat (SSR) markers of Xgwm639 and Xgwm1043. The Sb2 gene was later reported to be linked with the Tsn1 gene, which confers host-selective sensitivity to the fungal toxin ToxA produced by Pyrenophora tritici-repentis (Kumar et al., 2016). The Sb3 gene was discovered in the winter wheat line “621-7-1” based on its correlation with immune response to B. sorokiniana on leaves. Using bulked segregant analysis (BSA), Sb3 was mapped to chromosome 3BS, flanking SSR markers of Xbarc133 and Xbarc147 (Lu et al., 2016). The Sb4 gene was recently identified from two highly resistant wheat lines, “Zhongyu1211” and “GY17,” which prevented the infection of B. sorokiniana on both leaves and sheaths of wheat plants. Using RNA-based BSA and single-nucleotide polymorphism (SNP) mapping, Sb4 was delimitated to a 1.19 cM genetic interval region of chromosome 4BL, which contains 21 predicted genes in the corresponding “Chinese Spring” genome (Zhang et al., 2020). Future work should clone these Sb genes to further elucidate the mechanism of wheat resistance toward this devastating fungal pathogen.
Several other major QTLs have been discovered and preliminarily mapped using bi-parental populations. For example, two resistant QTLs derived from “Yangmai 6” were mapped to chromosomes 5B and 7D using microsatellite markers (Kumar et al., 2005). Three QTLs on chromosomes 5B, 6A, and 6D were identified based on analysis of SSR markers from the resistant genotype “G162” (Sharma et al., 2007). Four QTLs controlling resistance of wheat cultivar “Yangmai 6” to B. sorokiniana were mapped to chromosomes 2AL, 2BS, 5BL, and 6DL (Kumar et al., 2009). A total of seven QTLs providing resistance to B. sorokiniana infections were mapped in the wheat lines “Ning 8201” and “Chirya 3” (Kumar et al., 2010). Three QTLs on chromosomes 1BS, 3BS, and 5AS respectively explained 8.5, 17.6, and 12.3%, of the resistant effect in “SYN1,” a CIMMYT (International Maize and Wheat Improvement Center) synthetic-derived bread wheat line (Zhu et al., 2014b). From the Brazilian resistant cultivar “BH 1146,” two QTLs on chromosomes 7BL and 7DL were mapped using microsatellite markers (Singh et al., 2016). A prominent resistant QTL near the Vrn-A1 locus on chromosome 5AL was found in “BARTAI” and “WUYA” CIMMYT breeding lines (Singh et al., 2018). QTLs in Vrn-A1 and Sb2/Tsn1 loci were detected in two other CIMMYT breeding lines, “CASCABEL” and “KATH” (He et al., 2020).
Genome-wide association studies (GWAS) have been widely used to identify QTLs. Using 832 polymorphic Diversity Arrays Technology (DArT) markers, four QTLs resistant to spot blotch were mapped to chromosomes 1A, 3B, 7B, and 7D after analysis of 566 spring wheat germplasm (Adhikari et al., 2012). A phenotypic screening of 11 parental genotypes and 55 F2 lines identified “19HRWSN6” as a resistant source. Subsequent simple linear regression analysis revealed SSR markers on chromosomes 5B, 6A, and 7D associated with resistance to B. sorokiniana (Tembo et al., 2017). There has been recent progress in drafting the physical genome of hexaploid wheat (Appels et al., 2018), and high-throughput SNP toolkits are now available for GWAS on various complex traits of wheat (Sun et al., 2020). A total of 528 spring wheat genotypes from different geographic regions were tested for spot blotch resistance and eleven associated SNP markers were found by 9K SNP assay (Gurung et al., 2014). Another study evaluated the responses of 294 hard winter wheat genotypes to B. sorokiniana and performed GWAS by 15K SNP assay. Ten wheat genotypes with relatively high resistance were identified, and six major resistant QTLs were found to collectively explain 30% of the phenotypic variation (Ayana et al., 2018). A total of 159 spring wheat genotypes were screened for common root rot resistance and 24 QTLs were identified, with a major one on chromosome 7B that explained 14% of the phenotypic variation of spot blotch severity (Jamil et al., 2018). Another study profiled the resistant phenotype of 287 spring wheat germplasm and performed GWAS using 90K SNP array. Eight genetic loci were associated with incubation period, lesion number, and disease score of B. sorokiniana infection (Ahirwar et al., 2018). A recent study phenotyped 301 Afghan wheat germplasm and found that approximately 15% exhibited lower disease scores than the resistant control. A subsequent GWAS approach identified 25 marker-trait associations on more than 12 chromosomes, including previously identified Vrn-A1, and Sb2/Tsn1 loci (Bainsla et al., 2020). Another 141 spring wheat lines were collected for GWAS on spot blotch resistance. A total of 23 genomic loci were identified, including several stable QTLs on chromosomes 2B, 5B, and 7D, and a novel QTL on chromosome 3D (Tomar et al., 2020).
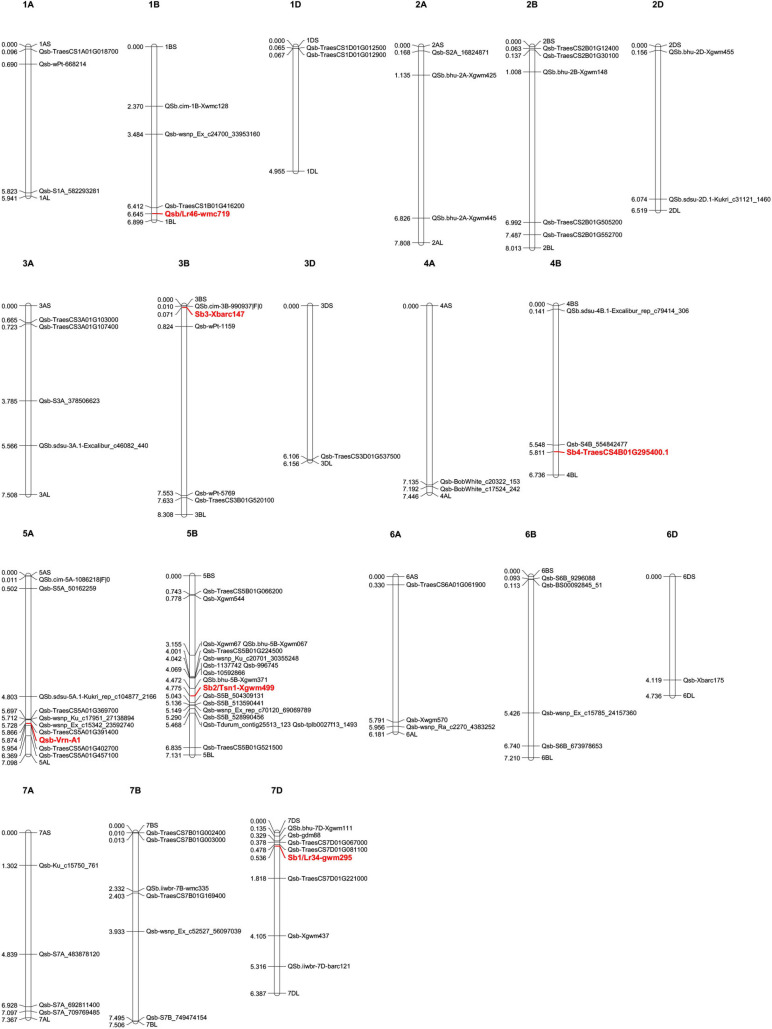
We have summarized the previously reported wheat germplasm with relatively higher resistance to B. sorokiniana (Table 1). These wheat materials may serve as valuable resources for the genetic improvement of wheat resistance to common root rot (spot blotch). We have also summarized detailed information of previously designated resistant QTLs (Table 1) and drafted their genomic distributions using the released genome of hexaploid wheat (Figure 2).
TABLE 1.
Genetics of resistance to common root rot (spot blotch) in wheat.
| QTL name | Associated markers or SNPs | Resistant wheat germplasms | References |
|
Sb1/Lr34* Qsb Qsb/Lr46/Yr29* |
7DS: Xgwm295, csLV34 7DS: wPt-7654, gdm88 1BL: wmc719, hbe248, ncw1-V |
Saar |
Lillemo et al., 2013 |
| Sb2/Tsn1* | 5BL: Xgwm499, Xgwm639, Xgwm1043 | YS116, CASCABEL |
Kumar et al., 2015, 2016; Bainsla et al., 2020; He et al., 2020 |
| Sb3* | 3BS: Xbarc147, XWGGC3957, XWGGC4320 | 621-7-1 | Lu et al., 2016 |
| Sb4* | 4B: TraesCS4B01G295400.1 | Zhongyu1211, GY17 | Zhang et al., 2020 |
| Qsb | 5B: Xgwm544 7D: Xgwm437 |
Yangmai 6 | Kumar et al., 2005 |
| Qsb | 5B: Xgwm67 | G162 | Sharma et al., 2007 |
|
QSb.bhu-2A QSb.bhu-2B QSb.bhu-5B QSb.bhu-6D |
2AL: Xbarc353, Xgwm445 2BS: Xgwm148, Xgwm374 5BL: Xgwm067, Xgwm371 6DL: Xbarc175, Xgwm732 |
Yangmai 6 |
Kumar et al., 2009 |
|
QSb.bhu-2A QSb.bhu-2B QSb.bhu-5B QSb.bhu-7D |
2AS: Xgwm425, Xbarc159 2BS: Xgwm148, Xbarc91 5BL: Xgwm067, Xgwm213 7DS:Xgwm111, Xgwm1168 |
Ning 8201 |
Kumar et al., 2010 |
|
QSb.bhu-2B QSb.bhu-2D QSb.bhu-3B QSb.bhu-7B QSb.bhu-7D |
2BS: Xgwm148, Xgwm129 2DS: Xgwm455, Xgwm815 3BS: Xgwm533, Xgwm1037 7BS: Xgwm263, Xgwm255 7DS: Xgwm111, Xswm008 |
Chirya 3 |
Kumar et al., 2010 |
|
QSb.cim-1B QSb.cim-3B QSb.cim-5A |
1B: Xwmc128, Xgwm374 3B: 990937| F| 0, 1123330| F| 0 5A: 1086218| F| 0, 982608| F| 0 |
SYN1, Mayoor, Tksn1081/Ae. squarrosa (222) |
Zhu et al., 2014b |
|
QSb.iiwbr-7B QSb.iiwbr-7D |
7BL: wmc758, wmc335 7DL: wmc653, barc121 |
BH 1146 | Singh et al., 2016 |
|
Qsb/Vrn-A1* |
5AL: Vrn-A1 |
BARTAI, WUYA, CASCABEL, KATH | Singh et al., 2018; Bainsla et al., 2020; He et al., 2020 |
|
Qsb |
1A: wPt-730148, wPt-668214 3B: wPt-1159, wPt-5769 7B: wPt-2838 7D: wPt-664459 |
Chirya 7, Forma Vinda de Varmland (PI 192569), IWA8600074 (PI 623098), Trigo (PI 477878), Soprimo (PI 479890), CI 10112 (PI 78814), Florentino (PI 565255), AW 6635A/86 (PI 572693), IWA8611737 (PI 625572), NW56A (PI 429667) |
Adhikari et al., 2012 |
|
Qsb |
1B: wsnp_Ex_c24700_33953160 5A: wsnp_Ex_c15342_23592740, wsnp_Ku_c17951_27138894 5B: wsnp_Ex_rep_c70120_69069789, wsnp_Ku_c20701_30355248 6B: wsnp_Ex_c15785_24157360 7B: wsnp_Ex_c52527_56097039 |
PI25989, PI384237, PI384239, PI479802, PI479890, PI576639, PI245377, PI366685, PI481715, PI624517, PI481574, PI91235, PI350795, PI565213 |
Gurung et al., 2014 |
|
Qsb |
5B:Xgwm544 6A:Xwgm570 7D:Xgwm437 |
19HRWSN6, 30SAWSN5 | Tembo et al., 2017 |
|
QSb.sdsu-2D.1 QSb.sdsu-3A.1 QSb.sdsu-4A.1 QSb.sdsu-4B.1 QSb.sdsu-5A.1 QSb.sdsu-7B.1 |
2D: Kukri_c31121_1460 3A: Excalibur_c46082_440 4A: IWA8475 4B: Excalibur_rep_c79414_306 5A: Kukri_rep_c104877_2166 7B: TA005844-0160 |
Duster, Colt, Custer, Intrada, MT0495, NE99495, OK04525, OK05122, OK05723W, Venango |
Ayana et al., 2018 |
|
Qsb |
1A: S1A_582293281 2A: S2A_16824871 3A: S3A_378506623 4B: S4B_554842477 5A: S5A_50162259 5B: S5B_513590441, S5B_504309131, S5B_528990456 6B: S6B_9296088, S6B_673978653 7A: S7A_483878120 7B: S7B_749474154 |
Chirya.3, Aust-53, Pak-13, SB12-6704, 7HTWSN-4516, 7HTWSN-4513, Aust-8, SB12-6703, Aust-66, SB12-6720, Aust-12, 7HTWSN-4522, 7HTWSN-4526, 7HTWSN-4412, 7HTWSN-4405, 7HTWSN-4517, H.Sat-8, Aust-59, Aust-29, 7HTWSN-4406, 7HTWSN-4510 |
Jamil et al., 2018 |
|
Qsb |
1B: BobWhite_c17559_105 4A: BobWhite_c20322_153, BobWhite_c17524_242 5B: Tdurum_contig25513_123, tplb0027f13_1493 6A: wsnp_Ra_c2270_4383252 6B: BS00092845_51 7A: Ku_c15750_761 |
N. A. |
Ahirwar et al., 2018 |
|
Qsb |
1B: TraesCS1B01G416200 5A: TraesCS5A01G391400, TraesCS5A01G369700 |
0KATIA, DE9, OK82282//BOW/NKT/3/F4105, PSN/BOW//ROEK/3/MILAN, KAUZ 2*/OPATA//KAUZ, ALTAR84/AE.SQ//2*, CNDO/R143//ENTE/MEXI- 2/3/…, PAMIR-94 x, NING9415, RENESANSA, VORONA/CUPE |
Bainsla et al., 2020 |
|
Qsb |
1A: TraesCS1A01G018700 1B: TraesCS1B01G424000, TraesCS1B01G423900 1D: TraesCS1D01G012500, TraesCS1D01G012900 2B: TraesCS2B01G505200, TraesCS2B01G552700, TraesCS2B01G12400, TraesCS2B01G30100 3A: TraesCS3A01G107400, TraesCS3A01G103000 3B: TraesCS3B01G520100 3D: TraesCS3D01G537500 5A: TraesCS5A01G402700, TraesCS5A01G457100 5B: TraesCS5B01G066200, TraesCS5B01G224500, TraesCS5B01G521500 6A: TraesCS6A01G061900 7A: TraesCS7A01G504700, TraesCS7A01G530700 7B: TraesCS7B01G002400, TraesCS7B01G003000, TraesCS7B01G169400 7D: TraesCS7D01G067000, TraesCS7D01G081100, TraesCS7D01G221000 |
N. A. |
Tomar et al., 2020 |
Genomic distribution of all these summarized resistant loci were drafted using associated markers and SNPs (bold labeled) that can be found in “Chinese Spring” wheat genome database. Stable QTLs with large effect or linked with designated genes were labeled with asterisk (*) and highlighted in Figure 2.
FIGURE 2.
Genetics of resistance to common root rot (spot blotch) in wheat. Molecular markers, SNPs, and genes associated with common root rot or spot blotch resistant QTLs were collected from previous publications and searched against the JBrowse-1.12.3-release of the common wheat “Chinese Spring” genome available from the “Triticeae Multi-omics Center (http://202.194.139.32/).” Physical positions (numbers indicated on the left side of each chromosome, in units of 100,000,000 bp) were used to generate a distribution map of all the collected QTLs using Mapchart v2.32 software. Stable QTLs with large effect or linked with designated genes are highlighted in red. Detailed information for these QTLs can be found in Table 1.
Genetic Loci Controlling Wheat Resistance to Fusarium Crown Rot
Since the causal agent of Fusarium head blight (FHB), Fusarium graminearum, can also induce the phenotype of Fusarium crown rot (Akinsanmi et al., 2006; Zhou et al., 2019), it is likely that FHB-resistant germplasm and genetic loci can be exploited to improve FCR resistance. For instance, the recently cloned FHB resistance gene Fhb7 encodes a glutathione S-transferase (GST) and provides broad-spectrum resistance to Fusarium diseases, including FCR induced by F. pseudograminearum, by detoxifying trichothecenes through de-epoxidation (Wang et al., 2020). However, an earlier investigation of the same wheat genotypes found no significant correlation of resistant phenotype or genetic loci conferring resistance to FHB and FCR (Li et al., 2010). A recent large-scale phenotyping of 205 Chinese wheat cultivars for resistance to both FHB and FCR also found no correlation in resistant phenotypes (Shi et al., 2020). Great efforts have also been made toward identification of FCR-resistant barley germplasm and genetic loci that control FCR resistance in barley (Liu and Ogbonnaya, 2015). Since recent review papers have already summarized QTLs conferring FHB resistance and susceptibility in wheat in detail (Buerstmayr et al., 2020; Fabre et al., 2020), here we have mainly focused on studies reporting wheat resistance to FCR induced by F. pseudograminearum and F. culmorum.
Genetic studies revealed a major FCR-resistant QTL on chromosome 3BL (Qcrs.cpi-3B). This resistant locus, Qcrs.cpi-3B, was identified in the wheat genotype “CSCR6” of the taxon Triticum spelta (Ma et al., 2010). In a wheat recombinant inbred line population of “Lang/CSCR6,” a QTL on chromosome 4B derived from “Lang” explained the soil-free FCR resistance (Yang et al., 2010). Another significant QTL on chromosome 6B was identified as responsible for FCR resistance during an introgression process for durum wheat using “CSCR6” as the donor parent (Ma et al., 2012b). Near-isogenic lines for the Qcrs.cpi-3B locus have been developed for both genetic research and breeding practice (Ma et al., 2012a). Subsequent transcriptome and allele specificity analysis revealed differentially expressed genes associated with the Qcrs.cpi-3B locus (Ma et al., 2014). Fine mapping of this QTL shortened the genetic interval to 0.7 cM, containing 63 coding genes in the reference wheat genome (Zheng et al., 2015). Future map-based cloning and identification of the functional gene in this large-effect QTL may help elucidate the molecular bases of wheat resistance to FCR.
Other resistant QTLs have been identified using bi-parental populations. Early investigation discovered a resistant locus near the dwarfing gene Rht1 on chromosome 4B from the wheat cultivar “Kukri” (Wallwork et al., 2004). Inherited from the wheat line “W21MMT70” with partial resistance to FCR, two QTLs were mapped to chromosomes 2D and 5D (Bovill et al., 2006). A major QTL on chromosome 1DL (QCr.usq-1D1) and several minor QTLs were identified in wheat line “2-49 (Gluyas Early/Gala)” using SSR markers (Collard et al., 2005, 2006). FCR resistance screening of 32 wheat genotypes identified “2-49,” “Aso zairai 11,” and “Ernie” as resistant sources. A QTL derived from “Ernie” was mapped to chromosome 3BL near markers wPt-1151 and wPt-1834 (Li et al., 2010). An Australian spring wheat cultivar “Sunco” showed partial resistance to FCR induced by F. pseudograminearum. Using bi-parental QTL mapping, a major QTL was identified on chromosome 3BL, between SSR markers Xgwm247 and Xgwm299 (Poole et al., 2012). These resistant sources of “W21MMT70,” “2-49,” and “Sunco” were then used for QTL pyramiding (Bovill et al., 2010). Four FCR-resistant QTLs were discovered, and their resistant alleles were derived from the bread wheat commercial variety “EGA Wylie.” Major QTLs on chromosomes 5DS and 2DL were consistently detected in all three populations and two minor QTLs were mapped to chromosome 4BS (Zheng et al., 2014). QTL mapping was also performed to find genetic loci controlling partial resistance to FCR in the four wheat germplasm “2-49,” “Sunco,” “IRN497,” and “CPI133817.” FCR resistance was evaluated in both seedlings and adult plants. Six QTLs among these resistant wheat genotypes were revealed (Martin et al., 2015). Stable QTLs on chromosomes 1DL and 3BL have been identified from wheat germplasm “2-49” and “Sunco,” respectively, in several studies.
A GWAS approach was used to screen 2,514 wheat genotypes for FCR resistance, and DArT and SSR markers identified two major QTLs on chromosome 3BL that explained 35 and 49% of the phenotypic variation (Liu et al., 2018). A set of 126 spring bread wheat lines from CIMMYT was phenotyped against FCR induced by F. culmorum and further genotyped using DArT markers, which resulted in the identification of three major QTLs on chromosomes 3B and 2D (Erginbasorakci et al., 2018). The use of GWAS for FCR resistance has greatly benefited from advanced high-throughput sequencing techniques and the released hexaploid wheat genome. A total of 234 Chinese wheat cultivars were evaluated for FCR resistance in four greenhouse experiments, with GWAS using a high-density 660K SNP assay. This revealed a major QTL on chromosome 6A, which was subsequently validated using a bi-parental population of “UC1110/PI610750” (Yang et al., 2019). The same team screened the FCR resistance of another 435 wheat introgression lines (generated by crossing of Yanzhan1 with other elite varieties) and performed GWAS using 660K SNP array. Most of the significant SNP associations were distributed on chromosome 4B and a gene encoding a dirigent protein (TaDIR-B1) was validated as a negative regulator of FCR resistance (Yang et al., 2021). A recent GWAS approach phenotyped 358 Chinese germplasm for FCR resistance, with less than 10% exhibiting a lower disease index. The wheat 55K SNP assay was applied for association analysis, resulting in detection of significant QTLs on chromosomes 1BS, 1DS, 5DS, 5DL, and 7BL (Jin et al., 2020). GWAS was also performed to evaluate FCR resistance of 161 wheat accessions under growth room and greenhouse conditions using F. culmorum as the pathogen. Using a 90K SNP array, a total of 15 QTLs for FCR resistance were detected with one major QTL on chromosome 3BS near the FHB resistance Fhb1 locus (Pariyar et al., 2020). A marker-assisted recurrent selection approach was next performed on two populations to pyramid minor FCR-resistant QTLs. Using 9K SNP array, a total of 23 marker-trait associations were identified by GWAS (Rahman et al., 2020).
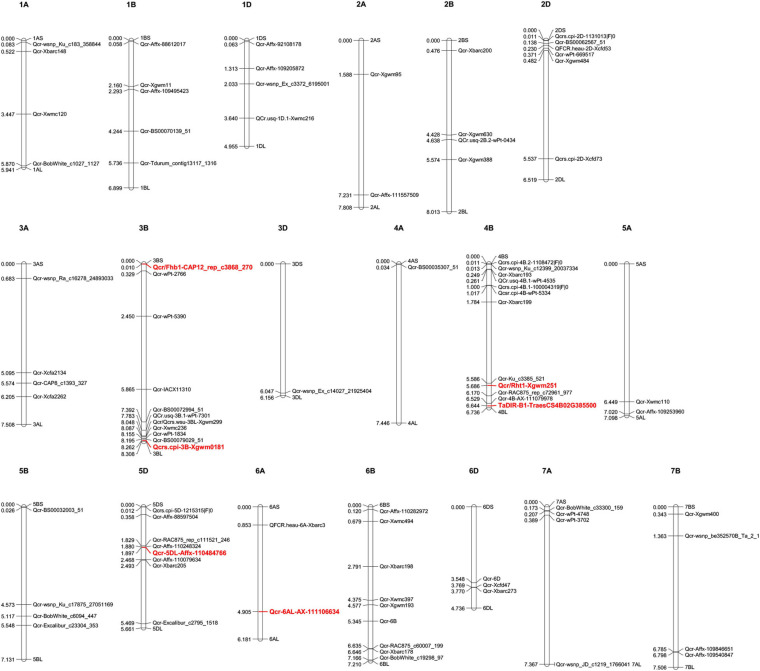
In Table 2, we summarize wheat germplasm resistant to FCR induced by either F. pseudograminearum or F. culmorum. Identified QTLs controlling FCR resistance are also highlighted (Table 2), with their genomic distributions annotated using the wheat genome database (Figure 3).
TABLE 2.
Genetic loci controlling wheat resistance to Fusarium crown rot.
| QTL name | Associated markers or SNPs | Resistant wheat germplasms | References |
|
Qcrs.cpi-3B* Qcsr.cpi-4B Qcr Qcr |
3BL: Xgwm0181, wPt-10505, wPt-2277 4BS: wPt-5334, wPt-4918, Xbarc199 5A: Xwmc110 6B: Xwmc494, Xgwm193, Xwmc397, Xbarc198, Xbarc178 |
CSCR6 (T. spelta), Lang, Kennedy | Ma et al., 2010, 2012a, b, 2014; Yang et al., 2010; Zheng et al., 2015 |
|
Qcr |
2BS: Xgdm086, Xbarc200 2D: Xwmc018, Xwmc190 5D: Xbarc205, barc143 |
W21MMT70, Mendos | Bovill et al., 2006 |
|
Qcr QCr.usq-1D.1 QCr.usq-2B.1 Qcr/Rht1* Qcr |
1AL: Xwmc120, Xwmc312 1DL: Xcfd19, Xwmc216 2BS: Xbarc349.1, Xgwm388 4BL: Xgwm165, Xgwm251 7BS: Xgwm400, Xwmc476 |
Kukri, 2-49 (Gluyas Early/Gala), Janz | Wallwork et al., 2004; Collard et al., 2005, 2006 |
|
QCr.usq-1D.1 QCr.usq-2B.2 QCr.usq-3B.1 QCr.usq-4B.1 Qcr |
1DL: Xcfd19, wPt-9380 2B: wPt-5374, wPt-0434 3BL: wPt-7301, wPt-0365 4BS: wPt-4535, Xgwm251 7AS: wPt-4748, wPt-8418 |
2-49, W21MMT70, Sunco | Bovill et al., 2010 |
| Qcr | 3B: wPt-1834, wPt-1151 | 2-49, Aso zairai 11, Ernie | Li et al., 2010 |
|
Qcrs.wsu-3BL Qcr Qcr |
3BL: Xgwm247, Xgwm299 3BS: wPt-5390, Xwmc777 7AS: wPt-3702 |
Sunco, Macon, Otis | Poole et al., 2012 |
|
Qcrs.cpi-2D Qcrs.cpi-4B.1 Qcrs.cpi-4B.2 Qcrs.cpi-5D |
2DL: 1131013| F| 0, 1246993| F| 0 4BS: 100004319| F| 0, 2324159| F| 0 4BS: 1108472| F| 0, 1093616| F| 0 5DS: 1215315| F| 0, 1237596| F| 0 |
EGA Wylie | Zheng et al., 2014 |
|
Qcr |
1AS: Xbarc148, Xgwm164 1BS: Xcfd65, Xgwm11 1DL: Xcfd19, Xwmc216 2A: Xgwm95, Xcfa2043 2B: Xgwm630, Xcfa2278 2DS: Xgwm484, Xgwm102 3AL: Xcfa2134, Xcfa2262 3BL: Xgwm299, wPt-0021, Xwmc236, wPt-0365 4BS: Xwmc467, Xgwm165 4BS: Xbarc193, Xwmc349 6DL: Xcfd188, Xcfd47 6DL: Xbarc196, Xbarc273 |
2-49, Sunco, IRN497, CPI133817 |
Martin et al., 2015 |
|
Qcr |
2DS: wPt-669517 3BS: wPt-2193, wPt-22988, wPt-732330, wPt-2766 |
2-49, Sunco, Altay-2000 | Erginbasorakci et al., 2018 |
|
QFCR.heau-2A QFCR.heau-2D Qcr-6AL* QFCR.heau-6A Qcr-6B Qcr-6D |
2AS: Xwms382, wPt-7462, wPt-3757 2DS: Xcfd53 6AL: AX-111106634, AX-94534539 6AS: Xbarc3, Xwmc754 6B: SNP position 534,514,143 6D: SNP position 354,819,336 |
Xunmai 118, Kaimai 26, Yanke 316, Xuke 732, Zhonglemai 9, Jinmai 1, Shenzhou 209, Fannong 1, Jiyanmai 7, UC1110, PI610750 | Yang et al., 2019 |
|
TaDIR-B1* Qcr |
4B: TraesCS4B02G385500 4B: AX-111079978, AX-110977572 |
Bainong64 | Yang et al., 2021 |
|
Qcr Qcr Qcr Qcr Qcr Qcr-5DL* Qcr Qcr |
1BS: Affx-88612017, Affx-109495423 1DS: Affx-92108178, Affx-109205872 2AL: Affx-111557509 5DS: Affx-88597504, Affx-110248324 5AL: Affx-109253960 5DL: Affx-110484766, Affx-110079634 6BS: Affx-110282972 7BL: Affx-109846651, Affx-109540847 |
Henong 982, Shiyou 17, Bao 6818, Quanmai 890, 04 Zhong 36, Junda 129, Xu 10054, Fanmai 5, Lian 0809, Shixin 733, Shi05-6678, Han 06-5170, Luomai 8, Zhongyuanzhixing, Yangao 21, Xumai 33 | Jin et al., 2020 |
|
Qcr Qcr Qcr/Fhb1* Qcr Qcr Qcr Qcr Qcr Qcr Qcr Qcr Qcr |
2AL: Kukri_c57491_156 3AS: wsnp_Ra_c16278_24893033, CAP8_c1393_327 3BS: CAP12_rep_c3868_270 3DL: wsnp_Ex_c14027_21925404 4BS: wsnp_Ku_c12399_20037334 4BL: RAC875_rep_c72961_977 5BS: wsnp_Ku_c17875_27051169, Excalibur_c23304_353 5DS: RAC875_rep_c111521_246 5DL: Excalibur_c2795_1518 6BS: RAC875_c17297_341 6BL: BobWhite_c19298_97 6DS: BS00021881_51 |
VICTORYA, Katea, KOLLEGA, DORADE-5/3/BOW”S”/GEN//SHAHI, 2180*K/2163//?/3/W1062A*HVA114/W3416, L 4224 K 12, NE04424, TX69A509.2//BBY/FOX/3/GRK//NO64/PEX/4/CER/5/KAUZ//ALTAR 84/AOS, ID800994.W/MO88 | Pariyar et al., 2020 |
| Qcr | 1A: BobWhite_c1027_1127, wsnp_Ku_c183_358844 1B: BS00070139_51, Tdurum_contig13117_1316 1D: wsnp_Ex_c3372_6195001 2D: BS00062567_51 3B: BS00072994_51, BS00079029_51, IACX11310 4A: BS00035307_51 4B: Ku_c3385_521 5B: BS00032003_51, BobWhite_c6094_447 6B: RAC875_c60007_199 7A: BobWhite_c33300_159, wsnp_JD_c1219_1766041 7B: wsnp_be352570B_Ta_2_1 |
AUS29529/2/2.49/Cunningham//Kennedy/3/Sunco, CSCR16/2/2.49/Cunningham//Kennedy/3/Sunco/2*Pastor | Rahman et al., 2020 |
| N. A. | N. A. | Cunmai633, LS4607, Pubing01, Hongyun2, Jimai216, Fengyunmai5, Huaihe15076, Luofeng2419, Yanfeng168, Zhengmai22, Zhoumai38, Zhoumai37, Lemai185, Xinmai38, Xinong733, Xinmai45, Guohemai12, Xinong625, Zhengmai162 | Shi et al., 2020 |
Genomic distribution of all these summarized resistant loci were drafted using associated markers and SNPs (bold labeled) that can be found in “Chinese Spring” wheat genome database. Stable QTLs with large effect or linked with designated genes were labeled with asterisk (*) and highlighted in Figure 3.
FIGURE 3.
Genetic loci controlling wheat resistance to Fusarium crown rot. Molecular markers, SNPs, and genes associated with FCR-resistant QTLs were collected from previous publications and searched against the JBrowse-1.12.3-release of the common wheat “Chinese Spring” genome available from the “Triticeae Multi-omics Center (http://202.194.139.32/).” Physical positions (numbers indicated on the left side of each chromosome, in units of 100,000,000 bp) were used to generate a distribution map of all the collected QTLs using Mapchart v2.32 software. Stable QTLs with large effect or linked with designated genes are highlighted in red. Detailed information for these QTLs can be found in Table 2.
Genetic Determinants of Wheat Resistance to Sharp Eyespot
Wheat resistance to sharp eyespot is controlled by QTLs. However, additional efforts should focus on identification of resistant germplasm and genetic loci conferring resistance to this fungal disease. A recent large-scale screening of sharp eyespot resistant germplasm in Chinese wheat cultivars revealed no immune or highly resistant germplasm, and only 4% exhibiting moderate resistance to R. cerealis (Ren et al., 2020). Introgression of exogenous chromosome segments from wheat relatives might help generate novel resistant germplasms. For example, a wheat-rye 4R chromosome disomic addition line gained high resistance to sharp eyespot (An et al., 2019). Wheat cultivars “Luke” and “AQ24788-83” showed high resistance to R. cerealis and subsequent genetic investigations revealed seven significant sharp eyespot resistant QTLs on chromosomes 1A, 2B, 3B, 4A, 5D, 6B, and 7B (Chen et al., 2013; Guo et al., 2017). Using 90 K SNP and SSR markers, five QTLs on chromosomes 2BS, 4BS, 5AL, and 5BS controlling resistance to R. cerealis were identified from the wheat cultivar “CI12633” (Wu et al., 2017). Three QTLs controlling resistance of wheat cultivars “Niavt14” and “Xuzhou25” to R. cerealis were mapped to chromosomes 2B and 7D (Jiang et al., 2016). A recent study using the same population of “Niavt14/Xuzhou25” and 55K SNPs revealed three novel stable QTLs on chromosomes 1D, 6D, and 7A (Liu et al., 2020).
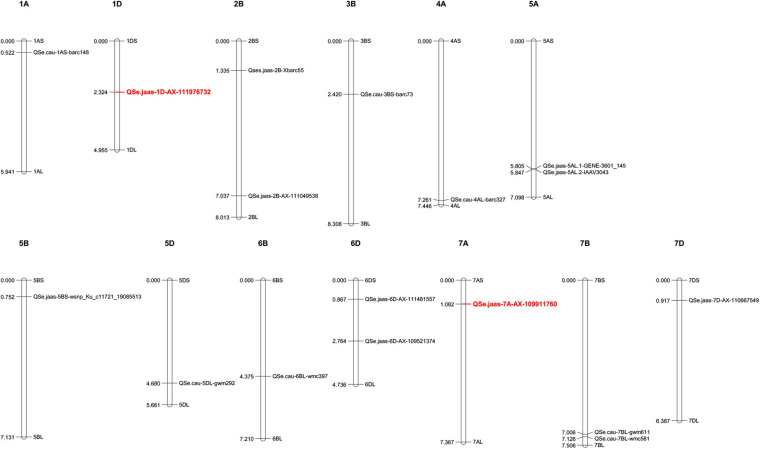
In Table 3, we summarize wheat germplasm resistant to R. cerealis. Reported QTLs controlling sharp eyespot resistance are highlighted (Table 3), with their genomic distributions annotated using the wheat genome database (Figure 4).
TABLE 3.
Genetic determinants of wheat resistance to sharp eyespot.
| QTL name | Associated markers or SNPs | Resistant wheat germplasms | References |
|
QSe.cau-1AS QSe.cau-2BS QSe.cau-3BS QSe.cau-4AL QSe.cau-5DL QSe.cau-6BL QSe.cau-7BL |
1AS: barc148, wmc120 2BS: wmc154, barc200 3BS: wmc777, barc73 4AL: barc327, wmc776 5DL: gwm292, cfd29, gwm212 6BL: gwm626, barc187, wmc397 7BL: gwm611, wmc166, wmc581 |
Luke, AQ24788-83 | Chen et al., 2013; Guo et al., 2017 |
|
QSe.jaas-2BS QSe.jaas-4BS QSe.jaas-5AL.1 QSe.jaas-5AL.2 QSe.jaas-5BS |
2BS: RAC875_c730_234, RAC875_c16697_1502 4BS: RAC875_c49792_228, Kukri_c34353_821 5AL: GENE-3601_145, Ku_c21002_908 5AL: IAAV3043, wsnp_Ex_c55777_58153636 5BS: wsnp_Ku_c11721_19085513, BS00068710_51 |
CI12633 | Wu et al., 2017 |
|
QSe.jaas-1D* QSe..jaas-2B QSe.jaas-6D QSe.jaas-7A* QSe.jaas-7D |
1D: AX-111976732, AX-110490771 2B: AX-111049538 6D: AX-111481557, AX-109521374 7A: AX-109911760, AX-110041698 7D: AX-110667549, AX-110559985 |
Niavt 14, Xuzhou 25 | Jiang et al., 2016; Liu et al., 2020 |
| N. A. | N. A. | Seedling resistance: CI12633, Banmangmai, Banjiemang, Ibis, Hongyouzi, Shaanhe6, Chinese Spring, Hongxingmai, Pingyuan 50, Linfen139, Chuanyu12, Yongfengnong2, Yunong202, Xinmai68, Huabei187, Jinmai50, Neixiang184 Adult plant resistance: Shaanhe6, CI12633, Banmangmai, Chinese Spring, Huomai, Banjiemang, Pingyuan50, Pingyang181, Yumai8, Qingfeng1, Hongyouzi, Hongxingmai, Libellula, Zhengmai8998 | Ren et al., 2020 |
Genomic distribution of all these summarized resistant loci were drafted using associated markers (bold labeled) that can be found in “Chinese Spring” wheat genome database. Stable QTLs with large effect or linked with designated genes were labeled with asterisk (*) and highlighted in Figure 4.
FIGURE 4.
Identified QTLs controlling wheat resistance to sharp eyespot. Molecular markers associated with Rc-resistant QTLs were collected from previous publications and searched against the JBrowse-1.12.3-release of the common wheat “Chinese Spring” genome available from the “Triticeae Multi-omics Center (http://202.194.139.32/).” Physical positions (numbers indicated on the left side of each chromosome, in units of 100,000,000 bp) were used to generate a distribution map of all the collected QTLs using Mapchart v2.32 software. Detailed information for these QTLs can be found in Table 3.
Discussion
We have described three rot diseases that commonly infect the stem base of wheat plants (Figure 1). These diseases are major threats to wheat productions in wheat-maize rotation areas with large-scale application of straw returning. Wheat breeding is the most efficient way to control these devastating fungal diseases. However, as summarized in this review (Tables 1–3), there are few wheat germplasm with relative high resistance to B. sorokiniana, F. pseudograminearum, or R. cerealis. Large-scale screenings of resistant wheat germplasm are still urgently needed for effective wheat breeding applications. New germplasm resources including wheat relatives (e.g., introgression lines using Thinopyrum ponticum, Triticum spelta, and rye) may have great potential to improve wheat resistance to these root and crown rot fungal diseases.
Genetic improvement of wheat resistance to these diseases requires exploring novel QTLs that control resistance. There are several previously reported resistant QTLs (Tables 1–3) and their genomic distributions have been mapped based on the released wheat genome (Figures 2–4). Stable QTLs with large effect or linked with designated genes were highlighted. Chromosome location data for all these reported QTLs was provided in Supplementary Table 1. Some identified QTLs that confer resistance to B. sorokiniana are associated with loci responsible for wheat resistance to other foliar fungal diseases, such as Lr34/Yr18/Pm38, Lr46/Yr29, and Tsn1. Wheat leaves might restrain the infection of different foliar fungal diseases using similar molecular approaches mediated by resistant genes. Wheat germplasm with broad-spectrum resistant loci should be evaluated for potential resistance to spot blotch or common root rot induced by B. sorokiniana. Of QTLs that control resistance to Fusarium crown rot, ones that also have resistance to FHB may be more valuable, since the major causal agents of these diseases (F. pseudograminearum, F. culmorum, and F. graminearum) are very likely to co-exist in a cultivation environment. For genetic studies on QTLs controlling resistance to sharp eyespot, the large-scale screening of resistant wheat germplasm would greatly accelerate the identification of novel QTLs correlated with resistance to sharp eyespot. There is also an urgent need to employ GWAS technique to screen for more sharp eyespot resistant QTLs at the genome-wide level. To explore QTLs with potential co-resistance effects to all these three stem base rot diseases, we combined the chromosome distribution maps of all the reported QTLs in Supplementary Figure 1. Chromosome regions on 1AS, 3BL, 4BL, 5AL, 5BL, and 7AS are enriched with QTLs conferring resistance to these soil-borne necrotrophic fungal diseases. Constructing near-isogenic lines and using residual heterozygotes allow the use of fine mapping and further positional cloning for key gene/loci that control resistance. With advanced genomic and capture-sequencing techniques such as MutRenSeq, AgRenSeq, and Exome Capture, fast-cloning approaches might accelerate this time-consuming process (Steuernagel et al., 2016; Krasileva et al., 2017; Arora et al., 2019). Gene editing may also increase the rate of genetic improvement of wheat resistance to these fungal diseases (Wang et al., 2018a). Both forward and reverse genetic studies will provide valuable targets for the application of CRISPR-Cas9 in wheat. Nevertheless, the main restraints for fine-mapping and cloning of genes/QTLs conferring resistance to these stem base rot diseases are accurate phenotyping of large-scale segregation populations and functional validation of candidate resistance genes.
Efforts should also be made to convert traditional markers used previously to identify resistant QTLs (microsatellite, SSR, and DrAT) to SNP markers, as SNP markers may serve as valuable tools for high-throughput marker-assisted selection in wheat breeding. Progress in wheat genome research and increased availability of high-density SNP toolkits will facilitate the use of GWAS on collected wheat germplasm to more efficiently identify novel resistant sources and genetic loci.
Author Contributions
XW, ZK, and WZ: conceptualization. JS, JZ, SZ, ML, and SP: data collection. XW: original draft preparation. SC and FC: review and editing. XW, ZK, and WZ: supervision. All authors read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Prof. Zaifeng Li from Hebei Agricultural University for discussion and input to this work.
Footnotes
Funding. This work was supported by Provincial Natural Science Foundation of Hebei (C2021204008), Open Project Program of National Key Laboratory of Wheat and Maize Crop Science, Provincial Supporting Program of Hebei for the Returned Oversea Scholars (C20190180), Open Project Program of State Key Laboratory of North China Crop Improvement and Regulation (NCCIR2020KF-4), National Key R&D Program of China (2017YFD0300906), and Provincial Innovation Program of Hebei for Post-graduate Student (CXZZSS2021070).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.699342/full#supplementary-material
Combined chromosome distribution map for all the QTLs conferring resistance to common root rot (spot blotch), Fusarium crown rot, and sharp eyespot in wheat.
Chromosome location data for all the reported QTLs.
References
- Adhikari T. B., Gurung S., Hansen J. M., Jackson E. W., Bonman J. M. (2012). Association mapping of quantitative trait loci in spring wheat landraces conferring resistance to bacterial leaf streak and spot blotch. Plant Genome 5 1–16. [Google Scholar]
- Ahirwar R. N., Mishra V. K., Chand R., Budhlakoti N., Mishra D. C., Kumar S., et al. (2018). Genome-wide association mapping of spot blotch resistance in wheat association mapping initiative (WAMI) panel of spring wheat (Triticum aestivum L.). PLoS One 13:e0208196. 10.1371/journal.pone.0208196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinsanmi O. A., Backhouse D., Simpfendorfer S., Chakraborty S. (2006). Genetic diversity of Australian Fusarium graminearum and F. pseudograminearum. Plant Pathol. 55 494–504. 10.1111/j.1365-3059.2006.01398.x [DOI] [Google Scholar]
- An D., Ma P., Zheng Q., Fu S., Li L., Han F., et al. (2019). Development and molecular cytogenetic identification of a new wheat-rye 4R chromosome disomic addition line with resistances to powdery mildew, stripe rust and sharp eyespot. Theor. Appl. Genet. 132 257–272. 10.1007/s00122-018-3214-3 [DOI] [PubMed] [Google Scholar]
- Appels R., Eversole K., Stein N., Feuillet C., Keller B., Rogers J., et al. (2018). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361:eaar7191. [DOI] [PubMed] [Google Scholar]
- Arora S., Steuernagel B., Gaurav K., Chandramohan S., Long Y., Matny O., et al. (2019). Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nat. Biotechnol. 37 139–143. 10.1038/s41587-018-0007-9 [DOI] [PubMed] [Google Scholar]
- Ayana G. T., Ali S., Sidhu J. S., Hernandez J. L. G., Turnipseed B., Sehgal S. K. (2018). Genome-wide association study for spot blotch resistance in hard winter wheat. Front. Plant Sci. 9:926. 10.3389/fpls.2018.00926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainsla N. K., Phuke R. M., He X., Gupta V., Bishnoi S. K., Sharma R. K., et al. (2020). Genome-wide association study for spot blotch resistance in Afghan wheat germplasm. Plant Pathol. 69 1161–1171. 10.1111/ppa.13191 [DOI] [Google Scholar]
- Bovill W. D., Horne M., Herde D. J., Davis M., Wildermuth G. B., Sutherland M. W. (2010). Pyramiding QTL increases seedling resistance to crown rot (Fusarium pseudograminearum) of wheat (Triticum aestivum). Theor. Appl. Genet. 121 127–136. 10.1007/s00122-010-1296-7 [DOI] [PubMed] [Google Scholar]
- Bovill W. D., Ma W., Ritter K., Collard B. C. Y., Davis M., Wildermuth G. B., et al. (2006). Identification of novel QTL for resistance to crown rot in the doubled haploid wheat population ‘W21MMT70’בMendos’. Plant Breed. 125 538–543. 10.1111/j.1439-0523.2006.01251.x [DOI] [Google Scholar]
- Buerstmayr M., Steiner B., Buerstmayr H. (2020). Breeding for Fusarium head blight resistance in wheat—progress and challenges. Plant Breed. 139 429–454. 10.1111/pbr.12797 [DOI] [Google Scholar]
- Burgess L. W., Backhouse D., Summerell B. A., Swan L. J. (2001). “Crown rot in wheat—-Chapter 20,” in Fusarium-Paul E Nelson Memorial Symposium, eds Summerell B. A., Leslie J. F., Backhouse D., Bryden W. L., Burgess L. W. (St Paul, MIN: The American Phytopathological Society; ), 271–294. [Google Scholar]
- Chen J., Li G. H., Du Z. Y., Quan W., Zhang H. Y., Che M. Z., et al. (2013). Mapping of QTL conferring resistance to sharp eyespot (Rhizoctonia cerealis) in bread wheat at the adult plant growth stage. Theor. Appl. Genet. 126 2865–2878. 10.1007/s00122-013-2178-6 [DOI] [PubMed] [Google Scholar]
- Cohen S. P., Leach J. E. (2020). High temperature-induced plant disease susceptibility: more than the sum of its parts. Curr. Opin. Plant Biol. 56 235–241. 10.1016/j.pbi.2020.02.008 [DOI] [PubMed] [Google Scholar]
- Collard B. C. Y., Grams R. A., Bovill W. D., Percy C. D., Jolley R., Lehmensiek A., et al. (2005). Development of molecular markers for crown rot resistance in wheat: mapping of QTLs for seedling resistance in a ‘2-49’בJanz’ population. Plant Breed. 124 532–537. 10.1111/j.1439-0523.2005.01163.x [DOI] [Google Scholar]
- Collard B. C. Y., Jolley R., Bovill W. D., Grams R. A., Wildermuth G. B., Sutherland M. W. (2006). Confirmation of QTL mapping and marker validation for partial seedling resistance to crown rot in wheat line ’2-49’. Crop Pasture Sci. 57 967–973. 10.1071/ar05419 [DOI] [Google Scholar]
- Conner R. L., Duczek L. J., Kozub G. C., Kuzyk A. D. (1996). Influence of crop rotation on common root rot of wheat and barley. Can. J. Plant Pathol. 18 247–254. 10.1080/07060669609500620 [DOI] [Google Scholar]
- Erginbasorakci G., Sehgal D., Sohail Q., Ogbonnaya F. C., Dreisigacker S., Pariyar S. R., et al. (2018). Identification of novel quantitative trait loci linked to crown rot resistance in spring wheat. Int. J. Mol. Sci. 19:2666. 10.3390/ijms19092666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre F., Rocher F., Alouane T., Langin T., Bonhomme L. (2020). Searching for FHB resistances in bread wheat: susceptibility at the crossroad. Front. Plant Sci. 11:731. 10.3389/fpls.2020.00731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Du Z., Chen J., Zhang Z. (2017). QTL mapping of wheat plant architectural characteristics and their genetic relationship with seven QTLs conferring resistance to sheath blight. PLoS One 12:e0174939. 10.1371/journal.pone.0174939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P. K., Chand R., Vasistha N. K., Pandey S. P., Kumar U., Mishra V. K., et al. (2018). Spot blotch disease of wheat: the current status of research on genetics and breeding. Plant Pathol. 67 508–531. 10.1111/ppa.12781 [DOI] [Google Scholar]
- Gurung S., Mamidi S., Bonman J. M., Xiong M., Brownguedira G., Adhikari T. B. (2014). Genome-wide association study reveals novel quantitative trait loci associated with resistance to multiple leaf spot diseases of spring wheat. PLoS One 9:e108179. 10.1371/journal.pone.0108179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada M. S., Yin Y., Chen H., Ma Z. (2011). The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest Manag. Sci. 67 1411–1419. 10.1002/ps.2236 [DOI] [PubMed] [Google Scholar]
- He X., Dreisigacker S., Sansaloni C., Duveiller E., Singh R. P., Singh P. K. (2020). QTL mapping for spot blotch resistance in two bi-parental mapping populations of bread wheat. Phytopathology 110 1980–1987. 10.1094/phyto-05-20-0197-r [DOI] [PubMed] [Google Scholar]
- Jamil M., Ali A., Gul A., Ghafoor A., Ibrahim A. M. H., Mujeeb-Kazi A. (2018). Genome-wide association studies for spot blotch (Cochliobolus sativus) resistance in bread wheat using genotyping-by-sequencing. Phytopathology 108 1307–1314. 10.1094/phyto-02-18-0047-r [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhu F., Cai S., Wu J., Zhang Q. (2016). Quantitative trait loci for resistance to Sharp Eyespot (Rhizoctonia cerealis) in recombinant inbred wheat lines from the cross Niavt 14× Xuzhou 25. Czech J. Genet. Plant Breed. 52 139–144. 10.17221/74/2016-cjgpb [DOI] [Google Scholar]
- Jin J., Duan S., Qi Y., Yan S., Li W., Li B., et al. (2020). Identification of a novel genomic region associated with resistance to Fusarium crown rot in wheat. Theor. Appl. Genet. 133 2063–2073. 10.1007/s00122-020-03577-1 [DOI] [PubMed] [Google Scholar]
- Joshi A. K., Kumar S., Chand R., Ortizferrara G. (2004). Inheritance of resistance to spot blotch caused by Bipolaris sorokiniana in spring wheat. Plant Breed. 123 213–219. 10.1111/j.1439-0523.2004.00954.x [DOI] [Google Scholar]
- Kazan K., Gardiner D. M. (2018). Fusarium crown rot caused by Fusarium Psudograminearum in cereal crops: recent progress and future prospects. Mol. Plant Pathol. 19 1547–1562. 10.1111/mpp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasileva K. V., Vasquez-Gross H. A., Howell T., Bailey P., Paraiso F., Clissold L., et al. (2017). Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. U.S.A. 114 E913–E921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger S. G., Lagudah E. S., Spielmeyer W., Singh R. P., Huerta-Espino J., Mcfadden H., et al. (2009). A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323 1360–1363. 10.1126/science.1166453 [DOI] [PubMed] [Google Scholar]
- Kumar J., Hückelhoven R., Beckhove U., Nagarajan S., Kogel K.-H. (2001). A compromised Mlo pathway affects the response of barley to the necrotrophic fungus Bipolaris sorokiniana (teleomorph: Cochliobolus sativus) and its toxins. Phytopathology 91 127–133. 10.1094/phyto.2001.91.2.127 [DOI] [PubMed] [Google Scholar]
- Kumar J., Schafer P., Huckelhoven R., Langen G., Baltruschat H., Stein E., et al. (2002). Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol. Plant Pathol. 3 185–195. 10.1046/j.1364-3703.2002.00120.x [DOI] [PubMed] [Google Scholar]
- Kumar S., Roder M. S., Singh R. P., Kumar S., Chand R., Joshi A. K., et al. (2016). Mapping of spot blotch disease resistance using NDVI as a substitute to visual observation in wheat (Triticum aestivum L.). Mol. Breed. 36:95. [Google Scholar]
- Kumar S., Roder M. S., Tripathi S. B., Kumar S., Chand R., Joshi A. K., et al. (2015). Mendelization and fine mapping of a bread wheat spot blotch disease resistance QTL. Mol. Breed. 35:218. [Google Scholar]
- Kumar U., Joshi A. K., Kumar S., Chand R., Roder M. S. (2009). Mapping of resistance to spot blotch disease caused by Bipolaris sorokiniana in spring wheat. Theor. Appl. Genet. 118 783–792. 10.1007/s00122-008-0938-5 [DOI] [PubMed] [Google Scholar]
- Kumar U., Joshi A. K., Kumar S., Chand R., Roder M. S. (2010). Quantitative trait loci for resistance to spot blotch caused by Bipolaris sorokiniana in wheat (T. aestivum L.) lines ‘Ning 8201’ and ‘Chirya 3’. Mol. Breed. 26 477–491. 10.1007/s11032-009-9388-2 [DOI] [Google Scholar]
- Kumar U., Kumar S., Tyagi K., Chand R., Joshi A. K. (2005). Microsatellite markers for resistance to spot blotch in spring wheat. Commun. Agric. Appl. Biol. Sci. 70:59. [PubMed] [Google Scholar]
- Lemańczyk G., Kwaśna H. (2013). Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur. J. Plant Pathol. 135 187–200. 10.1007/s10658-012-0077-3 [DOI] [Google Scholar]
- Li H., Conner R. L., Chen Q., Li H., Laroche A., Graf R. J., et al. (2004). The transfer and characterization of resistance to common root rot from Thinopyrum ponticum to wheat. Genome 47 215–223. 10.1139/g03-095 [DOI] [PubMed] [Google Scholar]
- Li H. B., Xie G. Q., Ma J., Liu G. R., Wen S. M., Ban T., et al. (2010). Genetic relationships between resistances to Fusarium head blight and crown rot in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 121 941–950. 10.1007/s00122-010-1363-0 [DOI] [PubMed] [Google Scholar]
- Lillemo M., Joshi A. K., Prasad R., Chand R., Singh R. P. (2013). QTL for spot blotch resistance in bread wheat line Saar co-locate to the biotrophic disease resistance loci Lr34 and Lr46. Theor. Appl. Genet. 126 711–719. 10.1007/s00122-012-2012-6 [DOI] [PubMed] [Google Scholar]
- Liu C., Guo W., Zhang Q., Fu B., Yang Z., Sukumaran S., et al. (2020). Genetic dissection of adult plant resistance to sharp eyespot using an updated genetic map of Niavt14× Xuzhou25 winter wheat recombinant inbred line population. Plant Dis. 105 997–1005. 10.1094/pdis-09-20-1924-re [DOI] [PubMed] [Google Scholar]
- Liu C., Ma J., Li H. B., Liu Y. X., Liu G. R., Wen S. M., et al. (2018). The homoeologous regions on long arms of group 3 chromosomes in wheat and barley harbour major crown rot resistance loci. Czech J. Genet. Plant Breed. 47 109–114. [Google Scholar]
- Liu C., Ogbonnaya F. C. (2015). Resistance to Fusarium crown rot in wheat and barley: a review. Plant Breed. 134 365–372. 10.1111/pbr.12274 [DOI] [Google Scholar]
- Lu P., Liang Y., Li D. F., Wang Z., Li W., Wang G., et al. (2016). Fine genetic mapping of spot blotch resistance gene Sb3 in wheat (Triticum aestivum). Theor. Appl. Genet. 129 577–589. 10.1007/s00122-015-2649-z [DOI] [PubMed] [Google Scholar]
- Ma J., Li H. B., Zhang C., Yang X. M., Liu Y., Yan G., et al. (2010). Identification and validation of a major QTL conferring crown rot resistance in hexaploid wheat. Theor. Appl. Genet. 120 1119–1128. 10.1007/s00122-009-1239-3 [DOI] [PubMed] [Google Scholar]
- Ma J., Stiller J., Zhao Q., Feng Q., Cavanagh C. R., Wang P., et al. (2014). Transcriptome and allele specificity associated with a 3BL locus for Fusarium crown rot resistance in bread wheat. PLoS One 9:e113309. 10.1371/journal.pone.0113309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Yan G., Liu C. (2012a). Development of near-isogenic lines for a major QTL on 3BL conferring Fusarium crown rot resistance in hexaploid wheat. Euphytica 183 147–152. 10.1007/s10681-011-0414-1 [DOI] [Google Scholar]
- Ma J., Zhang C. Y., Liu Y. X., Yan G. J., Liu C. J. (2012b). Enhancing Fusarium crown rot resistance of durum wheat by introgressing chromosome segments from hexaploid wheat. Euphytica 186 67–73. 10.1007/s10681-011-0492-0 [DOI] [Google Scholar]
- Martin A., Bovill W. D., Percy C. D., Herde D. J., Fletcher S., Kelly A., et al. (2015). Markers for seedling and adult plant crown rot resistance in four partially resistant bread wheat sources. Theor. Appl. Genet. 128 377–385. 10.1007/s00122-014-2437-1 [DOI] [PubMed] [Google Scholar]
- McBeath J. H., McBeath J. (2010). “Plant diseases, pests and food security,” in Environmental Change and Food Security in China. Advances in Global Change Research; (Dordrecht: Springer; ), 117–156. 10.1007/978-1-4020-9180-3_5 [DOI] [Google Scholar]
- Monds R. D., Cromey M. G., Lauren D. R., Menna M. E. D., Marshall J. W. (2005). Fusarium graminearum, F. cortaderiae and F. pseudograminearum in New Zealand: molecular phylogenetic analysis, mycotoxin chemotypes and co-existence of species. Fungal Biol. 109 410–420. 10.1017/s0953756204002217 [DOI] [PubMed] [Google Scholar]
- Obanor F., Chakraborty S. (2014). Aetiology and toxigenicity of Fusarium graminearum and F. pseudograminearum causing crown rot and head blight in Australia under natural and artificial infection. Plant Pathol. 63 1218–1229. 10.1111/ppa.12200 [DOI] [Google Scholar]
- Pariyar S. R., Erginbasorakci G., Dadshani S., Chijioke O. B., Leon J., Dababat A. A., et al. (2020). Dissecting the genetic complexity of Fusarium crown rot resistance in wheat. Sci. Rep. 10:3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta A. L., Sun Y., Mcdaniel M. D., Lennon J. T. (2018). Crop rotational diversity increases disease suppressive capacity of soil microbiomes. Ecosphere 9:e02235. 10.1002/ecs2.2235 [DOI] [Google Scholar]
- Poole G. J., Smiley R. W., Paulitz T. C., Walker C., Carter A. H., See D. R., et al. (2012). Identification of quantitative trait loci (QTL) for resistance to Fusarium crown rot (Fusarium pseudograminearum) in multiple assay environments in the Pacific Northwestern US. Theor. Appl. Genet. 125 91–107. 10.1007/s00122-012-1818-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J. J., Carere J., Fitzgerald T. L., Stiller J., Covarelli L., Xu Q., et al. (2017). The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.). Ann. Bot. 119 853–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao F., Yang X., Xu F., Huang Y., Zhang J., Song M., et al. (2021). TMT-based quantitative proteomic analysis reveals defense mechanism of wheat against the crown rot pathogen Fusarium pseudograminearum. BMC Plant Biol. 21:82. 10.1186/s12870-021-02853-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Davies P., Bansal U., Pasam R., Hayden M., Trethowan R. (2020). Marker-assisted recurrent selection improves the crown rot resistance of bread wheat. Mol. Breed. 40:28. [Google Scholar]
- Ren Y., Yu P.-B., Wang Y., Hou W.-X., Yang X., Fan J.-L., et al. (2020). Development of a rapid approach for detecting sharp eyespot resistance in seedling-stage wheat and its application in Chinese Wheat Cultivars. Plant Dis. 104 1662–1667. 10.1094/pdis-12-19-2718-re [DOI] [PubMed] [Google Scholar]
- Sharma R. C., Duveiller E. (2007). Advancement toward new spot blotch resistant wheats in South Asia. Crop Sci. 47 961–968. 10.2135/cropsci2006.03.0201 [DOI] [Google Scholar]
- Sharma R. C., Duveiller E., Jacquemin J. M. (2007). Microsatellite markers associated with spot blotch resistance in spring wheat. J. Phytopathol. 155 316–319. 10.1111/j.1439-0434.2007.01238.x [DOI] [Google Scholar]
- Shi S., Zhao J., Pu L., Sun D., Han D., Li C., et al. (2020). Identification of new sources of resistance to crown rot and Fusarium head blight in Wheat. Plant Dis. 104 1979–1985. 10.1094/pdis-10-19-2254-re [DOI] [PubMed] [Google Scholar]
- Singh P. K., He X., Sansaloni C., Juliana P., Dreisigacker S., Duveiller E., et al. (2018). Resistance to spot blotch in two mapping populations of common wheat is controlled by multiple QTL of Minor Effects. Int. J. Mol. Sci. 19:4054. 10.3390/ijms19124054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V., Singh G., Chaudhury A., Ojha A., Tyagi B. S., Chowdhary A. K., et al. (2016). Phenotyping at hot spots and tagging of QTLs conferring spot blotch resistance in bread wheat. Mol. Biol. Rep. 43 1293–1303. 10.1007/s11033-016-4066-z [DOI] [PubMed] [Google Scholar]
- Smiley R. W., Gourlie J. A., Easley S. A., Patterson L. (2005). Pathogenicity of fungi associated with the wheat crown rot complex in Oregon and Washington. Plant Dis. 89 949–957. 10.1094/pd-89-0949 [DOI] [PubMed] [Google Scholar]
- Steuernagel B., Periyannan S. K., Hernández-Pinzón I., Witek K., Rouse M. N., Yu G., et al. (2016). Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 34 652–655. 10.1038/nbt.3543 [DOI] [PubMed] [Google Scholar]
- Sun C., Dong Z., Zhao L., Ren Y., Zhang N., Chen F. (2020). The Wheat 660K SNP array demonstrates great potential for marker−assisted selection in polyploid wheat. Plant Biotechnol. J. 18 1354–1360. 10.1111/pbi.13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tembo B., Sibiya J., Tongoona P., Tembo L. (2017). Validation of microsatellite molecular markers linked with resistance to Bipolaris sorokiniana in wheat (Triticum aestivum L.). J. Agric. Sci. 155 1061–1068. 10.1017/s0021859617000144 [DOI] [Google Scholar]
- Tomar V., Singh R. P., Poland J., Singh D., Joshi A. K., Singh P. K., et al. (2020). Genome-wide association study and genomic prediction of spot blotch disease in wheat (Triticum aestivum L.) using genotyping by sequencing. Res. Square [Preprint]. [Google Scholar]
- Wallwork H., Butt M., Cheong J., Williams K. J. (2004). Resistance to crown rot in wheat identified through an improved method for screening adult plants. Aust. Plant Pathol. 33 1–7. 10.1071/ap03073 [DOI] [Google Scholar]
- Wang H., Sun S., Ge W., Zhao L., Hou B., Wang K., et al. (2020). Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 368:eaba5435. 10.1126/science.aba5435 [DOI] [PubMed] [Google Scholar]
- Wang J., Wang X., Xu M., Feng G., Zhang W., Yang X., et al. (2015). Contributions of wheat and maize residues to soil organic carbon under long-term rotation in north China. Sci. Rep. 5:11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Wang S., Liang Z., Shi W., Gao C., Xia G. (2018a). From genetic stock to genome editing: gene exploitation in wheat. Trends Biotechnol. 36 160–172. 10.1016/j.tibtech.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Wang X., Bi W., Gao J., Yu X., Wang H., Liu D. (2018b). Systemic acquired resistance, NPR1, and pathogenesis-related genes in wheat and barley. J. Integr. Agr. 17 2468–2477. 10.1016/s2095-3119(17)61852-5 [DOI] [Google Scholar]
- William M., Singh R. P., Huertaespino J., Islas S. O., Hoisington D. A. (2003). Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology 93 153–159. 10.1094/phyto.2003.93.2.153 [DOI] [PubMed] [Google Scholar]
- Wu X., Cheng K., Zhao R., Zang S., Bie T., Jiang Z., et al. (2017). Quantitative trait loci responsible for sharp eyespot resistance in common wheat CI12633. Sci. Rep. 7:11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ma J., Li H., Ma H., Yao J., Liu C. (2010). Different genes can be responsible for crown rot resistance at different developmental stages of wheat and barley. Eur. J. Plant Pathol. 128 495–502. 10.1007/s10658-010-9680-3 [DOI] [Google Scholar]
- Yang X., Pan Y., Singh P. K., He X., Ren Y., Zhao L., et al. (2019). Investigation and genome-wide association study for Fusarium crown rot resistance in Chinese common wheat. BMC Plant Biol. 19:153. 10.1186/s12870-019-1758-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhong S., Zhang Q., Ren Y., Sun C., Chen F. (2021). A loss−of−function of the dirigent gene TaDIR−B1 improves resistance to Fusarium crown rot in wheat. Plant Biotechnol. J. 19 866–868. 10.1111/pbi.13554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Guo G., Wu Q., Chen Y., Xie J., Lu P., et al. (2020). Identification and fine mapping of spot blotch (Bipolaris sorokiniana) resistance gene Sb4 in wheat. Theor. Appl. Genet. 133 2451–2459. 10.1007/s00122-020-03610-3 [DOI] [PubMed] [Google Scholar]
- Zhao R., Chen X., Zhang F., Zhang H., Schroder J. L., Romheld V. (2006). Fertilization and Nitrogen Balance in a Wheat–Maize Rotation System in North China. Agron. J. 98 938–945. 10.2134/agronj2005.0157 [DOI] [Google Scholar]
- Zheng Z., Kilian A., Yan G., Liu C. (2014). QTL conferring fusarium crown rot resistance in the elite bread wheat variety EGA Wylie. PLoS One 9:e96011. 10.1371/journal.pone.0096011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Ma J., Stiller J., Zhao Q., Feng Q., Choulet F., et al. (2015). Fine mapping of a large-effect QTL conferring Fusarium crown rot resistance on the long arm of chromosome 3B in hexaploid wheat. BMC Genomics 16:850. 10.1186/s12864-015-2105-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., He X., Wang S., Ma Q., Sun B., Ding S., et al. (2019). Diversity of the Fusarium pathogens associated with crown rot in the Huanghuai wheat−growing region of China. Environ. Microbiol. 21 2740–2754. 10.1111/1462-2920.14602 [DOI] [PubMed] [Google Scholar]
- Zhu X., Lu C., Du L., Ye X., Liu X., Coules A., et al. (2017). The wheat NB−LRR gene Ta RCR 1 is required for host defence response to the necrotrophic fungal pathogen Rhizoctonia cerealis. Plant Biotechnol. J. 15 674–687. 10.1111/pbi.12665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Qi L., Liu X., Cai S., Xu H., Huang R., et al. (2014a). The wheat ethylene response factor transcription factor pathogen-induced ERF1 mediates host responses to both the necrotrophic pathogen Rhizoctonia cerealis and freezing stresses. Plant Physiol. 164 1499–1514. 10.1104/pp.113.229575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Yang K., Wei X., Zhang Q., Rong W., Du L., et al. (2015). The wheat AGC kinase TaAGC1 is a positive contributor to host resistance to the necrotrophic pathogen Rhizoctonia cerealis. J. Exp. Bot. 66 6591–6603. 10.1093/jxb/erv367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Bonnett D., Ellis M., Singh P. K., Heslot N., Dreisigacker S., et al. (2014b). Mapping resistance to spot blotch in a CIMMYT synthetic-derived bread wheat. Mol. Breed. 34 1215–1228. 10.1007/s11032-014-0111-6 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Combined chromosome distribution map for all the QTLs conferring resistance to common root rot (spot blotch), Fusarium crown rot, and sharp eyespot in wheat.
Chromosome location data for all the reported QTLs.