Abstract
Introduction and importance
A patient presented with ipsilateral, synchronous primary malignancies of left upper back melanoma and left breast invasive ductal carcinoma. This complex presentation was managed with a multidisciplinary approach.
Case presentation
A 61-year-old female presented with multiple cutaneous lesions, revealed to be several foci of melanoma in situ as well as a T4b melanoma of the left upper back. On staging work up, a left breast malignancy was incidentally discovered. Genetic testing did not delineate a relevant mutation to explain the synchronous malignancies. Multidisciplinary surgical planning entailed consideration of the lymphatic drainage patterns of the lesions, with both the upper back melanoma and breast carcinoma expected to drain to the left axilla. Ultimately, simultaneous resections of both malignancies were performed as well as concomitant left sentinel lymph node biopsies utilizing dual tracer technique.
Clinical discussion
Currently, cases of synchronous primary cutaneous melanoma and independent, ipsilateral primary breast carcinoma have not been examined, and thus surgical considerations for axillary staging in this circumstance have not been discussed. The existing literature instead explores the incidence and operative challenges of one malignancy following the other after an interval of time.
Conclusion
This case highlights the utility of a multidisciplinary team for complex oncologic presentations and discusses a creative surgical approach to address two simultaneous primary malignancies involving the left breast and ipsilateral skin of the back. This case emphasizes an exceedingly rare presentation and serves as an important example to educate medical professionals on the innovative and team-based approach to treatment.
Keywords: Cutaneous melanoma, Breast cancer, Invasive ductal carcinoma, Sentinel lymph node biopsy, Synchronous primary malignancies, Case report
Highlights
-
•
Two simultaneous primary malignancies draining to the same lymph node basin is rare.
-
•
Post-surgical changes in lymph drainage should be considered for future management.
-
•
Dual tracer technique is recommended for sentinel lymph node biopsy.
-
•
Interdisciplinary collaboration in complex oncologic care improves patient outcomes.
1. Introduction
We report a rare presentation of primary cutaneous melanoma of the back with synchronous, ipsilateral invasive ductal carcinoma (IDC) of the breast. Multidisciplinary surgical planning at an academic cancer center entailed consideration of the lymphatic drainage patterns of the lesions, with both the upper back melanoma and breast carcinoma expected to drain to the left axilla; ultimately, the team performed simultaneous resection of both malignancies as well as concomitant left sentinel lymph node biopsies (SLNB) utilizing dual tracer technique.
This is the first report to discuss primary cutaneous melanoma and synchronous, independent, ipsilateral primary breast carcinoma. The existing literature has explored the incidence and operative challenges of one malignancy following the other after an interval of time, but surgical considerations for axillary staging with synchronous malignancies have not yet been discussed. This case highlights the utility of a multidisciplinary team for complex oncologic presentations and discusses a creative surgical approach.
2. Presentation of case
The patient is a 61-year-old female with no relevant medical or family history who presented to her dermatologist with multiple cutaneous lesions. Shave biopsies revealed several foci of melanoma, including a Clinical Stage IIC T4b Clark level IV 7.1 mm deep ulcerated lesion with 2 mitoses/mm2 of the left upper back (Fig. 1), for which she was referred to a surgical oncologist specializing in skin and soft tissue malignancy.
Fig. 1.
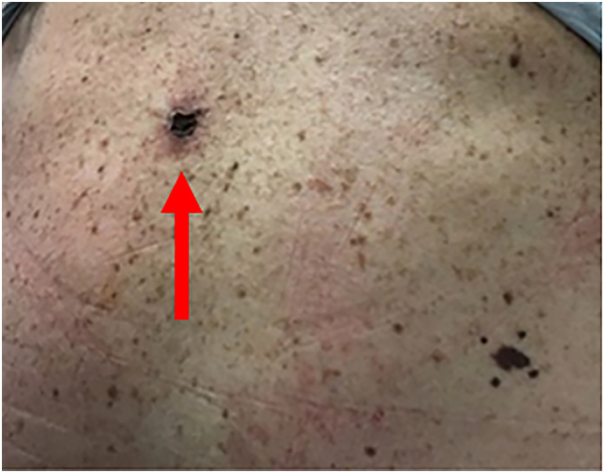
Left upper back melanoma (T4b, arrow).
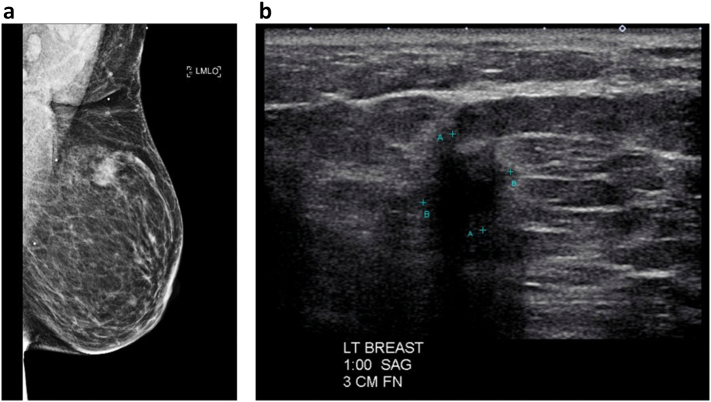
In her staging workup, computed tomography of the chest, abdomen, and pelvis showed a 2.1 cm left breast mass. Of note, the patient was not compliant with annual breast screening; her last mammogram was performed three years prior. On subsequent bilateral diagnostic mammogram (Fig. 2A) and left breast ultrasound (Fig. 2B), the mass measured 13 mm located at the 1:00 axis, 3 cm from the nipple, without evidence of axillary lymphadenopathy, BIRADS 4. Ultimately, she was referred to a breast surgical oncologist and underwent an ultrasound-guided core needle biopsy, revealing a Clinical Stage IA T1c N0 grade 1 IDC, Estrogen Receptor (ER) positive (91–100%), Progesterone Receptor (PR) negative (<1%), Human Epidermal Growth Factor 2 (HER2)/neu negative (1+ by immunohistochemistry). Genetic testing was performed given the synchronous malignancies, revealing a variant of uncertain significance in the Checkpoint Kinase 2 (CHEK2) and RAD50 Inhibitor 1 (RINT1) genes.
Fig. 2.
A. Left breast mammogram, mediolateral oblique view (left)
B. Left breast ultrasound, sagittal view (right).
Discussion at a multidisciplinary tumor board resulted in the suggestion of a surgery-first approach (no neoadjuvant treatment). The left upper back melanoma lesion was planned to be removed via wide local excision along with a left SLNB to assess nodal staging. For treatment of her breast cancer, she also needed a left breast lumpectomy with axillary SLNB. However, due to concern over the alteration of lymphatic drainage patterns if the surgeries were performed sequentially, the decision was made to perform simultaneous resections of the ipsilateral skin and breast lesions with concomitant SLNBs. The use of peri-operative lymphoscintigraphy for additional nodal mapping was considered, but given the proximity of the melanoma to the left axilla, the team ultimately determined that intra-operative radionuclide probe technology along with blue dye injection was appropriate. The treatment approach was discussed with the patient, and she was agreeable to the multidisciplinary approach.
Prior to surgery, the patient underwent wire localization of the left breast cancer, and subsequently, both the breast and back lesions were mapped with technetium-99 and methylene blue dye (dual tracer technique) for left axillary lymph node mapping. The radionuclide probe indicated drainage solely to the left axilla, with negative signaling from the contralateral axilla as well as bilateral cervical and inguinal nodes. Afterwards, a wide local excision of the left back melanoma was performed, followed by left axillary SLNB and finally the left breast lumpectomy. Final pathologic stage of the melanoma was Stage IIC T4bN0 with negative margins; final pathologic stage for the breast cancer was Stage IIA T2N0 2.2 cm grade 2 IDC with intermediate grade ductal carcinoma in situ, ER positive, PR and HER2/neu negative, with negative margins. Mapping revealed three sentinel lymph nodes removed from the superior and anterior aspect of the axilla, all negative for malignancy. Of note, there were no radioactive signals in the posterolateral axilla during the operation, which would have been the expected location of the lymphatic drainage for the back melanoma.
Postoperative genomic analysis of the breast cancer revealed an Oncotype DX score of 20, suggesting no benefit for adjuvant chemotherapy; thus, the patient received adjuvant whole breast irradiation and endocrine therapy with an aromatase inhibitor for treatment of her breast cancer. For treatment of her multiple melanomas, the patient subsequently had wide local excisions of the remaining four lesions concerning for melanoma on her right buttock, right upper back, and bilateral shoulders, all of which revealed melanoma in situ. No further nodal biopsy or adjuvant therapy was recommended for treatment of her melanoma.
3. Discussion
To date, the simultaneous occurrence of a primary cutaneous melanoma and independent, ipsilateral primary breast carcinoma has not been discussed. The existing literature has instead delineated the risk of a second primary malignancy after either a primary cutaneous melanoma or breast carcinoma [1], [2], [3], [4], as well as surgical considerations for axillary dissection in the treatment of one of the aforementioned malignancies following the other after an interval amount of time [5]. Though these two malignancies are common individually, a synchronous presentation demonstrates the importance of preoperative planning and a team-based approach to complex oncologic presentations.
There is a statistically significant increased risk of cutaneous melanoma in female breast cancer survivors; likewise, there is an increased risk of breast cancer in cutaneous melanoma survivors. One study reported that the risk of breast cancer in female patients with history of cutaneous melanoma is 11%, and the risk of subsequent cutaneous melanoma in breast cancer survivors is 16%. Mutations in BRCA2 and CDKN2A have been implicated with these two malignancies [3]. An additional relationship between an initial diagnosis of melanoma treated with immune checkpoint inhibitors and the development of a second primary malignancy, including breast cancer, has also been reported [6].
A case of primary melanoma with surgical excision, followed later by ipsilateral breast cancer with surgical excision and eventual lymphatic recurrence of both malignancies, has also been reported [7]. Another study discussed two cases of singular tumors in the breast containing “collision tumors” of primary combined melanoma and IDC [8]. However, neither of these cases involved distinct, independent primary malignancies of the skin and breast that occurred simultaneously.
Related to surgical techniques, it is known that cancer of the breast will drain to sentinel lymph nodes in the axilla, while cutaneous melanoma of the back and upper extremities will also most often drain to the axilla. Lymphatic drainage to the contralateral axilla as well as bilateral cervical and inguinal nodes was considered and assessed intraoperatively with the use of radionuclide probe technology, ultimately proving negative. Within the ipsilateral axilla, the location of sentinel lymph node drainage tends to differ for the two tumors, with the back melanoma draining more posteriorly, closer to the latissimus dorsi muscle, as compared to a more anterior location for breast tumors. Thus, mapping and excision of involved lymph nodes becomes more challenging when both primary malignancies occur simultaneously and ipsilaterally. Alterations in lymphatic drainage patterns after previous operations involving SLNBs or axillary dissections have been discussed using patient cases [5]. It is important to note that these operations were performed independently, unlike the case we have presented.
Performing a wide local excision of the back melanoma with SLNB before an ipsilateral wire-localized breast lumpectomy with SLNB, or vice versa, would have compromised the efficacy of the subsequent SLNB. Specifically, lymphatic drainage to the axilla would have been altered by an initial surgery, jeopardizing the ability of the second surgeon to accurately map and excise involved lymph nodes. Considerations to improve sentinel lymph node mapping include performing a formal nuclear lymphoscintigraphy with imaging to visually localize the sentinel lymph nodes just prior to the surgery. As discussed, this step was deemed unnecessary in light of the use of an intraoperative radionuclide probe.
While this case reflects simultaneous breast and melanoma cancers, given that both presented in early stages, primary surgical therapy was indicated prior to systemic treatment. However, if one of the cancers were more advanced at the time of initial presentation, then the decision to undergo neoadjuvant chemotherapy may have impacted the surgical plans or confounded the final pathology of the other cancer.
This work has been reported in line with the SCARE 2020 criteria [9].
4. Conclusion
This case describes a unique clinical presentation involving close collaboration between a breast surgical oncologist and a surgical oncologist subspecializing in skin and soft tissue. It highlights the utility of a multidisciplinary team and discusses a creative surgical approach in order to address two simultaneous, ipsilateral primary malignancies. We hope to emphasize the rarity of the presentation as well as educate medical professionals on the innovative and team-based approach to treatment.
Consent
Written and informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
None.
Ethical approval
This study is exempt from ethical approval in our institution.
Research registration
N/A.
Guarantor
Maria Kowzun, MD, FACS
Provenance and peer review
Not commissioned; externally peer-reviewed.
CRediT authorship contribution statement
Maria Kowzun, MD, FACS:
Patient care, surgical planning, drafting/editing the paper
Vadim Koshenkov, MD, FACS
Patient care, surgical planning, editing the paper
Catherine Davis, MD, MPH
Patient care, drafting/editing the paper
Nicole Fosko, BE
Patient care, initial data collection, drafting/editing the paper
Declaration of competing interest
None.
References
- 1.Bradford P.T., Freedman D.M., Goldstein A.M., Tucker M.A. Increased risk of second primary cancers after a diagnosis of melanoma. Arch. Dermatol. 2010;146(3) doi: 10.1001/archdermatol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caini S., Boniol M., Botteri E., Tosti G., Bazolli B., Russell-Edu W., Gandini S. The risk of developing a second primary cancer in melanoma patients: a comprehensive review of the literature and meta-analysis. J. Dermatol. Sci. 2014;75(1):3–9. doi: 10.1016/j.jdermsci.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Goggins W., Gao W., Tsao H. Association between female breast cancer and cutaneous melanoma. Int. J. Cancer. 2004;111(5):792–794. doi: 10.1002/ijc.20322. [DOI] [PubMed] [Google Scholar]
- 4.Gutman M., Shafir R., Rozin R.R., Klausner J.M., Cnaan A., Inbar M., Chaitchik S. Are malignant melanoma patients at higher risk for a second cancer? Cancer. 1991;68(3):660–665. doi: 10.1002/1097-0142(19910801)68:3<660::aid-cncr2820680337>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Johnson C., Intenzo C., Mastrangelo M.J., Feeney K., Berger A.C. Altered drainage patterns in patients with melanoma and previous axillary dissection. J. Dermatol. 2013;40(7):564–566. doi: 10.1111/1346-8138.12143. [DOI] [PubMed] [Google Scholar]
- 6.Deng W., Wang Y., Liu X., Liu J., Wang L., Yang Z., Jiang W. Assessment of trends in second primary cancers in patients with metastatic melanoma from 2005 to 2016. JAMA Netw. Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.28627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross M., Hadzikadic Gusic L., Dabbs D.J., Kelley J., Diego E. Simultaneous breast and axillary recurrence in a patient with a history of breast cancer and ipsilateral upper extremity melanoma: challenges in diagnosis and management. Tumori. 2014;100(4):136e–139e. doi: 10.1700/1636.17928. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- 8.Padmore R.F., Lara J.F., Ackerman D.J., Gales T., Sigurdson E.R., Ehya H., Patchefsky A.S. Primary combined malignant melanoma and ductal carcinoma of the breast: a report of two cases. Cancer. 1996;78(12):2515–2525. doi: 10.1002/(sici)1097-0142(19961215)78:12<2515::aid-cncr11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.for the SCARE Group. Agha R.A., Franchi T., Sohrabi C., Mathew G. The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]