Highlights
-
•
Condensed tannin and astringency properties of spine grapes and wines were studied.
-
•
Spine wines were rich in extension units of condensed tannins.
-
•
A positive correlation of mDp and astringency of spine wine was observed.
-
•
Spine grapes and wines might have potential for development.
Keywords: Astringency, Condensed tannin, Spine grapes, Spine Wine
Abstract
This study sought to determine the effects of variety on the astringency and chemistry of condensed tannins of spine grapes and wines. Fifteen varieties of red spine grape (Vitis davidii Foex) were used. Condensed tannin content, composition, and wine astringency were determined. The condensed tannin profiles were assessed by high-performance liquid chromatography coupled with diode array detector (HPLC-DAD). The condensed tannin content highly depended on the variety ranging from 0.30 mg/g to 7.80 mg/g (in skins), from 3.12 mg/g to 8.82 mg/g (in seeds), and from 62.60 mg/L to 225.90 mg/L (in wines). There were significant differences in proportions of certain constitutive subunits (as mole%) and mean degree of polymerization (mDp) among the varieties. Correlation analysis revealed that condensed tannin concentration and composition had a significant effect on the sensory evaluation and quantitative analysis of astringency. A positive correlation between mDp and astringency was also observed. The present results expand knowledge of the characterization of spine grape and wine condensed tannin chemistry and astringency.
1. Introduction
Condensed tannins play an important role in bitterness or mouth-feel perception, and this sensory perception defines red wine quality (Watrelot et al., 2018, Zarin et al., 2016). Condensed tannins are phenolic plant secondary compounds and formed from flavan-3-ol units, including (−)-epicatechin, (+)-catechin, (−)-epigallocatechin, and (−)-epicatechin-3-O-gallate, linked by carbon–carbon bonds (Li & Duan, 2018). These flavan-3-ol oligomers or polymers account for about 25%–50% of the total phenolics in young red wines, and the proportion may be higher in older wines (Waterhouse, 2002). Many recent studies have reported that condensed tannins in red wines may interact to a greater or lesser degree with salivary proteins depending on changes in the mean degree of polymerization (mDp) and the tannin concentration (Chira et al., 2012).
The characterization of condensed tannin chemistry and astringency in grapes and wines depends on the variety of grape and is affected by many factors, such as fermentation management, pH, and wine age (Watrelot et al., 2018). It is thus necessary to study the effects of grape variety on the astringency and condensed tannin chemistry of grapes and wines. In particular, some wild grape varieties, such as Vitis davidii and Vitis quinquangularis, have an important value for development and use, but researchers and winemakers know less about the characteristics of these wild varieties.
Spine grapes are native to China and belong to the East Asian Vitis spp. They are mainly grown in subtropical areas of China, such as the Yangtze River Basin and Yunnan-Guizhou Plateau (Kong, 2004). They are called spine grapes because the one- or two-year-old canes are densely covered with 1 mm–2 mm spines. Spine grapes have been found in different regions of China, including Yunnan Province, Jiangxi Province, Hunan Province (Meng et al., 2012). Many red spine grape varieties have been reported, such as Xiangzhenzhu, Junzi #1 and Junzi #2, and previous studies have also reported white spine grape varieties, called Baiputao (Meng et al., 2012). Spine grapes are rich in phenolics and have a distinctive aroma (Liang et al., 2013), but compared with V. vinifera grapes, greater processing of spine grapes for wine production is needed. In recent years, wine makers have tried to use spine grapes, especially Xiangzhenzhu, Zhilan, Junzi, etc., to make dry red, sweet red and rosé wines. These products are rich in phenolics and aromas, and are well consumed in China. However, there also some spine grape varieties are good to consume fresh, such as 5044. Understanding of the transformation from grape to industrial products (e.g., wine) thus not only protects nutrients but also increases the production value of the fruit. Previously, we found that spine grape varieties, such as Junzi #1 had the best health-promoting properties, and thus, higher use value and potential for development (Meng et al., 2012). To date, however, there have been few studies on spine grapes and wines, especially concerning their condensed tannin chemistry and astringency, therefore, further studies on this subject are necessary.
This study evaluated the varietal differences in condensed tannin chemistry and astringency among V. davidii Foex grapes and wines. The relationships between condensed tannin concentration, composition, and astringency in spine wines were also analyzed. From a practical standpoint, the results of this study might provide insights leading to better understanding of spine grape resources.
2. Materials and methods
2.1. Grapes and wine samples
Fifteen kinds of red spine grapes (V. davidii Foex) were collected at commercial maturity from different regions and used for winemaking (see Table S1 for sample information). The vineyard is located at 27°57′ N, 110°0′ E (Huaihua, Hunan, China), 29°10′ N, 107°5′ E (Chongqing, China) and 24°29′ N, 113°54′ E (Ganzhou, Jiangxi, China), respectively. The vines were spaced at 3.5 m × 4 m and were cultivated under high stem horizontal trellis. The vines were managed according to industry standards for nutrition and disease pest management (GB 12696–1990, GB/T 25393–2010). Vinification was performed using our previous methods (Ju et al., 2018). Briefly, about 50 kg grapes from each sample were sorted and crushed. After crushing, the grape mashes were transferred to a 50 l fermenter, and 35 mg/L SO2 and 20 mg/L pectinase were added. After 24 h, 200 mg/L of active dry yeast (Saccharomyces cerevisiae strain Rhone 2323, Lalvin, Denmark) was added. The mashes were kept at 23 °C–25 °C for about 10 d to allow fermentation and maceration. Throughout this period, daily mass homogenizations were performed to dissolve the cap. Temperature and density were also recorded daily. Once the fermentation and maceration processes were complete, the wine residue was removed from the vats and pressed with 2.0 bar pressure, discarding the marcs and recovering the wine, which was decanted 15 d later, discarding lees. At the end of alcoholic fermentation, 50 mg/L SO2 was added to each bottle. After fermentation, tartrate stabilization was performed by adding potassium tartrate into wine samples and the samples were filtered with 0.45 μm filter. Subsequently, wine was bottled in 750 mL bottles and stored until the analysis, and each wine bottle was opened immediately before the analysis. For each grape sample, three replicated vinifications were performed.
2.2. Reagents and standards
Methanol, acetonitrile, acetic acid, hydrochloric acid (HPLC grade) was purchased from Sigma-Aldrich (Shanghai, China). (−)-Epicatechin (≥98.0%, Sigma-Aldrich, Shanghai, China) was used as internal standard. Nutrient agar, Folin–Ciocalteu, acetone, ascorbic acid, sodium acetate, gallic acid equivalents, tannic acid and (+)-catechin was purchased from Kermel Chemical Reagent Co. Ltd. (Tianjin, China). The deionized water was obtained from a Milli-Q system (Merck Millipore, Darmstadt, Germany).
2.3. Analysis of physicochemical parameters of spine grapes skins and wines
To determine the total phenolic and total tannin contents, the grapes were deseeded, and the skins were peeled off under the protection of liquid nitrogen. Then, the grape skins and wines were used to determine the total phenolic contents using the Folin–Ciocalteu method, and the results were expressed as milligrams of gallic acid equivalents (GAE) per gram of grapes or per liter of wine (Jayaprakasha et al., 2001). The methylcellulose precipitation (MCP) method was used to measure the total tannin content of the grapes skins and wines (Zhang et al., 2012). Determination of the residual sugar, titratable acids, and pH values of the wine samples was conducted according to the methods of Yue et al., 2020, Yue et al., 2019.
2.4. Determination of condensed tannin concentration
The protein precipitation method was performed to determine the condensed tannin concentration of the grape skins, seeds, and wines (Harbertson et al., 2002), which were expressed in mg/g or mg/L (+)-catechin equivalent, respectively. Briefly, 50 grape berries were dissected into skin and seed fractions on ice; the grape skins and seeds were extracted with 50 mL of 70% acetone overnight with agitation, then the solids were filtered and acetone was removed from a 1 mL aliqout. Finally, the volumes were adjusted to 1 mL with ultra-pure water before the determination of protein-precipitable tannin. Condensed tannin concentration in wines were determined using the Adams-Harbertson (AH) protein precipitation assay method (Harbertson et al., 2002).
2.5. Analysis of constitutive subunits and mDp from spine grape skins, seeds, and wines
2.5.1. Extraction and purification of tannin from grape skins and seeds
The extraction of tannin was according to the methods of Meng et al., 2012, Meng et al., 2012 with some modified. Briefly, about 30 g (fresh weight) grape skins or seeds were grounded into powder with the help of liquid nitrogen, then freeze-dried at −50 °C. The powder of dried grape skins or seeds (1.00 g) was mixed with 5 mL acetone solution (acetone:water = 2:1), protecting with nitrogen; the mixtures were shaken at 35 °C for 24 h. The mixtures were then filtered with a funnel, evaporated at 35 °C to remove acetone, and diluted to 50 mL with deionized water. Finally, 9 mL of the extract was taken in a 15 mL centrifuge tube and concentrated to dryness by rotary evaporation at 35 °C. The residues were dissolved with 3 mL methanol; the solution was collected, protected from light, and stored at −40 °C for further analysis.
2.5.2. Extraction and purification of tannin from wine samples
The method for extraction of wine was according to previously methods reported by Watrelot et al., 2017, Duan et al., 2021 with modified. Briefly, a Hypersep C18 column (1 g, 6 mL column, ThermoFisher scientific) was slowly conditioned with methanol and deionized water, respectively. 1 mL of wine sample was added and slowly eluted with 5 mL of methanol. Four mL of methanol was then added to remove the methanol from the filling to obtain a tannin eluate. The tannin eluate was taken in a 15 mL centrifuge tube and concentrated to dryness by rotary evaporation at 35 °C. The residues were dissolved with 1 mL methanol; the solution was collected, protected from light, and stored at −40 °C for further analysis.
2.5.3. Determination of constitutive subunits composition and mDp
Determination of constitutive subunits composition and the mean degree of polymerization (mDp) of condensed tannin was performed by high performance liquid chromatography coupled with diode array detection (HPLC-DAD) at 280 nm according to the methods of Watrelot et al. (2018). Briefly, the phloroglucinol reagent solution (0.2 mol/L hydrochloric acid in methanol, containing 100 g/L phloroglucinol and 20 g/L ascorbic acid; 1:1, v/v) was added to the tannin extracts and the mixtures were then maintained at 50 °C for 20 min. Finally, 1000 μL of cold 40 mol/L aqueous sodium acetate was added to 200 μL of the sample to arrest the reaction. Before injection, the solution was filtered through a 0.45 μm organic filter. Injection volume was 20 μL. An HPLC system (HPLC, SHIMADZU, Japan) fitted with two Chromolith RP-18e (100 mm × 4.6 mm) columns connected in series and protected by a guard column containing the same material was used for analysis of condensed tannins. The column flow rate was 3.0 mL/min, and the temperature of column was 30 °C. Phase A: 1% v/v aqueous acetic acid; phase B: 1% v/v acetic acid in acetonitrile. The elution gradient was, time in min (%B): 0 (3%), 4.0 (3%), 14.0 (18%), 14.01 (80%), 16.0 (80%), 16.01 (3%), and 18.0 (3%). Detection was performed by a diode array detector (DAD) at 280 nm. The internal standard (−)-epicatechin (y = 4.9121x–16.388, R2 = 0.9995, recovery [%]: 93.61 ± 1.26, reproducibility [RSD/%]: 0.92, repeatability [RSD/%]: 0.81) (Sigma, Shanghai, China) was used for identification of the extension and terminal units. Their quantification were calculated by building the calibration curves of the area ratio of target compounds to the corresponding internal standard against the concentration ratio. The mDp was equal to the sum of all constitutive subunits (in moles)/ the sum of all terminal subunits (in moles).
2.6. Quantitative evaluation of spine wines astringency
The quantitative evaluation of wine astringency was carried out according to the method of Bao et al. (2015), which reported a quantitative analysis method for wine astringency and verified with 21 wines including spine wine and Cabernet Sauvignon wine. The results showed that the correlation coefficient between the slope of the logarithmic curve and the initial concentration of tannin reached 0.972, indicating that the method was reliable (Bao et al., 2015). Briefly, different volumes of the tannic acid solutions (2 g/L) were mixed with human oral protein solutions (3 g/L) and diluted to 6 mL with electrolyte to obtain the final protein solutions and tannic acid solutions (protein concentrations were 0.05 g/L, 0.10 g/L, 0.50 g/L, 1.00 g/L, and 1.50 g/L, respectively; tannic acid concentrations were 0 g/L, 0.1 g/L, 0.2 g/L, 0.3 g/L, 0.4 g/L, and 0.5 g/L, respectively). The above mixtures were maintained at 37 °C for 2 h following by centrifuging at 10,000 r/min for 6 min, and then the supernatant was collected to measure the absorbance value at 330 nm. In order to obtain the standard curve of tannic acid, a two-step test was performed: the first step, a known concentration of tannic acid reacted with a series of concentrations of protein, and the protein concentration was plotted against the absorbance value to obtain the slope of the curve of the concentration of tannic acid; the second step, the slope of the obtained curve was plotted with different concentrations of tannic acid to obtain a standard curve of tannic acid. To analyze wine astringency, we replaced the tannic acid solution in the above operation with a wine sample, and according to the above steps, reacted with the gradient protein, and plotted the logarithmic curve between the absorbance value at 330 nm and the protein concentration to obtain the slope of the logarithmic curve; then, substituting the slope into the standard curve of tannic acid to obtain a value for the sample’s astringency.
2.7. Sensory evaluation of spine wine samples
Sensory evaluation of spine wine samples was performed by 20 professionally trained tasters (10 males and 10 females). Before the analysis, the taster used tannin solutions (5 different concentrations of tannic acid in simulated wine solution) for training. Five gradient solutions were prepared using tannic acid as a training sample, and the five sensation levels were assigned to score values of 1, 2, 3, 4, and 5, respectively. The five solutions were: (1) 12% ethanol + 0 g/L tannic acid; (2) 12% ethanol + 0.2 g/L tannic acid; (3) 12% ethanol + 0.4 g/L tannic acid; (4) 12% ethanol + 0.6 g/L tannic acid; and (5) 12% ethanol + 0.8 g/L tannic acid. Pure water (24 °C-25 °C) was used as mouth rinsing. During the training process a test to access the panel's recognition accuracy of each tannic acid level was done once a week until the identification accuracy was above 95%. Then the panel was proceeded to perform wine sensory analysis.
Sensory analysis was carried out in a standard wine sensory laboratory at room temperature (20 °C) and humidity 70%. The international standard black blind cup was used for analysis. The sensory analysis was conducted between 9:30–11:00 am every day, and each time a maximum of 4 wine samples were tasted. Fifty millilitre of each wine was served for tasting. The panelists used a five-point scale method to score: one point was subtle sensation, and the tannin intensity was the weakest; two points meant perceptible, but not strong; three points meant clearly perceptible, medium tannin intensity; four points meant obviously perceptible and greater tannin intensity; and five points indicated the strongest tannin intensity. All wine samples were analyzed in duplicate.
2.8. Statistical analysis
Data were reported as mean ± standard deviation (SD, n = 3) values for the triplicate experiments. Tukey’s multiple range tests with a significance level at 0.05 or 0.01 and a two-tailed Pearson’s correlation test were used to analyze the data using SPSS 22.0 software (Inc., Chicago, IL, USA).
3. Results and discussion
3.1. Physicochemical parameters
The total phenolic content of the spine grapes ranged from 3.78 GAE mg/g to 21.07 GAE mg/g (Table S2). The highest total phenolic content was detected in the Xiangzhenzhu grape, reaching 21.07 GAE mg/g, followed by Gaoshan #4 and Gaoshan #2. The total phenolic content of the spine grapes tested here were comparable with the previous findings, which reported that the total phenolic content of four spine grape samples (JZ-1, JZ-2, BY, and LT) ranged from 1.57 GAE mg/g FW to 3.65 GAE mg/g (FW, fresh weight) (Meng et al., 2012). Xiangzhenzhu had the highest total tannin content, followed by Junzi #1 and Junzi #2. While the lowest total tannin contents appeared in 5061 grape skins. The total acid content of the wine samples ranged from 4.11 g/L to 6.24 g/L. The highest total acid content was detected in Gaoshan #2 wine, reaching 6.24 g/L, followed by 5055 wine. The lowest total acidity value was found for 5049 wine, at about 4.11 g/L. A previous study reported that the total acidity of the wine determined the ultimate pH of the wine and affected its color and stability (Meng et al., 2012), thus playing an important role in the sensory quality of the wine. The pH values of the spine wines ranged from 3.28 (Gaoshan #2 wine) to 3.58 (Junzi #1 wine), which are conducive to the stability of wine color, giving wine a good appearance, and promoting wine consumption. The total phenolics of the spine wine samples ranged from 614.13 GAE mg/L to 1229.90 GAE mg/L. The total phenolic content of the spine wines was lower than that of red V. Vinifera grape wines (Ju et al., 2016). Junzi #2 wine had the highest total phenolic value, while wine made from 5044 clone grapes had the lowest. The total tannins of the spine wine samples ranged from 140.99 mg/L to 451.18 mg/L. Junzi #1 wine had the highest total tannin value, while wine made from 5049 grapes had the lowest. The varietal differences in the total tannin content of the spine wines suggested that the tastes of these wines—including astringency—might vary.
3.2. Condensed tannin concentration, composition, and mDp in spine grape skins and seeds
Condensed tannins play an important role in the sensory characteristics of red wines, especially for the astringency and bitterness of wines (Kyraleou et al., 2020). The importance of the condensed tannins composition in the astringency properties is well known (Kyraleou et al., 2016). Previous report found that the tannins in grape skins might be helpful to protect anthocyanins from degradation during maceration, which can improve the color properties of wines (Chen et al., 2016). As Table 1 shows, condensed tannin concentration was significantly dependent on the variety of spine grape tested. The concentration of condensed tannins in spine grape skins ranged from 0.30 mg/g to 7.80 mg/g (Table 1). Gaoshan #4 grape skins had the highest condensed tannin concentration, reaching 7.80 mg/g, followed by Gaoshan #5 grape skins. However, the lowest condensed tannin concentration was found in 5055 grape skins, at about 0.30 mg/g. The varietal differences in the condensed tannin content of the spine grapes suggested that Gaoshan #4 and Gaoshan #5 grapes might yield more intense astringency (Ma et al., 2014). These results were similar to the findings from previous studies on spine grapes (Meng et al., 2012), and the differences between the varieties of spine grapes were reflected in the detailed phenolic profiles. There were significant differences in the proportions of certain constitutive subunits (as mole%) between varieties (Table 1). The (−)-epicatechin units were the most abundant subunits in all of the spine grape skins. Zhilan and Gaoshan #4 grape skins showed more (−)-epicatechin as extension units and mDp, while the proportions of (−)-epicatechin-3-O-gallate as extension and terminal units were lower in Zhilan and Gaoshan #4 grape skins. The highest proportion of (−)-epicatechin-3-O-gallate as extension and terminal units was detected in 5055 grape skins, while a lower mDp was observed in 5055 grape skins. Butkhup et al. (2010) also reported that (−)-epicatechin and (+)-catechin were the most common tannin compositions in wine grapes.
Table 1.
Average of constitutive unit content (mole %), mean degree of polymerization and concentrations of condensed tannin in spine grape skins.
| Grape skins | Extension units(%) |
Terminal units(%) |
mDP | Concentration | |||||
|---|---|---|---|---|---|---|---|---|---|
| ECG | EGC | C | EC | ECG | C | EC | mg/g | ||
| Xiangzhenzhu | 16.67 ± 0.24b | 0.67 ± 0.24ab | 1.85 ± 0.10d | 69.78 ± 0.15de | 4.91 ± 0.07f | 0.90 ± 0.06jk | 3.46 ± 0.33 k | 10.70 ± 0.16b | 6.00 ± 0.02b |
| Gaoshan #2 | 4.78 ± 0.15f | 0.03 ± 0.00e | 0.53 ± 0.33ef | 67.39 ± 0.28 g | 2.33 ± 0.23 k | 7.02 ± 0.02d | 14.44 ± 0.31c | 4.20 ± 0.14e | 5.70 ± 0.22b |
| Gaoshan #4 | 0.79 ± 0.15 l | 0.09 ± 0.06e | 1.60 ± 0.28d | 89.03 ± 0.02a | 2.62 ± 0.27jk | 0.43 ± 0.30 k | 1.44 ± 0.3j | 13.40 ± 0.25a | 7.80 ± 0.11a |
| Gaoshan #5 | 2.69 ± 0.22hi | 0.3 ± 0.21 cd | 0.89 ± 0.08e | 78.27 ± 0.19c | 1.43 ± 0.30 l | 1.09 ± 0.06ij | 13.19 ± 0.13d | 6.40 ± 0.26c | 7.40 ± 0.28a |
| Zhilan | 1.77 ± 0.16 k | 0.20 ± 0.14d | 0.18 ± 0.13f | 88.62 ± 0.27a | 3.37 ± 0.30hij | 2.26 ± 0.18 h | 1.57 ± 0.31 l | 14.00 ± 0.07a | 3.80 ± 0.14 cd |
| Junzi #1 | 17.20 ± 0.14b | 0.21 ± 0.15d | 4.04 ± 0.03a | 46.90 ± 0.07 k | 8.58 ± 0.30d | 11.6 ± 0.29b | 7.54 ± 0.32 h | 3.60 ± 0.28f | 2.20 ± 0.15e |
| Junzi #2 | 13.26 ± 0.18c | 0.32 ± 0.20 cd | 0.98 ± 0.02e | 68.83 ± 0.12f | 4.47 ± 0.33 fg | 0.85 ± 0.1jk | 9.19 ± 0.14 g | 6.90 ± 0.08c | 5.30 ± 0.20b |
| 5015 | 0.84 ± 0.11 l | 0.78 ± 0.15a | 0.68 ± 0.23ef | 81.83 ± 0.12b | 4.28 ± 0.20 fg | 5.07 ± 0.05f | 6.10 ± 0.06i | 6.50 ± 0.34c | 5.80 ± 0.15b |
| 5044 | 8.46 ± 0.32e | 0.76 ± 0.17a | 2.52 ± 0.34b | 69.13 ± 0.09ef | 6.05 ± 0.03e | 1.59 ± 0.30hi | 11.8 ± 0.12ef | 5.10 ± 0.10d | 3.00 ± 0.00d |
| 5049 | 9.42 ± 0.29d | 0.76 ± 0.12a | 2.08 ± 0.05 cd | 58.88 ± 0.09i | 6.49 ± 0.34e | 6.13 ± 0.09e | 14.15 ± 0.11c | 3.70 ± 0.19f | 4.50 ± 0.35c |
| 5059 | 13.22 ± 0.16c | 0.38 ± 0.30c | 0.74 ± 0.18e | 69.30 ± 0.21f | 4.13 ± 0.09fgh | 3.99 ± 0.00 g | 7.82 ± 0.13 h | 6.30 ± 0.19c | 5.70 ± 0.20b |
| 5055 | 19.28 ± 0.20a | 0.63 ± 0.206ab | 2.01 ± 0.01 cd | 35.99 ± 0.01 m | 22.16 ± 0.11a | 4.10 ± 0.07 g | 15.56 ± 0.31b | 2.40 ± 0.28 g | 0.30 ± 0.23 g |
| 5061 | 13.31 ± 0.22c | 0.59 ± 0.30b | 2.40 ± 0.28 cd | 56.53 ± 0.34j | 12.64 ± 0.26b | 1.80 ± 0.14 h | 12.10 ± 0.07e | 3.80 ± 0.16f | 3.40 ± 0.29d |
| 5063 | 3.27 ± 0.19 g | 0.36 ± 0.26c | 2.13 ± 0.09 cd | 63.10 ± 0.07 h | 9.70 ± 0.21c | 9.50 ± 0.35c | 7.72 ± 0.19 h | 3.71 ± 0.20f | 1.90 ± 0.08e |
| 5064 | 2.09 ± 0.06ij | 0.34 ± 0.24c | 3.35 ± 0.25b | 44.12 ± 0.08 l | 9.84 ± 0.12c | 14.60 ± 0.27a | 20.39 ± 0.28a | 2.23 ± 0.16 g | 1.44 ± 0.3ef |
Note: ECG = (−)-epicatechin-3-O-gallate, EGC = (−)-epigallocatechin, C = (+)-catechin, EC = (−)-epicatechin, different lowercase letters in the same column showed significant difference in Tukey multiple comparisons at P < 0.05 level.
Turning to the condensed tannin concentration of spine grape seeds, variety had a significant effect on their condensed tannin concentration (Table 2). The concentrations of condensed tannins in spine grape seeds ranged from 3.12 mg/g to 8.82 mg/g (Table 2). Zhilan and 5063 grape seeds had a higher concentration of condensed tannins than other varieties, followed by Xiangzhenzhu and Junzi #2 grape seeds. Junzi #2 and Xiangzhenzhu had a high condensed tannin content in both the grape skins and seeds (Table 1, Table 2). Zhilan, 5063, 5064, and 5055 had a higher condensed tannin content in the seeds than in the skins, while Gaoshan #4 and Gaoshan #5 had a higher condensed tannin content in the skins than in the seeds (Table 1, Table 2). For the condensed tannin composition of grape seeds, the content of the extension unit was higher than that of the terminal unit, and (−)-epicatechin as the extension unit was the most abundant subunit in all of the spine grape seeds (Table 2). Xiangzhenzhu and Junzi #2 grape seeds had more (−)-epicatechin as the extension unit and mDp, while the proportion of (−)-epicatechin as extension and terminal units was lower in 5064 grape seeds. In addition, 5055, 5063, and 5064 grape seeds had more (−)-epicatechin-3-O-gallate as the extension unit. A greater proportion of (−)-epicatechin-3-O-gallate as the terminal unit was detected in 5015 and 5044 grape seeds, but lower mDp was observed in 5015 and 5044 grape seeds (Table 2). The condensed tannin concentration, composition, and mDp in grape skins and seeds could thus be affected by spine grape varieties. Recently, more evidence suggests that the participation of tannins and changes in mDp may be associated with the softening of astringency perception (Chira et al., 2012). Spine grapes’ tannin and mDp diversity may thus give wines a different astringency range. The feeling of astringency is frequently felt alongside bitterness (Lee & Lawless, 1991), and Brossaud et al. (2001) reported that the seed fraction with a lower mDp was perceived to be bitterer than the skin fraction, but further interpretation remains unclear.
Table 2.
Average of constitutive unit content (mole %), mean degree of polymerization and concentrations of condensed tannin in spine grape seeds.
| Grape seeds | Extension units(%) |
Terminal units(%) |
mDP | Concentration | ||||
|---|---|---|---|---|---|---|---|---|
| ECG | C | EC | ECG | C | EC | mg/g | ||
| Xiangzhenzhu | 8.49 ± 0.35 g | 2.53 ± 0.33c | 69.43 ± 0.31a | 6.08 ± 0.05 g | 1.59 ± 0.29d | 11.88 ± 0.09gh | 5.12 ± 0.08a | 7.48 ± 0.34b |
| Gaoshan #2 | 10.30 ± 0.21f | 1.7 ± 0.23d | 58.52 ± 0.34d | 12.78 ± 0.16e | 2.06 ± 0.04b | 14.67 ± 0.23de | 3.39 ± 0.28c | 4.18 ± 0.12d |
| Gaoshan #4 | 1.34 ± 0.24 k | 2.10 ± 0.07c | 58.60 ± 0.28d | 21.87 ± 0.09b | 1.65 ± 0.25d | 14.44 ± 0.31de | 2.63 ± 0.26d | 4.32 ± 0.22d |
| Gaoshan #5 | 17.95 ± 0.03c | 1.75 ± 0.17d | 42.67 ± 0.23 l | 18.41 ± 0.29c | 3.70 ± 0.21a | 15.51 ± 0.35bc | 2.66 ± 0.24d | 5.76 ± 0.17c |
| Zhilan | 13.48 ± 0.34d | 2.43 ± 0.30c | 57.23 ± 0.16e | 12.79 ± 0.15e | 1.82 ± 0.13c | 12.25 ± 0.18 g | 3.72 ± 0.20bc | 8.43 ± 0.31a |
| Junzi #1 | 1.88 ± 0.08 k | 4.76 ± 0.17a | 60.53 ± 0.33c | 16.17 ± 0.12d | 2.17 ± 0.12bc | 14.48 ± 0.34de | 3.05 ± 0.03c | 4.84 ± 0.11d |
| Junzi #2 | 11.41 ± 0.29e | 1.91 ± 0.06d | 61.83 ± 0.12b | 7.82 ± 0.13f | 0.95 ± 0.04e | 16.08 ± 0.05b | 4.02 ± 0.02b | 7.61 ± 0.28b |
| 5015 | 3.80 ± 0.14j | 0.94 ± 0.04e | 50.12 ± 0.08 h | 26.05 ± 0.03a | 2.21 ± 0.15bc | 16.88 ± 0.08a | 2.22 ± 0.15e | 3.12 ± 0.08e |
| 5044 | 5.54 ± 0.32i | 1.19 ± 0.13de | 49.76 ± 0.17 h | 26.59 ± 0.29a | 1.91 ± 0.06c | 15.01 ± 0.00 cd | 2.30 ± 0.21e | 3.35 ± 0.25e |
| 5049 | 12.62 ± 0.27de | 3.69 ± 0.22b | 51.47 ± 0.33 g | 16.15 ± 0.1d | 2.76 ± 0.17b | 13.32 ± 0.23f | 3.10 ± 0.07c | 5.79 ± 0.15c |
| 5059 | 6.99 ± 0.01 h | 2.39 ± 0.28c | 55.58 ± 0.30f | 18.18 ± 0.13c | 2.06 ± 0.05bc | 14.8 ± 0.15cde | 2.85 ± 0.10d | 4.33 ± 0.24d |
| 5055 | 27.54 ± 0.33a | 1.10 ± 0.07de | 41.40 ± 0.29 k | 16.52 ± 0.34d | 1.40 ± 0.29d | 12.03 ± 0.02gh | 3.34 ± 0.24c | 5.98 ± 0.02c |
| 5061 | 13.41 ± 0.29d | 2.37 ± 0.27c | 48.36 ± 0.26i | 20.82 ± 0.13bc | 2.53 ± 0.33b | 12.50 ± 0.35 g | 2.79 ± 0.15d | 4.13 ± 0.09d |
| 5063 | 25.85 ± 0.10b | 1.29 ± 0.21de | 43.66 ± 0.24j | 12.88 ± 0.08e | 2.21 ± 0.15bc | 14.11 ± 0.08e | 3.43 ± 0.30c | 8.82 ± 0.13a |
| 5064 | 26.25 ± 0.17b | 0.83 ± 0.12e | 39.45 ± 0.32 l | 20.66 ± 0.24bc | 1.37 ± 0.26d | 11.43 ± 0.31 h | 2.99 ± 0.01d | 5.95 ± 0.04c |
Note: ECG = (−)-epicatechin-3-O-gallate, C = (+)-catechin, EC = (−)-epicatechin, different lowercase letters in the same column showed significant difference in Tukey multiple comparisons at P < 0.05 level.
3.3. Condensed tannin concentration, composition, and mDp in spine wines
The concentration and composition of condensed tannins in wines may be influenced by many factors, such as grape variety, fermentation, pH, and age (Watrelot et al., 2018). The effects of variety on the concentration and composition of condensed tannins in spine grape wines were determined in this study, and the detailed results are shown as Table 3. Junzi #2 wine had a higher concentration of condensed tannins than other varieties, followed by Junzi #1 and 5063 wines. Gaoshan #4, Gaoshan #5, and 5044 wines had lower condensed tannin contents than other wine samples. The varietal differences in tannin concentration in the wines might be due to tannin precipitation after their association with other components or to the formation of pigmented tannin (Watrelot et al., 2018). However, in Brazilian Vitis vinifera red wines, the amounts of total terminal (catechin, epicatechin, and epicatechin gallate) and extension units (phloroglucinol products of catechin, epicatechin, and epicatechin gallate) were reported as 48.1 mg/L to 94.6 mg/L and 215.9 mg/L to 568.3 mg/L, respectively (Gris et al., 2011). It thus appears that spine grape wines have higher extension units but lower terminal units than Brazilian V. vinifera red wines. On the other hand, the amounts of extension units (phloroglucinol products of epicatechin and epicatechin gallate) in spine wines were higher than those of Baboso Negro, Negramoll and Tintilla red wines (from Spanish), whereas, the amounts of extension units (phloroglucinol products of epigallocatechin and catechin) in spine wines were lower than those of Baboso Negro, Negramoll and Tintilla red wines (Juan et al., 2011). The amounts of extension units (phloroglucinol products of epicatechin gallate) in spine wines were higher than those of Baboso Negro, Negramoll and Tintilla red wines (from Spanish) (Juan et al., 2011). The astringency intensity of wines not only has a correlation with condensed tannins but also has a strong positive relationship with the condensed tannin composition and mDp (Ma et al., 2014). In this study, the proportion of extension units was higher than terminal units, and the proportion of (−)-epicatechin as the extension unit was the most common subunit among all of the spine wines (Table 3). The wines made with 5059 and 5015 spine grapes had more (−)-epicatechin as extension units, while the proportion of (−)-epicatechin as the extension unit was lower in the Junzi #1 wine. The proportion of (−)-epigallocatechin as the extension unit had a positive relationship with the mDp of spine wines. Xiangzhenzhu, Zhilan, and 5015 wines had a higher proportion of (−)-epigallocatechin as the extension unit and more mDp (Table 3). A previous study reported that the oxidation reactions of wine resulted in a decrease in mDp and (−)-epigallocatechin as the extension unit (McRae et al., 2015). The proportion of (−)-epicatechin-3-O-gallate as the extension unit in spine wines ranged from 0.08 to 37.06 (mole%) (Table 3). Junzi #1 and 5063 wines had a higher proportion of (−)-epicatechin-3-O-gallate as the extension unit in tannins than other varieties, followed by Junzi #2 and Zhilan wines. The wines made from 5044, 5059, and 5055 spine grape varieties had a lower proportion of (−)-epicatechin-3-O-gallate as the extension unit in tannins than the other wine samples. The varietal differences in condensed tannin concentration, composition, and mDp in spine wines may result in astringency variations (Ma et al., 2014).
Table 3.
Average of constitutive unit content (mole %), mean degree of polymerization and concentration of condensed tannin in spine wines.
| Wines | Extension units(%) |
Terminal units(%) |
mDP | Concentration | |||||
|---|---|---|---|---|---|---|---|---|---|
| ECG | EGC | C | EC | ECG | C | EC | mg/L | ||
| Xiangzhenzhu | 30.10 ± 0.07d | 0.67 ± 0.24b | 1.13 ± 0.09f | 49.60 ± 0.27e | 7.27 ± 0.19 g | 1.65 ± 0.25f | 9.57 ± 0.30 g | 5.41 ± 0.29a | 97.50 ± 0.33 g |
| Gaoshan #2 | 13.61 ± 0.28f | 0.03 ± 0.00f | 0.28 ± 0.20 g | 43.10 ± 0.07f | 8.02 ± 0.01 fg | 21.58 ± 0.30a | 13.41 ± 0.29f | 2.33 ± 0.23 h | 92.6 ± 0.23 h |
| Gaoshan #4 | 28.02 ± 0.01e | 0.09 ± 0.06f | 1.75 ± 0.2ef | 44.20 ± 0.16f | 13.41 ± 0.29c | 2.94 ± 0.04e | 9.57 ± 0.31 g | 3.86 ± 0.1d | 75.80 ± 0.34i |
| Gaoshan #5 | 9.77 ± 0.16 h | 0.30 ± 0.21d | 0.01 ± 0.00 h | 56.40 ± 0.25c | 13.74 ± 0.18c | 12.35 ± 0.25b | 7.47 ± 0.34 h | 2.98 ± 0.01 fg | 74.30 ± 0.15i |
| Zhilan | 31.64 ± 0.26c | 0.20 ± 0.14e | 2.02 ± 0.01e | 44.10 ± 0.09f | 8.35 ± 0.25f | 1.35 ± 0.25f | 12.32 ± 0.23f | 4.54 ± 0.32ab | 149.80 ± 0.14d |
| Junzi #1 | 34.07 ± 0.05b | 0.21 ± 0.14e | 3.35 ± 0.25 g | 36.84 ± 0.11 h | 7.37 ± 0.26b | 1.03 ± 0.02f | 17.34 ± 0.24e | 3.88 ± 0.08 cd | 178.70 ± 0.17c |
| Junzi #2 | 31.44 ± 0.31c | 0.21 ± 0.15e | 0.47 ± 0.33 g | 38.54 ± 0.33 g | 5.71 ± 0.21 h | 0.79 ± 0.15 g | 22.92 ± 0.06d | 3.40 ± 0.28de | 225.90 ± 0.06a |
| 5015 | 12.66 ± 0.24 g | 0.32 ± 0.23d | 2.94 ± 0.04d | 60.36 ± 0.26b | 4.77 ± 0.16i | 1.13 ± 0.09f | 17.81 ± 0.13e | 4.22 ± 0.15b | 105.50 ± 0.35f |
| 5044 | 0.08 ± 0.06 k | 0.78 ± 0.15a | 6.50 ± 0.35a | 54.67 ± 0.23d | 20.71 ± 0.21a | 3.16 ± 0.11d | 14.10 ± 0.07f | 2.63 ± 0.26gh | 62.60 ± 0.24j |
| 5049 | 13.15 ± 0.11f | 0.76 ± 0.24a | 1.16 ± 0.12c | 58.79 ± 0.23bc | 9.43 ± 0.30 g | 0.53 ± 0.33 g | 16.17 ± 0.12e | 3.83 ± 0.12d | 101.50 ± 0.28c |
| 5059 | 3.70 ± 0.21j | 0.38 ± 0.27d | 0.37 ± 0.26 g | 64.04 ± 0.03a | 7.40 ± 0.28 g | 1.06 ± 0.04f | 23.06 ± 0.04d | 3.17 ± 0.12e | 175.00 ± 0.01c |
| 5055 | 3.31 ± 0.22j | 0.63 ± 0.26b | 3.90 ± 0.07c | 33.61 ± 0.27 k | 10.28 ± 0.20d | 2.63 ± 0.26e | 45.63 ± 0.26a | 1.71 ± 0.21jk | 93.10 ± 0.05 h |
| 5061 | 10.21 ± 0.15 h | 0.59 ± 0.29bc | 5.29 ± 0.21b | 38.49 ± 0.35 g | 9.42 ± 0.29e | 3.51 ± 0.35d | 32.48 ± 0.34c | 2.20 ± 0.14ij | 116.20 ± 0.11e |
| 5063 | 37.06 ± 0.04a | 0.36 ± 0.15d | 0.65 ± 0.25 cd | 36.84 ± 0.11 h | 17.76 ± 0.17b | 0.50 ± 0.02 g | 6.83 ± 0.12 h | 3.99 ± 0.01c | 196.70 ± 0.21b |
| 5064 | 5.86 ± 0.10i | 0.34 ± 0.17d | 4.00 ± 0.01c | 37.32 ± 0.23 g | 7.28 ± 0.20 g | 7.36 ± 0.25c | 37.84 ± 0.11b | 1.91 ± 0.07jk | 178.10 ± 0.09c |
Note: ECG = (−)-epicatechin-3-O-gallate, EGC = (−)-epigallocatechin, C = (+)-catechin, EC = (−)-epicatechin, different lowercase letters in the same column showed significant difference in Tukey multiple comparisons at P < 0.05 level.
3.4. Quantitative analysis of astringency in spine wines
Tannin combined with oral salivary protein form astringent substances, which can be felt as a perceptible sensation in the mouth (Cliff et al., 2012). Researchers have established a quantitative evaluation system for astringency, which reported that the slope of the logarithmic curve reflected the amount of binding ability of the protein to the tannin, and the larger the absolute value of the slope, the stronger the feeling of astringency. The relative strength of the sample astringency could thus be measured by the slope. According to the absorbance value corresponding to the reaction of tannic acid with protein at a certain concentration, the slope of the logarithmic curve was obtained (Table 4). As shown in Fig. S1, the curve can be used as a tannic acid standard curve to measure the relative astringency values for the wine samples. The linear regression equation of the curve is y = 0.1354x – 0.0015, and the correlation R reached 0.9851, indicating that they exhibited a good linear relationship. As Table 4 shows, the relative astringency values of the spine wine samples were measured according to the standard tannic acid curve. Junzi #2 and Junzi #1 wines had higher relative astringency values, with higher condensed tannins and mDp observed in these wines (Table 3 and Table 4). Lower relative astringency values were determined in Gaoshan #2, Gaoshan #5, and 5061 wines, and the mDp of these wines was also low (Table 3, Table 4). The 5044 wine showed the lowest astringency and had the lowest tannin content and mDp value (Table 3, Table 4). As previously reported, the astringency of wine has a close relationship with the tannin mDp (Chira et al., 2012). Higher degrees of tannin polymerization and greater tannin concentration may benefit the binding ability of tannins and protein, which creates greater astringency in the wines, and makes the taste of wine more coordinated and layered (Ma et al., 2014).
Table 4.
Quantitative analysis of astringency in spine wines.
| Wines | Logarithmic curve and R | Absolute value | Relative astringency value |
|---|---|---|---|
| Xiangzhenzhu | y = −0.006ln(x) + 0.9924 R2 = 0.8205 | 0.006 | 0.55e |
| Gaoshan #2 | y = −0.004ln(x) + 0.7171 R2 = 0.965 | 0.004 | 0.41f |
| Gaoshan #4 | y = −0.009ln(x) + 0.3152 R2 = 0.9981 | 0.009 | 0.70d |
| Gaoshan #5 | y = −0.003ln(x) + 0.4849 R2 = 0.9583 | 0.003 | 0.33f |
| Zhilan | y = −0.006ln(x) + 1.1023 R2 = 0.6901 | 0.006 | 0.55e |
| Junzi #1 | y = −0.015ln(x) + 0.9677 R2 = 0.7742 | 0.015 | 1.22ab |
| Junzi #2 | y = −0.014ln(x) + 1.2333 R2 = 0.9999 | 0.014 | 1.14b |
| 5015 | y = −0.012ln(x) + 1.8913 R2 = 0.9407 | 0.012 | 1.00c |
| 5044 | y = −0.004ln(x) + 0.5970 R2 = 0.8059 | 0.001 | 0.18g |
| 5049 | y = −0.016ln(x) + 0.9624 R2 = 0.8349 | 0.016 | 1.29a |
| 5059 | y = −0.004ln(x) + 0.7171 R2 = 0.965 | 0.004 | 0.41f |
| 5055 | y = −0.01ln(x) + 1.87630 R2 = 0.6253 | 0.010 | 0.85d |
| 5061 | y = −0.003ln(x) + 1.8829 R2 = 0.7093 | 0.003 | 0.33f |
| 5063 | y = 0.0099ln(x) + 0.8214 R2 = 0.8316 | 0.010 | 0.85d |
| 5064 | y = −0.006ln(x) + 0.8028 R2 = 0.6042 | 0.006 | 0.55e |
Note: Different lowercase letters in the same column showed significant difference in Tukey multiple comparisons at P < 0.05 level.
3.5. Astringency sensory evaluation of spine wine samples
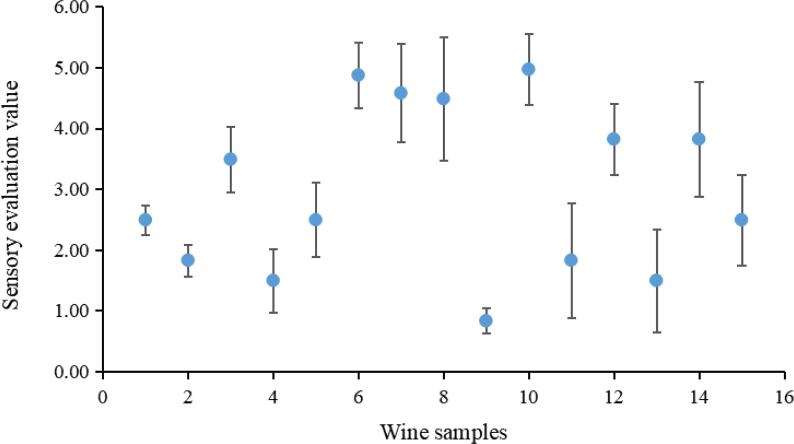
As Fig. 1 shows, the tasters found that the sensation of astringency produced by the spine wines varied greatly. The astringency sensory evaluation values ranged from 0.83 (5044 wine, No. 9) to 4.97 (5049 wine, No. 10). The wines of Junzi #1 (No. 6), Junzi #2 (No. 7), 5015 (No. 8), and 5049 (No. 10) spine grape varieties had significantly higher astringency sensory evaluation values than other wines, while 5044 (No. 9), 5059 (No. 11), 5061 (No. 13), Gaoshan #5 (No. 2) and Gaoshan #2 (No. 4) spine grape wines yielded lower astringency sensory evaluation values. These results suggest that variety has a significant effect on perceptible astringency. The correlation coefficient R between the astringency sensory evaluation value and the quantitative evaluation of the wine samples reached 0.9377, and the linear regression equation was y = 0.2446x – 0.089, indicating that the quantitative evaluation method for astringency used in this study was consistent with the results of the sensory evaluation (Fig. S2).
Fig. 1.
Sensory evaluation values of 15 spine wine samples. The X-axis numbers represent the wine samples (1-Xiangzhenzhu, 2-Gaoshan #2, 3-Gaoshan #4, 4-Gaoshan #5, 5-Zhilan, 6-Junzi #1, 7-Junzi #2, 8–5015, 9–5044, 10–5049, 11–5059, 12–5055, 13–5061, 14–5063, 15–5064). Wine samples were tasted by 20 professionally trained tasters (10 males and 10 females). Data were reported as mean ± standard deviation (SD, n = 3) values of triplicate experiments. P < 0.05.
3.6. Correlation analysis between condensed tannins and astringency in spine wines
As Table 5 shown that the total tannin levels were significantly and positively correlated with (−)-epigallocatechin and (+)-catechin as extension units, as well as condensed tannin concentration, relative astringency value, and astringency sensory evaluation value. The total tannin levels appeared to be negatively related with (−)-epicatechin-3-O-gallate as the terminal unit. There was a positive correlation between the proportion of (−)-epicatechin-3-O-gallate as the extension unit and mDp. It also appeared that mDp was significantly and negatively related with (+)-catechin and (−)-epicatechin as terminal units, but was positively related to wine astringency (Table 5). A positive correlation of mDp and astringency was also reported by Chira et al. (2009). Watrelot et al. (2018) observed a negative relationship between (−)-epicatechin as the terminal unit and mDp in aging wines, but a positive relationship between mDp and (−)-epigallocatechin as the extension unit. This might be explained by the multiple reactions (such as oxidation) between condensed tannins and anthocyanins (especially malvidin derivatives) in wines, resulting in widely varying tannin subunit compositions and mDp (McRae et al., 2015, Paissoni et al., 2020). Recently, more evidences revealed that changes of mDp may be associated with the softening process of PAs astringency perception (Ma et al., 2014). The condensed tannin concentration was significantly and positively related to (−)-epigallocatechin and (+)-catechin as the extension unit (Table 5). Correlation analysis revealed that the condensed tannin concentration had a significant effect on the astringency sensory evaluation and quantitative analysis of astringency (Table 5). As previous reported, there was a positively relationship between the intensity of astringency and the presence of galloylated subunits (%ECG) in the tannin structure (Rinaldi et al., 2014); however, not all researchers agreed with this phenomenon (Kyraleou et al., 2016). The positive relationship between %ECG of grape skins and astringency while in seeds the opposite was observed (Chira et al., 2012). Many studies revealed that the %ECG of grape skins was negatively related to the astringency of wines (Chira et al., 2012, Rinaldi et al., 2014, Kyraleou et al., 2020). In the present study, we found that the relative astringency values and astringency sensory evaluation values were significantly affected by the proportion of (−)-epigallocatechin as the extension unit (Table 5). The presence of (+)-catechin as the extension unit and the terminal unit were significantly related with the quantitative astringency values and the astringency sensory evaluation values, respectively. As expected, quantitative astringency values were significantly and positively related to the astringency sensory evaluation values. These results suggested that the condensed tannin concentration, composition, and mDp played important roles in spine grape wine astringency. Some previous results revealed that there was a positive relationship between PAs astringency and mDP, in contrast, others found a parabolic trend (Wei et al., 2020, Kyraleou et al., 2016). The mechanism of the influence of mDP on the PAs astringency of wine is not yet clear, and more further research is needed (Zhang et al., 2017).
Table 5.
Correlation between condensed tannins and astringency in spine wines.
| A | Monomer structure content |
C | D | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECG-ext | EGC-ext | C-ext | EC-ext | ECG-term | C-term | EC-term | mDP | B | |||||
| A | 1 | ||||||||||||
| Monomer structure content | ECG-ext | −0.07 | 1 | ||||||||||
| EGC-ext | 0.904** | −0.168 | 1 | ||||||||||
| C-ext | 0.539* | −0.444 | 0.508* | 1 | |||||||||
| EC-ext | −0.094 | −0.214 | 0.019 | −0.386 | 1 | ||||||||
| ECG-term | −0.504* | −0.106 | −0.379 | 0.063 | −0.135 | 1 | |||||||
| C-term | 0.423 | −0.363 | 0.39 | 0.338 | −0.259 | −0.08 | 1 | ||||||
| EC-term | −0.108 | −0.500* | −0.148 | 0.329 | −0.417 | −0.119 | −0.075 | 1 | |||||
| mDP | 0.039 | 0.716** | 0.014 | −0.442 | 0.427 | −0.246 | −0.476* | −0.692** | 1 | ||||
| B | 0.953** | −0.063 | 0.898** | 0.539* | −0.153 | −0.442 | 0.500* | −0.134 | −0.023 | 1 | |||
| C | 0.928** | −0.006 | 0.925** | 0.486* | −0.142 | −0.449 | 0.415 | −0.142 | 0.044 | 0.937** | 1 | ||
| D | 0.855** | −0.008 | 0.825** | 0.384 | −0.128 | −0.408 | 0.590** | −0.249 | 0.046 | 0.900** | 0.879** | 1 | |
* * was significantly correlated at the level of 0.01 and * was significantly correlated at the level of 0.05.
ECG = (−)-epicatechin-3-O-gallate, EGC = (−)-epigallocatechin, C = (+)-catechin, EC = (−)-epicatechin, Ext = extension unit, Term = terminal unit. A = total tannins of phenolic substances; B = quantitative analysis of condensed tannin concentration; C = relative astringency value; D = astringency sensory evaluation value.
4. Conclusions
Our results indicate that the cultivar had a statistically significant effect on the condensed tannin concentration, composition, and mDp in spine grapes and wines. Gaoshan #4 grape skins had the highest condensed tannin concentration. The content of (−)-epicatechin (as the extension unit) and the levels of mDp in Zhilan and Gaoshan #4 grape skins were greater than in other varieties. The extension unit content was higher than the terminal unit content, and (−)-epicatechin (as the extension unit) was the most common subunit in all of the spine grape seeds. The total acid content of spine wines was from 6.11 g/L to 8.24 g/L. The wines made from Junzi #1, Junzi #2 and 5015 grapes had the highest total phenolic content among all spine wines. The content of total tannin was higher in Junzi #1 and 5059 wine than in other varieties. The concentration and composition of the wine’s condensed tannins had a significant effect on wine astringency. We also observed a positive correlation between wine mDp level and astringency. These results suggest that spine grapes and wines, such as Junzi#1 and Gaoshan #4, might have potential for development based on their varied condensed tannin chemistry and astringency.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Key R&D Program on Monitoring, Early warning and Prevention of Major National Disaster (Grant No. 2017YFC1502806), the Innovation Team of Grape Modernization, Quality and Efficiency Cultivation Technology (2020TD-047), the National Key Research and Development Project (2019YFD1002500) and the China Agriculture Research System for Grape (Grant No. CARS-29-zp-6).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100125.
Contributor Information
Yan-lun Ju, Email: juyanlun2016@nwsuaf.edu.cn.
La Yang, Email: yangla369@sina.com.
Xiao-feng Yue, Email: yuexiaofeng@nwsuaf.edu.cn.
Rui He, Email: 18392387138@139.com.
Sheng-lin Deng, Email: D.shenglin@nwsuaf.edu.cn.
Xin Yang, Email: YangTrigold@163.com.
Xu Liu, Email: liuxu@nwsuaf.edu.cn.
Yu-lin Fang, Email: fangyulin@nwsuaf.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bao S.Y.N., Li S.Q., Lan Y.B., Pan Q.H. Quantitative evaluation method for wine astringency. China Brewing. 2015;34:152–156. [Google Scholar]
- Brossaud F., Cheynier V., Noble A.C. Bitterness and astringency of grape and wine polyphenols. Australian Journal of Grape and Wine Research. 2001;7(1):33–39. [Google Scholar]
- Chira K., Jourdes M., Teissedre P.-L. Cabernet Sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. European Food Research and Technology. 2012;234(2):253–261. [Google Scholar]
- Cliff M.A., Stanich K., Edwards J.E., Saucier C.T. Adding grape seed extract to wine affects astringency and other sensory attributes. Journal of Food Quality. 2012;35(4):263–271. [Google Scholar]
- Duan B., Ren Y., Zhao Y., Merkeryan H., Su-Zhou C., Li Y. An adequate regulated deficit irrigation strategy improves wine astringency perception by altering proanthocyanidin composition in Cabernet Sauvignon grapes. Scientia Horticulturae. 2021;285:110182. doi: 10.1016/j.scienta.2021.110182. [DOI] [Google Scholar]
- Gris E.F., Mattivi F., Ferreira E.A., Vrhovsek U., Pedrosa R.C., Bordignon-Luiz M.T. Proanthocyanidin profifile and antioxidant capacity of Brazilian Vitis vinifera red wines. Food Chemistry. 2011;126:213–220. doi: 10.1021/jf2008056. [DOI] [PubMed] [Google Scholar]
- Harbertson J.F., Kennedy J.A., Adams D.O. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot noir berries during ripening. American Journal of Enology and Viticulture. 2002;531:54–59. [Google Scholar]
- Jayaprakasha G.K., Singh R.P., Sakariah K.K. Antioxidant activity of grape seed (Vitis vinifera L.) extracts on peroxidation models in vitro. Food Chemistry. 2001;73(3):285–290. [Google Scholar]
- Ju Y.L., Liu M., Zhao H., Meng J.F., Fang Y.L. Effect of exogenous abscisic acid and methyl jasmonate on anthocyanin composition, fatty acids, and volatile compounds of Cabernet Sauvignon (Vitis vinifera L.) grape berries. Molecules. 2016;21(10):1354–1368. doi: 10.3390/molecules21101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y.-L., Liu M., Tu T.-Y., Zhao X.-F., Yue X.-F., Zhang J.-X. Effect of regulated deficit irrigation on fatty acids and their derived volatiles in ‘Cabernet Sauvignon’ grapes and wines of Ningxia. China. Food Chemistry. 2018;245:667–675. doi: 10.1016/j.foodchem.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Kong Q.S. Chinese Press of Agricultural Science and Technology; Beijing: 2004. Chinese ampelography. [Google Scholar]
- Kyraleou M., Kallithraka S., Gkanidi E., Koundouras S., Mannion D.T., Kilcawley K.N. Discrimination of five greek red grape varieties according to the anthocyanin and proanthocyanidin profiles of their skins and seeds. Journal of Food Composition and Analysis. 2020;92:103547. doi: 10.1016/j.jfca.2020.103547. [DOI] [Google Scholar]
- Kyraleou M., Kotseridis Y., Koundouras S., Chira K., Teissedre P.-L., Kallithraka S. Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L. cv. syrah grapes under semiarid conditions. Food Chemistry. 2016;203:292–300. doi: 10.1016/j.foodchem.2016.02.052. [DOI] [PubMed] [Google Scholar]
- Lee C.B., Lawless H.T. Time-course of astringent sensations. Chemical Senses. 1991;16(3):225–238. [Google Scholar]
- Li S.Y., Duan C.Q. Astringency, bitterness and color changes in dry red wines before and during oak barrel aging: An updated phenolic perspective review. Critical Reviews in Food Science and Nutrition. 2018:1–28. doi: 10.1080/10408398.2018.1431762. [DOI] [PubMed] [Google Scholar]
- Liang N.-N., Pan Q.-H., He F., Wang J., Reeves M.J., Duan C.-Q. Phenolic profiles of Vitis davidii and Vitis quinquangularis species native to China. Journal of Agricultural and Food Chemistry. 2013;61(25):6016–6027. doi: 10.1021/jf3052658. [DOI] [PubMed] [Google Scholar]
- Ma W., Guo A., Zhang Y., Wang H., Liu Y., Li H. A review on astringency and bitterness perception of tannins in wine. Trends in Food Science & Technology. 2014;40(1):6–19. [Google Scholar]
- McRae J.M., Day M.P., Bindon K.A., Kassara S., Schmidt S.A., Schulkin A. Effect of early oxygen exposure on red wine colour and tannins. Tetrahedron. 2015;71(20):3131–3137. [Google Scholar]
- Meng J.-F., Fang Y.-L., Qin M.-Y., Zhuang X.-F., Zhang Z.-W. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii foex) in Chongyi county (China) Food Chemistry. 2012;134(4):2049–2056. doi: 10.1016/j.foodchem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Meng J.-F., Xu T.-F., Qin M.-Y., Zhuang X.-F., Fang Y.-L., Zhang Z.-W. Phenolic characterization of young wines made from spine grape (Vitis davidii Foex) grown in Chongyi county (China) Food Research International. 2012;49(2):664–671. [Google Scholar]
- Paissoni M.A., Río Segade S., Carrero-Carralero C., Montanini C., Giacosa S., Rolle L. Role of anthocyanin traits on the impact of oenological tannins addition in the first stage of red winegrape skin simulated maceration. Food Chemistry. 2020;320:126633. doi: 10.1016/j.foodchem.2020.126633. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Jourdes M., Teissedre P.L., Moio L. A preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chemistry. 2014;164:142–149. doi: 10.1016/j.foodchem.2014.05.050. [DOI] [PubMed] [Google Scholar]
- Waterhouse, A.L. (2002). Wine phenolics. Annals of the New York Academy of Sciences, 957, 21-36. [DOI] [PubMed]
- Watrelot A.A., Badet-Murat M.L., Waterhouse A.L. Oak barrel tannin and toasting temperature: Effects on red wine anthocyanin chemistry. LWT-Food Science and Technology. 2018;91:330–338. [Google Scholar]
- Watrelot A.A., Schulz D.L., Kennedy J.A. Wine polysaccharides influence tannin-protein interactions. Food Hydrocolloids. 2017;63(2):571–579. [Google Scholar]
- Wei X., Ju Y.L., Ma T.T., Zhang J.X., Sun X.Y., Fang Y.L. New perspectives on the biosynthesis, transportation, astringency perception and detection methods of grape proanthocyanidins. Critical Reviews in Food science and Nutrition. 2020;3:1–27. doi: 10.1080/10408398.2020.1777527. [DOI] [PubMed] [Google Scholar]
- Yue X.F., Ma X., Tang Y.L., Wang Y., Wu B.W., Jiao X.L. Effect of cluster zone leaf removal on monoterpene profles of Sauvignon Blanc grapes and wines. Food Research International. 2020;131 doi: 10.1016/j.foodres.2020.109028. [DOI] [PubMed] [Google Scholar]
- Yue X.F., Ju Y.L., Tang Z.Z., Zhao Y.M., Jiao X.L., Zhang Z.W. Effects of the severity and timing of basal leaf removal on the amino acids profiles of Sauvignon Blanc grapes and wines. Journal of Integrative Agriculture. 2019;18(9):2052–2062. [Google Scholar]
- Zarin M.A., Wan H.Y., Isha A., Armania N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Science and Human Wellness. 2016;5(2):65–75. [Google Scholar]
- Zhang S., Li L., Cui Y., Luo L., Li Y., Zhou P. Preparative high-speed counter-current chromatography separation of grape seed proanthocyanidins according to degree of polymerization. Food Chemistry. 2017;219:399–407. doi: 10.1016/j.foodchem.2016.09.170. [DOI] [PubMed] [Google Scholar]
- Zhang Z.W., Ning P.F., Zhang J.X., Ai L.L., Wang X.N., Zhang X.Z. Comparison of two methods for the determination of condensed tannins in wine. Food Science. 2012;33(20):233–237. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.