Abstract
Practical methods for preventing embryotoxicity in chickens that are caused by aflatoxin-B1 (AFB1) are currently rare. Binding absorbers are commonly used in feeding stuff to reduce laying hens' exposure to off-contaminated diets, thus reducing residue exposure to fertilized eggs. Nonetheless, several adsorbents have been shown to affect the use of nutrients and the absorption of minerals in poultry. Thus, seeking an effective strategy to counter or control embryotoxicity in broiler chicks caused by AFB1 is a problem. A total of 180 embryonated eggs were injected with 36 ng AFB1 with or without 5.90 mg L-methionine (Met) 30 embryonated eggs each, followed by incubation in an incubator until hatching time. The in ovo injection of Met significantly reduced toxicity caused by AFB1 in broiler embryos by enhancing the liver and kidney functions, lipid profiles, and alleviated oxidative stress during the incubation period. Furthermore, the relative gene expressions (SSTR5, TSH-β, Bcl-2, GSH-Px, GST-a, and SOD in the liver) were up-regulated with in ovo injection of AFB1+Met compared to AFB1 alone. Moreover, there was a dowin-regulated trend in Bax, Caspases-3, Caspases-7, Caspases-9, CYP1A1, CYP2H1, and P53 gene expression with in ovo injection of AFB1+Met compared to AFB1 alone. The in ovo injection of Met led to less apoptotic cells in liver tissues. Such results might be necessary for the poultry industry as it is focused on managing the embryotoxicity of AFB1, which affecting poultry production and welfare. Results from this study demonstrated that in ovo Met injection could alleviate AF-induced toxicity in chicken embryos.
Key words: aflatoxin B1, embryotoxicity, methionine, gene expression, broiler
INTRODUCTION
Mycotoxins are one of the most widespread pollutants in poultry feed in tropical and subtropical areas (Abdallah et al., 2015). Mycotoxins' adverse effects on poultry health included ranges from lower poultry productivity and immune suppression, increased disease, and parasite susceptibility to overt disease and death (Ditta et al., 2018 and Ismail et al., 2020). Previous reports have confirmed that low fungal toxin levels are one of the major causes of deterioration in domestic bird performance. Hereof, any attempt to modify these toxins is based on various factors such as the types of poultry, the treatment time for fungal toxins and organisms (Ditta et al., 2018). Contrary to the prevailing idea regarding the saturation of previous studies regarding the fungal poisoning of chickens, there is still a need to explore many fungal poisoning ways and how to reduce their harmful effects.
Mycotoxins' effect on the embryotoxicity of chicks hatched under the influence of aflatoxins during incubation are extremely rare and inadequately studied. Aflatoxins (AFs), mainly produced by Aspergillus flavus and Aspergillus parasiticus is one of the most critical health hazard classes for humans and animals of naturally occurring mycotoxins (Gündüz and Oznurlu, 2014). Aflatoxin B1 (AFB1) is the most toxic and considered carcinogenic among the 4 major classes of aflatoxins, viz. B1, B2, G1, and G2. Acute or chronic poultry aflatoxicosis leads to a significant decrease in meat/egg production and immunosuppressant as well as hepatotoxicosis (Tessari et al., 2006; Gündüz and Oznurlu, 2014). Aflatoxins concentrations in poultry diets range from 10 to 20 µg/ kg diet, which may carry-over to the eggs produced in a ratio from 1/2000 to 1/2500 (Gündüz and Oznurlu, 2014). Contamination of poultry feed with AFB1 is a major concern for the poultry industry, as aflatoxicosis in chickens leads to substantial economic losses through the reduction in feed use, egg production, body weight gain, and subsequently increased mortality (Tessari et al., 2006; Oguz et al., 2011).
The mechanism of AFB1 intoxication in mature poultry is well known; however, there is less understanding of embryotoxicity and detoxification mechanisms (Hamilton and Bloom, 1986). The effect of AFB1 on DNA results from the toxin's interaction with the reactive sites of the macromolecule, and it distinguishes 2 ways of interaction. One result is a fast, reversible, noncovalent binding, whereas the other is an irreversible covalent binding that leads to aflatoxin-DNA (AF-DNA) adduct formation (Oguz et al., 2011). Another consequence of AFB1 is its biotransformation, which leads to the formation of several metabolic products, particularly hydroxylated derivatives. Additionally, AFB1 residues during the formation of eggs may be passed from the laying hens to the fertilized eggs, resulting in decreased viability and hatchability of the embryos (Qureshi et al., 1998) and causing multiple organ malformations (Cilievici et al., 1980). Furthermore, the transfer of AFB1 from layer feed to embryonic eggs caused retardation in chicken embryos development and inhibiting bone tissue growth, especially the tibia in chickens (Celik et al., 2000; Yin et al., 2017). In an in vitro experiment, chick embryos showed that the toxin halted mitosis and induced teratogenesis during early morphogenesis due to a decrease in cell proliferation (Joshi and Joshi 1981).
Methionine (Met) is restricted in vegetable protein sources and is necessary for feather growth and protein synthesis; it is still classified as the first limiting amino acid in poultry (NRC, 1994). Met has many physiological functions (Reda et al., 2020a,b), like giving the methyl group a vital methyl donor (Stipanuk, 2004). Similarly, like glutathione, Met is also well known to decrease oxidative stress and has antioxidant properties (Elnesr et al., 2019; Elwan et al., 2019). Met play a crucial role in biological processes including chemotherapy, detoxification, and anticancer as well as the effects on the congenital and immune system of poultry (NRC, 1994; Stipanuk, 2004; Elnesr et al., 2019; Elwan et al., 2019; Rehman et al., 2019). Met possesses significant functions in the body such as protein synthesis, a precursor to glutathione, reduction of reactive oxygen species (ROS), and DNA methylation reaction (Kidd, 2004). Due to the in ovo amino acid injection, several studies demonstrated an increase in protein synthesis rate within the prehatch growth period (Elnesr et al., 2019). It is further shown that prehatch embryos' development is impaired by the substances (carbohydrates, proteins, minerals…etc.) injected into the ova. Nevertheless, the effects of Met against AFB1 on embryotoxicity have yet to be published (Nazem et al., 2017). Our research hypothesis suggested that using Met in ovo injection may modulate harmful effects from AFB1. Therefore, the current study aimed to establish Met 's effectiveness in protecting broiler embryos from AFB1 toxicity by focusing on programmed cell death and cellular antioxidant status.
MATERIALS AND METHODS
The experimental procedures used in this investigation have been compiled along with the Chinese animal welfare guidelines and accepted by the Animal Care and Use Committee of the Zhejiang University Animal Science College (No. ZJU2013105002), Hangzhou, China.
Incubation Protocol
A commercial breeder flock in Hangzhou, Zhejiang, China, was used to obtain fertile broiler eggs (Ross 308). Eggs were moved to an incubator (440-egg size, ZF444 model, Zhengda Incubation Equipment Co. Ltd., Dezhou, China). Under optimum incubation temperature (37.8°C and 65 percent relative humidity), the eggs were incubated and turned automatically every 2 h, The eggs were then candled with a lamp after 10 d of incubation, and those containing dead embryos were removed from the incubator. The lived embryos (n = 180) were in ovo injected (in the amniotic cavity) and/or not injected with 5.90 mg L-methionine containing 36 ng AFB1/10 μL and/or 1.0 mL. At the end of the hatching period (d 21), each group of hatched chicks was subjected to taking blood and organs (liver, kidney, pectoral muscle, and duodenum) samples.
Experimental Designs
Pure aflatoxin B1 from Aspergillus flavus (AFB1 ≥ 98%, catalog no. A606874-0005, Sangon Biotech (Shanghai) Co., Ltd.) was diluted with 20% Methanol (20% methanol: 80 %saline 0.75% NaCl) to obtain final concentrations of 36 ng AFB1/L. A commercial enzyme-linked immunosorbent assay (ELISA) kit (Aflatoxin B1 (AFB1) ELISA kit, product code, MM-1911O1, Romer Labs, Union, CN) was used to evaluate level AFB1. A 99% purity L-methionine (C5H11NO2S) was purchased from Beijing Solarbio Sciences and Technology Co., Ltd. (Beijing, China). At d 10 of incubation (to avoiding early embryonic mortality), eggs (n = 180) 59.47 ± 4.78 were randomly distributed into 6 groups of 30 eggs/group using the AB204-N scale (METTLER TOLEDO equipment, Shanghai, Co., LTD, China), 0.0001 measurement accuracy. The first group was used as a control (free injected embryos), the second group was injected with only 1.0 mL of saline (0.75 % NaCl injected group), the third group was injected 20 µL/egg with a 20 % freshly prepared Methanol solution dissolved in 0.75 % NaCl saline solution (Methanol group), the fourth group was injected with a freshly prepared solution of 5.90 mg L-methionine dissolved in 1.0 mL saline solution (0.75% NaCl), the fifth group was injected with 20 µL/egg of 36 ng AFB1, the sixth group injected with 20 µL/egg of 36 ng AFB1 + 5.90 mg L-methionine dissolved in 1.0 mL saline solution. According to Ohta et al. (2001) and Bhanja et al. (2004), methionine concentration was measured as 2 percent of the methionine content (295 mg/egg) in the egg.
Embryonic Development Index
At the hatching date (d 21), the hatchability percentage and the chick body weight were recorded. The data of the dead embryos were excluded from each group. The mean relative weight of the hatched chicks and the relative weight of the residual yolk sac was expressed as a relative to the egg, while the tibia bone from each chick was cut, muscle and connective tissues cleaned, and weighed. The absolute tibia weight and tibia length were recorded; also, tibia weight was calculated as relative to chick weight. Also, the liver was dissected from each chick then weighed after dissecting the surrounded connective tissue. Then the relative liver weight was calculated.
Serum Biochemical Indicators and Antioxidant Biomarkers in the Tissues and Serum
Both serum biochemical indicators and antioxidant biomarkers in tissues (liver, kidney, pectoral muscle, and duodenum) and serum were evaluated using kits provided by the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Ten blood samples were collected randomly after slaughter and then centrifuged for 10 min (3,000 g) at room temperature, then separated serum was stored at −80°C in Eppendorf tubes (1.5 mL) until analysis. Serum total protein, albumin, glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), gamma-glutamyl transaminase (γGT), alkaline phosphate (AKP), urea nitrogen, uric acid, creatinine kinase, lipid profiles; the levels of triacylglycerides, total cholesterol, high-density lipoprotein (HDL-c), low-density lipoprotein (LDL-c) were determined. The levels of very-low-density lipoprotein (VLDL-c) was calculated according to Friedewald et al. (1972) equation by dividing the triglycerides by 5. Thyroid hormones in the serum triiodothyronine (T3), thyroxine (T4), thyroid-stimulating hormone (TSH) were detected by using ELISA kits.
The tissue laser-24 (Xin Jin Technology, Shanghai, China) was used at 65 Hz for 60 s, after diluted the tissue specimen ten times (0.1 g per mL) with isotonic physiological saline, and then centrifuged at 15294.24 × g at 4°C for 10 min; homogenated tissue samples (n = 10); then filtrated and stored at −80°C. Each specimen's total protein content was assessed using a total protein quantification kit (A045-2) after the homogenates centrifugation at 1295 × g at 4 ˚C. Total antioxidant capacity (TAOC), antioxidant oxidative biomarkers such as superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GSH-Px), catalase (CAT), and malondialdehyde (MDA) were determined. Dehydrogenase enzymes activities (lactate dehydrogenase (LDH), succinate dehydrogenase (SDH), glutamate dehydrogenase (GluDH) in the serum and tissues were determined.
Liver Histology Examination
The liver samples have been fixed in 4 percent paraformaldehyde and routinely processed in paraffin. Tissue was cut into thin sections (5 μm) and mounted on glass slides. Hematoxylin and eosin Y have been used to stained slides. A digital camera (NIKON, ECLIPSE 50I, JAPAN) was used to observe and photograph the tissues' histological structures.
TUNEL Assay
The apoptosis suggested fragmentation of DNA was computed using the terminal's deoxynucleotidyl transferase-mediated dUTP nick-end labeling method (TUNEL). TUNEL assays were performed using a detection kit (Cat. No. 11684817910, Roche Molecular Biochemicals, Germany), as described by Tayman et al. (2011). The slides were briefly rehydrated into a series of xylene and ethanol solutions and then incubated at room temperature for 20 min with proteinase K in a humidified chamber. The slices were then rinsed in saline (TBS) buffered with Tris. The whole specimens were coated with 3 percent H2O2 and then incubated at room temperature for 5 min, then rinsed slices in TBS. TUNEL enzyme and label solution were combined and added to slices in the moistened container that were again incubated at 37 °C for 1 h. The slices were thoroughly rinsed off with TBS and then added stop buffer, block buffer, and conjugate. Diaminobenzidine solution has been used to stain the apoptotic cell nuclei for 10 to 15 min. The hematoxylin was used to counteract the normal nuclei of the cells. Slides were dehydrated in 3 baths of ethanol and twice xylene baths for 5 min each. Apoptotic nuclei of the cells were either green (stained with fluorescein-dUTP FITC). The positive TUNEL cells (apoptotic cells) were counted using a computer-based imaging device connected with a light microscope (OLYMPUSAX70), with an objective X400 magnification. Then, Image-J image analysis software quantified the apoptotic cells. Five sections were measured and averaged in each category, and 5 fields in each section.
RNA Isolation and cDNA Synthesis
Total RNA was isolated from around 50 mg of tissue using TRIzol reagent kits (Invitrogen, Carlsbad, CA) and other reagents as instructed by the manufacturer. Nanodrop 2000 UV-Vis spectrophotometer (Thermo-fisher scientific, Wilmington, MA) was used to evaluate the purity of RNA at an optical ratio of OD260/OD280 in the range of 1.9 and 2.1. The cDNA synthesized using HIScriptIIQRT SuperMix by the reverse transcription protocols according to the qPCR manufacturer of (Vazyme, Nanjing, China).
Quantitative Real-Time PCR (qRT-PCR)
The mRNA gene expressions of SSTR5, TSH-β, Bcl-2, Caspases-3, Caspases-7, Caspases-9, P53, CYP1A1, CYP2H1, GSH-Px, GST-a, and SOD in newly hatched broiler chick liver tissues have been quantified by a quantitative real-time PCR system (ABI 7500, Applied Biosystems, Foster City, CA) according to the ChamQTM Universal SYBR qPCR Master Mix protocol (Vazume, Nanjing, China). Gene-specific primers of SSTR5, TSH-β, Bcl-2, Caspases-3, Caspases-7, Caspases-9, P53, CYP1A1, CYP2H1, GSH-Px, GST-a, and SOD and reference gene (β-actin) were described in Table 1. The PCR reaction was conducted as follows: 95 °C/30 sec, then 40 cycles of 95 °C/10 sec and 60 °C/30 sec, followed by 95 °C/15 sec, 60 °C/60 sec, and 95 °C/15 sec, respectively. There were 5 samples per group, each sample was done in duplicate, and there was no template control. Gene expression has been studied and the use of β-actin as control (Straus and Takemoto, 1991 and Kita et al., 2002). The primers series had been obtained from NCBI's GenBank. The primers were developed and synthesized by Sangon Biotech (Shanghai, China) (Table 2). The data from qRT-PCR were analyzed using a method of calculation 2-ΔΔCt defined by Livak and Schmittgen (2001).
Table 1.
Sequences of the nucleotide of specific primers used for real-time PCR.
| Gene symbol | Gene bank No. | Forward primers Sequence (5ʹ-3ʹ) | Reverse primers Sequence (5ʹ-3ʹ) | Amplicon length (bp) |
|---|---|---|---|---|
| β-actin | L08165 | ATGGCTCCGGTATGTG C AA | TGTCTTTCTGGCCCATACCAA | 178 |
| SSTR5 | XM_015294246.2 | GGTAGCGGTCCATGCTCATC | GCCACCCAGAATGCCATCT | 2432 |
| TSH-β | AF033495 | CCACCATCTGCGCTGGAT | GCCCGGAATCAGTGCTGTT | 474 |
| Bcl-2 | NM_205339 | CACCTGGATGACCGAGTACC | GTCCAAGATAAGCGCCAAGA | 205 |
| Bax | XM_422067 | TCCTCATCGCCATGCTCAT | CCTTGGTCTGGAAGCAGAAGA | 195 |
| Caspases-3 | NM_204725 | GAAGCAAGCAGTGGACCAGA | GTTCAAGTTTCCTGGCGTGT | 139 |
| Caspases-7 | XM_421764.3 | CATTTATGGCACCGATGGAC | CCGGTCCAGAGTCAGTTTGT | 2278 |
| Caspases-9 | AY057940 | AGATGAAACTTGCCGACGTT | CTTCAGAACGGGCGTAATGT | 87 |
| P53 | NM_205264.1 | TTACCACGACGACGAGACC | CCTCCAGTGTAAGGATGGTGA | 127 |
| CYP1A1 | X99454.1 | CACTTTCTGCCTGCTCCTG | GGTCCTTCCTCAGCTCCAG | 125 |
| CYP2H1 | M13454.1 | ATCCCCATCATTGGAAATGT | TCGTAGCCATACAGCACCAC | 137 |
| GSH-Px | NM001277853 | TTGTAAACATCAGGGGCAAA | ATGGGCCAAGATCTTTCTGTAA | 164 |
| GST-a | NM 205365.1 | GCCTGACTTCAGTCCTTGGT | CCACCGAATTGACTCCATCT | 131 |
| SOD | NM_205064 | AGGGGGTCATCCACTTCC | CCCATTTGTGTTGTCTCCAA | 122 |
β-actin = Reference gene; SSTR5 = somatostatin R5; TSH-β = thyroid stimulating hormone- β; Bcl-2 = B-cell lymphoma-2; Bax = Bcl-2-associated X protein; Caspases-3 = cysteine-aspartic acid protease-3; Caspases-7 = cysteine-aspartic acid protease-7; Caspases-9 = cysteine-aspartic acid protease-9; P53 = tumor protein p53; CYP1A1 = Cytochrome P450, family 1, subfamily A, polypeptide 1; CYP2H1 = Cytochrome P450, family 2, subfamily H, polypeptide 1; GSH-Px = Glutathione peroxidase; GST-a = Glutathione S transferase; SOD = Superoxide dismutase.
Table 2.
Total protein profile, enzymatic activities of γGT, AKP, GPT, GOT, urea nitrogen, uric acid creatinine kinase activity in serum of new hatched Ross broilers chicks exposed to AFB1and/or methionine during incubation.
| Treatments |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | Saline | Methanol | Met | AFB1 | AFB1+Met | SEM | P value |
| Total Protein (g/L) | 6.371ab | 6.151b | 6.211b | 6.475a | 4.907c | 5.769d | 0.1731 | 0.01 |
| Albumin (g/L) | 2.881b | 2.694b | 2.970b | 2.693b | 3.766a | 2.558b | 0.1446 | 0.01 |
| Globulin (g/L) | 3.490a | 3.457a | 3.240a | 3.781a | 1.141b | 3.211a | 0.4401 | 0.01 |
| Albumin/globulin ratio | 0.840bc | 0.799bc | 1.163b | 0.712c | 3.314a | 0.796bc | 0.1177 | 0.01 |
| γGT (IU/L) | 0.657bc | 0.620c | 0.677bc | 0.644bc | 1.512a | 0.757b | 0.0081 | 0.01 |
| AKP (IU/L) | 562.833d | 565.925d | 622.634c | 562.500d | 1600.010a | 855.568b | 11.1669 | 0.01 |
| GPT (IU/L) | 22.558e | 23.830d | 23.110de | 26.315c | 36.522a | 28.664b | 1.0293 | 0.01 |
| GOT (IU/L) | 2.738cd | 2.350e | 2.604d | 2.965c | 7.246a | 4.481b | 0.0795 | 0.01 |
| Urea nitrogen (µmol/L) | 1.300b | 1.323b | 1.267bc | 1.075c | 2.158a | 1.267bc | 0.0693 | 0.01 |
| Uric acid (µmol/L) | 1.853bc | 1.751bc | 1.863bc | 1.519c | 2.495a | 2.014b | 0.1402 | 0.01 |
| Creatine kinase (mmol/L) | 1.175b | 1.210b | 1.141bc | 0.950c | 2.033a | 1.356bc | 0.0658 | 0.01 |
a-eValues inside a row of different letters are substantially different at (P < 0.05).
Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1+Met = injected with 36 ng AFB1 + methionine (5.90 mg/L); γGT = Gamma-Glutamyl transferase; AKP = Alkaline Phosphatase; GPT = Glutamate-Pyruvate-Transaminase; GOT = Glutamic-Oxaloacetic-Transaminase.
Values are set to mean ± SEM (n = 10).
Statistical Analysis
Differences in statistics among measurements are considered significant at P = 0.05. After ANOVA, a post hoc study (Tukey- Kramer) was carried out. For all studies, JMP version 6.0 (SAS Institute, Cary, NC) was used. Data is viewed as a means, and SEM is pooled.
RESULTS
Effect of Met and/or AFB1 in Ovo Injection on Embryonic Mortality
Figure 1 demonstrates the mortality rate of embryos from all the treatment groups used in the current study. In control groups (free injected embryos, Saline, Methanol), Met, AFB1, and AFB1+Met mortalities ranged from 19.43 to 49.99%, with no developmental abnormalities observed. Embryo morphology has been examined; no abnormalities from any of the therapies have been found in developing embryos. The mortality rate in eggs injected with 36 ng AFB1 was 49.99%; however, significantly lower mortality of 27.77% when the eggs were exposed to 36 ng AFB1 + 5.90 mg/L Met. Met at 5.90 mg/L significantly reduced embryos mortality by 44.44% in the presence of 36 ng AFB1 (P < 0.05). Whereas the other controls recorded an average mortality rate (19.43–22.21%) with no significant differences among them.
Figure 1.
Effect of treatments on embryonic mortality. Control = free injected embryos; Saline = 0.75% NaCl-injected; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1 + Met = injected with 36 ng AFB1+ methionine (5.90 mg/L).
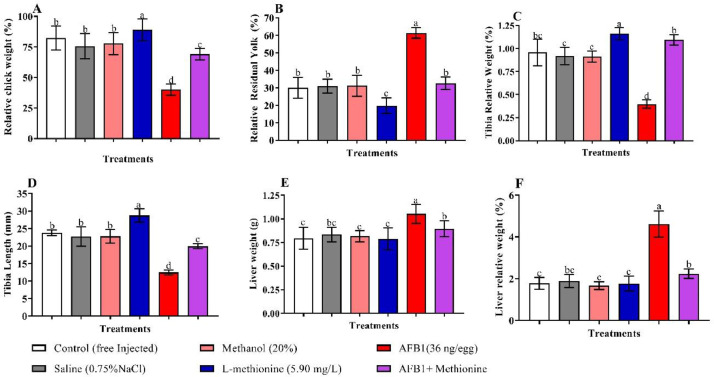
Effect of Met and/or AFB1 in Ovo Injection on Relative Chick Weight, Relative Yolk Sac Weight, Relative Tibia Weight, Tibia Length, Liver Weight, and Relative Liver Weight
Results in Figure 2 showed that eggs injected with the negative controls (saline and methanol) did not significantly affect the chick's relative weight and the relative weight of the yolk sac compared to the control. Eggs injected with 36 ng AFB1 displayed lower relative chick weight (P < 0.05) than controls. While substantial (P < 0.05) differences in relative chick weights were observed between group AFB1 and group AFB1 + Met, eggs injected with Met displayed higher relative chick weights in the presence of 36 ng AFB1 compared to those injected with 36 ng AFB1/egg alone (Figure 2A). The relative weight of the residual yolk sac of eggs injected with 36 ng AFB1/egg was found to be increased (P < 0.05) compared with the free injected group. Besides, the relative residual yolk sac weight of the eggs injected with 36 ng AFB1 decreased significantly by Met. However, negative controls (free injected group, saline, and methanol groups), showed no difference in relative yolk sac weights observed among eggs (Figure 2B). Figure 2 demonstrates the effects of Met and/or AFB1 injection on the tibia growth in embryos exposed to AFB1 on hatching day. Results showed that both the tibia's average relative weight (C), length (D) of the AFB1-injected embryos decreased (P < 0.05) compared with other groups.
Figure 2.
Relative chick weight (A), relative residual yolk sac (B), relative tibia weight (C), tibia length (D), liver weight (E), and liver relative weight (F) in the control and experimental groups. Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1+Met = injected with 36 ng AFB1 + methionine (5.90 mg/L).
Methionine alone increased the relative weight and length of the chick's tibia considerably compared with other groups. Nevertheless, injection of Met in the presence of 36 ng AFB1 increased (P < 0.05) the tibia weight substantially by 27.60 percent relative to embryos exposed to AFB1 alone. Besides, Met's injection in the presence of 36 ng AFB1 substantially increased the tibia length by 50.59 percent compared with the AFB1 group. There were usually no differences in the positive and negative control groups (saline, methanol). By comparison, in the AFB1 group, liver weight (E) and relative liver weight (F) increased markedly compared to other groups.
Effect of Met and/or AFB1 in Ovo Injection on Serum Total Protein Profiles, Liver Enzymes Activity, and Kidney Function
The effect of AFB1 on serum total protein profiles and liver enzyme activity was considered a clinical sign of liver injury. As shown in Table 2, serum albumin, albumin globulin ratio and enzymes activity of γGT, AKP, GPT, GOT, urea nitrogen, uric acid, and creatinine kinase were increased (P < 0.05) by 30.71%, 294.52%, 130.13%, 184.27%, 61.90%, 164.64 %, 66.00%, 34.64 % and 73.02 in AFB1 group compared with the control group respectively. Co-injection with Met (5.9 mg/L) plus AFB1 (36 ng AFB1/egg) alleviate AFB1-induced liver injury by significantly reducing the activity of γGT, AKP, GPT, GOT, levels of urea nitrogen, uric acid, and creatinine kinase activity by 49.93, 46.52, 21.51, 38.15, 41.28, 19.27 and 33.30 respectively, compared with AFB1-injected group alone. However, no significant difference in serum total protein profile, serum enzyme activity was observed among Saline, Methanol, Met, and control groups.
Effect of Met and/or AFB1 In Ovo Injection on Lipid Profiles and Glucose in the Serum
Lipid profiles (cholesterol, HDL-cholesterol, LDL-cholesterol, VLDL cholesterol, and triacylglycerols) and glucose in the serum of new hatched Ross broiler chicks exposed AFB1 during the incubation are shown in Table 3. Data illustrated that AFB1 in ovo injection leads to elevate (P < 0.05) bad cholesterol indices (LDL-cholesterol, VLDL-cholesterol) and triacylglycerols compared to other groups. However, the co-injection of Met enhances the harmful effects of AFB1 by decreasing bad cholesterol levels (LDL-cholesterol) and increasing good cholesterol (HDL-cholesterol) levels. Moreover, the in ovo injection of AFB1 leads to decreased glucose levels compared with other groups.
Table 3.
Lipid profiles and glucose in the serum of new hatched Ross broilers chicks exposed to AFB1and/or methionine during incubation.
| Treatments |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | Saline | Methanol | Met | AFB1 | AFB1+Met | SEM | P value |
| Cholesterol (mmol/L) | 13.276a | 12.789a | 12.703a | 11.344b | 9.653c | 10.344c | 0.2426 | 0.01 |
| HDL-cholesterol (mmol/L) | 4.709a | 4.132b | 4.834a | 4.959a | 2.007d | 3.175c | 0.0799 | 0.01 |
| LDL-cholesterol (mmol/L) | 8.214b | 8.493b | 7.864b | 6.540c | 9.849a | 7.920b | 0.2389 | 0.01 |
| VLDL-cholesterol(mmol/L) | 0.431c | 0.412c | 0.425c | 0.414c | 0.753a | 0.513b | 0.0198 | 0.01 |
| Triacylglycerols (mmol/L) | 2.158c | 2.062c | 2.129c | 2.072c | 3.769a | 2.569b | 0.0994 | 0.01 |
| Glucose (mg/dL) | 2.761a | 2.969a | 2.957a | 2.980a | 1.551c | 2.388b | 0.0877 | 0.01 |
a,bValues inside a row of different letters are substantially different at (P < 0.05).
Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1= injected with 36 ng AFB1; AFB1+Met = injected with 36 ng AFB1+ methionine (5.90 mg/L).
Values are set to mean ± SEM (n = 10).
Effect of Met and/or AFB1 in Ovo Injection on Thyroid Activity
The thyroid activity of new hatched Ross broiler chicks exposed to AFB1 during the incubation is shown in Table 4. The changes in serum concentrations of studied hormones (Т3 and Т4) showed that AFB1 in ovo injection leads to a markedly decrease in the thyroid gland activity by 44.60 and 41.74 % compared with the control group, respectively. Also, there were no significant differences among the control and NaCl, Methanol, and Met groups. However, there was no significant decrease (P > 0.05) in TSH levels between all groups.
Table 4.
Triiodothyronine, thyroxine and thyroid-stimulating hormone in serum of new hatched Ross broilers chicks exposed to AFB1and/or methionine during incubation.
| Treatments |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | Saline | Methanol | Met | AFB1 | AFB1+Met | SEM | P value |
| Triiodothyronine (ng/mL) | 1.065ab | 1.113a | 1.137a | 1.159a | 0.590c | 0.912b | 0.0544 | 0.01 |
| Thyroxine (ng/mL) | 57.853a | 57.877a | 57.874a | 57.879a | 33.705c | 42.549b | 0.6052 | 0.01 |
| TSH (ng/mL) | 0.676 | 0.691 | 0.689 | 0.693 | 0.584 | 0.656 | 0.0611 | 0.06 |
a,bValues inside a row of different letters are substantially different at (P < 0.05).
Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1 + Met = injected with 36 ng AFB1 + methionine (5.90 mg/L); TSH = Thyroid-stimulating hormone
Values are set to mean ± SEM (n = 10).
Effect of Met and/or AFB1 in Ovo Injection on Oxidative Stress Biomarkers
Data in Table 5 indicated that the in ovo injection of AFB1 decreased (P < 0.05) the enzymatic activities of oxidative stress biomarkers (SOD, GSH, GSH-Px activity, and Catalase) and total antioxidant capacity in tissues (liver, kidney, pectoral muscles, and duodenum) of newly hatched Ross broiler chicks. While MDA was increased with AFB1 in ovo injection. The in ovo injection of Met partially enhanced the adverse effects of AFB1 on oxidative stress biomarkers, by increasing (P < 0.05) the enzymatic activities of oxidative stress biomarkers (SOD, GSH, GSH-Px activity, and Catalase) and total antioxidant capacity in tested tissues and decreasing MDA.
Table 5.
Total antioxidant capacity, superoxide dismutase, glutathione, glutathione peroxidase, catalase, and malonaldehyde in tissues of new hatched Ross broilers chicks exposed to AFB1and/or Methionine during incubation.
| Treatments |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | Saline | Methanol | Met | AFB1 | AFB1+Met. | SEM | P value |
| Liver | ||||||||
| TAOC (U/mg prot.) | 226.067ab | 254.051b | 264.570ab | 273.531a | 112.992d | 211.972c | 3.2414 | 0.01 |
| SOD ((U/mg prot.) | 433.743a | 436.569a | 426.679a | 428.092a | 322.128c | 377.229b | 5.709 | 0.01 |
| GSH (µmol/g prot.) | 5.603a | 4.443b | 4.831b | 5.824a | 3.710c | 6.098a | 0.5069 | 0.01 |
| GSH-Px activity (U/mg prot.) | 406.112a | 399.655a | 398.947a | 408.250a | 263.448c | 302.214b | 5.4911 | 0.04 |
| Catalase (U/mg prot.) | 49.832a | 43.387a | 47.450a | 47.428a | 20.153b | 41.992a | 4.4343 | 0.01 |
| MDA (nmol/mg prot.) | 4.203c | 3.499c | 3.006c | 3.601c | 10.055a | 6.638b | 1.5086 | 0.03 |
| Kidney | ||||||||
| TAOC (U/mg prot.) | 107.932ab | 107.083b | 111.516a | 102.649c | 47.649e | 89.346d | 1.3662 | 0.01 |
| SOD ((U/mg prot.) | 182.822a | 184.014a | 179.845a | 180.440a | 85.772c | 159.002b | 2.4067 | 0.01 |
| GSH (µmol/g prot.) | 2.361a | 1.873b | 1.815b | 2.455a | 1.056c | 2.570a | 0.2431 | 0.01 |
| GSH-Px activity (U/mg prot) | 171.176a | 165.082ab | 163.941ab | 180.886a | 101.043 | 132.486b | 5.0251 | 0.04 |
| Catalase (U/mg prot.) | 21.004a | 19.288a | 20.006a | 19.991a | 8.494b | 21.492a | 1.8690 | 0.01 |
| MDA (nmol/mg prot.) | 1.771c | 1.475c | 1.267c | 1.518c | 4.238a | 2.484b | 0.6359 | 0.03 |
| Pectoral muscles | ||||||||
| TAOC (U/mg prot.) | 169.439b | 168.105b | 165.066b | 191.145a | 74.766d | 140.265c | 2.1444 | 0.01 |
| SOD ((U/mg prot.) | 287.008ab | 288.878ab | 282.333b | 293.268a | 113.152d | 249.613c | 3.7782 | 0.01 |
| GSH (µmol/g prot.) | 3.707a | 2.940b | 2.535b | 3.854a | 2.455c | 3.035ab | 0.3818 | 0.01 |
| GSH-Px activity (U/mg prot) | 268.724b | 259.158ab | 257.366ab | 281.173a | 174.324d | 218.665c | 3.1864 | 0.04 |
| Catalase (U/mg prot.) | 33.974a | 32.709a | 31.398a | 33.383a | 13.335b | 28.709a | 2.934 | 0.01 |
| MDA (nmol/mg prot.) | 2.781bc | 2.315c | 1.989c | 2.383c | 6.653a | 3.039b | 0.9982 | 0.03 |
| Duodenum | ||||||||
| TAOC (U/mg prot.) | 96.410b | 95.651c | 91.691d | 99.612a | 42.542e | 79.808d | 1.220 | 0.01 |
| SOD ((U/mg prot.) | 163.306b | 164.370b | 160.646b | 169.179a | 121.283d | 142.028c | 2.149 | 0.01 |
| GSH (µmol/g prot.) | 2.309a | 1.973b | 1.942b | 2.193a | 1.396c | 2.296a | 0.2172 | 0.01 |
| GSH-Px activity (U/mg prot) | 152.903a | 147.460a | 146.440a | 152.843a | 99.189c | 125.817b | 5.9528 | 0.02 |
| Catalase (U/mg prot.) | 18.762a | 16.335a | 17.865a | 17.857a | 7.587c | 15.198b | 1.669 | 0.01 |
| MDA (nmol/mg prot.) | 1.582c | 1.317c | 1.131c | 1.355c | 3.786a | 2.005b | 0.5680 | 0.03 |
a,bValues inside a row of different letters are substantially different at (P < 0.05).
Control = free injected embryos Saline = injected with 0.75% NaCl; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1 + Met = injected with 36 ng AFB1+ methionine (5.90 mg/L); TAOC = Total antioxidant capacity; SOD = superoxide dismutase; GSH = glutathione; GSH-Px = glutathione peroxidase; MDA = malonaldehyde.
Values are set to mean ± SEM (n = 10).
Effect of Met and/or AFB1 in Ovo Injection on LDH, SDH, and GluDH in Serum and Tissues
Data in Table 6 revealed that the in ovo injection of AFB1 increased the activity of LDH, SDH, and decreased the activity of GluDH compared with other groups either in the serum or tissues. However, the in ovo injection of Met plus AFB1 led to recovering dehydrogenase enzymes' activity as partially to be nearest to control.
Table 6.
Dehydrogenase enzymes activities (LDH, SDH, and GluDH) in the serum and tissues of new hatched Ross broilers chicks exposed to AFB1and/or methionine during incubation.
| Treatments |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | Saline | Methanol | Met | AFB1 | AFB1+Met | SEM | P value |
| Serum | ||||||||
| LDH (U/mL) | 844.94e | 924.50d | 943.02d | 898.95c | 1425.89a | 1196.66b | 13.713 | 0.01 |
| SDH (U/ mL) | 23.916d | 23.833d | 28.750c | 23.500d | 79.500a | 45.750b | 4.3486 | 0.01 |
| GluDH (U/ mL) | 33.250ab | 35.166ab | 39.916a | 30.083b | 24.583c | 31.916ab | 1.3626 | 0.01 |
| Liver | ||||||||
| LDH (U/mg prot.) | 633.71d | 693.37c | 707.27c | 761.15c | 1069.42a | 897.49b | 8.618 | 0.01 |
| SDH (U/mg prot.) | 11.479d | 11.832d | 14.835c | 13.245cd | 42.915a | 23.842b | 0.5676 | 0.01 |
| GluDH (U/mg prot.) | 180.138a | 162.477ab | 167.775ab | 171.307ab | 118.325c | 157.179b | 6.3266 | 0.01 |
| Kidney | ||||||||
| LDH (U/mg prot.) | 538.65d | 589.37d | 601.18c | 646.98c | 909.01a | 762.87b | 7.326 | 0.01 |
| SDH (U/mg prot.) | 4.838d | 4.987d | 6.253c | 5.583cd | 18.088a | 10.049b | 0.2392 | 0.01 |
| GluDH (U/mg prot.) | 75.928a | 68.484ab | 70.717ab | 72.206ab | 49.874c | 66.251b | 2.6666 | 0.01 |
| Pectoral muscles | ||||||||
| LDH (U/mg prot.) | 972.90e | 1064.503d | 1085.83d | 1168.56c | 1641.83a | 1377.88b | 13.232 | 0.01 |
| SDH (U/mg prot.) | 7.596d | 7.829d | 9.816c | 8.764cd | 28.396a | 15.776b | 0.3756 | 0.01 |
| GluDH (U/mg prot.) | 119.197a | 107.511ab | 111.017ab | 113.354ab | 78.296c | 104.005b | 4.186 | 0.01 |
| Duodenum | ||||||||
| LDH (U/mg prot.) | 362.59e | 396.73d | 404.68cd | 435.51c | 611.89a | 513.52b | 4.931 | 0.01 |
| SDH (U/mg prot.) | 4.322d | 4.455d | 5.585c | 4.986cd | 16.157a | 8.976b | 0.2137 | 0.01 |
| GluDH (U/mg prot.) | 67.823a | 61.173ab | 63.168ab | 64.498ab | 44.550c | 59.178b | 2.3820 | 0.01 |
a-eValues inside a row of different letters are substantially different at (P < 0.05).
Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol= injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1 + Met = injected with 36 ng AFB1 + methionine (5.90 mg/L); LDH = Lactate dehydrogenase; SDH = Succinate dehydrogenase; GluDH = Glutamate dehydrogenase.
Values are set to mean ± SEM (n = 10).
Effect of Met and/or AFB1 in Ovo Injection on SSTR5, and TSHB Relative mRNA Expression
Following the control group, in ovo injection of AFB1 significantly reduced liver mRNA expression of SSTR5 and TSHB (P < 0.05) on the day of the hatch (Figure 3). Interestingly, there was significant down-regulation of AFB1, showing that AFB1 has adverse modulatory effects on SSTR5 and TSHB (not significant) genes. However, in ovo injection of Met plus AFB1, upregulating the mRNA expression of these genes compared to AFB1 alone. Moreover, there were no significant differences among the control group, NaCl, and Methanol groups. However, the in ovo injection of Met alone led to the upregulation of SSTR5 and TSHB compared with other groups.
Figure 3.
SSTR5, and TSHB mRNA expression on the day of hatch detected by quantitative real-time PCR. Data are set to mean ± SD (n = 6). a, d Values with different letters differ significantly (P < 0.05) in relative expression levels of RNAs. Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol= injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1= injected with 36 ng AFB1; AFB1+Met=injected with 36 ng AFB1+ methionine (5.90 mg/L).
Effect of Met and/or AFB1 in Ovo Injection on GSH-px, GST-a, and SOD Relative mRNA Expression
The mRNA expressions of GSH-px, GST-a, and SOD in the AFB1 group on hatch day were significantly reduced compared to the control group (P < 0.05). What's more, the mRNA contents of GSH-px, GST-a, and SOD in Met either alone or plus AFB1 was higher (P = 0.01) than those in the control group (Figure 4).
Figure 4.
The relative expression level of GSH-px, GST-a, and SOD at the day of the hatch was detected by quantitative real-time PCR. Data are set to mean ± SD (n = 6). a,dValues with different letters differ significantly (P < 0.05) in relative expression levels of mRNAs. Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol= injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1+Met = injected with 36 ng AFB1+ methionine (5.90 mg/L).
Effect of Met and/or AFB1 in Ovo Injection on Bax, CASPASE-3, CASPASE-7, CASPASE-9, P53, CYP1A1, and CYP2H1 Relative mRNA Expression
The qRT-PCR study showed expressiveness levels of Bax, CASPASE-3, CASPASE-7, CASPASE-9, P53, CYP1A1, and CYP2H1 in the AFB1 group's liver were increased (P < 0.01), but BCl decreased significantly (P < 0.05) relative to those of the other groups. However, the mRNA contents of Bax, CASPASE-3, CASPASE-7, CASPASE-9, P53, CYP1A1, and CYP2H1 in Met either alone or plus AFB1 was lower (P < 0.05) than those in the control group (Figure 5). Also, BCl had the opposite direction.
Figure 5.
Effect of treatments on mRNA levels of mitochondrial apoptosis-associated genes (Bcl-2, Bax, CASPASE-3, CASPASE-7, CASPASE-9, P53, CYP1A1, and CYP2H1) liver mRNAs. All data were set as mean ± SD (n = 6). Columns with different letters (a–d) indicate a significant difference at (P < 0.05). Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol= injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1= injected with 36 ng AFB1; AFB1+Met=injected with 36 ng AFB1+ methionine (5.90 mg/L).
Effect of Met and/or AFB1 In Ovo Injection on Liver Histology and Apoptotic Percentage
Chicks liver sections from the control (Figure 6, 1A), NaCl (1B), Methanol (1C), Met (1D), AFB1 (1E), and AFB1+Met (1F) groups were stained with hematoxylin and eosin, examined by light microscopy. The initial magnification was projected at 400. AFB1 injected group (1E) showed an increase in intracytoplasmic vacuoles, pyknotic nucleus, and fibroblast cells. While the other groups generally showed normal hepatocytes compared with AFB1 group. The TUNEL assay demonstrated that the nuclei of TUNEL-positive cells were stained fluorescent green in all groups (Figure 5, 2A to 2F) with a different rate. More TUNEL-positive cells were detected in the AFB1 group than the control group (Figure 6, 2E). Furthermore, microscopic quantitative analysis revealed that the TUNEL-positive cells in the AFB1 group were significantly increased by 48% compared to the control group (Figure 6, 3). However, the in ovo injection of Met +AFB1 recorded the lowest TUNEL-positive cells to be 24.33%, with more than 50.68% better than the AFB1 group.
Figure 6.
Effect of Met and/or AFB1 in ovo injection on liver histology (1A, B, C, D, E, and F) and apoptotic percentage (2A, B, C, D, E, F, and 3) at the day of hatch. All data were set as mean ± SD (n = 6). Columns with different letters (a–c) indicate a significant difference at (P < 0.05). Control = free injected embryos; Saline = injected with 0.75% NaCl; Methanol = injected with 20 µL Methanol 20%; Met = injected with methionine (5.90 mg/L); AFB1 = injected with 36 ng AFB1; AFB1+Met = injected with 36 ng AFB1+ methionine (5.90 mg/L).
DISCUSSION
Aflatoxins also contaminate the chicken feed ingredients, causing birds to have aflatoxicosis, resulting in lower growth and increased susceptibility to infectious diseases. Moving AFs from hens to eggs, therefore, not only presents a public health threat, but residual AFs will adversely affect the viability and hatchability of embryos and potentially lead to organ dysfunction (Sur et al., 2011). AFs concentrations ranging from 5 to 100 μg/kg in broiler feed have been detected in different countries (Nizamlioglu, 1996; Pitet, 1998). Concentrations frequently build up below 50 μg/kg, but AFB1 is present at relatively low levels. 0.2–30.4 μg/kg AFB1 was found in poultry feed (Ozpinar et al., 1988). An egg can contain 1.3 ng AFB1 with a carry-over ratio of 1/2000 and an AFB1 sum of 10 μg/kg, as the hen consumes 130 g of the food every day and is laid every other day. Nevertheless, the egg's AFB1 content will increase to 2.15 ng with a 20 μg AFs/kg limit, since natural AFs consist of 83.06 percent AFB1, 12.98 percent AFB2, 2.84 percent AFG1, and 1.12 percent AFG2 (Oguz, 1997). Jelinek et al. (1985) used the chick embryotoxicity screening test-I (CHEST-I) to establish the embryotoxicity limits for AFB1 as 0.3–30 ng/egg and the teratogenicity limits as 3–30 ng/egg. Relatively small doses of 36 ng/egg have been used in the present analysis, although the limits are sometimes exceeded. Besides, previous researchers published various findings for concentrations of AFB1 in chicken eggs (Sur et al., 2011; Yin et al., 2017). Efficient methods for shielding fertilized eggs from aflatoxicosis are, therefore, important for the poultry industry's sustainability. Jacobson and Wiseman (1974) found 44 ng AFB1 in the eggs when the laying hens received 400 μg/kg of 9 d AFB1 dietary. Previous experiments using a chicken embryo model with in ovo AFB1 injections reported adverse effects on the development of chicken embryos in the presence of 10 to 100 ng of AFB1/egg (Celik et al., 2000; Oznurlu et al., 2012) in order to investigate the embryotoxicity of AFB1 to chickens further. Therefore, we investigated the effectiveness of Met in protecting chicken embryos from AFB1 toxicity at 36 ng AFB1/egg in the present test. Aflatoxin B1 is known to cause RNA and DNA synthesis inhibition, thereby reducing protein synthesis, which ultimately decreases growth (Khlangwiset et al., 2011). Our findings showed that the AFB1 dose caused substantial embryonic mortality. Also, the existence of Met substantially decreased the mortality rate when embryos were exposed to AFB1. The residual egg yolk is the main energy source for the developing embryo, which by oxidizing yolk lipids, provides more than 90 percent of the total energy needs of the embryo (R´ehault-Godbert et al., 2014). Besides, the yolk sac and yolk content is essential to promote embryo development in the embryo-genesis phase (Yalcin et al., 2008). Our study observed a substantial increase in relative yolk sac weight in eggs injected with 36 ng AFB1 compared with other groups, indicating a reduction in the development of embryos in AFB1-treated eggs, which reported a major reduction in the relative embryo weights in AFB1-injected eggs. Aflatoxin B1 has also been reported to inhibit the development and growth of bone tissue in chickens, thereby retarding the skeleton system's development, particularly tibia (Huff et al., 1980). In the present sample, as opposed to the other groups, 36 ng of AFB1/egg significantly reduced relative tibia weight and tibia length. In ovo injection of Met in the presence of 36 ng AFB1/egg, the tibia length was substantially improved compared to embryos injected with AFB1 alone. Chaudhry (1996) noted that the length of the femur and tibia and the femur's weight, tibia, radius, and ulna were significantly lower in birds continuously fed 5 mg/g AFs in feed than birds not receiving AFs for 6 wk. Khan et al. (2014) determined that significant mortalities, embryonic malformations, and hatchery of chicks with a defiant immune system result from embryo administration of AFB1 in ovo. Reduced body weight is one of the key effects of aflatoxicosis on livestock, directly affecting poultry industry productivity. As predicted, egg injection with AFB1 significantly reduced the relative weight of the embryo compared with controls. Nevertheless, following exposure to AFB1, Met increased the relative embryo weight, indicating the possible protective effect of Met to AFB1-injected embryos. Aydin et al. (2005) reported a dose-dependent decrease in hatching weights for aflatoxin Bl administered via in ovo. Oznurlu et al. (2012) reported that in ovo administered AFB1 adversely affected the embryonic development and growth of bone tissue resulting in delays in the skeletal system's development, with more pronounced effects in the tibia. Our findings also showed that the detrimental effects of AFB1 on the development of embryos decreased with Met addition. Likewise, the introduction of Met (0.8 percent) as an aflatoxin binder reduced the toxicity of AFB1 in broilers due to glutathione development (Yunianta et al., 2010). Marietto‐Gonçalves et al. (2017) also reported that the use of Met in rabbits could effectively treat the liver toxicity caused by AFs.
Methionine interaction with the AFB1 reduces the detrimental effects of AFB1. Besides, Blachier et al. (2013) stated that Met catabolism metabolites, such as taurine and glutathione, may have an antioxidant effect on the immune system's functioning. The mechanism(s) of the chicken embryo toxicity triggered by the protective effects of Met to AFB1 has not yet been identified. Nevertheless, the results from this study showed that Met significantly reduced the mortality rate of embryos treated with 36 ng AFB1/egg compared to the untreated dose and significantly increased Met's protective effect. While the mechanisms behind these Met enhanced protective effects against AFB1-induced mortality in AFB1-exposed chicken embryos are not clear, it may be that AFB1 36 ng/egg caused significantly higher mortality; also, the Met protective effect was better by decreasing mortality levels compared to AFB1 alone.
The liver is the primary organ for aflatoxin's toxic effect (Kubena et al., 1990). Impaired conversion of proteins, carbohydrates, amino acids, lipids, nucleic acids, and enzymes disturbs liver metabolism (Ellis et al., 1991). Increasing production of liver enzymes such as GPT, GOT, AKP, γGT, and LDH is used to assess the extent of aflatoxicosis in poultry, ducklings, and turkey poults (Cheng et al., 2000; Quist et al., 2000 and Yildirim et al., 2011) demonstrates the toxic effects of AFs. Aflatoxins are known to minimize protein production that can contribute to lower protein levels in the blood. Declining TP levels result in decreased efficiency of the immune system because the critical mechanisms of some immune responses are the production of factors that kill pathogens, such as antimicrobial peptides and proteins (Büchau and Gallo, 2007). It has been stated that the levels of total protein, cholesterol, triglyceride, and glucose decreased significantly by the AFs intoxications (Donmez and Keskin, 2008).
Aflatoxins are liposoluble compounds that are readily absorbed into the bloodstream via the liver at the exposure site (usually the gastrointestinal tract) where they are metabolized in the microsomal system to active or detoxified metabolites (Haschek et al., 2002). It is assumed that AFs change lipid synthesis, absorption, and transmission into extra-hepatic tissues. The composition of hepatic fatty acids in birds with aflatoxicosis is significantly altered (Agag, 2004). AFB1-8, 9- epoxy (formed by cytochrome P450 on AFB1), will significantly increase hepatic lipid peroxide rates lipid peroxidation starts negatively affecting the integrity of the membrane, the functioning of the membrane-bound enzyme, which leads to cell lysis. Oxidative damage to cells/tissues occurs when ROS concentration (O2, H2O2, and OH) predominates cells' ability to antioxidants. This may result from a substantial decrease of nonenzymatic antioxidants (e.g., vitamin E, and vitamin C) and enzymatic antioxidants (e.g., catalase, GSH-Px, SOD). Decreased protein biosynthesis can be responsible for reducing enzyme activity. The toxic effects of AFs are further compounded by considerably lower rates of GSH-Px (Verma, 2004). AFs promote free radical production leading to liver peroxidation that, in turn, leads to antioxidant degradation, oxidative stress, and apoptosis. They are all related to malabsorption evolution (Surai, 2002). The findings from the present study showed that AFB1+Met had significantly lower levels in the serum lipid profile. Kalinowski et al. (2003) clarified that Met as a donor to the methyl group plays a vital role in lipid metabolism and acts as a lipotropic agent. The drop in triglyceride levels may be attributed to higher hormone-sensitive lipase levels in adipose tissue (Zhan et al., 2006). Besides, Jariyahatthakij et al. (2018) pointed out that adding Met affects the depression of the synthesis of fatty acids.
Hormones in the thyroid gland are necessary to maintain the systemic physiological equilibrium of living beings (Bozakova and Popova-Ralcheva, 2007). The decreased rate of secretion of these hormones directly impacts organisms' general state (Rose, 2000). Our findings showed that AFB1 affects concentrations of serum T3 and T4 by decreasing these concentrations. Such changes were nevertheless directly related to TSH, as seen from the slight increases in TSH levels in all groups treated with AFB1 instead of controls. The modifications in T3 and T4 contribute to alterations in TSH, also indirectly. The lack of substantial blood TSH levels observed in this study can probably be due to the lower sensitivity of aflatoxin to the thyroid receptor (Graczyk et al., 2002; Eraslan et al., 2006). Aflatoxins are reported to induce the peroxidation of lipids in cells (Rastogi et al., 2001). The damage to thyroid receptors was possibly due to the enhanced generation of ROS produced by aflatoxins, which caused lipid peroxidation. Lower levels of the thyroid hormone suggest metabolic abnormalities develop. Thyroid hormone concentrations in the blood was reported to have an important regulatory role in growth, energy utilization, and several vital functions in chickens (Carew et al., 1999).
Aflatoxin B1 increases free radical production, leading to oxidative damage and lipid peroxidation that could eventually lead to cell damage and death (Surai, 2002). Eraslan et al. (2006) researched the effect of AFs on oxidative stress and observed decreased antioxidant activity relative to controls in chicks-fed AFs erythrocytes. The effects of AFs on antioxidant capacity, in particular AFB1, represent a major animal health issue. Aflatoxins increased the amount of MDA in chickens and decreased antioxidant enzymes (Assar et al., 2018). Increased ROS production after AFB1 toxicity may result from the biotransformation of AFB1 into a highly reactive intermediate metabolite-AFB1 8, 9‐epoxide, and free radicals cause oxidative damage (Shen et al., 1995). Alternatively, ROS can interfere with the cell membrane and cause its lipid peroxidation by allowing a gradual accumulation of lipid hydroperoxides in the plasma membrane, which is then decomposed to produce MDA under toxic or stressful conditions (Kandeil and Abu El-Saad, 2005). Such effects minimize the tissue's ability to scavenge the generated free radicals.
The primary detoxification route for AFB1 is enzymes that conjugate AFBO with GSH by GST. Cellular GSH is a crucial regulator for various biological processes, including DNA and protein synthesis, influencing cell growth and proliferation, apoptosis, immunity, transport of amino acids, xenobiotics and endogenous oxidizing metabolism/detoxification, redox sensitivity signal transduction, etc. (M´etayer et al., 2008; Del Vesco et al., 2015a). We hypothesize that Met injection with in ovo may have mechanisms for detoxification. Most studies are involved in ovo injection manipulations under standard incubation conditions, but not in AFB1 contamination, so the present study is designed to take advantage of the Met in the ovo injection method to reduce the negative effect of AFB1 on embryo production. Methionine serves as a precursor to GSH synthesis, which helps protect against oxidative stress (Del Vesco et al., 2015b). In the current research, AFB1 mediated embryotoxicity during incubation with Met injection (AFB1+Met) showed significantly lower MDA concentrations and higher levels of SOD, GSH-Px, and CAT GSH activity against lipid peroxidation in embryonic chicken tissues. The increases in enzyme activity (SOD, GSH-Px, and CAT) improve broiler chickens' antioxidant protection system. Dietary Met material mediated GSH-Px, GST-a, and SOD mRNA expression in the chicks exposed to AFB1 to regulate the antioxidant system to prevent increased ROS production (M´etayer et al., 2008). Met levels in broiler diets under contamination with AFB1 were also beneficial, and any damage to the embryos was recovered (Shen et al., 1995). Nemeth et al. (2004) reported that Met plays a vital role in promoting the chicks' synthesis of liver GSH-Px activity. Wen et al. (2017) also indicated that higher dietary Met rates would increase the breast muscle's antioxidant status by increasing GSH-Px and SOD activity.
Hepatic detoxification's primary processes include xenobiotic biotransformation (phase I metabolism) and subsequent conjugation of the resulting metabolites (phase II metabolism), rendering them more water-soluble and available for body excretion. Phase I metabolism is primarily comprised of the cytochrome enzymes P450 (CYP). Cytochrome P450 enzymes are related to several biological interactions, including hydroxylation, epoxidation, dehydrogenation, nitrogen dealkylation, and oxidative deamination (Kumar et al., 2006). While CYP-mediated reactions are essential to detoxify xenobiotics, they may also be producing ROS. The microsomal CYP-dependent mono-oxygenase system in the liver plays an integral part in xenobiotics metabolism (Akahori et al., 2005). CYP1A1 is known to metabolize different drugs and xenobiotics and cause these pro-mutagens in their carcinogenic forms (Hamilton et al., 1993). Equally, it is understood that CYP 2H1 is actively involved in xenobiotic metabolism (Klein et al., 2003). These CYP isoforms are involved in the biotransformation of AFB1 into AF-8, 9-epoxide, a highly toxic and carcinogenic poultry metabolite (Tiemersma et al., 2001). Aflatoxin-8, 9- epoxy, is detoxified by epoxy hydrolase (Tiemersma et al., 2001) and GST (Klein et al., 2003) for the enzymes. Due to the downregulation of CYP genes and the upregulation of epoxy hydrolase and GST-a genes in embryos injected with 36 ng AFB1 + 5.9 Met/egg, the risk of AF-8, 9-epoxide formation, and a greater risk of AFB1 detoxification may be decreased. Furthermore, over-expression of these CYP450 isoforms has been shown to cause chronic oxidative stress by generating more ROS, possibly leading to hepatocellular injury and death (Lee et al., 1999). Transcription activation of CYP1A1 and CYP2H1 isoforms as a response to AFB1 has the potential to increase oxidative stress from the results of this study. Besides, antioxidant genes such as GST-a and GSH-Px were de-regulated in embryos injected with 36 ng AFB1/egg, which could protect against oxidative stress, and this could further impede the ability of the bird to protect itself from oxidative damage. Such factors may all lead to AFB1′s toxicological and pathological effects.
P53 genes are a ROS sensor and play a role in redox regulation (Brahmi et al., 2011). The p53 is shown to be involved in embryonic growth and energetic metabolism. P53 structure can be redox-modified either directly or indirectly by redox-driven induction of kinase activity. In addition to this indirect antioxidant action, p53 stimulates the transcription of GSH-Px1, MnSOD (encoded by the SOD2 gene), and catalase antioxidant enzymes directly. As such, p53 is endowed with potent antioxidant activity like a cell survival drug.
These results are strongly associated with the resulting histopathological changes in the liver of exposed embryos induced by AFB1 and come online with the resulting oxidative stress effects of AFB1, where the antioxidant status was associated with immunosuppressive and anti-inflammatory properties (Lee et al., 1999). Apoptosis is the programmed cycle of decay and death that seeks to kill damaged, senescent, and harmful cells in the body; however, it can also occur as a reaction to various environmental stimuli, including toxicity. The available evidence indicates that AFB1 functions as a direct or indirect initiator and promoter of the apoptotic process (Deng et al., 2010). For example, AFB1 induced apoptosis of the hepatocytes (Wang et al., 2013), thymocytes (Kumar et al., 2006), splenocytes (Yang et al., 2012), bronchial epithelial cells (Peng et al., 2014), jejunal mucosal cells (Yuan et al., 2014), and Fabricius bursa (Yin et al., 2016). Early studies showed that 0.3 mg/kg of AFB1 in chickens’ diet-induced alterations in the expressions Bax, BCL-2, and CASPASE-3 mRNA was involved in apoptosis-related mitochondrial pathways in chickens' jejunum (Zheng et al., 2017). TUNEL assay can recognize DNA fragmentation and examine the topographical distribution of apoptotic cells. The quantitative microscopic analyses used to assess apoptotic concentrations under the microscope are the measurement of the positive reaction number and the combined optical strength of the TUNEL. Our current findings showed that AFB1 caused hepatic histopathological injury triggered excessive apoptosis based on TUNEL, in line with Yuan et al. (2014). Such experiments have demonstrated increased apoptosis caused by the AFB1. Apoptosis is a highly regulated cell death mechanism caused by mitochondria and death receptors. An early study showed that mitochondrial pathways, such as the BCL-2 and Bax genes, were associated with excessive apoptosis triggered by AFB1 (Waring and Müllbacher, 1999). Death receptor activation, which includes TNF-α, TNF-R1, and CASPASES (Peng et al., 2016), induces the death receptors' cascade. The downstream activation of CASPASES, including CASPASE-3, leads to cell death (Waring and Müllbacher, 1999); this study showed that AFB1 generally induced overexpression CASPASE-3, CASPASE-7, and CASPASE-9 mRNAs in the liver, suggesting that death receptor molecules involving excessive hepatic apoptosis. Similar results have also been observed for hepatocyte and chicken thymocyte apoptosis caused by AFB1 (Peng et al., 2016).
CONCLUSIONS
Our results indicated that AFB1 exercised broiler chicks embryotoxicity, as it caused oxidative stress and apoptosis. Also, the findings of histology showed that Met alleviated the embryotoxicity caused by AFB1. Therefore, Met prevented oxidative stress caused by AFB1 by reducing the ability of the antioxidant enzymes. Notably, in the liver of broiler new hatched chicks, Met attenuates excessive apoptosis caused by AFB1 through the mitochondrial-mediated apoptosis pathway. The current research results will provide valuable insight into the 5.9 mg/L of Met injection of in ovo as a therapeutic agent against embryotoxicity caused by AFB1.
ACKNOWLEDGMENTS
The authors extend their appreciation to the researchers supporting project number (RSP-2020/120) King Saud University, Riyadh, Saudi Arabia, and the earmarked fund supported the study for Modern Argo-Industry Technology Research System of China (No. CARS-40-K10) and Zhejiang Provincial Key Research and Development Program (2019C02051).
DISCLOSURES
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
REFERENCES
- Abdallah M.F., Girgin G., Baydar T. Occurrence, prevention and limitation of mycotoxins in feeds. Anim. Nutr. Technol. 2015;15:471–490. [Google Scholar]
- Agag B.I. Mycotoxins in foods and feeds. 1-Aflatoxins. Assiut. Univ. Bull. Environ. Res. 2004;7:173–206. [Google Scholar]
- Akahori M., Takatori A., Kawamura S., Itagaki S., Yoshikawa Y. No regional differences of cytochrome P450 expression in the liver of cynomolgus monkeys (Macaca fascicularis) Exp. Anim. 2005;54:131–136. doi: 10.1538/expanim.54.131. [DOI] [PubMed] [Google Scholar]
- Assar M.H., Attia K.M., Eid Y. Evaluation of the ability of a feed additive to ameliorate the adverse effects of aflatoxins in broiler chickens. Alex. J. Vet. Sci. 2018;56:1–18. [Google Scholar]
- Aydin M.F., Celik I., Sur E., Ozparlak H., Telatar T. Effects of In Ovo given aflatoxin Bı on the chick hatching weight. S.Ü. Veteriner Bilimleri. Dergisi. 2005;21:85–89. [Google Scholar]
- Bhanja S.K., Mandal A.B., Johri T.S. Standardization of injection site, needle length, embryonic age and concentration of amino acids for In Ovo injection in broiler breeder eggs. Indian J. Poult. Sci. 2004;39:105–111. [Google Scholar]
- Blachier F., Wu G., Yin Y. Springer; Vienna: 2013. Nutritional and Physiological Functions of Amino Acids in Pigs. [Google Scholar]
- Bozakova N., Popova-Ralcheva. S. Thyroid hormones level and relative liver weight in male turkeys in relationship with their welfare. Biotechnol. Anim. Husb. 2007;23:1511–1518. [Google Scholar]
- Brahmi D., Bouaziz C., Ayed Y., Mansour H.B., Zourgui L., Bacha H. Chemopreventive effect of cactus Opuntia ficusindica on oxidative stress and genotoxicity of aflatoxin B1. Nutr. Metab. 2011;8:73. doi: 10.1186/1743-7075-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchau A.S., Gallo. R.L. Innate immunity and antimicrobial defense systems in psoriasis. Clin. Dermatol. 2007;25:616–624. doi: 10.1016/j.clindermatol.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew L.B., Evarts K.G., Alster F.A. Growth, feed intake, and plasma thyroid hormone levels in chicks fed dietary excesses of essential amino acids. Poult. Sci. 1999;77:295–298. doi: 10.1093/ps/77.2.295. [DOI] [PubMed] [Google Scholar]
- Celik I., Oguz H., Demet O., Boydak M., Donmez H.H.M., Sur E.F., Nizamlioglu F. Embryotoxicity assay of aflatoxin produced by Aspergillus parasiticus NRRL 2999. Br. Poult. Sci. 2000;41:401–409. doi: 10.1080/713654961. [DOI] [PubMed] [Google Scholar]
- Chaudhry Z.I. PhD Thesis, Punjab University; Lahore, Pakistan: 1996. Effects of aflatoxicosis on chick muscle, fat and bone growth and possible reversal of aflatoxicosis by nandrolonedecanoate. [Google Scholar]
- Cheng Y.H., Shen T.F., Pang V.F., Chen B.J. Effects of aflatoxin and carotenoids on growth performance and immune response in mule ducklings. Comp. Biochem. Physiol. 2000;128:19–26. doi: 10.1016/s1532-0456(00)00173-3. [DOI] [PubMed] [Google Scholar]
- Cilievici O., Ghidus I.C.E., Moldovan A. The toxic and teratogenic effect of aflatoxin B1 on the chick embryo development. Morphol. Embryol. 1980;4:309–314. [PubMed] [Google Scholar]
- Del Vesco A.P., Gasparino E., Grieser O.D., Zancanela V., Soares M.A., Neto A.R. Effects of methionine supplementation on the expression of oxidative stress-related genes in acute heat stress exposed broilers. Br. J. Nutr. 2015;113:549–559. doi: 10.1017/S0007114514003535. [DOI] [PubMed] [Google Scholar]
- Del Vesco A.P., Gasparino E., Grieser O.D., Zancanela V., Soares M.A., Neto A.R. Effects of methionine supplementation on the expression of protein deposition-related genes in acute heat stressexposed broilers. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S.X., Tian L.X., Liu F.J., Jin S.J., Liang G.Y., Yang HJ. Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquac. 2010;307:233–240. [Google Scholar]
- Ditta Y.A., Mahad S., Bacha U. Mycotoxins-impact and Management Strategies. 2018. Aflatoxins: their toxic effect on poultry and recent advances in their treatment. IntechOpen. [Google Scholar]
- Donmez N., Keskin. E. The effect of aflatoxin and glucomannan on some antioxidants and biochemical parameters in rabbits. Acta. Vet. 2008;58:307–313. [Google Scholar]
- Ellis W.O., Smith J.P., Simpson B.K. Aflatoxin in food: Occurrence, biosynthesis, effects on organisms, detection and methods of control. Crit. Rev. Food Sci. Nutr. 1991;30:403–439. doi: 10.1080/10408399109527551. [DOI] [PubMed] [Google Scholar]
- Elnesr S.S., Elwan H.A.M., Xu Q.Q., Xie C., Dong X.Y., Zou X.T. Effects of In Ovo injection of sulfur-containing amino acids on heat shock protein 70, corticosterone hormone, antioxidant indices, and lipid profile of newly hatched broiler chicks exposed to heat stress during incubation. Poult. Sci. 2019;98:2290–2298. doi: 10.3382/ps/pey609. [DOI] [PubMed] [Google Scholar]
- Elwan H.A.M., Shaaban S.Elnesr., Qianqian Xu., Chao Xie., Xinyang Dong., Zou Xiaoting. Effects of In Ovo methionine-cysteine injection on embryonic development, antioxidant status, IGF-I and TLR4 gene expression, and jejunum histomorphometry in newly hatched broiler chicks exposed to heat stress during incubation. Animals. 2019;9:25. doi: 10.3390/ani9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraslan G., Akdoúan M.T., Lüman B.C., Kanbur M., Ýk Delübas N. Effects of dietary aflatoxin and hydrate sodium calcium aluminosilicate on triiodothyronine, thyroxine, thyrotrophin and testosterone levels in quails. Turk. J. Vet. Anim. Sci. 2006;30:41–45. [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Graczyk S., Kotoński B., Malicki A., Orda J., Zawadzki W. The thyroxine levels and blood serum profiles in ducklings after chronic aflatoxin B1 administration. Med. Vet. 2002;1:21–29. [Google Scholar]
- Gündüz N., Oznurlu. Y. Adverse effects of aflatoxin B1 on skeletal muscle development in broiler chickens. Brit. Poult. Sci. 2014;55:684–692. doi: 10.1080/00071668.2014.949621. [DOI] [PubMed] [Google Scholar]
- Hamilton J.W., Bloom. S.E. Correlation between induction of xenobiotic metabolism and DNA damage from chemical carcinogens in the chick embryo in vivo. Carcinogenesis. 1986;7:1101–1106. doi: 10.1093/carcin/7.7.1101. [DOI] [PubMed] [Google Scholar]
- Hamilton J.W., Louis C.A., Doherty K.A., Hunt S.R., Reed M.J., Treadwell M.D. Preferential alteration of inducible gene expression in vivo by carcinogens that induce bulky DNA lesions. Mol. Carcinog. 1993;8:34–43. doi: 10.1002/mc.2940080109. [DOI] [PubMed] [Google Scholar]
- Haschek, W. M., K. A. Voss, and V. R. Beasley. 2002. Selected Mycotoxins Affecting Animal and Human Health. Pages 645–699. 2nd ed. 1Academic Press, New York.
- Huff W.E., Doerr J.A., Hamilton P.B., Hamann D.D., Peterson R.E., Ciegler A. Evaluation of bone strength during aflatoxicosis and ochratoxicosis. Appl. Environ. Microbiol. 1980;40:102–107. doi: 10.1128/aem.40.1.102-107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail I.E., Farag M.R., Alagawany M., Mahmoud H.K., Reda FM. Efficacy of some feed additives to attenuate the hepato-renal damage induced by aflatoxin B1 in rabbits. J. Anim. Physiol. Anim. Nutr. (Berl) 2020;104:1343–1350. doi: 10.1111/jpn.13359. [DOI] [PubMed] [Google Scholar]
- Jacobson W.C., Wiseman. H.G. The transmission of aflatoxin B1 into eggs. Poult. Sci. 1974;53:1743–1745. doi: 10.3382/ps.0531743. [DOI] [PubMed] [Google Scholar]
- Jariyahatthakij P., Chomtee B., Poeikhampha T., Loongyai W., Bunchasak C. Effects of adding methionine in low-protein diet and subsequently fed low-energy diet on productive performance, blood chemical profile, and lipid metabolism-related gene expression of broiler chickens. Poult. Sci. 2018;97:2021–2033. doi: 10.3382/ps/pey034. [DOI] [PubMed] [Google Scholar]
- Jelinek R., Peterka M., Rychter Z. Chick embryotoxicity screening test –130 substances tested. Indian J. Exp. Biol. 1985;23:588–595. [PubMed] [Google Scholar]
- Joshi M.S., Joshi. M.V. Effects of aflatoxin B1 on early embryonic stages of chick Gallus domesticus cultured in vitro. Indian J. Exp. Biol. 1981;19:528–531. [PubMed] [Google Scholar]
- Kalinowski A., Moran E.T., Wyatt C.L. Methionine and cystine requirements of slow- and fast-feathering broiler males from three to six weeks of age. Poult. Sci. 2003;82:1428–1437. doi: 10.1093/ps/82.9.1428. [DOI] [PubMed] [Google Scholar]
- Kandeil M., Abu El-Saad. A. Fourth International Science Conference Faculty of Veterinary Medicine. Mansoura University; 2005. Biochemical effects of ascorbic acid on oxidative stress induced by aflatoxinB1 in male albino rats. [Google Scholar]
- Khan W.A., Khan M.Z., Khan A., Hassan Z.U., Rafique S., Saleemi M.K., Ahad A. Dietary vitamin E in White Leghorn layer breeder hens: a strategy to combat aflatoxin B1-induced damage. Avian Pathol. 2014;43:389–395. doi: 10.1080/03079457.2014.943691. [DOI] [PubMed] [Google Scholar]
- Khlangwiset P., Shephard G.S., Wu F. Aflatoxins and growth impairment: a review. Crit. Rev. Toxicol. 2011;41:740–755. doi: 10.3109/10408444.2011.575766. [DOI] [PubMed] [Google Scholar]
- Kidd M.T. Nutritional modulation of immune function in broilers. Poult. Sci. 2004;83:650–657. doi: 10.1093/ps/83.4.650. [DOI] [PubMed] [Google Scholar]
- Kita K., Nagao K., Taneda N., Inagaki Y., Hirano K., Shibata T., Yaman M.A., Conlon M.A., Okumura J. Insulin-like growth factor binding protein-2 gene expression can be regulated by diet manipulation in several tissues of young chickens. J. Nutr. 2002;132:145–151. doi: 10.1093/jn/132.2.145. [DOI] [PubMed] [Google Scholar]
- Klein P.J., VanVleet T.R., Hall J.O., Coulumbe R.A. Effects of dietary butylated hydroxytoluene on aflatoxin B1-relevant metabolic enzymes in turkeys. Food Chem. Toxicol. 2003;41:671–678. doi: 10.1016/s0278-6915(02)00332-0. [DOI] [PubMed] [Google Scholar]
- Kubena L.F., Harvey R.B., Huff W., Corrier D.E., Phillips T.D., Rottinghaus G.E. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and T-2 toxin. Poult. Sci. 1990;69:1078–1086. doi: 10.3382/ps.0691078. [DOI] [PubMed] [Google Scholar]
- Kumar H., Bhaskarannair K., Kuttan R. Inhibition of drug metabolizing enzymes (Cytochrome P450) in vitro as well as in vivo by Phyllanthus amarus. Schum. Thonn. Biol. Pharm. Bull. 2006;29:1310–1313. doi: 10.1248/bpb.29.1310. [DOI] [PubMed] [Google Scholar]
- Lee K.I., Rhee S.H., Park K.Y. Anticancer activity of phytol and eicosatrienoic acid identified from Perilla leaves. Korean J. Food Nutr. 1999;28:1107–1112. [Google Scholar]
- Livak K.J., Schmittgen. T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- M´etayer S.I., Seiliez A., Collin S., Duchˆene Y., Mercier P., Geraert A., Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J. Nutr. Biochem. 2008;19:207–215. doi: 10.1016/j.jnutbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Marietto-Gonçalves G.A., Brito M.B., Fiorentin E.L., Tonin A.A. Silymarin and methionine application on treatment of liver chronic diseases by aflatoxicosis in rabbit (Oryctolagus cuniculi)-case report. Comp. Clin. Path. 2017;26:719–722. [Google Scholar]
- National Research Council. 1994. Nutrient Requirements of Poultry. 9th rev. ed. National Academic Press, Washington, DC.
- Nazem M.N., Sayed M.S., Reza K., Hamideh M. Histomorphometric analysis of the duodenum of broiler chick embryos injected In Ovo with methionine. Anim. Prod. Sci. 2017;59:133–139. [Google Scholar]
- Nemeth K., M´ezes M., Ga´al T., Bartos A., Balogh K., Husv´eth F. Effect of supplementation with methionine and different fat sources on the glutathione redox system of growing chickens. Acta. Vet. Hung. 2004;52:369–378. doi: 10.1556/AVet.52.2004.3.12. [DOI] [PubMed] [Google Scholar]
- Nizamlioglu F. Determination of aflatoxin B1, B2, G1 and G2 in feeds and feedstuffs which were brought to Konya province laboratory. Veterinarium. 1996;7:42–45. [Google Scholar]
- Oguz H. PhD Thesis, University of Selçuk, Institute of Health Sciences; Konya: 1997. The preventive efficacy of polyvinylpolypyrrolidone (pvpp) alone and its combination with the other adsorbents into broiler feeds against aflatoxicosis. [Google Scholar]
- Oguz H., Nizamlıoglu F., Dinc I., Uney K., Aydın H. Determination of aflatoxin existence in mixed feed, wheat flour and bulgur samples. Eurasian J. Vet. Sci. 2011;27:171–175. [Google Scholar]
- Ohta Y., Kidd M.T., Ishibashi T. Embryo growth and amino acid concentration profiles of broiler breeder eggs, embryos, and chicks after In Ovo administration of amino acids. Poult. Sci. 2001;80:1430–1436. doi: 10.1093/ps/80.10.1430. [DOI] [PubMed] [Google Scholar]
- Oznurlu Y., Celik I., Sur E., Ozaydın T., Oguz H., Altunba K. Determination of the effects of aflatoxin B1 given In Ovo on the proximal tibial growth plate of broiler chickens: histological, histometric and immunohistochemical findings. Avian Pathol. 2012;41:469–477. doi: 10.1080/03079457.2012.712673. [DOI] [PubMed] [Google Scholar]
- Ozpinar H., Ozpinar A., Senel H.S. Die untersuchungen von aflatoxin und ochratoxin a in geflugellalleinfutter and futterrmittein im Marmara Geblet. Istanb. Univ. Vet. Fak. Derg. 1988;14:11–18. [Google Scholar]
- Peng X., Yu Z., Liang N., Chi X., Li X., Jiang M., Fang J., Cui H., Lai W., Zhou Y., Zhou S. The mitochondrial and death receptor pathways involved in the thymocytes apoptosis induced by aflatoxin B1. Oncotarget. 2016;7:12222–12234. doi: 10.18632/oncotarget.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Zhang S., Fang J., Cui H., Zuo Z., Deng J. Protective roles of sodium selenite against aflatoxin B1-induced apoptosis of jejunum in broilers. Inter. J. Env. Res. Pub. Heal. 2014;11:13130–13143. doi: 10.3390/ijerph111213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitet A. Natural occurrence of mycotoxins in foods and feeds – an updated review. Revue De Med. Vet. 1998;149:479–492. [Google Scholar]
- Quist C.F., Bounous D.I., Kilburn J.V., Nettles V.F., Wyatt R.D. The effect of dietary aflatoxin on wild turkey poults. J. Wildl. Dis. 2000;36:436–444. doi: 10.7589/0090-3558-36.3.436. [DOI] [PubMed] [Google Scholar]
- Qureshi M.A., Brake J., Hamilton P.B., Hagler W.M., Nesheim S. Dietary exposure of broiler breeders to aflatoxin results in immune dysfunction in progeny chicks. Poult. Sci. 1998;77:812–819. doi: 10.1093/ps/77.6.812. [DOI] [PubMed] [Google Scholar]
- R´ehault-Godbert S., Mann K., Bourin M., Brionne A., Nys Y. Effect of embryonic development on the chicken egg yolk plasma proteome after 12 d of incubation. J. Agric. Food Chem. 2014;62:2531–2540. doi: 10.1021/jf404512x. [DOI] [PubMed] [Google Scholar]
- Rastogi R., Srivastava A.K., Rastogi A.K. Long term effect of aflatoxin B1 on lipid peroxidation in rat liver and kidney: Effect of picroliv and silymarin. Phytother. Res. 2001;15:307–310. doi: 10.1002/ptr.722. [DOI] [PubMed] [Google Scholar]
- Reda F.M., Ismail Ismail E., El-Mekkawy Mohamed M., Farag M.R., Mahmoud Hemat K., Alagawany M. Dietary supplementation of potassium sorbate, hydrated sodium calcium almuniosilicate and methionine enhances growth, antioxidant status and immunity in growing rabbits exposed to aflatoxin B1 in the diet. J. Anim. Physiol. Anim. Nutr. 2020;104:196–203. doi: 10.1111/jpn.13228. [DOI] [PubMed] [Google Scholar]
- Reda F.M., Swelum A.A., Hussein E.O., Elnesr S.S., Alhimaidi A.R., Alagawany M. Effects of varying dietary DL-methionine levels on productive and reproductive performance, egg quality, and blood biochemical parameters of quail breeders. Animals. 2020;10:1839. doi: 10.3390/ani10101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A.U., Arif M., Husnain M.M., Alagawany M., Abd El-Hack M.E., Taha A.E., Elnesr S.S., Abdel-Latif M.A., Othman S.I., Allam A.A. Growth performance of broilers as influenced by different levels and sources of methionine plus cysteine. Animals. 2019;9:1056. doi: 10.3390/ani9121056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.R. Disorders of thyrotropin synthesis, secretion, and function. Curr. Opin. Pediatr. 2000;12:375–381. doi: 10.1097/00008480-200008000-00017. [DOI] [PubMed] [Google Scholar]
- Shen H.M., Ong C.N., Shi C.Y. Involvement of reactive oxygen species in aflatoxin B1induced cell injury in cultured rat hepatocytes. Toxicology. 1995;99:115–123. doi: 10.1016/0300-483x(94)03008-p. [DOI] [PubMed] [Google Scholar]
- Stipanuk M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- Straus D.S., Takemoto. C.D. Specific decrease in liver insulin-like growth factor-I and brain insulin-like growth factor-II gene expression in energy-restricted rats. J. Nutr. 1991;121:1279–1286. doi: 10.1093/jn/121.8.1279. [DOI] [PubMed] [Google Scholar]
- Sur E.I., Celik Y., Oznurlu M.F., Aydin H., Oguz V., Kurtoglu T. Enzyme histochemical and serological investigations on the immune system from chickens treated In Ovo with aflatoxin B1 (AFB1) Revue. Med. Vet. 2011;162:443–448. [Google Scholar]
- Surai, PF.2002. Natural antioxidants and mycotoxins. Page 455–489 in Natural Antioxidants in Avian Nutrition and Reproduction. Nottingham University Press, Nottingham, UK.
- Tayman C., Tonbul A., Kosus A., Hirfanoglu I.M., Haltas H., Uysal S., Tatli M.M., Andiran F. Protective effects of caffeic acid phenethyl ester (CAPE) on intestinal damage in necrotizing enterocolitis. Pediatr. Surg. Int. 2011;27:1179–1189. doi: 10.1007/s00383-011-2942-0. [DOI] [PubMed] [Google Scholar]
- Tessari E.N.C., Oliveira C.A.F., Cardoso A.L.S.P., Ledoux D.R., Rottinghaus G.E. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Briti. Poult. Sci. 2006;47:357–364. doi: 10.1080/00071660600756071. [DOI] [PubMed] [Google Scholar]
- Tiemersma E.W., Omer R.E., Bunschoten A., Van't veer P., Kok F.J., Idris M.O., Kadaru A.M., Fedail S.S., Kampman E. Role of genetic polymorphism of glutathione-S transferase T1 and microsomal epoxide hydrolase in aflatoxin-associated hepaticellular carcinoma. Cancer Epidemiol. Biomarkers Prev. 2001;10:785–791. [PubMed] [Google Scholar]
- Verma R.J. Aflatoxin cause DNA damage. Int. J. Human Genet. 2004;4:231–236. [Google Scholar]
- Wang F., Shu G., Peng X., Fang J., Chen K., Cui H., Chen Z., Zuo Z., Deng J., Geng Y., Lai W. Protective effects of sodium selenite against aflatoxin B1-induced oxidative stress and apoptosis in broiler spleen. Inter J. Env. Res. Pub. Heal. 2013;10:2834–2844. doi: 10.3390/ijerph10072834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring P., Müllbacher. A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell. Biol. 1999;77:312–317. doi: 10.1046/j.1440-1711.1999.00837.x. [DOI] [PubMed] [Google Scholar]
- Wen C.X.Y., Ding Jiang L.R., Wang T., Zhou Y.M. Effects of dietary methionine on growth performance, meat quality and oxidative status of breast muscle in fast- and slow-growing broilers. Poult. Sci. 2017;96:1707–1714. doi: 10.3382/ps/pew432. [DOI] [PubMed] [Google Scholar]
- Yalcin S., Ba˘gdatlio˘glu N., Bruggeman V., Babacano˘glu E., Uysal I., Buyse J., Decuypere E., Siegel P.B. Acclimation to heat during incubation. 2. Embryo composition and residual egg yolk sac fatty acid profiles in chicks. Poult. Sci. 2008;87:1229–1236. doi: 10.3382/ps.2007-00436. [DOI] [PubMed] [Google Scholar]
- Yang X.J., Lu HY., Li Z.Y., Bian Q., Qiu L.L., Li Z., Liu Q., Li J., Wang X., Wang S. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology. 2012;300:138–148. doi: 10.1016/j.tox.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Yildirim E., Yalchinkaya I., Kanbur M., Çnar M., Oruc E. Effects of yeast glucomannan on performance, some biochemical parameters and pathological changes in experimental aflatoxicosis in broiler chickens. Révue de Méd. Vét. 2011;162:413–420. [Google Scholar]
- Yin H., Jiang M., Peng X., Cui H., Zhou Y., He M., Zuo Z., Ouyang P., Fan J., Fang J. The molecular mechanism of G2/M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget. 2016;7:35592–35606. doi: 10.18632/oncotarget.9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H.B., Chen C.H., Darre M.J., Donoghue A.M., Donoghue D.J., Kumar V. Phytochemicals reduce aflatoxin-induced toxicity in chicken embryos. Poult. Sci. 2017;96:3725–3732. doi: 10.3382/ps/pex190. http://dx.doi.org/10.3382/ps/pex190. [DOI] [PubMed] [Google Scholar]
- Yuan S., Wu B., Yu Z., Fang J., Liang N., Zhou M., Huang C., Peng X. The mitochondrial and endoplasmic reticulum athways involved in the apoptosis of bursa of Fabricius cells in broilers exposed to dietary aflatoxin B1. Oncotarget. 2014;7:65295–65306. doi: 10.18632/oncotarget.11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunianta A., Agus A., Zupriza N. ISTAP-5 Proceedings. Universitas Gadjah Mada; Yogyakarta: 2010. The effect of methionine on glutathione production to eliminate Aflatoxin B1 toxicity. [Google Scholar]
- Zhan X.A., Li J.X., Xu Z.R., Zhao R.Q. Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br. Poult. Sci. 2006;47:576–580. doi: 10.1080/00071660600963438. [DOI] [PubMed] [Google Scholar]
- Zheng Zhixiang., Zhicai Zuo., Panpan Zhu., Fengyuan Wang., Heng Yin., Xi Peng., Jing Fang., Hengmin Cui1., Caixia Gao., Hetao Song., Ping Ouyang., Yi Zhou., Song Zhao. A study on the expression of apoptotic molecules related to death receptor and endoplasmic reticulum pathways in the jejunum of AFB1-intoxicated chickens. Oncotarget. 2017;8:89655–89664. doi: 10.18632/oncotarget.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]