Abstract
By some accounts, ducks were domesticated between 400 and 10,000 yr ago and have been a growing portion of the poultry industry for decades. Ducks specifically, and waterfowl in general, have unique health, housing, nutrition and welfare concerns compared to their galliform counterparts. Although there have been many research publications in regards to health, nutrition, behavior, and welfare of ducks there have been very few reviews to provide an overview of these numerous studies, and only one text has attempted to review all aspects of the duck industry, from breeders to meat ducks. This review covers incubation, hatching, housing, welfare, nutrition, and euthanasia and highlights the needs for additional research at all levels of duck production. The purpose of this review is to provide guidelines to raise and house ducks for research as specifically related to industry practices.
Key words: housing, lighting, welfare, wellbeing, duck
INTRODUCTION
Waterfowl, primarily ducks, are important commercial poultry worldwide. The global production of waterfowl is a rapidly growing industry, with total meat duck production increasing from 2.9 million tons in 2000 to nearly 4.4 million tons in 2013, a growth rate of 3.2% per year (Evans, 2015), and increased to a total of 7.2 million tons in 2018 with the USA producing nearly 31 million ducks per year (IndexBox & GlobalTrade information). Asia continues to be a leading producers of meat ducks (Evans, 2015), followed by France, Myanmar, and the USA and UK. The Pekin duck is the predominant breed followed by Muscovy and Mule ducks. A typical Pekin duck can reach market weight (3–4 kg) in 4 to 5 wk. The Muscovy duck is slower growing, with less fat and increased sexual dimorphism in body weight. Muscovy female ducks are generally marketed 2 to 3 wk earlier than males to limit carcass fat deposition. Mule ducks are a sterile hybrid cross between Pekin and Muscovy ducks and have the advantage of comparable weights between the 2 sexes, thus removing the obstacle associated with marketing females at a different age (Tai and Rouvier, 1998). Mule ducks are favored by certain markets for their good carcass composition with more meat and less fat than Pekin ducks. Both Muscovy and Mule ducks are usually grown to around 10 wk of age to a market weight of 2.75 to 3 kg.
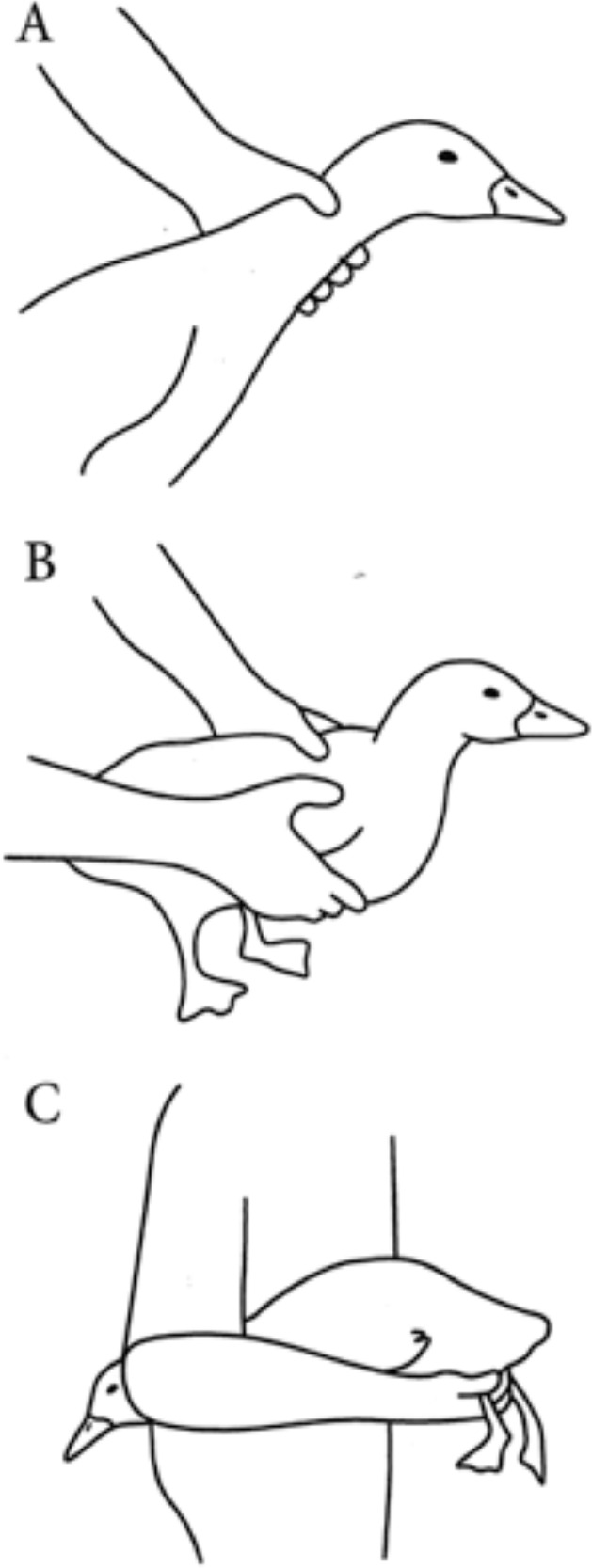
The purpose of this review is to provide guidelines for the use of ducks in research related to industry. As the Pekin duck is the predominate duck world-wide, we will focus on that breed for the purpose of this review. Excerpts from the current edition of the Ag Guide have been reproduced with permission from the Poultry Science Ag Guide Committee (Fraley et al., 2020); however, the purpose of this review is to focus on duck, research on ducks, and supporting information that may be required from other species due to the lack of research on commercial ducks. Figure 1 illustrates the proper way to handle Pekin ducks.
Figure 1.
Proper handling of ducks. Catching ducks by the wings and legs should be avoided. Adapted from Maple Leaf Farms (2011)
HISTORICAL AND CONTEMPORARY CONSIDERATIONS FOR INCUBATION
The biology of incubation is a fascinating process when one considers the unique differences among species; in particular, egg/embryonic temperature regulation, and correlated physiological adaptations (Webb, 1987). Although modern incubation temperature milieu may vary among industry members dependent upon needs and substrains, the basic elements of incubation have not changed substantially. It has been recognized for many yr that incubation temperature is only one of many factors that can influence day old hatchling quality (Tona et al., 2005; Yahav and Brake, 2014) and posthatch performance (Ducuypere, 1984; Yahav and Brake, 2014). Romanoff (1935) is the most frequently cited literature source for the widely accepted “optimal” incubation temperature of 37.5°C (99.50°F) for continuously incubated eggs for numerous species. Romanoff (1936) recommended that mean incubation temperature be decreased to approximately 36°C after 16 d of incubation in consideration of the metabolic heat produced by older embryos. Romanoff (1936) observed a linear decline in hatchability with each 1°C increase from 37.50 to 40.5°C which was the basis for his suggested 36.5°C as the optimal temperature from 16 d to hatch in chickens. Lourens et al. (2005) suggested that egg shell temperature (EST) was the most accurate assessment of actual embryo temperature. The practice of monitoring EST has been implemented in many commercial hatcheries with a target temperature of 37.8°. Incubation temperatures have been shown to affect physiological parameters in post hatch ducklings (Da Costa et al., 2015, 2016). However, novel research has focused on the use of lights in the incubator to improve posthatch performance (De Biasi et al., 2001; Rozenboim et al., 2004b, 2013; Archer et al., 2009; Huth and Archer, 2015). There are as many conflicting findings as there are corroborative and many of these differences could be explained by differences in incubators. However, there are many interesting findings but a much better understanding of lighting and photoreceptor biology of the developing embryo is needed for this exciting field.
Brooding Temperatures and Ventilation
Very little research has been done on brooding temperatures thus the following recommendations come from discussions with stakeholders and the limited published studies (Cherry and Morris, 2008; Jones and Dawkins, 2010a; Xie et al., 2019). Thermoregulatory mechanisms are poorly developed in birds, including ducklings thus higher environmental temperatures are required. Thermoregulation can be achieved by a variety of brooding environments such as floor pen housing with radiant heaters distributed in localized areas, battery brooders, or cage or pen units in heated rooms.
Within limits, ducklings can maintain appropriate body temperatures by moving away from or toward sources of heat when that is possible and by seeking or avoiding contact with other individuals. Huddling of young birds directly under the heat source usually indicates a need for more supplemental heat; dispersal associated with panting indicates that the environment is too warm. Brooding systems that allow birds to move toward or away from heat sources maintain a temperature of at least 20 to 25°C during the first few weeks but can cause the young birds to pant or show other signs of hyperthermia (Jones and Dawkins, 2010b). After ducks have fully feathered (about 23 d of age) they are comfortable at environmental temperatures of 10 to 15°C (Kaseloo and Lovvorn, 2003; Marais et al., 2011).
Ventilation is typically increased over the first few weeks of the brooding period. Whether ventilation is by a mechanical system or involves natural airflow, drafts should be avoided. In relatively open brooding facilities, as in barns with windows or curtains for ventilation, draft shields may prove beneficial up to 10 d after hatching. Ventilation rates for ducks (0.8–2.4 cfm per lb, max) are based upon earlier studies on chickens and turkeys (Davis and Dean, 1968). In order to control moisture content of bedding, water sources can be placed over a pit with slatted flooring (see below) to allow water to fall into pit or lagoon. Overall, a lower relative humidity is preferred in duck houses to help offset the increased drinking and urine production in duck (Jones and Dawkins, 2010b).
HISTORICAL AND CONTEMPORARY CONSIDERATIONS FOR HOUSING
Ducks should have sufficient freedom of movement to be able to turn around, get up, lie down, and preen (e.g., groom themselves [Brambell, 1965; Abo Ghanima et al., 2020]). Use of floor area by birds within groups follows a diurnal pattern and is influenced by the dimensions and design of the facilities. Ducks generally use less area during resting and grooming than during more active periods and will often seek the protection offered by the walls of the enclosure (Newberry and Hall, 1990; Cornetto and Estevez, 2001). Recommendations for minimum floor area for multiple-bird pens and cages as well as individually housed birds are presented in Table 1.
Table 1.
Minimum floor area for ducks raised in confinement.1
| Litter floor2 |
Wire floor |
|||
|---|---|---|---|---|
| Bird type and age (wk) | cm2 | in2 | cm2 | in2 |
| Growing ducks in multiple bird pens | ||||
| 1 | 232 | 36 | 232 | 36 |
| 2 | 464 | 72 | 439 | 68 |
| 3 | 839 | 130 | 651 | 101 |
| 4 | 1,116 | 173 | 974 | 151 |
| 5 | 1,393 | 216 | 1,187 | 184 |
| 6 | 1,671 | 259 | 1,413 | 219 |
| 7 | 1,858 | 288 | 1,625 | 252 |
| Developing breeders in multiple bird pens3 | ||||
| 7 to 28 | 2,322 | 360 | ||
| Breeders in multiple bird pens | ||||
| >28 | 3,251 | 504 | ||
| Individually caged breeder female or male4 | ||||
| >28 | 3,715 | 576 | ||
A duck should have sufficient freedom of movement to be able to turn around, get up, lie down, and groom itself. Space allocations may be slightly excessive for smaller breeds of ducks. The inside and outside areas for ducks in semiconfinement are totaled and equal the space allocations for confined ducks (Hawkins et al., 2014; RSPCA, 2015).
Space for drinkers is included. Drinkers are located on a wire-covered section with a cement drain underneath.
Developing breeders may be raised outdoors on well-drained soil (preferably sand) with open shelter. A minimum of 1,290 cm2 (200 in2) of shelter area /bird is recommended (Maple Leaf Farms, 2011).
An individual bird within a cage should be able to stand comfortably without hitting its head on the top of the cage. The cage door should be wide enough to allow for the easy removal of the bird. Does not include space for feeder, drinkers, or a hen's nest.
Flooring
Ducks may be kept on either solid floors with litter or in cages or pens with raised wire floors of appropriate gauge and mesh dimension. When poultry reside on solid floors litter provides a cushion during locomotion, resting and it absorbs water from droppings (Bell and Weaver Jr., 2002). Floor litter in duck barns is made up of either pine shavings, straw, or rice hulls although other substrates may be used (Bell and Weaver Jr., 2002). The ideal litter can absorb large quantities of water and release it quickly to promote rapid drying. Either a dusty litter or an overly wet litter will have a negative impact on the health, welfare, and performance of ducks (Jones and Dawkins, 2010a; Raud and Faure, 1994). The duck house should be ventilated to prevent litter from becoming moist. Excess moisture in the litter negatively impacts bird health by increasing dirty foot pads, foot pad dermatitis, hock lesions, leg defects, and corticosterone levels, an indirect measure of stress (Dawkins et al., 2004; Fraley et al., 2013a; Karcher et al., 2013a; Çavuşoğlu and Petek, 2019).
Footpad skin is considerably softer and thinner in ducks than other Galliformes species and, therefore, is more susceptible to injury (Koch, 1973). Dry litter floors are least irritating to the feet and hock joints of ducks, particularly if ducks are going to be kept for extended periods (Faridullah et al., 2009; Fraley et al., 2013a). However, litter floors that are not kept dry present a serious threat to the health of the flock. Nonirritating floor surfaces minimize or prevent injury to the foot pad and will minimize joint infection and lameness (Ying et al., 2016).
Lighting
Ducks, like all poultry, are seasonal breeders thus require a minimum of 14 h of light per day in order to maintain gonadal activity (Benoit et al., 1950). Ducks are typically housed under LED, incandescent, fluorescent and even kerosene lanterns in commercial settings around the world (Cherry and Morris, 2008; Porter et al., 2018). The decision for the light source is primarily economic and not a matter of welfare (Olanrewaju et al., 2016, 2018a, b). Fluorescent lights that allow light “flicker” may produce a stressful environment. Research in other avian species has indicated that the flicker fusion frequency is 120 Hz in brown-headed cowbirds (Ronald et al., 2017) and 80 to 105 Hz in chickens (Lisney et al., 2012). Thus, the use of modern fluorescent lights should eliminate concerns about “flicker” as they cycle at 20,000 Hz (National Lighting Product Information Program). The use of fluorescent, incandescent, or LED bulbs may not as important as the color of the light.
Specific wavelengths of light have been investigated to improve performance in turkeys (Leighton Jr. and Potter, 1969; Gill and Leighton Jr., 1988; Levenick and Leighton Jr., 1988; Felts et al., 1990; Hulet et al., 1992), laying chickens (Huber-Eicher et al., 2013), and broilers (Max et al., 1995; Rozenboim et al., 2004a; Bailey and Cassone, 2005; Halevy et al., 2006). The effect of red or blue lighting has been minimally studied in ducks, but recent studies have suggested that in grow-out ducks, there may be some small advantages to red light in terms of reduced activity and feather picking, but the reduced activity does not translate to improved growth rates or carcass quality (Campbell et al., 2015). Interestingly, unlike chickens, housing ducks under blue light may have negative impacts on health, fertility and welfare (Marchand and Sharp, 1977; Campbell, et al., 2015; Haas et al., 2017; House et al., 2018; Hua et al., 2020). The negative effects of blue light were also observed by an increase in corticosterone and decrease in growth hormone (Campbell et al., 2015). A single study has also shown that blue LED light is not appropriate for white Roman breeder geese as well (Chang et al., 2016). Thus, it appears that red or white light provide the best environmental conditions for ducks at any age and blue light should be avoided. However, the brightness of light may also be of concern.
When measuring the brightness of light, care must be given to the instrumentation. Devices that measure in lux or foot candles actually measure the “perceived brightness” of light at a given wavelength optimized for human vision, thus necessitating the use of a spectrophotometer to correctly assess lighting systems. Table 2 illustrates different color temperatures (Kelvin) for common lighting sources. A recent study suggested that light intensity below 15 lux may be insufficient to maintain fertility in adult ducks, and that this effect may be exaggerated in drakes compared to hens. To date no research has fully explored ducks’ needs for a scotophase, nor has a definition of “dark” been developed.
Table 2.
Color temperatures of different sources of white light.
| Temperature | Lighting source |
|---|---|
| 1,850 K | Candle flame, sunset/sunrise |
| 2,700 K | “Soft white” compact fluorescent and LED lamps |
| 3,000 K | Warm white compact fluorescent and LED lamps |
| 3,200 K | Studio lamps, photofloods, etc. |
| 5,000 K | Tubular fluorescent lamps or cool white / daylight compact fluorescent lamps, Horizon Daylight |
| 6,500 K | Daylight, overcast |
| 15,000 – 27,000 K | Clear blue Northern sky |
| These temperatures are merely characteristic. Considerable variation may be present. | |
Social Environment
Ducks are highly social animals and should be kept in groups when possible. Social behaviors have been described for wild ducks (de Lannoy, 1967; Hoffman et al., 1974; Desforges and Wood-Gush, 1975; Balthazart and Hendrick, 1976; Balthazart and Schoffeniels, 1979; Lickliter and Gottlieb, 1986). However, research on the behavior of ducks in commercial flocks is sparse. Ducks exhibit coordinated movements (Ramseyer et al., 2009; Liste et al., 2014), and can be observed sharing resources (Waitt et al., 2009; Makagon and Mench, 2011; Rice et al., 2014). Like other poultry species, ducks communicate using visual and vocal signals (McKinney, 1969; Miller, 1977). Tactile contact plays a key role in promoting social flexibility among ducklings (Gottlieb, 1993). Social experiences with age-matched ducklings within the first days of life have significant impacts on subsequent social preferences and behaviors (reviewed by (Lickliter et al., 1993), and sexual preferences (Kruijt et al., 1982). Therefore, captive ducks should have some means of social interaction starting at an early age.
When birds are kept within group housing, the fear response may result in birds trampling each other and piling up against barriers or in corners with resulting injury and mortality. Among waterfowl, Mule and Pekin ducks show a heightened fear response as compared to Muscovy ducks (Arnaud et al., 2010). Husbandry methods should be used to prevent death caused by smothering. Additionally, young birds should be habituated to conditions that are likely to be encountered later in life. Feather pecking and cannibalism can occur in duck flocks, more commonly among Muscovy ducks (Rodenburg et al., 2005). Although the specific causes leading to feather pecking are not known, high stocking density can be a contributing factor (as reviewed by Rodenburg et al., 2005). Other identified risk factors include genetic strain, light schedule and nutrition (Gustafson et al., 2007a,b). Injury to females resulting from excessive mounting by drakes is an additional concern for sexually mature breeder ducks.
Nest Areas
Nest boxes should be provided for all sexually mature breeder flocks as they provide for easier egg collection, cleaner eggs, and a decreased risk of cloacal cannibalism. Nesting motivation has not been assessed for ducks as it has for laying hens (Cooper, 1995, Cooper, 1997), however, most ducks will use nest boxes if allowed. Factors such as nest box design can affect nest use. When offered a choice among 4 nest boxes of varying levels of enclosure, ducks laid twice as many eggs as predicted by chance in nest boxes that are built with 3 opaque sides, an opaque top and entry curtains (Makagon et al., 2011). Nest boxes without tops were used half as often as predicted by chance suggesting that the presence of the top enhances nest attractiveness. Typically, approximately 1 nest box per 5 laying females is provided. Although ducks will lay eggs communally within individual nest boxes (Harun et al., 1998; Makagon and Mench, 2011) a further reduction of nest to bird ratios is not advised as it is likely to result in increased proportion of eggs laid on the floor (Makagon and Mench, 2011).
Water Systems
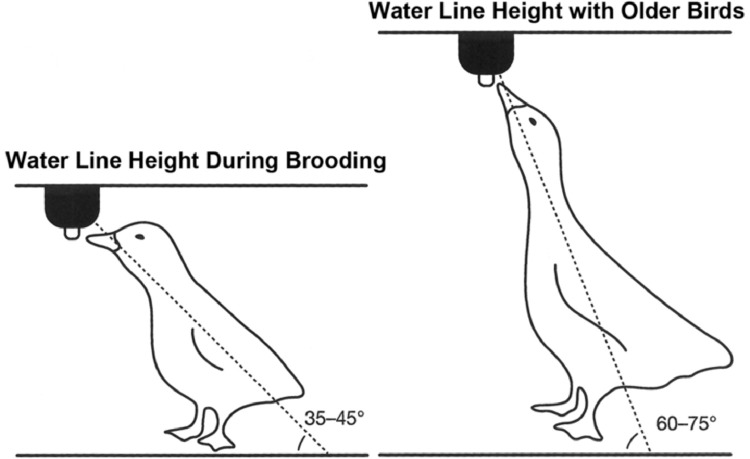
When housed in a research or commercial setting, Pekin ducks should be given access to water via pin-metered (nipple) water lines as open water sources may be a source of contamination of concern to ducks and humans (Kuhnt et al., 2004; Schenk et al., 2016). Pin-metered water lines should be arranged so that a maximum density of 3 adult ducks per pin is available for adequate hydration and to allow for social behaviors and preening to occur (Rice et al., 2014; Schenk et al., 2016). Water pressure in the lines should be adequate to allow for constant flow upon activation. Water lines should be flushed out daily and height of the lines adjusted daily during growth (Maple Leaf Farms, 2011). Figure 2 illustrates the appropriate water line height for young and adult waterfowl.
Figure 2.
Proper height of pin-metered water lines at different duck ages. Adapted from (Cherry and Morris, 2008; Maple Leaf Farms, 2011).
The use of pin-metered water lines has been criticized for not allowing ducks to perform behaviors such as dabbling, head-dipping, bathing, or swimming (for review see, Rodenburg et al., 2005). Others have suggested also that pin-metered water lines were not sufficient for the welfare of ducks (Jones et al., 2009; Jones and Dawkins, 2010b). However, conclusions drawn in those papers contradicted findings of yet other studies (Cooper et al., 2002; Knierim et al., 2004; Rice et al., 2014) that demonstrated wet preening did in fact occur with pin-metered water line systems. The purpose of preening is to maintain feather quality and cleanliness in healthy birds. A recent study showed that commercially housed ducks preen effectively and equally as often regardless of where they are in relation to the water source within a barn (Rice et al., 2014). Several studies have demonstrated that ducks with pin-metered water line systems showed excellent body condition, particularly with eye and feather quality, and feather cleanliness regardless of other differences in management or environmental conditions (Fraley et al., 2013b; Karcher et al., 2013b; Campbell et al., 2014; Colton and Fraley, 2014; Rice et al., 2014; Campbell et al., 2015). Studies have shown that open water systems increase bacterial contamination within open water containment systems as well as the local environment (Kuhnt et al., 2004; Schenk et al., 2016). The fact that waterfowl spread disease in open water has been well established in both commercial and natural water systems (Wolf and Burke, 1982; Kaleta et al., 1983; Spieker et al., 1996; Pearson and Cassidy, 1997; Hansen et al., 2000; Loudon et al., 2011; Green et al., 2012; Lebarbenchon et al., 2012; Dong et al., 2013; Kobayashi et al., 2013; Wozniakowski and Samorek-Salamonowicz, 2014). A recent study demonstrated that within 15 min of disinfection and cleaning, open water sources developed an excess of 1,000,000 colony forming units of bacteria per 50 mL of water, although the source for that water did not show viable bacteria (Schenk et al., 2016).
Ducks should have access to clean water for drinking at all times unless the experimental design specifically necessitates limited access. Table 3 lists the minimum requirement for water access in ducks. Ducks accessing pin-metered water lines should raise their heads up while standing to activate the trigger pins (Bell and Weaver Jr., 2002). Most conventional poultry drinkers may be used for ducks, except for cup drinkers that are smaller in diameter than the width of the duck's bill. If ducks are provided water for swimming or some other wet environment (such as showers), they should also have access to a clean and dry place; otherwise, the protection normally provided by their waterproof, insulated feathers may be lost. Therefore, given the fact that pin-metered water lines allow for adequate grooming behavior, protect against bacterial contamination and reduce the potential danger to human worker and student exposure from environmental pathogens introduced by open water sources, the use of pin-metered water lines may be sufficient to maintain the health and welfare of Pekin ducks. However, further studies on the welfare of ducks as related to water systems are required.
Table 3.
Minimum drinker space for ducks.
| Linear trough space1,3 |
Pin metered water lines | ||
|---|---|---|---|
| Bird type and age | cm2 | In | (maximum no. birds/pin)4 |
| Growing | |||
| 0–10 d | 8 | ||
| 11–21 d | >0.5 | 0.2 | 6 |
| >21 d | 4 | ||
| Breeders | |||
| 0–17 d | >0.5 | 0.2 | 5–6 |
| 18 d through Developer | >0.5 | 0.2 | 5 |
| >28 wk | >0.5 | 0.2 | 3 |
Linear trough space is when both sides of the trough are available. If only one side of the trough is available, double the amount of drinker space/bird (RSPCA, 2015).
At 14 d of age, water troughs must be >18 cm in width and at least 8 cm in depth. By 21 d of age water should be of sufficient depth to allow full body emersion (RSPCA, 2015).
Water trough dimensions are according to RSPCA Guidelines, however open water sources should be avoided as described in the WATER SYSTEMS section of this chapter.
Recommendations from (Cherry and Morris, 2008; Rice et al., 2014).
NUTRITION AND FEEDING
Feed troughs can be located either inside or outside the area where the birds are housed. If feed troughs are located outside the space in which the birds are housed (as is the case for most adult cages), then only one side of the trough is available to the birds. Minimum feeder space recommendations for ducks are shown in Table 4. Feeder space allocation is presented in the tables as linear trough space per bird when both sides of the trough are available. If only one side of the trough is available, then the amount of feeder space per bird must be doubled.
Table 4.
| Linear trough space3 |
||
|---|---|---|
| Bird type and age (wk) | cm | in |
| Growing ducks | ||
| 14 | 0.9 | 0.35 |
| 2 | 1.0 | 0.40 |
| 3 | 1.3 | 0.50 |
| 4 | 1.5 | 0.60 |
| 5 | 1.7 | 0.65 |
| 6 | 1.8 | 0.70 |
| 7 | 1.9 | 0.75 |
| Developing breeders (feed restricted)5 | ||
| 7–28 | 10.2 | 4.0 |
| Breeders | ||
| >28 | 2.0 | 0.8 |
Feed should be allocated and body weight routinely monitored to maintain the recommended body weight for a particular strain and age (Hawkins et al., 2014; RSPCA, 2015).
Feeder space allocations may be slightly excessive for smaller breeds of ducks.
Linear trough space is when both sides of the trough are available. If only one side of the trough is available, double the amount of feeder space/bird. Perimeter space for round feeders is obtained by multiplying linear trough space by 0.8.
During the first week, supplementary feed should be placed on some type of temporary feeders (such as egg flats) on the floor.
Feeder space during earlier ages is the same as for growing ducks.
Meat-type ducks have been bred for rapid growth to market age (Cherry and Morris, 2008). Therefore, their respective breeders have excessive body weight (BW) gain that might lead to problems unless energy intake is controlled beginning early in life. Breeders should be allocated limited feed to allow for a gradual increase in BW each week. Therefore, it is possible that breeders may show stereotypic pecking on non-nutritive objects, and excessive drinking of water. It should be noted that overfeeding or excessive food restriction may reduce fertility (Savory, 1998).
Feed should be allocated and BW routinely monitored to maintain the recommended BW for the particular stock and age. Rations may be either a fixed amount of feed allotted daily or under various alternate-day feeding schemes. Alternate-day feed restriction as opposed to limited feed each day allows more timid birds access to feed, resulting in better flock uniformity (Bell and Weaver Jr., 2002), although this method contradicts European welfare codes (RSPCA, 2015). Inhibition of feeding by subordinate birds is likely if feeder space is limited (Cunningham and van Tiehoven, 1984). Therefore, procedures that require restricted feeding should have enough feeder space so that all birds can eat concurrently. It may also be helpful to use low-density diets and to provide birds with environmental enrichment devices that they can manipulate to satisfy their feed-seeking behaviors (Colton and Fraley, 2014).
We recommended that all feeds for ducks be provided in pelleted form. Pellets no larger than 0.40 cm (5/32 in) in diameter and approximately 0.80 cm (5/16 in) in length should be fed to ducklings less than 2 wk of age. Pellets 0.48 cm (3/16 in) in diameter are suitable for ducks over 2 wk of age (Maple Leaf Farms, 2011). Pelleted feed is preferred to mash as our poultry ducks are dabbling ducks. Dabbling ducks filter feed from nonfeed through the lamellae in their mouths and tongue (Guillemain et al., 2000), and this behavior is not possible with mash feed. Further, the increased water consumption in ducks can cause mash feed to form a paste and not be able to be swallowed.
With the tremendous improvements in genetic selection of ducks driven by the demand for faster growth rate, higher breast meat yield, and better conversion ratio, nutrition is undoubtedly a key piece in commercial duck production to reach optimal genetic potential. The nutrient requirements of ducks can vary depending upon the species, age, production purpose (meat, egg, vs. reproduction), and environment. In addition to nutrient specifications, many other factors should be taken into consideration, including form of the feed and feed safety.
Nutrient Specification and Feeding Program: Meat Ducks
Commercial meat ducks are typically fed 2 or 3-phase diet: a starter diet from hatch to 14 or 21 d of age followed by a grower diet; a finisher diet can be fed if the ducks are to be held for additional days after wk 5. For any diets during the rearing period, energy and protein are the most expensive components.
The ME recommendation for Pekin ducks generally fall within the range of 2825 to 3000 kcal/kg for starters and 3000 to 3100 kcal/kg for growers (NRC, 1994; Adeola, 2006; Fan et al., 2008; Leeson and Summers, 2009). Notably, ducks are able to regulate energy intake via feed intake and are considered nonresponsive to variations in dietary energy level (Fan et al., 2008; Wen et al., 2017). Therefore, dietary energy level generally had no effect on carcass composition except for the proportion of abdominal fat (Fan et al., 2008; Xie et al., 2010).
Protein requirement can vary depending on the demands for end product and composition as protein level can significantly influence duck carcass quality (Chen et al., 2016a). The NRC (1994) recommended 22 and 16% for starter and grower ducks, respectively., while 19% was recommended by Zeng et al. (2015) in grower ducks to obtain the best growth performance and carcass traits. In the latter study, increasing dietary CP concentration from 15 to 19% showed a significant main effect of increasing breast meat yield from 18 to 20%, with a decrease of breast skin and fat yield.
The purpose of adding protein to the diet is to provide amino acids. In corn-soybean meal diet, methionine is likely to be the first limiting amino acid for ducks, followed by lysine, threonine, and tryptophan. In grower ducks, optimal Met was estimated to be 0.468, 0.408, and 0.484% for BW, breast meat yield, and feather; increases in Met improved carcass and breast meat yield, and also led to reduced breast skin and subcutaneous fat (Zeng et al., 2015). Because of the high amino acid concentration in keratin, which is the primary feather protein, the sulfur amino acid (Met + Cys) requirements for optimal feathering may be higher than that for optimal body weight or breast muscle yield (Zeng et al., 2015). Lysine requirement in starter ducks from recent studies ranged from 0.98 to 1.10% (Bons et al., 2002; Wang et al., 2006). Threonine, often the third limiting amino acid in ducks, has been shown to be critical for maintain intestinal structure and function (Horn et al., 2010). The recommended nutrient specifications for Pekin ducks at different growth phases are provided in Table 5.
Table 5.
Recommended range of nutrient specification for Pekin ducks (As-is basis).1
| Meat duck |
Breeding duck |
||||||
|---|---|---|---|---|---|---|---|
| Nutrient | Unit | Starter | Grower | Starter | Developer | Pre-Lay | Breeder |
| 0–14 d | 15–35 d | 0–7 wk | 8–16 wk | 17–20 wk | >20 wk | ||
| Moisture2 | % | 12 | 12 | 12 | 12 | 12 | 12 |
| Protein | % | 22–24 | 18–19 | 21–22 | 14–16 | 17–18 | 16–18.5 |
| Fat, Crude | % | 5–6 | 6–7 | 5–7 | 2.5–3.5 | 2.5–3.5 | 2.5–3.5 |
| Fiber, Crude | % | 2.0–2.5 | 2.0–2.5 | 2.0–2.5 | 2.5–3.0 | 2.5–3.0 | 2.5–3.0 |
| Metabolizable energy (ME) | Kcal/kg | 2900–3000 | 3000–3150 | 2900–3000 | 2600–3000 | 2630–2900 | 2450–2900 |
| Calcium | % | 0.85–1.2 | 0.75–1.2 | 0.85–1.2 | 0.75–1.15 | 1.8–2.0 | 3.0–3.75 |
| Nonphytate phosphorus | % | 0.40–0.48 | 0.38–0.55 | 0.40–0.48 | 0.35–0.6 | 0.35–0.6 | 0.38–0.65 |
| Sodium3 | % | 0.17–0.185 | 0.152–0.165 | 0.17–0.185 | 0.155– | 0.155–0.23 | 0.165–0.23 |
| Chloride3 | % | 0.20–0.25 | 0.20–0.28 | 0.20–0.28 | 0.18–0.28 | 0.18–0.25 | 0.18–0.25 |
| Lysine | % | 1.0–1.25 | 0.90–1.15 | 1.0–1.25 | 0.70–0.85 | 0.77–0.85 | 0.8–1.0 |
| Methionine | % | 0.42–0.65 | 0.40–0.60 | 0.42–0.65 | 0.58–0.65 | 0.4–0.42 | 0.40–0.50 |
| Total sulfur amino acids | % | 0.76–0.95 | 0.66–0.84 | 0.76–0.95 | 0.70–0.95 | 0.64–0.70 | 0.68–0.80 |
| Threonine | % | 0.76–0.98 | 0.62–0.80 | 0.76–0.98 | 0.48–0.50 | 0.48–0.54 | 0.58–0.6 |
| Tryptophan | % | 0.21–0.23 | 0.16–0.20 | 0.21–0.23 | 0.16–0.17 | 0.14–0.16 | 0.14–0.16 |
| Arginine | % | 0.94–1.0 | 0.76–0.89 | 0.94–1.0 | 0.90–1.10 | 0.90–1.10 | 0.90–1.10 |
| Isoleucine | % | 0.5–0.63 | 0.44–0.46 | 0.5–0.63 | 0.44–0.46 | 0.38 | 0.38 |
| Leucine | % | 1.26–1.5 | 0.91–1.33 | 1.26–1.5 | 0.91–1.33 | 0.76 | 0.76 |
| Valine | % | 0.77–0.8 | 0.56–0.71 | 0.77–0.8 | 0.56–0.71 | 0.47 | 0.47 |
| Vitamins (added) | |||||||
| Vitamin A | IU/kg | 12000–15000 | 12000–15000 | 12000–15000 | 10000–15000 | 10,000 | 12,000 |
| Vitamin D3 | IU/kg | 3000–5000 | 3000–5000 | 3000–5000 | 3000–4500 | 3,000 | 3,000 |
| Vitamin E | IU/kg | 40–80 | 40–80 | 40–80 | 50–100 | 50–100 | 50–100 |
| Vitamin K3 | IU/kg | 3–5 | 3–5 | 3–5 | 2–5 | 2–5 | 2–5 |
| Vitamin B1 | mg/kg | 2–3 | 2–3 | 2–3 | 2.5–3.5 | 2.5–3.5 | 2.5–3.5 |
| Vitamin B2 | mg/kg | 5–7 | 5–7 | 5–7 | 10–12 | ||
| Vitamin B6 | mg/kg | 5–7 | 5–7 | 5–7 | 5–6 | 5–6 | 5–6 |
| Vitamin B12 | mg/kg | 0.02–0.04 | 0.02–0.04 | 0.02–0.04 | 0.02–0.04 | 0.02–0.04 | 0.02–0.04 |
| Niacin | mg/kg | 60–80 | 60–80 | 60–80 | 45–60 | 45–60 | 45–60 |
| Pantothenic acid | mg/kg | 10–15 | 10–15 | 10–15 | 15–20 | 15–20 | 15–20 |
| Folic acid | mg/kg | 1–2 | 1–2 | 1–2 | 2–3 | 2–3 | 2–3 |
| Biotin | mg/kg | 0.2–0.25 | 0.2–0.25 | 0.2–0.25 | 0.25–0.40 | 0.25–0.40 | 0.25–0.40 |
| Vitamin C | mg/kg | 100–200 | 100–200 | 100–200 | 150–200 | 150–200 | 150–200 |
| Choline | mg/kg | 810–2000 | 810–2000 | 810–2000 | 810–2400 | 810–2400 | 810–2400 |
| Trace minerals4(Added) | |||||||
| Manganese | mg/kg | 20–50 | 20–50 | 20–50 | 20–50 | 20–50 | 20–50 |
| Iron | mg/kg | 40 | 40 | 40 | 40 | 40 | 40 |
| Copper | mg/kg | 8 | 8 | 8 | 8 | 8 | 8 |
| Zinc | mg/kg | 60 | 60 | 60 | 60 | 60 | 60 |
| Iodine | mg/kg | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Selenium | mg/kg | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
Values obtained from Leeson and Summers (2005); Maple Leaf Farms (2011); DSM (2016); Wen et al. (2014); Xie et al. (2010); Zeng et al. (2015); Zhang et al. (2014); Zhang et al. (2016) and from practical field experiences (Bold and Italic).
Moisture content is listed at 12 % to standardize all the nutrient values. This does not constitute an actual value or a requirement.
There is not a salt requirement as such. However; there are sodium and chloride requirements.
Common sources of trace minerals: iron from ferrous sulfate; copper from copper sulfate, organic forms, chelated forms or from tribasic copper chloride; manganese from manganous sulfate, manganese oxide (maximum of 50% of the total mineral), organic forms, or tribasic manganese chloride; zinc from zinc sulfate, zinc oxide (maximum of 50% of the total mineral), organic forms or tribasic zinc chloride; iodine from ethylene diamine hydroiodide; selenium from sodium selenite.
Nutrient Specification and Feeding Program: Breeder Ducks
Ducks raised for breeding purposes are typically fed a 4-phase diet: a starter diet from hatch to 7 wk, a developer diet from 8 to 16 wk, a prelay diet from 17 to 20 wk, and a breeder diet from 20 wk to the end of production.
During the developing stages (0–20 wk), it is recommended that restricted feed access be used to ensure that ducks follow a targeted body weight growth curve relative to the specific species and strain. Like other poultry, excess body weight gain in ducks during the developer period may lead to a decrease in fertility, egg production and hatchability (King‘Ori, 2011). The breeder diet is generally lower in protein and ME compared with growing meat duck diets. However, breeder diet must contain an increased level of calcium (3.0–3.75 %) to support egg production, eggshell formation, and skeletal system integrity (NRC, 1994). A prelay diet with an intermediate Ca concentration (approximately 1.8–2.0%) is usually fed to help the duck's transition to the high-Ca breeder diet and for the high egg production. Dietary Ca and P should be maintained at or close to a 2:1 ratio before the prelay phase as an imbalance of Ca and P can result in lameness and other bone health issues in ducks (Maple Leaf Farms, 2011).
Feed Safety
Ensuring feed safety is crucial when raising ducks. No anticoccidial medications should be present in duck feed, as they can be toxic to ducks and that ducks housed in clean conditions are less susceptible to coccidial infections compared with chickens (Gajadhar et al., 1983). In addition, Pekin ducks are extremely sensitive to mycotoxins such as aflatoxin. Levels as low as 0.1 mg aflatoxin B1/kg diet can lead to significantly impaired growth, liver function, and immune dynamics in Pekin ducklings (Chen et al., 2014). Notably, 0.1 mg/kg aflatoxin B1 is considered realistic concentration in commercial feed. A recent world mycotoxin survey has shown that aflatoxin concentration in main commodities and finished feed can be as high as 1.33 mg/kg. Also, the occurrence of co-contamination (more than one mycotoxin) from 21,287 samples and 86 countries was as high as 71% (Biomin, unpublished data, 2019). Given that it is a common practice to include multiple feedstuffs in typical diets, the risk of simultaneous exposure to multiple mycotoxins is possible, which may lead to synergistic effects and thus a greater potential threat to the animal (Grenier and Applegate, 2013). Feeding low protein diets can exacerbate the negative effects of mycotoxins; therefore, extra caution is needed when low protein diets are being fed (Chen et al., 2014, 2016b).
EUTHANASIA
Euthanasia is not equal to depopulation. Euthanasia, “kind death,” is appropriate for small numbers of individuals whereas depopulation is used to eliminate large populations quickly in response to disease or other disasters. The most commonly used reference is the Guidelines for Euthanasia of Animals by the American Veterinary Medical Association (Leary et al., 2013; Leary and Johnson, 2020). The only unconditionally approved method of euthanasia is an overdose of barbiturates. Methods that are conditionally accepted for non-neonatal birds include overdose of inhaled gases (CO2, CO, N, Ar or gas anesthetics), and physical methods (cervical dislocation, blunt force trauma, electrocution or decapitation with adjunctive methods, captive bolt or gunshot). Embryonated eggs and neonates under 72 h old may be euthanized via CO2, chilling and freezing, and maceration.
Advantages of overdose of injectable barbiturates for euthanasia include positive public perception and the ability to easily euthanize larger birds. The disadvantages include the need to use these agents in a secure area (most of these injectable drugs must be stored in a locked container with accurate record keeping, and evacuation of gases must be done in a way that does not injure human operators) and large doses are required for most bird species (Leary et al., 2013; Leary and Johnson, 2020). Accidental inoculation of the operator with barbiturates can result in significant injury. In addition, the purchase, storage, and record keeping of all scheduled pharmaceuticals require the supervision of an accredited and DEA-licensed individual.
Using gaseous agents is advantageous when euthanizing larger numbers of birds. Most adult birds will be killed quickly at CO2 concentrations over 50%. However, gas often requires longer exposure time for waterfowl due to the diving reflex. In addition, escaped gases can have implications for human and environmental health. Birds often react to rapid administration of gas by gasping, shaking heads and flapping wings; these reactions are attributed to the irritating effect of the gases on the mucosal epithelia (Leary and Johnson, 2020). Argon and nitrogen are usually used in combination with CO2 to reduce distress and reduce time to death.
Cervical dislocation, when performed by trained individuals, results in rapid death. However, there can be significant variations in time to death dependent upon the operator, so it is imperative that anyone performing cervical dislocation be well trained in the procedure. The American Veterinary Medical Association (AVMA) recommends that anyone performing cervical dislocation be trained using already deceased birds or birds destined for euthanasia that have been anesthetized prior to dislocation (Leary et al., 2013; Leary and Johnson, 2020). According to the AVMA guidelines for euthanasia, “the legs of the bird should be grasped (or wings if grasped at the base) and the neck stretched by pulling on the head while applying a ventrodorsal rotational force to the skull” (Leary et al., 2013; Leary and Johnson, 2020). However, grasping the wings instead of legs of waterfowl will cause wing breakage before euthanasia occurs, so gripping the legs is generally recommended for these species. The dislocation must be directly behind the skull at C1-C2 in order to avulse the brain stem from the spinal cord. Dislocation or crushing of lower cervical vertebrae are unacceptable methods of euthanasia. Large birds and birds with longer and thicker necks can pose injury to the operator and are more difficult to cervically dislocate, so other methods are preferred. The necks of all birds must be examined for separation of the vertebrae after euthanasia.
Nonpenetrating or penetrating captive bolt devices have been used for larger poultry and can be an alternative provided that the velocity and angle of impact are appropriate for the specific species. Proper restraint for this method of euthanasia is paramount in order to minimize stress to the bird, improper implementation and injury to the human operator.
All personnel performing euthanasia must be trained and proficient in whatever method is performed. It is also imperative to confirm death prior to disposal. Dilated pupils without a light reflex, and lack of audible or palpable heartbeat, respirations, corneal reflex and withdrawal during toe pinch should be used in combination in order to assure death. Ultimately, the method of euthanasia must be chosen based on what would cause the least distress to the bird, as well as the least potential harm to the human operator and the environment.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Maja Makagon (UC Davis) for material and critical comments on this review.
DISCLOSURES
The authors have no conflicts to declare.
REFERENCES
- Abo Ghanima M.M., Abd El-Hack M.E., Taha A.E., Tufarelli V., Laudadio V., Naiel M.A.E. Assessment of stocking rate and housing system on performance, carcass traits, blood indices, and meat quality of french pekin ducks. Agric. 2020;10:273–285. [Google Scholar]
- Adeola O. Review of research in duck nutrient utilization. Int. J. Poult. Sci. 2006;5:201–218. [Google Scholar]
- Archer G.S., Shivaprasad H.L., Mench J.A. Effect of providing light during incubation on the health, productivity, and behavior of broiler chickens. Poult. Sci. 2009;88:29–37. doi: 10.3382/ps.2008-00221. [DOI] [PubMed] [Google Scholar]
- Arnaud I., Gardin E., Sauvage E., Bernadet M.-D.D., Couty M., Guy G., Guemene D., Guémené D. Behavioral and adrenal responses to various stressors in mule ducks from different commercial genetic selection schemes and their respective parental genotypes. Poult. Sci. 2010;89:1097–1109. doi: 10.3382/ps.2009-00553. [DOI] [PubMed] [Google Scholar]
- Bailey M.J., Cassone V.M. Melanopsin expression in the chick retina and pineal gland. Brain Res Mol Brain Res. 2005;134:345–348. doi: 10.1016/j.molbrainres.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Balthazart J., Hendrick J. Annual variation in reproductive behavior, testosterone, and plasma FSH levels in the Rouen duck, Anas platyrhynchos. Gen Comp Endocrinol. 1976;28:171–183. doi: 10.1016/0016-6480(76)90169-6. [DOI] [PubMed] [Google Scholar]
- Balthazart J., Schoffeniels E. Pheromones are involved in the control of sexual behaviour in birds. Naturwissenschaften. 1979;66:55–56. doi: 10.1007/BF00369365. [DOI] [PubMed] [Google Scholar]
- Bell D.D., Weaver W.W., Jr . 5th ed. Kluwer Academic Publishers; Norwell, MA: 2002. Commercial Chicken Meat and Egg Production. [Google Scholar]
- Benoit J., Assenmacher I., Walter F.X. [Gonadotropic activity of the hypophysis of the domestic duck during seasonal testicular regression and prepuberty. C R Seances Soc. Biol. Fil. 1950;144:1403–1407. [PubMed] [Google Scholar]
- Bons A., Timmler R., Jeroch H. Lysine requirement of growing male Pekin ducks. Br. Poult. Sci. 2002;43:677–686. doi: 10.1080/0007166021000025073. [DOI] [PubMed] [Google Scholar]
- Brambell, F. W. R. 1965. The welfare of animals. Pages 9–15 in Report of the Technical Committee to Enquire into the Welfare of Animals Kept Under Intensive Livestock Husbandry Systems. Brambell, F.W.R. (Chairman), ed. Her Majesty's Stationery Office, London, UK.
- Campbell C.L., Colton S., Haas R., Rice M., Porter A., Schenk A., Meelker A., Fraley S.M., Fraley G.S. Effects of different wavelengths of light on the biology, behavior, and production of grow-out Pekin ducks. Poult. Sci. 2015;94:1751–1757. doi: 10.3382/ps/pev166. [DOI] [PubMed] [Google Scholar]
- Campbell C.L., Colton S., Porter A., Haas R., Gerometta E., Lindberg A., Fraley S.M., Fraley G.S. Descriptive analyses of gait characteristics in Pekin Ducks from hatch to market weight. J. Appl. Poult. Res. 2014;23:146–155. [Google Scholar]
- Çavuşoğlu E., Petek M. Effects of different floor materials on the welfare and behaviour of slow- and fast-growing broilers. Arch. Anim. Breed. 2019;62:335–344. doi: 10.5194/aab-62-335-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.C., Lin M.J., Zhuang Z.X., Huang S.Y., Lin T.Y., Jea Y.S., Fan Y.K., Lee T.T. Effect of Monochromic Light-emitting Diode Light with Different Color on the Growth and Reproductive Performances of Breeder Geese. Asian-Australas J. Anim. Sci. 2016;29:830–837. doi: 10.5713/ajas.15.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Horn N., Cotter P.F., Applegate T.J. Growth, serum biochemistry, complement activity, and liver gene expression responses of Pekin ducklings to graded levels of cultured aflatoxin B1. Poult. Sci. 2014;93:2028–2036. doi: 10.3382/ps.2014-03904. [DOI] [PubMed] [Google Scholar]
- Chen X., Naehrer K., Applegate T. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016;95:1312–1325. doi: 10.3382/ps/pew022. [DOI] [PubMed] [Google Scholar]
- Chen X., Murdoch R., Zhang Q., Shafer D.J., Applegate T.J. Effects of dietary protein concentration on performance and nutrient digestibility in Pekin ducks during aflatoxicosis. Poult. Sci. 2016;95:834–841. doi: 10.3382/ps/pev378. [DOI] [PubMed] [Google Scholar]
- Cherry P., Morris T.R. CABI, Wallingford, Oxfordshire, UK; Cambridge, MA: 2008. Domestic Duck Production: Science and Practice. [Google Scholar]
- Colton S., Fraley G.S. The effects of environmental enrichment devices on feather picking in commercially housed Pekin ducks. Poult. Sci. 2014;93:2143–2150. doi: 10.3382/ps.2014-03885. [DOI] [PubMed] [Google Scholar]
- Cooper J.J., McAfee L. Behavioural responses of domestic ducks to nipple drinkers, bell drinkers and water troughs. British Poult. Sci. 2002;43:S17–S18. [Google Scholar]
- Cooper J. Nesting behaviour of hens: effects of experience on motivation. Appl. Anim. Behav. Sci. 1995;98:533–547. [Google Scholar]
- Cooper J. Motivational aspects of individual variation in response to nestboxes by laying hens. Anim. Behav. 1997;54:1245–1253. doi: 10.1006/anbe.1997.0521. [DOI] [PubMed] [Google Scholar]
- Cornetto T., Estevez I. Influence of vertical panels on use of space by domestic fowl. Appl. Anim. Behav. Sci. 2001;71:141–153. doi: 10.1016/s0168-1591(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Cunningham D.L., van Tiehoven A. The effects of management program and social rank on behavior and productivity of white Leghorn Layers in cages. Poult. Sci. 1984;63:25–30. doi: 10.3382/ps.0630025. [DOI] [PubMed] [Google Scholar]
- Da Costa M.J., Oviedo-Rond E.O., Wineland M., Jeffrey D. Effects of eggshell conductance and incubation temperatures on duck footpad development. J. Appl. Poult. Res. 2015;5:830–837. [Google Scholar]
- Da Costa M.J., Oviedo-Rondón E.O., Wineland M., Jeffrey D. Pathogeny of Fatigued Walking Condition in Pekin Ducks. Avian. Dis. 2016;60:731–738. doi: 10.1637/11292-100315-RegR. [DOI] [PubMed] [Google Scholar]
- Davis H.R., Dean W.F. Environmental control of ducklings. Trans. ASAE. 1968;11:736–738. [Google Scholar]
- Dawkins M.S., Donnelly C.A., Jones T.A. Chicken welfare is influenced more by housing conditions than by stocking density. Nature. 2004;427:342–344. doi: 10.1038/nature02226. [DOI] [PubMed] [Google Scholar]
- De Biasi S.N., Apfelbaum L.I., Apfelbaum M.E. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur. J. Endocrinol. 2001;145:659–665. doi: 10.1530/eje.0.1450659. [DOI] [PubMed] [Google Scholar]
- de Lannoy J. [On the imprinting of instructive action patterns. (Studies on mallards Anas platyrhynchos L. and red-chested pochard Netta rufina Pallas)] Z Tierpsychol. 1967;24:162–200. [PubMed] [Google Scholar]
- Desforges M.F., Wood-Gush D.G. Behavioural differences between Aylesbury and wild mallard ducks: a study in domestication. Vet. Rec. 1975;96:509. doi: 10.1136/vr.96.23.509. [DOI] [PubMed] [Google Scholar]
- Dong B.B., Xu C.L., Dong L.B., Cheng H.J., Yang L., Zou S.M., Chen M., Bai T., Zhang Y., Gao R.B., Li X.D., Shi J.H., Yuan H., Yang J., Chen T., Zhu Y., Xiong Y., Yang S., Shu Y.L. A novel reassortant H3N8 influenza virus isolated from drinking water for duck in a domestic duck farm in Poyang Lake area. Biomed Env. Sci. 2013;26:546–551. doi: 10.3967/0895-3988.2013.07.005. [DOI] [PubMed] [Google Scholar]
- DSM. 2016. DSM Vitamin Supplementation Guidelines 2016 for Domestic Animals. DSM, Heerlen, the Netherlands.
- Ducuypere E. Incubation temperature in relation to post-natal performance in chickens. Arch. Exp. Vet. 1984;38:439–449. [PubMed] [Google Scholar]
- Evans, T. 2015. Global Poultry Trends - Asia Dominates Duck Production. The Poultry Site. Accessed March 2019. http://www.thepoultrysite.com/articles/3506/global-poultry-trends-asia-dominates-duck-production/.
- Fan H., Xie M., Wang W., Hou S., Huang W. Effects of dietary energy on growth performance and carcass quality of white growing pekin ducks from two to six weeks of age. Poult. Sci. 2008;87:1162–1164. doi: 10.3382/ps.2007-00460. [DOI] [PubMed] [Google Scholar]
- Faridullah M.Irshad, Yamamoto S., Honna T., Eneji A.E. Characterization of trace elements in chicken and duck litter ash. Waste Manag. 2009;29:265–271. doi: 10.1016/j.wasman.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Felts J.V, Leighton A.T., Jr., Denbow D.M., Hulet R.M. Influence of light sources on the growth and reproduction of large white turkeys. Poult. Sci. 1990;69:576–583. doi: 10.3382/ps.0690576. [DOI] [PubMed] [Google Scholar]
- Fraley, G. S., M. Lilburn, M. Sifri, X. Chen, D. Karcher, M. Makagon, P. Wakenell, and S. M. Fraley. 2020. Chapter 13, Waterfowl. Pages 199–207 in Guide for the Care and Use of Agricultural Animals in Research and Teaching. 4th ed. Poultry Science Association, Champagne, IL.
- Fraley S.M., Fraley G.S., Karcher D.M., Makagon M.M., Lilburn M.S. Influence of plastic slatted floors compared with pine shaving litter on Pekin Duck condition during the summer months. Poult. Sci. 2013;92:1706–1711. doi: 10.3382/ps.2012-02992. [DOI] [PubMed] [Google Scholar]
- Fraley S.M., Fraley G.S., Karcher D.M., Makagon M.M., Lilburn M.S. Influence of plastic slatted floors compared with pine shaving litter on Pekin Duck condition during the summer months. Poult. Sci. 2013;92:1706–1711. doi: 10.3382/ps.2012-02992. [DOI] [PubMed] [Google Scholar]
- Gajadhar A.A., Wobeser G., Stockdale P.H.G. Coccidia of domestic and wild waterfowl (Anseriformes) Can. J. Zool. 1983;61:1–24. [Google Scholar]
- Gill D.J., Leighton A.T., Jr. Effects of light environment and population density on growth performance of male turkeys: 2. Physiological changes. Poult. Sci. 1988;67:1518–1524. doi: 10.3382/ps.0671518. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. Social induction of malleability in ducklings: sensory basis and psychological mechanism. Anim. Behav. 1993;45:707–719. [Google Scholar]
- Green H.C., Dick L.K., Gilpin B., Samadpour M., Field K.G. Genetic markers for rapid PCR-based identification of gull, Canada goose, duck, and chicken fecal contamination in water. Appl. Env. Microbiol. 2012;78:503–510. doi: 10.1128/AEM.05734-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier B., Applegate T. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins (Basel) 2013;5:396–430. doi: 10.3390/toxins5020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemain M., Fritz H., Blais S. Foraging methods can affect patch choice: an experimental study in Mallard (Anas platyrhynchos) Behav. Process. 2000;50:123–129. doi: 10.1016/s0376-6357(00)00095-4. [DOI] [PubMed] [Google Scholar]
- Gustafson L., Cheng H., Garner J., Pajor E. Effects of bill-trimming Muscovy ducks on behavior, body weight gain, and bill morphopathology. Appl. Anim. 2007;103:59–74. [Google Scholar]
- Gustafson L.A., Cheng H.W., Garner J.P., Pajor E.A., Mench J.A. The effects of different bill-trimming methods on the well-being of Pekin ducks. Poult. Sci. 2007;86:1831–1839. doi: 10.1093/ps/86.9.1831. [DOI] [PubMed] [Google Scholar]
- Haas R., Alenciks E., Frazier K., Fraley G.S. The Maintenance of Reproductive Status in Pekin Drakes requires both red and blue wavelengths of light: relationship to Opsin-related proteins in the hypothalamus. Poult. Sci. 2017;96:2908–2919. [Google Scholar]
- Halevy O., Piestun Y., Rozenboim I., Yablonka-Reuveni Z. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1062–R1070. doi: 10.1152/ajpregu.00378.2005. [DOI] [PubMed] [Google Scholar]
- Hansen W.R., Nashold S.W., Docherty D.E., Brown S.E., Knudson D.L. Diagnosis of duck plague in waterfowl by polymerase chain reaction. Avian. Dis. 2000;44:266–274. [PubMed] [Google Scholar]
- Harun M., Veeneklaas R., Van Kampen M. Breeding biology of Muscovy duck Cairina moschata in natural incubation: the effect of nesting behavior on hatchability. Poult. Sci. 1998;77:1280–1286. doi: 10.1093/ps/77.9.1280. [DOI] [PubMed] [Google Scholar]
- Hawkins P., Brookes S., Parkin S., Clutton R.E., Gade P., Lane J., Proctor H., Edgar J., Vincent I., Weyer U. Report of the second RSPCA/AHVLA meeting on the welfare of agricultural animals in research: cattle, pigs, sheep and poultry. Anim. Technol. Welf. 2014;13:155–164. [Google Scholar]
- Hoffman H.S., Ratner A.M., Eiserer L.A., Grossman D.J. Aggressive behavior in immature ducklings. J Comp Physiol Psychol. 1974;86:569–580. doi: 10.1037/h0036161. [DOI] [PubMed] [Google Scholar]
- Horn N., Radcliffe J.S., Applegate T., Adeola O. Gut morphology and nutrient retention responses of broiler chicks and White Pekin ducklings to dietary threonine deciency. Can. J. Anim. Sci. 2010;90:513–520. [Google Scholar]
- House G., Sobotik E., Nelson J., Archer G. The effect of raising Pekin ducks two spectra of LED light on production, stress, and behavior. Page 347 in Poultry Science Association Annual Meeting, San Antonio, TX. 2018 [Google Scholar]
- Hua D., Xue F., Xin H., Zhao Y., Wang Y., Xiong B. Effects of monochromatic lights on the growth performance, carcass characteristics, eyeball development, oxidation resistance, and cecal bacteria of Pekin ducks. Asian-Australas J. Anim. Sci. 2020;34:931–940. doi: 10.5713/ajas.20.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Eicher B., Suter A., Spring-Stahli P. Effects of colored light-emitting diode illumination on behavior and performance of laying hens. Poult. Sci. 2013;92:869–873. doi: 10.3382/ps.2012-02679. [DOI] [PubMed] [Google Scholar]
- Hulet R.M., Denbow D.M., Leighton A.T., Jr. The effect of light source and intensity on turkey egg production. Poult. Sci. 1992;71:1277–1282. doi: 10.3382/ps.0711277. [DOI] [PubMed] [Google Scholar]
- Huth J.C., Archer G.S. Effects of LED lighting during incubation on layer and broiler hatchability, chick quality, stress susceptibility and post-hatch growth. Poult. Sci. 2015;94:3052–3058. doi: 10.3382/ps/pev298. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Dawkins M.S. Environment and management factors affecting Pekin duck production and welfare on commercial farms in the UK. Br. Poult. Sci. 2010;51:12–21. doi: 10.1080/00071660903421159. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Dawkins M.S. Effect of environment on Pekin duck behaviour and its correlation with body condition on commercial farms in the UK. Br Poult. Sci. 2010;51:319–325. doi: 10.1080/00071668.2010.499143. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Waitt C.D., Dawkins M.S. Water off a duck's back: showers and troughs match ponds for improving duck welfare. Appl. Anim. Behav. Sci. 2009;116:52–57. [Google Scholar]
- Kaleta E.F., Kaup F.J., Drommer W., Herbst W. [Duck plague in a water-bird flock] Zentralbl Vet. B. 1983;30:131–136. [PubMed] [Google Scholar]
- Karcher D.M., Makagon M.M., Fraley G.S., Fraley S.M., Lilburn M.S. Influence of raised plastic floors compared with pine shaving litter on environment and Pekin duck condition. Poult. Sci. 2013;92:583–590. doi: 10.3382/ps.2012-02215. [DOI] [PubMed] [Google Scholar]
- Karcher D.M., Makagon M.M., Fraley G.S., Fraley S.M., Lilburn M.S. Influence of raised plastic floors compared with pine shaving litter on environment and Pekin duck condition. Poult. Sci. 2013;92:583–590. doi: 10.3382/ps.2012-02215. [DOI] [PubMed] [Google Scholar]
- Kaseloo P.A., Lovvorn J.R. Heat increment of feeding and thermal substitution in mallard ducks feeding voluntarily on grain. J. Comp. Physiol. 2003;173:207–213. doi: 10.1007/s00360-002-0321-9. [DOI] [PubMed] [Google Scholar]
- King‘Ori A.M.K. Review of the factors that influence egg fertility and hatchabilty in poultry. Int. J. Poult. Sci. 2011;10:483–492. [Google Scholar]
- Knierim U., Bulheller M.A., Kuhnt K., Briese A., Hartung J. [Water provision for domestic ducks kept indoors–a review on the basis of the literature and our own experiences] Dtsch Tierarztl Wochenschr. 2004;111:115–118. [PubMed] [Google Scholar]
- Kobayashi A., Sano D., Hatori J., Ishii S., Okabe S. Chicken- and duck-associated Bacteroides-Prevotella genetic markers for detecting fecal contamination in environmental water. Appl Microbiol Biotechnol. 2013;97:7427–7437. doi: 10.1007/s00253-012-4469-2. [DOI] [PubMed] [Google Scholar]
- Koch T. Iowa State University Press; Ames, IA: 1973. Anatomy of the Chicken and Domestic birds. [Google Scholar]
- Kruijt J., Bossema I., Lammers G. Effects of early experience and male activity on mate choice in mallard females (Anas platyrhynchos) Behaviour. 1982;80:32–43. [Google Scholar]
- Kuhnt, K., M. A. Bulheller, J. Hartung, and U. Knierim. 2004. Hygienic aspects of provision of bathing water for Muscovy ducksin standard housing. Page 694 in Book of Abstracts XXII World's Poultry Science Congress.
- Leary, S., and C. L. Johnson. 2020. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. Members of the Panel on Euthanasia AVMA Staff Consultants. AVMA, Schaumburg, IL.
- Leary, S., W. Underwood, R. Anthony, and S. Cartner. 2013. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. AVMA, Schaumburg, IL.
- Lebarbenchon C., Sreevatsan S., Lefevre T., Yang M., Ramakrishnan M.A., Brown J.D., Stallknecht D.E. Reassortant influenza A viruses in wild duck populations: effects on viral shedding and persistence in water. Proc. Biol. Sci. 2012;279:3967–3975. doi: 10.1098/rspb.2012.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson S., Summers J.D. Commercial Poultry Nutrition. Nottingham University Press; Nottingham, England: 2005. [Google Scholar]
- Leeson S., Summers J.D. Commercial Poultry Nutrition. Nottingham University Press; Nottingham, England: 2009. [Google Scholar]
- Leighton A.T., Jr., Potter L.M. Reproductive performance of turkeys subjected to blackout versus brownout restricted light conditions. Poult. Sci. 1969;48:505–514. doi: 10.3382/ps.0480505. [DOI] [PubMed] [Google Scholar]
- Levenick C.K., Leighton A.T., Jr Effects of photoperiod and filtered light on growth, reproduction, and mating behavior of turkeys. 1. Growth performance of two lines of males and females. Poult. Sci. 1988;67:1505–1513. doi: 10.3382/ps.0671505. [DOI] [PubMed] [Google Scholar]
- Lickliter R., Dyer A., McBride T. Perceptual consequences of early social experience in precocial birds. Behav. Processes. 1993;30:185–200. doi: 10.1016/0376-6357(93)90132-B. [DOI] [PubMed] [Google Scholar]
- Lickliter R., Gottlieb G. Training ducklings in broods interferes with maternal imprinting. Dev. Psychobiol. 1986;19:555–566. doi: 10.1002/dev.420190607. [DOI] [PubMed] [Google Scholar]
- Lisney T.J., Ekesten B., Tauson R., Håstad O., Ödeen A. Using electroretinograms to assess flicker fusion frequency in domestic hens Gallus gallus domesticus. Vision. Res. 2012;62:125–133. doi: 10.1016/j.visres.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Liste G., Asher L., Broom D. When a duck initiates movement, do others follow? Testing preference in groups. Ethology. 2014;120:1199–1206. [Google Scholar]
- Loudon A.H., Shanmugasundaram R., Lilburn M.S., Selvaraj R.K. Intestinal physiology and regulatory T cell response to immediate or delayed access to feed and water in Pekin ducklings. Poult. Sci. 2011;90:2041–2046. doi: 10.3382/ps.2011-01347. [DOI] [PubMed] [Google Scholar]
- Lourens A., van den Brand H., Meijerhof R., Kemp B. Effect of eggshell temperature during incubation on embryo development, hatchability, and posthatch development. Poult. Sci. 2005;84:914–920. doi: 10.1093/ps/84.6.914. [DOI] [PubMed] [Google Scholar]
- Makagon M., Mench J. Floor laying by Pekin ducks: Effects of nest box ratio and design. Poult. Sci. 2011;90:1179–1184. doi: 10.3382/ps.2010-01287. [DOI] [PubMed] [Google Scholar]
- Makagon M., Tucker C., Mench J. Factors affecting nest choice by Pekin ducks. Appl. Anim. Behav. Sci. 2011;129:121–128. [Google Scholar]
- Maple Leaf Farms . Maple Leaf Farms Inc.; Leesburg, IN: 2011. Duck Management for Live Production. [Google Scholar]
- Marais M., Maloney S.K., Gray D.A. Ambient temperature modulates the magnitude of LPS-induced fevers in Pekin ducks. J. Therm. Biol. 2011;36:121–127. [Google Scholar]
- Marchand C.R., Sharp P.J. Immunofluorescent localization and ultrastructural characterization of gonadotrophe cells in the adenohypophysis of the barbary drake (Cairina moschata L.) using anti-chicken LH serum. Cell. Tissue. Res. 1977;181:531–544. doi: 10.1007/BF00221774. [DOI] [PubMed] [Google Scholar]
- Max M., McKinnon P.J., Seidenman K.J., Barrett R.K., Applebury M.L., Takahashi J.S., Margolskee R.F. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267:1502–1506. doi: 10.1126/science.7878470. [DOI] [PubMed] [Google Scholar]
- McKinney P. The behaviour of ducks. In: Hafez E.S.E., editor. The Behaviour of Domestic Animals. 2nd ed. Williams & Wilkins; Baltimore, MD: 1969. [Google Scholar]
- Miller D. Social displays of Mallard Ducks (Anas platyrhynchos): effects of domestication. J. Comp. Physiol. Psychol. 1977;91:221–232. [Google Scholar]
- Newberry R.C., Hall J.W. Use of pen space by broiler chickens: Effects of age and pen size. Appl. Anim. Behav. Sci. 1990;25:125–136. [Google Scholar]
- NRC. 1994. Nutrient Requirements of Poultry. National Academies Press, Washington, DC.
- Olanrewaju H.A., Collier S.D., Purswell J.L., Branton S.L. Effects of light-sources and photoperiod on hemato-physiological indices of broilers grown to heavy weights. Poult. Sci. 2018:1075–1082. doi: 10.3382/ps/pey466. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Miller W.W., Maslin W.R., Collier S.D., Purswell J.L., Branton S.L. Effects of light sources and intensity on broilers grown to heavy weights. Part 1: Growth performance, carcass characteristics, and welfare indices. Poult. Sci. 2016;95:727–735. doi: 10.3382/ps/pev360. [DOI] [PubMed] [Google Scholar]
- Olanrewaju H.A., Purswell J.L., Collier S.D., Branton S.L. Effect of light intensity adjusted for species-specific spectral sensitivity on blood physiological variables of male broiler chickens1. Poult. Sci. 2018;98:1090–1095. doi: 10.3382/ps/pey487. [DOI] [PubMed] [Google Scholar]
- Pearson G.L., Cassidy D.R. Perspectives on the diagnosis, epizootiology, and control of the 1973 duck plague epizootic in wild waterfowl at Lake Andes, South Dakota. J. Wildl. Dis. 1997;33:681–705. doi: 10.7589/0090-3558-33.4.681. [DOI] [PubMed] [Google Scholar]
- Porter L., Porter A., Potter H., Alenciks E., Fraley S.M., Fraley G.S. Low light intensity in Pekin duck breeder barns has a greater impact on the fertility of drakes than hens. Poult. Sci. 2018;97:4262–4271. doi: 10.3382/ps/pey289. [DOI] [PubMed] [Google Scholar]
- Ramseyer A., Petit O., Thierry B. Decision-making in group departures of female domestic geese. Behaviour. 2009;146:351–371. [Google Scholar]
- Raud H., Faure J.M. Welfare of ducks in intensive units. Rev Sci Tech. 1994;13:119–129. [PubMed] [Google Scholar]
- Rice M., Meelker A., Fraley S.M., Fraley G.S. Characterization of Pekin duck drinking and preening behaviors and comparison when housed on raised plastic versus pine litter flooring. J. Appl. Poult. Res. 2014;23:735–741. [Google Scholar]
- Rodenburg T., Bracke M., Berk J., Cooper J., Faure J., Guémené D., Ruis M., Rodenburg T.B., Bracke M.B.M., Berk J., Cooper J., Faure J.M., Guemene D., Guy G., Harlander A., Jones T., Kuhnt K., Pingel H., Reiter K., Serviere J., Ruis M.A.W. Welfare of ducks in European duck husbandry systems. World's Poult. Sci. J. 2005;61:633–646. [Google Scholar]
- Romanoff A. Influence of incubation temperature on the hatchability of eggs, post-natal growth and survival of turkeys. J. Agri. Sci. 1935;25:318–325. [Google Scholar]
- Romanoff A. Effects of different tempertures in the incubator on the prenatal and postnatal development of the chick. Poult. Sci. 1936;25:318–325. [Google Scholar]
- Ronald K.L., Sesterhenn T.M., Fernandez-Juricic E., Lucas J.R. The sensory substrate of multimodal communication in brown-headed cowbirds: are females sensory ‘specialists’ or ‘generalists’? J. Comp. Physiol. A Neuroethol. Sensory, Neural, Behav. Physiol. 2017;203:935–943. doi: 10.1007/s00359-017-1203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenboim I., Biran I., Chaiseha Y., Yahav S., Rosenstrauch A., Sklan D., Halevy O. The effect of a green and blue monochromatic light combination on broiler growth and development. Poult. Sci. 2004;83:842–845. doi: 10.1093/ps/83.5.842. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., El Halawani M.E., Kashash Y., Piestun Y., Halevy O. The effect of monochromatic photostimulation on growth and development of broiler birds. Gen Comp Endocrinol. 2013;190:214–219. doi: 10.1016/j.ygcen.2013.06.027. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Piestun Y., Mobarkey N., Barak M., Hoyzman A., Halevy O. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poult. Sci. 2004;83:1413–1419. doi: 10.1093/ps/83.8.1413. [DOI] [PubMed] [Google Scholar]
- RSPCA . Royal Society for the Prevention of Cruelty to Animals; Horsham, West Sussex, UK: 2015. RSPCA Welfare Standards for Domestic/Common Ducks. [Google Scholar]
- Savory C.J. Feather pecking damage in growing bantams is influenced by dietary tryptophan concentration but not dietary protein source. Br. Poult. Sci. 1998;39(Suppl):S17–18. doi: 10.1080/00071669888124. [DOI] [PubMed] [Google Scholar]
- Schenk A., Porter A.L.L., Alenciks E., Frazier K., Best A.A.A., Fraley S.M.M., Fraley G.S.S. Increased water contamination and grow-out Pekin duck mortality when raised with water troughs compared to pin-metered water lines using a United States management system. Poult. Sci. 2016;95:1–13. doi: 10.3382/ps/pev381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker J.O., Yuill T.M., Burgess E.C. Virulence of six strains of duck plague virus in eight waterfowl species. J. Wildl. Dis. 1996;32:453–460. doi: 10.7589/0090-3558-32.3.453. [DOI] [PubMed] [Google Scholar]
- Tai C., Rouvier R. Crossbreeding effect on sexual dimorphism of body weight in intergeneric hybrids obtained between Muscovy and Pekin duck. Genet. Sel. Evol. 1998;30:163. [Google Scholar]
- Tona K., Onagbesan O., Bruggeman V., Mertens K., Decuypere E. Effects of turning duration during incubation on embryo growth, utilization of albumen, and stress regulation. Poult. Sci. 2005;84:315–320. doi: 10.1093/ps/84.2.315. [DOI] [PubMed] [Google Scholar]
- Waitt C., Jones T., Dawkins M. Behaviour, synchrony and welfare of Pekin ducks in relation to water use. Appl. Anim. Behav. Sci. 2009;121:184–189. [Google Scholar]
- Wang Y., Hou S., Huang W., Zhao L., Fan H., Xie M. Lysine, methionine and tryptophan requirements of Beijing ducklings of 0–2 weeks of age. Agric. Sci. China. 2006;5:228–233. [Google Scholar]
- Webb D. Thermal tolerance of avian embryos : a review. Condor. 1987;89:874–898. [Google Scholar]
- Wen C., Chen Y., Wu P., Wang T., Zhou Y. MSTN, mTOR and FoxO4 are involved in the enhancement of breast muscle growth by methionine in broilers with lower hatching weight. PLoS One. 2014;9 doi: 10.1371/journal.pone.0114236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Rasolofomanana T., Tang J., Jiang Y., Xie M., Yang P., Hou S. Effects of dietary energy and lysine levels on growth performance and carcass yields of Pekin ducks from hatch to 21 days of age. Poult. Sci. 2017;96:3361–3366. doi: 10.3382/ps/pex122. [DOI] [PubMed] [Google Scholar]
- Wolf K., Burke C.N. Survival of duck plague virus in water from Lake Andes National Wildlife Refuge, South Dakota. J. Wildl. Dis. 1982;18:437–440. doi: 10.7589/0090-3558-18.4.437. [DOI] [PubMed] [Google Scholar]
- Wozniakowski G., Samorek-Salamonowicz E. First survey of the occurrence of duck enteritis virus (DEV) in free-ranging Polish water birds. Arch Virol. 2014;159:1439–1444. doi: 10.1007/s00705-013-1936-8. [DOI] [PubMed] [Google Scholar]
- Xie M., Sun P.X., Feng Y.L., Jiang Y., Tang J., Huang W., Zhang Q., Hou S.S. Effects of post-hatch brooding temperature on performance of starter and growing Pekin ducks. Poult. Sci. 2019;98:3647–3651. doi: 10.3382/ps/pez203. [DOI] [PubMed] [Google Scholar]
- Xie M., Zhao J.N., Hou S.S., Huang W. The apparent metabolizable energy requirement of White Pekin ducklings from hatch to 3 weeks of age. Anim. Feed Sci. Technol. 2010;157:95–98. [Google Scholar]
- Yahav S., Brake J. Chick embryogenesis: a unique platform to study the effects of environmental factors on embryo development. J. Stem. Cells. 2014;9:17–37. [PubMed] [Google Scholar]
- Ying S., Yang Z., Zhu B., Dai Z., Li Y., Zhao W., Lin Y., Ding W., Shi Z. Bio-bedding with automatically running plough system under slatted floor improving air quality of duck house and duck production performances. Nongye Gongcheng Xuebao/Transactions Chinese Soc. Agric. Eng. 2016;32:188–194. [Google Scholar]
- Zeng Q.F., Zhang Q., Chen X., Doster A., Murdoch R., Makagon M., Gardner A., Applegate T.J. Effect of dietary methionine content on growth performance, carcass traits, and feather growth of Pekin duck from 15 to 35 days of age. Poult. Sci. 2015;94:1592–1599. doi: 10.3382/ps/pev117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Cao J., Wang Z., Dong Y., Chen Y. Effect of a combination of green and blue monochromatic light on broiler immune response. J Photochem. Photobiol. B. 2014;138:118–123. doi: 10.1016/j.jphotobiol.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Chen X., Eicher S.D., Ajuwon K.M., Applegate T.J. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br. J. Nutr. 2016;116:2030–2043. doi: 10.1017/S0007114516003238. [DOI] [PubMed] [Google Scholar]