Abstract
Dysfunction of the intestinal mucosal barrier of chicks caused by Salmonella pullorum is of great harm to the poultry industry. Probiotics are recognized for their beneficial health-promoting properties, promoting maintenance of bowel epithelial integrity and host immune system homeostasis. Our previous research showed that Lactobacillus casei protects jejunal mucosa from injury in chicks infected with S. pullorum. However, the specific mechanisms underlying its protective properties are still not fully understood. In the present study, we aimed to explore the mechanisms underlying the protective effects of L. casei on the intestinal mucosal barrier of chicks infected with S. pullorum through histological, immunological, and molecular biology methods. The results indicated that L. casei significantly reduced the diarrhea rate, increased the daily weight gain, and maintained normal levels of IgA, IgM, and IgG in the serum of chicks infected with S. pullorum. Furthermore, we found that L. casei markedly improved the immunity of gut mucosa by regulating cytokine and chemokine receptor balance, elevating the number of intraepithelial lymphocytes, and hence effectively restraining bowel inflammation. Strikingly, feeding of infected chicks with L. casei notably boosted interleukin-22 expression to activate the Wingless-Int pathway, moderated diamine oxidase and D-lactic acid levels, diminished the generation of myosin light chain kinase, and expanded tight junction protein levels (Zonulin-1 and Claudin-1), strengthening the function of the gut mucosal epithelium. In addition, experiments using 16S rDNA sequencing also demonstrated that L. casei immensely weakened the adhesion of S. pullorum, mainly manifesting as improved diversity of the intestinal microbiota in the V4 area of infected chicks. Taken together, these results show that the application of L. casei may be a good strategy to regulate the intestinal inflammatory response of chicks infected with S. pullorum, providing new perspectives in producing antibiotic substitutes in poultry farms.
Key words: Lactobacillus casei, intestinal mucosal barrier, Salmonella pullorum, Wingless-Int pathway, chicks
INTRODUCTION
The intestinal mucosal barrier is regarded as the fundamental component that offers sufficient containment of luminal microorganisms and molecules while retaining the capability to absorb nutrients (Sánchez de Medina et al., 2014; Odenwald and Turner, 2016). Such barrier is constituted of multiple defensive products, including mucins, antimicrobial peptides, and immunoglobulins (sIgA, IgM, IgG) (Cohen et al., 2012). Increased permeability and/or bacterial translocation are caused by changes of the mucosal barrier function (MBF), as observed in a variety of situations, such as inflammatory bowel disease (IBD) and irritable bowel syndrome (Sperandio et al., 2015).
Two primary compositions of the MBF innate immune response system, namely, intestinal mucosal epithelial cells and innate immune cells such as intraepithelial lymphocytes (IELs), generate immediate protective signaling against pathogen invasion and stimulate the initiation of the adaptive immune response (Soderholm and Pedicord, 2019; Wang et al., 2010; Zhu et al., 2017). Accumulation of defensive mediators such as inflammatory cytokines and chemokines in innate immunity helps to develop a physical and chemical barrier with adequate antimicrobial properties to moderate microbial colonization and invasion processes (Sperandio et al., 2015; Ranhotra et al., 2016). It has been shown that after infection with Gram-negative bacterial strains, TLR4 is a major receptor for recognition, while TLR2 plays an important role in Gram-positive bacteria (Akira and Takeda, 2004). In MBF, Wingless-Int (Wnt) signaling molecules such as Wnt3, β-catenin, TCF-4, and c-Myc are necessary for the regeneration of intestinal epithelial cells, and interleukin-22 (IL-22) also participates in this process (Zhang et al., 2019). An increasing body of evidence shows that gut microbiota is the main determinant of health and disease, and it has also been considered to be part of the intestinal barrier, impacting the host's intestinal growth and immune regulation (Milani et al., 2013; McGuckin et al., 2011). But it remains to be illustrated whether these commensal microorganisms can transform into pathogens after disruption of the MBF.
Salmonella spp., which cause one of the most important gut diseases, are seriously harmful to human health, affecting 10 to 20 million people globally per year (Lettini et al., 2016; Kinsella and Stallings, 2020; Kupz et al., 2014). Among them, Salmonella pullorum can result in pullorum disease, which is extremely common in poultry, with a high incidence and mortality of chicks, persisting in adult chickens without evident clinical symptoms and leading to vertical and horizontal transmission (Li et al., 2019; Xie et al., 2017; Guo et al., 2017; Tadesse and Gebremedhin, 2015). In chicks, the gastrointestinal tract is the most susceptible to invasion of S. pullorum, which can further disturb intestinal homeostasis and contribute to the emergence of pullorum disease (Shivaprasad, 2000). Moreover, the richness of microbiota in the gut, particularly in the V4 zone, is vulnerable to destruction following S. pullorum invasion. For a long time, it has become the normal practice to add the antibiotics (such as tetracycline, gentamicin and kanamycin) to prevent and treat Salmonella infections in the poultry industry. It is nevertheless clear that colonization of the animal intestine by normal microbiota is also inhibited by the long-term large-scale use of antibiotics, which may also induce the generation of drug-resistant strains and veterinary drug residues, which pose a great threat to human health (Lettini et al., 2016; Michael and Schwarz, 2016). Studies have found that probiotics are expected to become alternative antibiotics in poultry farming (Gao et al., 2017).
Currently, an increasing body of evidence shows that Lactobacillus casei may improve IBD therapy by regulating the balance of intestinal microbiota and the host's immune response (Oka and Sartor, 2020; Biagioli et al., 2020). Several research groups, including our own, have also demonstrated that L. casei remarkably impede adhesion and invasion of harmful bacteria in the gut of food animals (Wang et al., 2019a; Wang et al., 2019b; Tabashsum et al., 2020). The results of clinical trials examining the use of L. casei in animal husbandry have pointed out notwithstanding that it is commonly safe, but the underlying mechanisms by which L. casei modulates the intestinal MBF are not fully understood. Based on this background, we speculated that L. casei protects the intestinal mucosa in chicks from damage caused by S. pullorum through enhancing the immunity and promoting the regeneration of intestinal epithelium. In the present study, we observed that administration of L. casei remarkedly strengthened the immunity, modulated the expression of inflammatory-associated factors, activated the Wnt signaling pathway, and increased the diversity of gut microbiota in chicks after S. pullorum challenge.
MATERIALS AND METHODS
Bacterial Strains
Lactobacillus casei DBN023 (CGMCC-16146), a productive probiotic strain, was obtained from the State Key Laboratory of Direct-Fed Microbial Engineering, Beijing DaBeiNong Science & Technology Group Co., Ltd. (DBN), Beijing, China. We fermented and freeze-dried L. casei DBN023 to prepare a powder that we added to the chicks’ basal diet at a dose of 108 CFU/g (Mathara et al., 2004; Wu et al., 2009).
Salmonella pullorum CMCC-533 was purchased from China Medical Microbial Culture Collection (CGMCC), Beijing. In this examination, S. pullorum CMCC-533 was used as a pathogenic strain to induce pullorum disease in chicks through oral administration at d 7; the dose was 109 CFU/mL at 1 mL per chick (Wang et al., 2019b).
Animal Experiments
All trials were conducted on newborn specific pathogen-free White Leghorn chicks purchased from Beijing Boehringer Ingelheim Weitong Biotechnology Co., Ltd., Beijing. A total of 450 newborn healthy chicks were randomly divided into 6 groups, each with 5 repeats and 15 chicks per repeat. All chicks received a nonantibiotic basal diet. We orally administered 1 mL of 109 CFU/mL S. pullorum CMCC-533 suspension per chick in the S. pullorum (SP), prevention (PV), treatment (TM), and prevention plus treatment (PT) groups at d 7. The blank control (CTL) group and the L. casei (LC) group were orally administered an equal amount of phosphate-buffered saline (PBS) instead of S. pullorum. Chicks in the L. casei group received a nonantibiotic diet supplemented with L. casei DBN023 at a dose of 108 CFU/g, and no S. pullorum infection was induced. In the prevention group, a nonantibiotic basal diet supplemented with L. casei DBN023 at a dose of 108 CFU/g was provided at d 1 to d 7. In the treatment group, a nonantibiotic basal diet supplemented with L. casei DBN023 at a dose of 108 CFU/g was provided at d 7 to d 14. In the prevention plus treatment group, we provided a nonantibiotic basal diet supplemented with L. casei DBN023 at a dose of 108 CFU/g throughout the entire experimental period, and chicks were infected with S. pullorum CMCC-533. Each repeat was reared independently in one isolator at a temperature of 20 ± 5°C. Each chick was free to eat and drink for 14 d. Diarrhea and weight were observed and recorded every day. On the 14th d, the sample of chick from each repetition of each group was collected.
Ethical Approval
The present research complied with the Guide for the Care and Use of Laboratory Animals published by the Animal Welfare Committee of the Agricultural Research Organization, China Agricultural University (Beijing, China), and was approved by the University Ethical Committee. An effort was made to minimize the suffering and the number of chicks used.
Sample Collection
On the 14th d, 1 chick from each repetition of each group was randomly selected, and the weight of each chick was recorded. After anesthesia was administered, the animals were sacrificed by cutting the carotid artery. Blood samples were collected and incubated at room temperature for 4 h to obtain the supernatant, which was subsequently stored at −20°C until the determination of antibody levels. Then, the abdominal cavity was dissected. We took 2 middle segments of the jejunum of approximately 1 cm long, and rinsed them with PBS to remove intestinal content. One was stored at −80°C until detection of protein or mRNA contents, while the other was fixed in 4% paraformaldehyde for morphologic observation. Another 3 cm middle segment of the jejunum was obtained and stored at −80°C until the assessment of the abundance of the microbiota. Simultaneously, we also collected the spleen to calculate the organ index.
Enzyme-Linked Immunosorbent Assay
The levels of IgM, IgG, IgA, diamine oxidase (DAO), and D-lactic acid (D-LA) in serum were detected using ELISA assay kits (Shanghai Jianglai Biological Technology Co., Ltd., Shanghai, China). Similarly, expressions of interferon-1β (IL-1β), interleukin-17 (IL-17), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-4, IL-10, IL-22, and myosin light chain kinase (MLCK) in the intestine were determined by ELISA assay kits (Shanghai Jianglai Biological Technology Co., Ltd.). All procedures were conducted in strict accordance with the manufacturer's instructions. The optical density (OD) value was measured at a wavelength of 450 nm, and the sample concentration was calculated from the standard curve.
Quantitative RT-PCR Analysis
Total RNA was extracted using TRIzol reagent (15596026; Invitrogen, Carlsbad, CA). RNA was reverse transcribed into cDNA using a SuperRT cDNA Synthesis Kit (CW0741; CWBIO, Beijing, China). Fecal genomic DNA was isolated by the TIANamp Stool DNA Kit (DP328; TIANGEN, Beijing, China). The relative mRNA expressions of Toll receptors (TLR2 and TLR4), chemokine receptors (CCR5 and CCR9), and Wnt-related genes (c-Myc and cyclin D1) in the intestine were measured using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Similarly, relative fecal DNA levels of S. pullorum and L. casei were also analyzed by qPCR. All gene expression levels were normalized with β-actin as the internal standard. The specific primers were designed by Perl Primer and are listed in Table 1. qRT-PCR and qPCR were performed using an UltraSYBR Mixture (CW0957; CWBIO, Beijing, China). The amplification procedure was 95°C for 10 min, followed by 42 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s, one cycle of 95°C for 30 s and 60°C for 30 s, and 30 s at 40°C. All operations were conducted in strict accordance with the manufacturer's instructions. The 2−△△Ct method was used to analyze the relative gene expression levels.
TABLE 1.
Specific primers for target genes.
| Gene | Primer sequences (5′-3′) | Product size (bp) |
Accession No. |
|---|---|---|---|
| TLR2 | F: ATCCTGCTGGAGCCCATTCAGAG R: TGCTCTTCATCAGGAGGCCACTC |
- | NM_204278.1/NM_001161650 |
| TLR4 | F: ATCTTTCAAGGTGCCACATC R: GGATATGCTTGTTTCCACCA |
167 | NM_001030693 |
| CCR5 | F: GTGGTCAACTGCAAAAAGCA R: GCCCGTTCAACTGTGTCG |
190 | NM_001045834.1 |
| CCR9 | F: GTGCCTCCCTGAGATCATGT R: TGTGCTTTTGGCATCTTTTG |
215 | NM_001045840.1 |
| c-Myc | F: CCCTGCGTGACCAGATACC R: TGCTCGTCCGATTGGATAGA |
106 | NM_001030952 |
| Cyclin D1 | F: CTGCTCAATGACAGGGTGC R: TCGGGTCTGATGGAGTTGT |
221 | NM_205381 |
| Salmonella pullorum | F: CGATAATGGCAACCGCACTG R: TGATGTCTGCCCCTTTCGAC |
356 | SEEP17495 |
| Lactobacillus casei | F: GTGCTTGCACTGAGATTCGACTTA R: TGCGGTTCTTGGATCTATGCG |
128 | - |
| β-actin | F: ACGTCGCACTGGATTTCGAG R: TGTCAGCAATGCCAGGGTAC |
282 | NM_205518 |
Histological Analysis
The fixed jejunum was dehydrated in gradient ethanol concentrations, cleared in xylol, and embedded in paraffin wax. Thin sections (4 μm thick) were cut, fixed on glass slides, and stained with H&E. The sections were first dewaxed in xylene and dehydrated in a gradient of alcohol. Then, nuclei were stained with hematoxylin (H9627; Sigma-Aldrich, St. Louis, MO) for 15 min and differentiated with 1% hydrochloric acid–alcohol solution for 30 s. The sections were blued using tap water for 10 min and dehydrated in alcohol. Next, the sections were submerged in 0.5% eosin (Beijing Chemical reagent company, China) for 40 s. Finally, the sections were routinely dehydrated, made transparent using xylene, and sealed using neutral balsam. Three sections were randomly selected for each group, and about 20 intact villi and crypts were randomly selected for each section. The entire intestinal section was imaged under a 40 × photographic microscope (Ni-U, Nikon, Tokyo, Japan), while the height of villi and the depth of crypts were assessed using Image-Pro Plus software (Media Cybernetics, Inc., Rockville, MD), and the ratio of villus/crypt (V/C) was calculated. Simultaneously, we selected 3 slices of each group and chose 50 cells of five intact villi of each slice to count the number of IELs per 100 cells.
Alcian Blue and Periodic Acid–Schiff Staining
The prepared jejunum paraffin sections were routinely dewaxed with water. The sections were placed in alcian blue staining solution (G1285; Solarbio, Beijing, China) for 15 min, washed with distilled water 3 times, and then submerged in 0.5% periodic acid solution to oxidize for 5 min. After oxidation, the sections were stained in Schiff reagent (G1285; Solarbio, Beijing, China) for 15 min and rinsed in tap water. Then, the sections were differentiated, dehydrated to transparency, and sealed using neutral balsam. Three slices were selected for each group, and five intact intestinal villi were selected for each slice. Fifty IECs were selected on each slide, and the number of goblet cells (GCs) in every 100 IECs was counted.
Immunohistochemistry
Immunohistochemistry was used to analyze the expression of proliferating cell nuclear antigen (PCNA). First, the paraffin sections were dewaxed with distilled water and submerged in 3% H2O2 for 10 min to remove the endogenous peroxidase activity. Then, sodium citrate buffer was used to restore antigen activity, and the sections were incubated in 10% donkey serum in a moist chamber for 25 min at room temperature. Polyclonal rabbit anti-PCNA (1:20; BS-2007R; Beijing Bioss Biotechnology Co., Ltd., Beijing, China) was added to the sections, which were incubated overnight at 4°C. Next, sections were incubated for 1 h at room temperature with a biotinylated goat anti-rabbit IgG (H&L) secondary antibody (1:200; K009; Kmbio, Beijing, China). ABC-peroxidase solution (PK-6102; Vector Laboratories, Burlingame, CA) was added, and the reaction was allowed to take place for 30 min. For color development, 3,3′-diaminobenzidine (DAB; D5637; Sigma-Aldrich, St. Louis, MO) was added, samples were incubated for 5 to 10 min, and hematoxylin was used to counterstain the nuclei for 25 s. The sections were finally dehydrated to transparency and sealed using neutral balsam. Three slices were randomly selected for each group to further choose five different regions of each slice. The average OD of PCNA immunopositive substances in the intestinal glands was calculated using Image-Pro Plus software (version 6.0).
Western Blotting
For the extraction of total protein, 100 μL RIPA lysis buffer (CW2334S; CWBIO, Beijing, China) and 1 μL Protease Inhibitor Cocktail (CW2200S; CWBIO, Beijing, China) were added to 100 mg of jejunal tissue, and the total protein was quantified using a bicinchoninic acid protein assay kit (CW0014; CWBIO, Beijing, China). Protein was separated by SDS-PAGE (Solarbio, Beijing, China) (20 μg per lane) and then transferred onto PVDF membranes (IPVH00010; Millipore, Danvers, MA). Next, the membranes were incubated with polyclonal goat anti-Wnt3 (ab116222; Abcam, UK), polyclonal rabbit anti-β-catenin (ab6302; Abcam, UK), monoclonal rabbit anti-TCF4/TCF7L2 (2565; CST, Boston, MA), polyclonal rabbit anti-ZO-1 (61-7300; Invitrogen, Camarillo, CA), and polyclonal rabbit anti-Claudin (ab129119; Abcam, UK) (all 1:2000 in Tris-buffered saline with Tween) overnight at 4°C. The membranes were incubated with HRP-conjugated rabbit anti-goat IgG (H&L) (1:5000; bs-0294R; Beijing Bioss Biotechnology Co., Ltd., Beijing, China) or HRP-conjugated goat anti-rabbit IgG (H&L) (1:5000; K008; Kmbio, Beijing, China) for 1 h at 37°C. Polyclonal anti-β-actin (1:5000; ab119716; Abcam, UK) was used for normalization of band intensities. The densitometric values of obtained immunoblot signals from 6 separate experiments were determined using Image J (National Institutes of Health, New York).
16S rDNA V4 Region Sequencing
The first step was to obtain bacterial genomic DNA from feces by the SDS method and measuring its concentration. DNA samples were PCR amplified using bar-coded specific primers flanking the V4 region (515F and 806R) of the 16S rRNA gene. The amplified procedure was performed using the following conditions: 95°C for 10 min, followed by 42 cycles of 95°C for 10 s, 60°C for 30 s, and 72°C for 30 s, one cycle of 95°C for 30 s, 60°C for 30 s, and 40°C for 30 s. Then, we purified the PCR product using the GeneJET Gel Extraction Kit (K0691; Thermo, Waltham, MA). We next constructed the genomic library by high-throughput pyrosequencing of the PCR products. Finally, the obtained reads of data were processed by cutting and removing the chimeric sequences, Uparse v7.0.1001 software was used to cluster the operational taxonomic units (OTUs), and then species were annotated.
Statistical Analysis
One-way ANOVA was performed using SPSS 17.0 (SPSS, Inc., an IBM Company, Chicago, IL) for statistical analysis. All data are shown as mean ± SEM. Multiple comparisons between 6 groups were performed using the least significant difference (LSD) test. A value of P < 0.05 was considered statistically significant.
RESULTS
Protective Capability of L. Casei in S. Pullorum-Infected Chicks
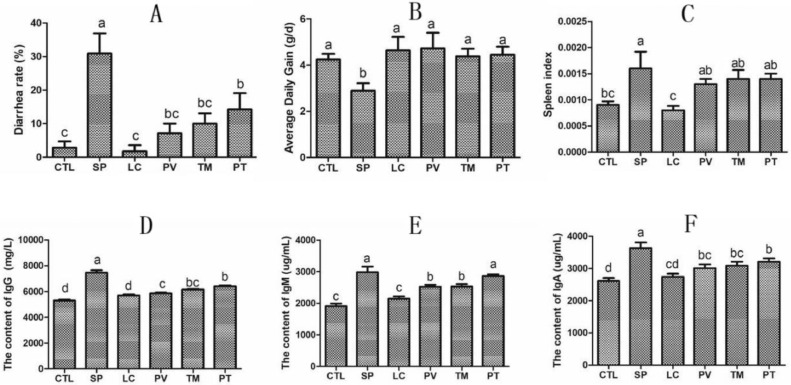
To explore whether L. casei protects chicks from S. pullorum damage, we first detected the diarrhea rate of infected chicks. After S. pullorum infection, the diarrhea rate of chicks in SP group increased by 28.09% (P < 0.05) when compared with the control group. But it was significantly reduced by 23.81%, 20.95%, and 16.67% (P < 0.05) in the PV, TM, and PT groups, respectively, compared with the SP group (Figure 1A). Furthermore, average daily weight gain was recorded to determine growth. Compared with the control group, the daily weight gain of the chicks in the SP group was diminished by 31.90% (P < 0.05). However, it was boosted by 63.29%, 51.42%, and 53.89% (P < 0.05) in the PV, TM, and PT groups, respectively, compared with the SP group (Figure 1B).
Figure 1.
Impact of L. casei on the diarrhea rate, the growth, and the immunity of S. pullorum-infected chicks in different groups. (A) Diarrhea rate. (B) Daily weight gain. (C) The index of the spleen. (D–F) ELISA analysis of serum levels of IgG (D), IgM (E), and IgA (F). CTL is the control group; SP is the S. pullorum group; LC is the L. casei group; PV is the prevention group; TM is the treatment group; PT is the prevention plus treatment group. Results are presented as the mean ± SEM. Different letters between two groups indicate a significant difference (P < 0.05).
In addition, we calculated the organ index of chicks in different groups. After S. pullorum infection, the spleen index in SP group increased by 77.8% (P < 0.05) when compared with the control group (Figure 1C). Although there was no significant difference in the three groups treated with L. casei, they all had a downward trend. Because the spleen is an immune organ, we speculated that L. casei may exert certain immunomodulatory effects. Thus, we analyzed the changes in serum antibody levels in chickens. The serum levels of IgM, IgG, and IgA increased by 56.37%, 40.85%, and 39.15% (P < 0.05) in the SP group, respectively, compared with the control; the levels of these 3 antibodies in the groups treated with L. casei were maintained in a relatively normal state (Figures 1D–1F).
Enhanced Intestinal Immune Function after L. Casei Administration
It is generally true that the mucosal barrier plays a crucial role in maintaining intestinal homeostasis, but it is vulnerable to damage when upon the challenge of S. pullorum. Innate immunity, the pioneering force of the gut, is initiated to identify the pathogen through pattern recognition receptor (PRR) (Kumar et al., 2011). TLR is of the greatest importance in PRR (Kawasaki and Kawai, 2014). To verify whether the protective effects of L. casei on chicks are exerted through modulating the intestinal MBF, we measured the levels of Toll receptors in the intestinal tissues in different groups. S. pullorum resulted in a reduction in TLR2 of 13.82% and an increase in TLR4 levels of 65.00% (P < 0.05) compared with the control group. Interestingly, the levels of TLR4 in the PV, TM, and PT groups were significantly decreased, by 14.39%, 18.09%, 22.61%, respectively, while the levels of TLR2 in the PV, TM, and PT groups were significantly augmented, by 52.57%, 53.27%, and 79.21% (P < 0.05), respectively, compared with the SP group (Figures 2A and 2B).
Figure 2.
Immunoregulatory effects of L. casei on the intestinal mucosal barrier of chicks infected with S. pullorum. (A, B, I, J) Quantitative RT-PCR analysis of (A) TLR4, (B) TLR2, (I) CCR5, and (J) CCR9 in the jejunum. (C–H) ELISA was applied to assess the levels of the proinflammatory cytokines (C) IL-1β, (D) TNF-α, (E) IFN-γ, and (F) IL-17 and the anti-inflammatory cytokines (G) IL-4 and (H) IL-10. (K) IELs counts per 100 IECs. Results are presented as the mean ± SEM. Different letters between two groups indicate a significant difference (P < 0.05).
TLR recognition of pathogen-associated molecular patterns triggers a series of intracellular reactions, eventually leading to the expression of related cytokines and chemokines. Therefore, we examined the levels of inflammatory cytokines in the intestinal tissues of the chicks. IL-1β, IL-17, TNF-α, and INF-γ levels increased by 86.83%, 37.20%, 19.33%, and 32.09% (P < 0.05), respectively, after S. pullorum infection, but their levels were significantly decreased in the PV, TM, and PT groups, which were treated with L. casei, to levels similar to the controls (Figures 2C–2F). By contrast, the expression of IL-4 and IL-10 was decreased by 23.11% and 25.68% (P < 0.05), respectively, upon S. pullorum infection, but they were greatly enhanced in the PV, TM, and PT groups (Figure 2G and 2H). It is worth mentioning that CCR5 and CCR9 levels were elevated by 150% and 90.61% (P < 0.05), respectively, in the S. pullorum group, and they were further increased by 96.58%, 100.4%, and 82.81% (P < 0.05) and 64.31%, 71.17%, and 39.44% (P < 0.05), respectively, in the PV, TM, and PT groups, which were treated with L. casei (Figures 2I and 2J).
The number of IELs in the intestinal epithelium in chicks in the SP group was increased by 13.76% compared with the control group (P < 0.05). In agreement with our results with respect to chemokine levels, a further increase in IEL numbers of 11.64%, 12.71%, and 15.77%, respectively, was observed in the PV, TM, and PT groups compared with the SP group (Figure 2K).
Administration of L. Casei Maintained Intestinal Mucosal Barrier Integrity of Chicks
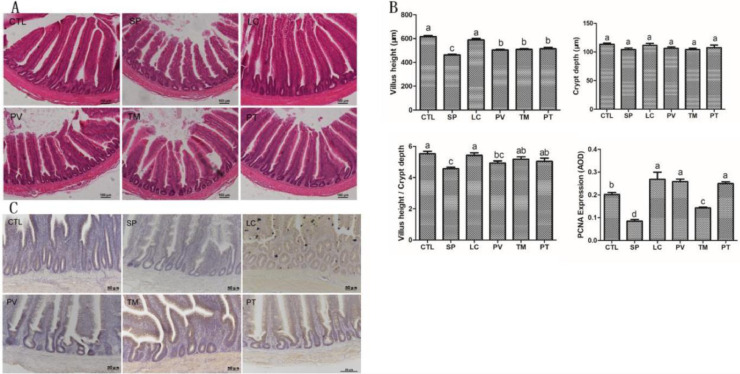
To investigate the effects of L. casei on the barrier function of intestinal mucosa of chicks, we first observed the changes in tissue morphology of the intestine. S. pullorum invasion caused a notable alteration of the epithelium, including exfoliated epithelial cells and damaged and incomplete villi. Of note, L. casei alleviated intestinal damage in the PV, TM, and PT groups, with lower exfoliative epithelial cell counts and relatively complete villi compared with the SP group (Figure 3A). According to our statistical analysis, compared with the control group, the height of the intestinal villi in the SP group was reduced by 33.40% (P < 0.05), but it was improved by 8.97%, 10.08%, and 11.31% (P < 0.05), respectively, in the PV, TM, and PT groups. Although no significant difference in crypt depth was found between different groups, the V/C value had greatly diminished by 17.30% (P < 0.05) in the SP group compared with the controls. Conversely, compared with the SP group, the V/C value of the TM and PT group was increased by 13.13% and 10.40% (P < 0.05), respectively, while there was no significant difference in the PV group (Figure 3B). Subsequently, we examined the mechanism by which L. casei maintains the integrity of the intestinal mucosal barrier. Analysis of PCNA content in intestinal tissues showed that it was mainly expressed in the intestinal crypts of chicks. We noted that in the SP group, the expression of PCNA was reduced by 57.96% (P < 0.05) compared with controls. Successful restoration of PCNA levels was observed in the PV, TM, and PT groups, with increases of 204.5%, 68.27%, and 194.7% (P < 0.05), respectively (Figure 3C).
Figure 3.
Effects of L. casei on the morphology of intestinal villi and the expression of PCNA in chick jejunum. (A) H&E staining analysis of the morphology of intestinal villi. (B) Statistics of villus height, crypt depth, V/C value, and PCNA content. (C) Immunohistochemistry assay of PCNA expression. Results are presented as the mean ± SEM. Different letters between two groups indicate a significant difference (P < 0.05). Abbreviation: PCNA, proliferating cell nuclear antigen.
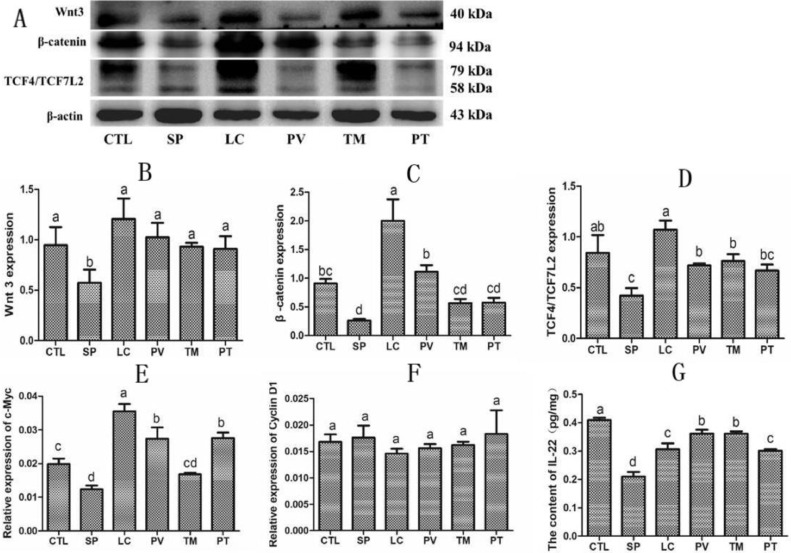
Studies have shown that IL-22 can promote intestinal stem cell-mediated epithelial regeneration (Lindemans et al., 2015), and normal intestinal stem cells require activation of the Wnt signaling pathway (Bradford et al., 2017; Nava et al., 2010). Analysis of IL-22 levels in the intestinal tissue in different groups showed that S. pullorum infection reduced the expression of IL-22 by 48.66% (P < 0.05), and that it was restored by treatment with L. casei in the PV, TM, and PT groups (P < 0.05). We then assessed the potential mechanisms whereby L. casei inhibits S. pullorum-induced intestinal damage. Our results revealed that S. pullorum infection depressed the expression of Wnt-associated molecules, including Wnt3, β-catenin, TCF4/TCF7L2, and c-Myc (P < 0.05). In the PV, TM, and PT groups, the expression of these molecules was hugely ameliorated (P < 0.05). However, neither S. pullorum infection nor L. casei administration caused a significant difference in cyclin D1 levels (Figure 4).
Figure 4.
Supplementation with L. casei increased the levels of Wnt pathway signaling molecules and IL-22 in S. pullorum-infected chicks. (A) Western blot analysis of Wnt3, β-catenin, and TCF4/TCFCL2. (B–D) Statistics of the data in (A). (E, F) Relative expression of (E) c-Myc and (F) cyclin D1 as measured by quantitative RT-PCR. (G) IL-22 levels as determined by ELISA assay. Results are presented as the mean ± SEM. Different letters between two groups indicate a significant difference (P < 0.05). Abbreviation: Wnt, Wingless-Int.
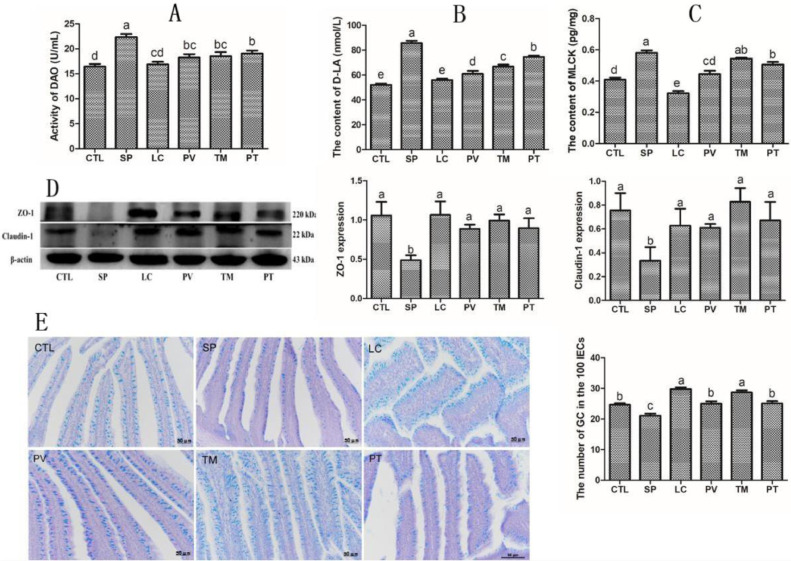
DAO and D-LA, which are regarded as important indicators of intestinal permeability, were also detected in our present research. For S. pullorum-infected chicks, the serum levels of DAO and D-LA were increased by 36.05% and 64.49% (P < 0.05), respectively, compared with the control group. Lactobacillus casei intervention reduced DAO and D-LA contents by 18.35%, 20.76%, and 14.79% (P < 0.05) and 28.77%, 22%, and 13.06% (P < 0.05), respectively, in the PV, TM, and PT groups (Figures 5A and 5B).
Figure 5.
Lactobacillus casei supplementation markedly increased the levels of tight junction-related proteins and the number of goblet cells in S. pullorum-infected chicks. (A–C) ELISA analysis of the levels of (A) DAO, (B) D-LA, and (C) MLCK. (D) Western blot analysis of the levels of ZO-1 and Claudin-1. (E) The number of goblet cells per 100 IECs as counted by alcian blue and periodic acid–Schiff staining. Results are presented as the mean ± SEM. Different letters between two groups indicate a significant difference (P < 0.05). Abbreviations: DAO, diamine oxidase; D-LA, D-lactic acid; MLCK, myosin light chain kinase.
To further examine whether L. casei could inhibit S. pullorum-induced changes in intestinal permeability, we evaluated the contents of MLCK and tight junction proteins (ZO-1 and Claudin-1) in intestinal tissue. Our results showed that the intestinal concentration of MLCK in the SP group was much higher than that in the controls (P < 0.05). MLCK levels were reduced by 23.35% and 13.01% (P < 0.05), respectively, in the PV and PT groups, although no significant difference was identified between the SP and TM groups. Besides, western blot analysis suggested that ZO-1 and Claudin-1 expression levels were reduced by 53.78% and 55.84% (P < 0.05) during S. pullorum infection, while in the PV, TM, and PT groups, this inhibitory effect was strongly reversed, with increases of 81.31%, 102.8%, and 83.44% (P < 0.05), and 82.49%, 147.60% and 101.0% (P < 0.05), respectively (Figures 5C and 5D).
Additionally, since an essential part of GCs is present in intestinal mucosal epithelium, we next assessed the number of GCs. We performed alcian blue and periodic acid–Schiff staining, and found that GCs were mainly present in small intestinal villi. Unfortunately, S. pullorum-infected chicks exhibit massive loss of GCs (P < 0.05) compared with the control group. However, in the PV, TM, and PT groups, the number of GCs was increased by 18.45%, 35.65%, and 18.93% (P < 0.05), reaching even higher levels than in the controls (Figure 5E).
Effect of L. Casei on Intestinal Microbiota Composition of S. Pullorum-Infected Chicks
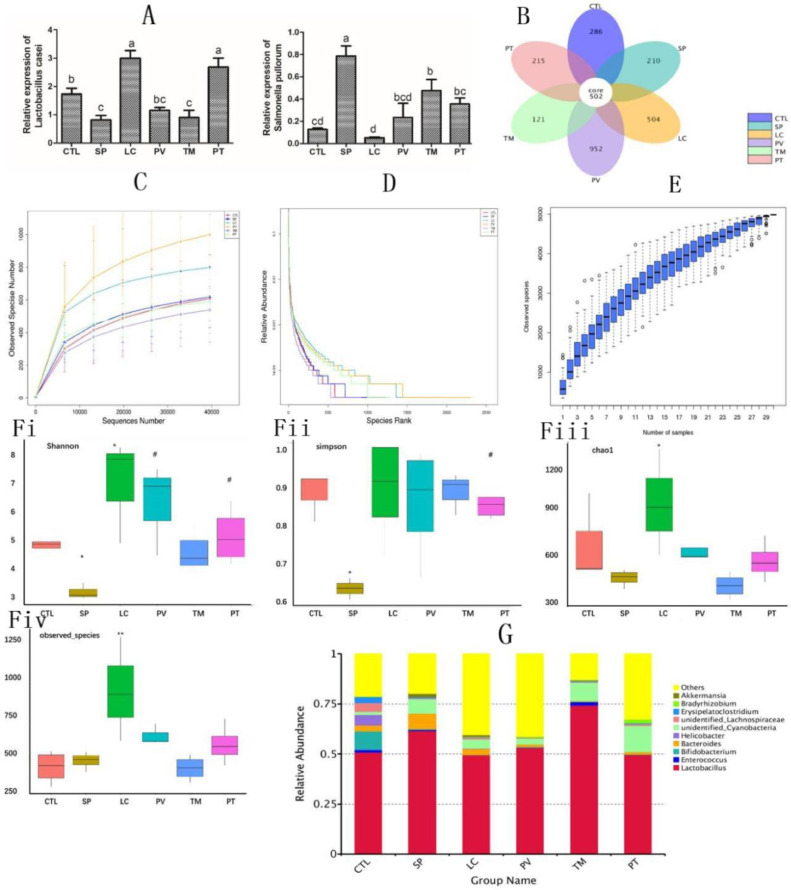
Next, we examined whether S. pullorum and L. casei supplementation could affect the intestinal microbiota. We first determined the colonization of the two bacteria in the gut of different groups. Compared with the control group, L. casei and S. pullorum counts in the LC and SP groups increased by 73.47% and 522% (P < 0.05), respectively. Intestinal S. pullorum counts in chicks in the PV, TM, and PT groups, fed with L. casei, were reduced by 70.03%, 39.14%, and 54.80% (P < 0.05), respectively (Figure 6A).
Figure 6.
Lactobacillus casei supplementation significantly inhibited the colonization of S. pullorum and modulated the balance of gut microbiota in chicks. (A) Quantitative RT-PCR analysis of the colonization of L. casei and S. pullorum. 16S rDNA high-throughput sequencing was applied to the V4 zone of the gut. (B) OTU number. (C) Dilution curves. (D) Hierarchical clustering curves. (E) Species accumulation box. (F) Alpha diversity including (i) the Shannon index, (ii) the Simpson index, (iii) the Chao 1 index, and (iv) observed species analysis of the abundance of the microbiota. (G) Relative abundance of top 10 bacteria. Results are presented as the mean ± SEM. Different letters between two groups indicate a significant difference (P < 0.05).
Furthermore, 16S rDNA high-throughput sequencing was performed to explore the diversity of microbiota in the gut. After removing low-quality sequences, 67,043 clean reads were obtained. Based on the 94.21% similarity level, all the effective reads were clustered into OTUs, and then species were annotated with the Silva132 database. From the petal map, it is clear that a total of 502 OTUs were shared between all groups. Further analysis indicated that the number of OTUs in the LC group (increased by 27.66% compared with the controls) was markedly different from that in the group subjected to S. pullorum infection alone (reduced by 9.64% compared with the controls). However, the number of OTUs was significantly increased in the groups given L. casei supplementation, except for the TM and PT groups, in which it was reduced by 12.5% and only increased by 0.702%, respectively, compared with the SP group (Figure 6B). Sequencing of chick feces genomic DNA amount in each group showed gradual increases, but the dilution curve steadily became flat (Figure 6C). Observing the results from hierarchical clustering, the curves of each group become increasingly smoother along the ordinate, while a certain span was found on the horizontal axis (Figure 6D). With increasing sampling amount of each group, the position of the box diagram tended to be flat, and no more significant boost in the number of bacteria was observed (Figure 6E). Using the Shannon and Simpson indexes, we further determined the abundance of microbes in the gut of chicks. The species diversity of the S. pullorum group was significantly reduced (P < 0.05), while in the L. casei group, it was increased markedly (P < 0.05) compared with the controls. Compared with the SP group, the number of gut microbiota that responded to L. casei supplementation was considerably higher in the PV and PT groups (P < 0.05), even though no significant difference was observed in the TM group (Figures 6Fi and Fii). Investigation of species and Chao 1 indexes showed that L. casei intervention alone significantly enhanced the richness of species when compared with the control group (Figure 6Fiii and Fiv). However, S. pullorum invasion vastly increased the relative abundance of Bacteroides by 164.4% (P < 0.05), while Bifidobacterium abundance was diminished by 95.56% (P < 0.05). Obviously, L. casei administration exerted productive effects in the PV, TM, and PT groups through notably impeding Bacteroides colonization, causing reductions of 83.57%, 94.52%, and 85.44% (P < 0.05), respectively, compared with the SP group (Figure 6G).
DISCUSSION
Previously, we showed the suppressive effect of L. casei on jejunal bacterial pathogens through in vivo studies (Wang et al., 2019a,b). Here, we verified the effectiveness of L. casei against the colonization of S. pullorum in a chick model. According to our in vivo study, L. casei significantly protected S. pullorum-infected chicks against damage, probably through regulating the Wnt signaling pathway. Additionally, L. casei intervention successfully inhibited the colonization of S. pullorum in the gut, maintaining the balance of microbiota.
Salmonella pullorum, which has been regarded as a serious pathogenic bacterium, is widely spread among poultry, causing great economic damage to the breeding industry (Foley and Lynne, 2008). It is currently recognized that long-term use of antibiotics to prevent Salmonella infection has not only resulted in drug-resistant strains but also led to drug residues, posing great threats to human and animal health (Lettini et al., 2016; Michael and Schwarz, 2016; Rowlands et al., 2014; Hu et al., 2014). An increasing body of evidence has shown that probiotics and in particular Lactobacilli have been indicated to exert strain-specific anti-inflammatory effects, becoming a promising alternative to antibiotics in poultry farming (Wang et al., 2019b; Macho Fernandez et al., 2011). It has also been demonstrated that L. casei improves the abundance of the microbiota in the gut following bacterial infection and affects the host's immune response, further maintaining bowel homeostasis (Gotteland et al., 2001; Ivory et al., 2008; Aktas et al., 2015). But the mechanisms underlying the effects of L. casei in the gut of chicks after S. pullorum invasion remain to be elucidated. In our current research, S. pullorum challenge in chicks led to pullorum disease and also lowered the average daily weight gain. After supplementation of L. casei, pullorum disease of chicks was greatly alleviated, and chick weight increased to levels that were even higher than the controls. Considering these positive impacts of L. casei, we next detected organ indexes of chicks in different treatment groups. Interestingly, a very significant effect was only observed in the spleen, rather than liver, heart, and bursa of Fabricius, following application of L. casei, which showed a smaller decrease in organ index. Seemingly, the most important focal point during S. pullorum challenge was the spleen, which is the largest secondary lymphoid organ in animals, with a crucial role in blood filtration and host defense (Noble et al., 2018). Hence, we further evaluated the serum levels of antibodies including IgA, IgM, and IgG in chicks after S. pullorum infection. Strikingly, administration of L. casei ameliorated the changes in serum antibody levels, modulating the host's immune function.
When facing a pathogenic bacterial challenge of the gut, the intestinal mucosal barrier defense is of utmost importance (Kim et al., 2010). Accumulating data, primarily in preclinical animal models, indicate that various probiotics, particularly L. casei, possess anti-inflammatory capacity (Vincenzi et al., 2020; Liu et al., 2020).
In our experiments, we uncovered that supplementation of L. casei markedly enhanced TLR2 content, but TLR4 showed a downwards trend, possibly because TLR4 is recognized as a major receptor of Gram-negative bacteria that is tightly associated with S. pullorum (McGuckin et al., 2011), and thus L. casei exerted protective effects through TLR2. Through further evaluation of inflammatory cytokines, we found that S. pullorum caused increased expression of IL-1β, IL-17, TNF-α, and IFN-γ, while it inhibited the release of IL-1 and IL-10, obviously weakening the immune system of chicks. These observations were significantly reversed in the PV and PT groups, rather than in the TM group, by L. casei supplementation. We hypothesize that preventive interference of L. casei would have greater efficacy. Intestinal levels of CCR5 and CCR9 of infected chicks were considerably elevated; however, L. casei further augmented their contents, further driving the immune response. Intestinal IELs are a large and diverse group of lymphoid cells located between the intestinal epithelial cells that form the intestinal mucosal barrier. IEL subsets communicate with each other and immune cells outside the epithelium, which then strengthens the MBF (Olivares-Villagómez and Van Kaer, 2018). In the present study, IEL numbers in the gut of chicks were greatly elevated after the consumption of L. casei, similarly to the trend of CCR5 and CCR9 secretion. However, the exact roles of IELs after the intervention of L. casei in the gut remain to be elucidated. By acting on those modulators and IELs, L. casei participates in intestinal immune regulation in chicks upon S. pullorum challenge, ensuring effective pathogen elimination.
The above results directly or indirectly support the surmise that L. casei contributes to the maintenance of the intestinal mucosal barrier of infected chicks. The intestinal mucosal epithelium is composed of microvilli, which contain villi and crypts (Barker, 2014; Clevers et al., 2014). The villus height and crypt depth are specific reflections of intestinal function. The villus height of the intestine can reflect the digestive function of the nutrient while the crypt depth can reflect the rate of cell production. That is, higher villi represent the stronger nutrient absorption capacity and shallower crypts indicate better cell maturity of the gut (Ding et al., 2020; Dunsford et al., 1989; Pluske et al., 1996). The structure of villi, which was significantly disrupted by S. pullorum, remarkably ameliorated following supplementation of L. casei, exhibiting increased height. Unfortunately, we observed no significant difference in crypt depth between different groups. The relative PCNA content, which is widely distributed in crypts of the gut, was strongly elevated after L. casei administration in our experiments, showing strong activation of cell proliferation. As is well known, Wnt pathway signaling molecules are necessary for the maintenance of intestinal stem cells, which then mediate intestinal epithelial regeneration, and IL-22 is of importance in this process (Lindemans et al., 2015). Our present experimental data indicate that L. casei strongly increased the expression of IL-22 and Wnt pathway-related regulators, including Wnt3, β-catenin, TCF4/TCF7L2, and c-Myc, but not cyclin D1. We, therefore, speculated that L. casei may play a role in the modulation of the Wnt signaling pathway. Moreover, secretion of DAO, D-LA, and MLCK was markedly reduced, manifesting that the application of L. casei was tightly related to the intestinal mucosal barrier. Besides, tight junction proteins such as ZO-1 and Claudin-1, as well as GC numbers, showed an increasing tendency in different groups supplemented with L. casei. Based on the protective properties of the intestinal mucosal barrier, L. casei may exert its effects through inducing the expression of IL-22 to act on the Wnt signaling pathway. The specific mechanism by which L. casei promotes the secretion of IL-22 and which types of cells take part in this process remain to be explored in future research.
It was reported that the cues from microbial product may influence the immunity and the epithelial cell proliferation, so we further explored the richness of microbiota in our current study. According to the literature, inflammation can provide a favorable environment for pathogens, thereby implicating the role of S. pullorum in disrupting the intestinal microbiota (Drumo et al., 2015; Sekirov et al., 2010). Although the efficacy of probiotics, particularly L. casei, in impeding colonization of enteric pathogenic bacteria is not yet fully established, a number of observations have suggested that the metabolites from probiotics may contribute to the overall health of the host, improving the resistance of pathogenic bacterial colonization (Peng and Biswas, 2017; Garner et al., 2009; Snel et al., 2010). Our recent research showed that by the exogenous addition of L. casei or S. pullorum, the abundance of the corresponding strains in the intestine increased with time, indicating that L. casei and S. pullorum multiply in the intestine and successfully colonize it. But the results from the PV, TM, and PT groups showed clearly that the relative expression of S. pullorum was significantly constrained. Based on our analysis, we speculate that L. casei may act as a disincentive, preventing S. pullorum from colonization in the gut of chicks. Moreover, L. casei notably increased the number of OTUs in infected chicks, in particular in the PV group, which showed more than three times as many OTUs than the controls. High-throughput 16S rRNA sequencing revealed that the introduction of L. casei in the intestine of chicks markedly improved the gut microbial ecosystem balance and the variety of microbes. Specifically, Shannon and Simpson index analysis indicated that the biodiversity of microbe populations was strongly enhanced in the PV and PT groups, rather than in the TM group, by the introduction of L. casei. It has been pointed out that in the chick gut, higher richness of beneficial bacteria including Lactobacillus and Bifidobacterium may be conducive to intestinal homeostasis in a proactive way, preventing against pathogenic bacterial invasion (Peng and Biswas, 2017). It is acknowledged, however, that the elevated abundance of Bacteroides signifies that the balance of the gut is disrupted, which further causes inflammation and leads to secondary infection of other pathogens (Ó Cuív et al., 2017). On the basis of the present findings, S. pullorum greatly enhanced the abundance of Lactobacillus and Bacteroides, and not that of Bifidobacterium. Interestingly, Bacteroides colonization was deeply restrained by the introduction of L. casei. Colonization by S. pullorum was inhibited by L. casei, which showed potent protective effects in infected chicks.
We would like to point out some limitations of the present study. As we only measured certain inflammation-related factors and IELs, the exact mechanisms by which L. casei exerts its anti-inflammatory effects remain to be explored. Our results clearly revealed that L. casei significantly increased the number of IELs, but the potential role of IELs also requires further study. By contrast, our data showed that introduction of L. casei could aid in the maintenance of the intestinal mucosal barrier and modulate the microbiota, but the underlying mechanisms, possibly involving enhanced production of metabolites from L. casei, remain to be explored in future studies.
CONCLUSIONS
Conclusively, here we report on the efficacy of L. casei protecting chicks against S. pullorum infection. Provision of L. casei significantly relieved diarrhea, increased the daily weight gain, and ameliorated the inflammation status. Moreover, L. casei may regulate the Wnt signaling pathway by stimulating IL-22 secretion, reduce MLCK expression, and promote the expression of tight junction proteins, further aiding in the maintenance of intestinal mucosal epithelium integrity. Additionally, L. casei supplementation successfully impeded the colonization of the gut by S. pullorum, modulating the balance of microbiota. Therefore, L. casei may possess beneficial properties with respect to the quality of the poultry facing S. pullorum challenges, providing new perspectives for the production of antibiotic substitutes for poultry farms.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to gratefully acknowledge the National Natural Science Foundation of China (No. 31772686) and the National Key Research and Development Program of China (No. 2018YFD0500606).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Akira S., Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Aktas B., De Wolfe T.J., Tandee K., Safdar N., Darien B.J., Steele J.L. The effect of Lactobacillus casei 32G on the mouse cecum microbiota and innate immune response is dose and time dependent. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat. Rev. Mol. Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- Biagioli M., Carino A., Di Giorgio C., Marchianò S., Bordoni M., Roselli R., Distrutti E., Fiorucci S. Discovery of a novel multi-strains probiotic formulation with improved efficacy toward intestinal inflammation. Nutrients. 2020;12:1945. doi: 10.3390/nu12071945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford E.M., Ryu S.H., Singh A.P., Lee G., Goretsky T., Sinh P., Williams D.B., Cloud A.L., Gounaris E., Patel V., Lamping O.F., Lynch E.B., Moyer M.P., De Plaen I.G., Shealy D.J., Yang G.Y., Barrett T.A. Epithelial TNF receptor signaling promotes mucosal repair in inflammatory bowel disease. Immunol. 2017;199:1886–1897. doi: 10.4049/jimmunol.1601066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Loh K.M., Nusse R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346 doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Cohen M., Varki N.M., Jankowski M.D., Gagneux P. Using unfixed, frozen tissues to study natural mucin distribution. Vis. Exp. 2012;67:e3928. doi: 10.3791/3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.Q., Li H.Y., Wen Z.W., Hou Yong., Wang G.L., Fan J.H., Qian L.C. Effects of fermented tea residue on fattening performance, meat quality, digestive performance, serum antioxidant capacity, and intestinal morphology in fatteners. Animals (Basel). 2020;10:185. doi: 10.3390/ani10020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumo R., Pesciaroli M., Ruggeri J., Tarantino M., Chirullo B., Pistoia C., Petrucci P., Martinelli N., Moscati L., Manuali E., Pavone S., Picciolini M., Ammendola S., Gabai G., Battistoni A., Pezzotti G., Alborali G.L., Napolioni V., Pasquali P., Magistrali C.F. Salmonella enterica Serovar Typhimurium Exploits Inflammation to Modify Swine Intestinal Microbiota. Front. Cell Infect. Microbiol. 2015;5:106. doi: 10.3389/fcimb.2015.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsford B.R., Knabe D.A., Haensly W.E. Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early-weaned pig. J. Anim. Sci. 1989;67:1855–1863. doi: 10.2527/jas1989.6771855x. [DOI] [PubMed] [Google Scholar]
- Foley S.L., Lynne A.M. Food animal-associated Salmonella challenges: pathogenicity and antimicrobial resistance. J. Anim. Sci. 2008;86:E173–87. doi: 10.2527/jas.2007-0447. [DOI] [PubMed] [Google Scholar]
- Gao P.F., Ma C., Sun Z., Wang L.F., Huang S., Su X.Q., Xu J., Zhang H.P. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome. 2017;5:91. doi: 10.1186/s40168-017-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C.D., Antonopoulos D.A., Wagner B., Duhamel G.E., Keresztes I., Ross D.A., Young V.B., Altier C. Perturbation of the small intestine microbial ecology by streptomycin alters pathology in a Salmonella enterica serovar typhimurium murine model of infection. Infect. Immun. 2009;77:2691–2702. doi: 10.1128/IAI.01570-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotteland M., Cruchet S., Verbeke S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment. Pharmacol. Ther. 2001;15:11–17. doi: 10.1046/j.1365-2036.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- Guo R.X., Li Z.Y., Jiao Y., Geng S.Z., Pan Z.M., Chen X., Li Q.C., Jiao X.N. O-polysaccharide is important for Salmonella Pullorum survival in egg albumen, and virulence and colonization in chicken embryos. Avian Pathol. 2017;46:535–540. doi: 10.1080/03079457.2017.1324197. [DOI] [PubMed] [Google Scholar]
- Hu Y.F., Yang X., Lu N., Zhu B.L. The abundance of antibiotic resistance genes in human guts has correlation to the consumption of antibiotics in animal. Gut Microbes. 2014;5:245–249. doi: 10.4161/gmic.27916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivory K., Chambers S.J., Pin C., Prieto E., Arqués J.L., Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin. Exp. Allergy. 2008;38:1282–1289. doi: 10.1111/j.1365-2222.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5:461. doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Ashida H., Ogawa M., Yoshikawa Y., Mimuro H., Sasakawa C. Bacterial interactions with the host epithelium. Cell Host Microbe. 2010;8:20–35. doi: 10.1016/j.chom.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Kinsella R.L., Stallings C.L. A flexible and deadly way to control salmonella infection. Immunity. 2020;53:471–473. doi: 10.1016/j.immuni.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akir S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Kupz A., Bedoui S., Strugnell R.A. Cellular requirements for systemic control of Salmonella enterica serovar Typhimurium infections in mice. Infect. Immun. 2014;82:4997–5004. doi: 10.1128/IAI.02192-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettini A.A., Vo Than T., Marafin E., Longo A., Antonello K., Zavagnin P., Barco L., Mancin M., Cibin V., Morini M., Dang Thi Sao M., Nguyen Thi T., Pham Trung H., Le L., Nguyen Duc T., Ricci A. Distribution of Salmonella serovars and antimicrobial susceptibility from poultry and swine farms in central Vietnam. Zoonoses Public Health. 2016;63:569–576. doi: 10.1111/zph.12265. [DOI] [PubMed] [Google Scholar]
- Li Q.C., Zhu Y., Yin K.Q., Xu L.J., Yin C., Li Y., Ren J.W., Yuan Y., Jiao X.N. Purification of recombinant IpaJ to develop an indirect ELISA-based method for detecting Salmonella enterica serovar Pullorum infections in chickens. BMC Vet. Res. 2019;15:3. doi: 10.1186/s12917-018-1753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemans C.A., Calafiore M., Mertelsmann A.M., O'Connor M.H., Dudakov J.A., Jenq R.R., Velardi E., Young L.F., Smith O.M., Lawrence G., Ivanov J.A., Y Y., Takashima S., Hua G., Martin M.L., O'Rourke K.P., Lo Y.H., Mokry M., Romera-Hernandez M., Cupedo T., Dow L., Nieuwenhuis E.E., Shroyer N.F., Liu C., Kolesnick R., van den Brink M.R.M., Hanash A.M. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.L., Ding J.H., Zhang H.M., Shen J., Hao Y.P., Zhang X.X., Qi W., Luo X.G., Zhang T.C., Wang N. Lactobacillus casei LH23 modulates the immune response and ameliorates DSS-induced colitis via suppressing JNK/p-38 signal pathways and enhancing histone H3K9 acetylation. Food Funct. 2020;11:5473–5485. doi: 10.1039/d0fo00546k. [DOI] [PubMed] [Google Scholar]
- Macho Fernandez E., Pot B., Grangette C. Beneficial effect of probiotics in IBD: are peptidogycan and NOD2 the molecular key effectors? Gut Microbes. 2011;2:280–286. doi: 10.4161/gmic.2.5.18255. [DOI] [PubMed] [Google Scholar]
- Mathara J.M., Schillinger U., Kutima P.M., Mbugua S.K., Holzapfel W.H. Isolation, identification and characterisation of the dominant microorganisms of kule naoto: the Maasai traditional fermented milk in Kenya. Int. J. Food Microbiol. 2004;94:269–278. doi: 10.1016/j.ijfoodmicro.2004.01.008. [DOI] [PubMed] [Google Scholar]
- McGuckin M.A., Lindén S.K., Sutton P., Florin T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- Michael G.B., Schwarz S. Antimicrobial resistance in zoonotic nontyphoidal Salmonella: an alarming trend? Clin. Microbiol. Infect. 2016;22:968–974. doi: 10.1016/j.cmi.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Milani C., Hevia A., Foroni E., Duranti S., Turroni F., Lugli G.A., Sanchez B., Martín R., Gueimonde M., van Sinderen D., Margolles A., Ventur M. Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS One. 2013;8:e68739. doi: 10.1371/journal.pone.0068739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava P., Koch S., Laukoetter M.G., Lee W.Y., Kolegraff K., Capaldo C.T., Beeman N., Addis C., Gerner-Smidt K., Neumaier I., Skerra A., Li L., Parkos C.A., Nusrat A. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32:392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble B.T., Brennan F.H., Popovich P.G. The spleen as a neuroimmune interface after spinal cord injury. J. Neuroimmunol. 2018;321:1–11. doi: 10.1016/j.jneuroim.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Ó Cuív P., de Wouters T., Giri R., Mondot S., Smith W.J., Blottière H.M., Begun J., Morrison M. The gut bacterium and pathobiont Bacteroides vulgatus activates NF-κB in a human gut epithelial cell line in a strain and growth phase dependent manner. Anaerobe. 2017;47:209–217. doi: 10.1016/j.anaerobe.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2016;14:9–21. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A., Sartor R.B. Microbial-based and microbial-targeted therapies for inflammatory bowel diseases. Dig. Dis. Sci. 2020;65:757–788. doi: 10.1007/s10620-020-06090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Villagómez D., Van Kaer L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. Trends Immunol. 2018;39:264–275. doi: 10.1016/j.it.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M., Biswas D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit. Rev. Food Sci. Nutr. 2017;57:3987–4002. doi: 10.1080/10408398.2016.1203286. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996;76:409–422. doi: 10.1079/bjn19960046. [DOI] [PubMed] [Google Scholar]
- Ranhotra H.S., Flannigan K.L., Brave M., Mukherjee S., Lukin D.J., Hirota S.A., Mani S. Xenobiotic receptor-mediated regulation of intestinal barrier function and innate immunity. Nucl. Receptor Res. 2016;3 doi: 10.11131/2016/101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands R.E., Ristori C.A., Ikuno A.A., Barbosa M.L., Jakabi M., Franco B.D. Prevalence of drug resistance and virulence features in Salmonella spp. isolated from foods associated or not with salmonellosis in Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2014;56:461–467. doi: 10.1590/S0036-46652014000600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez de Medina F., Romero-Calvo I., Mascaraque C., Martínez-Augustin O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014;20:2394–2404. doi: 10.1097/MIB.0000000000000204. [DOI] [PubMed] [Google Scholar]
- Sekirov I., Gill N., Jogova M., Tam N., Robertson M., de Llanos R., Li Y, Finlay B.B. Salmonella SPI-1-mediated neutrophil recruitment during enteric colitis is associated with reduction and alteration in intestinal microbiota. Gut Microbes. 2010;1:30–41. doi: 10.4161/gmic.1.1.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivaprasad H.L. Fowl typhoid and pullorum disease. Rev. Sci. Tech. 2000;19:405–424. doi: 10.20506/rst.19.2.1222. [DOI] [PubMed] [Google Scholar]
- Snel J., Born L., van der Meer R. Dietary fish oil impairs induction of gamma-interferon and delayed-type hypersensitivity during a systemic Salmonella enteritidis infection in rats. APMIS. 2010;118:578–584. doi: 10.1111/j.1600-0463.2010.02630.x. [DOI] [PubMed] [Google Scholar]
- Soderholm A.T., Pedicord V.A. Intestinal epithelial cells: at the interface of the microbiota and mucosal immunity. Immunology. 2019;158:267–280. doi: 10.1111/imm.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio B., Fischer N., Sansonetti P.J. Mucosal physical and chemical innate barriers: lessons from microbial evasion strategies. Semin. Immunol. 2015;27:111–118. doi: 10.1016/j.smim.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Tabashsum Z., Peng M.F., Alvarado-Martinez Z., Aditya A., Bhatti J., Romo P.B. Competitive reduction of poultry-borne enteric bacterial pathogens in chicken gut with bioactive Lactobacillus casei. Sci. Rep. 2020;10:16259. doi: 10.1038/s41598-020-73316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse G., Gebremedhin E.Z. Prevalence of Salmonella in raw animal products in Ethiopia: a meta-analysis. BMC Res. Notes. 2015;8:163. doi: 10.1186/s13104-015-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenzi A., Goettert M.I., Volken de Souza C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor Rev. 2020;57:27–38. doi: 10.1016/j.cytogfr.2020.10.004. [DOI] [PubMed] [Google Scholar]
- Wang Y.G., Koroleva E.P., Kruglov A.A., Kuprash D.V., Nedospasov S.A., Fu Y.X., Tumanov A.V. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity. 2010;32:403–413. doi: 10.1016/j.immuni.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Yan X., Han D.P., Liu Y.Y., Song W.P., Tong T.Q., Ma Y.F. Lactobacillus casei DBN023 protects against jejunal mucosal injury in chicks infected with Salmonella pullorum CMCC-533. Res. Vet. Sci. 2019;127:33–41. doi: 10.1016/j.rvsc.2019.09.010. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Yan X., Zhang W.W., Liu Y.Y., Han D.P., Teng K.D., Ma Y.F. Lactobacillus casei Zhang prevents jejunal epithelial damage to early-weaned piglets induced by Escherichia coli K88 via regulation of intestinal mucosal integrity, tight junction proteins and immune factor expression. J. Microbiol. Biotechnol. 2019;29:863–876. doi: 10.4014/jmb.1903.03054. [DOI] [PubMed] [Google Scholar]
- Wu R.N., Wang L.P., Wang J.C., Li H.P., Menghe B., Wu J.R., Guo M.R., Zhang H.P. Isolation and preliminary probiotic selection of lactobacilli from koumiss in inner Mongolia. J. Basic Microbiol. 2009;49:318–326. doi: 10.1002/jobm.200800047. [DOI] [PubMed] [Google Scholar]
- Xie X.L., Hu Y.C., Xu Y.H., Yin K.Q., Li Y., Chen Y., Xia J., Xu L.J., Liu Z.J., Geng S.Z., Li Q.C., Jiao X.N., Chen X., Pan Z.M. Genetic analysis of Salmonella enterica serovar Gallinarum biovar Pullorum based on characterization and evolution of CRISPR sequence. Vet. Microbiol. 2017;203:81–87. doi: 10.1016/j.vetmic.2017.02.010. [DOI] [PubMed] [Google Scholar]
- Zhang X.Y., Liu S.J., Wang Y.Q., Hu H.Q., Li L., Wu Y.B., Cao D., Cai Y.K., Zhang J.Q., Zhang X.L. Interleukin-22 regulates the homeostasis of the intestinal epithelium during inflammation. Int. J. Mol. Med. 2019;43:1657–1668. doi: 10.3892/ijmm.2019.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Ding S.Y., Wang P.H., Wei Z., Pan W., Palm N.W., Yang Y., Yu H., Li H.B., Wang G., Lei X.Q., de Zoete M.R., Zhao J., Zheng Y.J., Chen H.W., Zhao Y.J., Jurado K.A., Feng N.G., Shan L., Kluger Y., Lu J., Abraham C., Fikrig E., Greenberg H.B., Flavell R.A. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature. 2017;546:667–670. doi: 10.1038/nature22967. [DOI] [PMC free article] [PubMed] [Google Scholar]