Abstract
Melatonin (MEL) plays an important role in regulating growth and development of organisms and the cellular metabolism. This study was conducted to explore the role of MEL in mediating monochromatic light-induced secretion of somatostatin (SST) in the hypothalamus and pituitary in chicks. Pinealectomy models of newly hatched broilers were exposed to white (WL), red (RL), green (GL), and blue (BL) lights. The results showed that SST immunoreactive neurons and fibers were distributed in the hypothalamus. SST and SST receptor 2 (SSTR2) mRNA and protein levels in the hypothalamus and pituitary were higher in chicks exposed to RL than in chicks exposed to GL and BL. However, after pinealectomy, the mRNA and protein levels of SST and SSTR2 in the hypothalamus and pituitary in the different light groups were increased, and the differences between the groups disapeared. The expression trend of SSTR5 mRNA in the pituitary was the idential to that of SSTR2 mRNA in the pituitary. In vitro, exogenous SST inhibited growth hormone (GH) secretion, and selective antogonists of SSTR2 and SSTR5 promoted GH secretion. Selective antogonists of the melatonin receptor 1b (Mel1b) and Mel1c increased the relative concentrations of SST in the adenohypophysis cells. These results indicated that monochromatic light affects the expression of SST in chick hypothalamus and pituitary. MEL, via Mel1b and Mel1c, decreased SST secretion under GL, which was associated with the inhibition of SST, SSTR2, and SSTR5 in adenohypophysis cells.
Key words: melatonin, somatostatin, hypothalamus, pituitary, monochromatic light, chick
INTRODUCTION
Growth and development of birds are influenced by various light parameters such as the schedule, intensity, and wavelength. Light is transmitted to the visual center through the retinal ganglion cells (Cao et al., 2012) or acts on the photoreceptors in the brain through the skull (Perez et al., 2019). Light information is then converted into biological signals that affect the secretion of plasma hormones through the pituitary portal system and regulates physiological functions of the body (Yasuo et al., 2004). Previous studies found that green light (GL) significantly elevated liver insulin growth factor-1 (IGF-1) secretion (Li et al., 2016), skeletal satellite cell proliferation (Bai et al., 2016; Halevy et al., 1998), muscle growth (Cao et al., 2008; Liu et al., 2010), and improved meat quality in broilers (Ke et al., 2011; Zhang et al., 2014). While, growth hormone (GH) plays an important role in promoting growth and development, and improving the productive performance of broilers (Reiprich et al., 1995).
GH is one of the main regulatory hormones for avian growth, and is regulated by growth hormone releasing hormone (GHRH) and somatostatin (SST) (Harvey et al., 2014; Meng et al., 2014). SST plays a key biological role in combination with the five different subtypes of the somatostatin receptor (SSTR) subtypes (Meng et al., 2014). Studies have shown that GHRH immunoreactive neurons are distributed in the infundibular nucleus (IN) around the third ventricle, and GL improves GHRH mRNA and protein levels in the hypothalamus and promotes plasma GH secretion in the early stages in broilers (Zhang et al., 2016). However, chicken SST inhibited basal GH release and GHRH-stimulated GH secretion (Piper and Porter, 1997), and affected the secretion of GH in dose-dependent and receptor-specific patters (Cordoba-Chacon et al., 2012). In addition, SST expressed in the chick pituitary was different from that in mammals (Patel, 1999) and primates (Cordoba-Chacon et al., 2012). However, it is unclear whether monochromatic light affects SST levels in the hypothalamus and pituitary, and which types of SSTRs affect GH secretion under monochromatic light in chicks.
Melatonin (MEL) is ubiquitously present across animal species from single-cell organisms to higher vertebrates, and plays an important role in regulating the growth and development of organisms (van Dalum et al., 2020) and cellular metabolism (Majidinia et al., 2018). Previous studies have shown that plasma MEL is positively correlated with plasma GH and hypothalamic GHRH proteins. The levels of plasma GH, and mRNA and protein of hypothalamic GHRH decrease after pinealectomy (PINX) (Yue et al., 2019; Zhang et al., 2016); and MEL mediates the effects of monochromatic GL to trigger the expression of pituitary-specific transcription factor-1 to increase plasma GH through melatonin receptors 1b (Mel1b) and 1c (Mel1c) (Yue et al., 2019). However, whether MEL mediates the effects of monochromatic light on SST levels in the hypothalamus and pituitary, the subtypes of MEL receptors that affect pituitary SST secretion, and intracellular signal transduction involved in these processes in chicks needs to be explored further.
MATERIALS AND METHODS
Animal Treatments and Sampling
One hundred and forty-four newly hatched Arbor Acre male broilers (post-hatching d 0, P0) obtained from Beijing Huadu Breeding Corporation (Beijing) were randomly divided into four groups, and reared under different LED light conditions, including white (WL, 400–700 nm), red (RL, 626 nm), green (GL, 514 nm), and blue light (BL, 466 nm) until P14. The light intensity was -15 lx at the bird-head level, and the schedule was 23 h daily (L:D = 23 h:1 h, lights off at 2300). Food and water were available ad libitum. Sham operation (Sham) and PINX were performed at P3 as described in a previous study (Li et al., 2016). Each light group included intact (n = 10), sham (n = 10), and PINX (n = 10) treatments. The remaining 24 birds were reared under GL for in vitro testing. All animal procedures were approved by the Animal Welfare Committee of the Agricultural Research Organization, China Agricultural University (Beijing).
At P14, all the 120 birds in the light treatment groups were sacrificed by exsanguination under anesthesia with Nembutal (30–40 µg/g). The hypothalamus and anterior pituitary were removed and frozen in liquid nitrogen (−80°C) for quantitative reverse transcription polymerase chain reaction (RT-qPCR) and western blotting. The hypothalamus from the intact WL group of three birds were fixed in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS, pH 7.4) for 48 h, frozen (40 µm in thickness) and paraffin sections (10 µm in thickness) were used for immunohistochemical staining.
Immunohistochemical Staining
Sections were incubated overnight at 4°C with SST polyclonal antibodies (Enzo Life Scienes, New York, NY). Then, the sections were rinsed in 0.01 M PBS and incubated with a biotinylated goat anti-rabbit IgG (Cowin Bio., Taizhou, China) for 3 h at 25°C. After washing, the sections were incubated with streptavidin-conjugated horseradish peroxidase (Vector Laboratories, Burlingame, CA) for 2.5 h at 25°C. Immunoreactivitywas visualized by incubating in 0.01 M PBS containing 0.05% 3’,3-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, St Louis, MO) and 0.003% hydrogen peroxide for 15 min in the dark. The sections were then mounted and stained with hematoxylin, and the control slides without primary antibodies were examined (data not shown). Immunoreactive cells exhibited yellow-brown staining in the cytoplasm of the perikarya. The immunoreactive neurons and fibers were observed using an Olympus BX51 microscope (Japan) according to the stereotaxic atlas of the chick brain (Kuenzel and Masson, 1988).
RT-qPCR
RNA extraction and RT-qPCR amplification from tissues of the hypothalamus and pituitary were performed as described in a previous study (Yue et al., 2019). The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used as the internal standard, and the 2−△△Ct method was used to analyze each sample and gene. The PCR primers used are listed in Table 1.
Table 1.
Sequences of the primers for RT-qPCR.
| Genes | Primer sequences (5’-3’) | Produce size (bp) | Accession No. |
|---|---|---|---|
| SST | F: TCTGGAGTCGGAGGACTTGT | 124 | NM_205336.1 |
| R: GAAGTTCTTGCAGCCCGCTT | |||
| SSTR2 | F: TCTCCATCGTGGTTGCTGTC | 113 | NM_001030345.2 |
| R: GTCGAACATGCCCTTGAGGA | |||
| SSTR5 | F: TCGTGTTGGGTCTACAAGGC | 275 | NM_001024834.1 |
| R: CCTTTCGGAGGCAAAGGACT | |||
| GAPDH | F: ATCACAGCCACACAGAAGACG | 125 | NM_204305.1 |
| R: TGACTTTCCCCACAGCCTTA |
Western Blotting
Proteins were extracted from tissues of the hypothalamus and anterior pituitary, and the protein concentrations were determined as described in a previous study (Yue et al., 2019). Protein concentrations was adjusted to 2 mg/mL. Each sample of 100 µg of protein was separated using 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis, and then electrotransferred onto apolyvinylidene difluoride membrane (Millipore, Billerica, MA). After blocking with 5% skim milk/tris-buffered saline and Tween-20 (TBST) for 2 h at 25°C, the blots were probed with SST polyclonal antibodies (Enzo Life Scienes, New York, NY), SSTR2 (MyBioSource, San Diego, CA), or β-actinantibodies (Proteintech, Wuhan, China) overnight at 4°C. After washing with TBST thrice, the membranes were exposed to horseradish peroxidase-conjugated goat anti-rabbit IgG (Cowin Bio., Taizhou, China) for SST and SSTR2, or goat anti-mouse IgG (Cowin Bio., Taizhou, China) for β-actin for 2 h at 25°C. The protein bands were visualized with a chemiluminescence system (Millipore, Billerica, MA) and were analyzed using image analysis software (ImageJ software, NIH, Bethesda, MD). The results were expressed as the integral optical density (IOD) of the target bands versus the IOD of the β-actin bands, and were tested in triplicate.
Isolation and Primary Culture of Adenohypophysis Cells
Adenohypophysis cells were isolated from the anterior pituitaries of 24 birds in GL according to the protocol described in a previouse study (Yue et al., 2019), and cultured in an incubator at 37°C and 5% CO2 for 48 h. Then, some cells were incubated in 0.001% ethanol (as vehicle), 1.0 × 10−7M SST (Phoenix Biotech (Beijing) Co., Beijing, China), 1.0 × 10−6 μM CYN 154806 (SSTR2 antagonist; Abcam, Waltham, MA), or 1.0 × 10−7 M BIM 23056 (SSTR5 antagonist; Abcam, Waltham, MA), respectively. Another set of cells were incubated in 0.001% ethanol (as vehicle), 1.0 × 10−7 M luzindole (nonselective Mel1a/Mel1b antagonist; Santa Cruz Biotechnology Inc., Dallas, TX), 1.0 × 10−8 M 4-phenyl-2-propionamidotetralin (4P-PDOT, selective Mel1b antagonist; Tocris Bioscience, Abingdon, UK) or 1.0 × 10−7M prazosin (selective Mel1c antagonist; Santa Cruz Biotechnology Inc., Dallas, TX) for 30 min, followed by the addition of melatonin (dissolved inethanol 400 pg/mL; Sigma-Aldrich, St Louis, MO), respectively. The cells were cultured in an incubator at 37°C and 5% CO2 for 24 h. The control cells were incubated in serum-free Dulbecco's modified Eagle medium (DMEM; Gibco, Paisley, UK).
After 24 h, the culture suspernatant and the adenohypophysis cells were collected separately, and processed in two parts. The suspernatant of the adenohypophysis cells was used to measure SST (Jiangsu Meimian Industrial Co. Ltd., Yancheng, China) and GH (Jiangsu Meimian Industrial Co. Ltd., Yancheng, China) concentrations of chicks using enzyme-linked immunosorbent assay (ELISA) kits after centrifugation (5,000 g, 10 min, 4°C). The optical density (OD) was measured at 450 nm using an automated ELISA reader (Bio-Rad, Hercules, CA). The cells was used to determine the mRNA levels of SST by RT-qPCR.
Statistical Analysis
To normalize the values within each group, the values obtained were compared with WL or cell control (set at 1). Each sample was tested in triplicate. Three to 6 chicks from each treatment group were used for in vivo measurements. The data are expressed as the mean ± standard error (SEM). The differences in LED lights and treatments were analyzed using two-way analysis of variance (ANOVA), and the differences in the exogenous additions were analyzed using one-way ANOVA using SPSS 22.0 (SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant. All graphs were plotted using GraphPad Prism 7.0 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Distribution of SST Immunoreactive Neurons and Fibers in the Hypothalamus
Immunohistochemical staining of the hypothalamus of chicks showed that SST immunoreactive neurons were distributed in the paraventricular nucleus (PVN), ventromedial nucleus (VMN), dorsal supraoptic decussation (DSD), and IN. The SST immunoreactive neurons were fusiform, round or oval in shape. Dark brown cytoplasm with clearly demarcated nucleus was obvious in the neurons and some neurons had long streched out processes. SST immunoreactive fibers were distributed in the median eminence (ME) as well as in the PVN, VMN, DSD, and IN (Figure 1).
Figure 1.
Distribution of somatostatin (SST) immunoreactive neurons and fibers in the hypothalamus of chicks. (A) and (E), SST immunoreactive neurons in PVN; (B) and (F), SST immunoreactive neurons in VMN; (C) and (G), SST immunoreactive fibers in ME; (D) and (H), SST immunoreactive fibers in DSD. Scale bar = 100 μm in (A), (B), (C) and (D). Scale bar = 20 μm in (E), (F), (G) and (H). Abbreviations: DSD, dorsal supraoptic decussation; ME, median eminence; PVN, paraventricular nucleus; VNM, ventromedial nucleus.
Effects of Monochromatic Lights and Different Treatments (intact, sham, and PINX) on the Levels of SST mRNA in the Hypothalamus and Pituitary
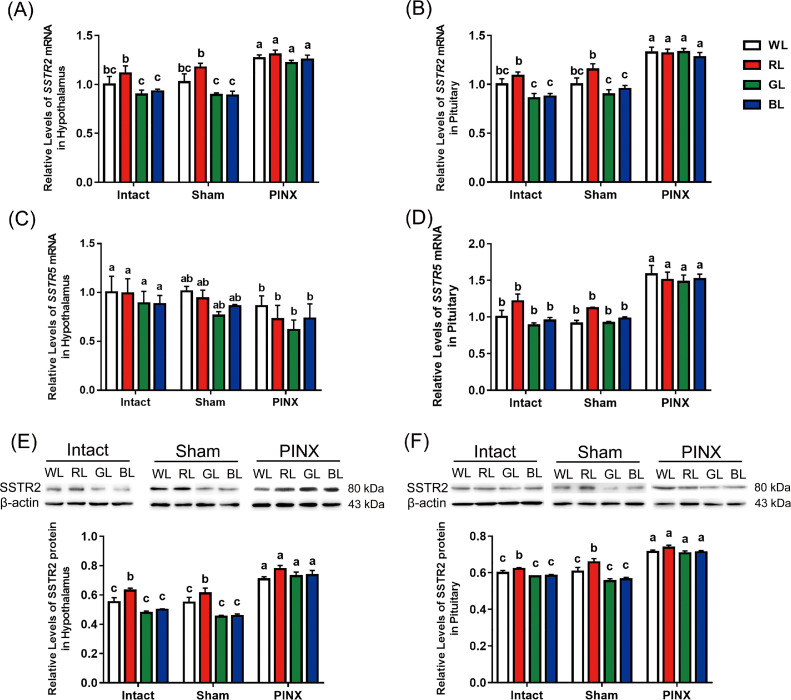
There was no interaction between monochromatic lights and different treatment on the levels of SST mRNA in the hypothalamus (F6,52 = 0.505, P = 0.801) or pituitary (F6,48 = 0.491, P = 0.812). The results showed that both monochromatic lights and other treatments significantly affected SST mRNA levels in the hypothalamus (monochromatic lights: F3,52 = 3.459, P = 0.023; treatments: F2,52 = 17.705, P = 0.000) and pituitary (monochromatic lights: F3,48 = 5.456, P = 0.003; treatments: F2,48 = 56.509, P = 0.000). Main effect analysis showed that SST mRNA was higher in the hypothalamus and pituitary in RL group than that in GL group by 25.40% (P = 0.025) and 13.60% (P = 0.001), respectively. However, no difference was observed between the monochromatic light groups. In the PINX group, SST mRNA was higher in the hypothalamus and pituitary by 37.45% to 39.88% (hypothalamus, P = 0.000) and 26.46% to 27.23% (pituitary, P = 0.000), compared to that in the intact and sham operation groups, respectively (Figures 2A and 2B).
Figure 2.
Effect of monochromatic lights and different treatments on the levels of somatostatin (SST) mRNA and SST protein in the hypothalamus (A and C) and pituitary (B and D) of chicks (n = 3–6). a-dValues within the intact, sham operation (Sham) or pinealectomy (PINX) groups with no common letters are significantly different (P < 0.05). Abbreviations: BL, blue light; GL, green light; RL, red light; WL, white light.
Effects of Monochromatic Lights and Different Treatments on the Levels of SST Protein in the Hypothalamus and Pituitary
There was no interaction between monochromatic lights and different treatments on the levels of SST protein in the hypothalamus (F6,45 = 0.727, P = 0.630).The results showed that the levels of SST protein in the hypothalamus was significantly affected by monochromatic lights (F3, 45 = 9.516, P = 0.000) and treatments (F2, 45 = 15.859, P = 0.000). Main effect analysis showed that SST protein levels were higher in the hypothalamic in RL group than those in GL group by 16.37% (P = 0.000) and in BL group by 14.17% (P = 0.001), respectively. However, there was no significant difference between GL and BL (P > 0.05). In the PINX group, SST protein levels were higher in the hypothalamus by 12.54% (P = 0.000) and 15.58% (P = 0.000), compared to those in the intact and sham operation groups, respectively (Figure 2C).
There was an interaction between monochromatic lights and different treatments on the levels of SST protein in the pituitary (F6,45 = 8.021, P = 0.000). Interaction analysis revealed that SST protein levels in the intact and sham operation groups in GL and BL were lower by 20.43% to 23.22% and 16.37% to 19.28% compared to that in RL group (P = 0.000), respectively. However, in the PINX group, SST protein levels were 1.38% higher in BL group compared to that in WL group (P > 0.05), and the difference was 16.80% (P = 0.000; Figure 2D).
Effects of Monochromatic Lights and Different Treatments on the Levels of SSTR2 and SSTR5 mRNA in the Hypothalamus and Pituitary
There was no interaction between monochromatic lights and different treatments on the levels of SSTR2 mRNA in the hypothalamus (F6,54 = 0.879, P = 0.516) or pituitary (F6,48 = 1.699, P = 0.142). SSTR2 mRNA levels in the hypothalamus and pituitary were significantly influenced by monochromatic light (hypothalamus: F3,54 = 7.515, P = 0.000; pituitary: F3,48 = 6.425, P = 0.001) and treatments (hypothalamus: F2,54 = 31.272, P = 0.000; pituitary: F2,48 = 62.088, P = 0.000). Main effect analysis showed that SSTR2 mRNA was higher in RL group than that in GL and BL groups by19.46% (P = 0.001) and 17.01% (P = 0.002) in the hypothalamus, and by 15.37% (P = 0.002) and 14.59% (P = 0.005) in the pituitary, respectively. In the PINX group, SSTR2 mRNA was higher than intact and sham operation groups by 28.15% (P = 0.000) and 26.86% (P = 0.000) in hypothalamus, and by 37.38% (P = 0.000) and 31.07% (P = 0.000) in pituitary, respectively (Figures 3A and 3B).
Figure 3.
Effects of monochromatic lights and different treatments on the levels of somatostatin receptor 2 (SSTR2), SSTR5 mRNA, and SSTR2 protein in the hypothalamus (A, C and E) and pituitary (B, D and F) of chicks (n = 3–6). a-cValues within the intact, sham operation (Sham) or pinealectomy (PINX) groups with no common letters are significantly different (P < 0.05). Abbreviations: BL, blue light; GL, green light; RL, red light; WL, white light.
There was no interaction between monochromatic lights and different treatments on the levels of SSTR5 mRNA in the hypothalamus (F6,45 = 0.121, P = 0.993) and pituitary (F6,45 = 2.010, P = 0.084). Statistical analysis showed that the levels of SST5 mRNA in the hypothalamus (F2,45 = 3.517, P = 0.038) and pituitary (F2,45 = 56.708, P = 0.000) were sifnificantly affected in the treatment groups. Main effect analysis showed that in the PINX group, SSTR5 mRNA was lower in the hypothalamus by 21.91% compared to that in intact group (P = 0.043). However, the unweighted marginal mean value of SSTR5 mRNA in the PINX group in the pituitary was 47.83% higher than that in the intact group (P = 0.000; Figures 3C and 3D).
Effects of Monochromatic Lights and Different Treatments on the Levels of SSTR2 Protein in the Hypothalamus and Pituitary
There was no interaction between monochromatic lights and different treatments on the levels of SSTR2 protein in the hypothalamus (F6,43 = 1.868, P = 0.108) and pituitary (F6,45 = 1.775, P = 0.126). However, the levels of SSTR2 protein in the hypothalamus and pituitary were influenced by monochromatic light (hypothalamus: F3,43 = 12.667, P = 0.000; pituitary: F3,45 = 10.250, P = 0.000) and treatment groups (hypothalamus: F2,43 = 85.650, P = 0.000; pituitary: F2,45 = 102.038, P = 0.000). Main effect analysis results showed that SSTR2 protein levels were higher in the hypothalamus and pituitary in RL than those in the other light groups by11.63% to 21.74% (P = 0.000–0.013) and 5.16%-9.62% (P = 0.000–0.038), respectively. In PINX group, SSTR2 protein levels in the hypothalamus and pituitary were higher by 36.73% to 42.64% (P = 0.000) and 20.13% to 20.34% (P = 0.000) compared to that in the other treatment groups, respectively (Figures 3E and 3F).
Effects of CYN 154806 and BIM 23056 on GH Secretion by Adenohypophysis Cells
Exogenous SST can significantly inhibited GH secretion by adenohypophysis cells. The relative concentration of GH was lower by 26.30 to 26.43% in the exogenous SST-treated group compared to that in the control (P = 0.000) and vehicle group (P = 0.000). When CYN 154806 (SSTR2 antagonist) and BIM 23056 (SSTR5 antagonist) were added separately, the GH secretion was significantly higher in adenohypophysis cells by 16.74% to 18.49% than that in the control (P = 0.000–0.001) and vehicle groups (P =0.000–0.001). When SST was combined with CYN 154806 and BIM 23056, GH levels in the supernatant of adenohypophysis cells were significantly lower than that in the CYN 154806 and BIM 23056 groups (18.45%–24.08%, P = 0.000), but higher than that in SST group alone (22.07–29.40%, P = 0.000–0.003; Figure 4).
Figure 4.
Effect of somatostatin (SST) and somatostatin receptors (SSTRs) antagonists on growth hormone secretion in primary adenohypophysis cells (n = 24).CYN 154806 is a selective SSTR2 antagonist; BIM 23056 is a selective SSTR5 antagonist. a-cValues with no common letters are significantly different (P < 0.05).
Melatonin Receptors Mediated the Expression and Secretion of SST in Adenohypophysis Cells
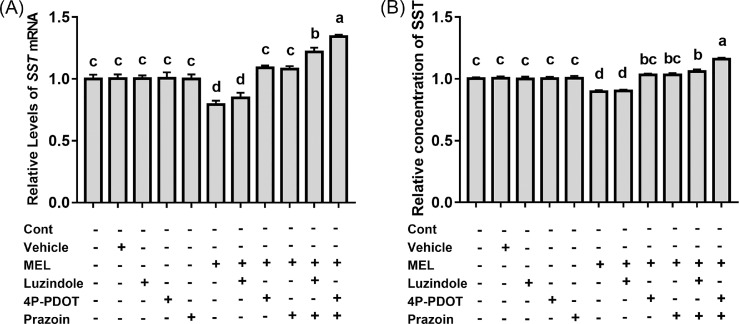
MEL and melatonin receptors (Mel1a, Mel1b, and Mel1c) antagonists affected SST mRNA (F10,33 = 22.002, P = 0.000) and protein concentraitons (F10,55 = 23.961, P = 0.000) in chick adenohypophysis cells (Figure 5). Addition of melatonin receptor antagonist alone did not affect the synthesis and secretion of SST (P > 0.05). Compared to the control group, the SST mRNA and protein levels decreased following addition of MEL by 20.78% (P = 0.000) and 10.33% (P = 0.000), respectively. Compared to the MEL group, the levels of SST mRNA and protein increased by 37.68 % (P = 0.000) and 14.95 % (P = 0.000) in MEL + 4P-PDOT, 36.26 % (P = 0.000) and 15.11 % (P = 0.000) in MEL + Prazosin, 53.75 % (P = 0.000) and 18.08 % (P = 0.000) in MEL + Luzindole + Prazosin, and 69.41 % (P = 0.000) and 29.09 % (P = 0.000) in MEL + 4P-PDOT + Prazosin, respectively. However, there was no difference in SST mRNA and protein levels between the MEL + Luzindole and MEL groups (P > 0.05).
Figure 5.
Effect of melatonin and melatonin receptor antagonists on the levels of somatostatin(SST) mRNA (A) and concentration of SST (B) in primary adenohypophysis cells (n = 24).Luzindole is a nonselective melatonin receptor subtype 1a/1b (Mel1a/Mel1b) antagonist; 4-phenyl-2-propionamidotetralin (4P-PDOT) is a selective Mel1b antagonist; prazosin is a selective melatonin receptor subtype 1c (Mel1c) antagonist. a-cValues with no common letters are significantly different (P < 0.05).
DISCUSSION
A previous study showed that the level of SST was the highest in the hypothalamus of the chick brain (Geris et al., 2000). During the chick embryo development, the level of SST in the hypothalamus is maintained at a low level from embryonic d 14 (E14) to E17. The level of SST in the hypothalamus at E18 is twice that of E17, and reaches a peak at the time of hatching followed by a gradual decrease (Geris et al., 1998). Using radioimmunoassay to analyze SST concentration in one-day-old male layer chicks, Geris et al. (2000) found that SST was expressed in the hypothalamic ME, DMN, VMN, PHN, AM, POP, POM, PVN, and nCPa, and its concentration was highest in the ME. However, SST positive neurons and fibers were only detected in PVN, DSD, VMN, IN, and ME at P14 by immunohistochemistry in this study (Figure 1). The differences in the results of the two studies may be due to differences in the breed and age of the chicks, and the detection methods.
SST has been relatively conserved throughout evolution in vertebrates. For example, SST-14 is an effective inhibitor of GHRH-stimulated GH secretion in frog (Jeandel et al., 1998); SST-14 and SST-28 inhibit GH secretion in adenohypophysis in goldfish (Klein and Sheridan, 2008); SST inhibits thyrotropin releasing hormone stimulated release of GH from pituitary in amphibian and reptile (Hall and Chadwick, 1984); while SST inhibits the basal GH release and GHRH-stimulated GH secretion in chicken (Piper and Porter, 1997). In constrast to mammals, SST is expressed in the hypothalamus and pituitary in chicks (Geris et al., 2000). To identify the mechanisms that mediated the effect of monochromatic light on growth and development of chicks, the levels of SST mRNA and protein in the hypothalamus and pituitary were analyzed by RT-qPCR and western blotting. The results showed that the color of light affected the levels of SST in the hypothalamus and pituitary of chicks, which were the highest in RL group and the lowest in GLgroup (Figure 2). These results are consistent with those of Zhang et al.(2016) and Yue et al.(2019). GL promotes the expression of GHRH in the hypothalamus and secretion of GH in plasma, while RL has the opposite effect. SST is an important regulator of GH, GHRH and SST in the hypothalamus, and SST and GH in the pituitary play an important role in growth and development of chicks exposed to monochromatic light. However, the biological function of SST is mediated through 5 different SSTR subtypes, namely SSTR1-5. Meng et al.(2014) analyzed the expression of SSTRs in different tissues of adult Roman chicks by PCR, and found that the expression levels of SSTR2 in the hypothalamus, and those of SSTR2 and SSTR5 in the pituitary were higher compared to those in the other tissues. Similarly, the changes in SSTR2 in the hypothalamus, SSTR2 and SSTR5 in the pituitary under different light groups were similar to that of SST in the hypothalamus and pituitary (Figure 3). It has been reported that selective agonists of SSTR2 strongly inhibit both basal and GHRH-stimulated GH release at low nanomolar concentrations, while selective agonists of SSTR5 inhibited GH release only under basal conditions (Bossis and Porter, 2001). To validate the in vivo data, adenohypophysis cells were cultured and treated with exogenous antagonists of SSTR2 and SSTR5 to determine the effect of SST on GH secretion in adenohypophysis cells under monochromatic GL. The results showed that exogenous addition of SSTR2 (CYN154806) and SSTR5 (BIM23056) antagonists alleviated the inhibitory effect of SST on GH secretion in adenohypophysis cells. When SST was combined with the receptor antagonists of SSTR2 and SSTR5, separately, the inhibited effect of SST on GH secretion in adenohypophysis cells decreased (Figure 4). These results suggest that the inhibition of GH secretion by SST is mediated via SSTR2 and SSTR5.
MEL is a multieffect hormone secreted by the pineal gland, and regulate a wide variety of physiological functions in the organism (Cipolla-Neto and Amaral, 2018). Previous studies found that monochromatic light influences the level of MEL in the plasma (Jiang et al., 2016; Jin et al., 2011), which is associated with growth and development in chicks (Li et al., 2016). Therefore, PINX was used to reduce the level of MEL circulating blood and explore the role of MEL on the effect of monochromatic light on the secretion of SST in the hypothalamus and pituitary. The results showed that the levels of SST in the hypothalamus and pituitary increased after PINX (Figure 2), suggesting that MEL-mediated the expression of SST in the hypothalamus and pituitary of chicks under monochromatic light. This result is consistent with the decrease in GH mRNA in the pituitary and GH in the plasma after PINX (Yue et al., 2019). Therefore, the above results illustrated that MEL, secreted by pineal glands, inhibited the synthesis and secretion of SST in the hypothalamus and pituitary, thus weakening the inhibition of SST on the synthesis and secretion of GH in adenohypophysis, leading to increased level of GH in the plasma, and promotion of growth. After pinealectomy, the levels of SSTR2 in the hypothalamus, and SSTR2 and SSTR5 in the pituitary increased in all the light groups, and the differences between various light groups disappeared (Figure 3). These results demonstrated that MEL specifically affects the expression of SSTR in the hypothalamus and pituitary.
Studies suggest that MEL regulates physiological functions in an organism through different types of receptors on the target cells, and has tissue specificity in chicks. For example, Mel1a and Mel1c mediate monochromatic light-induced proliferation of B lymphocytes in the bursa of Fabricius (Li et al., 2013). Mel1b and Mel1c mediate monochromatic light-induced proliferation of T lymphocytes in chick thymus (Chen et al., 2016), and the secretion of GH in adenohypophysis cells (Yue et al., 2019), Mel1c mediates monochromatic light-induced secretion of IGF-1 in chick liver (Li et al., 2016). However, Mel1c inhibits GnRH expression via GnIH neurons in the chick hypothalamus (Zhang et al., 2017). We sought to investigate whether MEL receptors mediate the secretion of SST in the same manner as they mediate GH secretion in adenohypophysis under monochromatic GL. We found that the exogenous addition of MEL decreased SST secretion in adenohypophysis cells (Figure 5). When 4P-PDOT (Mel1b selective antagonist) or prazosin (Mel1c selective antagonist) were added, the secretion of SST by adenohypophysis cells increased. When 4P-PDOT and prazosin were added together, the secretion of SST in adenohypophysis cells increased more significantly. The above results indicate that Mel1b and Mel1c mediate the secretion of SST in chick adenohypophysis cells. Combined with our previous data (Yue et al., 2019), our results demonstrate that MEL has dual effects on GH secretion in adenohypophysis cells, by acting both directly and indirectly; MEL can directly promotes the secretion of GH in adenohypophysis cells through Mel1b and Mel1c, and also indirectly promotes the secretion of GH by inhibiting SST in the hypothalamus and pituitary under GL.
ACKNOWLEDGMENTS
We thank for Dr.Xuejin Wang (College of Science, China Agricultural University, Beijing) for testing the emission spectra of LED lamps. The authors would like to thank all the members of neurobiology laboratory. This work was supported by the National Natural Science Foundation of China (31972632, 31572474, 31672501 and 31873000), the Natural Science Foundation of Beijing Municipality (6192012 and 6182018), and the Fundamental Research Funds for the Central Universities (2020TC008).
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Bai X., Wang Y., Wang Z., Cao J., Dong Y., Chen Y. In ovo exposure to monochromatic lights affect posthatch muscle growth and satellite cell proliferation of chicks: role of IGF-1. Growth Factors. 2016;34:107–118. doi: 10.1080/08977194.2016.1199553. [DOI] [PubMed] [Google Scholar]
- Bossis I., Porter T.E. Identification of the somatostatin receptor subtypes involved in regulation of growth hormone secretion in chickens. Mol. Cell Endocrinol. 2001;182:203–213. doi: 10.1016/s0303-7207(01)00561-5. [DOI] [PubMed] [Google Scholar]
- Cao J., Liu W., Wang Z., Xie D., Jia L., Chen Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poult. Res. 2008;17:211–218. [Google Scholar]
- Cao J., Naito J., Chen Y. Retrograde tracing with fluorescent microspheres reveals bifurcating projections from central retina to tectum and thalamus in chicks. Anat. Histol. Embryol. 2012;41:306–310. doi: 10.1111/j.1439-0264.2011.01131.x. [DOI] [PubMed] [Google Scholar]
- Chen F., Reheman A., Cao J., Wang Z., Dong Y., Zhang Y., Chen Y. Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens. J. Photochem. Photobiol. B. 2016;161:9–16. doi: 10.1016/j.jphotobiol.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Cipolla-Neto J., Amaral F.G.D. Melatonin as a hormone: new physiological and clinical insights. Endocr. Rev. 2018;39:990–1028. doi: 10.1210/er.2018-00084. [DOI] [PubMed] [Google Scholar]
- Cordoba-Chacon J., Gahete M.D., Culler M.D., Castano J.P., Kineman R.D., Luque R.M. Somatostatin dramatically stimulates growth hormone release from primate somatotrophs acting at low doses via somatostatin receptor 5 and cyclic AMP. J. Neuroendocrinol. 2012;24:453–463. doi: 10.1111/j.1365-2826.2011.02261.x. [DOI] [PubMed] [Google Scholar]
- Geris K.L., Berghman L.R., Kühn E.R., Darras V.M. Pre- and posthatch developmental changes in hypothalamic thyrotropin-releasing hormone and somatostatin concentrations and in circulating growth hormone and thyrotropin levels in the chicken. J. Endocrinol. 1998;159:219–225. doi: 10.1677/joe.0.1590219. [DOI] [PubMed] [Google Scholar]
- Geris K.L., Meeussen G., Kühn E.R., Darras V.M. Distribution of somatostatin in the brain and of somatostatin and thyrotropin-releasing hormone in peripheral tissues of the chicken. Brain Res. 2000;873:306–309. doi: 10.1016/s0006-8993(00)02550-6. [DOI] [PubMed] [Google Scholar]
- Halevy O., Biran I., Rozenboim I. Various light source treatments affect body and skeletal muscle growth by affecting skeletal muscle satellite cell proliferation in broilers. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998;120:317–323. doi: 10.1016/s1095-6433(98)10032-6. [DOI] [PubMed] [Google Scholar]
- Hall T.R., Chadwick A. Effects of synthetic mammalian thyrotrophin releasing hormone, somatostatin and dopamine on the secretion of prolactin and growth hormone from amphibian and reptilian pituitary glands incubated in vitro. J. Endocrinol. 1984;102:175–180. doi: 10.1677/joe.0.1020175. [DOI] [PubMed] [Google Scholar]
- Harvey S., Gineste C., Gaylinn B.D. Growth hormone (GH)-releasing activity of chicken GH-releasing hormone (GHRH) in chickens. Gen. Comp. Endocrinol. 2014;204:261–266. doi: 10.1016/j.ygcen.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Jeandel L., Okuno A., Kobayashi T., Kikuyama S., Tostivint H., Lihrmann I., Chartrel N., Conlon J.M., Fournier A., Tonon M.C., Vaudry H. Effects of the two somatostatin variants somatostatin-14 and [Pro2, Met13]somatostatin-14 on receptor binding, adenylyl cyclase activity and growth hormone release from the frog pituitary. J. Neuroendocrinol. 1998;10:187–192. doi: 10.1046/j.1365-2826.1998.00188.x. [DOI] [PubMed] [Google Scholar]
- Jiang N., Wang Z., Cao J., Dong Y., Chen Y. Role of monochromatic light on daily variation of clock gene expression in the pineal gland of chick. J. Photochem. Photobiol. B. 2016;164:57–64. doi: 10.1016/j.jphotobiol.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Jin E., Jia L., Li J., Yang G., Wang Z., Cao J., Chen Y. Effect of monochromatic light on melatonin secretion and arylalkylamine N-acetyltransferase mRNA expression in the retina and pineal gland of broilers. Anat. Rec. 2011;294:1233–1241. doi: 10.1002/ar.21408. [DOI] [PubMed] [Google Scholar]
- Ke Y.Y., Liu W.J., Wang Z.X., Chen Y.X. Effects of monochromatic light on quality properties and antioxidation of meat in broilers. Poult. Sci. 2011;90:2632–2637. doi: 10.3382/ps.2011-01523. [DOI] [PubMed] [Google Scholar]
- Klein S.E., Sheridan M.A. Somatostatin signaling and the regulation of growth and metabolism in fish. Mol. Cell Endocrinol. 2008;286:148–154. doi: 10.1016/j.mce.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Kuenzel W.J., Masson M. A stereotaxic atlas of the brain of the chick (Gallus domesticus) Johns Hopkins University Press; Baltimore, MD: 1988. [Google Scholar]
- Li J., Wang Z., Cao J., Dong Y., Chen Y. Melatonin receptor subtypes Mel1a and Mel1c but not Mel1b are associated with monochromatic light-induced B-lymphocyte proliferation in broilers. Domest. Anim. Endocrinol. 2013;45:206–215. doi: 10.1016/j.domaniend.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Li S., Cao J., Wang Z., Dong Y., Wang W., Chen Y. Melatonin mediates monochromatic light-induced insulin-like growth factor 1 secretion of chick liver: involvement of membrane receptors. Photochem. Photobiol. 2016;92:595–603. doi: 10.1111/php.12594. [DOI] [PubMed] [Google Scholar]
- Liu W., Wang Z., Chen Y. Effects of monochromatic light on developmental changes in satellite cell population of pectoral muscle in broilers during early posthatch period. Anat. Rec. 2010;293:1315–1324. doi: 10.1002/ar.21174. [DOI] [PubMed] [Google Scholar]
- Majidinia M., Reiter R.J., Shakouri S.K., Mohebbi I., Rastegar M., Kaviani M., Darband S.G., Jahanban-Esfahlan R., Nabavi S.M., Yousefi B. The multiple functions of melatonin in regenerative medicine. Ageing Res. Rev. 2018;45:33–52. doi: 10.1016/j.arr.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Meng F., Huang G., Gao S., Li J., Yan Z., Wang Y. Identification of the receptors for somatostatin (SST) and cortistatin (CST) in chickens and investigation of the roles of cSST28, cSST14, and cCST14 in inhibiting cGHRH1-27NH2-induced growth hormone secretion in cultured chicken pituitary cells. Mol. Cell Endocrinol. 2014;384:83–95. doi: 10.1016/j.mce.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Patel Y.C. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- Perez J.H., Tolla E., Dunn I.C., Meddle S.L., Stevenson T.J. A comparative perspective on extra-retinal photoreception. Trends Endocrinol. Metab. 2019;30:39–53. doi: 10.1016/j.tem.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Piper M.M., Porter T.E. Responsiveness of chicken embryonic somatotropes to somatostatin (SRIF) and IGF-I. J. Endocrinol. 1997;154:303–310. doi: 10.1677/joe.0.1540303. [DOI] [PubMed] [Google Scholar]
- Reiprich K., Muhlbauer E., Decuypere E., Grossmann R. Characterization of growth hormone gene expression in the pituitary and plasma growth hormone concentrations during posthatch development in the chicken. J. Endocrinol. 1995;145:343–353. doi: 10.1677/joe.0.1450343. [DOI] [PubMed] [Google Scholar]
- van Dalum J., Melum V.J., Wood S.H., Hazlerigg D.G. Maternal photoperiodic programming: melatonin and seasonal synchronization before birth. Front. Endocrinol. 2020;10:901. doi: 10.3389/fendo.2019.00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuo S., Watanabe M., Tsukada A., Takagi T., Iigo M., Shimada K., Ebihara S., Yoshimura T. Photoinducible phase-specific light induction of Cry1 gene in the pars tuberalis of Japanese quail. Endocrinology. 2004;145:1612–1616. doi: 10.1210/en.2003-1285. [DOI] [PubMed] [Google Scholar]
- Yue L., Qin X., Liu X., Wang Z., Dong Y., Chen Y., Cao J. Melatonin receptor Mel1b- and Mel1c-mediated green light induced the secretion of growth hormone in anterior pituitary of chicks. Photochem. Photobiol. 2019;95:1387–1394. doi: 10.1111/php.13127. [DOI] [PubMed] [Google Scholar]
- Zhang L., Cao J., Wang Z., Dong Y., Chen Y. Melatonin modulates monochromatic light-induced GHRH expression in the hypothalamus and GH secretion in chicks. Acta. Histochem. 2016;118:286–292. doi: 10.1016/j.acthis.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Zhang L., Chen F., Cao J., Dong Y., Wang Z., Hu M., Chen Y. Green light inhibits GnRH-I expression by stimulating the melatonin-GnIH pathway in the chick brain. J. Neuroendocrinol. 2017;29:12468. doi: 10.1111/jne.12468. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Wang J., Wu S.G., Qiao X., Yue H.Y., Yao J.H., Qi G.H. Stimulation with monochromatic green light during incubation alters satellite cell mitotic activity and gene expression in relation to embryonic and posthatch muscle growth of broiler chickens. Animal. 2014;8:86–93. doi: 10.1017/S1751731113001882. [DOI] [PubMed] [Google Scholar]