Abstract
Background
The current desirable endpoint of treatment against chronic hepatitis B virus infection (cHBV) is to achieve a functional cure, which is defined as HBsAg loss (sAg-L) with or without anti-HBs seroconversion. However, the immunological features that are associated with functional cure have not been studied in detail.
Methods
172 cHBV patients (67 HBeAg+ and 105 HBeAg-), including 141 HBsAg retained (sAg-R) patients (115 chronic hepatitis and 26 asymptomatic carriers), 31 sAg-L patients, and 24 healthy individuals (vaccinated but not infected with HBV) were examined for their T cell phenotypic profile and HBV-specific T cell responses by flow cytometry. 18 cHBV patients with low serum HBsAg levels were also longitudinally followed for their T cell phenotypic profile and HBV-specific T cell responses up to 60 weeks.
Findings
sAg-L patients showed distinct CD4+ and CD8+ T cell phenotype fingerprints compared to those of sAg-R patients, as mainly indicated by the upregulation of HLA-DR on both CD4+ and CD8+ T cells, and a potent HBcAg-specific CD8+ T cell response. The changes in the T cell phenotype in cHBV patients were even more profound during rapid HBsAg decrease and was associated with interferon α treatment. The expression of HLA-DR (r = 0·3269, p = 0·0037), CD95 (r = 0·2796, p = 0·0151), CD40L (r = 0·2747, p = 0·0156), CTLA-4 (r = 0·2786, p = 0·0148), TIM-3 (r = 0·3082, p = 0·0068), CD107a (r = 0·3597, p = 0·0013) on CD4+ T cells, and HLA-DR (r = 0·3542, p = 0·0016), CD69 (r = 0·2507, p = 0·0279), CD107a (r = 0·2875, p = 0·0112) on CD8+ T cells were positively correlated with the rate of HBsAg decrease. The expression of HLA-DR (r = 0·2846, p = 0·0009) and CD95 (r = 0·2442, p = 0·0049) on CD8+ T cells were positively correlated with the magnitude of the HBcAg-specific T cell responses in cHBV patients. Importantly, CTLA-4, CD95 and CD107a expression on CD4+ T cells, as well as HLA-DR and TIM-3 expression on CD8+ T cells in combination with HBsAg quantification were identified as potential predictive factors for sAg-L within 48 weeks in cHBV patients.
Interpretation
The onset of HBsAg decrease and subsequent loss in cHBV patients on treatment is associated with significant alterations of both CD4+ and CD8+ T cell phenotypes. Characterization of the T cell phenotype in cHBV patients may present predicative value for sAg-L.
Funding
National Natural Science Foundation of China, National Scientific and Technological Major Project of China, Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College, “Double-First Class” Project for the International Cooperation Center on Infection and Immunity, HUST.
Keywords: Chronic HBV infection, Functional cure, HBsAg, T cell phenotype, HBV-specific T cell
Research in context.
Evidence before this study
T cells are believed to play an irreplaceable role in HBV clearance and determine the outcome of HBV infection. Previous studies demonstrate that the quantity and function of HBV-specific T cells are associated with the outcome of HBV infection. Thus, a better understanding of HBV-specific T cell biology is believed to have significant implications for guiding clinical practice in cHBV treatment and for developing effective immunotherapy to cure cHBV. However, available data on T cell responses related to cHBV functional cure mainly come from cross-sectional studies, there is currently a lack of longitudinal analysis of the T cell phenotype and function during the course of HBsAg loss and seroconversion in cHBV patients.
Added value of this study
Our study analyzed T cell phenotype profiles and HBV-specific T cell responses in a group of 31 HBsAg loss patients and compared with 141 HBsAg-positive patients. We also characterized T cell phenotype profiles and HBV-specific T cell responses during the course of HBsAg loss, rapid decrease and seroconversion in the peripheral blood mononuclear cells (PBMCs) of 18 cHBV patients. The results indicate that cHBV patients with HBsAg loss showed distinct CD4+ and CD8+ T cell phenotype profiles and the alteration of T cell phenotype in cHBV patients with HBsAg loss was associated with IFN-α therapy. Monitoring T cell phenotype combined with HBsAg quantification possesses potential predictive value for predicting HBsAg loss in cHBV patients.
Implications of all the available evidence
The findings of our study provide incremental evidence to monitor T cell phenotype during the course of HBsAg decrease and subsequent loss in cHBV patients. This knowledge may assist in developing assays to precisely evaluate immune status and to improve treatment strategies for achieving functional cure in cHBV patients.
Alt-text: Unlabelled box
1. Introduction
The World Health Organization has implemented a Global Strategy on Viral Hepatitis to declare that the elimination of hepatitis B virus (HBV) is possible [1]. Currently, most clinical practice guidelines recommend that the optimal endpoint of chronic HBV infection (cHBV) treatment is to achieve functional cure, which is defined as HBsAg seroclearance with or without anti-HBs seroconversion [2,3]. This is based on the observation that the HBsAg seroclearance is associated with a beneficial effect on disease progression and reduced risk of hepatocellular carcinoma (HCC) development [4]. Spontaneous HBsAg seroclearance is common in acute HBV infection in immunocompetent adults, but it is a rare clinical event in untreated chronic HBV infected patients with annual clearance rates ranging from 0·7% to 2·26% [4,5].
T cells are believed to play an irreplaceable role in HBV clearance and determine the outcome of HBV infection as demonstrated in the HBV chimpanzee model [6,7]. Therefore, T cells from patients at different stages of HBV infection have been intensively characterized for their phenotypic profile and function [8,9]. Available data indicate that the quantity and function of HBV-specific T cells are associated with the outcome of HBV infection [10,11]. Strong HBV-specific CD4+ and CD8+ T cell responses were frequently detected in patients who spontaneously cleared the virus during acute HBV infection, but were not found in cHBV patients [10,12]. During cHBV infection, an association between increased HBcAg/HBeAg-specific T cell responses and HBeAg seroconversion was also reported [13]. Moreover, the recovery of HBV-specific T cell responses was observed in cHBV patients after long-term effective therapy with nucleos(t)ide analogues (NUCs), and in patients who achieved a functional cure either spontaneously or under antiviral treatment [14]. A recent study also demonstrated that the presence of functional HBV-specific T cells may serve as a potential immune system biomarker to safely discontinue NUC therapy in chronic hepatitis B (CHB) patients [11]. Thus, a better understanding of HBV-specific T cell biology is believed to have significant implications for guiding clinical practice in cHBV treatment and for developing effective immunotherapy to cure cHBV. However, current available data on T cell responses related to cHBV functional cure mainly come from cross-sectional studies [[15], [16], [17]], and a longitudinal analysis of the T cell phenotype and function during the course of HBsAg loss (sAg-L) and seroconversion in cHBV patients is still lacking. In this study, T cell phenotype profiles and HBV-specific T cell responses were longitudinally analyzed in a group of cHBV patients prior to, during and after achieving functional cure upon treatment and compared with HBsAg-positive patients. Furthermore, T cell features correlating with rapid HBsAg decrease, loss and seroconversion in cHBV patients were characterized.
2. Methods
2.1. Subjects
A total of 24 healthy controls (HC) vaccinated but not infected with HBV, 141 HBsAg retained (sAg-R) cHBV patients, and 31 sAg-L cHBV patients were recruited by the Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from June 2016 to March 2019. The diagnosis and phase classification of chronic HBV infection were based on the EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection [3]. All patients tested negative for HIV, HCV, hepatitis E virus, and hepatitis delta virus. Patients with alcoholic liver disease, autoimmune disease, malignancy, or serious illness of other systems were excluded. Written informed consent was obtained from each patient, and the study protocol was approved by the local medical ethics committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (S016) in accordance with the guidelines of the Declaration of Helsinki.
2.2. Characteristics of study cohort
Demographic profiles and detailed patient characteristics are listed in Tables 1 and S1. There were 67 HBeAg-positive and 105 HBeAg-negative patients. At the time of recruitment, serum HBV DNA levels of 6 cHBV patients surpassed 200,000 IU/mL, 10 patients were between 2000 and 200,000 IU/mL, 9 patients were between 100 and 2000 IU/mL, and 147 patients were below 100 IU/mL. 11 patients were analyzed for HBV genotypes, 7 were genotype B and 4 were genotype C, which is consistent with previous reports that the dominant genotypes of HBV prevalence in China are B and C [18]. All cHBV patients were monitored for serum HBV virology markers during the observation period between 2 and 18 times. Eighteen cHBV patients who met the following criteria have been longitudinally monitored for their T cell phenotype profile and HBV-specific T cell response for 60 weeks: (1) NUCs treated patients with undetected serum HBV DNA; (2) experienced sharp decline of serum HBeAg levels (over 500 index/mL) or HBeAg seroconversion after NUCs treatment; (3) serum HBsAg below 1000 IU/ml. All patients in the sAg-R group remained serum HBsAg positive during the observation period of 60 weeks. Six patients in the sAg-L patient group were serum HBsAg positive at the start of the study and experienced HBsAg loss during the observation period within 48 weeks, and 3 of them showed HBsAg seroconversion. Twenty-five patients were already serum HBsAg negative at the time of recruitment, including 5 became sAg-L within 3 months, 10 within 12 months, 7 within 2 years, and 3 within 5 years. A significantly higher percentage of patients in the sAg-L group compared to the sAg-R group (58·07% vs 9·93%) had received interferon α (IFN-α) treatment either alone or in combination with NUCs for more than 24 weeks. For cHBV patients, 107 of them received NUCs monotherapy treatment, 32 received interferon α (IFN-α) combined or monotherapy treatment and the others received no antiviral treatment.
Table 1.
Demographics and baseline characteristics of study subjects.
| Healthy controls | HBsAg retained | HBsAg loss |
||
|---|---|---|---|---|
| sAg-L at enrolment | sAg-L during follow up | |||
| Number of subjects | 24 | 141 | 25 | 6 |
| Age, years, median (IQR) | 25 (24–27) | 32 (27–39) | 40 (30–43·50) | 30 (24·75–46) |
| Male sex,% (n) | 33·33% (8) | 76·60% (108) | 88·00% (22) | 66·67% (4) |
| ALT, U/L, median (IQR) | n.d. | 24 (14–36·50) | 23 (17–46·50) | 24 (16–72·75) |
| HBeAg-positive,% (n) | n.d. | 47·52% (67) | n.d. | n.d. |
| HBeAg, log10 index/mL, median (IQR) | n.d. | 1·73 (1·30–2·96) | n.d. | n.d. |
| HBsAg, log10 IU/mL, median (IQR) | n.d. | 2·61 (1·64–2·92) | n.d. | 0·78 (−0·35–1·30) |
| HBV DNA- negative% (n) | n.d. | 82·98% (117) | n.d. | 83·33% (5) |
| HBV DNA, log10 IU/mL, median (IQR) | n.d. | 3·60 (3·18–4·98) | n.d. | 5·86 |
| anti-HBs-positive,% (n) | n.d. | n.d. | 52·00% (13) | n.d. |
| Treatment, IFN,% (n) | n.d. | 1·42% (2) | 20·00% (5) | 0 (0) |
| Treatment, NUCs+IFN,% (n) | n.d. | 8·51% (12) | 28·00% (7) | 100% (6) |
| Treatment, NUCs,% (n) | n.d. | 71·63% (101) | 24·00% (6) | 0 (0) |
| Without treatment,% (n) | n.d. | 18·44% (26) | 28·00% (7) | 0 (0) |
The cutoff values between positive and negative for HBsAg, anti-HBs, and HBeAg are 0·1 IU/mL, 10 mIU/mL, and 2·5 index/mL, respectively.
Abbreviations: sAg-L, HBsAg loss; IQR, interquartile range; ALT, alanine aminotransferase; IFN, interferon; NUCs, nucleos(t)ide analogues; n.d., not detectable.
2.3. Serological assays
Sera of study subjects were tested for routine hepatitis B serological markers (HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc) by commercial methods (Maglumi X8, SNIBE Co. Ltd., Shenzhen, China). A quantitative assay for HBsAg was conducted using the fully automated MAGLUMI HBsAg (SNIBE Co. Ltd., Shenzhen, China) assay with a detection range of 0·02- 250 IU/mL. If the HBsAg level was higher than 250 IU/mL, the samples were diluted 1:100–1:1000 to obtain a reading within the range of the calibration curve. A concentration higher than 0·10 IU/mL was considered HBsAg positive.
2.4. Isolation of peripheral blood mononuclear cells (PBMCs)
PBMCs of healthy controls and patients were isolated using Ficoll density gradient centrifugation (DAKEWE Biotech, Beijing) and were freshly used for flow cytometry analysis.
2.5. Flow cytometry
Surface and intracellular staining for flow cytometry analysis were performed as described previously [19,20]. The antibodies used for surface and intracellular staining are listed and their Research Resource Identifiers (RRID) tags are provided in Table S3. For surface staining, cells were incubated with relevant fluorochrome-labelled antibodies for 20 min at 4 °C in the dark. For intracellular cytokine staining (ICS), cells were fixed and permeabilized using the Intracellular Fixation & Permeabilization Buffer Set (Invitrogen, USA) and stained with FITC-anti-IFN-γ, PE-anti-IL-2, or APC-anti-TNF-α (eBioscience, San Diego, USA). Freshly isolated cells were used for all assays, and approximately 20 000–40 000 T cells were acquired for each sample using a BD FACS Canto II flow cytometer. Data analysis was performed using Flow Jo software V10.0.7 (Tree Star, Ashland, OR, USA). Cell debris and dead cells were excluded from the analysis based on scatter signals and Fixable Viability Dye eFluor 506 (Thermo Fisher Scientific).
2.6. Analysis of the HBV-specific CD8+ T cell response in patients
The HBV-specific CD8+ T cells were detected after antigen-specific expansion as previously described [21]. Briefly, PBMCs were resuspended in complete medium (RPMI 1640 containing 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μM HEPES) and stimulated with overlapping peptide pools covering the entire sequences of HBcAg or HBsAg (genotype B and C, GeneBank accession number: AF121243 and AF112063), anti-CD28/CD49d (0.5 μg/ml; BD Biosciences), and recombinant interleukin-2 (20 U/ml; Hoffmann-La Roche). Fresh medium containing IL-2 was added twice per week. On day 10, the cells were tested for the expression of IFN-γ, TNF-α and IL-2 after re-stimulation with corresponding peptide pools by intracellular cytokine staining and subsequent flow cytometry analysis.
2.7. Statistical analysis
Statistical significance was determined by GraphPad Prism version 8.3 (GraphPad Software, Inc., CA, USA) or SPSS version 26 (IBM, Chicago, IL, USA) using tests as stated in the figure legends. Significant differences between three or more groups were determined by the Kruskal-Wallis test followed by Dunn's multiple comparisons test. Significant differences between two groups were determined by the Mann-Whitney test. The percentages of T cell markers were log2-transformed and presented as z-scores in the heat maps to demonstrate the expression intensities of the markers. Correlations between variables were performed by Spearman's Rank correlation test. Binary logistic regression analysis was performed to fit immunological parameters with HBsAg quantification, and the power of the selected parameters for predicting HBsAg loss in cHBV patients were assessed by receiver operating characteristic (ROC) and area under the ROC curve (AUC). All reported P values were two-sided, and a P value less than 0·05 was considered statistically significant.
2.8. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Alteration of the T cell phenotype in sAg-L patients and patients experiencing rapid HBsAg decrease
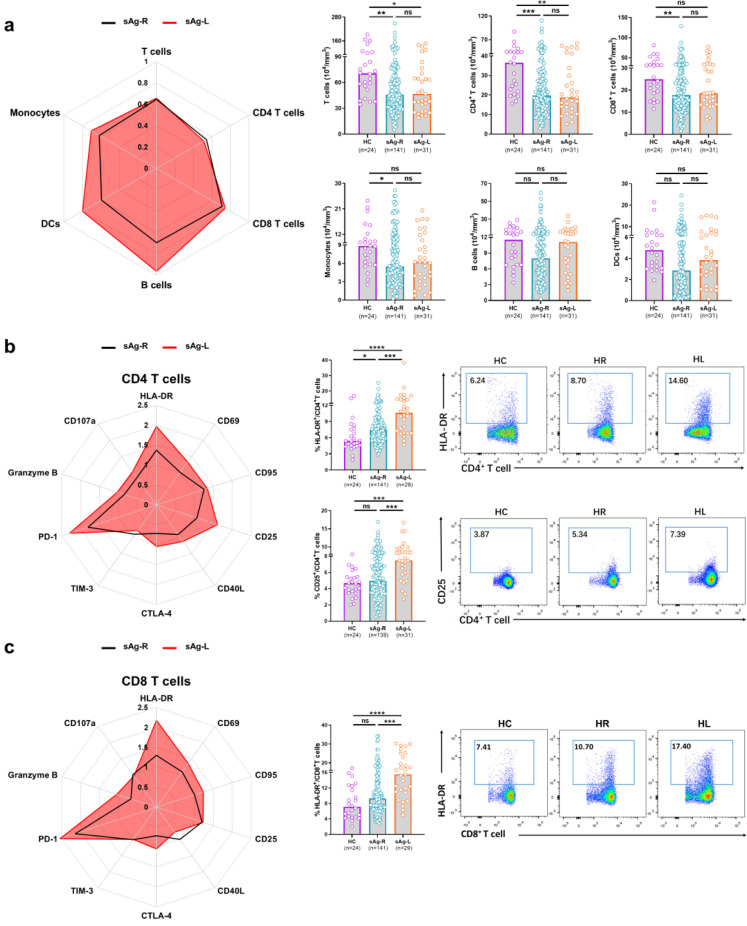
First, we characterized ex vivo whether immune cell populations and the T cell phenotype in PBMCs changed in sAg-L patients after HBsAg loss compared to sAg-R patients and HC by flow cytometry (as depicted in Figs. 1a and S1). There were no significant differences in age, ALT levels, and sex between the sAg-R and sAg-L patient groups. However, the HC group had significantly lower age and higher percentage of females than the other two groups. The absolute numbers of total T cells as well as CD4+ T cells in sAg-R (45·80×104/mm3 vs 70·70×104/mm3, p = 0·0010; 19·77×104/mm3 vs 36·52×104/mm3, p = 0·0004, Kruskal-Wallis test) and sAg-L (46·64×104/mm3 vs 70·70×104/mm3 p = 0·0290; 18·74×104/mm3 vs 36·52×104/mm3, p = 0·0059, Kruskal-Wallis test) patients were significantly lower than those in HC. sAg-R but not sAg-L patients also showed significantly lower frequencies of total T cells (31·68% vs 39·99%, p = 0·0126, Kruskal-Wallis test) as well as absolute numbers of CD8+ T cells (17·74×104/mm3 vs 24·94×104/mm3, p = 0·0097, Kruskal-Wallis test) and monocytes (5·46×104/mm3 vs 8·82×104/mm3, p = 0·0200, Kruskal-Wallis test) than HC. sAg-L patients showed significantly higher CD8+ T cell frequencies than HC (46·70% vs 39·10%, p = 0·0280, Kruskal-Wallis test) (Fig. 1a and S1c). sAg-R patients also showed lower absolute numbers and frequencies of B cells and dendritic cells (DCs) than sAg-L patients and HC, however, the differences were not statistically significant (Fig. 1a and S1c).
Fig. 1.
Characterization of lymphocyte subsets and T cell phenotype profiles in cHBV patients with HBsAg loss. Radar plots depicting the fold changes of the median numbers of lymphocyte subsets (a), median percentages of CD4+T cells (b) and CD8+T cells (c) expressing different T cell markers in the PBMC for HBsAg retained patients (sAg-R, n = 141) and HBsAg loss patients (sAg-L, n = 31) compared to those of healthy controls (HC, n = 24). The median values of all detection indexes of HC are defined as 1. Representative flow cytometry analyses showing HLA-DR, CD25 expression on CD4+T cells (b) and HLA-DR expression on CD8+T cells (c) obtained from HC, sAg-R and sAg-L patients. Data are presented as median and statistical analysis was performed by GraphPad Prism using Kruskal-Wallis test followed by Dunn's multiple comparisons test; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001. Abbreviation: ns, not significant.
Next, we analyzed the T cell phenotype by staining cell surface and intracellular markers associated with T cell activation (CD69, HLA-DR, CD95, CD25 and CD40L), exhaustion (PD-1, TIM-3 and CTLA-4) and effector function (Granzyme B and CD107a). As shown in Fig. 1b and 1c, both CD4+ and CD8+ T cell phenotype fingerprints of sAg-L patients were distinct from those of sAg-R patients and HC. Statistical analysis of the percentage of cells expressing each marker revealed that CD4+ T cells of sAg-L patients had significantly increased expression of the activation markers HLA-DR (sAg-L vs sAg-R and HC: 10·60% vs 7·40% and 5·38%, p<0·0001, Kruskal-Wallis test) and CD25 (sAg-L vs sAg-R and HC: 7·48% vs 4·95% and 4·66%, p<0·0001, Kruskal-Wallis test) compared to those from sAg-R patients and HC (Fig. 1b). HLA-DR expression on CD8+ T cells in sAg-L patients was also significantly increased compared to sAg-R patients and HC (sAg-L vs sAg-R and HC: 15·50% vs 9·25% and 7·11%, p<0·0001, Kruskal-Wallis test) (Fig. 1c). The expression of the T cell exhaustion marker PD-1 on both CD4+ (sAg-R and sAg-L vs HC: 5·43% and 6·89% vs 3·01%, p = 0·0005, Kruskal-Wallis test) and CD8+ T cells (sAg-R and sAg-L vs HC: 5·24% and 6·22% vs 2·45%, p = 0·0001, Kruskal-Wallis test) and CD95 (sAg-R and sAg-L vs HC: 46·15% and 49·20% vs 36·70%, p = 0·0027, Kruskal-Wallis test) on CD4+ T cells in sAg-R and sAg-L patients was significantly higher than those in HC (Fig. S2). No significant differences in CD69, TIM-3, Granzyme B, CD40L, CTLA-4 and CD107a expression on both CD4+ and CD8+ T cells were observed between sAg-L, sAg-R patients, and HC (Fig. S2). Moreover, no significant differences in CD4+ and CD8+ T cell phenotypes were observed between sAg-L patients with or without seroconversion to anti-HBs (Fig. S3).
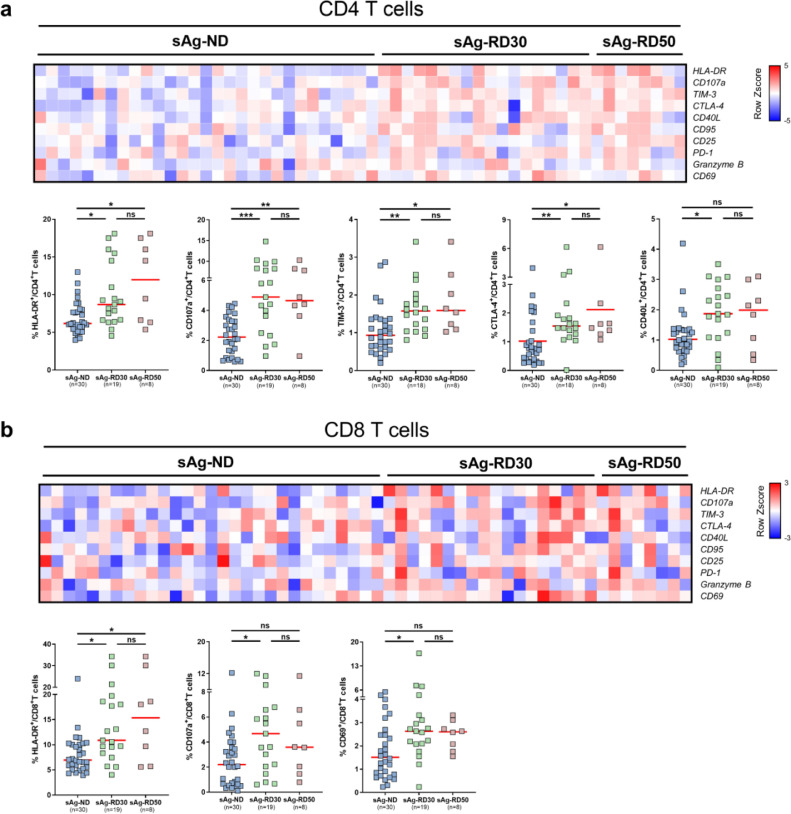
We subsequently examined whether cHBV patients experiencing a rapid HBsAg decrease (sAg-RD) also had an alteration of the T cell phenotype compared to cHBV patients with no decrease of serum HBsAg levels during the observational period. There were 19 sAg-R patients whom sustained greater than 30% decrease in HBsAg levels from the baseline within 6 months (sAg-RD30), 30 sAg-R patients demonstrated comparable or higher serum HBsAg levels during the follow-up period compared to the baseline were considered as HBsAg no decrease group (sAg-ND) (Table S1). As shown in Fig. 2, sAg-RD30 patients showed significant increases in HLA-DR (8·70% vs 6·18%, p = 0·0141; 10·90% vs 6·97%, p = 0·0127, Kruskal-Wallis test) and CD107a (4·89% vs 2·22%, p = 0·0003; 4·67% vs 2·21%, p = 0·0225, Kruskal-Wallis test) expression on both CD4+ and CD8+ T cells compared to sAg-ND patients. TIM-3 (1·58% vs 0·94%, p = 0·0047, Kruskal-Wallis test), CD40L (1·87% vs 1·03%, p = 0·0213, Kruskal-Wallis test) and CTLA-4 (1·55% vs 1·02%, p = 0·0052, Kruskal-Wallis test) expression on CD4+ T cells as well as CD69 (2·63% vs 1·52%, p = 0·0240, Kruskal-Wallis test) expression on CD8+ T cells in sAg-RD30 patients were also significantly higher than those in sAg-ND patients (Fig. 2). No significant differences in CD25, CD95, PD-1, and Granzyme B expression on T cells were observed between the two groups of patients (Fig. S4). The differences in the T cell phenotype became even more evident when we examined a cohort of 8 patients with a more profound HBsAg decrease (greater than 50% decrease, sAg-RD50). sAg-RD50 patients showed significant increases in HLA-DR (11·99% vs 6·18%, p = 0·0306, Kruskal-Wallis test), TIM-3, (1·59% vs 0·94%, p = 0·0195, Kruskal-Wallis test), CTLA-4 (2·12% vs 1·02%, p = 0·0318, Kruskal-Wallis test), and CD107a (4·65% vs 2·22%, p = 0·0065, Kruskal-Wallis test) expression on CD4+, as well as HLA-DR expression on CD8+ T cells (15·35% vs 6·97%, p = 0·0488, Kruskal-Wallis test) compared to sAg-ND patients (Fig. 2). No significant differences in CD95, CD25, CD69, CD40L, PD-1 and Granzyme B expression on T cells were observed between the two groups of patients (Fig. S4).
Fig. 2.
Characterization of T cell phenotype profiles in cHBV patients with HBsAg rapid decrease. Percentages of HLA-DR, CD107a, TIM-3, CTLA-4, CD40L, CD95, CD25, PD-1, Granzyme B, CD69 were analyzed on CD4+T cells (a) and CD8+T cells (b) through flow cytometry and were compared between cHBV patients who experienced more than 30% decrease of HBsAg levels (sAg-RD30, n = 19), patients who experienced more than 50% decrease of HBsAg levels (sAg-RD50, n = 8) compared to the baseline within 6 months and patients with no decrease of serum HBsAg levels (sAg-ND, n = 30). Percentages are represented by the heat map as Z scores of log2 transformed. Each column represents a single patient and patients with undetected T cell markers did not show on the heatmap. Data are presented as median and statistical analysis was performed by GraphPad Prism using Kruskal-Wallis test followed by Dunn's multiple comparisons test; *P < 0·05; **P < 0·01; ***P < 0·001; ns, not significant.
Overall, these data demonstrated that cHBV patients experiencing a rapid HBsAg decrease or loss have altered T cell phenotypes compared to HBsAg retaining patients mainly associated with cell activation.
3.2. Association of HBV-specific T cell responses with alteration of T cell phenotype in sAg-L patients
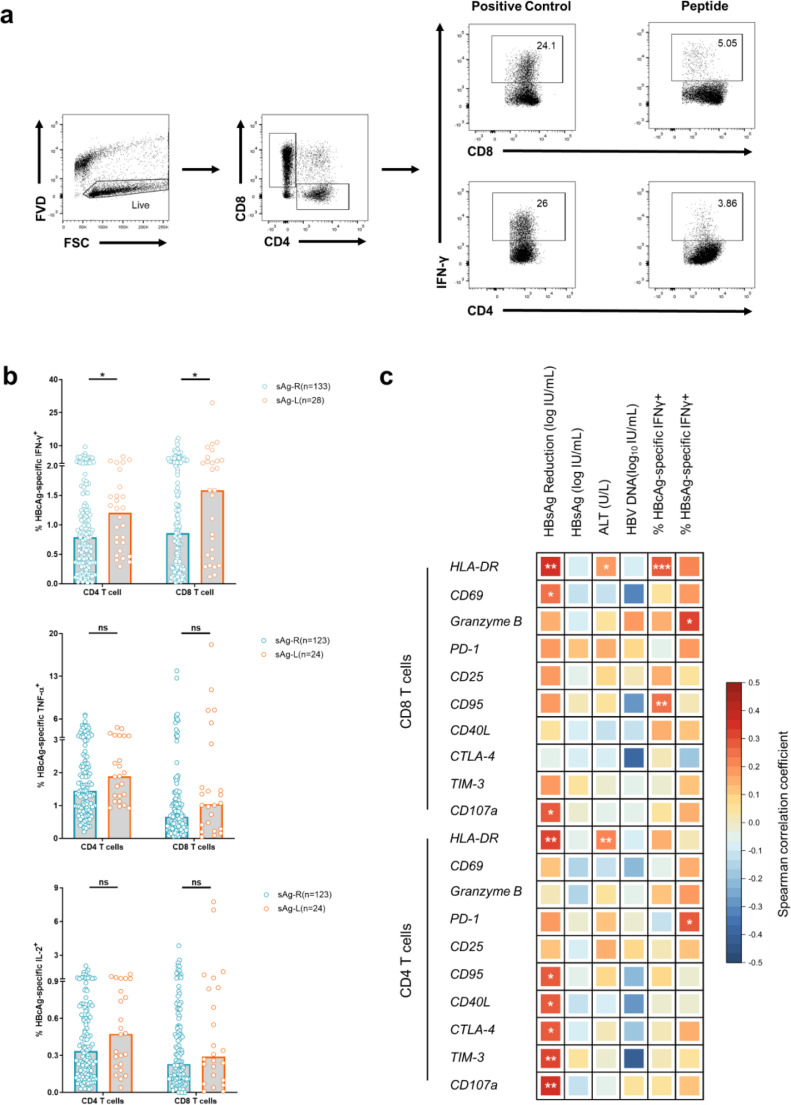
During the natural history of cHBV, the quantity and function of HBV-specific T cells are shown to correlate with HBV control [22]. Thus, we next examined how HBV-specific CD4+ and CD8+ T cell response change in our patient cohorts and how this correlated with T cell phenotypes. The PBMCs from cHBV patients were stimulated for 10 days with overlapping peptide pools spanning the HBcAg or HBsAg to induce HBV-specific CD4+ and CD8+ T cell expansion. Subsequently, the frequencies of IFN-γ, TNF-α or IL-2-producing HBV-specific CD4+ or CD8+ T cells were detected by FACS. sAg-L patients showed significantly increased frequencies of IFN-γ (1·21% vs 0·79%, p = 0·0442; 1·59% vs 0·86%, p = 0·0490, Mann-Whitney test) but not TNF-α, or IL-2-producing HBcAg-specific CD4+and CD8+ T cells, compared to sAg-R patients (Fig. 3b). Although sAg-L patients that seroconverted to anti-HBs showed higher frequencies of IFN-γ, TNF-α, or IL-2-producing HBcAg-specific CD8+ T cells than non-seroconverters, the differences were not statistically significant (Fig. S5a). Upon HBV core peptide stimulation, sAg-ND, sAg-RD30 and sAg-RD50 patients showed no differences in their capability of CD4+ or CD8+ T cells to produce IFN-γ, TNF-α and IL-2 (Fig. S5b). No significant increases in the frequencies of IFN-γ, TNF-α, or IL-2-producing HBsAg-specific CD4+ and CD8+ T cells were observed in sAg-L patients compared to sAg-R patients (Fig. S5c).
Fig. 3.
Characterization of HBV specific T cell responses in cHBV patients with HBsAg loss and rapid decrease. PBMCs from chronic hepatitis B patients were stimulated with overlapping peptide pools covering the entire sequences of HBcAg or HBsAg for 10 days. Cells were analyzed for IFN-γ, TNF-α, and IL-2 production by intracellular cytokine staining. (a) Gating strategy of intracellular cytokine staining analysis. (b) HBcAg-specific T cell responses were compared between HBsAg retained patients (sAg-R) and HBsAg loss (sAg-L) patients. (c) Pairwise spearman correlations between 10 indicated phenotypical T cell markers in chronic hepatitis B patients (CHB) with HBsAg reduction, HBsAg, HBV DNA, ALT levels and HBcAg- and HBsAg- specific IFN-γ responses. colour intensities of squares indicate correlation coefficients according to the legends. Significant (P < 0·05) correlations are highlighted within squares. Data are presented as median and statistical analysis was performed by GraphPad Prism using Mann-Whitney test (b); Spearman's Rank correlation (c); *P < 0·05; **P < 0·01; ***P < 0·001; ns, not significant.
Next, we set out to explore potential correlations between the alterations in T cell phenotype and the virological and clinical markers by Spearman's Rank correlation test (Fig. 3c). We observed that HLA-DR (r = 0·3269, p = 0·0037), CD95 (r = 0·2796, p = 0·0151), CD40L (r = 0·2747, p = 0·0156), CTLA-4 (r = 0·2786, p = 0·0148), TIM-3 (r = 0·3082, p = 0·0068), and CD107a (r = 0·3597, p = 0·0013) expression levels on CD4+ T cells, as well as HLA-DR (r = 0·3542, p = 0·0016), CD69 (r = 0·2507, p = 0·0279), and CD107a (r = 0·2875, p = 0·0112) on CD8+ T cells, were positively correlated with the intensity of HBsAg reduction in cHBV patients (Fig. 3c). We also found that HLA-DR expression on both CD4+ (r = 0·2150, p = 0·0058) and CD8+ (r = 0·1710, p = 0·0290) T cells, were positively correlated with serum ALT levels (Fig. 3c). No correlations between the T cell phenotype and serum HBV-DNA levels were observed (Fig. 3c). Moreover, the correlation between HBV-specific T cell responses and T cell phenotype was analyzed. We found that HLA-DR (r = 0·2846, p = 0·0009) and CD95 (r = 0·2442, p = 0·0049) expression on CD8+ T cells were positively correlated with the intensities of HBcAg-specific CD8+ T cell responses (Fig. 3c). Besides, PD-1 expression on CD4+T cells and Granzyme B expression on CD8+T cells were positively correlated with the intensities of HBsAg-specific responses of corresponding CD4+ and CD8+ T cells (Fig. 3c).
Collectively, these data show that the activated phenotype of T cells is associated with the intensities of HBsAg reduction and HBV-specific T cell responses during the course of rapid HBsAg decrease and loss.
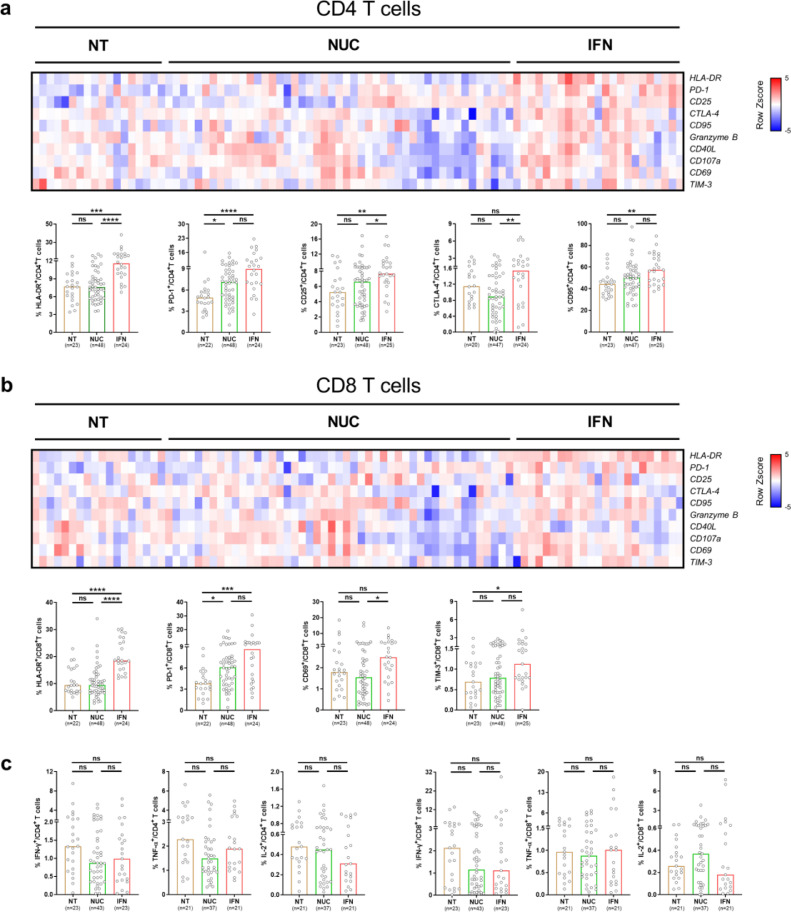
3.3. Alteration of the T cell phenotype in sAg-L patients is associated with IFN-α treatment
Accumulating clinical experience and increasing data from clinical trials have demonstrated that peg-IFN-α-containing therapy in cHBV significantly increased the rates of functional cure in selected cHBV patient cohorts [23]. Our data also shows an increased percentage of patients receiving peg-IFN-α-containing therapy in the sAg-L group compared to the sAg-R group (58·07% vs 9·93%, Table 1). Therefore, we next examined whether the treatment strategy with IFN-α had an influence on the T cell phenotype and function. In total, 97 HBeAg-cHBV patients with undetectable serum HBV-DNA levels were divided into 3 groups based on their treatment strategies, including no treatment group (NT, n = 24), NUC treatment alone group (NUC, n = 48), and peg-IFN-α-containing treatment group (either alone or in combination with NUCs, IFN, n = 25).The IFN group showed significant increases in HLA-DR (IFN group vs NT and NUC group: 11·60% vs 7·69% and 5·75%, p<0·0001, Kruskal-Wallis test), CD25 (IFN group vs NT and NUC group: 7·64% vs 5·24% and 6·61%, p = 0·0079, Kruskal-Wallis test) expression on CD4+ T cells, as well as HLA-DR (IFN group vs NT and NUC group: 18·55% vs 9·41% and 9·48%, p<0·0001, Kruskal-Wallis test) expression on CD8+ T cells, compared to the NT or NUC group (Figs. 4a and 4b, S6). The IFN group also showed significantly higher CTLA-4 (1·54% vs 0·89%, p = 0·0071, Kruskal-Wallis test) expression on CD4+T cells and CD69 (2·49% vs 1·55%, p = 0·0124, Kruskal-Wallis test) expression on CD8+T cells than the NUC group, but not the NT group (Fig. 4a and 4b, Fig. S6). We also observed that the NUC and IFN group expressed higher levers of PD-1 on both CD4+ (NUC and IFN group vs NT group: 7·10% and 8·97% vs 4·92%, p<0·0001, Kruskal-Wallis test) and CD8+ (NUC and IFN group vs NT group: 6·12% and 8·65% vs 3·83%, p = 0·0004, Kruskal-Wallis test) T cells than the NT group. However, no changes in the frequency of HBcAg-specific T cell responses were detected between the three groups (Fig. 4c).
Fig. 4.
Characterization of T cell phenotype and HBcAg-specific T cell responses in cHBV patients received IFN-α treatment. (a and b) CD4+ and CD8+T cell phenotypes were compared between HBV patients received no therapy (NT, n = 24), NUC monotherapy (NUC, n = 48) and peg-IFN-α contained therapy (IFN, n = 25). Percentages are represented by the heat map as Z scores of log2 transformed. Each column represents a single patient and patients with undetected T cell markers did not show on the heatmap. (c) HBcAg-specific T cell responses were compared between HBV patients received no therapy (NT, n = 24), NUC monotherapy (NUC, n = 48) and peg-IFN-α contained therapy (IFN, n = 25). Data are presented as median and statistical analysis was performed by GraphPad Prism using Kruskal-Wallis test followed by Dunn's multiple comparisons test; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001; ns, not significant.
These results suggest that alterations of the T cell phenotype in sAg-L patients is partially associated with peg-IFN-α treatment, which is known to exhibit immunomodulatory function and known to be beneficial for achieving HBsAg loss in cHBV patients.
3.4. Kinetics of the T cell phenotype during rapid HBsAg decrease, loss, and seroconversion
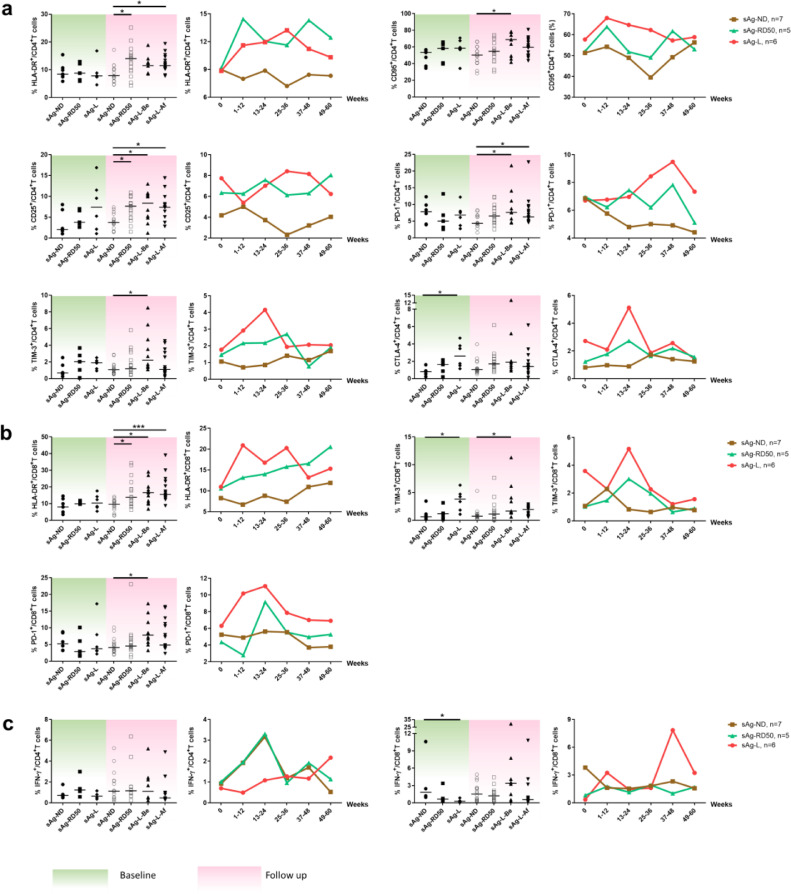
Next, the kinetics of T cell phenotype changes during rapid HBsAg decrease and loss was characterized by analyzing the longitudinal data obtained from 7 sAg-ND, 5 sAg-RD50, and 6 sAg-L patients (Table S2, Fig. 5). These patients were followed up to 60 weeks, monitored for virological and clinical markers, and sampled 2–8 times for T cell phenotype and HBV-specific T cell response analysis. No significant differences in CD69, HLA-DR, CD95, PD-1, CD25, CD40L, Granzyme B, and CD107a expression on CD4+ and CD8+ T cells were observed between the sAg-ND, sAg-RD50, and sAg-L groups at baseline (Figs. 5 and S7). Interestingly, the sAg-L group showed significantly higher CTLA-4 expression on CD4+ T cells and TIM-3 expression on CD8+ T cells compared to the sAg-ND group at baseline (Fig. 5a and 5b). The phenotypes of CD4+ and CD8+ T cells in the sAg-ND patient group remained relatively stable during the entire observational period. In contrast, the phenotypes of CD4+ and CD8+ T cells in both the sAg-RD50 and sAg-L patients group showed significant alterations during the follow-up period (Fig. 5a and 5b). The expressions of HLA-DR, CD25 on CD4+ T cells and HLA-DR on CD8+ T cells from sAg-RD50 patients were significantly higher than sAg-ND patients during the follow-up, which was the period of rapid HBsAg reduction (Fig. 5a and 5b). Significant increases in the expression of CD25, PD-1 on CD4+ T cells, as well as HLA-DR on CD8+ T cells were observed in sAg-L patients both before and after HBsAg loss during the follow-up period. Furthermore, sAg-L patients showed significant upregulation of CD95, TIM-3 on CD4+ T cells and TIM-3, PD-1 on CD8+ T cells only before but not after HBsAg loss compared to sAg-ND patients (Fig. 5a and 5b). An upregulation of HLA-DR on CD4+ T cells was observed in sAg-L patients only after but not before HBsAg loss (Fig. 5a and 5b). Next, the kinetics of the HBcAg-specific CD4+ and CD8+ T cell response were also analyzed. In general, no significant differences in HBcAg-specific CD4+ T cell responses were observed during the entire observation period. Intriguingly, the sAg-ND group showed even significantly increased HBcAg-specific CD8+T cell responses compared to the sAg-L group at baseline (Fig. 5c). The HBcAg-specific CD8+ T cell responses in sAg-ND and sAg-RD50 patients remained relatively stable during the entire observation period, while sAg-L patients showed increases in HBcAg-specific CD8+ T cell responses only at later time points of the observation period (37–48 weeks of follow-up; Fig. 5c). However, no statistically significant differences in HBcAg-specific CD8+ T cell responses were observed in sAg-L patients before and after HBsAg loss (Fig. 5c).
Fig. 5.
Kinetic analysis of T cell phenotypes and HBcAg-specific T cell responses in cHBV patients during HBsAg rapid decrease and loss. The phenotypes of CD4+ T cells (a), CD8+ T cells (b), and HBcAg-specific T cell responses (c) were longitudinally analyzed during the observation period. Left: Data were pooled and compared at either baseline (green background) and follow-up period (pink background) between cHBV patients with no HBsAg decrease (sAg-ND), HBsAg rapid decrease (sAg-RD50), and HBsAg loss (sAg-L). Right: Kinetic changes of T cell phenotype and HBcAg-specific T cell responses were demonstrated at indicated time points for sAg-ND, sAg-RD50, and sAg-L patients. Data are presented as median and statistical analysis was performed by GraphPad Prism using Kruskal-Wallis test followed by Dunn's multiple comparisons test; sAg-l-Be, before HBsAg loss; sAg-l-Af, after HBsAg loss; *P < 0·05; ***P < 0·001.
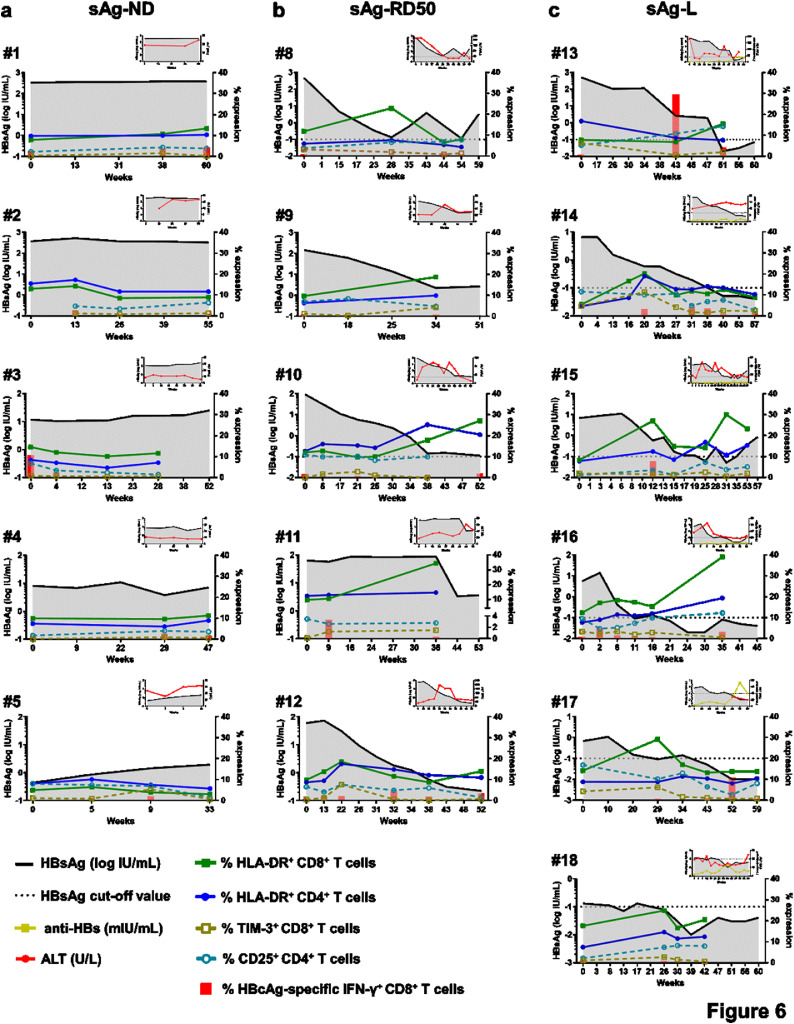
Next, the kinetics of T cell phenotype and serum HBsAg level were plotted individually for representative patients from each group. As shown in Fig. 6a and S8a, sAg-ND patients showed a minor alteration of the T cell phenotype and weak or undetectable HBcAg-specific CD8+ T cell responses. An association of altered T cell phenotypes, as mainly represented by increasing HLA-DR expression on both CD4+ and CD8+ T cells, with a decrease in serum HBsAg levels was observed in sAg-RD50 patients. However, HBcAg-specific CD8+ T cell response was also barely detected in sAg-RD50 patients during rapid HBsAg decrease (Fig. 6b). In sAg-L patients, an alteration of the T cell phenotype was observed during the course of HBsAg loss. Additionally, HBcAg-specific CD8+ T cell responses were more frequently detected in sAg-L patients during and after HBsAg loss (Fig. 6c). The correlation of the T cell phenotype change with the HBsAb titer was also kinetically analyzed in 6 cHBV patients with seroconversion to anti-HBs. In most of the cases, the upregulation of HLA-DR expression on CD4+ and CD8+ T cells was accompanied by an increase in HBsAb levels (Fig. S8b).
Fig. 6.
Kinetic analysis of T cell phenotypes and HBcAg-specific T cell responses in individual cHBV patients. HBsAg, HBcAg-specific T cell response, frequency of HLA-DR+CD8+T cells, TIM-3+CD8+T cells, HLA-DR+CD4+T cells and CD25+CD4+T cells were longitudinal analyzed with 5 representative patients with no HBsAg reduction (a, sAg-ND), 5 patients who experienced greater than 50% decrease of HBsAg levels than the baseline within 6 months (b, sAg-RD50) and 6 patients who experienced HBsAg loss (c, sAg-L). Graphical inserts are depicting the quantity of HBsAg, anti-HBs and ALT.
3.5. Predicting HBsAg loss in cHBV patients by T cell surface markers
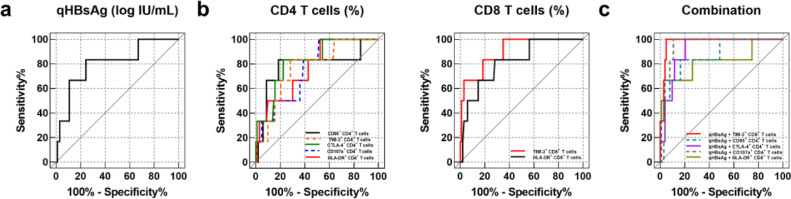
Our data demonstrate that the alteration of T cell phenotype might occur prior to HBsAg loss, therefore, we next evaluated the predictive performance of T cell phenotype, as well as the frequencies of IFN-γ producing HBcAg-specific CD8+ T cells in predicting HBsAg loss within 48 weeks of follow-up, which was the time frame that all incidents of HBsAg loss in our patient cohort occurred. Patients with low levels of serum HBsAg (<1000 IU/ml) are more likely to sustain HBsAg loss [24]. The receiver operator characteristic (ROC) curve analysis was performed in a total of 109 cHBV patients with HBsAg less than 1000 IU/ml. Since previously it has been demonstrated that serum HBsAg level is a predictive biomarker for HBsAg loss [25], we firstly examined the predictive efficiency of HBsAg quantification (qHBsAg) in our patient cohort. Our result showed that the area under the ROC curve (AUC) of qHBsAg for predicting 48-week HBsAg loss was 0·806 (95% CI, 0·620–0·992; p = 0·0120) (Fig. 7a and Table 2), suggesting qHBsAg is a significant predictor for 48-week HBsAg loss. Our further analysis with all detected immune markers showed that HLA-DR (AUC, 0·769; 95% confidence interval [CI], 0·601 to 0·938; p = 0·0270), CTLA-4 (AUC, 0·821; 95% CI, 0·668 to 0·974; p = 0·0084), CD95 (AUC, 0·785; 95% CI, 0·549 to 1·000; p = 0·0194), CD107a (AUC, 0·759; 95% CI, 0·598 to 0·920; p = 0·0335), and TIM-3 (AUC, 0·769; 95% CI, 0·600 to 0·939; p = 0·0270) expression of CD4 T cells, as well as HLA-DR (AUC, 0·819; 95% CI, 0·658 to 0·980; p = 0·0089), and TIM-3 (AUC, 0·903; 95% CI, 0·794 to 1·000; p = 0·0009) expression of CD8+ T cells were also significant predictors for 48-week HBsAg loss (Fig. 7b and Table 2). Then we investigated whether combining both virological and immunological biomarkers possess a greater power to predict HBsAg loss. ROC curves for the combined detection of each marker for HBsAg loss in cHBV subjects were constructed based on binary logistic regression. The combination of the aforementioned immune markers with qHBsAg resulted in increased AUC values to predict HBsAg loss, in comparison to when either was analyzed independently, and among which TIM-3 expression of CD8+ T cells plus qHBsAg possessed the highest predictive efficiency (AUC, 0·976; 95% CI, 0·948–1·000; p<0·0001) (Fig. 7c and Table 2).
Fig. 7.
Predictive factors for HBsAg loss within 48 weeks in cHBV patients. Receiver operator characteristic (ROC) curve analyses of HBsAg quantification (a), the expression of indicated CD4+ and CD8+ T cell markers (b), and a combination of both HBsAg quantification and indicated T cell markers (c) were performed to predict HBsAg loss by using SPSS.
Table 2.
The area under the receiver operating characteristic curve (AUC) of predictive parameters.
| Parameters | AUC | 95% CI | P |
|---|---|---|---|
| Virological marker | |||
| qHBsAg (log IU/mL) | 0·806 | 0·620 to 0·992 | 0·0120 |
| Immunological markers | |||
| CD4+ T cells | |||
| HLA-DR+ CD4+ T cells (%) | 0·769 | 0·601 to 0·938 | 0·0270 |
| CTLA-4+ CD4+ T cells (%) | 0·821 | 0·668 to 0·974 | 0·0084 |
| CD95+ CD4+ T cells (%) | 0·785 | 0·549 to 1·000 | 0·0194 |
| CD107a+ CD4+ T cells (%) | 0·759 | 0·598 to 0·920 | 0·0335 |
| TIM-3+ CD4+ T cells (%) | 0·769 | 0·600 to 0·939 | 0·0270 |
| PD-1+ CD4+ T cells (%) | 0·667 | 0·405 to 0·928 | 0·1712 |
| CD25+ CD4+ T cells (%) | 0·622 | 0·360 to 0·884 | 0·3158 |
| Granzyme B+ CD4+ T cells (%) | 0·547 | 0·274 to 0·820 | 0·7000 |
| CD40L+ CD4+ T cells (%) | 0·702 | 0·521 to 0·884 | 0·0968 |
| CD69+ CD4+ T cells (%) | 0·502 | 0·327 to 0·677 | 0·9894 |
| CD8+ T cells | |||
| HLA-DR+ CD8+ T cells (%) | 0·819 | 0·658 to 0·980 | 0·0089 |
| TIM-3+ CD8+ T cells (%) | 0·903 | 0·794 to 1·000 | 0·0009 |
| PD-1+ CD8+ T cells (%) | 0·686 | 0·435 to 0·937 | 0·1265 |
| CD25+ CD8+ T cells (%) | 0·694 | 0·469 to 0·919 | 0·1109 |
| CTLA-4+ CD8+ T cells (%) | 0·652 | 0·374 to 0·930 | 0·2117 |
| CD95+ CD8+ T cells (%) | 0·694 | 0·481 to 0·907 | 0·1109 |
| Granzyme B+ CD8+ T cells (%) | 0·594 | 0·321 to 0·867 | 0·4409 |
| CD40L+ CD8+ T cells (%) | 0·557 | 0·386 to 0·727 | 0·6419 |
| CD107a+ CD8+ T cells (%) | 0·670 | 0·508 to 0·832 | 0·1630 |
| CD69+ CD8+ T cells (%) | 0·633 | 0·415 to 0·851 | 0·2759 |
| Combination | |||
| qHBsAg + CTLA-4+ CD4+ T cells | 0·917 | 0·850 to 0·985 | 0·0010 |
| qHBsAg + CD95+ CD4+ T cells | 0·871 | 0·731 to 1·000 | 0·0020 |
| qHBsAg + CD107a+ CD4+ T cells | 0·945 | 0·900 to 0·990 | <0·0001 |
| qHBsAg + HLA-DR+ CD8+ T cells | 0·825 | 0·606 to 1·000 | 0·0080 |
| qHBsAg + TIM-3+ CD8+ T cells | 0·976 | 0·948 to 1·000 | <0·0001 |
Abbreviations: CI, confidence interval.
4. Discussion
In the current study, we longitudinally characterized T cell phenotype profile and HBV-specific response during HBsAg rapid decrease, loss and seroconversion. Importantly, all analyses were performed directly ex vivo, since freezing and thawing T cells before analysis may induce profound changes in their functionality [11]. Our findings reveal that the courses of HBsAg rapid decrease and loss in cHBV patients is associated with significant alteration of both CD4+ and CD8+ T cell phenotypes. We identify that T cells upregulate the expression of a panel of surface molecules, including CD25 expression on CD4+ T cells, and HLA-DR on both CD4+ and CD8+ T cells. The expression of HLA-DR, CD95, CD40L, CTLA-4, CD107a, TIM-3 on CD4+ T cells, and HLA-DR, CD69, CD107a on CD8+ T cells are positively correlated with the extent of HBsAg decrease. Our data demonstrates that the courses of HBsAg rapid decrease and loss are associated with T cell activation as indicated by the upregulation of HLA-DR and CD107a on T cells. This is in line with a recent report showing that global T cells of patients with subsequent HBsAg loss showed a more activated phenotype compared to patients with retained HBsAg [26]. Our data also suggested that the detection of T cell phenotype demonstrated an excellent predictive value for 48-week HBsAg loss in cHBV patients, especially when combined with qHBsAg.
Successful HBV clearance in patients is associated with robust and broad HBV-specific proliferative and IFN-γ+ effector T cell responses compared with weak, dysfunctional responses in cHBV patients. We found that HBcAg-specific responses were more likely to be detected in the blood of patients with HBsAg loss. A significant higher frequency of HBcAg-specific IFN-γ producing CD4+ and CD8+ T cells were found in patients with HBsAg loss in comparison with HBsAg positive patients, suggesting that strong HBcAg-specific IFN-γ producing T cell responses are required for HBsAg loss, as observed by previous study which analyzed HBV specific T cell response in cHBV patients with different outcomes [27]. Further analysis revealed that the expression of HLA-DR and CD95 on CD8+ T cells were positively correlated with the intensity of HBcAg-specific T cell response.
The presence of functional HBV-specific T cells is considered as a potential immunological biomarker to evaluate the immune status of cHBV patients and to guide clinical practice, such as safe NUC therapy discontinuation [11]. However, the process of detecting HBV-specific CD8+ T cell responses in cHBV patients is relatively complicated to be broadly applied for diagnostic purposes. In this study, we have identified that HLA-DR expression on CD8+ T cells is positively correlated with the intensity of HBcAg-specific CD8+ T cell response, and thus it might serve as an easy surrogate detection marker for HBcAg-specific CD8+ T cell response in cHBV patients. Besides, we have also identified HLA-DR, CTLA-4, CD95, CD107a and TIM-3 expression on CD4+ T cells, as well as HLA-DR and TIM-3 expression on CD8+ T cells, as candidate immunological biomarkers for predicting HBsAg loss within 48 weeks. Previous studies have indicated that a lower HBsAg level and HBV DNA level at baseline and older age may predict HBsAg loss and seroconversion [28,29]. Our data also indicate that HBsAg quantification is a promising diagnostic marker for HBsAg loss. Further evaluation of biomarker combination using binary logistic regression model showed that the combination of CTLA-4, CD95, CD107a expression on CD4+ T cells or HLA-DR, TIM-3 expression on CD8+ T cells and qHBsAg had a higher predictive accuracy. Therefore, it appears plausible that utilizing immunological biomarkers combination were complimentary to clinical parameters and contributed significantly to the prediction of HBsAg loss.
Functional cure is a rare clinical event in cHBV patients, with an annual clearance rate of 0·33% taking on average more than 50 years to clear the virus [30]. Previous studies have shown that improved peg-IFN-α-containing treatment strategies, such as “adding-on” or “switching to” peg-IFN-α in patients who achieved long-term effective virological remission by NUCs, may significantly increase the HBsAg loss rates to more than 20% [23,31]. In line with these reports, an increased rate of receiving peg-IFN-α-containing therapy in sAg-L patients than sAg-R patients was observed in the patient cohort of this study. Peg-IFN-α has been shown to have both direct antiviral and immunomodulatory effects for the treatment of CHB, and it is conceivable that treatment outcome may be mostly triggered by the immunomodulatory effects of peg-IFN-α on the innate and adaptive immune responses [32]. The mechanisms responsible for the peg-IFN-α mediated restoration of anti-HBV immune functions are not fully understood. Treatment with peg-IFN-α in CHB patients has been shown to lead to a significant functional augmentation of NK cells, however, quite different effects were observed on T cells [33]. IFN-α therapy led to a striking reduction of CD8+ T cells and showed no effect on the restoration of frequency and early effector functions of HBV-specific CD8+ T cells [33,34]. In our study, we observed patients who received IFN-α treatment demonstrated a more active phenotype of global T cells than IFN-α untreated patients, although no significant increases in HBcAg-specific CD8+ T cell responses were found in patients who received IFN-α treatment compared to those without. This is probably due to the majority (> 70%) of the IFN-α treated patient in our study also received NUCs treatment, while all patients in previous studies [33,34] only received IFN-α monotherapy. It has been shown that HBV-specific CD8+ T cell functions could be restored, at least in part, after long-term treatment with NUCs [14]. Therefore, it is assumed that peg-IFN-α could improve the immunomodulatory action of NUCs and vice versa [35]. Our observation supports this assumption and indicates that IFN-α therapy may also be favorable for the T cell arm of immune system to control HBV infection in long-term NUC treated patients. However, analysis of larger prospective group of patients is needed to define more precisely the immunomodulatory power of peg-IFN-α therapy to predict clinical outcome.
Interestingly, we observed that markers usually used to represent CD8+T cell exhaustion statues during chronic viral infection, such as PD-1 and TIM-3, were also upregulated on CD8+ T cells during peg-IFN-α therapy. Previous studies have shown that PD-1 is not a definitive marker for complete functional exhaustion but can also be expressed on at least partially functional T cells in the context of a chronic infection [11,36,37]. Patients with partial immune control of HBV infection display higher levels of intrahepatic PD-1+CD39+ tissue-resident CD8+ T cells that possess the capacity to mount immediate and strong cytokine responses [38]. PD-1 has also been shown to prevent virus-specific CD8+ T cells from terminal exhaustion and to contribute to the survival of memory T cell populations [39]. Intriguingly, a recent study has demonstrated that almost all HBV core- and polymerase-specific CD8+ T cells in cHBV patients were PD-1 positive [40]. Similar results were observed by us in an HBV replication mouse model. Also, the upregulation of PD-1 and TIM-3 expression on T cells during acute-resolving HBV infection has been reported [41], [42], [43], [42], [44], and higher TIM-3 expression on CD8+ T cells was found to be associated with elevated serum anti-HBs production in HBV-carrying mice [52]. The reasons underlying these discrepancies are not fully understood, further studies are needed to elucidate these processes underlying peg-IFN-α treatment.
There are several limitations of the current study. First is the lack of access to hepatic tissue samples, and thus we cannot compare the phenotypes of intrahepatic T cells with their peripheral counterparts. However, previous reports have shown that the immunologic changes of T cells and NK cells in peripheral blood could closely mirror those in the liver in cHBV patients [45, 46]. Second is the lack of analyzing phenotypes of HBV-specific T cells. Due to the low frequency of HBV-specific T cells present in cHBV patients, phenotype analysis of such rare cell populations requires large volume of peripheral blood and applying a pMHC tetramer-based technic to enrich the cells [36,40]. We were unable to perform such an analysis due to the limitation of sample volume we were allowed to take from patients. It would be important to compare whether the phenotype change of global T cells represents that of the HBV-specific T cells. It has been previously shown by Miller et al. that the CD8+ T cells specific for yellow fever vaccine can be mapped by CD38 and HLA-DR co-expression [47]. Therefore, the changes in the fingerprint phenotype of global T cells that we observed in sAg-L patients are most likely due to the expansion and activation of HBV-specific T cells, rather than T cell bystander activation. Thirdly, the majority of the study subjects are Chinese. It remains unclear whether the findings of our study can be generalized to other populations, such as Caucasians and Africans. Previous studies have demonstrated that there is an association of specific HLA-DP polymorphisms with spontaneous HBsAg seroclearance in various populations [48], [49], [50], suggesting that the immunological mechanisms of HBV clearance might be universal. Future studies are necessary to demonstrate the validity of our current findings in diverse populations. Moreover, we observed that sAg-L patients had higher absolute numbers and frequencies of B cells and DCs than sAg-R patients, which implies that T cells may not be the only driver of HBsAg loss in CHB patients. The differences in B cells and DCs between sAg-L and sAg-R patients were not statistically significant, which is probably due to the sAg-R patient group in our cohort being more heterogeneous in B cells and DCs than the sAg-L patient group and the HC group. It was recently reported that maturation of HBsAg-specific B cells also played a vital role in HBV clearance [51]. Thus, a thorough examination on other components of the immune system and their complex network should also be considered in future studies.
In the current study, we present the longitudinal characteristics of T cell phenotypes and responses during the course of HBsAg rapid decrease, loss and seroconversion in the peripheral blood of cHBV patients and identified potential immunological biomarkers to predict HBsAg loss. This knowledge may assist in developing assays to precisely evaluate immune status and to improve treatment strategies for achieving functional cure in cHBV patients.
5. Contributors
SEX, DZ, JL and XZ conceived and designed the experiments. SEX, DZ, BYL, MYL, WP and JYH performed the experiments. SEX, DZ, JL and XZ analyzed and interpreted the data. HW, MJL, DLY, JL and XZ contributed reagents/materials/analysis tools. SEX, DZ, MJL, KS, UD, DL, XZ and JL drafted the manuscript. SEX, DZ, JL and XZ have verified the underlying data. All authors reviewed and approved the final version of the manuscript.
6. Data sharing statement
The raw data of the study are deposited in the database of the Department of Infectious Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, and can be provided to inquirers upon reasonable requests.
Declaration of Competing Interest
The authors have declared that no conflict of interest exists.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (81861138044, 91742114, 81271884 and 81461130019), and the National Scientific and Technological Major Project of China (2017ZX10202203, 2018ZX10723203, 2018ZX10302206, 2017ZX10202201, 2017ZX10202202 and 2017YFC0908100), the Integrated Innovative Team for Major Human Diseases Program of Tongji Medical College and the “Double-First Class” Project for the International Cooperation Center on Infection and Immunity, HUST.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2021.103464.
Contributor Information
Jia Liu, Email: jialiu77@hust.edu.cn.
Xin Zheng, Email: Xinz@hust.edu.cn.
Appendix. Supplementary materials
References
- 1.World Health Organization . World Health Organization; Geneva: 2016. Global health sector strategy on viral hepatitis 2016–2021. [Google Scholar]
- 2.Terrault N.A., Lok A.S.F., McMahon B.J. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Simonetti J., Bulkow L., McMahon B.J. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51(5):1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 5.Liu J., Yang H.I., Lee M.H. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139(2):474–482. doi: 10.1053/j.gastro.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Thimme R., Wieland S., Steiger C. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77(1):68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asabe S., Wieland S.F., Chattopadhyay P.K. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83(19):9652–9662. doi: 10.1128/JVI.00867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoletti A., Gehring A.J. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87(Pt 6):1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- 9.Webster G.J., Reignat S., Brown D. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J Virol. 2004;78(11):5707–5719. doi: 10.1128/JVI.78.11.5707-5719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maini M.K., Boni C., Ogg G.S. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology. 1999;117(6):1386–1396. doi: 10.1016/s0016-5085(99)70289-1. [DOI] [PubMed] [Google Scholar]
- 11.Rivino L., Le Bert N., Gill U.S. Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Investig. 2018;128(2):668–681. doi: 10.1172/JCI92812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boni C., Fisicaro P., Valdatta C. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai S.L., Chen P.J., Lai M.Y. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. Implications for hepatitis B e antigen seroconversion. J Clin Investig. 1992;89(1):87–96. doi: 10.1172/JCI115590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boni C., Laccabue D., Lampertico P. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143(4):963-73 e9. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Rehermann B., Lau D., Hoofnagle J.H., Chisari F.V. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest. 1996;97(7):1655–1665. doi: 10.1172/JCI118592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boni C., Laccabue D., Lampertico P. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143(4):963–973.e9. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Vyas A.K., Sharma B.C., Sarin S.K., Trehanpati N. Immune correlates of hepatitis B surface antigen spontaneous seroconversion in hepatitis B e antigen negative chronic hepatitis B patients. Liver Int Off J Int Assoc Stud Liver. 2018;38(1):38–49. doi: 10.1111/liv.13475. [DOI] [PubMed] [Google Scholar]
- 18.Chu C.J., Lok A.S. Clinical significance of hepatitis B virus genotypes. Hepatology. 2002;35(5):1274–1276. doi: 10.1053/jhep.2002.33161. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q., Pan W., Liu Y. Hepatitis B virus-specific CD8+ T cells maintain functional exhaustion after antigen reexposure in an acute activation immune environment. Front Immunol. 2018;9:219. doi: 10.3389/fimmu.2018.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S., Wang L., Pan W. MMP2/MMP9-mediated CD100 shedding is crucial for inducing intrahepatic anti-HBV CD8 T cell responses and HBV clearance. J Hepatol. 2019 doi: 10.1016/j.jhep.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Kefalakes H., Budeus B., Walker A. Adaptation of the hepatitis B virus core protein to CD8(+) T-cell selection pressure. Hepatology. 2015;62(1):47–56. doi: 10.1002/hep.27771. [DOI] [PubMed] [Google Scholar]
- 22.Bertoletti A., Ferrari C. Adaptive immunity in HBV infection. J Hepatol. 2016;64(1 Suppl):S71–S83. doi: 10.1016/j.jhep.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Ren H., Huang Y. Effects of pegylated interferon-alpha based therapies on functional cure and the risk of hepatocellular carcinoma development in patients with chronic hepatitis B. J Viral Hepat. 2019;26(Suppl 1):5–31. doi: 10.1111/jvh.13150. [DOI] [PubMed] [Google Scholar]
- 24.Cornberg M., Wong V.W., Locarnini S., Brunetto M., Janssen H.L.A., Chan H.L. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66(2):398–411. doi: 10.1016/j.jhep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Tseng T.C., Liu C.J., Su T.H. Serum hepatitis B surface antigen levels predict surface antigen loss in hepatitis B e antigen seroconverters. Gastroenterology. 2011;141(2):517–525. doi: 10.1053/j.gastro.2011.04.046. 25 e1-2. [DOI] [PubMed] [Google Scholar]
- 26.Rinker F., Zimmer C.L., Honer Zu Siederdissen C. Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol. 2018;69(3):584–593. doi: 10.1016/j.jhep.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Le Bert N., Gill U.S., Hong M. Effects of hepatitis B surface antigen on virus-specific and global T cells in patients with chronic hepatitis B virus infection. Gastroenterology. 2020;159(2):652–664. doi: 10.1053/j.gastro.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Chan H.L., Wong G.L., Tse C.H., Chan H.Y., Wong V.W. Viral determinants of hepatitis B surface antigen seroclearance in hepatitis B e antigen-negative chronic hepatitis B patients. J Infect Dis. 2011;204(3):408–414. doi: 10.1093/infdis/jir283. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y.C., Jeng W.J., Chu C.M., Liaw Y.F. Decreasing levels of HBsAg predict HBsAg seroclearance in patients with inactive chronic hepatitis B virus infection. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10(3):297–302. doi: 10.1016/j.cgh.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 30.Kim G.A., Lim Y.S., An J. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63(8):1325–1332. doi: 10.1136/gutjnl-2013-305517. [DOI] [PubMed] [Google Scholar]
- 31.Hu P., Shang J., Zhang W. HBsAg loss with peg-interferon alfa-2a in hepatitis B patients with partial response to nucleos(t)ide analog: new switch study. J Clin Transl Hepatol. 2018;6(1):25–34. doi: 10.14218/JCTH.2017.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thimme R., Dandri M. Dissecting the divergent effects of interferon-alpha on immune cells: time to rethink combination therapy in chronic hepatitis B? J Hepatol. 2013;58(2):205–209. doi: 10.1016/j.jhep.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Micco L., Peppa D., Loggi E. Differential boosting of innate and adaptive antiviral responses during pegylated-interferon-alpha therapy of chronic hepatitis B. J Hepatol. 2013;58(2):225–233. doi: 10.1016/j.jhep.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 34.Penna A., Laccabue D., Libri I. Peginterferon-α does not improve early peripheral blood HBV-specific T-cell responses in HBeAg-negative chronic hepatitis. J Hepatol. 2012;56(6):1239–1246. doi: 10.1016/j.jhep.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 35.Vigano M., Grossi G., Loglio A., Lampertico P. Treatment of hepatitis B: is there still a role for interferon? Liver Int Off J Int Assoc Stud Liver. 2018;38(Suppl 1):79–83. doi: 10.1111/liv.13635. [DOI] [PubMed] [Google Scholar]
- 36.Wieland D., Kemming J., Schuch A. TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun. 2017;8:15050. doi: 10.1038/ncomms15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Im S.J., Hashimoto M., Gerner M.Y. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pallett L.J., Davies J., Colbeck E.J. IL-2(high) tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J Exp Med. 2017;214(6):1567–1580. doi: 10.1084/jem.20162115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odorizzi P.M., Pauken K.E., Paley M.A., Sharpe A., Wherry E.J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J Exp Med. 2015;212(7):1125–1137. doi: 10.1084/jem.20142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuch A., Salimi Alizei E., Heim K. Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut. 2019;68(5):905. doi: 10.1136/gutjnl-2018-316641. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z., Zhang J.Y., Wherry E.J. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134(7):1938–1949. doi: 10.1053/j.gastro.2008.03.037. 49 e1-3. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z., Jin B., Zhang J.Y. Dynamic decrease in PD-1 expression correlates with HBV-specific memory CD8 T-cell development in acute self-limited hepatitis B patients. J Hepatol. 2009;50(6):1163–1173. doi: 10.1016/j.jhep.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 43.Wu W., Shi Y., Li J., Chen F., Chen Z., Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J. 2011;8:113. doi: 10.1186/1743-422X-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu W., Shi Y., Li S. Blockade of tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. Eur J Immunol. 2012;42(5):1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z., Zhang S., Zou Z. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology. 2011;53(1):73–85. doi: 10.1002/hep.23977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisicaro P., Valdatta C., Massari M. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology. 2010;138(2):682–693. doi: 10.1053/j.gastro.2009.09.052. 93 e1-4. [DOI] [PubMed] [Google Scholar]
- 47.Miller J.D., Van der Most R.G., Akondy R.S. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28(5):710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 48.Kamatani Y., Wattanapokayakit S., Ochi H. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41(5):591–595. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 49.Thomas R., Thio C.L., Apps R. A novel variant marking HLA-DP expression levels predicts recovery from hepatitis B virus infection. J Virol. 2012;86(12):6979–6985. doi: 10.1128/JVI.00406-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koukoulioti E., Fischer J., Schott E. Association of HLA-DPA1 and HLA-DPB1 polymorphisms with spontaneous HBsAg seroclearance in Caucasians. Liver Int. 2019;39(4):646–654. doi: 10.1111/liv.14008. [DOI] [PubMed] [Google Scholar]
- 51.Salimzadeh L., Le Bert N., Dutertre C.A. PD-1 blockade partially recovers dysfunctional virus-specific B cells in chronic hepatitis B infection. J Clin Invest. 2018;128(10):4573–4587. doi: 10.1172/JCI121957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying Ju, Nan Hou, Xiaoning Zhang. Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell Mol Immunol. 2009;6(1):35–43. doi: 10.1038/cmi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.