Abstract
Cisplatin is a chemotherapy drug commonly used in cancer treatment. Tumour cells are more sensitive to cisplatin than normal cells. Cisplatin exerts an antitumour effect by interfering with DNA replication and transcription processes. However, the drug-resistance properties of tumour cells often cause loss of cisplatin efficacy and failure of chemotherapy, leading to tumour progression. Owing to the large amounts of energy and compounds required by tumour cells, metabolic reprogramming plays an important part in the occurrence and development of tumours. The interplay between DNA damage repair and metabolism also has an effect on cisplatin resistance; the molecular changes to glucose metabolism, amino acid metabolism, lipid metabolism, and other metabolic pathways affect the cisplatin resistance of tumour cells. Here, we review the mechanism of action of cisplatin, the mechanism of resistance to cisplatin, the role of metabolic remodelling in tumorigenesis and development, and the effects of common metabolic pathways on cisplatin resistance.
Keywords: tumour metabolism, cisplatin, resistance, DNA damage repair, ROS
Introduction
Cancers are systemic and complex diseases that seriously endanger human health, and their incidence is increasing because of the influence of environmental pollution and modern living habits. Current treatments for tumours include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy (McCarthy, 2006; Englinger et al., 2019). In recent years, molecular-mechanism-based targeted therapies and immunotherapy have shown great progress. However, the targeted therapy approach is limited by the low frequencies of gene mutations and small experimental population sizes. The use of immunotherapy also encounters many challenges, with few definitive answers on how to select the population likely to benefit, optimize the immunotherapy regimen, evaluate the effects of immunotherapy, and solve drug resistance and other urgent problems (Nishino et al., 2013; Carbone et al., 2017; Sharma et al., 2017). Chemotherapy remains the most widely used adjuvant treatment for cancer. Platinum drugs, including cisplatin, carboplatin, and oxaliplatin, are commonly used for most tumour types. Their basic pharmacological mechanism involves internal and interchain crosslinks created by binding to DNA, inhibition of DNA replication and transcription, and then induction of damage to double-stranded DNA. Cisplatin, a first-generation platinum drug, is used as a first-line therapy in clinical practice and has a good inhibitory effect on solid tumours including testicular cancer, ovarian cancer, lung cancer, stomach cancer, head and neck tumours, cervical cancer, and breast cancer (Dasari and Tchounwou, 2014). However, it is associated with numerous undesirable side effects including severe kidney problems, allergic reactions, decreased immunity to infections, gastrointestinal disorders, haemorrhage, and hearing loss especially in younger patients (Dasari and Tchounwou, 2014). Moreover, drug resistance of tumour cells often leads to loss of cisplatin efficacy and failure of chemotherapy, resulting in tumour progression (Q. Wu et al., 2014; Cree and Charlton, 2017). Previous in-depth studies on cisplatin resistance have addressed the contribution of genetics, epigenetics, and signal transduction pathways. Increasing attention has been given to the role of tumour metabolism in cisplatin resistance.
Tumour tissue is composed of cancer cells with different genetic and/or epigenetic backgrounds and surrounding stromal cells, a condition known as intra-tumoral heterogeneity (Hanahan and Weinberg, 2011; L. V. Nguyen et al., 2012). The microenvironment around cancer cells is completely different from that around normal cells. Therefore, tumour cells must demonstrate a rapid adaptive response to hypoxia and hypotrophic conditions. This phenomenon of bioenergetics in tumour cells, known as “metabolic reprogramming” (Yoshida, 2015), has been identified as one of the 10 characteristics of cancer. Remodelling of glucose, amino acid, and lipid metabolism is an important factor for promoting tumour development (Biswas, 2015). Metabolic reprogramming, on the one hand, meets the energy and material requirements of tumours; on the other hand, it involves epigenetic regulation, thereby playing an important part in tumour formation, metastasis, drug resistance, and other processes (Biswas, 2015; Wettersten et al., 2017). The processes by which cisplatin induces tumour cell death and by which tumour cells resist cisplatin-induced death are accompanied by metabolic reprogramming. Targeting metabolic processes therefore represents a potential novel strategy to reverse cisplatin resistance. The role of metabolic reprogramming in cisplatin resistance is reviewed in this paper.
Discovery and Mechanism of Cisplatin Action
The antitumour effect of cisplatin was discovered in the 1960s by American physicist B. Rosenberg (Rosenberg et al., 1965), who associated the shapes of cell mitotic filaments with an electric or magnetic dipole field direction map and studied the effects of electric fields on bacterial division to discover disinfectants. In the course of this research, he found that when the platinum electrode was energized, Escherichia coli cells were 300-fold longer than the corresponding non-energized normal cells (Muggia et al., 2015; Rancoule et al., 2017). To study this phenomenon, T. Krigas tested the bacteria with all the products isolated from the culture and eventually found that [Pt (Ⅳ) (NH3)2Cl4] was a platinum-activated complex produced by electrolysis in nutrient solution that contributed to E. coli filamentation (Rosenberg et al., 1967). Krigas then asked, “Does only tetravalent platinum have this activity?” To answer this question, Krigas synthesized divalent platinum [Pt (Ⅱ) (NH3)2Cl4] and found that its activity was stronger than that of tetravalent platinum. [Pt (Ⅱ) (NH3)2Cl4] has both cis- and trans-structures, and its cis-structures have active trans-structures; the cis-structure is cisplatin (ROSENBERG et al., 1965). Rosenberg then applied cisplatin in antitumour research and found that it had good antitumour activity (Rosenberg et al., 1967). In 1978, the United States Food and Drug Administration approved cisplatin as a new anticancer drug, ushering in a new era of platinum drug development and applications (Prestayko et al., 1979). Later, a large number of studies demonstrated the antitumour effects of cisplatin on solid tumours including ovarian cancer, testicular cancer, and head and neck tumours (Kaye et al., 1992; Tanaka et al., 2018; de Vries et al., 2020).
Following the discovery of the anticancer effect of cisplatin, its mechanism of action was studied. The biochemical mechanism by which cisplatin crosses the cell membrane is still not completely understood. Recently, many studies have demonstrated that cisplatin enters cells through the copper trafficking system, which includes members of the copper transport (Ctr) protein family such as Ctr1 and Ctr2 (Kilari et al., 2016). The ATP7A and ATP7B copper pumps are also associated with the pumping of cisplatin. Cisplatin is inert and must be intracellularly activated by a series of aquation reactions that consist of the substitution of one or both cis-chloro groups with water molecules. Therefore, the activation of cisplatin depends on environmental conditions. In the blood or extracellular tissue fluid, the physiological chloride concentration is approximately 100 mmol/L, and the activity of cisplatin is low. Inside cells, the concentration of chloride ions decreases to only a few mmol/L, enabling the generation of highly reactive mono-and bi-aquated cisplatin forms (Chu et al., 1994).
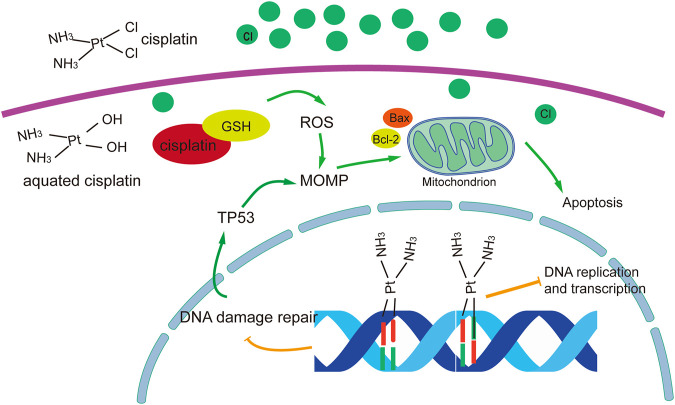
The anticancer mechanism of cisplatin can be divided into nuclear and cytoplasmic modules according to localization. Aquated cisplatin avidly binds DNA, with a predilection for nucleophilic N7-sites on purine bases, resulting in 1,2- or 1,3-intrastrand crosslinks (P. Chen et al., 2016) and a lower percentage of interstrand crosslinks (Ming et al., 2017). These interactions lead to damage to the DNA double helix structure and interfere with replication and transcription. In particular 1,2-intrastrand ApG and CpG crosslinks have been identified as the most prominent cisplatin-induced DNA lesions and have been suggested to account for most, if not all, cisplatin cytotoxicity. In addition, the altered structure of the DNA makes it unrecognisable by DNA-damage-repair proteins. In general, the main roles of cisplatin in the nucleus are to inhibit DNA replication and RNA transcription, arrest the cell cycle, and cause programmed cell death. However, only ∼1% of intracellular cisplatin binds to nuclear DNA (Gonzalez et al., 2001), and cisplatin has been shown to have significant cytotoxicity to enucleated cells (Berndtsson et al., 2007). The larger proportion of intracellular cisplatin interacts with cytoplasm nucleophiles such as glutathione (GSH), mitochondrial DNA, proteins, phospholipids and phosphatidylserine in membranes, sulphur donors, and other mitochondrial structures (Galluzzi et al., 2014). These interactions result in reduced isotonic consumption and/or direct maintenance of reactive oxygen species (ROS) production. ROS have a dual role in cisplatin cytotoxicity, directly triggering mitochondrial outer membrane permeabilization (MOMP) or aggravating DNA damage induced by cisplatin (Sancho-Martinez et al., 2012).
The most effective mode of action of cisplatin involves the DNA damage response and mitochondrial apoptosis. Cisplatin-induced lesions cause distortions in DNA that can be identified by multiple repair pathways. Among these, the nucleotide excision repair (NER) and mismatch repair (MMR) systems are the most prominent mechanisms for the removal of cisplatin. If the damage cannot be repaired, cells become committed to (usually apoptotic) death. This involves the sequential activation of the ATR (ataxia telangiectasia mutated and RAD3-related protein, a sensor of DNA damage) and checkpoint kinase 1 (the most prominent substrate and downstream effector of ATR), which in turn phosphorylates the tumour suppression protein TP53. TP53 activates several genes whose products promote MOMP, thereby triggering endogenous apoptosis, as well as genes encoding components of exogenous apoptotic pathways. The extrinsic pathway is activated when the ligand binds to members of the tumour necrosis factor-α receptor superfamily and then forms the death-inducing signalling complex through oligomerization of the connector molecule and recruitment of procaspase-8 (Kischkel et al., 1995). The intrinsic pathways are initiated by cellular stresses such as DNA damage, leading to the release of cytochrome C by mitochondria, which activates procaspase-9. Bcl-2 family proteins regulate DNA-damage-induced apoptosis by regulating the release of mitochondrial cytochrome C in response to DNA damage (Nunez et al., 1998) (Figure 1).
FIGURE 1.
Overview of molecular mechanisms of cisplatin in cancer treatment. The figure was drawn in adobe illustrator.
Clinical Applications of Cisplatin in Tumour Treatment
Cisplatin is widely used in the treatment of various cancers owing to its excellent anticancer effects (Table 1). Induction chemotherapy followed by radiation therapy (RT) is an organ-sparing treatment approach targeted to selected sub-sites of locally advanced head and neck squamous cell carcinoma. Induction regimens originally included cisplatin and 5-fluorouracil (5-FU) (PF) (Forastiere et al., 2003). More recent phase III trials have shown that the addition of docetaxel to PF results in superior efficacy in patients treated with RT (Vermorken et al., 2007) or carboplatin and RT (Posner et al., 2007). The standard chemotherapy for the initial treatment of ovarian cancer is a combination of a platinum analogue with paclitaxel (McGuire et al., 1996; Ozols et al., 2003); Deborah K found that intravenous paclitaxel plus intraperitoneal cisplatin and paclitaxel improved survival in patients with optimally debulked stage III ovarian cancer compared with intravenous paclitaxel plus cisplatin (Armstrong et al., 2006). In 1993, Housset and colleagues reported encouraging results with regard to bladder preservation and patient compliance with a hypofractionated twice-a-day radiation approach employing concurrent cisplatin and 5-FU that could be safely given as an outpatient regimen (Housset et al., 1993). Treatment of TC depends on stage and tumour type, i.e., seminoma or non-seminoma. Patients with disseminated non-seminoma (intermediate or low risk IGCCC) are treated with four courses of the BEP (bleomycin, etoposide and cisplatin) or VIP (etoposide, ifosfamide and cisplatin) regimen after surgical removal of the affected testicle. In cases of residual disease after completion of chemotherapy, patients undergo surgical removal of affected lymph nodes and/or metastases that had not completely disappeared after chemotherapy. Approximately 10–15% of patients with disseminated disease will need second-line treatment as a consequence of relapse or refractory disease (Adra and Einhorn, 2017). Various effective salvage strategies are currently available. The choice of standard-dose salvage treatment depends on which drugs were initially used in combination with cisplatin. Some common and effective standard-dose salvage treatments have been reported, with long-term remission rates ranging from 23 to 54% using VIP (McCaffrey et al., 1997; Miller et al., 1997), 63% using TIP (paclitaxel, ifosfamide and cisplatin) (Kondagunta et al., 2005), 24% using VeIP (vinblastine, ifosfamide, and cisplatin (Loehrer et al., 1998), and 51% using GIP (gemcitabine, ifosfamide and cisplatin) (Fizazi et al., 2014). In patients with advanced non-small-cell lung cancer, cisplatin plus etoposide was more effective but also more toxic than carboplatin plus etoposide (Klastersky et al., 1990; Faivre-Finn et al., 2017). Patients with limited-stage small-cell lung cancer were treated with concurrent twice-daily chest radiotherapy and etoposide/cisplatin followed by cyclophosphamide, doxorubicin, and vincristine (Johnson et al., 1996 Mar). Compared with paclitaxel plus cisplatin, paclitaxel plus carboplatin is not inferior and should be a standard treatment option for metastatic or recurrent cervical cancer; however, cisplatin is still the key drug for patients who have not received platinum agents (Kitagawa et al., 2015). 5- Fluorouracil combining with cisplatin (FP), capecitabine plus cisplatin (XP) regimen, epirubicin/cisplatin–5-FU (ECF), as well as 5-FU, an anthracycline and cisplatinis are adopted as standard reference regimens for patients with gastric cancer (Kang et al., 2009; Lee et al., 2012). Brandes et al. found that cisplatin plus temozolomide appeared to be effective in chemotherapy-naive patients with recurrent glioblastoma multiforme, with an acceptable level of toxicity (Brandes et al., 2004). The CBCSG006 trial reported the superior efficacy of a cisplatin plus gemcitabine (GP) regimen compared with paclitaxel plus gemcitabine as a first-line treatment for metastatic triple-negative breast cancer (Hu et al., 2015; J. Zhang et al., 2018a).
TABLE 1.
The chemotherapy regimens of cisplatin in various tumours.
| Tumour | Cisplatin chemotherapy regimens |
|---|---|
| Head and neck squamous cell carcinoma | cisplatin and 5-fluorouracil Armstrong et al. (2006); Kelland, (2007) |
| Ovarian cancer | cisplatin and paclitaxel McGuire et al. (1996); Ozols et al. (2003) |
| Bladder cancer | cisplatin and 5-fluorouracil Kaufman et al. (2000) |
| Testicular cancer | cisplatin, ifosfamide and etoposide Housset et al. (1993) |
| Lung cancer | cisplatin and etoposide Klastersky et al. (1990); Faivre-Finn et al. (2017) |
| Cervical cancer | cisplatin and paclitaxel Kitagawa et al. (2015) |
| Stomach cancer | cisplatin and capecitabine Lee et al. (2012) |
| Glioblastoma cancer | cisplatin and temozolomide Brandes et al. (2004) |
| Breast cancer | cisplatin and gemcitabine Hu et al. (2015); Zhang et al. (2018a) |
Molecular Mechanism of Cisplatin Resistance
Despite the successful application of cisplatin treatment against several cancer types, the effectiveness of the therapy is often limited by resistance, leading to therapeutic failure. DNA-damage-mediated apoptotic signals can be attenuated, and the resistance that ensues is a major limitation of cisplatin-based chemotherapy. Drug resistance is still the major obstacle to successful chemotherapy. Given the multiple mechanisms of cytotoxicity exerted by cisplatin, the cisplatin-resistant phenotype of cancer can be due to alterations in one or more of these molecular circuits (Galluzzi et al., 2012). The mechanism of cisplatin resistance includes the following main aspects.
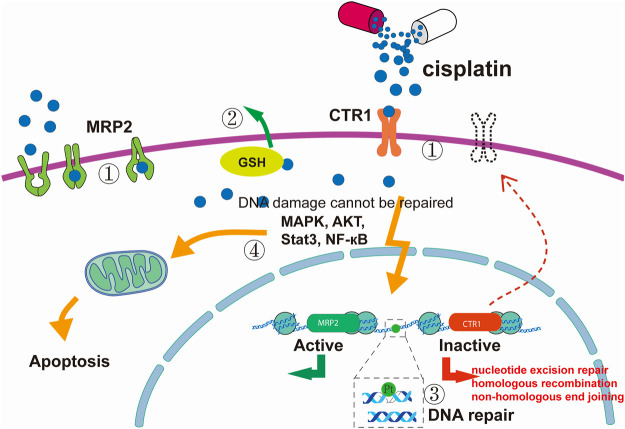
1) Decreased drug uptake or increased drug effusion. This can significantly reduce cisplatin adducts, resulting in reduced toxicity and resistance. Ctr1 is a transmembrane protein with an important role in the cellular uptake of cisplatin. Studies have shown that cisplatin at clinical concentrations reduces the expression of CTR1, leading to a reduction in cisplatin uptake (Holzer et al., 2006). Overexpression of the ABC family transporter MRP2 leads to cisplatin being pumped from the cell into the extracellular space, reducing the intracellular cisplatin concentration (Borst et al., 2000; Liedert et al., 2003).
2) Increased sequestration of cisplatin by GSH and other cytoplasmic scavengers with nucleophilic properties. Aquated cisplatin binds to cytoplasmic nucleophilic substances, including GSH, methionine, metallothionein, and other cysteine-rich proteins. The activation of GSH detoxification and metallothionein systems by nucleophilic substances serving as cytoplasmic scavenging agents also accelerates the removal of cisplatin from cells (Mandic et al., 2003).
3) The sensitivity of tumour cells to cisplatin is related to the molecular damage caused by the direct binding of cisplatin to its target. Once cisplatin reacts with DNA, the cell must clear or tolerate the lesions to survive. Thus, cisplatin resistance is also related to DNA damage repair ability. The repair of damage to double-stranded DNA caused by cross-linking requires the joint participation of different repair mechanisms. Previous studies have found that NER (Shuck et al., 2008), homologous recombination (Telli et al., 2016), non-homologous end joining (Diggle et al., 2005; Sears and Turchi, 2012), and other repair mechanisms are involved.
4) The continuous action of cisplatin leads to abnormalities in the signal regulation networks in tumour cells, resulting in strong anti-apoptosis ability and resistance of cells to cisplatin. For example, TP53 is inactivated in approximately one-half of human tumours, endowing tumour cells with anti-apoptotic ability (Vousden and Lane, 2007). Other studies have found that the MAPK pathway (which has a critical role in regulating cisplatin-induced apoptosis) cannot be activated in cisplatin-tolerant cells, and, as a result, the FAS/FASL system (an inducer of extrinsic apoptosis) cannot be activated to enable cell survival (Spierings et al., 2003). In addition, PI3K/AKT, NF-κB, Stat3, and other signalling pathways are also involved in the regulation of cisplatin resistance (Mitsuuchi et al., 2000) (Figure 2).
FIGURE 2.
Cisplatin resistance mechanism. (1) Reduced intracellular accumulation of cisplatin. (2) Increased sequestration of cisplatin by GSH and other cytoplasmic scavengers with nucleophilic properties. (3) Enhanced DNA damage repair ability. (4) Defects in apoptotic signal transduction pathways. The figure was drawn in Adobe Illustrator.
These resistance mechanisms explain the changes in the genome, proteome, and signal transduction pathways that occur under the action of cisplatin. This does not fully explain the resistance mechanism of cisplatin. New research fields such as metabolic reprogramming are gradually being developed; we will elaborate on these in detail below.
The Metabolic Remodelling Process of Tumours
In recent years, the understanding of malignant tumours has gradually changed from the concept of a “genetic disease” to one of a “metabolic disease” (Wishart, 2015), and metabolic remodelling has been recognized as one of the ten characteristics of tumours. Obesity, diabetes, dyslipidaemia, and other metabolic diseases are related to the development of tumours. Metabolic changes create selective advantages for tumour growth, proliferation, and survival. Metabolic processes produce energy and anabolic growth substrates to sustain cell survival and proliferation. Tumour cells meet the energy, biosynthesis, and oxidation-reduction reaction requirements for rapid and continuous proliferation through metabolic remodelling, which involves glycometabolism, amino acid metabolism, lipid metabolism, and other processes (Mathieu and Ruohola-Baker, 2017). Normal cells dissipate glucose energy mainly through the glycolytic–tricarboxylic acid (TCA) cycle–phosphorylation pathway under aerobic conditions; however, owing to their higher demand for energy, tumour cells rely on glycolysis as the main pathway of energy production.
This remodelling of glucose metabolism in tumour cells is known as the Warburg effect (Warburg et al., 1927) and also involves changes in the metabolic intermediates that provide biosynthetic materials for the rapid growth and division of tumour cells. These changes in metabolite levels vary among different cancers; for instance, a high-glycine diet may prevent breast cancer (Y. Wang et al., 2018b), serine metabolism is dysregulated in many tumours (Mattaini et al., 2016), asparaginase is an integral component of multiagent chemotherapy regimens for the treatment of acute lymphoblastic leukaemia (Salzer et al., 2018), histone hypermethylation can be induced in V600EBRAF melanoma cells by withdrawing glutamine (Cluntun et al., 2017), and high lactate levels are predictive of metastasis and restricted patient survival (Choi et al., 2013). Cellular glucose metabolism and cancer metabolism in general were not previously considered to be major branches of cancer biology, and high cellular glucose metabolism has only recently been recognized as one of the hallmarks of cancer by biologists (Hay, 2016). Lipid metabolism remodelling has an important role in the occurrence and development of hepatocellular carcinoma (HCC), mainly involving lipid biosynthesis and desaturation caused by upregulation of numerous crucial enzymes [ACL, acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN), and stearoyl-coenzyme A desaturase-1 (SCD1)] in fatty acid biosynthesis. Monosaturated fatty acids, produced from saturated fatty acids by SCD1, are vital for membrane synthesis and prostaglandin synthesis and serve as sources for triacylglycerols. They influence cancer cell survival by contributing to autophagy activation, promoting cell membrane turnover, influencing intracellular signalling and gene transcription, and enhancing energy production (Pope et al., 2019). The three major metabolisms are not only independent of each other but are also related to each other through the TCA cycle. Below we will introduce the link between metabolic reprogramming and cisplatin resistance.
Roles of Metabolic Processes in DNA Damage Repair
As mentioned earlier, the direct target of cisplatin is DNA, which can cause DNA cross-linking and double-strand damage. Metabolism may be involved in tumour development because it influences DNA damage repair. The first connection between cell metabolism and DNA repair involves DNA folding (Jeggo and Downs, 2014). Chromatin packaging and remodelling are accomplished through various histone post-translational modifications and DNA modifications, the most common types being methylation (Niculescu and Zeisel, 2002) and acetylation. Most methyl donors are produced during S-adenosylmethionine (SAM) metabolism, which is correlated with methionine, one-carbon metabolism, tetrahydrofolate (THF), and the choline pathway (Niculescu and Zeisel, 2002; Mehrmohamadi et al., 2016). Changes in methionine, THF, and choline concentrations directly affect SAM and thus DNA or histone methylation. The only acetyl donor is acetyl-coenzyme A, which is closely related to TCA (Choudhary et al., 2014; Sivanand et al., 2017). Limiting the number of acetyl donors can destroy normal DNA in affected tissue and influence DNA folding and DNA remodelling (Sivanand et al., 2017). Therefore, regulating methyl and acetyl donors through different metabolic pathways affects the DNA repair process. Second, the availability of metabolites and other nutrients affects the amount and the proportion of nucleotides produced in cells (Rao et al., 2015), thereby affecting DNA repair and replication. Various amino acids are closely related to raw DNA synthesis materials. Glutamine and glycine are involved in purine synthesis (Cory and Cory, 2006; Lane and Fan, 2015), while aspartic acid is related to pyrimidine synthesis. Finally, the regulation of ROS metabolism is also related to DNA damage repair. Strict regulation of cellular oxidation-reduction reaction stress is necessary because high levels of ROS can lead to oxidative stress and oxidative damage to proteins, DNA, and lipids; however, a certain level of ROS is essential to activate signalling pathways involved in multiple biological processes (Shanware et al., 2011; Alleman et al., 2014).
Cells have evolved several ways to balance ROS levels, and GSH is one of the main molecules that scavenge ROS. In addition, studies have found that an important regulator of ROS levels is the transcription factor NRF2, which has been proven to regulate key enzymes of serine metabolism (PHGDH, PSAT1, and ATF4) (DeNicola et al., 2015). In our previous work, we found that reducing the concentration of serine in the medium or inhibiting the activity of PHGDH could reduce levels of H3K4 methylation and promote DNA repair, leading to resistance to cisplatin (X. Zhao et al., 2020). Although tumour metabolic remodelling can affect DNA repair pathways, DNA damage caused by endogenous and exogenous genotoxicity may also lead to cellular metabolic remodelling (Mirzayans et al., 2006). Knowledge of the associations between tumour metabolism and DNA damage and repair is growing, providing opportunities to study the mechanistic basis behind potential metabolic defects in tumours.
Glycometabolism and Cisplatin Resistance
Glucose is the most demanded nutrient. After ingestion, glucose undergoes glycolysis to produce pyruvate; this can be catalysed to produce lactic acid, which is the final product under hypoxic conditions, or catalysed into acetyl-CoA under normal conditions, ultimately entering the TCA cycle. Tumour cells undergo glycolysis as the main way to generate energy, even under aerobic conditions, owing to the Warburg effect, which is also involved in cisplatin resistance (Warburg et al., 1927). Evidence indicates that increased glucose uptake and enhanced aerobic glycolysis induce intrinsic or acquired resistance to cisplatin in several cancer cell types (X. Y. Zhang et al., 2018b).
In a cisplatin-resistant gastric cancer cell model, glycolysis levels were shown to be significantly increased. Gastric cancer cells were significantly more sensitive to cisplatin after the inhibition of glycolysis via treatment with 2-deoxy-D-glucose, a glucose-competitive substrate (Qian et al., 2017; Varghese et al., 2020), which had the same effect in head and neck cancer cells (Simons et al., 2007). Glucose transporter 1 (GLUT1) is closely related to cell metabolism and is mainly involved in transport of glucose across the membrane to provide energy for cells. Studies have found that inhibiting GLUT1 can improve the sensitivity of oesophageal and head and neck cancer cells to cisplatin (Sawayama et al., 2019). In breast and cervical cancer cells, cisplatin inhibited the expression of GLUT1, GLUT4, LDHA (Manerba et al., 2015), and other glycolysis-related proteins, thereby inhibiting glycolysis.
Alterations in glycolysis affecting cisplatin resistance have been shown to be associated with several enzymes. Enolase1 catalyses the conversion of glycerate-2-phosphate into phosphoenolpyruvate in the ninth step of glycolysis (Qiao et al., 2019). The expression of enolase1 in drug-resistant cells is significantly increased, as proven by proteomic screening. Knocking down enolase1 expression resulted in increased sensitivity of gastric cancer cells to cisplatin (Qian et al., 2017). In this resistance model, higher expression of PDK3 was discovered through gene chip technology; PDK3 functions by preventing pyruvate from being catabolized into acetyl-CoA, which forms a positive feedback loop with HSF1, driving cisplatin resistance (J. Xu et al., 2019). Xu et al. found that cisplatin resistance in ovarian cancer involved higher glucose uptake; moreover, oxidative phosphorylation was modulated by Bcl-2, and targeting Bcl-2 reversed cisplatin resistance by inhibiting glucose metabolism (Y. Xu et al., 2018). M2 pyruvate kinase (PKM2) (X. Wang et al., 2017) catalyses phosphoenolpyruvate to produce pyruvate; in dimer form, it typically supplies energy for tumour cells. In osteosarcoma tumour stem cells, PKM2 is highly expressed and downregulated with metformin, which reduces the uptake of glucose and the production of lactic acid and ATP, thereby increasing cell sensitivity to cisplatin (Shang et al., 2017). The oncogene ALC1 can promote cisplatin resistance in oesophageal cancer cells by activating glycolysis (F. Li et al., 2019). Glycolysis levels increased significantly in cisplatin-resistant T24 cells of bladder cancer, promoting acetate and fatty acid synthesis (Wen et al., 2019).
Inhibition of the glycolytic pathway was shown to increase the sensitivity of drug-resistant ovarian cancer cells to cisplatin (Xintaropoulou et al., 2018). In cisplatin-resistant A549 lung cancer cells, the expression of G6PD (Hong et al., 2018), a key enzyme involved in bypassing the pentose phosphate pathway, was increased, and reducing G6PD could increase cisplatin sensitivity (Hong et al., 2018; Giacomini et al., 2020). Pyruvate participates in oxidative phosphorylation via conversion to acetyl-CoA. Pyruvate dehydrogenase kinase (PDK) can inhibit the conversion of pyruvate to acetyl-CoA (Z. Sun and Xu, 2019; G. Wang et al., 2019). Dichloroacetate (DCA), an inhibitor of PDK, can facilitate the transition from glycolysis to oxidative phosphorylation (Stacpoole et al., 2019; Tataranni and Piccoli, 2019). In drug-resistant ovarian cancer cells, PDK1 expression was significantly upregulated, and knocking down PDK1 expression significantly increased cisplatin sensitivity (Zhang et al., 2019). In cisplatin-resistant head and neck cancer cells, glycolysis enhanced cisplatin resistance and PDK2 expression was increased, whereas DCA reversed this enhancement of resistance (Roh et al., 2016). In addition, microRNAs are involved in the regulation of glucose metabolism during cisplatin resistance. Study have found that the expression of miR-5787 is downregulated in cisplatin-resistant tongue squamous cell carcinoma, promoting the transition from oxidative phosphorylation to aerobic glycolysis; in contrast, high expression of miR-5787 can improve the sensitivity of these cells to cisplatin (W. Chen et al., 2019).
Amino Acid Metabolism and Cisplatin Resistance
A large number of studies have shown that amino acids are used not only as substrates for protein synthesis but also as metabolites and metabolic regulators to support the growth of cancer cells (Z. Li and Zhang, 2016; L. Sun et al., 2018; G. Wu, 2009). Among the amino acids used in this way, glutamine, serine, and glycine have been widely studied (Nikiforov et al., 2002; Labuschagne et al., 2014). Amino acid uptake and metabolism are abnormal in many cancers that show addiction to specific amino acids. Amino acids promote the survival and proliferation of cancer cells under genotoxicity, oxidative stress, and nutritional stress. Thus, targeting amino acid metabolism is a potential cancer treatment strategy (Wei et al., 2020). Amino acid metabolites and metabolic enzymes also affect cisplatin resistance.
Glutamine, the most abundant amino acid in blood and muscle, maintains the high bioenergy requirements of tumour cells and serves as a precursor for macromolecular biosynthesis (Windmueller and Spaeth, 1974). Glutamine has a pleiotropic role, providing not only carbons but also nitrogen for nucleic acid synthesis. This amino acid can serve as a respiratory substrate that enters the TCA cycle in mitochondria, thereby driving ATP production (Fan et al., 2013). In addition, glutamine supports GSH biosynthesis and NADPH production and is involved in cellular redox homeostasis (Jiang et al., 2019). From the perspective of the current review, the glutamine dependence of cancer cells may represent a metabolic vulnerability of cancer; therefore, enzymes that inhibit the glutamine metabolic pathway could be used in cancer therapy (Obrist et al., 2018). Glutamine intake affects the sensitivity of cells to cisplatin. ASCT2 (SLC1A5) (Liu et al., 2018), a glutamine transporter, was found to be highly expressed in A549 wild-type cells and cisplatin-resistant cells but was negligibly expressed in normal lung fibroblasts (J. Wu et al., 2018). By simulating a polyglutamine delivery system with glutamine macromolecules, SLC1A5 was used to deliver specific therapeutic compounds to glutamine-dependent cancer cells, further sensitizing these cancer cells to cisplatin (C. Wang et al., 2018a). The rapid catabolic metabolism of glutamine mediated by the oncogene KRAS continuously enhances the antioxidant capacity of cisplatin-resistant cells, thus enabling them to tolerate cytotoxicity. However, excessive consumption of glutamine also impedes the growth of cisplatin-resistant cells. Compared with cisplatin-sensitive cells, cisplatin-resistant cells show increased autophagy and are susceptible to glutamine deprivation. In the case of glutamine deficiency, the G1 phase is significantly blocked, and the apoptosis rate is increased (Duan et al., 2018a). Inhibition of glutamine metabolism can increase the sensitivity of drug-resistant ovarian cancer cells to cisplatin (Duan et al., 2018b). Combining the glutaminase inhibitor BPTES with cisplatin significantly increased the apoptosis induction rate of cisplatin-sensitive and cisplatin-resistant ovarian cancer cells (Hudson et al., 2016; Masamha and LaFontaine, 2018).
In addition to glutamine, there are other amino acids that contribute to cisplatin resistance. Cisplatin-resistant lung cancer cells do not primarily use glucose but instead consume amino acids such as glutamine and tryptophan to survive. Compared with cisplatin-sensitive lung cancer cells, IDO1 activity and ROS levels in cisplatin-resistant cells were increased. Inhibition of IDO1 with shRNAs or IDO1 inhibitors increased ROS levels and produced significant growth inhibition only in cisplatin-resistant cells (D. J. M. Nguyen et al., 2020). In our previous work, we found that serine deficiency or insufficiency (12.5, 25, or 50% of serine contained in RPMI-1640 complete medium) during cell culture inhibited the toxicity and pro-apoptotic effects of cisplatin on gastric cancer cells by reducing H3K4 tri-methylation. The addition of serine could reverse the sensitivity of gastric cancer cells to cisplatin (X. Zhao et al., 2020).
Lipid Metabolism and Cisplatin Resistance
Lipid metabolic reprogramming is a newly recognized hallmark of malignancy. Increased lipid uptake, storage, and fat production in various cancers contribute to rapid tumour growth. Lipids form the basic structure of cell membranes and also function as signalling molecules and energy sources (Cheng et al., 2018; Long et al., 2018). Abnormal lipid metabolism is closely related to the occurrence of cancer (Ameer et al., 2014). Lipids affect cell survival, membrane fluidity and dynamics, and response to chemotherapy; therefore, lipid metabolism is relevant to tumour therapy (Qiu et al., 2015). In recent years, lipid metabolism has also been shown to be closely related to cisplatin resistance. Specifically, metabolic enzymes related to lipid metabolism have an effect on cisplatin resistance. In T24R drug-resistant bladder cancer cells, enzymes involved in acetic acid use (ACSS2) and fatty acid synthesis (ACC) and fatty acid synthesis precursors (acetyl-CoA) levels are increased, leading to higher yields of glucose-derived acetic acid and fatty acids. ACSS2 is highly expressed in cisplatin-resistant tissues, and targeted inhibition of fatty acid synthesis was shown to inhibit bladder cancer cell resistance (Jin et al., 2018). In addition, ω-3 polyunsaturated fatty acids induce apoptosis by ADORA1 and enhance the effect of cisplatin on gastric cancer cells, human lung cancer cells, and melanoma cells (Zajdel et al., 2014; Sheng et al., 2016). In primary HCC cells, carnitine palmitoyltransferase-2 is downregulated to promote adipogenesis of cancer cells, inducing cisplatin resistance of HCC cells and enhancing their oncogenic activity and metastasis potential (Lin et al., 2018). AGPS is a key enzyme in the synthesis of ether-based lipids and is highly expressed in cisplatin-resistant glioma cells (U87MGDDP). Reducing AGPS levels can inhibit cell proliferation and increase cisplatin sensitivity (Zhu et al., 2014). FASN is essential for initiating long-chain fatty acid synthesis, which is necessary to meet the ever-increasing demands of cancer cells for membrane, energy, and protein production. FASN is highly expressed in cancer tissues compared with normal fallopian tubes. Bauerschlag et al. found that inhibition of FASN could increase the sensitivity of ovarian cancer cells to cisplatin and induce apoptosis, and reverse cisplatin resistance (Bauerschlag et al., 2015).
Other Forms of Metabolism and Cisplatin Resistance
In addition to the three major metabolites, other metabolites can affect cisplatin resistance. Vitamin D supplements can reduce the risk of many cancers. Vitamin D sensitizes oral cancer cells to cisplatin and partially reverses cisplatin resistance. Cisplatin enhances the expression of lipocalin 2 (LCN2) by decreasing methylation at the promoter, whereas vitamin D inhibits the expression of LCN2 by increasing methylation and promoting cisplatin chemotherapy (Huang et al., 2019). Vitamin D can also inhibit GPX1; reduce the migration, invasion, and proliferation of oesophageal cancer cells; and reduce cisplatin resistance (Gan et al., 2014).
Summary and Prospects
Cisplatin is an important tool in the treatment of some solid tumours, including ovarian cancer, testicular cancer, lung cancer, and head and neck cancer. Unfortunately, owing to intrinsic or acquired drug resistance, patients treated with platinum often experience relapses and treatment failures. As discussed in this review, cisplatin-resistant cancer cells have been shown to evade drug toxicity by reprogramming their metabolism. Reprogramming involves all major pathways including cell biosynthesis, energy substrates, redox homeostasis, and signal transduction. Now that the regulatory role of metabolism in cisplatin sensitivity is recognized, further attention should be directed to determining how to reverse tumour resistance to cisplatin by intervening in metabolic processes. As mentioned above, many inhibitors targeting metabolic enzymes combined with cisplatin have synergistic antitumour effects (Table 2). It is noteworthy that an effective combination therapy can be developed by linking the findings of basic research to translational research.
TABLE 2.
The inhibitor targeting metabolic alterations of cisplatin-resistant tumours.
| Metabolic pathway | Target | Inhibitor | Type of study |
|---|---|---|---|
| Glucose metabolism | GLUT1 | BAY-876 Sawayama et al. (2019) | In vitro |
| LDHA | Galloflavin oxamate Manerba et al. (2015) | In vitro | |
| PKM2 | Shinkonin Wang et al. (2017) | In vitro | |
| G6PD | 6AN, DHEA Hong et al. (2018) | In vitro | |
| PDK | Dichloroacetate (DCA) Tataranni and Piccoli, (2019) | In vitro | |
| Amino acid metabolism | ASCT2(SLC1A5) | Resveratrol Liu et al. (2018) | In vitro |
| Glutaminase | BPTES Masamha and LaFontaine, (2018) | In vitro | |
| IDO1 | Epacadostat Nguyen et al. (2020) | In vitro | |
| Lipid metabolism | ACSS2 | 1–2 urea Wen et al. (2019) | In vitro |
| FASN | C75 Bauerschlag et al. (2015) | In vitro |
The cost of research and development of inhibitors is high and the cycle is long, which leads to a lack of effective intervention strategies for many of the mechanisms currently recognized; therefore, interventions need to be derived from other perspectives. Nutrients are sources of metabolites and regulating the nutritional status of the body can improve the effect of tumour therapeutics. Diet directly determines the nutritional status of the body; therefore, the effect of regulating diet on tumour metabolism has attracted increasing attention. Many studies have also shown that diet can influence the effectiveness of drugs by altering the metabolic state of tumours. Prevention and blocking of drug resistance through diet control will be a new direction for the further development of cisplatin and other platinum-based treatment strategies. Two aspects of diet are notable: diet affects DNA repair and the rate of tumour cell apoptosis by changing the metabolic state of tumour cells; and diet can reduce the toxicity and side effects of cisplatin by changing the organism’s environment, for example, by co-administering sulphur-containing “chemoprotective agents” with cisplatin (Sooriyaarachchi et al., 2016).
In summary, we have systematically summarized the role and mechanism of tumour metabolism in cisplatin resistance and described prospective drug resistance reversal strategies based on tumour metabolism, thus providing new perspectives for the clinical applications of cisplatin.
Author Contributions
LW wrote the article, XZ was in charge of the literature review, JF, WX, JY directed the writing.
Funding
This work was supported by the Jinhua Science and Technology Research Program (2020-3-028, 2020-3-046, 2020-3-049) and the Doctor Foundation of Jinhua Hospital (JY 2019-3-001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ACSS2, acyl-CoA synthetase short-chain family member 2; ACL, ATP-citrate lyase; ACC, acetyl-CoA carboxylase; ADORA1, adenosine A1 receptor; AGPS, alkylglycerone phosphate synthase; ALL, acute lymphoblastic leukaemia; ASS1, argininosuccinate synthase 1; ATF, activating transcription factor 4; ATM, ataxia telangiectasia mutated; Bim, BCL2 like 11 belongs to the BCL-2 protein family; BPTES, glutaminase inhibitor; CPT2, carnitine palmitoyltransferase-2; CHEK1, checkpoint kinase 1; CTR1, copper transporter 1; DCA, dichloroacetate; DDR, DNA damage repair; DNA, deoxyribonucleic acid; DNL, de novo lipogenesis; FASN fatty acid synthase; FDA, food and drug administration; G6PD, glucose-6-phosphate dehydrogenase; PHGDH, phosphoglycerate dehydrogenase; PSAT1, phosphoserine aminotransferase 1; GPX1, glutathione peroxidase 1; GSH, glutathione; H3K4 tri-methylation di-methyl-histone H3; Lys4 tri-methylation; HR, homologous recombination; HSF1, heat shock transcription factor 1; LCN2, lipocalin 2; MAST1, microtubule-associated serine/threonine kinase 1; MAPK, mitogen-activated protein kinase; MOMP, mitochondrial outer membrane permeabilization; NER, nucleotide excision repair; NHEJ, non-homologous end joining; NRF2, nuclear factor E2-related factor 2; PDK, pyruvate dehydrogenase kinase; PKM2, pyruvate kinase isoenzyme M2; REDOX, oxidation reduction reaction; RNA, ribonucleic acid; ROS, reactive oxygen species; SAM, S-adenosylmethionine; SAT1, spermidine/spermine N1-acetyltransferase; SCD1, stearoyl-coenzyme A desaturase-1; TAGs, triacylglycerols; TCA, tricarboxylic acid cycle; THF, tetrahydrofolate.
References
- Adra N., Einhorn L. H. (2017). Testicular Cancer Update. Clin. Adv. Hematol. Oncol. 15 (5), 386–396. [PubMed] [Google Scholar]
- Alleman R. J., Katunga L. A., Nelson M. A. M., Brown D. A., Anderson E. J. (2014). The “Goldilocks Zone” from a Redox perspective—Adaptive vs. Deleterious Responses to Oxidative Stress in Striated Muscle. Front. Physiol. 5, 358. 10.3389/fphys.2014.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameer F., Scandiuzzi L., Hasnain S., Kalbacher H., Zaidi N. (2014). De Novo lipogenesis in Health and Disease. Metabolism 63 (7), 895–902. 10.1016/j.metabol.2014.04.003 [DOI] [PubMed] [Google Scholar]
- Armstrong D. K., Bundy B., Wenzel L., Huang H. Q., Baergen R., Lele S., et al. Gynecologic Oncology Group (2006). Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N. Engl. J. Med. 354 (1), 34–43. 10.1056/nejmoa052985 [DOI] [PubMed] [Google Scholar]
- Bauerschlag D. O., Maass N., Leonhardt P., Verburg F. A., Pecks U., Zeppernick F., et al. (2015). Fatty Acid Synthase Overexpression: Target for Therapy and Reversal of Chemoresistance in Ovarian Cancer. J. Transl Med., 13, 146. 10.1186/s12967-015-0511-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndtsson M., Hagg M., Panaretakis T., Havelka A. M., Shoshan M. C., Linder S. (2007). Acute Apoptosis by Cisplatin Requires Induction of Reactive Oxygen Species but Is Not Associated with Damage to Nuclear DNA. Int. J. Cancer 120 (1), 175–180. 10.1002/ijc.22132 [DOI] [PubMed] [Google Scholar]
- Biswas S. K., (2015). Metabolic Reprogramming of Immune Cells in Cancer Progression[Journal Article; Research Support, Non-U.S. Gov't; Review], Immunity. 43 (3), 435–449. 10.1016/j.immuni.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Borst P., Evers R., Kool M., Wijnholds J. (2000). A Family of Drug Transporters: the Multidrug Resistance-Associated proteinsResearch Support, Non-U.S. Gov't; Review]. J Natl Cancer Inst. 92 (16), 1295–1302. 10.1093/jnci/92.16.1295 [DOI] [PubMed] [Google Scholar]
- Brandes A. A., Basso U., Reni M., Vastola F., Tosoni A., Cavallo G., et al. (2004). First-line chemotherapy with cisplatin plus fractionated temozolomide in recurrent glioblastoma multiforme: a phase II study of the Gruppo Italiano Cooperativo di Neuro-Oncologia. J. Clin. Oncol. 22 (9), 1598–1604. 10.1200/jco.2004.11.019 [DOI] [PubMed] [Google Scholar]
- Carbone D. P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., et al. (2017). First-Line Nivolumab in Stage IV or Recurrent Non-small-cell Lung Cancer. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Comment]. N. Engl. J. Med. 376, 2415–2426. 10.1056/nejmoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Li J., Chen Y. C., Qian H., Chen Y. J., Su J. Y., et al. (2016). The Functional Status of DNA Repair Pathways Determines the Sensitization Effect to Cisplatin in Non-small Cell Lung Cancer Cells. [Journal Article]. Cel Oncol (Dordr) 39 (6), 511–522. 10.1007/s13402-016-0291-7 [DOI] [PubMed] [Google Scholar]
- Chen W., Wang P., Lu Y., Jin T., Lei X., Liu M., et al. (2019). Decreased Expression of Mitochondrial miR-5787 Contributes to Chemoresistance by Reprogramming Glucose Metabolism and Inhibiting MT-CO3 translation. [Journal Article; Research Support, Non-U.S. Gov't]. Theranostics 9 (20), 5739–5754. 10.7150/thno.37556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Geng F., Cheng X., Guo D. (2018). Lipid Metabolism Reprogramming and its Potential Targets in Cancer. Cancer Commun. (Lond) 38 (1), 27. 10.1186/s40880-018-0301-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. Y., Collins C. C., Gout P. W., Wang Y. (2013). Cancer-generated Lactic Acid: a Regulatory, Immunosuppressive Metabolite? [Journal Article; Research Support, Non-U.S. Gov't; Review]. J. Pathol. 230 (4), 350–355. 10.1002/path.4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C., Weinert B. T., Nishida Y., Verdin E., Mann M. (2014). The Growing Landscape of Lysine Acetylation Links Metabolism and Cell signalling. [Journal Article; Research Support, Non-U.S. Gov't; Review]. Nat Rev. Mol. Cel Biol 15 (8), 536–550. 10.1038/nrm3841 [DOI] [PubMed] [Google Scholar]
- Chu G. (1994). Cellular Responses to Cisplatin. The Roles of DNA-Binding Proteins and DNA Repair. Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.; Review]. J. Biol. Chem. 269, 787–790. 10.1016/s0021-9258(17)42175-2 [DOI] [PubMed] [Google Scholar]
- Cluntun A. A., Lukey M. J., Cerione R. A., Locasale J. W. (2017). Glutamine Metabolism in Cancer: Understanding the Heterogeneity. [Journal Article; Review; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Trends Cancer 3, 169–180. 10.1016/j.trecan.2017.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory J. G., Cory A. H. (2006). Critical Roles of Glutamine as Nitrogen Donors in Purine and Pyrimidine Nucleotide Synthesis: Asparaginase Treatment in Childhood Acute Lymphoblastic Leukemia. [Journal Article; Review]. In. Vivo 20 (5), 587–589. [PubMed] [Google Scholar]
- Cree I. A., Charlton P. (2017). Molecular Chess? Hallmarks of Anti-cancer Drug Resistance. [Journal Article; Review]. BMC Cancer 17 (1), 10. 10.1186/s12885-016-2999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S., Tchounwou P. B. (2014). Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. [Journal Article; Research Support, N.I.H., Extramural; Review]. Eur J. Pharmacol. 740, 364–378. 10.1016/j.ejphar.2014.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries G., Rosas-Plaza X., van Vugt M. A. T. M., Gietema J. A., de Jong S. (2020). Testicular Cancer: Determinants of Cisplatin Sensitivity and Novel Therapeutic Opportunities. [Journal Article; Review]. Cancer Treat Rev 88, 102054. 10.1016/j.ctrv.2020.102054 [DOI] [PubMed] [Google Scholar]
- DeNicola G. M., Chen P. H., Mullarky E., Sudderth J. A., Hu Z., Wu D., et al. (2015). NRF2 Regulates Serine Biosynthesis in Non-small Cell Lung Cancer. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Nat. Genet. 47, 1475–1481. 10.1038/ng.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle C. P., Bentley J., Knowles M. A., Kiltie A. E. (2005). Inhibition of Double-Strand Break Non-homologous End-Joining by Cisplatin Adducts in Human Cell extracts. [Journal ArticleResearch Support, Non-U.S. Gov't]. Nucleic Acids Res. 33 (8), 2531–2539. 10.1093/nar/gki528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G., Shi M., Xie L., Xu M., Wang Y., Yan H., et al. (2018a). Increased Glutamine Consumption in Cisplatin-Resistant Cells Has a Negative Impact on Cell Growth. [Journal Article; Research Support, Non-U.S. Gov't]. Sci. Rep. 8 (1), 4067. 10.1038/s41598-018-21831-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan G., Song Z., Qi M., Bai X., Wang J., Zhang Y., et al. (2018b). Increased Autophagy Levels Mediate Cisplatin Resistance in Cisplatin-Resistant Cells while Also Rendering Them Vulnerable to Autophagy Induction. [Journal Article]. Biomed. Res. Int. 2018, 1736738. 10.1155/2018/1736738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englinger B., Pirker C., Heffeter P., Terenzi A., Kowol C. R., Keppler B. K., et al. (2019). Metal Drugs and the Anticancer Immune Response. [Journal Article; Research Support, Non-U.S. Gov't; Review]. Chem. Rev. 119 (2), 1519–1624. 10.1021/acs.chemrev.8b00396 [DOI] [PubMed] [Google Scholar]
- Faivre-Finn C., Snee M., Ashcroft L., Appel W., Barlesi F., Bhatnagar A., et al. (2017). Concurrent Once-Daily versus Twice-Daily Chemoradiotherapy in Patients with Limited-Stage Small-Cell Lung Cancer (CONVERT): an Open-Label, Phase 3, Randomised, Superiority Trial. Lancet Oncol. 18 (8), 1116–1125. 10.1016/s1470-2045(17)30318-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Kamphorst J. J., Mathew R., Chung M. K., White E., Shlomi T., et al. (2013). Glutamine-driven Oxidative Phosphorylation Is a Major ATP Source in Transformed Mammalian Cells in Both Normoxia and Hypoxia. Mol. Syst. Biol. 9, 712. 10.1038/msb.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K., Gravis G., Flechon A., Geoffrois L., Chevreau C., Laguerre B., et al. (2014). Combining Gemcitabine, Cisplatin, and Ifosfamide (GIP) Is Active in Patients with Relapsed Metastatic Germ-Cell Tumors (GCT): a Prospective Multicenter GETUG Phase II Trial. Ann. Oncol. 25 (5), 987–991. 10.1093/annonc/mdu099 [DOI] [PubMed] [Google Scholar]
- Forastiere A. A., Goepfert H., Maor M., Pajak T. F., Weber R., Morrison W., et al. (2003). Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N. Engl. J. Med. 349 (22), 2091–2098. 10.1056/nejmoa031317 [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Senovilla L., Vitale I., Michels J., Martins I., Kepp O., et al. (2012). Molecular Mechanisms of Cisplatin Resistance. Oncogene 31 (15), 1869–1883. 10.1038/onc.2011.384 [DOI] [PubMed] [Google Scholar]
- Galluzzi L., Vitale I., Michels J., Brenner C., Szabadkai G., Harel-Bellan A., et al. (2014). Systems Biology of Cisplatin Resistance: Past, Present and Future. Cell Death Dis 5, e1257. 10.1038/cddis.2013.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X., Chen B., Shen Z., Liu Y., Li H., Xie X., et al. (2014). High GPX1 Expression Promotes Esophageal Squamous Cell Carcinoma Invasion, Migration, Proliferation and Cisplatin-Resistance but Can Be Reduced by Vitamin D. [Journal Article]. Int. J. Clin. Exp. Med. 7 (9), 2530–2540. [PMC free article] [PubMed] [Google Scholar]
- Giacomini I., Ragazzi E., Pasut G., Montopoli M. (2020). The Pentose Phosphate Pathway and its Involvement in Cisplatin Resistance. Int. J. Mol. Sci. 21 (3). 937. 10.3390/ijms21030937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez V. M., Fuertes M. A., Alonso C., Perez J. M. (2001). Is Cisplatin-Induced Cell Death Always Produced by Apoptosis?. Mol. Pharmacol. 59 (4), 657–663. 10.1124/mol.59.4.657 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of Cancer: the Next Generation. Cell 144 (5), 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Hay N. (2016). Reprogramming Glucose Metabolism in Cancer: Can it Be Exploited for Cancer Therapy? Nat. Rev. Cancer 16 (10), 635–649. 10.1038/nrc.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer A. K., Manorek G. H., Howell S. B., (2006). Contribution of the Major Copper Influx Transporter CTR1 to the Cellular Accumulation of Cisplatin, Carboplatin, and Oxaliplatin. [Journal Article; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, Non-P.H.S.]. Mol. Pharmacol. 70, 1390–1394. 10.1124/mol.106.022624 [DOI] [PubMed] [Google Scholar]
- Hong W., Cai P., Xu C., Cao D., Yu W., Zhao Z., et al. (2018). Inhibition of Glucose-6-Phosphate Dehydrogenase Reverses Cisplatin Resistance in Lung Cancer Cells via the Redox System. [Journal Article]. Front. Pharmacol. 9, 43. 10.3389/fphar.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housset M., Maulard C., Chretien Y., Dufour B., Delanian S., Huart J., et al. (1993). Combined Radiation and Chemotherapy for Invasive Transitional-Cell Carcinoma of the Bladder: a Prospective Study. J. Clin. Oncol. 11 (11), 2150–2157. 10.1200/jco.1993.11.11.2150 [DOI] [PubMed] [Google Scholar]
- Hu X. C., Zhang J., Xu B. H., Cai L., Wang Z. H., Ragaz J., et al. (2015). Cisplatin Plus Gemcitabine versus Paclitaxel Plus Gemcitabine as First-Line Therapy for Metastatic Triple-Negative Breast Cancer (CBCSG006): a Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 16 (4), 436–446. 10.1016/s1470-2045(15)70064-1 [DOI] [PubMed] [Google Scholar]
- Huang Z., Zhang Y., Li H., Zhou Y., Zhang Q., Chen R., et al. (2019). Vitamin D Promotes the Cisplatin Sensitivity of Oral Squamous Cell Carcinoma by Inhibiting LCN2-Modulated NF-kappaB Pathway Activation through RPS3. [Journal Article; Research Support, Non-U.S. Gov't]. Cel Death Dis 10 (12), 936. 10.1038/s41419-019-2177-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C. D., Savadelis A., Nagaraj A. B., Joseph P., Avril S., DiFeo A., et al. (2016). Altered Glutamine Metabolism in Platinum Resistant Ovarian Cancer. [Journal Article]. Oncotarget 7 (27), 41637–41649. 10.18632/oncotarget.9317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Downs J. A. (2014). Roles of Chromatin Remodellers in DNA Double Strand Break Repair. [Journal Article; Research Support, Non-U.S. Gov't; Review]. Exp. Cel Res 329 (1), 69–77. 10.1016/j.yexcr.2014.09.023 [DOI] [PubMed] [Google Scholar]
- Jiang J., Srivastava S., Zhang J. (2019). Starve Cancer Cells of Glutamine: Break the Spell or Make a Hungry Monster?. Cancers (Basel) 11 (6). 10.3390/cancers11060804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L., Chun J., Pan C., Li D., Lin R., Alesi G. N., et al. (2018). MAST1 Drives Cisplatin Resistance in Human Cancers by Rewiring cRaf-independent MEK Activation. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.]. Cancer Cell 34, 315–330. 10.1016/j.ccell.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. E., Bridges J. D., Sobczeck M., Gray J., Linnoila R. I., Gazdar A. F., et al. (1996). Mar). Patients with Limited-Stage Small-Cell Lung Cancer Treated with Concurrent Twice-Daily Chest Radiotherapy and Etoposide/cisplatin Followed by Cyclophosphamide, Doxorubicin, and Vincristine. J. Clin. Oncol. 14 (3), 806–813. 10.1200/jco.1996.14.3.806 [DOI] [PubMed] [Google Scholar]
- Kang Y. K., Kang W. K., Shin D. B., Chen J., Xiong J., Wang J., et al. (2009). Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as First-Line Therapy in Patients with Advanced Gastric Cancer: a Randomised Phase III Noninferiority Trial. Ann. Oncol. 20 (4), 666–673. 10.1093/annonc/mdn717 [DOI] [PubMed] [Google Scholar]
- Kaufman D. S., Winter K. A., Wu S., Heney N. M., Chetner M. P., Souhami L., et al. (2000). The Initial Results in Muscle-Invading Bladder Cancer of RTOG 95-06: Phase I/II Trial of Transurethral Surgery Plus Radiation Therapy with Concurrent Cisplatin and 5-fluorouracil Followed by Selective Bladder Preservation or Cystectomy Depending on the Initial Response. Oncologist 5 (6), 471–476. 10.1634/theoncologist.5-6-471 [DOI] [PubMed] [Google Scholar]
- Kaye S. B., Lewis C. R., Paul J., Duncan I. D., Gordon H. K., Kitchener H. C., et al. (1992). Randomised Study of Two Doses of Cisplatin with Cyclophosphamide in Epithelial Ovarian Cancer. [Clinical Trial; Journal Article; Multicenter Study; Randomized Controlled Trial; Research Support, Non-U.S. Gov't]. Lancet 340 (8815), 329–333. 10.1016/0140-6736(92)91404-v [DOI] [PubMed] [Google Scholar]
- Kelland L. (2007). The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 7 (8), 573–584. 10.1038/nrc2167 [DOI] [PubMed] [Google Scholar]
- Kilari D., Guancial E., Kim E. S. (2016). Role of Copper Transporters in Platinum Resistance. World J. Clin. Oncol. 7 (1), 106–113. 10.5306/wjco.v7.i1.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kischkel F. C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P. H., et al. (1995). Cytotoxicity-dependent APO-1 (Fas/CD95)-Associated Proteins Form a Death-Inducing Signaling Complex (DISC) with the Receptor. EMBO J. 14 (22), 5579–5588. 10.1002/j.1460-2075.1995.tb00245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R., Katsumata N., Shibata T., Kamura T., Kasamatsu T., Nakanishi T., et al. (2015). Paclitaxel Plus Carboplatin versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J. Clin. Oncol. 33 (19), 2129–2135. 10.1200/jco.2014.58.4391 [DOI] [PubMed] [Google Scholar]
- Klastersky J., Sculier J. P., Lacroix H., Dabouis G., Bureau G., Libert P., et al. (1990). A Randomized Study Comparing Cisplatin or Carboplatin with Etoposide in Patients with Advanced Non-small-cell Lung Cancer: European Organization for Research and Treatment of Cancer Protocol 07861. J. Clin. Oncol. 8 (9), 1556–1562. 10.1200/jco.1990.8.9.1556 [DOI] [PubMed] [Google Scholar]
- Kondagunta G. V., Bacik J., Donadio A., Bajorin D., Marion S., Sheinfeld J., et al. (2005). Combination of Paclitaxel, Ifosfamide, and Cisplatin Is an Effective Second-Line Therapy for Patients with Relapsed Testicular Germ Cell Tumors. J. Clin. Oncol. 23 (27), 6549–6555. 10.1200/jco.2005.19.638 [DOI] [PubMed] [Google Scholar]
- Labuschagne C. F., van den Broek N. J., Mackay G. M., Vousden K. H., Maddocks O. D. (2014). Serine, but Not glycine, Supports One-Carbon Metabolism and Proliferation of Cancer cells. [Journal Article; Research Support, Non-U.S. Gov't]. Cell Rep. 7 (4), 1248–1258. 10.1016/j.celrep.2014.04.045 [DOI] [PubMed] [Google Scholar]
- Lane A. N., Fan T. W. (2015). Regulation of Mammalian Nucleotide Metabolism and Biosynthesis. [Journal Article; Research Support, N.I.H., Extramural; Review]. Nucleic Acids Res. 43 (4), 2466–2485. 10.1093/nar/gkv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Lim D. H., Kim S., Park S. H., Park J. O., Park Y. S., et al. (2012). Phase III Trial Comparing Capecitabine Plus Cisplatin versus Capecitabine Plus Cisplatin with Concurrent Capecitabine Radiotherapy in Completely Resected Gastric Cancer with D2 Lymph Node Dissection: the ARTIST Trial. J. Clin. Oncol. 30 (3), 268–273. 10.1200/jco.2011.39.1953 [DOI] [PubMed] [Google Scholar]
- Li F., Zhang Z., Wang P., Wen P., Xu Q., Wang Y., et al. (2019). ALC1 Knockdown Enhances Cisplatin Cytotoxicity of Esophageal Squamous Cell Carcinoma Cells by Inhibition of Glycolysis through PI3K/Akt Pathway. [Journal Article]. Life Sci. 232, 116679. 10.1016/j.lfs.2019.116679 [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang H. (2016). Reprogramming of Glucose, Fatty Acid and Amino Acid Metabolism for Cancer progression. [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cell Mol. Life Sci. 73 (2), 377–392. 10.1007/s00018-015-2070-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedert B., Materna V., Schadendorf D., Thomale J., Lage H. (2003). Overexpression of cMOAT (MRP2/ABCC2) Is Associated with Decreased Formation of Platinum-DNA Adducts and Decreased G2-Arrest in Melanoma Cells Resistant to cisplatin. [Journal Article; Research Support, Non-U.S. Gov't]. J Invest. Dermatol. 121 (1), 172–176. 10.1046/j.1523-1747.2003.12313.x [DOI] [PubMed] [Google Scholar]
- Lin M., Lv D., Zheng Y., Wu M., Xu C., Zhang Q., et al. (2018). Downregulation of CPT2 Promotes Tumorigenesis and Chemoresistance to Cisplatin in Hepatocellular Carcinoma. [Journal Article]. Onco Targets Ther. 11, 3101–3110. 10.2147/ott.s163266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Peng Q., Li Y., Gao Y. (2018). Resveratrol Enhances Cisplatin-Induced Apoptosis in Human Hepatoma Cells via Glutamine Metabolism Inhibition. BMB Rep. 51 (9), 474–479. 10.5483/bmbrep.2018.51.9.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loehrer P. J., Sr, Gonin R., Nichols C. R., Weathers T., Einhorn L. H. (1998). Vinblastine Plus Ifosfamide Plus Cisplatin as Initial Salvage Therapy in Recurrent Germ Cell Tumor. J. Clin. Oncol. 16 (7), 2500–2504. 10.1200/jco.1998.16.7.2500 [DOI] [PubMed] [Google Scholar]
- Long J., Zhang C. J., Zhu N., Du K., Yin Y. F., Tan X., et al. (2018). Lipid Metabolism and Carcinogenesis, Cancer Development. Am. J. Cancer Res. 8 (5), 778–791. [PMC free article] [PubMed] [Google Scholar]
- Mandic A., Hansson J., Linder S., Shoshan M. C. (2003). Cisplatin Induces Endoplasmic Reticulum Stress and Nucleus-independent Apoptotic signaling. [Journal Article; Research Support, Non-U.S. Gov't]. J Biol. Chem. 278 (11), 9100–9106. 10.1074/jbc.m210284200 [DOI] [PubMed] [Google Scholar]
- Manerba M., Di Ianni L., Fiume L., Roberti M., Recanatini M., Di Stefano G. (2015). Lactate Dehydrogenase Inhibitors Sensitize Lymphoma Cells to Cisplatin without Enhancing the Drug Effects on Immortalized normal Lymphocytes. Eur. J. Pharm. Sci. 74, 95–102. 10.1016/j.ejps.2015.04.022 [DOI] [PubMed] [Google Scholar]
- Masamha C. P., LaFontaine P. (2018). Molecular Targeting of Glutaminase Sensitizes Ovarian Cancer Cells to Chemotherapy. [Journal Article; Research Support, Non-U.S. Gov't]. J. Cel Biochem 119 (7), 6136–6145. 10.1002/jcb.26814 [DOI] [PubMed] [Google Scholar]
- Mathieu J., Ruohola-Baker H. (2017). Metabolic Remodeling during the Loss and Acquisition of Pluripotency. Journal Article; Review; Research Support, N.I.H., Extramural]. Development 144, 541–551. 10.1242/dev.128389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaini K. R., Sullivan M. R., Vander H. M. G. (2016). The Importance of Serine Metabolism in Cancer. [Journal Article; Review; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.; Research Support, N.I.H., Extramural]. J. Cel Biol 214, 249–257. 10.1083/jcb.201604085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey J. A., Mazumdar M., Bajorin D. F., Bosl G. J., Vlamis V., Motzer R. J. (1997). Ifosfamide- and Cisplatin-Containing Chemotherapy as First-Line Salvage Therapy in Germ Cell Tumors: Response and Survival. J. Clin. Oncol. 15 (7), 2559–2563. 10.1200/jco.1997.15.7.2559 [DOI] [PubMed] [Google Scholar]
- McCarthy E. F. (2006). The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. [Biography; Historical Article; Journal Article; Portrait]. Iowa Orthop J 26, 154–158. [PMC free article] [PubMed] [Google Scholar]
- McGuire W. P., Hoskins W. J., Brady M. F., Kucera P. R., Partridge E. E., Look K. Y., et al. (1996). Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 334 (1), 1–6. 10.1056/nejm199601043340101 [DOI] [PubMed] [Google Scholar]
- Mehrmohamadi M., Mentch L. K., Clark A. G., Locasale J. W. (2016). Integrative Modelling of Tumour DNA Methylation Quantifies the Contribution of Metabolism. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Nat. Commun. 7, 13666. 10.1038/ncomms13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. D., Loehrer P. J., Gonin R., Einhorn L. H. (1997). Salvage Chemotherapy with Vinblastine, Ifosfamide, and Cisplatin in Recurrent Seminoma. J. Clin. Oncol. 15 (4), 1427–1431. 10.1200/jco.1997.15.4.1427 [DOI] [PubMed] [Google Scholar]
- Ming X., Groehler A. Th., Michaelson-Richie E. D., Villalta P. W., Campbell C., Tretyakova N. Y. (2017). Mass Spectrometry Based Proteomics Study of Cisplatin-Induced DNA-Protein Cross-Linking in Human Fibrosarcoma (HT1080) Cells. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Chem. Res. Toxicol. 30 (4), 980–995. 10.1021/acs.chemrestox.6b00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayans R., Severin D., Murray D. (2006). Relationship between DNA Double-Strand Break Rejoining and Cell Survival after Exposure to Ionizing Radiation in Human Fibroblast Strains with Differing ATM/p53 Status: Implications for Evaluation of Clinical Radiosensitivity. [Journal Article; Research Support, Non-U.S. Gov't]. Int. J. Radiat. Oncol. Biol. Phys. 66 (5), 1498–1505. 10.1016/j.ijrobp.2006.08.064 [DOI] [PubMed] [Google Scholar]
- Mitsuuchi Y., Johnson S. W., Selvakumaran M., Williams S. J., Hamilton T. C., Testa J. R., et al. (2000). The Phosphatidylinositol 3-kinase/AKT Signal Transduction Pathway Plays a Critical Role in the Expression of p21WAF1/CIP1/SDI1 Induced by Cisplatin and Paclitaxel. [Journal Article; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, P.H.S.]. Cancer Res. 60, 5390–5394. [PubMed] [Google Scholar]
- Muggia F. M., Bonetti A., Hoeschele J. D., Rozencweig M., Howell S. B. (2015). Platinum Antitumor Complexes: 50 Years since Barnett Rosenberg's Discovery. [Biography; Historical Article; Journal Article]. J. Clin. Oncol. 33 (35), 4219–4226. 10.1200/jco.2015.60.7481 [DOI] [PubMed] [Google Scholar]
- Nguyen D. J. M., Theodoropoulos G., Li Y. Y., Wu C., Sha W., Feun L. G., et al. (2020). Targeting the Kynurenine Pathway for the Treatment of Cisplatin-Resistant Lung Cancer. Mol. Cancer Res. 18 (1), 105–117. 10.1158/1541-7786.mcr-19-0239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. V., Vanner R., Dirks P., Eaves C. J. (2012). Cancer Stem Cells: an Evolving Concept. [Research Support, Non-U.S. Gov't; Review]. Nat. Rev. Cancer 12 (2), 133–143. 10.1038/nrc3184 [DOI] [PubMed] [Google Scholar]
- Niculescu M. D., Zeisel S. H. (2002). Diet, Methyl Donors and DNA Methylation: Interactions between Dietary Folate, Methionine and Choline. [Journal Article; Research Support, U.S. Gov't, P.H.S.]. J. Nutr. 132, 2333S–2335S. 10.1093/jn/132.8.2333s [DOI] [PubMed] [Google Scholar]
- Nikiforov M. A., Chandriani S., O'Connell B., Petrenko O., Kotenko I., Beavis A., et al. (2002). A Functional Screen for Myc-Responsive Genes Reveals Serine Hydroxymethyltransferase, a Major Source of the One-Carbon Unit for Cell Metabolism. [Journal Article; Research Support, U.S. Gov't, P.H.S.]. Mol. Cel Biol 22, 5793–5800. 10.1128/mcb.22.16.5793-5800.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M., Giobbie-Hurder A., Gargano M., Suda M., Ramaiya N. H., Hodi F. S., et al. (2013). Developing a Common Language for Tumor Response to Immunotherapy: Immune-Related Response Criteria Using Unidimensional Measurements. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't], Clin. Cancer Res. 19, 3936–3943. 10.1158/1078-0432.ccr-13-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez G., Benedict M. A., Hu Y., Inohara N. (1998). Caspases: the Proteases of the Apoptotic Pathway. Oncogene 17 (25), 3237–3245. 10.1038/sj.onc.1202581 [DOI] [PubMed] [Google Scholar]
- Obrist F., Michels J., Durand S., Chery A., Pol J., Levesque S., et al. (2018). Metabolic Vulnerability of Cisplatin-Resistant Cancers. EMBO J. 37 (14). 10.15252/embj.201798597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols R. F., Bundy B. N., Greer B. E., Fowler J. M., Clarke-Pearson D., Burger R. A., et al. (2003). Phase III Trial of Carboplatin and Paclitaxel Compared with Cisplatin and Paclitaxel in Patients with Optimally Resected Stage III Ovarian Cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 21 (17), 3194–3200. 10.1200/jco.2003.02.153 [DOI] [PubMed] [Google Scholar]
- Pope E. D. Rd., Kimbrough E. O., Vemireddy L. P., Surapaneni P. K., Copland J. A. Rd., Mody K., et al. (2019). Aberrant Lipid Metabolism as a Therapeutic Target in Liver cancer. Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Expert Opin. Ther. Targets 23, 473–483. 10.1080/14728222.2019.1615883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M. R., Hershock D. M., Blajman C. R., Mickiewicz E., Winquist E., Gorbounova V., et al. (2007). Cisplatin and Fluorouracil Alone or with Docetaxel in Head and Neck Cancer. N. Engl. J. Med. 357 (17), 1705–1715. 10.1056/nejmoa070956 [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., D'Aoust J. C., Issell B. F., Crooke S. T. (1979). Cisplatin (Cis-diamminedichloroplatinum II). [Clinical Trial; Comparative Study; Journal Article; Review]. Cancer Treat Rev 6. 17–39. 10.1016/s0305-7372(79)80057-2 [DOI] [PubMed] [Google Scholar]
- Qian X., Xu W., Xu J., Shi Q., Li J., Weng Y., et al. (2017). Enolase 1 Stimulates Glycolysis to Promote Chemoresistance in Gastric Cancer. [Journal Article]. Oncotarget 8 (29), 47691–47708. 10.18632/oncotarget.17868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Wang Y., Zhu B., Jiang L., Yuan W., Zhou Y., et al. (2019). Enolase1 Overexpression Regulates the Growth of Gastric Cancer Cells and Predicts Poor survival. [Journal Article; Research Support, Non-U.S. Gov't]. J Cel Biochem 120 (11), 18714–18723. 10.1002/jcb.29179 [DOI] [PubMed] [Google Scholar]
- Qiu B., Ackerman D., Sanchez D. J., Li B., Ochocki J. D., Grazioli A., et al. (2015). HIF2alpha-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov. 5 (6), 652–667. 10.1158/2159-8290.cd-14-1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancoule C., Guy J. B., Vallard A., Ben Mrad. M., Rehailia A., Magne N. (2017). [50th Anniversary of Cisplatin]. [Historical Article; Journal Article; Review]. Bull. Cancer 104 (2), 167–176. 10.1016/j.bulcan.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Rao X., Duan X., Mao W., Li X., Li Z., Li Q., et al. (2015). O-GlcNAcylation of G6PD Promotes the Pentose Phosphate Pathway and Tumor growth. [Journal Article; Research Support, Non-U.S. Gov't]. Nat Commun. 6, 8468. 10.1038/ncomms9468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J. L., Park J. Y., Kim E. H., Jang H. J., Kwon M. (2016). Activation of Mitochondrial Oxidation by PDK2 Inhibition Reverses Cisplatin Resistance in Head and Neck Cancer. [Journal Article; Research Support, Non-U.S. Gov't]. cancer Lett. 371, 20–29. 10.1016/j.canlet.2015.11.023 [DOI] [PubMed] [Google Scholar]
- Rosenberg B., Van Camp L., Grimley E. B., Thomson A. J. (1967). The Inhibition of Growth or Cell Division in Escherichia coli by Different Ionic Species of Platinum(IV) Complexes. [Journal Article]. J. Biol. Chem. 242 (6), 1347–1352. 10.1016/s0021-9258(18)96186-7 [DOI] [PubMed] [Google Scholar]
- Rosenberg B., Vancamp L., Krigas T. (1965). inhibition of cell division in escherichia coli by electrolysis products from a platinum electrode. [journal article]. Nature 205, 698–699. 10.1038/205698a0 [DOI] [PubMed] [Google Scholar]
- Salzer W., Bostrom B., Messinger Y., Perissinotti A. J., Marini B., Gov't Non-U. S. (2018). “Asparaginase Activity Levels and Monitoring in Patients with Acute Lymphoblastic Leukemia. [Journal Article; Research Support, Non-U.S. Gov't; Review]. Leuk. Lymphoma, 59, 1797–1806. 10.1080/10428194.2017.1386305 [DOI] [PubMed] [Google Scholar]
- Sancho-Martinez S. M., Prieto-Garcia L., Prieto M., Lopez-Novoa J. M., Lopez-Hernandez F. J. (2012). Subcellular Targets of Cisplatin Cytotoxicity: an Integrated View. Pharmacol. Ther. 136 (1), 35–55. 10.1016/j.pharmthera.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Sawayama H., Ogata Y., Ishimoto T., Mima K., Hiyoshi Y., Iwatsuki M., et al. (2019). Glucose Transporter 1 Regulates the Proliferation and Cisplatin Sensitivity of Esophageal Cancer. Cancer Sci. 110 (5), 1705–1714. 10.1111/cas.13995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears C. R., Turchi J. J. (2012). Complex Cisplatin-Double Strand Break (DSB) Lesions Directly Impair Cellular Non-homologous End-Joining (NHEJ) Independent of Downstream Damage Response (DDR) Pathways. [Journal Article; Research Support, N.I.H., Extramural]. J. Biol. Chem. 287 (29), 24263–24272. 10.1074/jbc.m112.344911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang D., Wu J., Guo L., Xu Y., Liu L., Lu J. (2017). Metformin Increases Sensitivity of Osteosarcoma Stem Cells to Cisplatin by Inhibiting Expression of PKM2. [Journal Article]. Int. J. Oncol. 50 (5), 1848–1856. 10.3892/ijo.2017.3950 [DOI] [PubMed] [Google Scholar]
- Shanware N. P., Mullen A. R., DeBerardinis R. J., Abraham R. T. (2011). Glutamine: Pleiotropic Roles in Tumor Growth and Stress Resistance. [Journal Article; Review]. J. Mol. Med. (Berl) 89 (3), 229–236. 10.1007/s00109-011-0731-9 [DOI] [PubMed] [Google Scholar]
- Sharma P., Hu-Lieskovan S., Wargo J. A., Ribas A., Extramural N. I. H. (2017), Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. [Journal Article; Review; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cell 168, 707–723. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H., Chen X., Liu B., Li P., Cao W. (2016). Omega-3 Polyunsaturated Fatty Acids Enhance Cisplatin Efficacy in Gastric Cancer Cells by Inducing Apoptosis via ADORA1. [Journal Article]. Anticancer Agents Med. Chem. 16 (9), 1085–1092. 10.2174/1871520616666160330104413 [DOI] [PubMed] [Google Scholar]
- Shuck S. C., Short E. A., Turchi J. J. (2008). Eukaryotic Nucleotide Excision Repair: from Understanding Mechanisms to Influencing Biology. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Review]. Cell Res 18, 64–72. 10.1038/cr.2008.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons A. L., Ahmad I. M., Mattson D. M., Dornfeld K. J., Spitz D. R. (2007). 2-Deoxy-D-glucose Combined with Cisplatin Enhances Cytotoxicity via Metabolic Oxidative Stress in Human Head and Neck Cancer Cells. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Cancer Res. 67, 3364–3370. 10.1158/0008-5472.can-06-3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanand S., Rhoades S., Jiang Q., Lee J. V., Benci J., Zhang J., et al. (2017). Nuclear Acetyl-CoA Production by ACLY Promotes Homologous Recombination. [Journal Article]. Mol. Cel 67 (2), 252–265. 10.1016/j.molcel.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooriyaarachchi M., George G. N., Pickering I. J., Narendran A., Gailer J. (2016). Tuning the Metabolism of the Anticancer Drug Cisplatin with Chemoprotective Agents to Improve its Safety and Efficacy. Journal Article; Review; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, Non-P.H.S.; Research Support, Non-U.S. Gov't]. Metallomics 8, 1170–1176. 10.1039/c6mt00183a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spierings D. C., de Vries E. G., Vellenga E., de Jong S. (2003). Loss of Drug-Induced Activation of the CD95 Apoptotic Pathway in a Cisplatin-Resistant Testicular Germ Cell Tumor Cell Line. [Journal Article; Research Support, Non-U.S. Gov't]. Cell Death Differ 10 (7), 808–822. 10.1038/sj.cdd.4401248 [DOI] [PubMed] [Google Scholar]
- Stacpoole P. W., Martyniuk C. J., James M. O., Calcutt N. A. (2019). Dichloroacetate-induced Peripheral Neuropathy. [Journal Article; Review]. Int. Rev. Neurobiol. 145, 211–238. 10.1016/bs.irn.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Sun L., Suo C., Li S. T., Zhang H., Gao P. (2018). Metabolic Reprogramming for Cancer Cells and Their Microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta Rev. Cancer 1870 (1), 51–66. 10.1016/j.bbcan.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Sun Z., Xu L. (2019). Expression of PDK-1 and DMBT1 in the Thyroid Carcinoma and its Clinicopathological Significance. [Journal Article]. Oncol. Lett. 18 (3), 2819–2824. 10.3892/ol.2019.10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N., Osman A. A., Takahashi Y., Lindemann A., Patel A. A., Zhao M., et al. (2018). Head and Neck Cancer Organoids Established by Modification of the CTOS Method Can Be Used to Predict In Vivo Drug Sensitivity. [Evaluation Study; Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't]. Oral Oncol. 87, 49–57. 10.1016/j.oraloncology.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni T., Piccoli C. (2019).Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. [Journal Article; Review]. Oxid Med Cell Longev 2019, 8201079. 10.1155/2019/8201079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telli M. L., Timms K. M., Reid J., Hennessy B., Mills G. B., Jensen K. C., et al. (2016). Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple-Negative Breast Cancer. [Clinical Trial, Phase II; Journal Article]. Clin. Cancer Res. 22 (15), 3764–3773. 10.1158/1078-0432.ccr-15-2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese E., Samuel S. M., Liskova A., Samec M., Kubatka P., Busselberg D. (2020). Targeting Glucose Metabolism to Overcome Resistance to Anticancer Chemotherapy in Breast Cancer. Cancers (Basel) 12 (8). 10.3390/cancers12082252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermorken J. B., Remenar E., van Herpen C., Gorlia T., Mesia R., Degardin M., et al. (2007). Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer. N. Engl. J. Med. 357 (17), 1695–1704. 10.1056/nejmoa071028 [DOI] [PubMed] [Google Scholar]
- Vousden K. H., Lane D. P. (2007). p53 in Health and Disease. [Journal Article; Review]. Nat. Rev. Mol. Cel Biol 8 (4), 275–283. 10.1038/nrm2147 [DOI] [PubMed] [Google Scholar]
- Wang C., Wu J., Wang Z., Yang Z., Li Z., Deng H., et al. (2018a). Glutamine Addiction Activates Polyglutamine-Based Nanocarriers Delivering Therapeutic siRNAs to Orthotopic Lung Tumor Mediated by Glutamine Transporter SLC1A5. [Journal Article; Research Support, Non-U.S. Gov't]. Biomaterials 183, 77–92. 10.1016/j.biomaterials.2018.08.035 [DOI] [PubMed] [Google Scholar]
- Wang G., Liu X., Xie J., Meng J., Ni X. (2019). PDK-1 Mediated Hippo-YAP-IRS2 Signaling Pathway and Involved in the Apoptosis of Non-small Cell Lung Cancer Cells. [Journal Article]. Biosci. Rep. 39 (5). BSR20182099. 10.1042/BSR20182099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang F., Wu X. R. (2017). Inhibition of Pyruvate Kinase M2 Markedly Reduces Chemoresistance of Advanced Bladder Cancer to Cisplatin. Sci. Rep. 7, 45983. 10.1038/srep45983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu L., Ji F., Jiang J., Yu Y., Sheng S., et al. (2018b). Soybean (Glycine max) Prevents the Progression of Breast Cancer Cells by Downregulating the Level of Histone Demethylase JMJD5. [Journal Article]. J. Cancer Res. Ther. 14 (Suppl. ment), S609–S615. 10.4103/0973-1482.187292 [DOI] [PubMed] [Google Scholar]
- Warburg O., Wind F., Negelein E. (1927). THE METABOLISM of TUMORS IN the BODY. [Journal Article]. J. Gen. Physiol. 8 (6), 519–530. 10.1085/jgp.8.6.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Liu X., Cheng C., Yu W., Yi P. (2020). Metabolism of Amino Acids in Cancer. Front Cel Dev Biol 8, 603837. 10.3389/fcell.2020.603837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Lee S., Zhu W. G., Lee O. J., Yun S. J., Kim J., et al. (2019). “Glucose-derived Acetate and ACSS2 as Key Players in Cisplatin Resistance in Bladder Cancer. [Journal Article; Research Support, N.I.H., Extramural; Research Support, Non-U.S. Gov't; Research Support, U.S. Gov't, Non-P.H.S.; Research Support, U.S. Gov't, P.H.S.]. Biochim. Biophys. Acta Mol. Cel Biol Lipids, 1864, 413–421. 10.1016/j.bbalip.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Wettersten H. I., Aboud O. A., Lara P. N., Jr, Weiss R. H. (2017). Metabolic Reprogramming in clear Cell Renal Cell Carcinoma. [Journal Article; Review]. Nat. Rev. Nephrol. 13 (7), 410–419. 10.1038/nrneph.2017.59 [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. (1974). Uptake and Metabolism of Plasma Glutamine by the Small Intestine. J. Biol. Chem. 249 (16), 5070–5079. 10.1016/s0021-9258(19)42329-6 [DOI] [PubMed] [Google Scholar]
- Wishart D. S. (2015). Is Cancer a Genetic Disease or a Metabolic Disease? [Journal Article]. EBioMedicine 2 (6), 478–479. 10.1016/j.ebiom.2015.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. (2009). Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 37 (1), 1–17. 10.1007/s00726-009-0269-0 [DOI] [PubMed] [Google Scholar]
- Wu J., Li Z., Yang Z., Guo L., Zhang Y., Deng H., et al. (2018). A Glutamine-Rich Carrier Efficiently Delivers Anti-CD47 siRNA Driven by a "Glutamine Trap" to Inhibit Lung Cancer Cell Growth. [Journal Article; Research Support, Non-U.S. Gov't]. Mol. Pharm. 15 (8), 3032–3045. 10.1021/acs.molpharmaceut.8b00076 [DOI] [PubMed] [Google Scholar]
- Wu Q., Yang Z., Nie Y., Shi Y., Fan D., (2014), Multi-drug Resistance in Cancer Chemotherapeutics: Mechanisms and Lab Approaches. [Journal Article; Research Support, Non-U.S. Gov't; Review]. Cancer Lett 347, 159–166. 10.1016/j.canlet.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Xintaropoulou C., Ward C., Wise A., Queckborner S., Turnbull A., Michie C. O., et al. (2018). Expression of Glycolytic Enzymes in Ovarian Cancers and Evaluation of the Glycolytic Pathway as a Strategy for Ovarian Cancer Treatment. [Journal Article]. BMC Cancer 18 (1), 636. 10.1186/s12885-018-4521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Shi Q., Xu W., Zhou Q., Shi R., Ma Y., et al. (2019). Metabolic Enzyme PDK3 Forms a Positive Feedback Loop with Transcription Factor HSF1 to Drive chemoresistance. [Journal Article; Research Support, Non-U.S. Gov't]. Theranostics 9 (10), 2999–3013. 10.7150/thno.31301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Gao W., Zhang Y., Wu S., Liu Y., Deng X., et al. (2018). ABT737 Reverses Cisplatin Resistance by Targeting Glucose Metabolism of Human Ovarian Cancer Cells. [Journal Article]. Int. J. Oncol. 53 (3), 1055–1068. 10.3892/ijo.2018.4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida G. J. (2015). Metabolic Reprogramming: the Emerging Concept and Associated Therapeutic Strategies. [Journal Article; Review]. J. Exp. Clin. Cancer Res. 34, 111. 10.1186/s13046-015-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel A., Wilczok A., Latocha M., Tarkowski M., Kokocinska M., Dzierzewicz Z. (2014). Polyunsaturated Fatty Acids Potentiate Cytotoxicity of Cisplatin in A549 Cells. [Journal Article; Research Support, Non-U.S. Gov't]. Acta Pol. Pharm. 71 (6), 1060–1065. [PubMed] [Google Scholar]
- Zhang J., Lin Y., Sun X. J., Wang B. Y., Wang Z. H., Luo J. F., et al. (2018a). Biomarker Assessment of the CBCSG006 Trial: a Randomized Phase III Trial of Cisplatin Plus Gemcitabine Compared with Paclitaxel Plus Gemcitabine as First-Line Therapy for Patients with Metastatic Triple-Negative Breast Cancer. Ann. Oncol. 29 (8), 1741–1747. 10.1093/annonc/mdy209 [DOI] [PubMed] [Google Scholar]
- Zhang M., Cong Q., Zhang X. Y., Zhang M. X., Lu Y. Y., Xu C. J. (2019). Pyruvate Dehydrogenase Kinase 1 Contributes to Cisplatin Resistance of Ovarian Cancer through EGFR activation. [Journal Article; Research Support, Non-U.S. Gov't]. J Cel Physiol 234 (5), 6361–6370. 10.1002/jcp.27369 [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Zhang M., Cong Q., Zhang M. X., Zhang M. Y., Lu Y. Y., et al. (2018b). Hexokinase 2 Confers Resistance to Cisplatin in Ovarian Cancer Cells by Enhancing Cisplatin-Induced Autophagy. Int. J. Biochem. Cel Biol 95, 9–16. 10.1016/j.biocel.2017.12.010 [DOI] [PubMed] [Google Scholar]
- Zhao X., Fu J., Tang W., Yu L., Xu W. (2020). Inhibition of Serine Metabolism Promotes Resistance to Cisplatin in Gastric Cancer. Onco Targets Ther. 13, 4833–4842. 10.2147/ott.s246430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Liu X. J., Yang P., Zhao M., Lv L. X., Zhang G. D., et al. (2014). Alkylglyceronephosphate Synthase (AGPS) Alters Lipid Signaling Pathways and Supports Chemotherapy Resistance of Glioma and Hepatic Carcinoma Cell lines. [Journal Article; Research Support, Non-U.S. Gov't]. Asian Pac. J. Cancer Prev. 15 (7), 3219–3226. 10.7314/apjcp.2014.15.7.3219 [DOI] [PubMed] [Google Scholar]