Abstract
Objective
Sex steroids are thought to contribute to the pathogenesis of osteoarthritis (OA). This study investigated the causal role of sex steroids in site- and sex-specific OA and risk of joint replacement surgery using the Mendelian randomization (MR) method.
Methods
Instrumental variables for estradiol, dehydroepiandrosterone sulfate, testosterone (T), and dihydrotestosterone (DHT) were selected. We used the inverse variance weighting (IVW) approach as the main MR method to estimate causal effects based on the summary-level data for OA and joint replacement surgery from genome-wide association studies (GWAS).
Results
A positive causal association was observed between serum T level and risks of hip OA (odds ratio [OR]=1.558, 95% confidence interval [CI]: 1.193–2.034; P=0.001) and hip replacement (OR=1.013, 95% CI: 1.008–1.018; P=2.15×10−8). Serum DHT level was also positively associated with the risk of hip replacement (OR=1.011, 95% CI: 1.006–1.015; P=4.03×10−7) and had potential causality with hip OA (OR=1.398, 95% CI: 1.054–1.855; P=0.020).
Conclusions
Serum T and DHT levels may play causal roles in the development of hip OA and contribute to the risk of hip replacement, although the underlying mechanisms require further investigation.
Keywords: Mendelian randomization, osteoarthritis, dehydroepiandrosterone sulfate, estradiol, testosterone, dihydrotestosterone
Introduction
Osteoarthritis (OA) is the most common degenerative joint disorder worldwide (1) and one of the main causes of years lived with disability according to the 2010 World Health Organization Global Burden of Disease study (2, 3). OA is clinically characterized by chronic pain, morning stiffness, and crepitus along with radiographic findings in diarthrodial joints such as the knee and hip. The pathogenesis of OA is not fully understood, but excessive physiologic activity and an overload of pathologic factors such as inflammatory cytokines (4) and matrix degradation (5) are known to contribute. Standard treatment for OA includes early prevention and pharmacotherapy, while joint replacement surgery (6) is effective for end-stage disease (7). Additionally, hormone replacement therapy (HRT) was shown to reduce the revision rate after total knee or hip arthroplasty by almost 40% (8). Middle-aged women are more likely than men to be affected by OA, especially in the hip or knee (9). Low plasma androstenedione concentration was shown to be associated with an increased risk of knee and hip arthroplasty in overweight men (10). The evidence to date indicates that sex steroids play an important role in the development of OA. However, a causal relationship between sex steroids and OA risk has not been established.
We recently reported a positive causal relationship between circulating sex hormone-binding globulin (SHBG) concentration (11) and calcium level (12) and risk of OA. Testosterone (T) and estradiol (E2) are active forms of sex steroids in males and females that are derived from inactive precursors such as dehydroepiandrosterone sulfate (DHEAS) and androstenedione in the circulation. E2 can also be formed from the aromatization of T, which can be converted to the more potent hormone dihydrotestosterone (DHT).
Genome-wide association studies (GWASs) have identified multiple genetic loci represented by single nucleotide polymorphisms (SNPs) that are closely associated with sex steroid concentrations (13). The Mendelian randomization (MR) (14) approach is widely used to evaluate the causal relationship between exposures and clinical outcomes based on summary data from GWASs with SNPs as instrumental variables. The fundamental principle of MR analysis is that genetic variants are randomly inherited at conception; because their distribution in a population is natural, it is presumed that the results of MR analyses are less susceptible to environmental influence and confounds. In the present study, we used validated SNPs and summary statistics from publicly available GWAS datasets to investigate the causal association between sex steroids and OA development with the MR method.
Methods
Selection of Instrumental Variables
E2, DHEAS, T, and DHT were selected as exposures. SNPs associated with each sex steroid were identified from GWAS datasets of European cohorts of the following sizes: E2, number(N)=11,907 (15); DHEAS, N=14,846 (16); T, N=3239 (17); and DHT, N=3239 (17). All SNPs selected as instrumental variables were correlated with the corresponding exposure at a genome-wide significance level (P<5×10−8). A linkage disequilibrium (LD) test ( Supplementary Table 1 ) was performed on the LD-link website (https://ldlink.nci.nih.gov/; European, r2<0.2). Detailed information of the association between the selected SNPs and exposures is shown in Table 1 . The potential confounders associated with the selected SNPs were analyzed in the PhenoScanner database(http://www.phenoscanner.medschl.cam.ac.uk/) ( Supplementary Table 2 ).
Table 1.
Characteristics of SNPs for exposures from GWAS.
| Exposure | Gene | SNP | Chromosome: Position | EA | Association with exposure | |
|---|---|---|---|---|---|---|
| β (SE) | P value | |||||
| E2 | CYP19A1 | rs727479 | 15:51534547 | A | 1.39 (0.12) | 8.2×10-30 |
| E2 | CYP19A1 | rs16964258 | 15:51605408 | G | 2.13 (0.25) | 8.2×10-15 |
| E2 | FAM9B | rs5934505 | X:8913826 | C | 0.67 (0.12) | 3.4×10-8 |
| E2 | MIR | rs5951794 | X:146432188 | G | 0.68 (0.11) | 3.1×10-10 |
| DHEAS | BCL2L11 | rs6738028 | 2:111949327 | G | -0.04 (0.01) | 1.72×10-8 |
| DHEAS | ARPC1A | rs740160 | 7:98957880 | T | 0.15 (0.02) | 1.56×10-16 |
| DHEAS | TRIM4 | rs17277546 | 7:99489571 | A | -0.11 (0.02) | 4.50×10-11 |
| DHEAS | HHEX | rs2497306 | 10:94485211 | C | -0.04 (0.01) | 4.64×10-9 |
| DHEAS | CYP2C9 | rs2185570 | 10:96751270 | C | -0.06 (0.01) | 2.29×10-8 |
| DHEAS | BMF | rs7181230 | 15:40360741 | G | 0.05 (0.01) | 5.44×10-11 |
| DHEAS | SULT2A1 | rs2637125 | 19:48401893 | A | -0.09 (0.01) | 2.61×10-19 |
| T | JMJD1C | rs10822186 | 10:65350383 | A | -0.06 (0.01) | 1.20×10-8 |
| T | SHBG | rs4239258 | 17:7397043 | A | -0.16 (0.03) | 4.47×10-8 |
| T | SHBG | rs34790908 | 17:7451110 | T | 0.07 (0.01) | 1.66×10-9 |
| T | SHBG | rs727428 | 17:7537792 | T | -0.07 (0.01) | 1.26×10-12 |
| DHT | SHBG | rs4151121 | 17:7342294 | G | 0.10 (0.02) | 7.96×10-10 |
| DHT | SHBG | rs4265880 | 17:7396267 | T | -0.24 (0.04) | 1.89×10-8 |
| DHT | SHBG | rs4227 | 17:7491177 | G | 0.09 (0.02) | 3.68×10-8 |
Gene, nearest gene to the SNP; EA, effect allele; β, per allele effect on the exposure; SE, standard error; P value, p-value for the genetic association.
Genetic Associations With Outcomes
Because both knee and hip are common sites of OA (3), summary data for overall OA and hip and knee OA were derived from a GWAS meta-analysis of UK Biobank and Arthritis Research UK Osteoarthritis Genetics datasets (18) that included 455,221; 393,873; and 403,124 European individuals. Given that sex is a risk factor of OA, summary-level data for OA in each sex was extracted from the UK Biobank (http://www.nealelab.is/uk-biobank), which included 30,046 cases of OA (19,397 women and 10,649 men) among 361,141 European subjects (194,153 women and 166,988 men). Summary data for hip and knee replacement surgery were also obtained from UK Medical Research Council Integrative Epidemiology Unit OpenGWAS Project (https://gwas.mrcieu.ac.uk/), which included 7322 cases of hip and 5657 cases of knee replacement among 462,933 European individuals. The association data of the selected instrumental variables and sex steroids along with outcomes are provided in Supplementary Tables 3 – 5 . All studies contributing data to our analyses were approved by the relevant ethics committees, and all study participants provided written, informed consent ( Supplementary Table 6 ).
Statistical Analysis
A 2-sample MR approach was adopted in our study. Causal associations between sex steroids and risks of OA and joint replacement were estimated based on the random-effects inverse variance weighting (IVW) model (19). Since four separate outcomes are being tested in our study, the threshold for adjusted p-value was 0.0125. The analytical results with a p-value between 0.125 and 0.05 are considered nominally significant results.
A weighted median (WM) analysis, which involved calculating the median value of ratio instrumental variable estimates, was also performed as sensitivity analysis. The MR-Egger and MR pleiotropy residual sum and outlier (MR-PRESSO) (20) methods were used to account for pleiotropic effects (21) to exclude bias observed in the sensitivity analysis. Outlying genetic variants were identified by MR-PRESSO and used to correct the results. Moreover, SNPs associated with included outcomes (P<1×10−4) were removed from IVW in the sensitivity analysis. The estimated effects are reported as odds ratios (ORs) with 95% confidence intervals (CIs). We used R v3.6.1 and the R MendelianRandomization package (22) for all statistical analyses.
Results
Causal Associations Between Sex Steroid Levels and Overall and Site-Specific OA
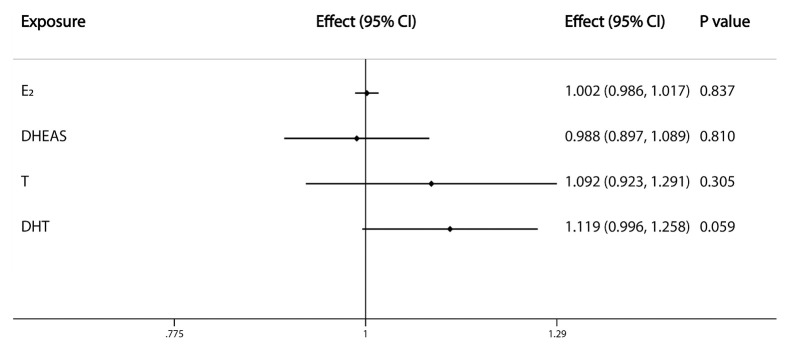
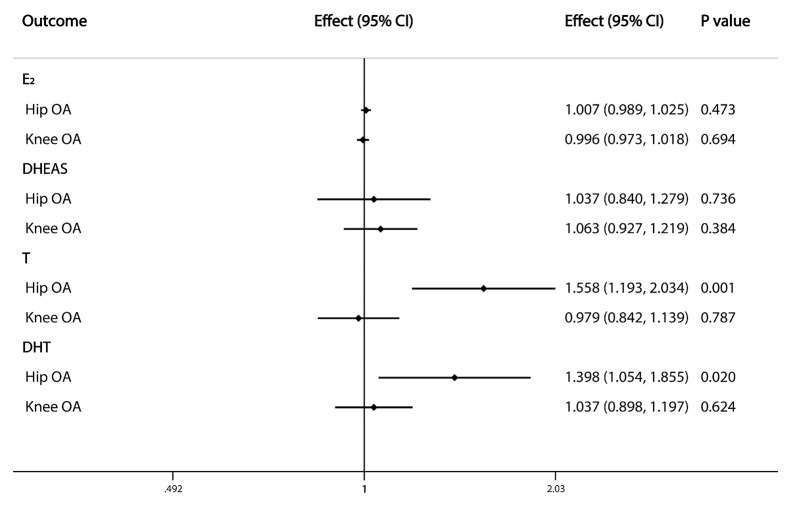
The primary IVW analyses provide no evidence for the causal relationship between the included sex steroids and overall OA ( Figure 1 ). Nevertheless, the risk of hip OA was causally influenced by serum T levels (OR=1.558, 95% CI: 1.193–2.034; P=0.001). And the nominally significant results suggested a positive association between serum DHT levels (OR=1.398, 95% CI: 1.054–1.855; P=0.020) and hip OA. However, there was no evidence for associations between the levels of other sex steroids and hip and knee OA ( Figure 2 ).
Figure 1.
Causal effects of sex steroids on the risk of overall OA. The estimated effects, 95% confidence intervals and p-values of associations were contained. Effect, the combined causal effect; CI, confidence interval; P value, p-value of the causal estimate.
Figure 2.
Causal effects of sex steroids on the risk of OA of hip and knee. The estimated effects, 95% confidence intervals and p-values of associations were contained. Effect, the combined causal effect; CI, confidence interval; P value, p-value of the causal estimate.
Causal Association Between Sex Steroid Levels and OA by Sex
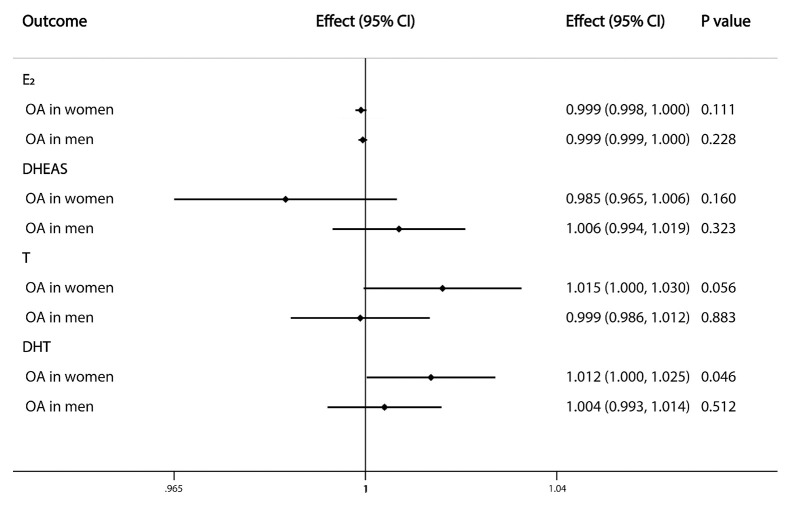
The nominally significant results of IVW analyses showed that serum DHT level tended to have a causal effect on the risk of OA in women (OR=1.012, 95% CI: 1.000–1.025; P=0.046), while no relationship was observed between sex steroid levels and OA risk in men ( Figure 3 ).
Figure 3.
Causal effects of sex steroids on the risk of OA in single sex. The estimated effects, 95% confidence intervals and p-values of associations were contained. Effect, the combined causal effect; CI, confidence interval; P value, p-value of the causal estimate.
Causal Association Between Sex Steroid Levels and Joint Arthroplasty
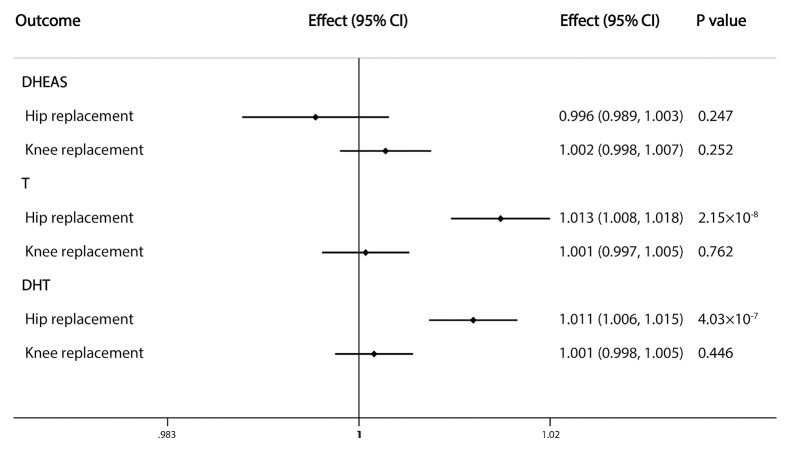
Because of a lack of corresponding outcome data for E2-associated SNPs, the association between serum E2 level and joint replacement was not estimated by IVW analysis in our study. The results of the analysis showed that the risk of hip replacement was causally influenced by serum T (OR=1.013, 95% CI: 1.008–1.018; P=2.15×10−8) and DHT (OR=1.011, 95% CI: 1.006–1.015; P=4.03×10−7) levels. However, we found no evidence of an association between the exposures and knee replacement ( Figure 4 ).
Figure 4.
Causal effects of sex steroids on the risk of joint replacement of hip and knee. The estimated effects, 95% confidence intervals and p-values of associations were contained. Effect, the combined causal effect; CI, confidence interval; P value, p-value of the causal estimate.
Sensitivity Analysis
First, the WM method was used for sensitivity analysis. Similar with the results of main IVW analyses, the WM analyses suggested a positive causal relationship between serum DHT (P=0.006) and T (P=4.97×10−4) levels and hip OA and the causal effects of serum DHEAS (P=0.017) and T (P=4.10×10−5) levels on the risk of hip replacement were statistically significant ( Supplementary Tables 7 – 9 ). However, positive associations were found between serum DHT level and overall OA and between serum T level and OA in women, which were inconsistent with our primary findings. Second, in the MR-Egger analysis, nearly all of the intercept terms were centered around the origin, suggesting that there was no horizontal pleiotropy ( Supplementary Tables 7 – 9 ). Nevertheless, there were some exceptions such as the P value of the pleiotropy estimate of the relationships between E2 and overall OA and between DHT and overall or hip OA.
The MR-PRESSO analysis was performed to identify outlying SNPs when the number of genetic variants for specific exposure was >3. In the association between serum E2 level and overall OA, rs5934505 was identified as an outlier and removed; rs747279 and rs5934505 were also identified as outliers and were excluded from the MR-PRESSO analysis of the association between serum E2 level and knee OA. Rs34790908 was another outlying SNP in the association between serum T level and overall OA. After removing these outliers, the causal association between serum T level and hip OA was still observed in the MR-PRESSO analysis (OR=1.558, 95% CI: 1.193–2.034; P=0.047). Additionally, after removing genetic variants that were directly associated with the outcome measures (rs5934505 for overall OA; rs34790908 for hip OA and hip replacement; and rs4227 for hip OA and hip replacement), the positive causal effects of serum T (hip OA: P=0.001; hip replacement: P=4.52×10−5) and DHT (hip OA: P=0.011; hip replacement: P=0.002) levels on risk of hip OA and hip replacement remained significant in the IVW analyses ( Supplementary Table 10 ).
Discussion
This MR study was conducted to investigate the causal relationship between sex steroids levels and OA risk. Our results revealed positive causal effects of serum concentrations of T on the risk of hip OA. There was potential positive association between DHT and hip OA as well while little evidence of the association between sex steroids levels and overall OA was found. Interestingly, males were reported to have higher scores for cartilage damage than age-matched females in a murine OA model induced by destabilization of the medial meniscus (23). Orchiectomized males had less severe damage than their intact counterparts whereas the opposite was true for ovariectomized females, indicating that sex hormones play varied roles in the progression of OA. In fact, the steroid hormones 17β-estradiol, DHEA, and T were shown to promote articular cartilage integration (24).
In a cohort of healthy middle-aged men with no symptoms of knee OA or risk factors, serum free T level was associated with the rate of tibial cartilage loss leading to the development of arthritis 2 years later (25). In a cross-sectional study, a higher concentration of serum T was associated with higher cartilage volume (26), possibly due to greater physical activity and stress on the articular cartilage. Thus, chronic stress and damage to weight-bearing joints—especially the hip and knee—can contribute to OA. However, the protective effects of E2 and DHEAS on OA occurrence are unclear. In a cohort of community-dwelling older subjects, a lower DHEAS was associated with OA irrespective of site and sex (27); and histomorphometric studies in a rabbit model of progressive OA showed that DHEAS treatment reduced cartilage lesions and delayed cartilage degeneration (28, 29). DHEAS was shown to modulate the imbalance between matrix metalloproteinases (MMPs) and its inhibitors (30–33); and intra-articular administration of DHEA reduced aggrecanase expression in vivo (34).
In our study, a nominally significant result for the relationship between serum DHT levels and risk of OA in women was found, while little evidence of the sex-specific association between serum T levels and OA was provided. However, a 2-year double-blind cross-sectional analysis of 273 seniors with severe knee OA at an average age of 70.3 years found that a higher T level was associated with less knee disability in non-operated women and less pain (as determined by the Western Ontario and McMaster Universities Osteoarthritis Index) in normal-weight men (35). In another longitudinal investigation on the association between endogenous sex hormone levels and knee OA features and pain, T level was inversely associated with effusion-synovitis volume and pain score in female OA patients (36). However, these findings can only explain the relationship between T level and severity of knee OA symptoms because all of the subjects were OA patients. Besides, our results were based on a non–age-stratified population. A clinical study reported that middle-aged menopausal women with generalized OA had a slightly higher T level and lower circulating SHBG level than control subjects (37).
Unexpectedly, we did not observe any causal association between E2 level and OA risk in women, which may be caused by the male-specific GWAS from which the SNPs for E2 were selected. The marked increase in OA incidence during or soon after menopause is well established. Moreover, estradiol is a known protective factor against OA. The use of oral estrogen was found to be associated with a decreased incidence of radiographic hip OA in elderly Caucasian women (38). A case–control study of women aged ≥45 years found that short-term HRT (up to 5 years) was associated with an increased risk of hip OA, while long-term treatment had a nonsignificant protective effect (39). In premenopausal Caucasian women with knee grade ≥2, a clinical diagnosis of OA was positively correlated with serum estradiol level (40). These findings indicate that the effects of reproductive hormones vary according to age and concentration.
E2 level was shown to be negatively correlated with OA severity and positively correlated with interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α concentrations in the synovial fluid of postmenopausal women (41). In vitro studies have demonstrated that 17β-estradiol treatment enhanced proliferation and viability in chondrocytes by inhibiting mitophagy via the G protein-coupled estrogen receptor (GPER/GPR30) and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways (42, 43), and induced chondrocyte redifferentiation via the estrogen receptor (ER)α66/specificity protein (Sp)1/Sp3/SRY-box (SOX)9/p300 protein complex (44). In experiments with mice, a reduction in serum E2 caused by ovariectomy induced severe OA, while supplementary administration of β-estradiol rescued bone turnover and tissue degradation (45). Lower levels of endogenous estrogen and its metabolites were significantly associated with the development of knee OA (46, 47). E2 was shown to inhibit activation of the NOD-, LRR-, and pyrin domain-containing protein (NLRP)3 inflammasome, IL-1β and IL-18 expression, and the catabolic activity of MMPs via estrogen receptor or miR-140 (48–52). Our findings suggested that women are more susceptible to OA than men because of differences in sex steroid profiles.
Our results showed a positive causal association between serum T and DHT levels and risk of hip replacement while no relationship was found between E2 and arthroplasty due to data deficiencies. A long-term study with a mean follow-up of 12.7 years reported that oral contraceptive use, current HRT use, and longer duration of HRT were associated with increased risk of total knee arthroplasty for OA in women aged 40–69 years (53). However, in another prospective cohort study, the association between incidence of total knee replacement for OA and lower E2 concentration was independent of established risk factors for knee OA, and there was no relationship between E2 and total hip replacement (54). In spite of the exclusion of LD-linkage, rs5934505 for E2 and rs727428 for DHT were found to be confounded with T, and rs4227 for T was found to be confounded with DHT by the analysis in PhenoScanner database. Both of them are all located in the same chromosome locus named SHBG. Previous studies suggested that SHBG was significantly associated with T and DHT levels in European ancestry (17) but was not associated with T in Japanese men (55). Moreover, a mutant allele of rs727428 was found to be positively correlated with PCOS in Mediterranean women (56). rs4227 was found to be positively associated with IgA nephropathy in Han Chinese which located in 17p13 7431901 coding MPDU1 gene (57).
Compared to traditional retrospective analyses and case–control prospective studies, MR is less likely to introduce artificial errors and bias. Sex steroid levels fluctuate over time and measurements are often imprecise and experiment design cannot always be perfect. The cost of data collection and analysis can be high, and the MR method can to some extent mitigate data migration; moreover, sequential application of different algorithms in the sensitivity analysis can increase the accuracy and reliability of the results. Nonetheless, our study had several limitations. First, the outcome datasets and representative SNPs were sometimes too limited for us to carry out sensitivity analyses. Second, age-adjusted IVW analyses are necessary to exclude the influence of aging on OA and sex steroid levels, but we did not find any age-stratified OA datasets to perform adjusted IVW analyses. Third, since the GWASs on E2, T and DHT included only male participants in this study, the results of sex-stratified analyses may be influenced by the exposure data. Forth, there might be some dependencies between T and DHT. Finally, our study focused on the causal role of sex steroids in the pathogenesis of OA, but the underlying mechanisms remain to be elucidated.
In conclusion, serum T and DHT levels were causally related to the risk of hip replacement surgery and T was positively associated with risk of hip OA. Further, a nominally significant relationship was found between serum DHT levels and OA in women as well as hip OA. Thus, these sex steroids may contribute to the development of OA. Our findings can guide the development of effective clinical management strategies to maintain joint health and prevent OA, especially in women.
Data Availability Statement
Summary data used in our study was downloaded from GWAS Catalog (https://www.ebi.ac.uk/gwas/), Neale’s lab (http://www.nealelab.is/uk-biobank) and IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/).
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Y-SY, ZQ, D-QY, WW, SY, H-FH desinged the study. ZQ analyzed the data. Y-SY wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 81772360).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All cited projects and datasets were approved by the relevant ethics committees. We thank the authors of the cited studies for making their datasets available for download.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.683226/full#supplementary-material
References
- 1. Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet (2015) 386:376–87. 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 2. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years Lived With Disability (Ylds) for 1160 Sequelae of 289 Diseases and Injuries 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380:2163–96. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The Global Burden of Hip and Knee Osteoarthritis: Estimates From the Global Burden of Disease 2010 Study. Ann Rheum Dis (2014) 73:1323–30. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 4. Wang T, He C. Pro-Inflammatory Cytokines: The Link Between Obesity and Osteoarthritis. Cytokine Growth Factor Rev (2018) 44:38–50. 10.1016/j.cytogfr.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 5. Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and Osteoarthritis: Central Role of the Extracellular Matrix. Ageing Res Rev (2017) 40:20–30. 10.1016/j.arr.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 6. Harris WH, Sledge CB. Total Hip and Total Knee Replacement (2). N Engl J Med (1990) 323:801–7. 10.1056/NEJM199009203231206 [DOI] [PubMed] [Google Scholar]
- 7. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet (2019) 393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 8. Prieto-Alhambra D, Javaid MK, Judge A, Maskell J, Cooper C, Arden NK. Hormone Replacement Therapy and Mid-Term Implant Survival Following Knee or Hip Arthroplasty for Osteoarthritis: A Population-Based Cohort Study. Ann Rheum Dis (2015) 74:557–63. 10.1136/annrheumdis-2013-204043 [DOI] [PubMed] [Google Scholar]
- 9. Ding C, Cicuttini F, Blizzard L, Scott F, Jones G. A Longitudinal Study of the Effect of Sex and Age on Rate of Change in Knee Cartilage Volume in Adults. Rheumatol (Oxford) (2007) 46:273–9. 10.1093/rheumatology/kel243 [DOI] [PubMed] [Google Scholar]
- 10. Hussain SM, Cicuttini FM, Giles GG, Graves SE, Wang Y. Relationship Between Circulating Sex Steroid Hormone Concentrations and Incidence of Total Knee and Hip Arthroplasty Due to Osteoarthritis in Men. Osteoarthr Cartil (2016) 24:1408–12. 10.1016/j.joca.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 11. Qu Z, Huang J, Yang F, Hong J, Wang W, Yan S. Sex Hormone-Binding Globulin and Arthritis: A Mendelian Randomization Study. Arthritis Res Ther (2020) 22:118. 10.1186/s13075-020-02202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qu Z, Yang F, Hong J, Wang W, Li S, Jiang G, et al. Causal Relationship of Serum Nutritional Factors With Osteoarthritis: A Mendelian Randomization Study. Rheumatol (Oxford) (2020) 60(5):2383–90. 10.1093/rheumatology/keaa622 [DOI] [PubMed] [Google Scholar]
- 13. Harroud A, Richards JB. Mendelian Randomization in Multiple Sclerosis: A Causal Role for Vitamin D and Obesity? Multiple Sclerosis J (2018) 24:80–5. 10.1177/1352458517737373 [DOI] [PubMed] [Google Scholar]
- 14. Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA (2017) 318:1925–6. 10.1001/jama.2017.17219 [DOI] [PubMed] [Google Scholar]
- 15. Eriksson AL, Perry JRB, Coviello AD, Delgado GE, Ferrucci L, Hoffman AR, et al. Genetic Determinants of Circulating Estrogen Levels and Evidence of a Causal Effect of Estradiol on Bone Density in Men. J Clin Endocrinol Metab (2018) 103:991–1004. 10.1210/jc.2017-02060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhai G, Teumer A, Stolk L, Perry JRB, Vandenput L, Coviello AD, et al. Eight Common Genetic Variants Associated With Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms. PloS Genet (2011) 7:e1002025. 10.1371/journal.pgen.1002025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin G, Sun J, Kim S-T, Feng J, Wang Z, Tao S, et al. Genome-Wide Association Study Identifies a New Locus JMJD1C at 10q21 That may Influence Serum Androgen Levels in Men. Hum Mol Genet (2012) 21:5222–8. 10.1093/hmg/dds361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. Identification of New Therapeutic Targets for Osteoarthritis Through Genome-Wide Analyses of UK Biobank Data. Nat Genet (2019) 51:230–6. 10.1038/s41588-018-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pierce BL, Burgess S. Efficient Design for Mendelian Randomization Studies: Subsample and 2-Sample Instrumental Variable Estimators. Am J Epidemiol (2013) 178:1177–84. 10.1093/aje/kwt084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verbanck M, Chen C-Y, Neale B, Do R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred From Mendelian Randomization Between Complex Traits and Diseases. Nat Genet (2018) 50:693–8. 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stearns FW. One Hundred Years of Pleiotropy: A Retrospective. Genetics (2010) 186:767–73. 10.1534/genetics.110.122549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yavorska OO, Burgess S. MendelianRandomization: An R Package for Performing Mendelian Randomization Analyses Using Summarized Data. Int J Epidemiol (2017) 46:1734–9. 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis Severity is Sex Dependent in a Surgical Mouse Model. Osteoarthr Cartil (2007) 15:695–700. 10.1016/j.joca.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 24. Englert C, Blunk T, Fierlbeck J, Kaiser J, Stosiek W, Angele P, et al. Steroid Hormones Strongly Support Bovine Articular Cartilage Integration in the Absence of Interleukin-1beta. Arthritis Rheumatism (2006) 54:3890–7. 10.1002/art.22250 [DOI] [PubMed] [Google Scholar]
- 25. Hanna F, Ebeling PR, Wang Y, O’Sullivan R, Davis S, Wluka AE, et al. Factors Influencing Longitudinal Change in Knee Cartilage Volume Measured From Magnetic Resonance Imaging in Healthy Men. Ann Rheum Dis (2005) 64:1038–42. 10.1136/ard.2004.029355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cicuttini FM, Wluka A, Bailey M, O’Sullivan R, Poon C, Yeung S, et al. Factors Affecting Knee Cartilage Volume in Healthy Men. Rheumatol (Oxford) (2003) 42:258–62. 10.1093/rheumatology/keg073 [DOI] [PubMed] [Google Scholar]
- 27. Veronese N, Maggi S, Noale M, Trevisan C, De Rui M, Bolzetta F, et al. Serum Dehydroepiandrosterone Sulfate and Osteoarthritis in Older People: The Pro.V.A. Study. Clin Rheumatol (2016) 35:2609–14. 10.1007/s10067-016-3213-1 [DOI] [PubMed] [Google Scholar]
- 28. Huang K, Bao J-p, Jennings GJ, Wu L-d. The Disease-Modifying Effect of Dehydroepiandrosterone in Different Stages of Experimentally Induced Osteoarthritis: A Histomorphometric Study. BMC Musculoskelet Disord (2015) 16:178. 10.1186/s12891-015-0595-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu LD, Yu HC, Xiong Y, Feng J. Effect of Dehydroepiandrosterone on Cartilage and Synovium of Knee Joints With Osteoarthritis in Rabbits. Rheumatol Int (2006) 27:79–85. 10.1007/s00296-006-0238-9 [DOI] [PubMed] [Google Scholar]
- 30. Jo H, Park JS, Kim EM, Jung MY, Lee SH, Seong SC, et al. The In Vitro Effects of Dehydroepiandrosterone on Human Osteoarthritic Chondrocytes. Osteoarthr Cartil (2003) 11:585–94. 10.1016/S1063-4584(03)00094-3 [DOI] [PubMed] [Google Scholar]
- 31. Sun JS, Wu CX, Tsuang YH, Chen LT, Sheu SY. The In Vitro Effects of Dehydroepiandrosterone on Chondrocyte Metabolism. Osteoarthr Cartil (2006) 14:238–49. 10.1016/j.joca.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 32. Jo H, Ahn HJ, Kim EM, Kim HJ, Seong SC, Lee I, et al. Effects of Dehydroepiandrosterone on Articular Cartilage During the Development of Osteoarthritis. Arthritis Rheumatism (2004) 50:2531–8. 10.1002/art.20368 [DOI] [PubMed] [Google Scholar]
- 33. . Bao J-p, . Chen W-p, Feng J, Zhao J, . Shi Z-l, Huang K, et al. Variation Patterns of Two Degradation Enzyme Systems in Articular Cartilage in Different Stages of Osteoarthritis: Regulation by Dehydroepiandrosterone. Clin Chim Acta; Int J Clin Chem (2009) 408:1–7. 10.1016/j.cca.2009.06.040 [DOI] [PubMed] [Google Scholar]
- 34. Huang K, Zhang C, Zhang X-W, Bao J-P, Wu L-D. Effect of Dehydroepiandrosterone on Aggrecanase Expression in Articular Cartilage in a Rabbit Model of Osteoarthritis. Mol Biol Rep (2011) 38:3569–72. 10.1007/s11033-010-0467-6 [DOI] [PubMed] [Google Scholar]
- 35. Freystaetter G, Fischer K, Orav EJ, Egli A, Theiler R, Münzer T, et al. Total Serum Testosterone and WOMAC Pain and Function Among Older Men and Women With Severe Knee OA. Arthritis Care Res (Hoboken) (2019) 72(11):1511–8. 10.1002/acr.24074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jin X, Wang BH, Wang X, Antony B, Zhu Z, Han W, et al. Associations Between Endogenous Sex Hormones and MRI Structural Changes in Patients With Symptomatic Knee Osteoarthritis. Osteoarthr Cartil (2017) 25:1100–6. 10.1016/j.joca.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 37. Spector TD, Perry LA, Jubb RW. Endogenous Sex Steroid Levels in Women With Generalised Osteoarthritis. Clin Rheumatol (1991) 10:316–9. 10.1007/BF02208698 [DOI] [PubMed] [Google Scholar]
- 38. Nevitt MC, Cummings SR, Lane NE, Hochberg MC, Scott JC, Pressman AR, et al. Association of Estrogen Replacement Therapy With the Risk of Osteoarthritis of the Hip in Elderly White Women. Study Osteoporotic Fractures Res Group Arch Intern Med (1996) 156:2073–80. 10.1001/archinte.156.18.2073 [DOI] [PubMed] [Google Scholar]
- 39. Dennison EM, Arden NK, Kellingray S, Croft P, Coggon D, Cooper C. Hormone Replacement Therapy, Other Reproductive Variables and Symptomatic Hip Osteoarthritis in Elderly White Women: A Case-Control Study. Br J Rheumatol (1998) 37:1198–202. 10.1093/rheumatology/37.11.1198 [DOI] [PubMed] [Google Scholar]
- 40. Sowers MF, Hochberg M, Crabbe JP, Muhich A, Crutchfield M, Updike S. Association of Bone Mineral Density and Sex Hormone Levels With Osteoarthritis of the Hand and Knee in Premenopausal Women. Am J Epidemiol (1996) 143:38–47. 10.1093/oxfordjournals.aje.a008655 [DOI] [PubMed] [Google Scholar]
- 41. Liu YP, Li J, Xin SB, JX. Study the Relevance Between Inflammatory Factors and Estradiol and Their Association With Knee Osteoarthritis in Postmenopausal Women. Eur Rev Med Pharmacol Sci (2018) 22:472–8. 10.26355/eurrev_201801_14197 [DOI] [PubMed] [Google Scholar]
- 42. Fan D-X, Yang X-H, Li Y-N, Guo L. 17β-Estradiol on the Expression of G-Protein Coupled Estrogen Receptor (Gper/Gpr30) Mitophagy, and the PI3K/Akt Signaling Pathway in ATDC5 Chondrocytes In Vitro. Med Sci monit Int Med J Exp Clin Res (2018) 24:1936–47. 10.12659/MSM.909365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang JG, Xia C, Zheng XP, Yi TT, Wang XY, Song G, et al. 17β-Estradiol Promotes Cell Proliferation in Rat Osteoarthritis Model Chondrocytes Via PI3K/Akt Pathway. Cell Mol Biol Lett (2011) 16:564–75. 10.2478/s11658-011-0023-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maneix L, Servent A, Porée B, Ollitrault D, Branly T, Bigot N, et al. Up-Regulation of Type II Collagen Gene by 17β-Estradiol in Articular Chondrocytes Involves Sp1/3, Sox-9, and Estrogen Receptor α. J Mol Med (Berlin Germany) (2014) 92:1179–200. 10.1007/s00109-014-1195-5 [DOI] [PubMed] [Google Scholar]
- 45. Yang J-H, Kim J-H, Lim D-S, Oh K-J. Effect of Combined Sex Hormone Replacement on Bone/Cartilage Turnover in a Murine Model of Osteoarthritis. Clin Orthop Surg (2012) 4:234–41. 10.4055/cios.2012.4.3.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sowers MR, McConnell D, Jannausch M, Buyuktur AG, Hochberg M, Jamadar DA. Estradiol and its Metabolites and Their Association With Knee Osteoarthritis. Arthritis Rheumatism (2006) 54:2481–7. 10.1002/art.22005 [DOI] [PubMed] [Google Scholar]
- 47. Gao W, Zeng C, Cai D, Liu B, Li Y, Wen X, et al. Serum Concentrations of Selected Endogenous Estrogen and Estrogen Metabolites in Pre- and Post-Menopausal Chinese Women With Osteoarthritis. J Endocrinological Invest (2010) 33:644–9. 10.1007/BF03346664 [DOI] [PubMed] [Google Scholar]
- 48. Shi J, Zhao W, Ying H, Zhang Y, Du J, Chen S, et al. Estradiol Inhibits NLRP3 Inflammasome in Fibroblast-Like Synoviocytes Activated by Lipopolysaccharide and Adenosine Triphosphate. Int J Rheumatic Dis (2018) 21:2002–10. 10.1111/1756-185X.13198 [DOI] [PubMed] [Google Scholar]
- 49. Liang Y, Duan L, Xiong J, Zhu W, Liu Q, Wang D, et al. E2 Regulates MMP-13 Via Targeting miR-140 in IL-1β-Induced Extracellular Matrix Degradation in Human Chondrocytes. Arthritis Res Ther (2016) 18:105. 10.1186/s13075-016-0997-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Claassen H, Steffen R, Hassenpflug J, Varoga D, Wruck CJ, Brandenburg LO, et al. 17β-Estradiol Reduces Expression of MMP-1, -3, and -13 in Human Primary Articular Chondrocytes From Female Patients Cultured in a Three Dimensional Alginate System. Cell Tissue Res (2010) 342:283–93. 10.1007/s00441-010-1062-9 [DOI] [PubMed] [Google Scholar]
- 51. Lee YJ, Lee EB, Kwon YE, Lee JJ, Cho WS, Kim HA, et al. Effect of Estrogen on the Expression of Matrix Metalloproteinase (MMP)-1, MMP-3, and MMP-13 and Tissue Inhibitor of Metalloproternase-1 in Osteoarthritis Chondrocytes. Rheumatol Int (2003) 23:282–8. 10.1007/s00296-003-0312-5 [DOI] [PubMed] [Google Scholar]
- 52. Tsai CL, Liu TK, Chen TJ. Estrogen and Osteoarthritis: A Study of Synovial Estradiol and Estradiol Receptor Binding in Human Osteoarthritic Knees. Biochem Biophys Res Commun (1992) 183:1287–91. 10.1016/S0006-291X(05)80330-4 [DOI] [PubMed] [Google Scholar]
- 53. Hussain SM, Wang Y, Giles GG, Graves S, Wluka AE, Cicuttini FM. Female Reproductive and Hormonal Factors and Incidence of Primary Total Knee Arthroplasty Due to Osteoarthritis. Arthritis Rheumatol (Hoboken N.J.) (2018) 70:1022–9. 10.1002/art.40483 [DOI] [PubMed] [Google Scholar]
- 54. Hussain SM, Cicuttini FM, Bell RJ, Robinson PJ, Davis SR, Giles GG, et al. Incidence of Total Knee and Hip Replacement for Osteoarthritis in Relation to Circulating Sex Steroid Hormone Concentrations in Women. Arthritis Rheumatol (Hoboken N.J.) (2014) 66:2144–51. 10.1002/art.38651 [DOI] [PubMed] [Google Scholar]
- 55. Sato Y, Tajima A, Katsurayama M, Nozawa S, Yoshiike M, Koh E, et al. An Independent Validation Study of Three Single Nucleotide Polymorphisms at the Sex Hormone-Binding Globulin Locus for Testosterone Levels Identified by Genome-Wide Association Studies. Hum Reprod Open (2017) 2017(1):hox002. 10.1093/hropen/hox002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martinez-Garcia MA, Gambineri A, Alpanes M, Sanchon R, Pasquali R, Escobar-Morreale HF. Common Variants in the Sex Hormone-Binding Globulin Gene (SHBG) and Polycystic Ovary Syndrome (PCOS) in Mediterranean Women. Hum Reprod (2012) 27(12):3569–76. 10.1093/humrep/des335 [DOI] [PubMed] [Google Scholar]
- 57. Yu XQ, Li M, Zhang H, Low HQ, Wei X, Wang JQ, et al. A Genome-Wide Association Study in Han Chinese Identifies Multiple Susceptibility Loci for IgA Nephropathy. Nat Genet (2011) 44(2):178–82. 10.1038/ng.1047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Summary data used in our study was downloaded from GWAS Catalog (https://www.ebi.ac.uk/gwas/), Neale’s lab (http://www.nealelab.is/uk-biobank) and IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/).