Abstract
In Hunter syndrome (mucopolysaccharidosis II [MPS-II]), systemic accumulation of glycosaminoglycans (GAGs) due to a deficiency of iduronate-2-sulfatase (IDS), caused by mutations in the IDS gene, leads to multiple somatic manifestations and in patients with the severe (neuronopathic) phenotype, also to central nervous system (CNS) involvement. These symptoms cannot be effectively treated with current enzyme-replacement therapies, as they are unable to cross the blood-brain barrier (BBB). Pabinafusp alfa, a novel IDS fused with an anti-human transferrin receptor antibody, was shown to penetrate the BBB and to address neurodegeneration in preclinical studies. Subsequent phase 1/2 and 2/3 clinical studies in Japan have shown marked reduction of GAG accumulation in the cerebrospinal fluid (CSF), along with favorable clinical responses. A 26-week, open-label, randomized, parallel-group phase 2 study was conducted in Brazil to further evaluate the safety and efficacy of intravenously administered pabinafusp alfa at 1.0, 2.0, and 4.0 mg/kg/week in MPS-II patients. The safety profiles in the three dosage groups were similar. Neurodevelopmental evaluation suggested positive neurocognitive signals despite a relatively short study period. The 2.0-mg/kg group, which demonstrated marked reductions in substrate concentrations in the CSF, serum, and urine, was considered to provide the best combination regarding safety and efficacy signals.
Keywords: mucopolysaccharidosis II, Hunter syndrome, pabinafusp alfa, JR-141, blood-brain barrier, iduronate-2-sulfatase, neurocognitive impairment, anti-human transferrin receptor antibody, heparan sulfate, enzyme-replacement therapy
Graphical abstract
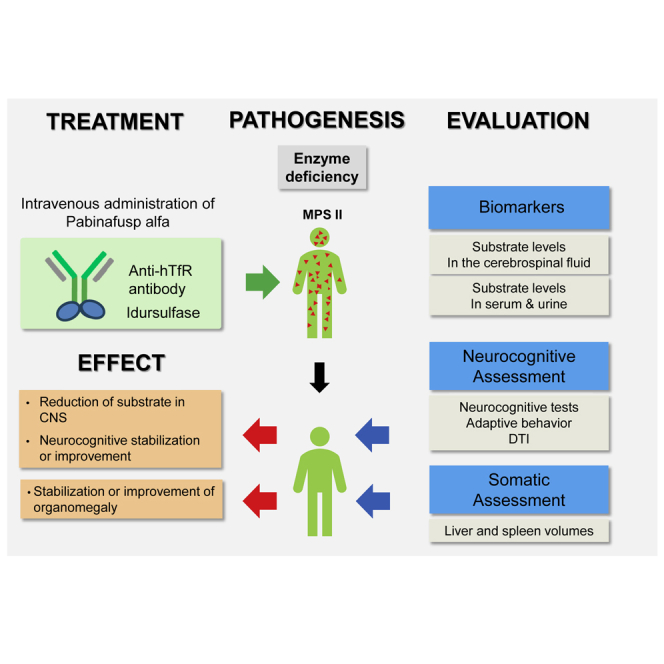
To address neurodegeneration in mucopolysaccharidosis II by drug delivery across the blood-brain barrier, pabinafusp alfa has been evaluated in two clinical trials in Japan with positive somatic and neurocognitive results. This article reports a phase 2 study in Brazil to replicate and further substantiate its safety and efficacy.
Introduction
Hunter syndrome (mucopolysaccharidosis II [MPS-II]) is an X-linked recessive lysosomal storage disorder, caused by mutations in the iduronate-2-sulfatase (IDS) gene leading to a deficiency of IDS,1 an essential enzyme for the catabolism of glycosaminoglycans (GAGs) such as heparan sulfate (HS) and dermatan sulfate (DS). The consequent pathological accumulation of GAGs in the lysosomes in most cells throughout the body results in a broad spectrum of somatic symptoms1, 2, 3 in patients with the severe (neuronopathic) or the attenuated (non-neuronopathic) phenotype. Furthermore, central nervous system (CNS) manifestations resulting from complex progressive neurodegeneration4 brought about by the accumulation of substrates are present in the patients with the severe phenotype that affects around 2/3 of the MPS-II population.2,3
Enzyme replacement therapy (ERT) with recombinant human IDS (idursulfase and idursulfase beta) has been used to treat the somatic symptoms in patients with MPS-II.5, 6, 7, 8 However, intravenously administrated idursulfase does not cross the blood-brain barrier (BBB) to address the associated neurodegeneration. Much research still focuses on clarifying and devising treatment for the resulting CNS manifestations.3
Drug delivery across the BBB9, 10, 11, 12 by way of transcytosis has been explored, focusing on endogenous insulin13,14 and transferrin15 receptors on the cerebrovascular endothelial cells, the latter being focused on for their ubiquitous expressions in most organs,16 far beyond the well-known expressions on hepatocytes and erythroid precursors.17 Positive findings have been reported in preclinical and clinical studies of patients with MPS-I.14 Other administration routes (e.g., intrathecal18 and intracerebroventricular19), as well as transient disruption of the BBB by ultrasound20 and hyperthermia,21 have also been attempted but are more invasive, less convenient, and with limited tangible clinical benefit so far.
JCR Pharmaceuticals has developed pabinafusp alfa (JR-141), which consists of human IDS fused to the C terminus of the heavy chain of an anti-human transferrin receptor (hTfR) antibody.22 Its successful delivery across the BBB into the CNS via TfR-mediated transcytosis has been demonstrated in preclinical studies22,23 and subsequently confirmed in phase 1/2 and 2/3 clinical trials in Japan,24,25 which evaluated its pharmacokinetics, safety, and efficacy in patients with MPS-II. Significant reductions in HS concentrations in the cerebrospinal fluid (CSF) were observed along with favorable behavioral changes, both of which suggest successful delivery of the drug across the BBB with salient clinical efficacy against both somatic and CNS symptoms. A subsequent phase 2 study was carried out in Brazil with the aim of replicating the findings of the clinical trials in Japan and of further investigating the safety, pharmacokinetics, and exploratory efficacy of the drug in MPS-II patients, thereby substantiating its effects on neurodegenerative CNS disorders. This study also examined the effects of a high dosage of pabinafusp alfa not previously tested in humans to acquire further dose-response evidence and provided neurodevelopmental data for detailed analysis.
Results
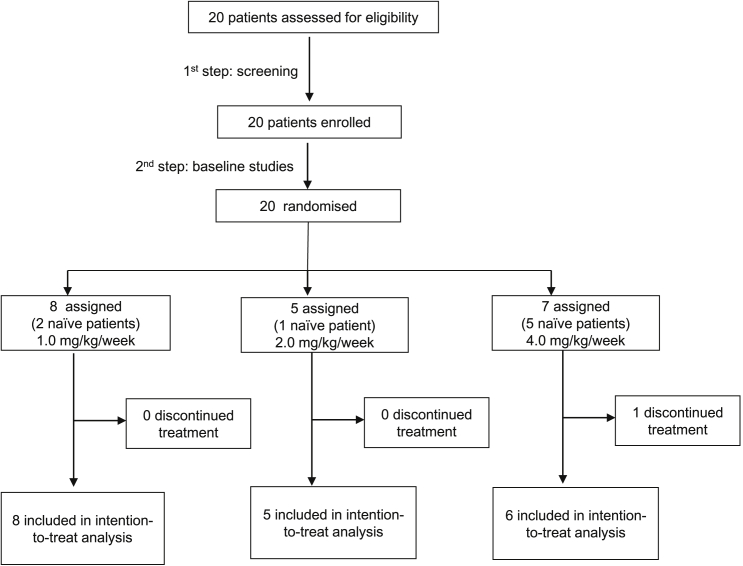
Twenty patients diagnosed with MPS-II were screened and randomized in this study (Figure 1). Pabinafusp alfa was then administered to all 20, and 19 of them completed the study. Eleven of these patients had been previously treated with conventional ERT with idursulfase (treatment duration: 1 year 3 months to 14 years, with an average of circa 4 years), whereas eight were naive to treatment. The baseline demographics and clinical characteristics of the patients are shown in Table 1. The subtype classification of MPS-II, attenuated or severe, was by clinical judgment of the investigators based on the symptomatology and severity of each patient.
Figure 1.
Trial profile
Table 1.
Baseline demographics and clinical characteristics of the study patients
| 1.0 mg/kg (n = 8) | 2.0 mg/kg (n = 5) | 4.0 mg/kg (n = 6) | Total (n = 19) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 8.76 ± 8.95 | 12.93 ± 14.15 | 19.56 ± 18.82 | 13.27 ± 14.01 |
| Weight (kg) at screening | ||||
| Mean ± SD | 28.75 ± 15.53 | 39.34 ± 19.01 | 41.52 ± 25.12 | 35.57 ± 19.64 |
| Height standing (cm) at screening | ||||
| Mean ± SD | 115.65 ± 23.20 | 128.00 ± 18.76 | 128.62 ± 33.69 | 122.99 ± 25.38 |
| BMI (kg/m2) at screening | ||||
| Mean (SD) | 20.04 ± 3.29 | 23.01 ± 6.72 | 22.38 ± 4.37 | 21.56 ± 4.63 |
| Race, n (%) | ||||
| White (%) | 6 (75.00) | 3 (60.00) | 4 (66.67) | 13 (68.42) |
| African American (%) | 2 (25.00) | 0 | 1 (16.67) | 3 (15.79) |
| Asian (%) | 0 | 0 | 0 | 0 |
| Native American or Inuit (%) | 0 | 0 | 1 (16.67) | 1 (5.26) |
| Native Hawaiian or other Pacific Islander (%) | 0 | 0 | 0 | 0 |
| Other (%) | 0 | 2 (40.00) | 0 | 2 (10.53) |
| Anti-IDS antibody at screening, n (%) | ||||
| Positive (%) | 5 (62.50) | 2 (40.00) | 1 (16.67) | 8 (42.11) |
| Negative (%) | 3 (37.50) | 3 (60.00) | 5 (83.33) | 11 (57.89) |
| MPS-II subtype, n (%) | ||||
| Attenuated (%) | 1 (12.50) | 1(20.00) | 3(50.00) | 5 (26.32) |
| Neuronopathic (%) | 7 (87.50) | 4 (80.00) | 3 (50.00) | 14 (73.68) |
| Previous ERT, n (%) | ||||
| Yes (%) | 6 (75.00) | 4 (80.00) | 1 (16.67) | 11 (57.89) |
| No (%) | 2 (25.00) | 1 (20.00) | 5 (83.33) | 8 (42.11) |
Table 2 summarizes the adverse drug reactions (ADRs) observed in the study. The ADR profiles in the 1.0- and 2.0-mg/kg dosage groups were comparable. Most of the observed ADRs were mild to moderate in severity; the highest rate of ADRs was observed in the 4.0-mg/kg group. No cessation of test drug administration was necessitated in any other patients, and no serious adverse events were considered to be related to the test drug. Although all three dosages were safely administered, given the higher rate of ADRs in the 4.0-mg/kg group, we deemed 2.0 mg/kg to be the best weekly dosage for further clinical trials aimed at establishing the efficacy of pabinafusp alfa.
Table 2.
Incidence of adverse drug reactions
| 1.0 mg/kg (n = 8) | 2.0 mg/kg (n = 5) | 4.0 mg/kg (n = 7) | |
|---|---|---|---|
| Subjects with ADRs | 4 | 1 | 6 |
| Skin and subcutaneous tissue disorders | 2 | 0 | 5 |
| Urticaria | 0 | 0 | 4 |
| Dermatitis acneiform | 1 | 0 | 0 |
| Erythema | 0 | 0 | 1 |
| Hyperhidrosis | 0 | 0 | 1 |
| Skin plaque | 1 | 0 | 0 |
| General disorders and administration site conditions | 1 | 0 | 4 |
| Pyrexia | 1 | 0 | 3 |
| Chills | 1 | 0 | 0 |
| Infusion site urticaria | 0 | 0 | 1 |
| Pain | 0 | 0 | 1 |
| Gastrointestinal disorders | 1 | 0 | 3 |
| Vomiting | 1 | 0 | 2 |
| Nausea | 1 | 0 | 2 |
| Injury, poisoning, and procedural complications | 1 | 1 | 2 |
| Infusion-related reaction | 1 | 1 | 2 |
| Central nervous system disorders | 2 | 0 | 2 |
| Tremor | 0 | 0 | 1 |
| Burning sensation | 0 | 0 | 1 |
| Headache | 1 | 0 | 0 |
| Somnolence | 1 | 0 | 0 |
| Investigations | 0 | 0 | 2 |
| Body temperature increased | 0 | 0 | 2 |
| Immune system disorders | 0 | 0 | 1 |
| Anaphylactic reaction | 0 | 0 | 1 |
ADRs, adverse drug reactions. MedDRA version: 22.0.
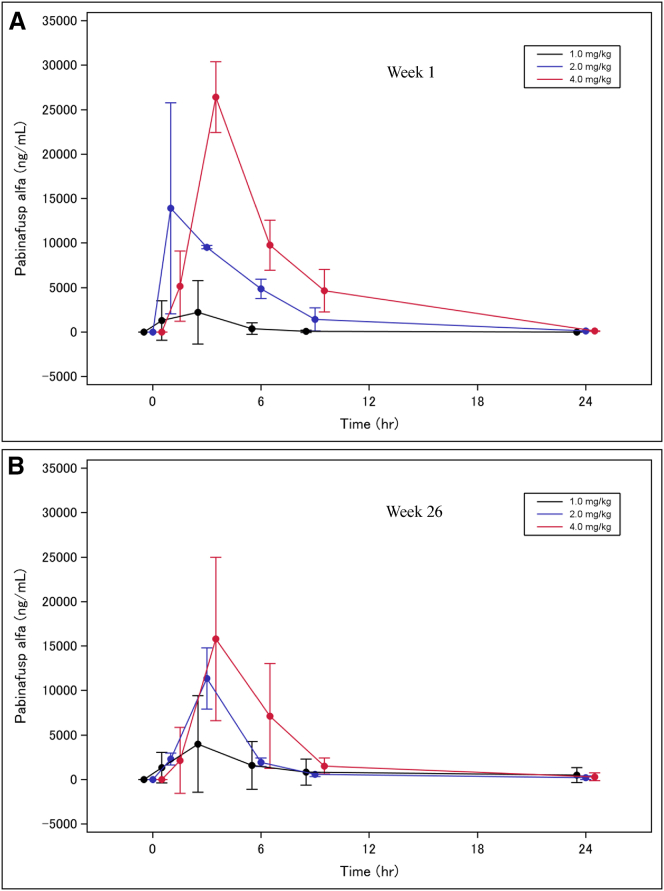
Figure 2 shows the time courses of plasma concentrations of pabinafusp alfa at weekly dosages of 1.0, 2.0, and 4.0 mg/kg at week 1 (Figure 2A) and week 26 (Figure 2B). Maximum plasma concentrations increased in a dose-dependent manner. Plasma concentration profiles were similar in the first and the final administrations, indicating no drug accumulation in any of the dosage groups. Plasma half-lives of pabinafusp alfa at 1.0, 2.0, and 4.0 mg/kg at week 1 and week 26 are listed in Table S1.
Figure 2.
Time courses of mean plasma concentrations of pabinafusp alfa at weekly dosages of 1.0, 2.0, and 4.0 mg/kg at weeks 1 and 26
(A) Week 1. (B) Week 26). The data represent mean ± SD.
The liver and spleen volumes adjusted for body weight in the patients previously treated with conventional ERT were maintained in the 1.0- and 2.0-mg/kg groups until week 26, whereas they increased in one patient in the 4.0-mg/kg group. In the naive patients, however, a pattern of decreasing volumes was observed in all dosage groups. As evaluation of changes in organomegaly needs to take into consideration large variability in original organ volumes by subjects, individual relative volume changes by subjects at week 26 have been calculated, in which the baseline volume is counted as 100% (Figure S1). Marked improvement in hepatosplenomegaly was observed in all naive patients and most of the switched patients, suggesting notable somatic efficacy of pabinafusp alfa against organomegaly even in a relatively short study period.
Cardiac function, as assessed by echocardiography, generally remained stable throughout the study period in terms of left ventricular ejection fraction and left ventricular mass index.
The urine HS (Figure S2) and DS (Figure S3) concentrations showed no changes in all dosage groups in the patients previously treated with conventional ERT (Figures S2A-1 and S3A-1). In the naive patients, the urine HS concentrations at weeks 13 and 26 decreased in the 2.0- and 4.0-mg/kg groups but not in the 1.0-mg/kg group (Figure S2B-1). The serum HS concentrations in the previously treated patients (Figure S2A-2) remained stable with some decrease at weeks 13 and 26 in the 1.0- and 4.0-mg/kg groups, whereas the serum DS concentrations (Figure S3A-2) were generally stable at weeks 13 and 26 in all dosage groups. In the naive patients, the serum HS and DS concentrations were decreased in all dosage groups, albeit with a high standard deviation in the 1.0 mg/kg group (Figures S2B-2 and S3B-2).
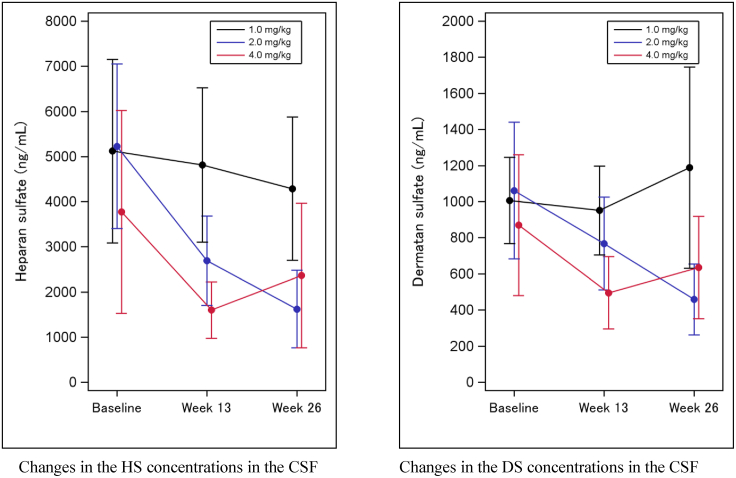
The HS and DS concentrations in the CSF continuously decreased in the 2.0-mg/kg and 4.0-mg/kg dosage groups (Figure 3). The 2.0-mg/kg group demonstrated a statistically significant reduction from baseline in both HS and DS concentrations, based on the upper limits of 95% confidence intervals for the ratio of HS concentrations at week13 and week 26 to baseline, both below 1 in the 2.0-mg/kg group.
Figure 3.
HS and DS concentrations in the CSF
The data represent mean ± SD.
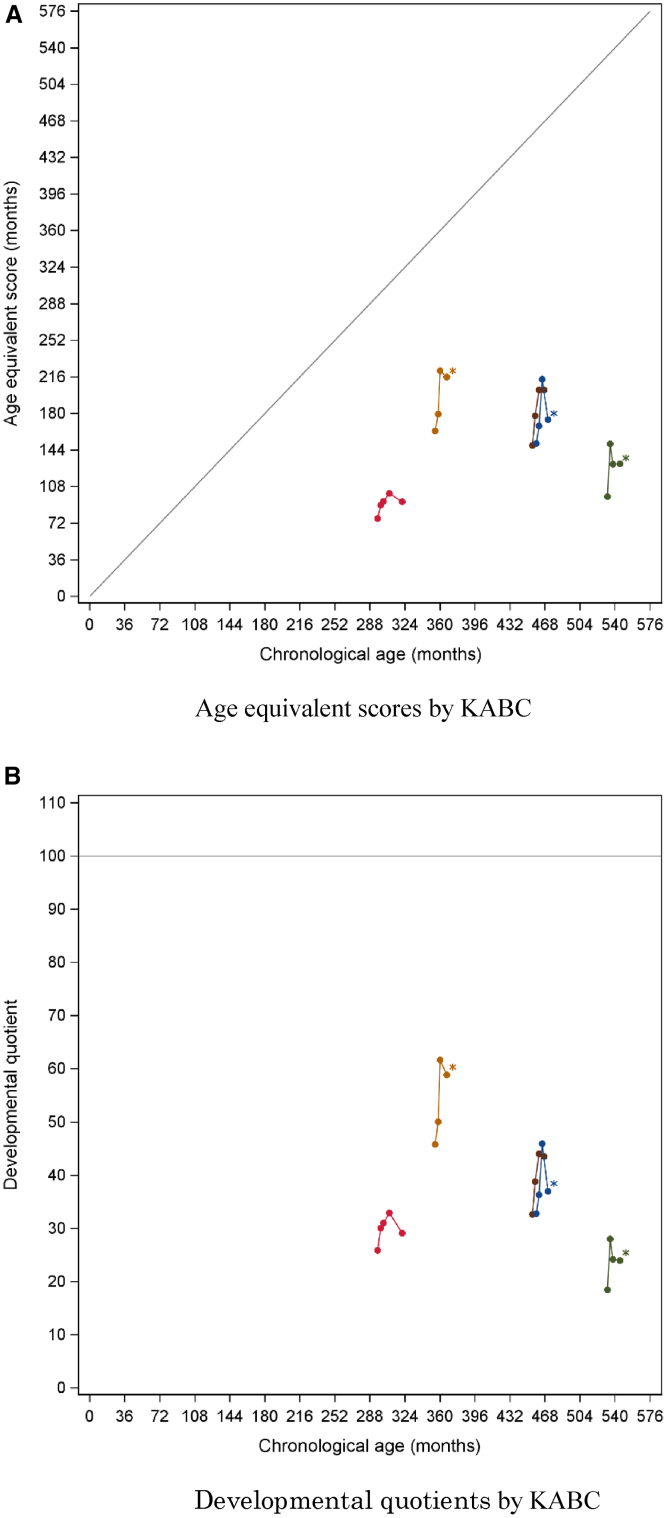
The efficacy of pabinafusp alfa on the neurocognitive signs and symptoms was evaluated by way of standardized neurocognitive assessments and descriptive reports on behavioral changes. An extension study following this trial has generated data for a further 26 weeks so far, allowing for a total of 52 weeks of neurodevelopmental data to be analyzed. The Bayley Scales of Infant and Toddler Development (BSID-III) was used for neurocognitive evaluations of the patients younger than 42 months of developmental age (age-equivalent [AE] score), whereas the Kaufman Assessment Battery for Children, 2nd edition (KABC-II), was used for the older patients. The Vineland Adaptive Behavior Scale, second edition (VABS-II), was also used to assess a potential influence of pabinafusp alpha on the adaptive behavioral functions.
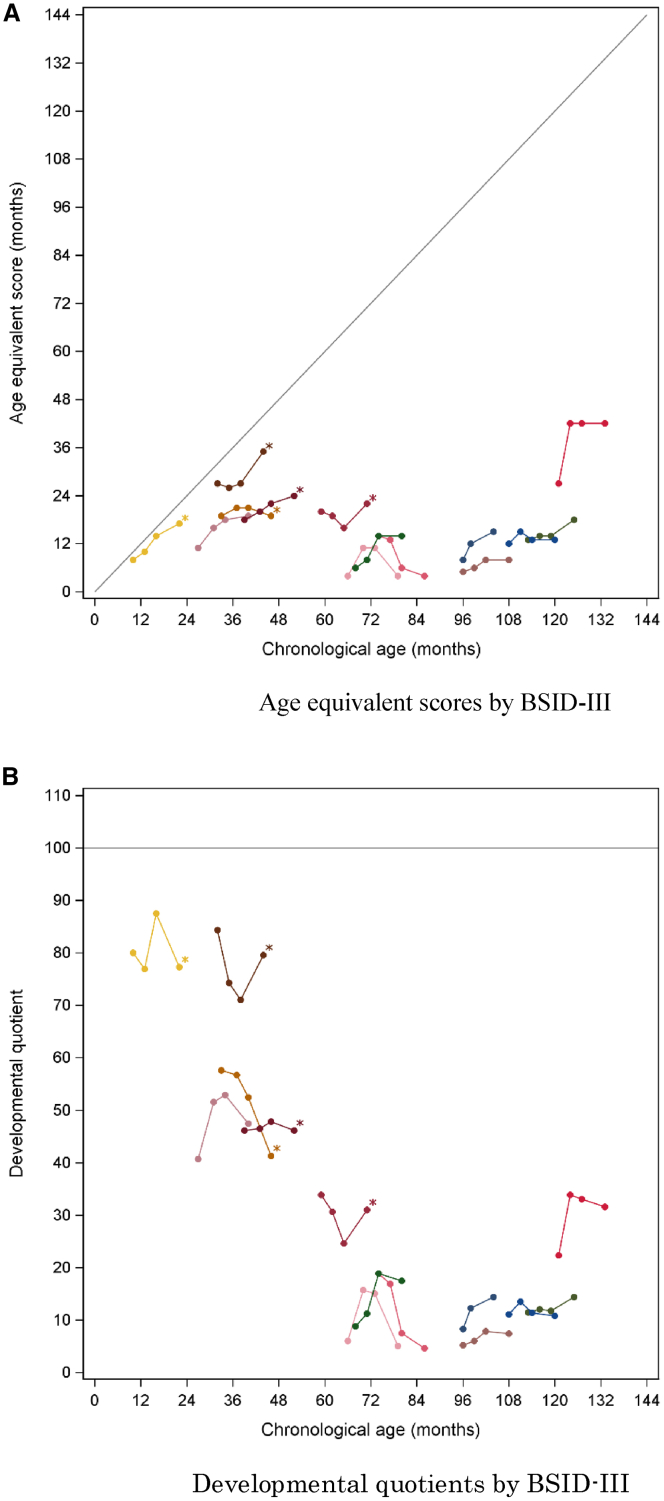
Figures 4 and 5 show the changes in AE scores and developmental quotient (DQ) scores (i.e., cognitive AE score divided by chronological age × 100) in individual patients against their chronological ages at baseline and at weeks 13, 26, and 52, as determined with BSID-III/KABC-II, respectively. The very young patients and the ones with the severe subtype were assessed by BSID-III; those with the attenuated by KABC-II. Improvements were shown in the patients younger than 6 years of age with severe MPS-II and also in those older than 10 years. DQ scores were maintained in the patients younger than 6 years of age, especially in those two who showed maintenance of DQ beyond 70. Four patients older than 25 years of age had a DQ between 18 and 45 at baseline but nonetheless improved in their DQ and AE score.
Figure 4.
Age-equivalent scores and developmental quotients against chronological age as determined with BSID-III
(A) Age-equivalent scores. (B) Developmental quotients. All patients belong to a severe (neuronopathic) subtype. The curves with asterisks denote the patients naive to ERT.
Figure 5.
Age-equivalent scores and developmental quotients against chronological age as determined with KABC
(A) Age-equivalent scores. (B) Developmental quotients. All patients belong to the attenuated subtype. The curves with asterisks denote the patients naive to ERT.
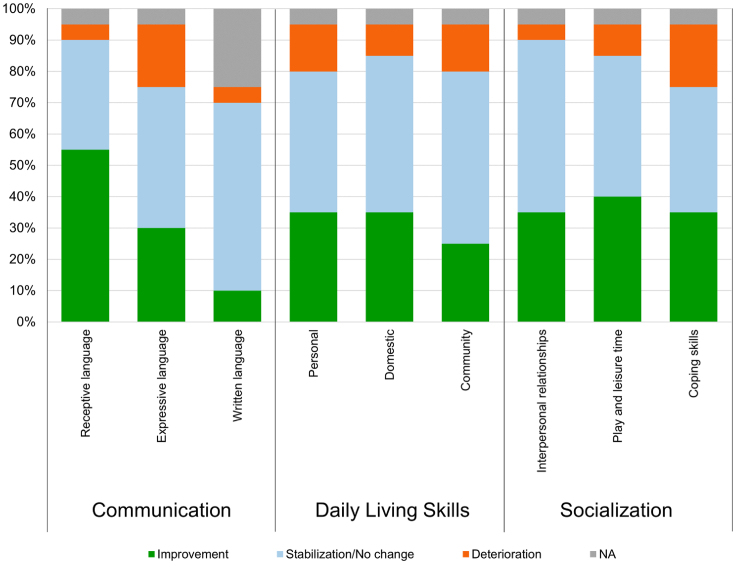
VABS-II also provided neurodevelopmental data supporting the changes observed with BSID and KABC. Subdomain analysis of VABS-II results with respect to the changes over 52 weeks has revealed improvement or stabilization across the 11 subdomains in the majority of the patients with severe MPS-II. Figure 6 shows the changes observed in the three major domains, i.e., communication, daily living skills, and socialization, in which most of the patients with severe MPS-II improved or stabilized. The motor skill domain is not included in Figure 6 for a methodological issue in VABS-II in that the patients with skeletal deformities often cannot respond appropriately to the test.
Figure 6.
Changes seen over 52 weeks in three subdomains in VABS-II in the MPS-II patients
The patients include both attenuated and severe subtypes.
The neurodevelopmental parameters from the three test batteries remained stable in all dosage groups, suggesting general maintenance of overall neurocognitive development and apparent amelioration in some young patients. The most notable positive changes were observed in the receptive language domain (>50% of the patients improved) and play and leisure time (almost 50% of the patients improved).
The behavioral changes in 19 patients, as recognized by their families and the investigators, were collected in the narrative records (Table S2) in order to register subtle changes that standardized assessments may fail to capture but which can nonetheless be clinically informative. The findings in three major areas—language, motor skills, and liveliness/expression—indicate generally positive changes throughout, e.g., improvement in vocabulary and walking, increased smiling, and stabilized mood. Favorable changes were more frequently observed among the younger patients than the adults. It is also notable that the patients without marked improvements in language or motor functions showed positive changes in liveliness/expression. These subjective observational changes are generally in accordance with the objective results from the aforementioned neurocognitive assessments.
Magnetic resonance imaging (MRI) of the brain was used to assess cortical gray matter volume, ventricular volumes, diffusion tensor imaging (DTI), and other parameters. No significant changes were observed in the volumes of the cortical gray matter or the ventricles. DTI remained generally stable in both the previously treated and naive patients in all dosage groups. Overall, the neuroimaging results suggested no consistently meaningful changes.
Taken together, the exploratory efficacy data suggest first that pabinafusp alfa possesses somatic efficacy comparable to that of idursulfase. Second, the marked reduction of HS concentrations in the CSF together with the positive changes revealed in the neurocognitive assessments suggests its efficacy also on CNS manifestations.
Discussion
Following on from the preceding phase1/2 study in Japan,22 this study carried out in Brazil further investigated the safety, pharmacokinetics, and efficacy of pabinafusp alfa for severe MPS-II, focusing in particular on the highest dosage of 4.0 mg/kg and including a detailed analysis of neurocognitive parameters.
As far as safety was concerned, no drug-related severe adverse events were observed across the three dosages evaluated. Drug-related ADRs were unsurprisingly greatest in the 4.0-mg/kg group, but they were mostly mild to moderate in severity and except for some manageable infusion-associated reactions, did not interfere with administration of the drug to the patients. Of the three dosages, 2.0 mg/kg seems to be the safest and effective: the efficacy of the drug was most notable in the 2.0- and 4.0-mg/kg groups, but there were more infusion-associated reactions in the 4.0-mg/kg group. The pharmacokinetic evaluation results showed no accumulation of the drug, irrespective of dosages, so the suggested dosage of pabinafusp alfa for late-phase clinical trials, as well as for wide-scale future usage, is 2.0 mg/kg.
Comparisons of the pharmacokinetic parameters investigated in this study and the preceding phase 1/2 study22 in Japan are not possible due to the limited number of subjects assigned to each of the three doses tested in this study with its wide variability, but the time courses of mean plasma concentrations of pabinafusp alfa at 1.0 and 2.0 kg/mg did not show notable differences between the two studies. Relatively low plasma concentrations of pabinafusp alfa after the 26-week administration in this study are interpreted to be due to the said wide variability of the concentrations in each patient within a small sample, in addition to development of antidrug antibodies in one patient in both 2.0 and 4.o mg/kg groups.
The efficacy of pabinafusp alfa against somatic symptoms was evaluated on the basis of substrate concentrations in the urine and serum, as well as liver and spleen volumes and cardiac functions. Marked reductions in substrate concentrations were observed in the naive patients, and levels were generally maintained in the patients who switched from idursulfase to the test drug. Liver and spleen volumes and cardiac functions remained generally stable throughout the study period in patients previously on the conventional ERT, whereas improvements in organomegaly were seen in ERT-naive patients. Taken together, these results suggest that the somatic efficacy of pabinafusp alfa is comparable to that of idursulfase.
In terms of the drug’s efficacy against CNS symptoms, the Brazil study showed most notable reductions of HS levels in the CSF in the 2.0-mg/kg and 4.0-mg/kg dosage groups, successfully replicating the findings of the clinical trials24,25 conducted in Japan and providing further evidence that pabinafusp alfa is delivered into the brain through the BBB to address the initial process of the complex cascade of neurodegenerative events that take place in severe MPS-II.4
As neurodegeneration causes multifaceted functional and structural damage that is neither specific nor pathognomonic to MPS-II, clinical manifestations vary significantly, making them difficult to evaluate clinically. One of the most devastating CNS manifestations of severe MPS is neurocognitive impairment, which is known to differ in severity and progression according to the specific MPS-II phenotypes.26 Furthermore, neurocognitive evaluation needs to take account of both normal development, which may be sustained in some patients for several years after birth, and disease-derived developmental delays that differ considerably from patient to patient. Evaluation of neurocognitive development is thus hard to conduct in a limited number of patients within a short study period.
The neurodevelopmental data of 52 weeks from this study and its extension study showed overall stabilization of neurocognitive function as well as adaptive behavior, along with positive changes in developmental trajectories in most patients, irrespective of the severity of their condition.
Neurodevelopmental tests (BSID-III/KABC-II) showed improvements in the patients younger than 6 years of age with severe MPS-II and also in those older than 10 years, although the changes in the very young patients are difficult to differentiate from normal development before neurodegeneration manifests itself, and need to be evaluated in the light of long-term observation. This study included five patients aged 24 years or older, whose DQ was in a range between 18 and 42 at baseline, a value that would normally not assign them to the attenuated phenotype. These low scores may be due to limitations inherent in the KABC-II, whereby older patients score much lower than their true developmental status. The notable improvement in their AE score and DQ, however, may suggest that at least some CNS symptoms can still respond to pabinafusp alfa even in older patients, probably those symptoms caused chiefly by a neurotransmission defect that is not as irreversible as the neuronal loss.4 On the other hand, the early introduction of ERT with pabinafusp alfa seems to be very important for patients with the severe subtype who will probably benefit most from its potential prophylactic effects before neurodegeneration leads to irreversible damage.
Analysis of VABS-II in the subdomains revealed that even in the relatively short observation period of 52 weeks, most of the patients showed positive changes in terms of their receptive and expressive language skills, their daily living skills (interpersonal and personal), and their motor skills (gross and fine). The narrative behavioral reports also described favorable changes over the 52 weeks, irrespective of the patients’ age or disease severity, including notable improvements that are only perceived non-verbally (i.e., changes in liveliness, facial expressions, and smiling, the last being a well-established attachment behavior27 in child psychiatry). Accordance of these subjective observations with the objective findings from the three assessment scales further suggests the neurocognitive efficacy of pabinafusp alfa. Despite the limited study period and sample size, the present study provides salient developmental data, both objective and subjective, suggesting positive neurocognitive changes by pabinafusp alfa.
As long-term functional and structural assessment necessary to evaluate drug efficacy for the CNS is impractical in the case of a rare and progressive disease in pediatric patients such as MPS-II, a realistic compromise had to be made in this study to capture both biochemical surrogate endpoints on the one hand, i.e., HS levels in the CSF, and clinical endpoints reflecting CNS manifestations, i.e., neurodevelopmental assessments, on the other. In other words, this study examined both the initial process of neurodegeneration and at the same time, some of the clinical neuropsychiatric manifestations as a final outcome of the long and complex pathological process.
There were several limitations to this study. First, it was an open study with no comparator arm. Second, the study duration was 26 weeks with another 26 weeks of extension, which was sufficient to show significant reductions in HS concentrations in the CSF but was not long enough to substantiate neurodevelopmental effects, even with a total of 52 weeks of data. A longer comparative study with idursulfase as well as comparative analysis with natural history data28 is expected to address these two limitations and help establish the efficacy of pabinafusp alfa against CNS disorders, in particular, progressive neurocognitive impairment.
In conclusion, the present study has established the safety profile and pharmacokinetics of pabinafusp alfa up to a weekly dose of 4.0 mg/kg in patients with MPS-II. It has also shown that the somatic efficacy of the drug is comparable to that of idursulfase. Regarding its efficacy in relation to CNS symptoms, the significant reduction of substrate concentrations in the CSF and the positive results of the neurocognitive batteries strongly suggest that the drug was successfully delivered across the BBB, taking enzymatic activity to the brain and leading to positive clinical effects. 2.0 mg/kg has been suggested to be the best weekly dosage, with which we plan to further illustrate these effects in another multinational comparative trial.29 Furthermore, our success in achieving drug delivery across the BBB is a clinically significant achievement and is hoped to be applied to other large molecules to address a wide range of CNS disorders.
Materials and methods
Study design
This was a 26-week, open-label, parallel group, randomized clinical trial to evaluate the safety, pharmacokinetics, and efficacy of pabinafusp alfa administered intravenously to patients with MPS-II. The study was conducted in two hospitals in Brazil and complied with the Declaration of Helsinki. The protocol and procedures regarding informed consent were reviewed and approved by the Institutional Review Board at each participating institution. All patients or their legal guardians submitted a signed, informed consent form prior to enrolment.
The study consisted of four periods: screening and confirmation of eligibility, baseline studies, randomization and washout, and treatment/assessment. The patients who had been receiving idursulfase underwent a washout period of 1 week before switching to the test drug, thereby ensuring no interruption of the weekly enzyme replacement. The patients were intravenously administered the assigned dosage weekly for 26 weeks, after which, efficacy and safety of the test drug were evaluated.
Participants and procedures
A total of 20 patients were screened at the two study sites and randomly allocated to three pabinafusp alfa dosage groups, with eight in the 1.0-mg/kg group, five in the 2.0-mg/kg group, and seven in the 4.0-mg/kg group. Nineteen of the patients received the test drug weekly for the entire study period, whereas one died of exacerbation of disease-associated respiratory arrest (unrelated to the study drug). The study protocol, including the full inclusion and exclusion criteria, is available online (https://clinicaltrials.gov/ct2/show/NCT03359213; ClinicalTrials.gov: NCT03359213).
Randomization and masking
After screening, the patients were divided into three age groups (0 to 3 years 11 months, 4 to 7 years 11 months, and 8 years or older) and randomly assigned to one of the three dosage groups, ensuring that at least two members of each age group were assigned to each dosage group so that overall randomization to the treatment arms was at a ratio of 1:1:1.
Outcomes
Primary endpoint evaluations of the safety of weekly intravenous administrations of pabinafusp alfa at the assigned dosages were based on the type and severity of adverse events, vital signs (heart rate, respiratory rate, body temperature, and blood pressures), anti-pabinafusp alpha antibodies, electrocardiography results, routine blood tests (hematology, liver function, renal function, and iron-related parameters), and urinalysis.
The secondary efficacy endpoints were the following: (1) changes between baseline and week 26 in serum and urine HS and DS concentrations, liver and spleen volumes by MRI, and left ventricular mass index by echocardiography and (2) changes between baseline and week 26 in cortical gray matter, ventricular volumes and DTI results by MRI, HS and DS concentrations in the CSF, results of neurocognitive and adaptive behavioral tests (BSID-III, KABC-II, and VABS-II), quality-of-life measurements, and actigraphy readings. High-sensitivity liquid chromatography-tandem mass spectrometry23 was used to measure HS and DS concentrations in the CSF, serum, and urine.
Pharmacokinetic evaluations were performed at all dosing points and after the first and the last administrations of pabinafusp alfa in the patients aged 8 years or older who had been administered at least one dose of the test drug and from whom at least one blood sample had been collected. Blood samples were collected at six time points: 10 min prior to infusion; 1 h after the start of dosing; immediately after the last administration; and then 3, 6, and 21 h post-infusion. To provide pharmacokinetic parameters (AUC0-t, Cmax, AUC0-inf, tmax, kel, t1/2, and MRT0-t), electrochemiluminescence assay was used to measure plasma concentrations of the test drug.
Statistical analysis
All data analyses followed the intention-to-treat principle, whereby the analyses included all patients who had received at least one dose of pabinafusp alfa with at least one post-baseline efficacy assessment. Pharmacokinetics analysis was done only for the patients older than 8 years of age who were administered with at least one dose of pabinafusp alfa and for whom plasma drug concentration data were available.
The somatic efficacy endpoints were analyzed separately in the patients with and without prior ERT with idursulfase. The liver and spleen volume MRI results were adjusted for body weight and expressed as the volume per kilogram of body weight. The urinary HS and DS concentrations were adjusted by dividing the original concentrations by the urinary creatinine concentrations.
Regarding HS and DS concentrations in the CSF and neurocognitive testing, the patients with and without prior ERT were analyzed together. In the neurocognitive testing result analyses, AE scores and DQ scores were calculated for the cognitive domain of the BSID-III and the nonverbal index of the KABC-II. For the VABS-II, patients were classified in terms of improvement, stabilization, or deterioration according to the absolute change in AE scores for each subdomain at 52 weeks.
All statistical analyses were performed with the SAS version 9.4 statistical software package (SAS Institute, Cary, NC, USA).
Acknowledgments
The authors are grateful to all investigators, sub-investigators, study coordinators, clinical research team, and patients involved for their contribution and commitment to the study. Helpful suggestions and guidance by Elsa Shapiro of University of Minnesota, Minneapolis, on the neurodevelopmental assessment are most appreciated. We thank Shunichi Kawasaki, Naoki Kawata, Kohtaro Minami, Maiko Kokado, Naoko Takasao, Minako Kobayashi, Hideyuki Ide, Kohtaro Hamauchi, and Kimika Nakamura of JCR Pharmaceuticals for their support at various stages of the study. Special thanks are due to Timothy Minton of Keio University, Tokyo, for his immense editorial help. This clinical trial was funded by JCR Pharmaceuticals. The funder participated in the design of the trial; collection, analysis, and interpretation of the data; and writing of the report; it also dealt with all of the regulatory requirements for carrying out the trial. The funder is also the sole intellectual property holder, as well as the manufacturer of the test drug, pabinafusp alfa (JR-141).
Author contributions
R.G., S.S., and K.T. conceived and designed the study, and all other authors assisted in its design. R.G. and A.M.M. conducted the trial as its principal investigators. T.I. and Y.S. wrote the first draft of the manuscript. M.Y. and T.I. designed and conducted all statistical analyses. K.T., T.I., and M.S. analyzed the developmental data. All authors were involved in the interpretation and critical review of the data and in drafting and revising the manuscript for important intellectual content; all approved the final version proposed by Y.S. All authors had full access to the data used in the study, and the corresponding author had final responsibility for the completion of the manuscript and the decision to submit it for publication.
Declaration of interests
R.G. has been an investigator, consultant, and/or speaker within the last 12 months for Abeona, Allevex, Amicus, BioMarin, Chiesi, Denali, Idorsia, Inventiva, JCR, Lysogene, Novartis, PassageBio, PTC, RegenxBio, Sanofi-Genzyme, Sigilon, Sobi, Takeda, and Ultragenyx. A.M.M. has received honors and support for travels and congresses from BioMarin, Sanofi Genzyme, Takeda, and Ultragenyx. A.M.M. has received research fundings from Alexion, BioMarin, Sanofi Genzyme, and Takeda.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.03.019.
Supplemental information
References
- 1.Wilson P.J., Morris C.P., Anson D.S., Occhiodoro T., Bielicki J., Clements P.R., Hopwood J.J. Hunter syndrome: isolation of an iduronate-2-sulfatase cDNA clone and analysis of patient DNA. Proc. Natl. Acad. Sci. USA. 1990;87:8531–8535. doi: 10.1073/pnas.87.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliani R. The mucopolysaccharidoses. In: Mehta A., Winchester B., editors. Lysosomal Storage Disorders: A Practical Guide. First Edition. Wiley-Blackwell; 2012. pp. 94–100. [Google Scholar]
- 3.Giugliani R., Vairo F., Kubaski F., Poswar F., Riegel M., Baldo G., Saute J.A. Neurological manifestations of lysosomal disorders and emerging therapies targeting the CNS. Lancet Child Adolesc. Health. 2018;2:56–68. doi: 10.1016/S2352-4642(17)30087-1. [DOI] [PubMed] [Google Scholar]
- 4.Sato Y., Okuyama T. Novel Enzyme Replacement Therapies for Neuropathic Mucopolysaccharidoses. Int. J. Mol. Sci. 2020;21:E400. doi: 10.3390/ijms21020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muenzer J., Wraith J.E., Beck M., Giugliani R., Harmatz P., Eng C.M., Vellodi A., Martin R., Ramaswami U., Gucsavas-Calikoglu M. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet. Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 6.Muenzer J., Gucsavas-Calikoglu M., McCandless S.E., Schuetz T.J., Kimura A. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol. Genet. Metab. 2007;90:329–337. doi: 10.1016/j.ymgme.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama T., Tanaka A., Suzuki Y., Ida H., Tanaka T., Cox G.F., Eto Y., Orii T. Japan Elaprase Treatment (JET) study: idursulfase enzyme replacement therapy in adult patients with attenuated Hunter syndrome (Mucopolysaccharidosis II, MPS II) Mol. Genet. Metab. 2010;99:18–25. doi: 10.1016/j.ymgme.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Muenzer J., Beck M., Eng C.M., Giugliani R., Harmatz P., Martin R., Ramaswami U., Vellodi A., Wraith J.E., Cleary M. Long-term, open-labeled extension study of idursulfase in the treatment of Hunter syndrome. Genet. Med. 2011;13:95–101. doi: 10.1097/GIM.0b013e3181fea459. [DOI] [PubMed] [Google Scholar]
- 9.Yu Y.J., Zhang Y., Kenrick M., Hoyte K., Luk W., Lu Y., Atwal J., Elliott J.M., Prabhu S., Watts R.J., Dennis M.S. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y.J., Atwal J.K., Zhang Y., Tong R.K., Wildsmith K.R., Tan C., Bien-Ly N., Hersom M., Maloney J.A., Meilandt W.J. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 2014;6:261ra154. doi: 10.1126/scitranslmed.3009835. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q.H., Boado R.J., Lu J.Z., Hui E.K., Pardridge W.M. Brain-penetrating IgG-iduronate 2-sulfatase fusion protein for the mouse. Drug Metab. Dispos. 2012;40:329–335. doi: 10.1124/dmd.111.042903. [DOI] [PubMed] [Google Scholar]
- 12.Niewoehner J., Bohrmann B., Collin L., Urich E., Sade H., Maier P., Rueger P., Stracke J.O., Lau W., Tissot A.C. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- 13.Boado R.J., Ka-Wai Hui E., Zhiqiang Lu J., Pardridge W.M. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol. Bioeng. 2014;111:2317–2325. doi: 10.1002/bit.25289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giugliani R., Giugliani L., de Oliveira Poswar F., Donis K.C., Corte A.D., Schmidt M., Boado R.J., Nestrasil I., Nguyen C., Chen S., Pardridge W.M. Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): an open label phase 1-2 trial. Orphanet J. Rare Dis. 2018;13:110. doi: 10.1186/s13023-018-0849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couch J.A., Yu Y.J., Zhang Y., Tarrant J.M., Fuji R.N., Meilandt W.J., Solanoy H., Tong R.K., Hoyte K., Luk W. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005338. 183ra57, 1–12. [DOI] [PubMed] [Google Scholar]
- 16.Ono H., Ogasawara O., Okubo K., Bono H. RefEx, a reference gene expression dataset as a web tool for the functional analysis of genes. Sci. Data. 2017;4:170105. doi: 10.1038/sdata.2017.105. http://refex.dbcls.jp/gene_info.php?lang=en&db=human&geneID=7037&refseq=NM_001128148&unigene=Hs.529618&probe=208691_at [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawabata H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019;133:46–54. doi: 10.1016/j.freeradbiomed.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Muenzer J., Hendriksz C.J., Fan Z., Vijayaraghavan S., Perry V., Santra S., Solanki G.A., Mascelli M.A., Pan L., Wang N. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet. Med. 2016;18:73–81. doi: 10.1038/gim.2015.36. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka K., Tamura T., Tsuji D., Dohzono Y., Kitakaze K., Ohno K., Saito S., Sakuraba H., Itoh K. Therapeutic potential of intracerebroventricular replacement of modified human β-hexosaminidase B for GM2 gangliosidosis. Mol. Ther. 2011;19:1017–1024. doi: 10.1038/mt.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipsman N., Meng Y., Bethune A.J., Huang Y., Lam B., Masellis M., Herrmann N., Heyn C., Aubert I., Boutet A. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018;9:2336. doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuthardt E.C., Duan C., Kim M.J., Campian J.L., Kim A.H., Miller-Thomas M.M., Shimony J.S., Tran D.D. Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier. PLoS One. 2016;11:e0148613. doi: 10.1371/journal.pone.0148613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoda H., Morimoto H., Yoden E., Koshimura Y., Kinoshita M., Golovina G., Takagi H., Yamamoto R., Minami K., Mizoguchi A. A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018;26:1366–1374. doi: 10.1016/j.ymthe.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka N., Kida S., Kinoshita M., Morimoto H., Shibasaki T., Tachibana K., Yamamoto R. Evaluation of cerebrospinal fluid heparan sulfate as a biomarker of neuropathology in a murine model of mucopolysaccharidosis type II using high-sensitivity LC/MS/MS. Mol. Genet. Metab. 2018;125:53–58. doi: 10.1016/j.ymgme.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Okuyama T., Eto Y., Sakai N., Minami K., Yamamoto T., Sonoda H., Yamaoka M., Tachibana K., Hirato T., Sato Y. Iduronate-2-Sulfatase with Anti-human Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: A Phase 1/2 Trial. Mol. Ther. 2019;27:456–464. doi: 10.1016/j.ymthe.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuyama T., Eto Y., Sakai N., Nakamura K., Yamamoto T., Yamaoka M., Ikeda T., So S., Tanizawa K., Sonoda H., Sato Y. A phase 2/3 trial of pabinafusp alfa, IDS fused with anti-human transferrin receptor antibody, targeting neurodegeneration in MPS-II. Mol. Ther. 2021;29:671–679. doi: 10.1016/j.ymthe.2020.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro E.G., Escolar M.L., Delaney K.A., Mitchell J.J. Assessments of neurocognitive and behavioral function in the mucopolysaccharidoses. Mol. Genet. Metab. 2017;122S:8–16. doi: 10.1016/j.ymgme.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Bowlby J. Second Edition. Volume 1. Basic Books; 1969. (Attachment and Loss: Attachment). [Google Scholar]
- 28.Seo J.-H., Okuyama T., Shapiro E., Fukuhara Y., Kosuga M. Natural history of cognitive development in neuronopathic mucopolysaccharidosis type II (Hunter syndrome): Contribution of genotype to cognitive developmental course. Mol. Genet. Metab. Rep. 2020;24:100630. doi: 10.1016/j.ymgmr.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JCR Pharmaceuticals Co., Ltd. 2020. A Phase III Study of JR-141 in Patients With Mucopolysaccharidosis II. ClinicalTrials.gov: NCT04573023.https://clinicaltrials.gov/ct2/show/NCT04573023 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.