Abstract
Niche partitioning of time, space or resources is considered the key to allowing the coexistence of competitor species, and particularly guilds of predators. However, the extent to which these processes occur in marine systems is poorly understood due to the difficulty in studying fine-scale movements and activity patterns in mobile underwater species. Here, we used acceleration data-loggers to investigate temporal partitioning in a guild of marine predators. Six species of co-occurring large coastal sharks demonstrated distinct diel patterns of activity, providing evidence of strong temporal partitioning of foraging times. This is the first instance of diel temporal niche partitioning described in a marine predator guild, and is probably driven by a combination of physiological constraints in diel timing of activity (e.g. sensory adaptations) and interference competition (hierarchical predation within the guild), which may force less dominant predators to suboptimal foraging times to avoid agonistic interactions. Temporal partitioning is often thought to be rare compared to other partitioning mechanisms, but the occurrence of temporal partitioning here and similar characteristics in many other marine ecosystems (multiple predators simultaneously present in the same space with dietary overlap) introduces the question of whether this is a common mechanism of resource division in marine systems.
Keywords: competition, behavioural plasticity, intra-guild predation, circadian rhythm, elasmobranch, accelerometer
1. Introduction
Niche partitioning is one of the main mechanisms allowing sympatric competitors to coexist through the division of resources. Niche partitioning commonly takes several forms, including resource partitioning, where species specialize in different food or prey items, spatial partitioning, where species use different areas to forage or hunt, and temporal partitioning, where sympatric species rotate peak foraging times on a diel or seasonal scale [1]. Some partitioning mechanisms are promoted by morphological specializations of the species involved. For example, the evolution of different dentition and jaw morphologies in carnivore guilds promotes specialization of different prey resources [2,3], and adaptations of co-occurring species that restrict diurnal or nocturnal activity promote temporal partitioning (e.g. [4,5]). Additionally, within morphological or physiological constraints, partitioning regimes can also shift behaviourally based on environmental variation or changes in assemblages of competitor guilds or predator and prey species [6–9].
It is particularly important to understand the partitioning mechanisms that drive coexistence of predator populations; predators generally disproportionately affect ecosystems through top-down control and behaviourally mediated impacts on prey species [10,11], and the maintenance of predator populations is necessary for the preservation of healthy ecosystems [12]. Given the high rates of anthropogenically driven environmental change, it is also crucial to understand the current patterns and drivers of partitioning in predator guilds; shifts in temperature or weather patterns from climate change (e.g. [13,14]), destruction of habitats (e.g. [15]), the introduction of alien predators (e.g. [16,17]), overexploitation of prey resources (e.g. [18]) or depletion of predator populations (e.g. [19,20]) may drive predators to occupy or forage in new areas, potentially changing the assemblage of species co-occurring within a predator guild. Over the last decade, studies have started to unravel the partitioning mechanisms of some terrestrial predator guilds, such as the intact African large carnivore guild (e.g. [21,22]). However, the few studies that have examined the mechanisms allowing coexistence of large marine predator guilds (including in elasmobranchs and seabirds) have generally focused on resource-level partitioning (e.g. [23,24]), spatial partitioning (e.g. [24–27]) or seasonal partitioning where allopatric predators partition occupancy of an area on an annual basis (e.g. [28–30]). In particular, the occurrence of diel temporal partitioning of sympatric marine predators is poorly studied (although see [26]), probably due to the difficulty in determining diel foraging patterns in highly mobile underwater species.
The present study examined diel activity patterns and the potential for diel temporal niche partitioning in a guild of large coastal sharks. Activity patterns of six shark species in the Gulf of Mexico (Florida, USA) were determined using accelerometers deployed on free-ranging sharks. Because these six species seasonally co-occur (all present during winter months) [31] and show overlap in their prey as well as evidence of hierarchical predation within the guild (larger species are known to prey on some smaller species; [32,33] and references therein), we hypothesized that there would be a degree of diel temporal niche partitioning to limit interspecific competition and promote coexistence of all species.
2. Results
Sufficient accelerometer data (more than 5 individuals each tracked for more than 24 h) for analyses of activity patterns were obtained from six sympatric species of large coastal sharks: blacktip sharks (Carcharhinus limbatus), bull sharks (Carcharhinus leucas), sandbar sharks (Carcharhinus plumbeus), tiger sharks (Galeocerdo cuvier), great hammerhead sharks (Sphyrna mokarran) and scalloped hammerhead sharks (Sphyrna lewini) (table 1). In total, 3766 h of acceleration data from 172 individuals were used in our analyses (table 1). All species were caught in winter months (November–April), at water temperatures spanning approximately 16–26°C (table 1 and figure 1). Tiger sharks from a large size range were caught and tagged, but smaller animals (less than 150 cm total length, TL) showed different activity patterns compared to larger animals (greater than 150 cm TL). Few small tiger sharks were tagged (n = 3), and therefore robust analysis of activity patterns was not feasible for this group. As a result, small individuals were removed from analysis and activity patterns of only larger sharks (greater than 150 cm TL) were included in the analyses.
Table 1.
Tagging metadata for each shark species. The number and sizes of individuals of each species used in analyses, along with the total data volume used, the range of water temperatures recorded, the range of hourly mean overall dynamic body acceleration (ODBA) and the approximate timing of peak activity (greater than 80% of the difference between their minimum and maximum ODBA) identified by generalized additive mixed models. Note that only data from winter months when all species concurrently inhabit the study area were used (approx. 16–26°C, see data temperature range below), but most species were also present in the study area at warmer temperatures.
| species | N | hours of data | TL range (cm) | data temperature range used (°C) | ODBA range (g) | timing of peak activity |
|---|---|---|---|---|---|---|
| blacktip shark Carcharhinus limbatus | 21 | 500 | 126–186 | 18.8–27.2 | 0.02–0.19 | 18.00–21.00 |
| bull shark Carcharhinus leucas | 11 | 260 | 181–269 | 19.8–26.0 | 0.02–0.11 | 4.00–10.00 |
| sandbar shark Carcharhinus plumbeus | 71 | 1676 | 162–227 | 16.2–26.5 | 0.02–0.08 | 13.00–19.00 |
| tiger shark Galeocerdo cuvier | 39 | 827 | 154–264 | 16.2–23.5 | 0.02–0.10 | 9.00–15.00 |
| great hammerhead Sphyrna mokarran | 15 | 264 | 205–292 | 23.5–26.3 | 0.06–0.17 | 21.00–03.00 |
| scalloped hammerhead Sphyrna lewini | 15 | 239 | 154–224 | 21.2–26.4 | 0.05–0.18 | 22.00–04.00 |
Figure 1.

Study site and capture locations. Map of capture locations of large coastal sharks caught during winter months (November–April; water temperature approx. 16–26°C) in the current study. Capture locations for all individuals caught (tagged and untagged) are shown as an indication of their spatial distribution within the study area. (Online version in colour.)
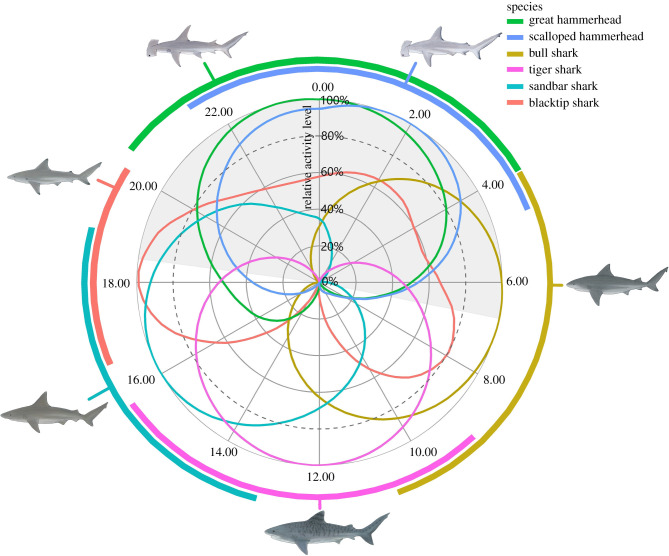
Generalized additive mixed models (GAMMs) were used to examine diel activity patterns. The top GAMM included time of day as a smoothed term, species as a factor within the smoother, and temperature and TL as fixed predictors (table 2). Temperature and TL influenced the intercept of the models, with higher overall dynamic body acceleration (ODBA) values generally observed at higher temperatures and in smaller sharks (see electronic supplementary material, figure S1). The exclusion of hour of deployment as either a fixed predictor or smoothed term in the model confirms that results were not affected by any extended recovery of animals throughout the deployment. Each of the six large coastal shark species showed a different diel activity pattern with a limited overlap of peak activity times (figure 2), suggesting that temporal partitioning is occurring. Bull sharks were most active in early morning hours, tiger sharks during midday, sandbar sharks during the afternoon, blacktip sharks during evening hours and both scalloped and great hammerhead sharks during night-time hours, the only two species with substantial overlap in timing of peak activity (figure 2 and table 1). The hourly mean acceleration values recorded for individuals of each species alongside the best-fit GAMM trend are plotted in electronic supplementary material, figure S1.
Table 2.
Generalized additive mixed model selection table. Model selection table for generalized additive mixed models (GAMMs) used to determine diel patterns of activity. The top five models (based on the corrected Akaike's information criterion; AICc) are shown, with the best-fit model in bold. A single model was used for all species, with species included as a factor (×) in the smoother (denoted by ‘s()’) with hour of day (HOD). Temp, temperature; TL, total length; DH, hour of deployment.
| model formula | AICc | ΔAICc | d.f. | log likelihood | R2 |
|---|---|---|---|---|---|
| ODBA ∼ s(HOD × species) + Temp + TL | −11143.9 | — | 22 | 5585.1 | 0.11 |
| ODBA ∼ s(HOD × species) + Temp + TL + DH | −11138.5 | 5.4 | 23 | 5583.4 | 0.12 |
| ODBA ∼ s(HOD × species) + Temp | −11134.7 | 9.2 | 21 | 5579.4 | 0.11 |
| ODBA ∼ s(HOD × species) + TL | −11134.4 | 9.5 | 21 | 5579.3 | 0.02 |
| ODBA ∼ s(HOD × species) + Temp + DH | −11133.1 | 10.8 | 22 | 5579.6 | 0.12 |
Figure 2.
Diel activity patterns of co-occurring shark species. Diel activity patterns of six species of co-occurring large coastal sharks found in the Eastern Gulf of Mexico, Florida, USA. The shaded region indicates the approximate night-time period. Because of different levels and degrees of change of overall dynamic body acceleration (ODBA) recorded for different species, activity patterns are plotted here as a percentage of the difference between the minimum (0%) and maximum (100%) ODBA level recorded for each species. The coloured bars in the outer circle show the time span of peak activity (greater than or equal to 80% of maximum activity) of each species, with this 80% threshold indicated with a dotted line on the figure. For individual species trends, error and hourly data, see electronic supplementary material, figure S1. (Online version in colour.)
3. Discussion
The minimal overlap in diel timing of peak activity in the six large coastal shark species examined (with the exception of the two hammerhead species) provides evidence for the occurrence of temporal partitioning. To our knowledge, these results are the first example of diel temporal partitioning in a marine predator guild. Such partitioning is likely to be driven by a combination of physiological and morphological constraints of each species and behavioural mechanisms, including a species's potential for behavioural plasticity.
The six species examined here have been concurrently captured in the present study and in past work (e.g. [31]). It is also of note that in past studies and in the present study spinner sharks (Carcharhinus brevipinna; similar trophic position to blacktip sharks) were commonly caught alongside the six species examined here and may play a role in temporal partitioning patterns, although we obtained insufficient accelerometer data from this species to be included in the analyses. Past studies have defined most of the species studied here as generalist teleost/elasmobranch predators with dietary overlap (e.g. [34–37]), suggesting that there is limited spatial or resource-level partitioning. Thus it is perhaps not surprising that temporal partitioning exists, although it has been found to be rare in predator guilds compared with spatial or resource partitioning [6,38]. There is also the potential that a degree of spatial or seasonal temporal partitioning exists within this guild. For example, within the study area, sandbar sharks were typically caught further offshore, and blacktip, great hammerhead and scalloped hammerhead sharks more often caught close to shore (figure 1); and tiger sharks were more commonly caught in the lower half of the study temperature range, while great hammerheads were more common in the upper half of the range (table 1). However, all six species were caught within the same longline set (i.e. within approx. 10 km of each other at the same time) at some point during the study. Furthermore, all species studied here range widely throughout the same general area, suggesting they display considerable spatial overlap. It is also notable that a degree of resource partitioning may also exist and may result directly from temporal partitioning, as the assemblage of available prey species probably varies throughout the diel cycle [39,40]. Diet analyses have not been conducted on the sub-adult/adult populations of these species found within the specific area of the study; however, past work in nearby locations has determined that all species here are generalist teleost, elasmobranch and cephalopod predators [32,33,41–43]. The only notable differences in diets between species in past studies are that marine reptiles, seabirds and invertebrates (incl. molluscs and bivalves), in addition to teleosts and elasmobranchs, may be important parts of the diet of tiger sharks [44].
There are two main factors identified as potential reasons that temporal partitioning is rare compared with resource or spatial partitioning. First, circadian rhythms are often assumed to be heavily evolutionarily constrained [6,45–47], limiting the potential for co-occurring species to change their activity patterns to promote diel partitioning. However, recent studies have shown that within the bounds of evolutionary adaptations to diurnal rhythmicity (e.g. eye types or neurological adaptations that promote vision in either dark or light) many animals, including sharks, have the capacity to behaviourally shift their diel activity patterns in reaction to shifts in environmental variables, prey assemblages or the introduction/removal of a predator or competitor [7,8,48–51]. Second, if diel rhythmicity is constrained, temporal partitioning may represent a greater drop in potential energy acquisition compared with other partitioning mechanisms; if animals are unable to shift their diel activity patterns, they would partition time instead by ceasing to forage during certain times of the day, trading potential energy acquisition for no energy acquisition [1,6]. Conversely, with spatial or resource partitioning, if animals shift from optimal foraging conditions to foraging in a suboptimal habitat or on a suboptimal prey source, they theoretically trade optimal energy intake for lower energy intake. Because of the relatively large drop in potential energy intake theorized from temporal partitioning, previous work has suggested that for this type of partitioning to occur either competition for resources must be severe, or interference competition is present (often assumed more likely), where hierarchical predation within the predator guild exists and predators partition time to avoid agonistic attacks [6,52,53]. Elasmobranchs (including large sharks) have been identified as a main prey source for great hammerhead (but not scalloped hammerhead) sharks [37,54], tiger sharks in Hawaii and South Africa [55,56], and bull sharks in South Africa [57]. Elasmobranchs (but mainly rays or small sharks) have also been identified as an infrequent prey source for sandbar sharks [36,58] and blacktip sharks [34]. Therefore, interference competition may be a significant driver of activity patterns and behaviour in less dominant shark species. This may be particularly relevant for blacktip sharks, the smallest species examined here and most likely to be predated upon by larger sharks. By reducing activity at times where higher-order predators are most active, competitors which are lower on the trophic scale (i.e. blacktip sharks) can decrease their probability of detection by a predator at those times [59,60]. It is also possible that refuging and foraging occur in slightly different microhabitats, and that temporal partitioning of foraging times results in spatial separation of lower trophic order competitors that are also prey sources for more dominant species.
The diel temporal partitioning observed here in large coastal sharks is likely to be the result of a combination of constraints imposed by morphological or physiological limitations as well as behavioural plasticity. The larger or more dominant predators, including tiger sharks, bull sharks and great hammerhead sharks, may be active and forage during the times of day that best suit them physiologically. For example, hammerhead sharks (most active here at night) are known to have superior binocular vision compared to carcharhinid species which may put them at an advantage in low light environments [61], while tiger sharks (most active here during midday) have been proposed to use visual silhouettes of prey on the surface as a main foraging mechanism [62,63], requiring higher light levels. Conversely, the less dominant species such as blacktip sharks may shift their diel foraging patterns in order to avoid interactions with larger species. This is supported by previous studies that show similar diurnal activity in tiger sharks and nocturnal activity in hammerhead sharks across different oceans and populations [31,49,64–67]. The maintenance of foraging rhythms across populations of hammerhead and tiger sharks experiencing different prey species, competitors and environmental conditions suggests that these foraging times are optimal for these species and are potentially highly regulated by conserved physiological mechanisms.
Conversely, diel rhythms of blacktip sharks (the lowest trophic level of sharks in the current study) have varied across populations, with previous work indicating that blacktip sharks forage at night [68,69] or during the morning [31], while here blacktip sharks were most active during the evening. When the same methods used in this study are applied to accelerometer data collected from blacktip sharks using the same area during summer months (n = 12) and in Florida Bay in October (n = 9), peaks of activity occur in the early morning and midday, respectively (N.M.W., K.O.L. & J.J.M. 2014–2017, unpublished data). This indicates a high degree of plasticity in diel rhythmicity in this species even over relatively small spatial or seasonal scales, most likely in response to shifting assemblages of predator, prey or competitor species. Such plasticity may be key in allowing this species to succeed in a variety of environments, and may provide a stronger buffer for deleterious trophic impacts stemming from environmental change compared to species with stricter diel patterns. Plastic diel rhythms may be especially important for mesopredator species in mid or low trophic levels, as these animals would be more likely to shift diel patterns as a result of interference competition. Whether diel foraging and activity patterns in specific species are driven more by morphological constraints or behavioural shifts requires further investigation, for example by examining diel activity patterns in assemblages of similar species across time and space.
4. Conclusions
Regardless of the relative contribution of morphological versus behavioural mechanisms in driving patterns of diel activity, this study has demonstrated the importance of diel temporal variability as a niche partitioning axis in a marine predator guild for the first time. Recent and historical work suggests that healthy marine ecosystems are almost always characterized by abundant and diverse predator populations, although such healthy or pristine systems are unfortunately becoming increasingly rare [70,71]. Considering that many species of sharks tend to share space with other species as both juveniles and adults, and often display an overlap in prey and hierarchical predation that would incite interference competition, diel temporal niche partitioning may be an important factor in allowing multiple shark species to coexist in a variety of ecosystems. Documenting the occurrence of temporal niche partitioning and determining the mechanistic drivers and interspecific relationships of such partitioning are essential to understanding the community ecology of marine ecosystems and to forecasting the direct and indirect repercussions of environmental change. This is particularly important considering that many predator and prey species in marine systems are exploited for human consumption, and rapid environmental change such as temperature shifts and habitat destruction may alter the assemblage of predators or competitors present. Understanding the mechanisms that allow marine predators to coexist will help to preserve and restore healthy, predator-rich marine systems.
5. Methods
(a) . Animal capture and tagging
Large coastal sharks were caught in the Gulf of Mexico in open coastal waters near Madeira Beach, FL, using bottom longlines. Longline sets were deployed between 2013 and 2017, using 18/0 circle hooks baited with local teleost species including bonito (Scombridae) and ladyfish (Elops saurus), and with soak times ranging from 2 to 15 h. Sharks were briefly restrained onboard the vessel and gills irrigated with seawater during the tagging process. Sharks were measured (total length, TL), sexed and tagged with an acceleration data logger (model G6A+, Cefas, Lowestoft, UK), which recorded triaxial acceleration at 25 Hz, depth at 1 Hz and water temperature at 0.03 Hz. These data loggers were built into positively buoyant tag packages (see [72]), which were secured to the first dorsal fin of sharks with an attachment incorporating a galvanic timed release, set to dissolve and break the attachment at a predetermined time. Once tagging was complete, sharks were released at the site of capture. The tagging and measurement process took approximately 3–6 min. The galvanic timed releases were set to release the tag package after approximately 1–3 days, allowing the tag package to float to the surface. Tag packages were relocated using VHF telemetry (M130b transmitter and R410 receiver with 3-element yagi antenna; Advanced Telemetry Systems, Isanti, MN, USA) and physically recovered from a vessel following methods described by Lear & Whitney [73].
(b) . Data processing and analysis
Once acceleration data logger packages were recovered, the data were downloaded and analysed using Igor Pro (v. 6.8, Wavemetrics, Lake Oswego, OR, USA) and R (v. 3.3.1, R Foundation for Statistical Computing, Vienna, Austria). Data from the first 12 h after tagging were excluded to prevent biasing analyses with behavioural effects of the capture and tagging process [74]. Static acceleration was separated from dynamic acceleration using a 3 s box smoother, which was sufficient to remove tailbeat signals from the static acceleration for all species. The activity was calculated as ODBA, the sum of the absolute value of the dynamic acceleration from all three axes [75], which has widely been used as a measurement of activity in fish and other taxa (e.g. [76–78]) as it reflects the total movement of the animal in three dimensions.
To determine diel patterns of activity, mean ODBA was calculated for each hour of the deployment for each individual. Subsequently, a GAMM was constructed using the ‘mgcv’ package [79] in R (v. 1.8–12; R Foundation for Statistical Computing, Vienna, Austria). This model predicted activity (as ODBA) by hour of the day as a cyclic smoothed term, with species included as an interaction within the smoother. It is well established that temperature influences activity levels in ectothermic animals [78,80], and that ODBA and tailbeat frequency scale with body size [75,81,82]. Therefore, the total length of sharks and water temperature were also included as fixed predictors of activity in the GAMM. Additionally, the hour of the deployment was included as either a fixed predictor (allowing a change in the magnitude of activity) or a smoothed term (allowing change in the pattern of activity) to ensure that no effects of tagging or extended recovery biased results. Inclusion of these fixed predictors and smoothed terms in the final model was tested using the Akaike information criterion corrected for small sample size (AICc), with fixed predictors maintained if their inclusion in the GAMM produced models with an AICc > 2 lower than models without the predictors included. The individual was included in all models as a random effect. Serial dependence of data was accounted for in the GAMMs by including an auto-regressive process of order 1 [83] using the CorAR1 function. Following the establishment of activity patterns, the most active period of the day for each species was identified as the time where activity was greater than or equal to 80% of the difference between the maximum and minimum activity levels observed for the species. This peak activity time was assumed to represent peak foraging time based on previous studies in similar animals (e.g. [59,84–88]).
Supplementary Material
Acknowledgements
We are grateful to many people who assisted with the collection of data for this study, including commercial fishing captains R. Lauser, L. Hill, D. Campo, and J. Bonnell, captains D. Dougherty, G. Byrd, C. Jelicks, P. Hull and R. Kane, and pilots F. Casey, G. Roam and B. Powell who assisted with tag recovery, and numerous Mote Marine Laboratory staff and volunteers who assisted with capture and tagging, including H. Marshall, A. Andres, A. Ontkos and R. Hueter.
Ethics
All work with animals was approved by Mote Marine Laboratory Institutional Animal Care and Use Committee (protocol no. 13-11-NW2).
Data accessibility
The processed hourly acceleration and related data for individuals used in this study are available in the electronic supplementary material [89].
Authors' contributions
K.O.L.: conceptualization, data curation, formal analysis, methodology, writing—original draft, writing—review and editing; N.M.W.: data curation, funding acquisition, writing—review and editing; J.J.M.: data curation, writing—review and editing; A.C.G.: conceptualization, formal analysis, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors declare no conflict of interests.
Funding
Funding for this study was provided by the National Oceanic and Atmospheric Administration Bycatch Reduction and Cooperative Research Programmes.
References
- 1.Schoener TW. 1974. Resource partitioning in ecological communities. Science 185, 27-39. ( 10.1126/science.185.4145.27) [DOI] [PubMed] [Google Scholar]
- 2.Van Valkenburgh B. 1989. Carnivore dental adaptations and diet: a study of trophic diversity within guilds. In Carnivore behavior, ecology, and evolution (ed. JL Gittleman), pp. 410-436. Berlin, Germany: Springer. [Google Scholar]
- 3.Meloro C, O'Higgins P. 2011. Ecological adaptations of mandibular form in fissiped Carnivora. J. Mamm. Evol. 18, 185-200. ( 10.1007/s10914-011-9156-z) [DOI] [Google Scholar]
- 4.Narendra A, Greiner B, Ribi WA, Zeil J. 2016. Light and dark adaptation mechanisms in the compound eyes of Myrmecia ants that occupy discrete temporal niches. J. Exp. Biol. 219, 2435-2442. ( 10.1242/jeb.142018) [DOI] [PubMed] [Google Scholar]
- 5.Bennie JJ, Duffy JP, Inger R, Gaston KJ. 2014. Biogeography of time partitioning in mammals. Proc. Natl Acad. Sci. USA 111, 13 727-13 732. ( 10.1073/pnas.1216063110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kronfeld-Schor N, Dayan T. 2003. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 153-181. ( 10.1146/annurev.ecolsys.34.011802.132435) [DOI] [Google Scholar]
- 7.McCauley DJ, Hoffmann E, Young HS, Micheli F. 2012. Night shift: expansion of temporal niche use following reductions in predator density. PLoS ONE 7, e38871. ( 10.1371/journal.pone.0038871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey S, Fisher JT, Burton AC, Volpe JP. 2017. Investigating animal activity patterns and temporal niche partitioning using camera-trap data: challenges and opportunities. Remote Sens. Ecol. Conserv. 3, 123-132. ( 10.1002/rse2.60) [DOI] [Google Scholar]
- 9.Monterroso P, Alves PC, Ferreras P. 2014. Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: implications for species coexistence. Behav. Ecol. Sociobiol. 68, 1403-1417. ( 10.1007/s00265-014-1748-1) [DOI] [Google Scholar]
- 10.Heithaus MR, Frid A, Wirsing AJ, Worm B. 2008. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202-210. ( 10.1016/j.tree.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 11.Williams TM, Estes JA, Doak DF, Springer AM. 2004. Killer appetites: assessing the role of predators in ecological communities. Ecology 85, 3373-3384. ( 10.1890/03-0696) [DOI] [Google Scholar]
- 12.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301-306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 13.Harley CDG. 2011. Climate change, keystone predation, and biodiversity loss. Science 334, 1124-1127. ( 10.1126/science.1210199) [DOI] [PubMed] [Google Scholar]
- 14.Hazen EL, et al. 2013. Predicted habitat shifts of Pacific top predators in a changing climate. Nat. Clim. Chang. 3, 234-238. ( 10.1038/nclimate1686) [DOI] [Google Scholar]
- 15.Thuiller W, Broennimann O, Hughes G, Alkemade JRM, Midgley GF, Corsi F. 2006. Vulnerability of African mammals to anthropogenic climate change under conservative land transformation assumptions. Glob. Change Biol. 12, 424-440. ( 10.1111/j.1365-2486.2006.01115.x) [DOI] [Google Scholar]
- 16.Griffen BD, Guy T, Buck JC. 2008. Inhibition between invasives: a newly introduced predator moderates the impacts of a previously established invasive predator. J. Anim. Ecol. 77, 32-40. ( 10.1111/j.1365-2656.2007.01304.x) [DOI] [PubMed] [Google Scholar]
- 17.Valverde MP, Sharpe DMT, Torchin ME, Buck DG, Chapman LJ. 2020. Trophic shifts in a native predator following the introduction of a top predator in a tropical lake. Biol. Invasions 22, 643-661. ( 10.1007/s10530-019-02119-1) [DOI] [Google Scholar]
- 18.Bearzi G, Politi E, Agazzi S, Azzellino A. 2006. Prey depletion caused by overfishing and the decline of marine megafauna in eastern Ionian Sea coastal waters (central Mediterranean). Biol. Conserv. 127, 373-382. ( 10.1016/j.biocon.2005.08.017) [DOI] [Google Scholar]
- 19.Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS. 2009. The rise of the mesopredator. Bioscience 59, 779-791. ( 10.1525/bio.2009.59.9.9) [DOI] [Google Scholar]
- 20.Ripple WJ, Wirsing AJ, Wilmers CC, Letnic M. 2013. Widespread mesopredator effects after wolf extirpation. Biol. Conserv. 160, 70-79. ( 10.1016/j.biocon.2012.12.033) [DOI] [Google Scholar]
- 21.Cozzi G, Broekhuis F, McNutt JW, Turnbull LA, Macdonald DW, Schmid B. 2012. Fear of the dark or dinner by moonlight? Reduced temporal partitioning among Africa's large carnivores. Ecology 93, 2590-2599. ( 10.1890/12-0017.1) [DOI] [PubMed] [Google Scholar]
- 22.Schuette P, Wagner AP, Wagner ME, Creel S. 2013. Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biol. Conserv. 158, 301-312. ( 10.1016/j.biocon.2012.08.008) [DOI] [Google Scholar]
- 23.Shipley ON, Gallagher AJ, Shiffman DS, Kaufman L, Hammerschlag N. 2019. Diverse resource-use strategies in a large-bodied marine predator guild: evidence from differential use of resource subsidies and intraspecific isotopic variation. Mar. Ecol. Prog. Ser. 623, 71-83. ( 10.3354/meps12982) [DOI] [Google Scholar]
- 24.Papastamatiou YP, Wetherbee BM, Lowe CG, Crow GL. 2006. Distribution and diet of four species of carcharhinid shark in the Hawaiian Islands: evidence for resource partitioning and competitivie exclusion. Mar. Ecol. Prog. Ser. 320, 239-251. ( 10.3354/meps320239) [DOI] [Google Scholar]
- 25.Humphries NE, Simpson SJ, Wearmouth VJ, Sims DW. 2016. Two's company, three's a crowd: fine-scale habitat partitioning by depth among sympatric species of marine mesopredator. Mar. Ecol. Prog. Ser. 561, 173-187. ( 10.3354/meps11937) [DOI] [Google Scholar]
- 26.Navarro J, Votier SC, Aguzzi J, Chiesa JJ, Forero MG, Phillips RA. 2013. Ecological segregation in space, time and trophic niche of sympatric planktivorous petrels. PLoS ONE 8, e62897. ( 10.1371/journal.pone.0062897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwyer RG, Campbell HA, Cramp RL, Burke CL, Micheli-Campbell MA, Pillans RD, Lyon BJ, Franklin CE. 2020. Niche partitioning between river shark species is driven by seasonal fluctuations in environmental salinity. Funct. Ecol. 34, 2170-2185. ( 10.1111/1365-2435.13626) [DOI] [Google Scholar]
- 28.Matich P, et al. 2017. Ecological niche partitioning within a large predator guild in a nutrient-limited estuary. Limnol. Oceanogr. 62, 934-953. ( 10.1002/lno.10477) [DOI] [Google Scholar]
- 29.Granroth-Wilding HMV, Phillips RA. 2019. Segregation in space and time explains the coexistence of two sympatric sub-Antarctic petrels. Ibis 161, 101-116. ( 10.1111/ibi.12584) [DOI] [Google Scholar]
- 30.Monteiro LR, Furness RW. 1998. Speciation through temporal segregation of Madeiran storm petrel (Oceanodroma castro) populations in the Azores? Phil. Trans. R. Soc. Lond. B 353, 945-953. ( 10.1098/rstb.1998.0259) [DOI] [Google Scholar]
- 31.Grace M, Henwood T. 1997. Assessment of the distribution and abundance of coastal sharks in the US Gulf of Mexico and eastern seaboard, 1995 and 1996. Mar. Fish Rev. 59, 23-32. [Google Scholar]
- 32.Clark E, von Schmidt K. 1965. Sharks of the central Gulf coast of Florida. Bull. Mar. Sci. 15, 13-83. [Google Scholar]
- 33.Castro JI. 2011. The sharks of North America. New York, NY: Oxford University Press. [Google Scholar]
- 34.Castro JI. 1996. Biology of the blacktip shark, Carcharhinus limbatus, off the southeastern United States. Bull. Mar. Sci. 59, 508-522. [Google Scholar]
- 35.Snelson FF, Mulligan TJ, Williams SE. 1984. Food habits, occurrence, and population structure of the bull shark, Carcharhinus leucas, in Florida coastal lagoons. Bull. Mar. Sci. 34, 71-80. [Google Scholar]
- 36.Ellis JK, Musick JA. 2007. Ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in lower Chesapeake Bay and Virginia (USA) coastal waters. Environ. Biol. Fishes 80, 51-67. ( 10.1007/s10641-006-9116-2) [DOI] [Google Scholar]
- 37.Gallagher AJ, Klimley AP. 2018. The biology and conservation status of the large hammerhead shark complex: the great, scalloped, and smooth hammerheads. Rev. Fish Biol. Fish. 28, 777-794. ( 10.1007/s11160-018-9530-5) [DOI] [Google Scholar]
- 38.Ross ST. 1986. Resource partitioning in fish assemblages: a review of field studies. Copeia 1986, 352-388. ( 10.2307/1444996) [DOI] [Google Scholar]
- 39.Castillo-Rivera M, Zárate-Hernández R, Ortiz-Burgos S, Zavala-Hurtado J. 2010. Diel and seasonal variability in the fish community structure of a mud-bottom estuarine habitat in the Gulf of Mexico. Mar. Ecol. 31, 633-642. ( 10.1111/j.1439-0485.2010.00394.x) [DOI] [Google Scholar]
- 40.Hagan SM, Able KW. 2008. Diel variation in the pelagic fish assemblage in a temperate estuary. Estuaries Coasts 31, 33-42. ( 10.1007/s12237-007-9018-3) [DOI] [Google Scholar]
- 41.Plumlee JD, Wells RJD. 2016. Feeding ecology of three coastal shark species in the northwest Gulf of Mexico. Mar. Ecol. Prog. Ser. 550, 163-174. ( 10.3354/meps11723) [DOI] [Google Scholar]
- 42.Dodrill JW. 1977. A hook and line survey of the sharks of Melbourne Beach, Brevard County, Florida. Melbourne, FL: Florida Institute of Technology. [Google Scholar]
- 43.Hoffmayer ER, Parsons GR. 2003. Food habits of three shark species from the Mississippi Sound in the northern Gulf of Mexico. Southeast. Nat. 2, 271-280. ( 10.1656/1528-7092(2003)002[0271:FHOTSS]2.0.CO;2) [DOI] [Google Scholar]
- 44.Aines AC, Carlson JK, Boustany A, Mathers A, Kohler NE. 2018. Feeding habits of the tiger shark, Galeocerdo cuvier, in the northwest Atlantic Ocean and Gulf of Mexico. Environ. Biol. Fishes 101, 403-415. ( 10.1007/s10641-017-0706-y) [DOI] [Google Scholar]
- 45.Daan S. 1981. Adaptive daily strategies in behavior. In Biological rhythms (ed. V Kumar), pp. 275-298. Berlin, Germany: Springer. [Google Scholar]
- 46.Kronfeld-Schor N, Dayan T. 2008. Activity patterns of rodents: the physiological ecology of biological rhythms. Biol. Rhythm. Res. 39, 193-211. ( 10.1080/09291010701683268) [DOI] [Google Scholar]
- 47.Kronfeld-Schor N, Dayan T, Elvert R, Haim A, Zisapel N, Heldmaier G. 2001. On the use of the time axis for ecological separation: diel rhythms as an evolutionary constraint. Am. Nat. 158, 451-457. ( 10.1086/321991) [DOI] [PubMed] [Google Scholar]
- 48.Tambling CJ, Minnie L, Meyer J, Freeman EW, Santymire RM, Adendorff J, Kerley GIH. 2015. Temporal shifts in activity of prey following large predator reintroductions. Behav. Ecol. Sociobiol. 69, 1153-1161. ( 10.1007/s00265-015-1929-6) [DOI] [Google Scholar]
- 49.Cunningham CX, Scoleri V, Johnson CN, Barmuta LA, Jones ME. 2019. Temporal partitioning of activity: rising and falling top-predator abundance triggers community-wide shifts in diel activity. Ecography 42, 2157-2168. ( 10.1111/ecog.04485) [DOI] [Google Scholar]
- 50.Payne NL, van der Meulen DE, Gannon R, Semmens JM, Suthers IM, Gray CA, Taylor MD. 2013. Rain reverses diel activity rhythms in an estuarine teleost. Proc. R. Soc. B 280, 20122363. ( 10.1098/rspb.2012.2363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenn MGP, Macdonald DW. 1995. Use of middens by red foxes: risk reverses rhythms of rats. J. Mammal. 76, 130-136. ( 10.2307/1382321) [DOI] [Google Scholar]
- 52.Amarasekare P. 2007. Trade-offs, temporal variation, and species coexistence in communities with intraguild predation. Ecology 88, 2720-2728. ( 10.1890/06-1515.1) [DOI] [PubMed] [Google Scholar]
- 53.Carothers JH, Jaksić FM. 1984. Time as a niche difference: the role of interference competition. Oikos 42, 403-406. ( 10.2307/3544413) [DOI] [Google Scholar]
- 54.Raoult V, Broadhurst MK, Peddemors VM, Williamson JE, Gaston TF. 2019. Resource use of great hammerhead sharks (Sphyrna mokarran) off eastern Australia. J. Fish Biol. 95, 1430-1440. ( 10.1111/jfb.14160) [DOI] [PubMed] [Google Scholar]
- 55.Lowe CG, Wetherbee BM, Crow GL, Tester AL. 1996. Ontogenetic dietary shifts and feeding behavior of the tiger shark, Galeocerdo cuvier, in Hawaiian waters. Environ. Biol. Fishes 47, 203-211. ( 10.1007/BF00005044) [DOI] [Google Scholar]
- 56.Dicken ML, Hussey NE, Christiansen HM, Smale MJ, Nkabi N, Cliff G, Wintner SP. 2017. Diet and trophic ecology of the tiger shark (Galeocerdo cuvier) from South African waters. PLoS ONE 12, e0177897. ( 10.1371/journal.pone.0177897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cliff G, Dudley SFJ. 1991. Sharks caught in the protective gill nets off Natal, South Africa. 4. The bull shark Carcharhinus leucas Valenciennes. S. Afr. J. Mar. Sci. 10, 253-270. ( 10.2989/02577619109504636) [DOI] [Google Scholar]
- 58.McElroy WD, Wetherbee BM, Mostello CS, Lowe CG, Crow GL, Wass RC. 2006. Food habits and ontogenetic changes in the diet of the sandbar shark, Carcharhinus plumbeus, in Hawaii. Environ. Biol. Fishes 76, 81-92. ( 10.1007/s10641-006-9010-y) [DOI] [Google Scholar]
- 59.Lima SL. 2002. Putting predators back into behavioral predator–prey interactions. Trends Ecol. Evol. 17, 70-75. ( 10.1016/S0169-5347(01)02393-X) [DOI] [Google Scholar]
- 60.Houston AI, McNamara JM. 2014. Foraging currencies, metabolism and behavioural routines. J. Anim. Ecol. 83, 30-40. ( 10.1111/1365-2656.12096) [DOI] [PubMed] [Google Scholar]
- 61.McComb DM, Tricas TC, Kajiura SM. 2009. Enhanced visual fields in hammerhead sharks. J. Exp. Biol. 212, 4010-4018. ( 10.1242/jeb.032615) [DOI] [PubMed] [Google Scholar]
- 62.Andrzejaczek S, Gleiss AC, Lear KO, Pattiaratchi C, Chapple TK, Meekan MG. 2020. Depth-dependent dive kinematics suggest cost-efficient foraging strategies by tiger sharks. R. Soc. Open Sci. 7, 200789. ( 10.1098/rsos.200789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heithaus M, Dill L, Marshall G, Buhleier B. 2002. Habitat use and foraging behavior of tiger sharks (Galeocerdo cuvier) in a seagrass ecosystem. Mar. Biol. 140, 237-248. ( 10.1007/s00227-001-0711-7) [DOI] [Google Scholar]
- 64.Hearn A, Ketchum J, Klimley AP, Espinoza E, Penaherrera C. 2010. Hotspots within hotspots? hammerhead shark movements around wolf island, Galapagos Marine Reserve. Mar. Biol. 157, 1899-1915. ( 10.1007/s00227-010-1460-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heithaus MR. 2001. The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environ. Biol. Fishes 61, 25-36. ( 10.1023/A:1011021210685) [DOI] [Google Scholar]
- 66.Klimley AP, Butler SB, Nelson DR, Stull AT. 1988. Diel movements of scalloped hammerhead sharks, Sphyrna lewini Griffith and Smith, to and from a seamount in the Gulf of California. J. Fish Biol 33, 751-761. ( 10.1111/j.1095-8649.1988.tb05520.x) [DOI] [Google Scholar]
- 67.Hounslow JL, Jewell OJD, Fossette S, Whiting S, Tucker AD, Richardson A, Edwards D, Gleiss AC. 2020. Animal-borne video from a sea turtle reveals novel anti-predator behaviors. Ecology 102, e03251. ( 10.1002/ecy.3251) [DOI] [PubMed] [Google Scholar]
- 68.Driggers WB III, Campbell MD, Hoffmayer ER, Ingram GW Jr. 2012. Feeding chronology of six species of carcharhinid sharks in the western North Atlantic Ocean as inferred from longline capture data. Mar. Ecol. Prog. Ser. 465, 185-192. ( 10.3354/meps09901) [DOI] [Google Scholar]
- 69.Legare B, Skomal G, DeAngelis B. 2018. Diel movements of the blacktip shark (Carcharhinus limbatus) in a Caribbean nursery. Environ. Biol. Fishes 101, 1011-1023. ( 10.1007/s10641-018-0755-x) [DOI] [Google Scholar]
- 70.MacNeil MA, et al. 2020. Global status and conservation potential of reef sharks. Nature 583, 801-806. ( 10.1038/s41586-020-2519-y) [DOI] [PubMed] [Google Scholar]
- 71.Pacoureau N, et al. 2021. Half a century of global decline in oceanic sharks and rays. Nature 589, 567-571. ( 10.1038/s41586-020-03173-9) [DOI] [PubMed] [Google Scholar]
- 72.Whitmore BM, White CF, Gleiss AC, Whitney NM. 2016. A float-release package for recovering data-loggers from wild sharks. J. Exp. Mar. Biol. Ecol. 475, 49-53. ( 10.1016/j.jembe.2015.11.002) [DOI] [Google Scholar]
- 73.Lear KO, Whitney NM. 2016. Bringing data to the surface: recovering data loggers for large sample sizes from marine vertebrates. Anim. Biotelementry 4, 12. ( 10.1186/s40317-016-0105-8) [DOI] [Google Scholar]
- 74.Whitney NM, Lear KO, Gleiss AC, Payne NL, White CR. 2018. Advances in the application of high-resolution biologgers to elasmobranch fishes. In Shark research: emerging technologies and applications to the field (eds Carrier JC, Heithaus MR, Simpfendorfer CA), pp. 45-69. Boca Raton, FL: CRC Press. [Google Scholar]
- 75.Wilson RP, White CR, Quintana F, Halsey LG, Liebsch N, Martin GR, Butler PJ. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J. Anim. Ecol. 75, 1081-1090. ( 10.1111/j.1365-2656.2006.01127.x) [DOI] [PubMed] [Google Scholar]
- 76.Wright S, Metcalfe JD, Hetherington S, Wilson R. 2014. Estimating activity-specific energy expenditure in a teleost fish, using accelerometer loggers. Mar. Ecol. Prog. Ser. 496, 19-32. ( 10.3354/meps10528) [DOI] [Google Scholar]
- 77.Payne NL, et al. 2016. Temperature dependence of fish performance in the wild: links with species biogeography and physiological thermal tolerance. Funct. Ecol. 30, 903-912. ( 10.1111/1365-2435.12618) [DOI] [Google Scholar]
- 78.Lear KO, Whitney NM, Morgan DL, Brewster LR, Whitty JM, Poulakis GR, Scharer RM, Guttridge TL, Gleiss AC. 2019. Thermal performance responses in free-ranging elasmobranchs depend on habitat use and body size. Oecologia 191, 829-842. ( 10.1007/s00442-019-04547-1) [DOI] [PubMed] [Google Scholar]
- 79.Wood S. 2019. Package ‘mgcv’. R package, version 1.8-31.
- 80.Angilletta MJ, Niewiarowski PH, Navas CA. 2002. The evolution of thermal physiology in ectotherms. J. Therm. Biol 27, 249-268. ( 10.1016/S0306-4565(01)00094-8) [DOI] [Google Scholar]
- 81.Whitney NM, Papastamatiou YP, Gleiss AC. 2012. Integrative multisensor tagging: emerging techniques to link elasmobranch behavior, physiology, and ecology. In Biology of sharks and their relatives (eds Carrier JC, Musick JA, Heithaus MR), pp. 265-289, 2nd edn. Boca Raton, FL: CRC Press. [Google Scholar]
- 82.Sato K, Mitani Y, Cameron MF, Siniff DB, Naito Y. 2003. Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J. Exp. Biol. 206, 1461-1470. ( 10.1242/jeb.00265) [DOI] [PubMed] [Google Scholar]
- 83.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. GLM and GAM for count data. In Mixed effects models and extensions in ecology with R (eds AF Zuur, EN Ieno, NJ Walker, AA Saveliev, GM Smith), pp. 209-243. Berlin, Germany: Springer. [Google Scholar]
- 84.Gleiss AC, Morgan DL, Whitty JM, Keleher JJ, Fossette S, Hays GC. 2017. Are vertical migrations driven by circadian behaviour? Decoupling of activity and depth use in a large riverine elasmobranch, the freshwater sawfish (Pristis pristis). Hydrobiologia 787, 181-191. ( 10.1007/s10750-016-2957-6) [DOI] [Google Scholar]
- 85.Gleiss AC, Wright S, Liebsch N, Wilson RP, Norman B. 2013. Contrasting diel patterns in vertical movement and locomotor activity of whale sharks and Ningaloo Reef. Mar. Biol. 160, 2981-2992. ( 10.1007/s00227-013-2288-3) [DOI] [Google Scholar]
- 86.Papastamatiou YP, Watanabe YY, Bradley D, Dee LE, Weng K, Lowe CG, Caselle JE. 2015. Drivers of daily routines in an ectothermic marine predator: hunt warm, rest warmer? PLoS ONE 10, e0127807. ( 10.1371/journal.pone.0127807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitney NM, Papastamatiou YP, Holland KN, Lowe CG. 2007. Use of an acceleration data logger to measure diel activity patterns in captive whitetip reef sharks, Triaenodon obesus. Aquat. Living Resour. 20, 299-305. ( 10.1051/alr:2008006) [DOI] [Google Scholar]
- 88.Byrnes EE, Daly R, Leos-Barajas V, Langrock R, Gleiss AC. 2021. Evaluating the constraints governing activity patterns of a coastal marine top predator. Mar. Biol. 168, 1-15. ( 10.1007/s00227-020-03803-w) [DOI] [Google Scholar]
- 89.Lear KO, Whitney NM, Morris JJ, Gleiss AC. 2021. Temporal niche partitioning as a novel mechanism promoting co-existence of sympatric predators in marine systems. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lear KO, Whitney NM, Morris JJ, Gleiss AC. 2021. Temporal niche partitioning as a novel mechanism promoting co-existence of sympatric predators in marine systems. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The processed hourly acceleration and related data for individuals used in this study are available in the electronic supplementary material [89].