Abstract
Adenomatoid tumor (AT) is an uncommon benign neoplasm of mesothelial origin, usually occurring in the female and male genital tracts. Extragenital localization such as the adrenal gland is extremely rare. Until now, only 39 cases of adrenal AT have been reported in the English literature. Here we report two novel cases of adrenal AT that occurred in male patients aged 30 and 31 years. The tumors were discovered incidentally by computed tomography (CT). Macroscopically, the tumors were unilateral and solid, and the greatest dimension of the tumors was 3.5 and 8.0 cm, respectively. Histologically, the tumors consisted of angiomatoid, cystic, and solid patterns and infiltrated the adrenal cortical or medullary tissue. The tumor cells had low nuclear/cytoplasmic ratio, with no pathological mitosis or nuclear pleomorphism. Thread-like bridging strands and signet-ring-like cells could be seen. Immunohistochemically, the tumor cells were positive for epithelial markers (AE1/AE3, CK7) and mesothelial markers (D2-40, calretinin, and WT-1). The Ki-67 index was approximately 1 and 2%, respectively. The differential diagnosis of adrenal AT includes a variety of benign and malignant tumors. The patients had neither local recurrence nor distant metastasis at 21 and 8 months after removal of the tumor. In the literature review, we comprehensively summarized the clinical, morphological, immunohistochemical, and prognostic features of adrenal AT. Adrenal ATs are morphologically and immunophenotypically identical to those that occur in the genital tracts. Combining the histology with immunohistochemical profiles is very supportive in reaching the diagnosis of this benign tumor, helping to avoid misdiagnosis and overtreatment.
Keywords: adenomatoid tumor, adrenal gland, clinicopathological features, differential diagnosis, case report
Introduction
Adenomatoid tumor (AT) is a benign neoplasm originating from mesothelial cells. It is commonly encountered in the genital tracts, especially the uterus and fallopian tube in females and paratesticular sites in males (1, 2). Extragenital localization such as the adrenal gland is extremely rare. Until now, only 39 cases of adrenal AT have been reported in the English literature (3–32). In this paper, we report two novel cases and review the literature to summarize the clinical presentation, histological features, immunophenotype, differential diagnosis, and prognostic features of adrenal AT in order to comprehend this rare tumor better and avoid misdiagnosis.
Materials and Methods
Case No. 1: A 30-year-old man admitted to the hospital complained of palpitation and dizziness for one year. The laboratory results, including routine blood examination, serum cortisol, and ketosteroid levels, were within the normal range. Computed tomography (CT) examination showed a relatively well-demarcated, oval mass measuring 34 × 24 mm in the right adrenal gland. A laparoscopic right adrenalectomy was performed. After surgery, the patient was discharged from the hospital and had an uneventful recovery. No recurrence or metastasis was observed during 21 months follow-up.
Case No. 2: A 31-year-old man presented to the hospital for a routine physical examination, and a mass lesion was incidentally detected by CT. He was asymptomatic, and CT demonstrated a left adrenal mass measuring 77 × 43 mm which was regarded as an adrenal adenoma. Preoperative laboratory examinations revealed that the urinary vanillylmandelic acid, serum cortisol, and ketosteroid levels were within normal limits. A laparoscopic left adrenalectomy was performed. The postsurgical recovery was also uneventful. The patient had no signs of recurrence or metastatic lesion after 8 months follow-up.
All the resected specimens were routinely fixed in 10% neutral buffered formalin and embedded in paraffin. Sections of 4 μm in thickness were stained with hematoxylin and eosin for histopathological examination. Immunohistochemical analyses were performed using the Ventana Ultra View Universal DAB Detection Kit, and primary antibodies were obtained from different companies (see Table 1 ). Clinical data were obtained, including patients’ gender, age, clinical symptom, tumor size, anatomic site of involvement, and follow-up. A review of the literature was also done via electronic searches through PubMed for articles that contained the following search terms, adenomatoid tumor and adrenal gland. Only those written in English were included.
Table 1.
Summary of primary antibodies.
| Antibody | Clone | Source | Dilution |
|---|---|---|---|
| AE1/AE3 | AE1/AE3 | Novocastra | 1:100 |
| CK7 | OV-TL12/30 | Maixin | 1:100 |
| Calretinin | 5A5 | Novocastra | 1:100 |
| D2-40 | D2-40 | Maixin | 1:100 |
| HBME-1 | HBME-1 | Maixin | 1:50 |
| WT-1 | MX012 | Maixin | 1:50 |
| HMB45 | HMB45 | Novocastra | 1:100 |
| Melan-A | A103 | Novocastra | 1:50 |
| Desmin | DE-R-11 | Novocastra | 1:100 |
| Actin | αsm-1 | Novocastra | 1:100 |
| S100 | 4C4.9 | Maixin | 1:200 |
| Ki-67 | MIB-1 | Maixin | 1:150 |
| Syn | 27G12 | Novocastra | 1:200 |
| CgA | MX018 | Maixin | 1:200 |
| CD31 | JC/70A | Maixin | 1:100 |
| CD34 | QBEnd/10 | Novocastra | 1:200 |
Results
Pathological Findings
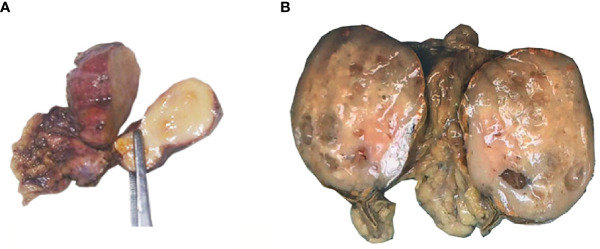
The excised adrenal tumor specimens measured 3.5 × 2.0 × 1.0 cm and 8.0 × 6.5 × 3.0 cm in size, respectively. The tumors were well circumscribed. On cut surface ( Figure 1 ), the tumors were solid, grayish yellow without hemorrhage or necrosis. Some tiny thin-walled cysts were identified in the bigger mass. Small remnants of normal adrenal tissues were perceived at the periphery in two cases.
Figure 1.
Gross examination of adrenal AT. (A) The tumor of case No. 1 measured 3.5 × 2.0 × 1.0 cm in size. Cut surface showed a solid, grayish yellow tumoral lesion. (B) The tumor of case No. 2 measured 8.0 × 6.5 × 3.0 cm in size. On cut surface, the tumor was solid, grayish yellow with some tiny thin-walled, translucent cysts. Small remnants of normal adrenal tissues were perceived at the periphery.
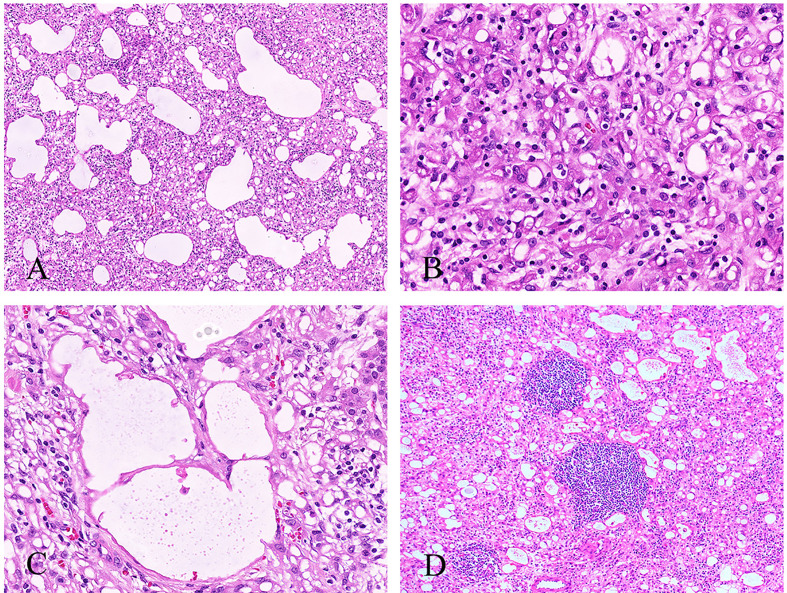
Histologically, the tumor cells infiltrated and compressed the normal adrenal cortical or medullary tissue, imparting an appearance of an infiltrative growth pattern. The invasion of the capsular or periadrenal adipose tissue was not detected. The tumors consisted of different patterns: angiomatoid, cystic, or solid. The angiomatoid pattern was composed of anastomosing glands or small to medium sized tubules lined by flattened or cuboidal cells with scant-to-moderate amounts of eosinophilic cytoplasm ( Figure 2A ). The cystic pattern was composed of large cysts lined by flattened or cuboidal cells. The solid area was covered with epithelial cells with abundant eosinophilic cytoplasm, and signet-ring-like cells could also be seen ( Figure 2B ). All the tumor cells had low nuclear/cytoplasmic ratio, with no appreciable mitotic activity or nuclear pleomorphism. Thread-like bridging strands were found in two cases ( Figure 2C ). No necrosis was observed. Lymphocytes infiltrated and aggregated in the stroma of two cases ( Figure 2D ).
Figure 2.
Histological features of the adrenal AT. (A) The angiomatoid pattern of tumor composed of anastomosing, variably sized tubules lined by flattened or cuboidal cells (100×). (B) The solid pattern of tumor composed of epithelioid cells with eosinophilic cytoplasm, and signet-ring-like cells can be seen (400×). (C) Thread-like bridging strands were found (400×). (D) Some lymphocytes were infiltrated and aggregated in the stroma (100×).
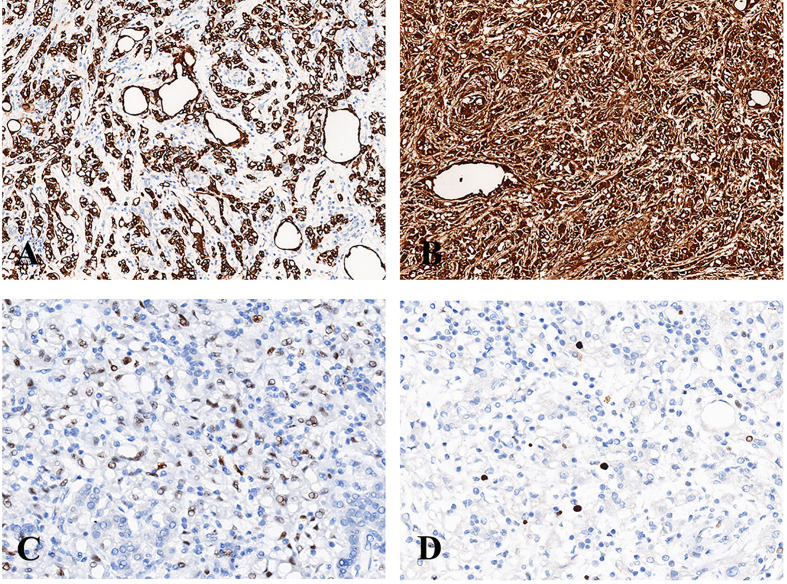
Immunohistochemically, the tumor cells of case No. 1 were positive for AE1/AE3, calretinin, D2-40, focal positive for HBME-1 and negative for HMB45, Melan-A, Desmin, Actin, and S100. The Ki-67 index was about 1%. Tumor cells of case No. 2 were positive for AE1/AE3 ( Figure 3A ), CK7, calretinin ( Figure 3B ), D2-40, focal positive for WT-1 ( Figure 3C ), and negative for HMB45, Melan-A, Desmin, S100, Syn, CgA, CD31, and CD34. The Ki-67 index was approximately 2% ( Figure 3D ).
Figure 3.
Immunohistochemistry of adrenal AT. (A) The tumor cells were diffusely positive for AE1/AE3 (200×). (B) The tumor cells were diffusely positive for calretinin (200×). (C) The tumor cells were focally positive for WT-1 (400×). (D) The Ki-67 index was approximately 2% (400×).
In summary, two cases were diagnosed as adenomatoid tumor of the adrenal gland definitely when the clinical findings were combined with morphology and immunohistochemical results.
Review of the Literature
By searching PubMed for adenomatoid tumor originating from the adrenal gland, we could find only 39 cases reported in the English medical literature to date. The clinicopathological features of this 39 previously reported adrenal ATs and our two cases are presented in Table 2 .
Table 2.
Clinicopathological features of adenomatoid tumors of the adrenal gland.
| NO. | Author and citation | Age-gender-side | Greatest dimension (cm) | Clinical Findings and symptoms | Gross | Histologic patterns | Extension | Lymphocytes infiltrate/aggregate | Signet-ring-like cells | Positive IHC staining | Negative IHC staining | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Evans et al. (3) | 36-M-L | 11.0 | IRF (painless gross hematuria) | Solid-cystic | Papillary, glandular, cystic | No | Not stated | Yes | Not stated | Not stated | 8 months |
| 2 | Travis et al. (4) | 24-M-L | 1.1 | IRF (Cushing syndrome) | Solid-cystic | Cystic | Cortex and periadrenal adipose tissue | Not stated | Not stated | AE1/AE3, vimentin, EMA (weakly positive) | Not stated | 6 months (died of pulmonary carcinoid) |
| 3 | Simpson (5) | 44-M-L | 3.2 | IRF (hypertension) | Solid-cystic | Adenoid, angiomatoid, cystic, solid | Cortex, medulla and periadrenal adipose | Yes | Yes | AE1/AE3, CAM 5.2, CK7, vimentin, CK5/6 (weak and focal) | CD15, CD31, CD34, CK20, MOC31, CEA-P | 177 months |
| 4 | Raaf et al. (6) | 49-M-R | 1.3 | IFA | Solid | Classic | No | Not available | Not available | MAK-6, AE1/AE3, vimentin | Not stated | Found at autopsy |
| 5 | Raaf et al. (6) | 57-M-L | 3.8 | IFA | Solid | Classic | No | Not available | Not available | MAK-6, AE1/AE3, vimentin | Not stated | Found at autopsy |
| 6 | Raaf et al. (6) | 50-F-R | 0.5 | IFA | Solid | Classic | No | Not available | Not available | Not stated | Not stated | Found at autopsy |
| 7 | Raaf et al. (6) | 40-M-L | 6.0 | IRF (CT scan during sarcoma staging) | Cystic | Cystic | No | Not available | Not available | MAK-6, AE1/AE3, vimentin | Not stated | Not stated |
| 8 | Angeles-Angeles et al. (7) | 34-M-R | 3.0 | IFA (AIDS) | Solid | Round, oval, irregular, or tubular spaces, papillary | Cortex/medulla | Not stated | Not stated | Low molecular weight cytokeratin CKAE-3, vimentin (weak reaction) | CD34, FVIII | Found at autopsy (died of acute bilateral pneumonia) |
| 9 | Gasque et al. (8) | 28-M-R | 9.0 | IRF (acute cholecystitis) | Solid-cystic | Adenoid, cystic | No | Yes | Not stated | CAM 5.2 | Neuroendocrine markers | 16 months |
| 10 | Glatz et al. (9) | 54-M-L | 6.5 | IRF (pneumonia) | Solid-cystic | Papillae, tubular spaces and gland-like, solid | Cortex | Yes | Yes | Cam5.2, Lu-5, calretinin thrombomodulin(weakly) | CEA, MOC-31, BerEP4, CD34 | Not stated |
| 11 | Isotalo et al. (10) | 37-M-L | 3.1 | IFS (rectal adenocarcinoma) | Solid-cystic | Adenoid, angiomatoid, cystic, solid | Cortex, medulla and periadrenal adipose | Yes | Yes | AE1/AE3, CAM 5.2, CK7, vimentin, CK5/6 (weak and focal) | CD15, CD31, CD34, CK20, MOC31, CEA-P | 40 months |
| 12 | Isotalo et al. (10) | 31-M-R | 3.2 | IRF (asymptomatic) | Solid | Adenoid, angiomatoid, solid | Cortex/medulla | Yes | Yes | AE1/AE3, CAM 5.2, CK7, vimentin, CK5/6 (weak and focal) | CD15, CD31, CD34, CK20, MOC31, CEA-P | Not stated |
| 13 | Isotalo et al. (10) | 31-M- Not stated | 3.5 | IRF (hypertension) | Solid | Adenoid, angiomatoid, solid | Cortex | Yes | Yes | AE1/AE3, CAM 5.2, CK7, vimentin, CK5/6 (weak and focal) | CD15, CD31, CD34, CK20, MOC31, CEA-P | 50 months |
| 14 | Isotalo et al. (10) | 64-M-L | 1.2 | IFA | Solid | Adenoid, angiomatoid, cystic | Cortex, medulla and periadrenal adipose | Yes | Yes | AE1/AE3, CAM 5.2, CK7, vimentin, CK5/6 (weak and focal) | CD15, CD31, CD34, CK20, MOC31, CEA-P | Found at autopsy |
| 15 | Chung-Park et al. (11) | 51-M-R | 3.0 | IRF (hypertension of primary aldosteronism) | Solid | Anastomosing glands and cleft-like spaces, multiple microcysts and canalicular spaces | Cortex | Yes | Yes | Cytokeratin, calretinin | Syn, CgA, factor VIII, CD34, S-100 | Not stated |
| 16 | Kim et al. (12) | 33-M-L | 1.7 | IRF (hypertension) | Solid | Variably sized cystic spaces | Adrenal parenchyma | Not stated | Yes | Cytokeratin, calretinin | CD34 | Not stated |
| 17 | Denicol et al. (13) | 42-M-L | 19.0 | IRF (renal lithiasis, hypertension) | Solid-cystic | Small tubules, cysts or string-shaped | No | Not stated | Not stated | AE1/AE3, vimentin | CEA, CD31 | 3 years |
| 18 | Garg et al. (14) | 46-M-L | Microscopical | IRF (central and right flank abdominal pain) | Cystic | Anastomosing glands and tubules | No | Yes | Yes | Calretinin, CK5/6 | HMB45, myeloperoxidase | Not stated |
| 19 | Garg et al. (14) | 33-M-L | 1.7 | IRF (hypertension) | Solid | Anastomosing glands and tubules | No | Yes | Yes | Calretinin, CK5/6 | HMB45, CD34, myeloperoxidase | Not stated |
| 20 | Garg et al. (14) | 33-M-R | 4.2 | IRF (asymptomatic) | Solid | Anastomosing glands and tubules | No | Yes | Yes | Calretinin | HMB45, CD34, myeloperoxidase | 1 year |
| 21 | Varkarakis et al. (15) | 54-M-R | 3.6 | IRF (renal lithiasis) | Solid | Sheets, tubules (with heterotopic ossification) | Cortex | Not stated | Not stated | Calretinin | Not stated | 1 year |
| 22 | Hamamatsu et al. (16) | 30-M-L | 3.0 | IFA | Solid | Anastomosing glands and various sized tubules | Cortex | Yes | No | Calretinin, D2-40, WT1, MC, CA125, vimentin, thrombomodulin, AE1/AE3, OV-TL 12/30, CAM5.2, MNF116 | CD31, CD34, factor VIII-related antigen, CD56 ER, AR | Found at autopsy (acute coronary thrombosis) |
| 23 | Fan et al. (17) | 42-M-L | 2.5 | IRF (hypertension, left renal cyst and nephrolithiasis) | Solid | Anastomosing, variably sized tubules, channels and small cystic | Cortex/medulla | Not stated | Yes | CK7, calretinin, EMA, antimesothelial cell antibody, vimentin | CEA, CD34, CD105, F8, VEGFR3 | Not stated |
| 24 | Timonera et al. (18) | 47-M-R | 7.0 | IRF (diverticulitis) | Solid | Anastomosing tubules and cystic spaces | Cortex | Yes | Not stated | D2-40, calretinin, CK5/6(weak reactivity) | Not stated | Not stated |
| 25 | Timonera et al. (18) | 52-M-R | 5.5 | IRF (hypertension) | Solid-cystic | Anastomosing tubules and cystic spaces | Cortex | Yes | Not stated | D2-40 and calretinin, CK5/6(weak reactivity) | Not stated | Not stated |
| 26 | Hoffmann et al. (19) | 26-M-R | 15.0 | IRF (asymptomatic) | Cystic | Glandular formations | No | Not stated | Not stated | Cytokeratin, calretinin | CD31, CD34, CD56 | Not stated |
| 27 | Bisceglia et al. (20) | 39-M-R | 5.5 | IRF (asymptomatic, cancer of the left colon 4 years ago) | Cystic | Variably sized, anastomosing tubules, channels, and small cystic spaces | Cortex/medulla | Yes | Yes | Pan keratins, CK5/6, calretinin | CD34, FVIII-RAg, CD31 | Not stated |
| 28 | Chaudhry et al. (21) | 60-M-R | 11.0 | IRF (hypertension, hyperlipidemia, and impaired fasting glucose) | Solid-cystic | Large cysts, fenestrated channels, and anastomosing tubules | No | Not stated | No | Calretinin, WT-1, pan-epithelial markers | Factor VIII, CD31, CD34 | Not stated |
| 29 | Białas et al. (22) | 29-M-R | 4.0 | IRF (asymptomatic) | Solid | Gland-like spaces and focal cystic dilation and solid nests | Cortex | Yes | Yes | AE1/AE3, CK7, calretinin, D2-40, vimentin, CK5/6(focal) | CD31, CD34, Factor VIII, CK20 | Not stated |
| 30 | El-Daly et al. (23) | 51-M-L | Not stated | IRF (asymptomatic) | Solid-cystic | Variably sized tubules and fenestrated channels | Cortex, capsule and periadrenal fat | Yes | Not stated | Calretinin, Cam5.2, CK7, vimentin, EMA (focally) | ER, CD31, CD34, Factor 8, CgA, Syn, S100, inhibin | Not stated |
| 31 | Liu et al. (24) | 44-M-L | 17.0 | IRF (asymptomatic) | Cystic | Glandular and nest-like formations | No | Not stated | Not stated | Calretinin, EMA | CD34, CD56, HMB45 | 3 months |
| 32 | Phitayakorn et al. (25) | 22-M-R | 2.5 | IRF (HIV infection) | Solid | Nests, cords, and tubules | enwrapped the ipsilateral renal artery and vein | Not stated | Yes | Calretinin, AE1/AE3, CAM 5.2 | CD31, CD34, Factor VIII | 7 months |
| 33 | Limbach et al. (26) | 24-M-L | 3.6 | IRF (asymptomatic, SDHD mutation) | Solid | Anastomosing tubules and channels | Cortex/medulla, adrenal capsule and periadrenal fat | Yes | Yes | AE1/AE3, calretinin, WT-1, S100(focally) | Syn, CgA, CD31, CD34 | 6 months |
| 34 | Li et al. (27) | 32-M-L | 4.0 | IRF (asymptomatic) | Solid | Cystic and sinusoid-like channels | Adjacent adrenal tissues | Yes | Yes | CK5/6, calretinin, D2-40, MC, vimentin, EMA (focally) | CgA, NSE, CD10 | 2.5 years |
| 35 | Zhao et al. (28) | 62-M-R | 3.0 | IRF (hypertension) | Solid-cystic | Tubules, fenestrated channels, and small cystic spaces | Cortex, adrenal capsule and periadrenal adipose tissue | Yes | Not stated | AE1/AE3, CK5/6, calretinin,vimentin | CD31, CD34, FVIII, CEA, SMA, HMB45, Melan-A, S100 | 8 months |
| 36 | Sağlıcan et al. (29) | 40-M-R | 5.5 | IRF (asymptomatic) | Solid-cystic | Papillary, microcysts and tubules | Cortex | Yes | Yes | AE1/AE3, calretinin | CD34, CD31 | 1 year |
| 37 | Krstevska et al. (30) | 30-F-R | 8.0 | IRF (asymptomatic) | Cystic | Cystic spaces | No | Yes | Not stated | Vimentin, S100, MCA mesothelial Ag, CD 69 | Actin, CK7, CD3 | 4 years |
| 38 | Jiang et al. (31) | 26-M-R | 4.0 | IRF (asymptomatic) | Solid | Variably sized tubules and fenestrated channels | No | Not stated | Yes | CK5/6, calretinin, WT-1, D2-40, vimentin | CD34, CgA, Syn, S100 | Not stated |
| 39 | Dietz et al. (32) | 28-M-R | 4.8 | IRF (chronic abdominal pain) | Solid | Tubular, pseudoangiomatous | No | Yes | Yes | CK7, D2-40, BAP1, calretinin | Not stated | Not stated |
| 40 | our case | 30-M-R | 3.5 | IRF (palpitation and dizziness) | Solid | angiomatoid, cystic and solid | Cortex/medulla | Yes | Yes | AE1/AE3, calretinin, D2-40, HBME-1 (focal) | HMB45, Melan-A, Desmin, Actin, S100 | 21 months |
| 41 | our case | 31-M-L | 8.0 | IRF (asymptomatic) | Solid | angiomatoid, cystic and solid | Cortex/medulla | Yes | Yes | AE1/AE3, CK7, calretinin, D2-40, WT-1 | HMB45, Melan-A, Desmin, S100, Syn, CgA, CD31, CD34 | 8 months |
M, male; F, female, R, right adrenal gland; L, left adrenal gland; IRF, incidental radiographic finding; IFA, incidental finding during autopsy; IFS, incidental finding during surgery for unrelated reasons; Syn, synaptophysin; CgA, chromogranin, CK5/6, cytokeratin 5/6.
Patient’s age at diagnosis ranged from 22 to 64 years (mean 39 years, median 37 years), with more than half of the patients younger than 40 years of age. There are 18 cases of adrenal AT located in the left adrenal gland and 22 cases located in the right adrenal gland, and the location of one case was unavailable. ATs primarily occurred in males, accounting for 39 of 41cases.
All tumors presented in patients as incidental radiological, surgical, or autopsy findings. The tumor in thirty-four patients was discovered incidentally during radiological examinations, six patients during autopsy and one patient during surgery for resection of rectal adenocarcinoma. Most of the patients were asymptomatic and physical examination was non-specific. Some patients presented with symptoms or comorbidities. Ten cases of adrenal AT were found to be associated with hypertension. Three patients had nephrolithiasis. Two patients had a history of chronic abdominal pain. One patient suffered from painless gross hematuria. One patient of our case complained of palpitation and dizziness. The tumors in other patients were discovered during the investigation in the course of Cushing syndrome, sarcoma staging, acute cholecystitis, pneumonia, diverticulitis, inguinal hernia, and acquired immune deficiency syndrome (AIDS). Six cases of adrenal AT were found during autopsy, and the cause of death of four patients was not available. One patient with HIV-1 infection developed acute bilateral pneumonia with severe respiratory failure and died. The immediate cause of death was disseminated coccidioidomycosis. Another patient died of acute coronary thrombosis resulting in heart failure after drinking alcohol.
Macroscopically, the tumors ranged from 0.5 to 19.0 cm (mean 5.3 cm, median 3.8 cm) in greatest dimension. The dimension of two cases was unavailable. Twenty-three cases were presented as a solid mass, whereas twelve cases have been described as mixed solid and cystic, and only six cases as entirely cystic.
Histologically, extension of tumor into cortex/medulla or periadrenal adipose tissue was observed in 58.5% (24/41) of cases and one tumor enwrapped the ipsilateral renal artery and vein. Most of tumors contained multiple histologic patterns, such as adenoidal, angiomatoid, cystic, papillary, and solid patterns. Adenoidal, angiomatoid or cystic patterns were common in AT. Only 11 cases of tumor had solid patterns, and four cases had papillary patterns. All the tumor cells had low nuclear/cytoplasmic ratio, with no pathological mitosis or nuclear pleomorphism. Signet-ring-like cells were described in 23 cases, and lymphocytes infiltration or aggregates were observed in 25 cases. Ultrastructural study was done in 10 cases, and all displayed numerous long, bushy, and slender microvilli that were typical of mesothelial-derived cells.
Immunohistochemically, the tumor cells were positive for epithelial markers, such as AE1/AE3, CAM5.2, and CK7, and mesothelial markers, such as calretinin, D2-40, and WT-1. However, the tumor cells were weakly or focally positive for CK5/6, which was also the marker of mesothelial cells. Negative staining was observed for CD34, CD31, HMB45, Melan-A, Actin, Desmin, Syn, and CgA.
No local recurrence or metastasis of adrenal AT has ever been reported in 18 patients who were followed up for 3–177 months, and one patient died of pulmonary carcinoid.
Discussion
Adenomatoid tumor (AT) of the adrenal gland is extremely rare. From 1988 to 2020, only 39 cases of adrenal AT were reported in the English medical literature, among which only four authors reported more than one case of adrenal AT (6, 10, 14, 18), and the rest was a single case report. Here we report two novel cases and review the literature to summarize the clinical, morphological, immunohistochemical, and prognostic features of adrenal AT more comprehensively than before.
Adrenal ATs tend to occur in adults in their fourth decade of life, but there is a wide age range. The tumor has a significant male predilection (male–female ratio, 39:2). A possible explanation related to the different roles of mesonephric ducts in males and females in embryological development together with the hypothesis that ATs arise from primitive mesenchymal cells associated with the Mullerian tract (33, 34). The mesonephric duct slowly transforms into a duct of epididymis and the paramesonephric ducts disappear in male, whereas the mesonephric ducts regress early during the embryogenesis in female (35). Therefore, it is conceivable that there is developing tissue that persists in males, so that this embryological difference between male and female systems may explain for the male predominance of adrenal AT. The ratio of adrenal AT located in the left and right adrenal gland was 18:22. It seems that the right adrenal gland was involved more frequently than the left adrenal gland which is contrary to the previous reports (11, 21). However, more cases are needed to draw a definite conclusion.
Usually, adrenal AT is non-functioning, asymptomatic and discovered incidentally during radiological examinations, surgical procedures for other unrelated reasons, or at autopsy. Radiological examinations are not specific for adrenal ATs. In rare occurrences, it is associated with symptoms or comorbidities, such as hypertension, AIDS, disseminated coccidioidomycosis, hematuria, Cushing syndrome, adrenal cysts, and kidney stones. About 24.4% (10/41) of adrenal AT was associated with hypertension. We speculate that adrenal AT may be associated with hypertension. However, the inherent relationship of hypertension and adrenal AT needs further investigation. Angeles-Angeles et al. (7) reported an autopsy case of an incidentally discovered AT arising in the right adrenal gland of a 34-year-old man with AIDS, and the immediate cause of death was disseminated coccidioidomycosis. Phitayakorn et al. (25) described another case of a 22-year-old man with AIDS who denied any symptoms and underwent a laparoscopic right adrenalectomy with no post-operative complications on follow-up examination seven months later. Patients with AIDS frequently present with a number of associated neoplasms, such as Kaposi’s sarcoma or lymphoma, often as a result of their immunosuppression. It is very unusual for patients with AIDS to present with adrenal gland neoplasms. One patient suffered from painless gross hematuria which was attributed to compression of the upper pole of the kidney by the adrenal AT (3). Fan et al. (17) reported a case of adrenal AT with a concurrent left kidney cyst and left nephrolithiasis in a man with moderate hypertension. Moreover, two cases of adrenal AT in a man who sought evaluation of renal colic or acute right flank pain due to nephrolithiasis were reported by Denicol et al. (13) and Varkarakis et al. (15), respectively. A few patients had a history of sarcoma (6), inguinal hernia (18), colon cancer (20), and mucoepidermoid carcinoma (11), and adrenal AT was incidentally discovered.
Macroscopically, the tumors are usually solid, but rarely, they present as solid-cystic or completely cystic tumors, which may probably be misdiagnosed as lymphangioma (10, 36). On the cut surface, the tumor is well circumscribed and grayish yellow. The average greatest dimension was 5.3 cm; only eight cases had reported with a greatest dimension of more than or equal to 8.0 cm, including one case reported by us.
Microscopically, the tumors may extend into the adrenal capsule, cortex, medulla, or extra-adrenal adipose tissue. We should note that this is not the diagnostic criterion of malignancy. The tumors have multiple patterns of growth, including adenoid, angiomatoid, cystic, solid, and papillary. About half of the cases showed two or more patterns (36). In our cases, the angiomatoid pattern is dominant, and solid or cystic areas can be found. Angiomatoid or cystic structures are often covered by flattened or cuboidal cells. In the solid structure, the tumor cells are epithelioid with eosinophilic cytoplasm, and signet-ring-like cells always can be seen. The papillary patterns are uncommon which are covered by flat or cuboidal cells (3, 7, 9, 29). There is no cellular atypia, pathological mitosis, or necrosis. An article proposed that thread-like bridging strands were a morphologic feature present in all adenomatoid tumors (37). Lymphocyte infiltration and aggregates can be found in most cases which is also the histological feature of adrenal ATs. Other histological characteristics have been noted including dystrophic calcifications (6, 10, 15–17, 19), intratumoral adipose tissue (14, 18, 22) and metaplastic ossification (15). Timonera et al. (18) reported two cases of composite AT and myelolipoma with unclear pathogenesis. In the non-neoplastic adrenal tissue, adrenocortical hyperplasia was found in two cases (4, 11), and cytomegalovirus (CMV) infections with classical viral inclusions were seen in a patient with AIDS (7).
Immunostaining of tumor cells is usually diffusely positive for epithelial markers (AE1/AE3, CAM5.2, CK7) (10, 36, 38) and is also positive for mesothelial markers, such as D2-40, calretinin, WT-1, and HBME-1. D2-40 and calretinin had been shown to be expressed in 100% of the cases (38–40), and the positive rate of WT-1 reached 95.5% (38). Therefore, calretinin, D2-40 and WT-1 are relatively specific markers of ATs. There is little research on molecular genetics of adrenal ATs. Only one reported adrenal AT case was found in a patient with a personal and family history of succinate dehydrogenase complex subunit D (SDHD) mutation (26). Further studies are required to explore the molecular genetics of ATs.
Because of the unusual location, lack of specific clinical and radiological features, the blurred boundary between the tumor and the adjacent tissue, existence of various growth patterns, adrenal ATs may lead to difficulties in diagnosis and need to be differentiated from other tumors. Differential diagnosis covers a variety of entities of benign and malignant tumors.
Benign tumors mainly include lymphangioma and angiomyolipoma. Lymphangioma is a benign hamartomatous tumor which is characterized by abnormal proliferation of lymphatic vessels that usually present as cystic masses, with hemangioma, cystic architecture, and lymphocyte aggregates similar to ATs, but lymphangioma shows absence of a solid pattern and is negative for epithelial markers, WT-1 and calretinin (41). Angiomyolipoma is a benign mesenchymal neoplasm composed of an admixture of thick dysmorphic blood vessels, smooth muscle, and adipose tissue. It is similar to the angiomatoid pattern of AT if the main component of angiomyolipoma is adipose tissue. However, tumor cells of angiomyolipoma are positive for HMB45, Melan-A, Actin, Desmin, and S100 (42).
The malignant tumors include epithelioid hemangioendothelioma, malignant mesothelioma (MM), and metastatic adenocarcinoma. Epithelioid hemangioendothelioma is a vascular neoplasm with cords or small nests of round endothelial cells with abundant eosinophilic cytoplasm (43). Tumor cells often have intracytoplasmic vacuoles representing small vascular lumina, like the signet-ring-like cells. Immunohistochemistry will be indicative of vascular origin which is positive for ERG, CD31, CD34, and Fli-1. MM is a malignant neoplasm of mesothelial origin. The immunohistochemistry of MM is the same as that of ATs. However, MM shows more atypia, easily visible mitosis, and higher proliferation index. P16/CDKN2A deletions are common genetic alterations in MM (44). Finally, the glandular-like pattern and signet-ring-like cells of ATs possibly raise suspicion of metastatic adenocarcinoma. However, the absence of significant atypia and the mesothelial immunoprofile of ATs help in avoiding misdiagnosis.
As for treatment, surgery is the main treatment, and postoperative adjuvant treatment is not required. Although only limited clinical follow-up data is available, no local recurrence or metastasis has been reported in adrenal ATs. In our two cases, patients have a good prognosis after surgery.
Conclusion
Adrenal AT is a benign neoplasm of mesothelial derivation. The tumors tend to occur in adults in their fourth decade of life and have a significant male predilection. Most of the tumors are asymptomatic, discovered incidentally by imaging during surgery or at autopsy. The tumors are well circumscribed and solid, with a grayish yellow cut surface. Histologically, the tumor may extend into the adrenal capsule, cortex, medulla, or extra-adrenal adipose tissue. There is a mixture of multiple patterns of growth, including adenoid, angiomatoid, cystic, solid, and papillary. No cellular atypia, pathological mitosis, or necrosis can be seen. Signet-ring-like cells and thread-like bridging strands can be found. Lymphocyte infiltration and aggregates also exist. The tumor cells are positive for epithelial markers (AE1/AE3, CK7, CAM5.2) and mesothelial markers (D2-40, calretinin and WT-1). The differential diagnosis includes lymphangioma, angiomyolipoma, epithelioid hemangioendothelioma, malignant mesothelioma, and metastatic adenocarcinoma. Complete excision is the main treatment. No recurrence or metastasis has been reported. Histology and immunohistochemical profiles are very supportive in reaching the diagnosis of adrenal ATs, helping to avoid misdiagnosis and overtreatment.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JG and CZ: conceived the idea of the work, contributed significantly to analysis and manuscript preparation, performed the data analyses, and wrote the manuscript. HL: contributed to the manuscript preparation and reference collection, performed the data analyses. WZ and WL: stained sections of 4 μm in thickness with hematoxylin and eosin, performed the experiment of immunohistochemical analyses. LT: provided revision opinions and revised the manuscript. JC: supervised the study, funding support, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province (2018A030313650), the Guangzhou Science and Technology Project (202102010156) and the NSFC cultivating grant of The Third Affiliated Hospital, Sun Yat-sen University (2020GZRPYMS01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Lyon: International Agency for Research on Cancer (IARC) (2017). [Google Scholar]
- 2. Lack EE. Tumors of the Adrenal Gland and Extra Adrenal Paraganglia – AFIP Atlas of Tumor Pathology. In: Silverberg SG, Sobin LH, editors. Washington: American Registry of Pathology, Armed Forces Institute of Pathology; (2007). [Google Scholar]
- 3. Evans CP, Vaccaro JA, Storrs BG, Christ PJ. Suprarenal Occurrence of an Adenomatoid Tumor. J Urol (1988) 139(2):348–9. 10.1016/S0022-5347(17)42410-4 [DOI] [PubMed] [Google Scholar]
- 4. Travis WD, Lack EE, Azumi N, Tsokos M, Norton J. Adenomatoid Tumor of the Adrenal Gland With Ultrastructural and Immunohistochemical Demonstration of a Mesothelial Origin. Arch Pathol Lab Med (1990) 114(7):722–4. [PubMed] [Google Scholar]
- 5. Simpson PR. Adenomatoid Tumor of the Adrenal Gland. Arch Pathol Lab Med (1990) 114(7):725–7. [PubMed] [Google Scholar]
- 6. Raaf HN, Grant LD, Santoscoy C, Levin HS, Abdul-Karim FW. Adenomatoid Tumor of the Adrenal Gland: a Report of Four New Cases and a Review of the Literature. Modern Pathol (1996) 9(11):1046–51. [PubMed] [Google Scholar]
- 7. Angeles-Angeles A, Reyes E. Munoz-Fernandez L and Angritt P. Adenomatoid Tumor of the Right Adrenal Gland in a Patient With AIDS. Endocr Pathol (1997) 8(1):59–64. 10.1007/BF02739708 [DOI] [PubMed] [Google Scholar]
- 8. Gasque CR, Martí-Bonmatí L, Dosdá R, Gonzalez Martinez A. MR Imaging of a Case of Adenomatoid Tumor of the Adrenal Gland – Case Report. Eur Radiol (1999) 9(3):552–4. 10.1007/s003300050708 [DOI] [PubMed] [Google Scholar]
- 9. Glatz K, Wegmann W. Papillary Adenomatoid Tumour of the Adrenal Gland. Histopathology (2000) 37(4):376–7. 10.1046/j.1365-2559.2000.00997.x [DOI] [PubMed] [Google Scholar]
- 10. Isotalo PA, Keeney GL, Sebo TJ, Riehle DL, Cheville JC. Adenomatoid Tumor of the Adrenal Gland. A Clinopathologic Study of Five Cases and Review of the Literature. Am J Surg Pathol (2003) 27(7):969–77. 10.1097/00000478-200307000-00012 [DOI] [PubMed] [Google Scholar]
- 11. Chung-Park M, Yang JT, McHenry CR, Khiyami A. Adenomatoid Tumor of the Adrenal Gland With Micronodular Adrenal Cortical Hyperplasia. Hum Pathol (2003) 34(8):818–21. 10.1016/s0046-8177(03)00243-0 [DOI] [PubMed] [Google Scholar]
- 12. Kim MJ, Ro JY. Pathologic Quiz Case: A 33-Year-Old Man With an Incidentally Found Left Adrenal Mass During Workup for Hypertension. Arch Pathol Lab Med (2003) 127(12):1633–4. 10.5858/2003-127-1633-PQCAYM [DOI] [PubMed] [Google Scholar]
- 13. Denicol NT, Lemos FR, Koff WJ. Adenomatoid Tumor of Supra-Renal Gland. Int Braz J Urol (2004) 30(4):313–5. 10.1590/S1677-55382004000400008 [DOI] [PubMed] [Google Scholar]
- 14. Garg K, Lee P, Ro JY, Qu Z, Troncoso P, Ayala AG. Adenomatoid Tumor of the Adrenal Gland: A Clinopathologic Study of 3 Cases. Ann Diag Pathol (2005) 9(1):11–5. 10.1053/j.anndiagpath.2004.10.003 [DOI] [PubMed] [Google Scholar]
- 15. Varkarakis IM, Mufarrij P, Studeman KD, Jarrett TW. Adenomatoid of the Adrenal Gland. Urology (2005) 65(1):175. 10.1016/j.urology.2004.08.026 [DOI] [PubMed] [Google Scholar]
- 16. Hamamatsu A, Arai T, Iwamoto M, Kato T, Sawabe M. Adenomatoid Tumor of the Adrenal Gland: Case Report With Immunohistochemical Study. Pathol Int (2005) 55(10):665–9. 10.1111/j.1440-1827.2005.01887.x [DOI] [PubMed] [Google Scholar]
- 17. Fan SQ, Jiang Y, Li D, Wei QY. Adenomatoid Tumour of the Left Adrenal Gland With Concurrent Left Nephrolithiasis and Left Kidney Cyst. Pathology (2005) 37(5):398–400. 10.1080/00313020500252721 [DOI] [PubMed] [Google Scholar]
- 18. Timonera ER, Paiva ME, Lopes JM, Eloy C, van der Kwast T, Asa SL. Composite Adenomatoid Tumor and Myelolipoma of Adrenal Gland: Report of 2 Cases. Arch Pathol Lab Med (2008) 132(2):265–7. 10.5858/2008-132-265-CATAMO [DOI] [PubMed] [Google Scholar]
- 19. Hoffmann M, Yedibela S, Dimmler A, Hohenberger W, Meyer T. Adenomatoid Tumor of the Adrenal Gland Mimicking an Echinococcus Cyst of the Liver – A Case Report. Int J Surg (2008) 6(6):485–7. 10.1016/j.ijsu.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 20. Bisceglia M, Carosi I, Scillitani A, Pasquinelli G. Cystic Lymphangioma-Like Adenomatoid Tumor of the Adrenal Gland: Case Presentation and Review of the Literature. Adv Anat Pathol (2009) 16(6):424–32. 10.1097/PAP.0b013e3181bb6c09 [DOI] [PubMed] [Google Scholar]
- 21. Chaudhry S, Lubitz S, Newman K, Pei ZH, Shepard T, Danoff A. Adenomatoid Tumor of the Adrenal Glandcase Report of a Rare Adrenal Lesion. Endocrinologist (2009) 19(5):233–6. 10.1097/TEN.0b013e3181b5a01f [DOI] [Google Scholar]
- 22. Białas M, Szczepański W, Szpor J, Okoń K, Kostecka-Matyja M, Hubalewska-Dydejczyk A, et al. Adenomatoid Tumour of the Adrenal Gland: A Case Report and Literature Review. Pol J Pathol (2010) 61(2):97–102. [PubMed] [Google Scholar]
- 23. El-Daly H, Rao P, Palazzo F, Gudi M. A Rare Entity of an Unusual Site: Adenomatoid Tumour of the Adrenal Gland: A Case Report and Review of the Literature. Patholog Res Int (2010) 15:702472. 10.4061/2010/702472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu YQ, Zhang HX, Wang GL, Ma LL, Huang Y. A Giant Cystic Adenomatoid Tumor of the Adrenal Gland: A Case Report. Chin Med J (Engl) (2010) 123(3):372–4. [PubMed] [Google Scholar]
- 25. Phitayakorn R, Maclennan G, Sadow P, Wilhelm S. Adrenal Adenomatoid Tumor in a Patient With Human Immunodeficiency Virus. Rare Tumors (2011) 3(2):e21. 10.4081/rt.2011.e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Limbach AL, Ni Y, Huang J, Eng C, Magi-Galluzzi C. Adenomatoid Tumour of the Adrenal Gland in a Patient With Germline SDHD Mutation: A Case Report and Review of the Literature. Pathology (2011) 43(5):495–8. 10.1097/PAT.0b013e3283486bb9 [DOI] [PubMed] [Google Scholar]
- 27. Li S, Wang X, Zhang S. Adenomatoid Tumor of Adrenal Gland: A Rare Case Report. Indian J Pathol Microbiol (2013) 56(3):319–21. 10.4103/0377-4929.120413 [DOI] [PubMed] [Google Scholar]
- 28. Zhao M, Li C, Zheng J, Yan M, Sun K and Wang Z. Cystic Lymphangioma-Like Adenomatoid Tumor of the Adrenal Gland: Report of a Rare Case and Review of the Literature. Int J Clin Exp Pathol (2013) 6(5):943–50. [PMC free article] [PubMed] [Google Scholar]
- 29. Sağlıcan Y, Kurtulmus N, Tunca F, Süleyman E. Mesothelial Derived Adenomatoid Tumour in a Location Devoid of Mesothelium: Adrenal Adenomatoid Tumour. BMJ Case Rep (2015) 2015:bcr2015211147. 10.1136/bcr-2015-211147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krstevska B, Mishevska SJ, Jovanovic R. Adenomatoid Tumor of the Adrenal Gland in Young Woman: From Clinical and Radiological to Pathological Study. Rare Tumors (2016) 8(4):6506. 10.4081/rt.2016.6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang G, Zhao Y, Fan C. An Adenomatoid Tumor of the Right Adrenal Gland: A Rare Case Report and Review of the Literature. Int J Clin Exp Med (2018) 11(2):1055–60. [Google Scholar]
- 32. Dietz M, Neyrand S, Dhomps A, Decaussin-Petrucci M, Tordo J. 18F-FDG PET/CT of a Rare Case of an Adenomatoid Tumor of the Adrenal Gland. Clin Nucl Med (2020) 45(7):e331–3. 10.1097/RLU.0000000000003097 [DOI] [PubMed] [Google Scholar]
- 33. Söderström KO. Origin of Adenomatoid Tumor. A Comparison Between the Structure of Adenomatoid Tumor and Epididymal Duct Cells. Cancer (1982) 49(11):2349–57. [DOI] [PubMed] [Google Scholar]
- 34. Stephenson TJ, Mills PM. Adenomatoid Tumors. An Immunohistochemical and Ultrastructural Appraisal of Their Histogenesis. J Pathol (1986) 148(4):327–35. 10.1002/path.1711480409 [DOI] [PubMed] [Google Scholar]
- 35. Moore KL, Persaud TVN. The Developing Human. Clinically Oriented Embryology. Philadelphia: Saunders; (2003). [Google Scholar]
- 36. Karpathiou G, Hiroshima K, Peoc’h M. Adenomatoid Tumor: A Review of Pathology With Focus on Unusual Presentations and Sites, Histogenesis, Differential Diagnosis, and Molecular and Clinical Aspects With a Historic Overview of Its Description. Adv Anat Pathol (2020) 27(6):394–407. 10.1097/PAP.0000000000000278 [DOI] [PubMed] [Google Scholar]
- 37. Hes O, Perez-Montiel DM, Alvarado Cabrero I, Zamecnik M, Podhola M, Sulc M, et al. Thread-Like Bridging Strands: A Morphologic Feature Present in All Adenomatoid Tumors. Ann Diagn Pathol (2003) 7(5):273–7. 10.1016/s1092-9134(03)00085-6 [DOI] [PubMed] [Google Scholar]
- 38. Sangoi AR, McKenney JK, Schwartz EJ, Rouse RV, Longacre TA. Adenomatoid Tumors of the Female and Male Genital Tracts: A Clinicopathological and Immunohistochemical Study of 44 Cases. Mod Pathol (2009) 22:1228–35. 10.1038/modpathol.2009.90 [DOI] [PubMed] [Google Scholar]
- 39. Kuroda N, Toi M, Hiroi M, Lee GH. Diagnostic Pitfall of D2-40 in Adenomatoid Tumour. Histopathology (2007) 51(5):719–21. 10.1111/j.1365-2559.2007.02839.x [DOI] [PubMed] [Google Scholar]
- 40. Ordóñez NG. D2-40 and Podoplanin are Highly Specific and Sensitive Immunohistochemical Markers of Epithelioid Malignant Mesothelioma. Hum Pathol (2005) 36(4):372–80. 10.1016/j.humpath.2005.01.019 [DOI] [PubMed] [Google Scholar]
- 41. Ellis CL, Banerjee P, Carney E, Sharma R, Netto GJ. Adrenal Lymphangioma: Clinicopathologic and Immunohistochemical Characteristics of a Rare Lesion. Hum Pathol (2011) 42(7):1013–8. 10.1016/j.humpath.2010.10.023 [DOI] [PubMed] [Google Scholar]
- 42. Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB, et al. Update on the Diagnosis and Management of Renal Angiomyolipoma. J Urol (2016) 195(4 Pt 1):834–46. 10.1016/j.juro.2015.07.126 [DOI] [PubMed] [Google Scholar]
- 43. Rosenberg A, Agulnik M. Epithelioid Hemangioendothelioma: Update on Diagnosis and Treatment. Curr Treat Options Oncol (2018) 19(4):19. 10.1007/s11864-018-0536-y [DOI] [PubMed] [Google Scholar]
- 44. Husain AN, Colby TV, Ordóñez NG, Allen TC, Attanoos RL, Beasley MB, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med (2018) 142(1):89–108. 10.5858/arpa.2017-0124-RA [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.